Abstract
BACKGROUND
Eukaryotic chromosomal ends are linear and are protected by nucleoprotein complexes known as telomeres. The complex structural anatomy and the diverse functions of telomeres as well as the unique reverse transcriptase enzyme, telomerase that maintains telomeres are under intensive scientific scrutiny. Both are involved in many human diseases including cancer, but also in ageing and chronic disease such as diabetes. Their intricate involvement in many cellular processes and pathways is being dynamically deciphered in many organs including the endometrium. This review summarizes our current knowledge on the topic of telomeres and telomerase and their potential role in providing plausible explanations for endometrial aberrations related to common gynaecological pathologies.
OBJECTIVE AND RATIONALE
This review outlines the recent major findings in telomere and telomerase functions in the context of endometrial biology. It highlights the contemporary discoveries in hormonal regulation, normal endometrial regeneration, stem cells and common gynaecological diseases such as endometriosis, infertility, recurrent reproductive failure and endometrial cancer (EC).
SEARCH METHODS
The authors carried out systematic PubMed (Medline) and Ovid searches using the key words: telomerase, telomeres, telomere length, human telomerase reverse transcriptase, telomeric RNA component, with endometrium, hormonal regulation, endometrial stem/progenitor cells, endometrial regeneration, endometriosis, recurrent miscarriage, infertility, endometrial hyperplasia, EC and uterine cancer. Publications used in this review date from 1995 until 31st June 2016.
OUTCOMES
The human endometrium is a unique somatic organ, which displays dynamic telomerase activity (TA) related to the menstrual cycle. Telomerase is implicated in almost all endometrial pathologies and appears to be crucial to endometrial stem cells. In particular, it is vital for normal endometrial regeneration, providing a distinct route to formulate possible curative, non-hormonal therapies to treat chronic endometrial conditions. Furthermore, our current understanding of telomere maintenance in EC is incomplete. Data derived from other malignancies on the role of telomerase in carcinogenesis cannot be extrapolated to EC because unlike in other cancers, TA is already present in proliferating healthy endometrial cells.
WIDER IMPLICATIONS
Since telomerase is pivotal to endometrial regeneration, further studies elucidating the role of telomeres, telomerase, their associated proteins and their regulation in normal endometrial regeneration as well as their role in endometrial pathologies are essential. This approach may allow future development of novel treatment strategies that are not only non-hormonal but also potentially curative.
Keywords: endometrium, telomerase, telomere, stem cells, endometriosis, endometrial cancer, infertility, recurrent miscarriage, progesterone, estrogen
Introduction
All eukaryotic chromosomal ends consist of specialized heterochromatin nucleoprotein complexes, termed telomeres, containing repeated nucleotide sequences ((TTAGGG)n) and associated specific proteins (Blackburn and Gall, 1978). The intact telomeres prevent the chromosomal ends from being recognized as DNA strand break and protects the loss of genomic DNA as well as end-to-end fusion and degradation of chromosomes. Telomeric DNA is lost with each round of DNA replication (Olovnikov, 1971; Watson, 1971; Lundblad, 2012) and shortening of telomeres beyond a critical length results in a permanent cell cycle arrest. This is due to initiation of sustained DNA damage signalling, resulting in activation of either senescence or apoptosis pathways (Blackburn and Gall, 1978; Blackburn et al., 2015). Telomere shortening and telomerase dysfunction are therefore implicated as universal features of cellular senescence and ageing as well as the age-related decrease in tissue regeneration and lifespan restriction in long lived mammals (Djojosubroto et al., 2003; Mikhelson and Gamaley, 2012).
The action of the reverse transcriptase enzyme telomerase is the main mechanism that counteracts telomere shortening in cells. Human cells such as embryonic stem cells, germ line cells and cancer cells with unlimited replicative capacity express high levels of telomerase activity (TA) which maintains and elongates telomeres, compensating for telomeric erosion (Counter et al., 1992; Yang and Huang, 2014; Meena et al., 2015). In contrast, adult stem/progenitor cells (SPCs) have the potential to up-regulate telomerase but these cells also undergo telomere shortening with age (Hiyama and Hiyama, 2007; Flores et al., 2008; Rane et al., 2016). Most human somatic cells do not express significant levels of TA (Opresko and Shay, 2016) and age-related telomere shortening is commonly described in many human proliferative tissues (Djojosubroto et al., 2003) while telomere shortening in post-mitotic tissues is negligible (Benetos et al., 2011). Therefore, most work on the functional relevance of telomerase is confined to the aforementioned specialized cells that express telomerase, such as cancer and stem cells.
The human endometrium is a unique organ in terms of regeneration and ageing. It is a dynamic somatic tissue that undergoes repetitive monthly cycles of growth, differentiation, shedding and regeneration throughout a woman's reproductive lifespan. These endometrial cycles of are regulated by ovarian steroid hormones (Hapangama et al., 2015). Every month, the endometrium grows from 1 mm in thickness, at the end of the menstrual shedding, to 15 mm in thickness, measured in the mid-secretory phase of the cycle (Dallenbach-Hellweg and Dallenbach, 2010), thus the endometrial regeneration capacity is unparalleled amongst other adult tissues. At the menopause, with the cessation of ovarian steroid hormone synthesis, the endometrium becomes proliferatively quiescent. However, a fully functional endometrium can be regenerated from the remaining thin postmenopausal endometrium with the provision of exogenous ovarian steroid hormones (Paulson et al., 2002). Thus, it is the only female reproductive organ not showing irreversible age-related changes. Although it is a somatic organ, endometrium expresses dynamic TA associated with the menstrual cycle. Therefore, the apparent endometrial age-defiance might include a physiological regulation of telomeres and telomerase distinct from other human tissues.
This review focuses particularly on recent findings in endometrial telomere and telomerase biology in the context of the inexhaustible proliferative and regenerative capacity of the human endometrium. This may provide an explanation for the seemingly eternal ‘youthfulness’ retained by the endometrium throughout a woman's life when compared with other female reproductive organs. Furthermore, there is mounting evidence that telomerase and telomere dysfunctions might play important roles in endometrial pathologies.
Method
We performed systematic PubMed (Medline) and Ovid searches using key words: telomerase, telomeres, telomere length (TL), telomerase reverse transcriptase (TERT), telomeric RNA component (TERC), with endometrium, endometrial SPCs, endometrial regeneration, endometriosis, recurrent miscarriage, infertility, endometrial hyperplasia (EH), endometrial cancer (EC) and uterine cancer. All studies investigating telomerase or telomere biology in endometrium in women or animals or respective cell lines, either primary cells or tissue explants in culture, and published from 1995 until 30th July 2016, were considered. Further manuscripts published before 1995 were also reviewed for specific topic areas and are included as appropriate.
Telomeres
Structure
The mammalian telomere complex consists of a tandemly repeated telomeric DNA sequence d(TTAGGG)n and its complementary strand. This is, followed by a short (35–600 nucleotide) single-stranded 3′ guanosine-rich protruding overhang; known as the G-tail (Royale 2006; Blackburn et al., 2015). Telomeres are associated with a complex of six well-described shelterin proteins: Telomeric Repeat Factor 1 and 2 (TRF1, TRF2), Repressor/Activator Protein I (RAP1, encoded by TERF2IP gene), TRF1-Interacting Nuclear Protein 2 (TIN2), Tripeptidyl Peptidase I (TPP1) and Protection of Telomeres I (POT1). TRF1 and TRF2 bind directly to the double-stranded telomeric sequence, and POT1 binds the single-stranded overhang; these proteins are therefore telomere DNA binding proteins. They interact with and bind to the remaining three shelterin proteins: TIN2 to TRF1, RAP1 to TRF2 and TPP1 to POT1.
The single-stranded overhang forms a D-loop (displacement) (Griffith et al., 1999) that prevents the access of telomerase outside of late S-phase when the overhang becomes accessible (Fig. 1A) (de Lange 2005; Palm and de Lange 2008; Shay 2016). In addition, the whole telomere forms a large duplex structure (T-loop) via the strand invasion from the 3′ single-stranded overhang (Griffith et al., 1999; Blackburn et al., 2000) providing proper telomere capping. The T-loop size is believed to be proportional to the length of the respective telomere (Cimino-Reale et al., 2001). Thus, in addition to telomere shortening, the dysfunction of telomere capping can also initiate a DNA damage response (DDR) (Griffith et al., 1999; Yoo and Chung 2011; Bodvarsdottir et al., 2012).
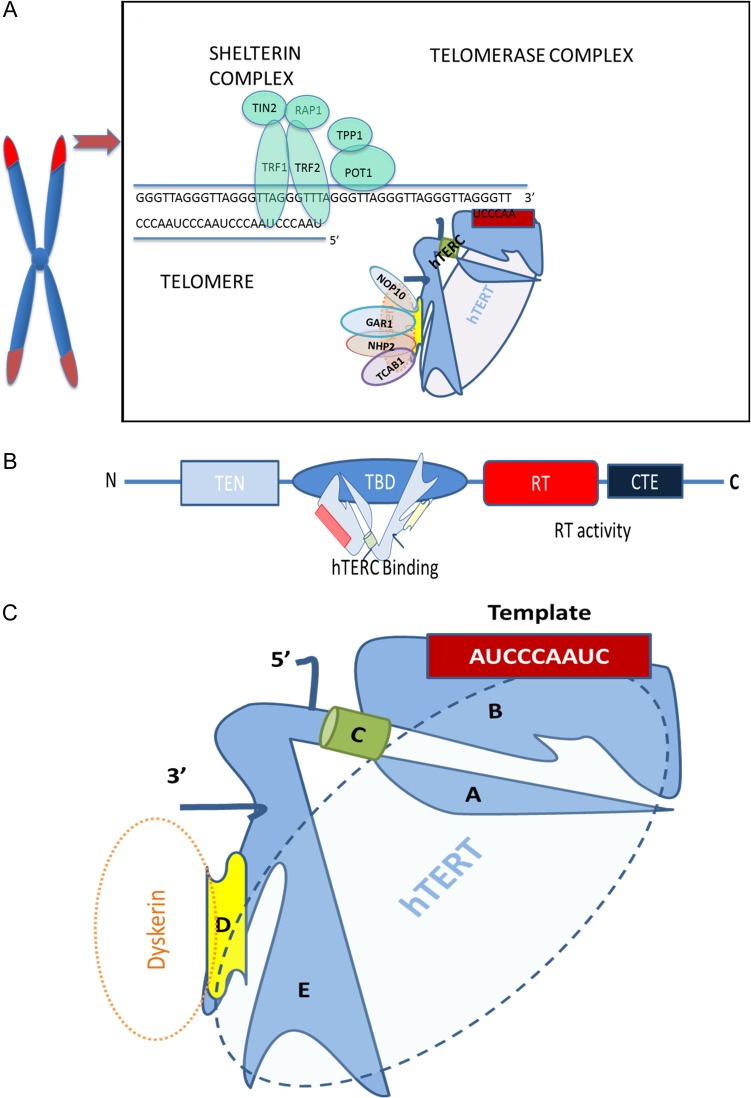
Figure 1.
Schematic illustration of the main telomerase subunits and their interaction with the telomere complex. (A) Illustration of the human telomere complex and telomerase (only one half of the dimeric holoenzyme complex is shown for clarity). Out of the shelterin proteins, telomere repeat binding factors 1 (TRF1) and 2 (TRF2) bind directly to the double-stranded telomeric sequence, and protection of telomeres protein-1 (POT1) binds to the single-stranded overhang; hence these are named telomere binding proteins and interact with remaining shelterin proteins TIN2 (binds to TRF1), RAP1 (binds to TRF2) and TPP1 (binds to POT1). Telomerase associated proteins NOP10, NHP2 and GAR1 form the H/ACA motif-of the human telomerase reverse transcriptase (hTERT) associated tetramer with dyskerin. (B) The four functional domains of hTERT: the telomerase N-terminal (TEN) domain has roles in recruiting telomerase to telomeres as well as telomeric repeat synthesis; telomeric RNA component (TERC)-binding domain (TBD) interacts with hTERC; and both the reverse transcriptase (RT) domain and C-terminal extension (CTE) contribute to the reverse transcriptase enzyme activity (Nakamura et al., 1997; Blackburn and Collins, 2011). (C) Diagram of the core elements of hTERC: 5′ region containing (A) the pseudoknot domain and (B) stem terminus element-loop that contains the 11 nucleotide RNA template and (C) the template boundary element (Theimer and Feigon, 2006). Both A and B domains are important for in vivo stability of hTERC and they interact with hTERT. The RNA stabilizing 3′ region contains (D) an H/ACA motif, which interacts with dyskerin or any of the other three H/ACA RNP components (NOP10, NHP2 and GAR1), and (E) trans-activating domain containing CR4/5 C that also binds hTERT (Webb and Zakian, 2016). The template boundary element together with the 3′ end prevents DNA synthesis beyond the template (Feng et al., 1995; Fu and Collins, 2003; Kiss et al., 2010).
The shelterin complex is ubiquitously expressed and remains associated with telomeres during the cell cycle (Royale 2006; Takai et al., 2011). In addition to the six shelterin proteins, there are various additional proteins (e.g. NBS1/MRE11/Rad50, tankyrase, PinX1, Ku) located at the telomere that are involved in DDR and repair processes but also have non-telomeric functions (Kuimov 2004; De Boeck et al., 2009).
Telomere shortening
Telomere shortening with age is a general observation in human proliferating tissues. In cell culture, with each cell division, about 20–50 base pairs (bp) of telomeric DNA is lost due to ‘the end replication problem’ (Olovnikov, 1973; Watson, 1972). This term describes the fact that DNA polymerases can only synthesize in 5′–3′ direction and thus can only synthesize the leading DNA strand un-interrupted. The lagging strand is synthesized by a series of Okazaki fragments which requires the help of short RNA primers and that are finally ligated together by ligases. However, at the very end of the lagging strand, the terminal RNA primer is removed resulting in the 3′ overhang and loss of the DNA in the next round of replication (Watson, 1972; Olovnikov, 1973; Blackburn, 1984; Lundblad, 2012; Mikhelson and Gamaley, 2012; Blackburn et al., 2015). In addition to the end replication, environmental conditions such as oxidative stress are an additional mechanism of telomere shortening (von Zglinicki et al., 1995; von Zglinicki, 2000). The progressive loss of mean TL is a hallmark of replicative senescence of proliferating cells while the amount of telomere shortening can vary in different tissues and organs during ageing and disease conditions depending on cell proliferation (Benetos et al., 2011; Benetos et al., 2011) and oxidative stress (von Zglinicki et al., 2000). Human mean TLs are 12–15 kb at birth and shorten down to a minimal TL of around 5 kb when a DDR and cell cycle arrest are signalled, which can lead to cellular senescence (Kipling and Cooke, 1990; Calado and Dumitriu, 2013). Shorter telomeres in lymphocytes have been associated with mortality, disease and poor-survival as well as reproductive ageing in humans (von Zglinicki et al., 2000; Cawthon et al., 2003; Shay, 2016). Thus, TL in human peripheral blood monocytes (PBMCs) has been proposed as a useful biomarker for human ageing and disease (von Zglinicki et al., 2000; von Zglinicki, 2002; Bekaert et al., 2005).
Although the mean TL of PMBCs had been employed in age determination in forensic medicine, the veracity of this approach is questionable due to the fact that TL is also inherited. In addition, more subtle methods for TL measurements considering initial TL as well as telomere shortening rates have been proposed (Benetos et al., 2011; Benetos et al., 2013). TL shortening starts during early gestation in many human tissue types such as heart, kidney and brain due to the down-regulation of TA (Ulaner and Giudice, 1997; Ulaner et al., 1998, 2001) and fast postnatal organ-specific growth accounts for most of the observed differential organ-specific telomere shortening rates (Carneiro et al., 2016).
Functions of telomeres
Prevention of recognition of linear chromosomal ends as double-stranded DNA breaks
The shelterin complex and the telomeric loop structure prevent telomeres from being identified as a DNA break that would signal a DDR. TRFs and POT1 prevent DDR activation due to the formation of the T-loop (Palm and de Lange 2008); TRF2 prevents end-to-end fusion (Cesare and Karlseder 2012) and POT1 helps to prevent the single-stranded telomeric 3′ end from being recognized by the DDR complex by forming a displacement (D-) loop with the remaining double strand (Jacob et al., 2007; Yang et al., 2008; Baumann and Price, 2010; Kibe et al., 2010).
Protection of chromosome ends from degradation and end-to-end fusion
Telomeres protect chromosomal ends from degradation by nucleases. Different DNA damage checkpoint proteins act together with EXO1 and MRE11 nucleases to inhibit proliferation of cells undergoing telomere attrition (Keijzers et al., 2016; Xue et al., 2016). Without the protective capping structure of telomeres, chromosomal ends would fuse together and form anaphase bridges duringmitosis leading to a fuse-breakage-fuse cycle. This process would greatly increase the risk of genomic instability and may result in tumourigenesis (Djojosubroto et al., 2003; Meena et al., 2015; Shay, 2016; Terali and Yilmazer, 2016).
Sentinels for DNA damage
Telomeres are more susceptible to DNA damage than genomic DNA (Petersen et al., 1998) due to their high guanine content (Henle et al., 1999; Wang et al., 2010) and lack of DNA repair mechanisms (Petersen et al., 1998). Telomere-associated DNA damage in the form of TIFs (telomere dysfunction-induced foci) or TAFs (telomere-associated foci) is hardly ever repaired (d'Adda di Fagagna et al., 2003; Takai et al., 2003; Takai et al., 2011; Fumagalli et al., 2012; Hewitt et al., 2012). This telomere-associated damage can have the same function as critically shortened telomeres in signalling cell cycle arrest. As the ‘first responders’ to hazards of genomic instability, the damaged telomeric DNA initiates a sustained DDR, resulting in a cell cycle arrest and inducing senescence or apoptosis, thereby protecting the organism from dangerous genetic aberrations and mutations (Blackburn et al., 2015; Shay 2016). Telomeres have thus been proposed to be sentinels for DNA damage (von Zglinicki 2002) and epigenetic sensors of general stress in DNA metabolism (Cesare and Karlseder 2012).
Recruitment of telomerase and regulation of its access to telomeres
Shelterin (Fig. 1) has a dual role in recruitment of telomerase and blocking its access to telomeres (Smogorzewska and de Lange 2004; Palm and de Lange 2008; Nandakumar and Cech 2013; Zhang et al., 2013; Schmidt and Cech 2015). POT1 prevents telomerase accessing an intact telomere complex but after hetero-dimerization with TPP1, it allows telomerase to become active at telomeres and to extend the 3′ overhang in late S-phase (Wang et al., 2007; Zhang et al., 2013; Chu et al., 2016).
Regulation of gene transcription/telomere position effect
Telomeres may also regulate gene transcription via a telomere position effect (TPE) (Robin et al., 2014), whereby genes located close to the telomeres are transcribed at a reduced rate. This allows changeable epigenetic transcriptional repression permitting genes the ability to switch their transcription rate. TPE has been reported to affect the expression of genes involved in stress, growth and recognition by the immune system in various invertebrate organisms and in cultured human cells (Robin et al., 2014). Recently it has also been connected to human diseases (Stadler et al., 2013). Further work examining the role of TPE in gene regulation in human tissues and during telomere shortening is needed to unravel its involvement in endometrial diseases.
Non-telomeric functions of telomere-associated proteins
Some shelterin proteins also have non-telomeric, genomic binding sites that allow extra-telomeric functions, such as regulating transcription of various genes. For example, RAP1 has been shown to regulate female obesity, a function unrelated to telomeres (Martinez et al., 2013). Cell type, subcellular localization and development stage specific pathways may regulate the shelterin complex. TIN2 has been found in mitochondria (Chen et al., 2012) and a reduction in TIN2 expression inhibits glycolysis and reactive oxygen species production and enhances ATP levels and oxygen consumption in cancer cells. This suggests a link between some shelterin proteins and metabolic control, providing an additional mechanism by which telomeric proteins might regulate the cellular processes beyond their function at telomeres. Additional non-telomeric functions in embryonic development have also been described for TIN2 (Chiang et al., 2004).
Telomere maintenance
Cells can maintain their telomeres via a telomerase dependent pathway or a telomerase independent alternative lengthening of telomeres (ALT) pathway (Brien et al., 1997; Bryan et al., 1997). Although activation of the latter pathway has been limited to particular types of cancers (sarcomas) and immortalized cell lines, there are suggestions that the ALT process may occur under physiological conditions in undifferentiated cells such as stem cells or even normal somatic cells (Neumann et al., 2013; Bojovic et al., 2015). There is a general consensus that in telomerase competent cells and in most normal cells, the ALT process is redundant and hence repressed (Henson et al., 2002). Therefore, in the context of the endometrium, ALT is less likely to be relevant and this review focuses mainly on telomerase dependent telomere maintenance.
Telomerase
Telomerase is a reverse transcriptase (RNA dependent DNA polymerase) that employs an integral RNA subunit harbouring a template sequence to add G-rich telomeric repeats to the 3′ single-stranded overhang of telomeres (Lingner et al., 1997). The telomerase holoenzyme has a dimeric structural configuration, where each half contains a human TERT (hTERT) and human TERC (hTERC) connected by a hinge region in the middle (Blackburn, 2011; Blackburn and Collins, 2011) (Figs 1B and C). The main components of the telomerase complex are hTERT, hTERC and dyskerin (DKC1) (Venteicher et al., 2009) (Fig. 1C). In addition, there are various telomerase associated proteins that interact with these core components (Fig. 1A). There are 184 telomeres and approximately 250 molecules of telomerase and in a cancer cell in late S-phase, when telomerase is actively recruited to telomere ends (Mozdy and Cech, 2006; Xi and Cech, 2014; Schmidt and Cech, 2015). When not active at telomeres, telomerase is localized to Cajal bodies in the nucleus for most of the cell cycle. After the telomerase/telomere interaction, every single telomerase activation event is thought to add 50–60 nucleotides to most telomeres in cancer cells with short TLs in vitro (Zhao et al., 2009; Schmidt and Cech 2015). Since the telomere lengthening action is limited to the nucleus, shuttling of the telomerase protein hTERT out of the nucleus prevents any telomeric extension. This shuttling is regulated by different domains on the hTERT protein; for example, a nuclear localization signal at amino acid residues 222–240 of hTERT (Chung et al., 2012) and a nuclear export signal (Seimiya et al., 2000) as well as a mitochondrial localization signal (Santos et al., 2004) have been described. Furthermore, recent data also suggest that the ability of telomerase in extending telomeres may be dependent on pH levels (Ge et al., 2016). Acidic pH (6.8) encourages preferential lengthening of short telomeres yet telomerase lengthens telomeres independent of their lengths at higher pH levels (7.2, 7.4).
Telomerase components
Telomerase reverse transcription activity has been demonstrated in an in vitro cell free system with just hTERT and hTERC (Weinrich et al., 1997). However, some compounds such as dyskerin actively associate with the telomerase complex in a cellular environment and are important for stability, maturation and function of the enzyme (Cohen et al., 2007).
Human telomerase reverse transcriptase
hTERT is the catalytic subunit of telomerase and is often the main rate-limiting factor for telomerase enzyme activity (Zhou et al., 2006; Zhang et al., 2013). The hTERT gene is located on chromosome 5p15.33 (Shay 2010) and consists of 16 exons and 15 introns spanning ∼35 kb (Cong et al., 1999). There are over 20 spliced variants of hTERT but only the wild type (full lengths protein, Fig. 1B) exhibits reverse transcriptase activity (Hrdlickova et al., 2012). The balance between the full lengths hTERT and its different splice variants has been shown to affect its function (Listerman et al., 2014; Radan et al., 2014). In addition to telomere maintenance, hTERT is implicated in increasing the anti-apoptotic capacity of cells, maintaining pluripotency of stem cells and regulating gene expression (for review see Saretzki, 2014).
Human telomeric RNA component
TERCs are species specific in size and sequence, but highly conserved in their structure and all contain a short sequence complementary to the telomeric TTAGGG hexanucleotide repeat sequence. Human TERC is relatively short (451 nucleotides (nt) compared with >1000 nt in yeast) (Theimer and Feigon 2006). The 3′ stabilizing element shares an H/ACA motif with small nucleolar and small Cajal body RNAs (snoRNA, scaRNA), and in turn associates with all four H/ACA RNP components, dyskerin, NOP10, NHP2 and GAR1 (Egan and Collins 2010) (Fig. 1A). hTERC is a non-coding RNA transcribed by RNA polymerase II (Gallardo and Chartrand 2008; Smekalova et al., 2012). It undergoes subsequent exonucleolytic cleavage up to the boundary formed by the H/ACA domain, meaning its co-transcriptional association with dyskerin is essential for stabilization, preventing further cleavage and nuclear retention (Feng et al., 1995; Fu and Collins 2003; Kiss, Fayet-Lebaron and Jady 2010). This H/ACA domain is mutated in dyskeratosis congenita, where the disease-associated hTERC variants impair hTERC accumulation. Disease-associated hTERC variants with sequence changes outside the H/ACA domain do not affect hTERC RNA processing or stability; they instead impose a catalytic defect (Fu and Collins 2003). The tetrameric complex of the accessory proteins dyskerin, NOP10, NHP2 and chaperone NAF1 (which later is replaced by GAR1) bind to hTERC and this association is crucial for normal TA.
Dyskerin
Dyskerin is an evolutionarily conserved 58 kDa, 514-amino-acid large protein (Knight et al., 1999). In humans, it is encoded by the DKC1 gene located on chromosome Xq28 (Cerrudo et al., 2015) and it is generally located in the nucleus. Dyskerin is an essential protein for cellular survival; thus DKC1 deletion is lethal (Rocchi et al., 2013; Angrisani et al., 2014). In the context of telomerase, dyskerin plays an established role in the maintenance of telomere integrity by stabilizing hTERC in the telomerase holoenzyme that is assembled in Cajal bodies (Cohen et al., 2007; Gallardo and Chartrand 2008; Gardano et al., 2012). Dyskerin is the only component to co-purify with active, endogenous human telomerase (Gardano et al., 2012). Loss of dyskerin binding leads to hTERC degradation and reduction in TA in vivo (Shukla et al., 2016). Furthermore, dyskerin has other non-telomerase associated functions essential to elementary cellular events such as mRNA translation, growth and proliferation. Dyskerin may regulate these functions via directing the isomerization of specific uridines to pseudouridines by acting as a catalytic pseudouridine synthase and by acting through the snoRNA-derived miRNA regulatory pathway, thus affecting different biological processes (reviewed in Angrisani et al., 2014).
Other accessory proteins of telomerase
Apart from NOP10, NHP2 and GAR1 which form the H/ACA motif-associated tetramer with dyskerin (Fig. 1), there are a plethora of other proteins (some listed in Table I) associated with telomerase with roles including: assembly, processing of telomerase, localization and accessibility to telomeres. In addition to these proteins, telomerase interacts with many others which are required for the formation of the appropriate structure and its stabilization, however their importance in TA is unknown (Smekalova et al., 2012). It is important also to appreciate the close relationship of telomerase with many cell cycle regulating, tumour suppressor, pluripotency and epithelial–mesenchymal transition (EMT) related proteins and pathways, such as Wnt/β-catenin, Cyclin D1, BCL-2, OCT-4, p53, EGFR, etc. (Ding et al., 2011; Listerman et al., 2014; Tang et al., 2016; Xue et al., 2016). Interestingly, recent data has suggested that the DDR kinases Ataxia Telangiectasia Mutated (ATM) and Ataxia Telangiectasia and Rad3-Related Protein (ATR) are required to recruit telomerase to telomeres via a TRF1 regulated pathway (Tong et al., 2015), while a central role of the ATM pathway in regulating telomere addition has been further highlighted (Lee, Bohrson, et al., 2015). In yeast, there exists a counting mechanism involving the shelterin RAP1, which prevents ATM accessing/activating telomerase on long telomeres thereby regulating TL (Yuan et al., 2013). However, whether a similar feedback mechanism exists in human cells is not yet known (Runge and Lustig, 2016). These findings are only beginning to unravel the intricate cellular pathways that are converging to regulate telomerase and telomere biology.
Table I.
Telomerase associated proteins.
| hTERT associated proteins | Function |
|---|---|
| P23, hsp90 | Assist in ribonucleoprotein (RNP) assembly (Elmore et al., 2008). |
| Protein 14-3-3 |
|
| DHX36 (DEAH-Box Helicase 36) Protein |
|
| Nuclear VCP-Like protein 2 (NVL2) |
|
| Pontin and reptin |
|
| hTERC associated proteins | Function |
| GAR1, NAP57, NOP10, NHP2 (members of H/ACA ribonucleoproteins complex) |
|
| Dyskerin (DKC1, a member of H/ACA ribonucleoproteins complex) |
|
| A1, UP1 | Help in accessibility of telomerase to telomeres (Fiset and Chabot, 2001; Nagata et al., 2008) |
| TEP1 (telomerase protein component 1) | Not essential for telomerase activity or telomere length maintenance in vivo, essential for telomere replication (Harrington et al., 1997) |
| La antigen | Direct and specific interaction between La and TERC influences telomere homeostasis (Aigner et al., 2003) |
| STAU (human homologue of staufen), RPL22 (Ribosomal Protein L22) | Assist in telomerase processing, localization and telomerase assembly (Le, Sternglanz and Greider, 2000) |
| TCAB1 (telomerase and Cajal body protein 1, encoded by WRAP53) | May license the catalytically active hTERT–hTERC holoenzyme for recruitment to telomeres (Venteicher et al., 2009) |
Functions of telomerase
Telomere maintenance
In eukaryotic cells, TA counteracts the end-replication problem and elongates the 3′ single strand in the absence of a DNA template. The subsequent replication of the complementary C-rich strand then will be possible by the conventional DNA replication in the next S-phase. The human telomerase complex consisting of hTERC and hTERT is targeted to telomeres specifically in the late S-phase of the cell cycle (Hug and Lingner 2006). Recent work has suggested that hTERT remains bound to hTERC for most of the cell cycle (Vogan and Collins 2015). The telomerase holoenzyme Cajal body-associated protein, TCAB1, is released from hTERC during cell cycle progression in mitotic cells coincident with TCAB1 delocalization from Cajal bodies (Vogan and Collins 2015). This observation proposes that TCAB1 and hTERC association may license the catalytically active hTERT–hTERC holoenzyme for recruitment to telomeres in the G1 phase of the cell cycle. TRF1 is the shelterin protein that is primarily responsible for regulation of an efficient replication of telomeric DNA (Sfeir et al., 2009). Apparently not all telomeres are required to be elongated by telomerase in each DNA replication round but there might be preferential lengthening of the shortest telomeres in telomerase active cells to ensure all the TLs remain above a critical length that would otherwise initiate activation of apoptotic and cell cycle arresting pathways (Fakhoury et al., 2010; D'Souza et al., 2013). The exact mechanism by which telomerase differentially extends telomeres with various lengths is still not well understood.
Non-telomeric functions of the telomerase component hTERT
Although telomere protection/lengthening is the most widely studied function of telomerase, there has been a considerable amount of evidence on non-telomeric functions of the protein subunit hTERT, such as promoting cellular proliferation/growth, survival, retaining an undifferentiated status, as well as increasing motility and metabolism (reviewed in Saretzki, 2014; Terali and Yilmazer, 2016). Recent evidence also suggests that hTERT binds to and stimulates ribosomal DNA transcription, particularly under hyper-proliferative conditions (Gonzalez et al., 2014). Protection of mitochondrial function under oxidative stress has been proposed as an important role of hTERT in various cell types, it was associated with reduction in oxidative stress and sensitivity to apoptosis as well as with a reduction in DNA damage (Ahmed et al., 2008; Singhapol et al., 2013). While initial studies on the beneficial role of mitochondrial hTERT were conducted mainly in vitro in cell culture, recent data describe beneficial effects also in vivo, for example, in vascular function (Beyer et al., 2016). Telomerase may interact with various well-established proliferative pathways including EGFR signalling (Smith et al., 2003), MYC and Wnt/β-catenin pathways (Park et al., 2009; Hoffmeyer et al., 2012). Telomerase has been shown to promote cell survival by blocking the death receptor (Dudognon et al., 2004) as well as by down regulating pro-apoptotic genes such as BAX and BCL-2 (Del Bufalo et al., 2005; Massard et al., 2006) in addition to suppressing mitochondrial and endoplasmic reticulum stress induced cell death (Zhou et al., 2006). Telomerase inhibition in stem cells has induced differentiation and loss of pluripotency genes, suggesting a role as a pluripotency gene in embryonic stem cells maintaining an undifferentiated status (Saretzki et al., 2008; Yang et al., 2008; Liu et al., 2013).
The endometrium
Human endometrium lines the uterine cavity and is organized into two functionally distinct layers: the superficial functionalis and deeper basalis (Valentijn et al., 2013); (Hapangama, Kamal and Bulmer 2015). The transient, exquisitely hormone responsive, functionalis exists only during the reproductive life of a woman, whereas the permanent, relatively hormonally unresponsive basalis layer persists throughout her whole life. The endometrial menstrual cycle is an exclusive phenomenon to upper order primates and is regulated by ovarian steroid hormonal signals (Hapangama et al., 2015; Kamal et al., 2016; Kamal et al., 2016) (Fig. 2A).
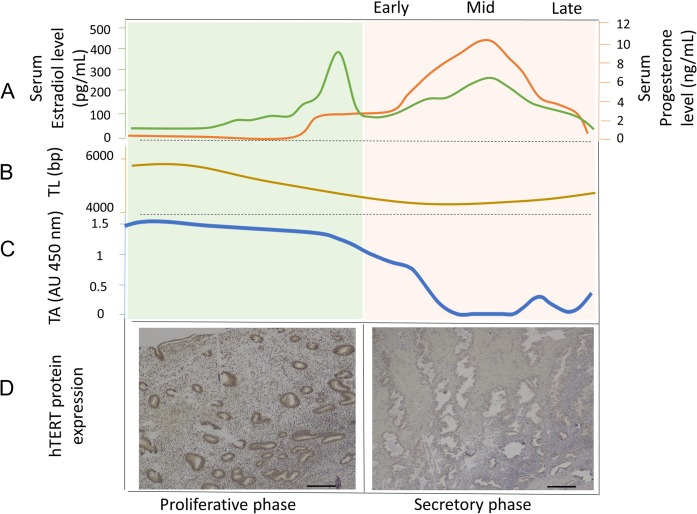
Figure 2.
Correlation of typical ovarian hormonal changes with the observed changes in endometrial telomerase activity (TA), mean telomere length (TL) and endometrial hTERT protein expression. (A) Estrogen (green line) and progesterone (orange line) (ovarian hormones) show typical cyclical variations during the menstrual cycle in premenopausal women. (B) Endometrial TA increases steadily under the influence of estrogen in the proliferative phase, whereas the levels plummet in the progesterone-dominant secretory phase of the cycle (Kyo et al., 1997; Saito et al., 1997; Williams et al., 2001; Hapangama, Turner, Drury, Quenby, et al., 2008; Hapangama et al., 2009). (C) Our recent work further demonstrates similar dynamic changes in the mean endometrial TL across the menstrual cycle (Valentijn et al., 2015). (D) In full thickness endometrial tissue sections, hTERT protein expression studied with immunohistochemistry employing a monoclonal mouse anti-human telomerase antibody (ab27573, Abcam, Cambridge UK), detection with ImmPRESS anti-mouse/rabbit polymer and visualization with ImmPACT DAB (Vector Laboratories, Peterborough, UK). Positive hTERT staining was observed in functionalis and basalis epithelial cells in the proliferative phase but the brown positive staining is limited to the basalis epithelium in the secretory phase. Magnification ×200, Scale bar 10 μm.
Endometrium is the primary target organ for ovarian steroid hormones and the endometrial cell cycle is intricately regulated by them. The reproductive lifespan of a woman is dictated by ovarian function. It commences with menarche and finishes with menopause. During that period, an average woman endures about 400 menstrual cycles in which the functionalis layer of the endometrium undergoes a well-defined cycle of proliferation, differentiation and menstrual shedding followed by regeneration. Ovarian steroid hormones regulate this endometrial cycle via their cognate receptors (reviewed in Hapangama et al., 2015; Kamal et al., 2016). It is generally accepted that estrogen is the trophic hormone of the endometrium, where it induces cellular growth and proliferation; while progesterone influences cellular differentiation and counteracts proliferation and other estrogenic effects (Hapangama, 2003). The third ovarian steroid hormone, androgen, is also postulated to impact the endometrial cycle, yet unlike the aforementioned hormones, the exact details of androgenic regulation of the endometrium are yet to be fully elucidated. The huge regenerative potential seen with the monthly endometrial cycle is unparalleled by other tissues, and the exact reason for this menstrual shedding, which is biologically a very expensive process, is yet unknown.
Telomerase and telomeres in endometrial tissue: functional relevance
TA is high in the premenopausal endometrial functionalis (Kyo et al., 1997) (Fig. 2C and D). Its dynamic changes regulated by the ovarian cycle are well established and correlate with glandular proliferation (Williams et al., 2001; Hapangama et al., 2008; Hapangama et al., 2009). Our recent work further demonstrates similar dynamic changes in the mean endometrial TLs across the menstrual cycle (Valentijn et al., 2015) (Fig. 2B). Once the ovarian hormone production has ceased, the relatively quiescent postmenopausal endometrium expresses low levels of TA (Brien et al., 1997; Tanaka et al., 1998).
When examining distinct cellular compartments within the endometrium, stromal cells, regardless of the cycle phase, maintain longer TLs compared with the epithelial cells. However, they demonstrate absent or significantly lower TA (Fig. 3A–C) and hTERC expression compared with the epithelial cells (Tanaka et al., 1998; Yokoyama et al., 1998; Valentijn et al., 2015), (Vidal et al., 2002). Data from a previous study which employed in situ assessment of endometrial TLs also demonstrated that glandular epithelium of the endometrial functionalis possesses the shortest TL (Cervello et al., 2012) (Fig. 3D). Furthermore, the proliferating endometrial epithelial cells have the highest TA that correlates negatively with TL (Valentijn et al., 2015) (Fig. 3B). This suggests that in the epithelial cells, the high TA preferentially preserves the short telomeres in order to avoid a critically short length. In rodents, estrogen increases the pH in the uterine fluid, while progesterone has the opposite effect (Chinigarzadeh et al., 2016). It can therefore be speculated that low pH in the proliferative phase may preferentially direct telomerase function to the short telomeres in endometrial epithelial cells. This is in accordance with the recent evidence regarding pH dependent telomerase function (Ge et al., 2016). Further studies would be required to fully investigate and confirm this possibility.
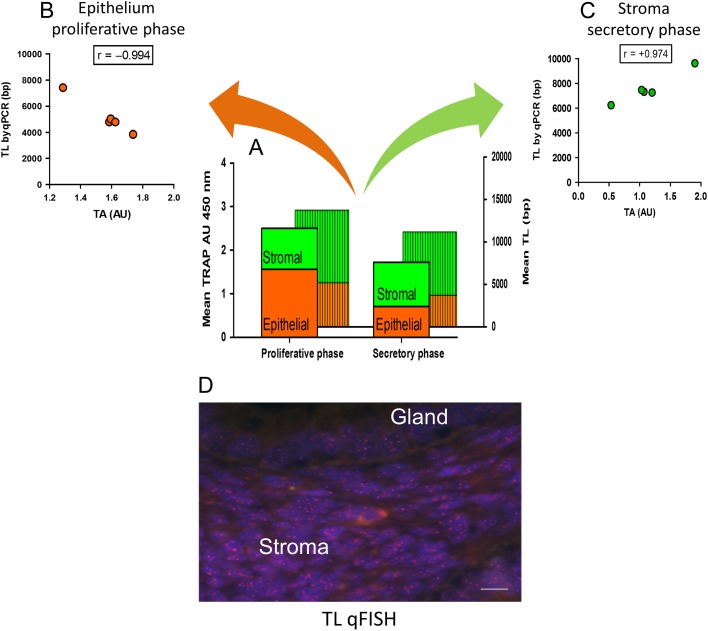
Figure 3.
TLs and TA in the human endometrium. (A) Endometrial TA (measured by TRAP assay) with endometrial TL (measured by qPCR) during the proliferative and the secretory phase of the cycle in healthy women with proven fertility (Valentijn et al., 2015). (B) TA correlated negatively with TL in isolated epithelial cells in the proliferative phase (n = 5, r = −0.994, ***P = 0.0005). (C) TA correlated positively with TL in endometrial stromal cells in the secretory phase (n = 5, r = +0.974, ***P = 0.0005); no correlation was seen between these parameters during the proliferative phase in the stroma or the secretory phase of the epithelium. Epithelia represent Epcam +ve epithelial fraction (positive selection) and stroma represents Epcam −ve stromal cell fraction from the dissociated endometrial biopsies. Single cell suspensions were purified using Epcam microbeads (negative selection) (Valentijn et al., 2015). (D) Telomeres identified in an endometrial sample during the proliferative phase by fluorescence in situ hybridization (FISH) using a peptide nucleic acid telomere probe (Panagene, Japan). Note the brighter (red) telomere signal in the stromal cells compared to the epithelial cells. Scale bar 50 µM.
Moreover, endometrial hTERT may have extra-telomeric functions. Direct in vitro inhibition of TA with the TERC inhibitor ‘imetelstat’ inhibited endometrial cell proliferation and disrupted gland formation by healthy epithelial cells (Valentijn et al., 2015). In contrast, overexpressing hTERT in endometrial stromal cells did not increase cell proliferation rate or hormone responsiveness (Barbier et al., 2005) similar to the other non-endometrial fibroblasts (Ahmed et al., 2008). Thus, there might be a specific co-regulation of TA and proliferative capacity limited to the endometrial epithelial cells or to epithelial cells in general. Other groups have found a correlation between TA in ovarian granulosa cells and their proliferation and differentiation status which is also under the control of growth factors and steroid hormones, similar to endometrial epithelium (Chronowska 2012). Importantly, although it has been possible to immortalize benign endometrial stromal cells by overexpressing hTERT (Krikun et al., 2006), immortalization of endometrial epithelial cells using a similar process has not been equally successful. This might be due to the fact that epithelial cells are likely to require an additional inhibition of the p16INK4a tumour suppressor in order to be immortalized by hTERT overexpression (Kiyono et al., 1998; Farwell et al., 2000; Shao et al., 2008; Novak et al., 2009).
The previously reported immortalization of benign human endometrial epithelial cells with hTERT overexpression (Kyo et al., 2003) has not been successfully replicated. This is an important fact, as the only other supposedly immortalized benign endometrial epithelial cell line that was generated by telomerase overexpression (Hombach-Klonisch et al., 2005) was later confirmed to be the misidentified breast cell line MCF-7 (Korch et al., 2012). This particular cell line was widely available to several groups leading to many publications (King et al., 2010). Unfortunately, the reportedly immortalized epithelial cell line generated by Kyo and colleagues (Kyo et al., 2003) has not undergone similar scrutiny and has not been available to other groups for further confirmatory studies for authenticity. In summary, we conclude that there are fundamental differences in telomerase function between endometrial epithelial and stromal cells. The mere presence of telomerase may result in a survival advantage for stroma, while epithelial cell proliferation may be regulated by telomerase. Additional factors than TA seem to be necessary for the long term survival and immortalization of epithelial cells in culture.
We are just beginning to understand the importance of extra-telomeric functions of telomere and telomerase components; for example, RAP1 has an extra-telomeric function on stromal cell decidualization in the rat endometrium (Kusama et al., 2014). Further examination of extra-telomeric functions of telomerase/telomeric proteins in the endometrium is warranted to further reveal their interplay with other cell cycle regulators specific to the endometrium.
Hormonal regulation of telomerase in epithelial cells
TA can be regulated at multiple levels, e.g. transcription, splicing, epigenetic and post-translational modification (reviewed in Fojtova and Fajkus, 2014; Lewis and Tollefsbol, 2016; Akincilar et al., 2016). Most human somatic tissue has absent or low levels of TA which is tightly regulated compared with the high and easily detectable levels seen in cancer cells and in germ line/stem cells. Thus, the initial studies on telomerase regulation were conducted in the context of developmental or stem cell biology or in cancer cells (reviewed in Batista, 2014; Huang et al., 2014; Gladych et al., 2011). However, in this review, we focus primarily on the hormonal regulation of telomerase at a normal, physiological level in the endometrium which is pivotal to its function.
Estrogen
Early work on the hormone responsive breast cancer cell line MCF-7 showed that estrogen up-regulates TA and hTERT gene expression via direct and indirect effects on the hTERT promoter (Kyo et al., 1999). Gel shift assays on MCF-7 cells further revealed that there is an imperfect palindromic estrogen-responsive element in the hTERT promoter that specifically binds to estrogen receptor (ER) and is responsible for transcriptional activation by ligand-activated ER (Kyo et al., 1999). Further confirmation of 17β-estradiol (E2) induced transcription of hTERT via ERα was also reported in various other cell types, including ovarian epithelial cells (Kimura et al., 2004; Gladych et al., 2011), ovarian stromal cells (Misiti et al., 2000), mesenchymal stem cells (MSCs) (Cha et al., 2008) and human umbilical vein endothelial cells (Hiyama and Hiyama 2007). Although ChIP assays in prostate cells suggested a recruitment of both ER subtypes to the hTERT promotor, its induction by ERβ in other cells remains controversial (Nanni et al., 2002).
There is some evidence that longer exposure to endogenous estrogen (length of reproductive years of life) might correlate with greater TLs and TA in PBMCs (Pines 2013). In other words, longer TLs seem to be present in different tissues and may be associated with longer reproductive life. E2 increased TA and TERT mRNA in heart, liver and brain tissue in an ovariectomized rat model (Cen et al., 2015). However, mature peripheral T cells do not respond to E2 with changes in expression or function of telomerase (Benko et al., 2012) suggesting that the effect of estrogen on telomerase is tissue/cell specific. Finally, the relative longevity of women compared with men has been speculated to be related to the effects of estrogen induced telomerase on telomere protection (Leri et al., 2000; Calado et al., 2009; Barrett and Richardson 2011; Gopalakrishnan et al., 2013; Cen et al., 2015). More active telomerase was found in cardiac myocytes from female rats which seems to correspond to higher myocyte numbers in older women compared to myocyte loss in older men (Leri et al., 2000). Others have suggested that reduction of oxidative stress by estrogens may result in longer telomeres in tissues such as brain and liver (Vina et al., 2005). Greater female longevity is suggested to possibly be connected to the female exposure to estrogens (Muezzinler et al., 2013). However, a recent longitudinal study reports a higher rate of PBMC TL attrition in the premenopausal period than in the postmenopausal period (Dalgard et al., 2015) with the authors proposing the opposite effect of estrogen on leucocyte turnover and menstrual bleeding. Thus, the influence of estrogen on TL and female longevity is still controversial.
There is in vivo and in vitro evidence suggesting that estrogens are able to induce TA and hTERT expression in the endometrium (Tanaka et al., 1998; Kyo et al., 1999; Vidal et al., 2002). In contrast, postmenopausal endometrium and endometrium treated with anti-estrogen drugs exhibited decreased TA (Tanaka et al., 1998). Furthermore, long term treatment with clinically relevant doses of conjugated E2 increased TERC expression preferentially in endometrial glands of ovariectomized female cynomolgus macaques (Macaca fascicularis) (Vidal et al., 2002). Increased TERC levels also correlate with higher proliferation and progesterone receptor expression in the endometrium of treated animals (Vidal et al., 2002). Both of these parameters are known to be regulated by estrogen in the endometrium (Hapangama et al., 2015). In the ER positive, hormone responsive endometrial epithelial adenocarcinoma cell line (Ishikawa cells), E2 induced TA and hTERT mRNA levels via a MAPK dependent pathway in an ERα dependent fashion (Zhou et al., 2006). In contrast, isolated primary epithelial cells (Tanaka et al., 1998) or intact endometrium in short-term explant culture did not show a significant response to E2 on TA (Valentijn et al., 2015). Conversely, co-cultured primary endometrial epithelial and stromal cells responded to E2 or a mitogenic fibroblast growth factor stimulus, suggesting that the E2 effect on telomerase induction may be enhanced or mediated by stroma and/or the duration of E2 treatment (Oshita et al., 2004).
Progesterone
Although progesterone has diverse effects on hTERT mRNA expression in progesterone receptor (PR) expressing breast and EC cell lines, the mechanisms by which hTERT expression is regulated by progesterone appear to be complex. The hTERT promoter lacks a canonical progesterone-responsive element (Wang et al., 2000), therefore classical PR mediated direct effects are less likely. The role of recently described progesterone receptor membrane components 1 and 2 on telomerase regulation has not yet been demonstrated (Bunch et al., 2014). In a breast cancer cell line, synthetic progestogen, medroxyprogesterone acetate inhibited hTERT mRNA transcription even in the presence of estrogen (Wang et al., 2000; Lebeau et al., 2002) and arrested cells in the late G1 phase (Lebeau et al., 2002) with the induction of p21 (Lange et al., 1999; Wang et al., 2000). There is also evidence for cell cycle-dependent regulation of telomere synthesis and telomerase gene expression in healthy hormone responsive human cells by progesterone (Wang et al., 2000; Tomlinson et al., 2006).
Since endometrial TA, hTERT mRNA/protein and hTERC levels reach their nadir during the progesterone-dominant mid-secretory phase, progesterone is thought to negatively regulate endometrial telomerase (Williams et al., 2001; Hapangama et al., 2008; Hapangama et al., 2009; Valentijn et al., 2015). The shortest endometrial TLs were also measured in the mid-secretory phase, indicating a telomere lengthening/maintenance function for endometrial TA (Valentijn et al., 2015). Exogenous progestogen administration is known to inhibit endometrial epithelial proliferation (Kurita et al., 1998; Shimizu et al., 2010) and we have recently shown this progestogen-induced decreased endometrial cell proliferation to be associated with a significant decrease in TA (Valentijn et al., 2015). Interestingly, the inhibition of TA in the progesterone-dominant secretory phase is associated with an induction of endometrial p21 and corresponds to a non-DNA damage induced cell cycle arrest function of p21 (Toki et al., 1998; Yoshimura et al., 2007). We can speculate that progesterone induced telomerase suppression might result in short endometrial epithelial telomeres (Hapangama et al., 2009; Valentijn et al., 2015) and perhaps influence changes in the endometrial epithelial cell cycle via p21 induction (Aix et al., 2016). Taken together, the hTERT gene may be a target of progesterone and the well-established progesterone induced down-regulation of the endometrial cell cycle may involve telomerase.
Androgens
Androgens such as dihydrotestosterone (DHT) induced TA at the G1 phase of the cell cycle in the androgen sensitive prostate cancer cell line LnCAP (Thelen et al., 2004). However, there was no modulation of TA by androgens in either androgen insensitive prostate cancer cell lines (TSU-Pr1, DU145) or in normal human prostate cells (Soda et al., 2000). In a recent study in men, serum DHT and E2 levels were shown to correlate with TL in PMBC, suggesting that both hormones may have a synergistic influence on TA (Yeap et al., 2016). However, caution should be taken when interpreting this observation as the authors have not demonstrated a direct regulatory effect. Oral treatment with Danazol (a synthetic steroid with weak androgenic properties) for 2 years resulted in universal leucocyte telomere elongation in both male and female patients with diseases such as bone marrow failure, liver cirrhosis and pulmonary fibrosis known to involve telomeres (Townsley et al., 2016). The intra-cellular metabolism of testosterone to estrogens is well described (Sasano et al., 2008). Androgens appear to regulate telomerase expression and activity mainly by aromatization of testosterone to estrogens through ERα in normal peripheral blood lymphocytes and human bone marrow-derived CD34(+) cells in vitro (Calado et al., 2009). Therefore, it is difficult to clearly ascertain if the observed effects of androgenic compounds were related to the direct effects on the androgens receptor (AR) or indirectly mediated via ER.
Endometrium expresses AR yet the direct specific effects of androgens in normal endometrium are only beginning to be understood. There is no published work yet examining the effects of androgens in endometrial telomerase regulation.
Other hormones relevant for the endometrium
Melatonin appears to regulate hTERT and hTERC expression in MCF-7 cells (Leon-Blanco et al., 2004) while dexamethasone reduced TA through the inhibition of TERT expression before induction of apoptosis (Akiyama et al., 2002). In contrast, hydrocortisone did not affect TA in human leucocytes (Calado et al., 2009). Therefore, the evidence for other non-ovarian steroidal hormones having a potential regulatory function of endometrial telomerase is limited and they will not be further discussed in this review.
Endometrial stem cells and telomerase
The involvement of SPCs in the endometrial regenerative process has been suggested for a long time (Prianishnikov 1978). After menstrual shedding, a new functionalis layer is thought to regenerate from the remaining SPC rich basalis (Valentijn et al., 2013; Hapangama et al., 2015) and SPCs in many other tissues have the potential to activate telomerase (Hiyama and Hiyama 2007). Interestingly, the available evidence for the differences in TA between the endometrial basalis and the functionalis is controversial. A study in which different endometrial layers were crudely isolated by scraping (using a curette or a scalpel) suggested that TA is lower in the basalis (Bonatz et al., 1998); whereas isolated basalis epithelial cells, identified by expression of the surface marker SSEA1 from primary endometrial epithelial cells in short-term culture, showed higher TA than functionalis epithelial cells (Valentijn et al., 2013). Our study examined only sorted endometrial epithelial cells and telomerase expression and TA were limited mainly to the epithelial cells (Tanaka et al., 1998; Yokoyama et al., 1998; Valentijn et al., 2015). Therefore both studies should have demonstrated similar results. The reasons for this contradictory observation could be due to the fact that epithelial SPC cells are likely to be activated during isolation and cultivation in the latter study, which removed the epithelial SPC cells from its niche, a process known to induce telomerase (Engelhardt et al., 1997). Furthermore, the presumed basalis tissue obtained by scraping the myometrium in the former study might have contained a higher proportion of myometrial tissue with low TA levels.
The available evidence suggests that the endometrium contains multiple progenitor cell populations. Cells with some stem cell properties have been isolated from the human endometrium expressing phenotypical markers of epithelial, stromal, leucocyte and vascular origin (Chan et al., 2004; Masuda et al., 2010; Cervello et al., 2011). Freshly isolated undifferentiated side population cells containing all these primitive cell types from human endometrium also expressed TA (Cervello et al., 2011). The presence of TA in the most widely characterized and studied endometrial SPC cell subtype, the endometrial stromal (mesenchymal) SPCs (Gargett et al., 2016), is yet to be fully described. Human MSCs (hMSC) from other locations are known to have negative or very low TA (Zimmermann et al., 2003; Tichon et al., 2009; Ogura et al., 2014). However, there is a report suggesting that early passages of endometrial stromal SPCs isolated on the basis of their expression of the putative MSC marker CD146 express hTERT protein and mRNA but the authors did not measure TA (Yang et al., 2011). Furthermore, isolated primary endometrial stromal cells had low but measurable TA in our recent study (Valentijn et al., 2015). Our unpublished data also suggest that TA in isolated primary stromal cells positively correlated with mean TLs, suggesting that telomerase expression may have a telomere lengthening function in these cells (Fig. 3). It will be interesting in the future to confirm these preliminary findings and examine the functional relevance of TA to TL in the different endometrial SPC subtypes. However, the low amount of TA in stromal cells and problems with the specificity of the currently available anti-hTERT antibodies that are suitable for IHC/IF make this task difficult. Interestingly, in a recent study, there were rare telomerase expressing cells in murine endometrial stroma that may represent SPC cells (Deane et al., 2016). However, due to the fact that telomerase is expressed constitutively in many mouse tissues unlike in humans, it is difficult to evaluate the significance/relevance of this finding in the context of the human endometrium.
The only characterized human endometrial epithelial cell subpopulation (cells that express surface marker SSEA-1, nuclear SOX9 and nuclear β-catenin) that exhibits progenitor properties in vitro, also showed high TA and longer TL compared with their more differentiated epithelial cell counterparts (Valentijn et al., 2013; Gargett et al., 2016). Importantly, these cells with high TA were able to produce endometrial gland like structures in 3D in vitro culture and when confronted with a 2D environment they were able to produce a monolayer, functionally akin to the re-epithelialization of the denuded endometrial surface after shedding of the functionalis (Valentijn et al., 2015). Finally, in a study employing immunofluorescence microscopy, the potential stem cell marker Mushashi1 also co-localized with the telomerase protein hTERT in the endometrial epithelium (Gotte et al., 2008). However, Mushashi1 expressing cells have not been shown yet to have stem cell characteristics in functional studies.
Taken together, the above data suggests that endometrial SPC cells (basalis SSEA1+ epithelial cells and possibly stromal SPCs) have TA (Valentijn et al., 2015). The exact function of telomerase in the epithelial and in stromal SPCs is not fully understood yet. Similar to the intestine, epidermis and other epithelial tissues and organs, endometrium may also have multiple, heterogeneous epithelial stem cell populations with or without a functional hierarchy (Schepers et al., 2011; Goodell et al., 2015; Pirvulet 2015) and they may have corresponding differential telomerase activation states. Active, more differentiated progenitor cells involved in normal physiological regeneration might have higher TA, while the true, dormant/quiescent stem cell population may not express any or very low levels of TA until they are activated. The quiescent stem cell population may have low TA during normal physiological regeneration of endometrium and only show high TA if challenged by extensive tissue disruption or when progenitors are compromised, such as after iatrogenic endometrial ablation (Hiyama and Hiyama 2007; Biswas et al., 2015). Further studies on telomere biology and telomerase function in the endometrial stem cell population are required in order to elucidate altered pathways relevant to endometrial proliferative diseases. Since stem cells are hypothesized to harbour defects specific to chronic endometrial pathologies (Figueira et al., 2011; Gargett et al., 2014; Sourial et al., 2014), treatment strategies directed towards them may prove to be curative.
Role of telomerase in pathological conditions of the endometrium
Endometriosis
Endometriosis is a common chronic inflammatory disease, defined by the existence of endometrial like stroma and epithelial tissue in ectopic sites, outside the uterine cavity. Since endometrial tissue is intensely responsive to ovarian hormones, the main stimulus for the growth of ectopic endometriotic lesions is estrogen (Sourial et al., 2014; Hapangama et al., 2015; Hapangama and Bulmer 2016; Kamal et al., 2016) and progesterone resistance has been proposed as a fundamental feature of ectopic endometriotic lesions (Bulun et al., 2006). The endometrium of women with endometriosis has been shown to be different to that of healthy fertile women (Hapangama et al., 2012; Mathew et al., 2016).
There is a large body of evidence demonstrating that high TA, expression of hTERT and protein levels associated with longer mean endometrial TLs are features of the eutopic secretory endometrium of women with endometriosis (Kim et al., 2007; Hapangama et al., 2008; Hapangama et al., 2009; Hapangama et al., 2010; Valentijn et al., 2013; Mafra et al., 2014; Valentijn et al., 2015). These changes have been proposed to contribute to the functional endometrial abnormalities that result in the clinical manifestation of subfertility and propagate ectopic lesions. Considering the known pro-survival function of telomerase, the high TA in late-secretory endometrium of women with endometriosis (Hapangama et al., 2008) might be responsible for the survival of cells that are shed into the peritoneal cavity during retrograde menstruation (Hapangama et al., 2010). The preferential survival of these cells and their enhanced replicative capacity due to high TA could facilitate implantation and/or establishment of ectopic lesions (Hapangama et al., 2010; Valentijn et al., 2015) (Fig. 4). This corresponds well with the finding of high TA and hTERT mRNA/protein levels in active peritoneal ectopic endometriotic lesions. In addition, ectopic epithelial cells display longer relative TL compared with eutopic epithelial cells from the same patient (Hapangama et al., 2008; Valentijn et al., 2015). This observation seems to be in agreement with the progesterone resistance described in the pathogenesis of endometriosis (Bulun et al., 2006; Sourial et al., 2014), where the development of ectopic endometriotic lesions may increase TA due to the failure of endogenous progesterone to inhibit telomerase at the ectopic site. We have already shown that dysregulation of telomerase is an important early change in endometriotic cells since high TA was required for the early establishment of ectopic lesions in a baboon model of induced endometriosis (Hapangama et al., 2010; Afshar et al., 2013) (Fig. 4).
Figure 4.
Telomerase is suggested to play a key role in our proposed model for the pathogenesis of endometriosis: (1) Ectopic endometriotic deposits are initiated by an increase in retrograde menstruation or an increased activity in genes that promote angiogenesis and adhesion. (2) The ectopic endometriotic deposits induce a local inflammatory response and secrete various cytokines. (3) The cytokines (or other substances) act on the eutopic endometrium to induce the pro-proliferative markers. (4) The induced eutopic endometrial cells express telomerase and adopt the pro-proliferative, apoptosis-resistant phenotype, which has a survival advantage in the peritoneal cavity. (5) Finally, retrograde menstruation of induced eutopic endometrium with the pro-proliferative phenotype together with other genes that also promote cell survival gives rise to further endometriotic deposits and maintains the disease (adapted from Hapangama et al., 2010).
Furthermore, in the baboon model, establishment of ectopic lesions was associated with induction of high TA and TERT expression in the eutopic endometrium (Hapangama et al., 2010). Interestingly, the initial induction of endometriosis was associated with activation of epidermal growth factor (EGF) signalling in the eutopic endometrium of the baboon model (Afshar et al., 2013) and EGF signalling was associated with up-regulation of TA in normal ovarian surface epithelial cells (Bermudez et al., 2008). A similar scenario might be happening in the eutopic endometrium in the baboon model. Eutopic endometrial cells with high TA can subsequently initiate more ectopic lesions after retrograde menstruation contributing to a self-propagation cycle of the disease (Hapangama et al., 2010) (Fig. 4). Ovarian endometriotic epithelial cells were successfully immortalized by combinatorial transfection of human cyclin D1, cdk4 and hTERT genes, whereas the introduction of hTERT alone, or together with cdk4, was insufficient for immortalization of these cells (Bono et al., 2012). Therefore, telomerase alone may not be sufficient for the apparent survival advantage displayed by the endometriotic cells (Fig. 4).
SPCs are suggested to play a key role in the pathogenesis of endometriosis (Figueira et al., 2011; Gargett et al., 2014; Sourial et al., 2014). Intriguingly, the epithelial cells of ectopic lesions show phenotypic similarities with SSEA1 expressing basalis epithelial cells (Valentijn et al., 2013; Valentijn et al., 2015). Recent data also suggests that the tumour suppressor protein ARID1A might negatively regulate hTERT transcription and TA. Induction of ARID1A repressed transcription of hTERT via binding to a regulatory element on the hTERT promoter, and promoted a repressive histone mode via occupying SIN3A and H3K9me3 (Rahmanto et al., 2016). ARID1A is a member of the SWI/SNF chromatin remodelling complex, and is reported to be frequently mutated in two epithelial ovarian carcinoma subtypes: ovarian clear cell carcinomas and endometrioid ovarian carcinomas (Samartzis et al., 2013; Grandi et al., 2015). These cancers have been molecularly and epidemiologically linked to endometriosis with approximately 20% of benign ovarian endometriosis lesions having a loss of BAF250a (encoded by ARID1A) expression (Xiao et al., 2012). Therefore, it is conceivable that hTERT expression may be potentially involved in carcinogenesis associated with the loss of ARID1A. However, the seemingly vigorous endometrial regulation of telomerase via ovarian hormones and the fact that TA levels are high and constitutively expressed in proliferating endometrial epithelial cells may be responsible for the apparently rare incidence of such transformation (Zafrakas et al., 2014; Ness et al., 2015). TL in peripheral lymphocytes of women with endometriosis compared to healthy controls did not differ in our studies (Hapangama et al., 2008; Hapangama et al., 2009) while others have reported longer telomeres in PBMCs from women with endometriosis (Dracxler et al., 2014). The reason for this observed difference could be attributed to the influence of different demographic features known to affect PMBC TL such as age, BMI and ethnicity. These factors were not accounted for and significantly differ between the two patient groups in the latter study and may account for the different TLs observed rather than endometriosis. Our studies controlled for these demographical parameters as well as the menstrual cycle phase and did not show an endometriosis associated significant difference in PMBC TLs (Hapangama et al., 2008; Hapangama et al., 2009).
Endometriosis shares some of the typical features of increased synthesis of pro-inflammatory cytokines and the imbalance between pro-inflammatory and anti-inflammatory cytokines with other chronic inflammatory diseases (Fig. 4, Souriel et al.2014). Interestingly, telomere attrition and decreased TA have been associated with many chronic inflammatory diseases (Zhang et al., 2016). Although opposite changes in endometrial telomere regulation and involvement of high TA in endometriosis have been confirmed by various studies to date (Kim et al., 2007; Hapangama et al., 2008, 2009, 2010; Valentijn et al., 2013; Mafra et al., 2014; Valentijn et al., 2015), this knowledge is yet to be translated into a therapeutic solution. Since endometriosis is postulated to be a progesterone resistant condition (Bulun et al., 2006) and since telomerase inhibition is a downstream effector of progesterone (Valentijn et al., 2015), the option of telomerase inhibition must be further explored as an attractive, non-hormonal treatment for endometriosis.
Endometrial polyps
Endometrial polyps are defined as abnormal outgrowth of hypertrophic endometrial tissue consisting of a monoclonal overgrowth of endometrial stromal cells with inclusion of a non-neoplastic glandular component (Hapangama and Bulmer, 2016). Endometrial stimulation by estrogen is postulated as the main driving force for endometrial polyp formation and this is supported by the observation that the use of tamoxifen, which acts as an ER agonist on the endometrium, increases the risk of developing endometrial polyps. Lower levels of hTERT protein in endometrial polyps have been reported compared with normal endometrium in the proliferative phase (Hu and Yuan, 2011). CD146 expressing mesenchymal SPCs derived from endometrial polyps did not express any hTERT (Ding et al., 2011). Jointly these studies suggest that benign endometrial polyps with increased stromal growth have low telomerase and that TA is less likely to play an essential role in them. However, telomerase biology in polyps with epithelial hyperplasia/atypia remains to be explored in future studies.
Reproductive failure
Embryo implantation occurs during the window of implantation in the mid-secretory phase. The mid-secretory phase is defined by the dominant action of progesterone with maximum cell differentiation in an environment where endometrial glandular proliferation indices are at their nadir. This period is also associated with the lowest endometrial TA and the shortest mean TL (Williams et al., 2001; Valentijn et al., 2015) suggesting a requirement of low endometrial TA for the establishment of an early pregnancy. This suppression of TA in the endometrium of fertile women has been proposed as a necessary process in order to allow endometrial cells to undergo differentiation with cellular apoptosis/senescence required to make space for the invading embryo (Williams et al., 2001; Hapangama et al., 2008; Hapangama et al., 2009; Hapangama et al., 2010). It is therefore not surprising that significantly high telomerase expression and TA was observed in endometrial tissue of women with recurrent reproductive failure (Hapangama et al., 2008; Long et al., 2016). The mid-secretory endometria of women with recurrent miscarriages, recurrent embryo loss and recurrent implantation failure all showed high endometrial TA (Hapangama et al., 2008; Long et al., 2016) and a trend for longer mean endometrial TLs in endometrial epithelium (Hapangama et al., 2008). However, this preliminary evidence needs to be confirmed in independent studies. It is plausible that persistent proliferation and high TA of endometrial cells may interfere with the embryo implantation and trophoblastic invasion, all of which are known to be involved in the establishment of early pregnancy. The question of why TA is down-regulated in the endometrium of successful pregnancies but not so in unsuccessful cases implies a difference in telomerase regulation. So far, the underlying mechanisms for the differential (dys)regulation are not understood. In a further case controlled study, infertile women with deep infiltrating endometriosis also had high endometrial telomerase expression further suggesting a detrimental effect of high levels of TA on conception/embryo implantation (Mafra et al., 2014). Progesterone is commonly employed to treat women with a variety of reproductive failures, from infertility, to luteal phase defects to recurrent miscarriages, yet the available evidence on the effectiveness of this therapy is inconclusive (Coomarasamy et al., 2016). Most of these conditions are multifactorial and the lesions from women included in clinical trials therefore are heterogeneous. This prevents elucidation of the true effectiveness of progesterone treatment in subgroups of women with an apparently similar clinical manifestation. Further examination of downstream effectors of progesterone treatment, such as telomerase, may enable the stratification of women in the future in order to identify those who may benefit from the administration of the hormone.
Polycystic Ovarian Syndrome
PCOS is a common gynaecological condition defined by the clinical manifestations of hormonal aberrations of hyperandrogenism and insulin resistance (Clark et al., 2014; Lizneva et al., 2016). It is often associated with anovulation and a subsequently increased risk of EH with risk of progression to EC (Hapangama et al., 2015; Hapangama and Bulmer, 2016; Kamal et al., 2016). A genome-wide association study in Korean women with PCOS has identified susceptibility loci for polycystic ovarian syndrome (PCOS) (Lee et al., 2015). The authors reported the strongest signal to be located upstream of KH domain containing, RNA binding, signal transduction associated 3 (KHDRBS3). KHDRBS3 was found to regulate TA in colon cancer cells (Zhang et al., 2006). With this evidence, the authors concluded that telomerase may be an important driving force in developing PCOS and related phenotypes (Lee et al., 2015). Considering the suggested role that unopposed estrogen and excessive androgens have on TA (Boggess et al., 2006; Nourbakhsh et al., 2010) and cellular proliferation in various carcinoma cells including EC cells (Chao et al., 2013; Dumesic and Lobo, 2013; Plaza-Parrochia et al., 2014; Hapangama and Bulmer, 2016; Kamal et al., 2016), studies examining the involvement of telomerase in the endometrium of women with PCOS may unravel novel avenues for therapeutic manipulation.
Malignant conditions of the endometrium
Constitutively high levels of hTERT expression and TA have been identified in over 90% of human cancers including hepatocellular carcinoma, colorectal cancer and EC (Lehner et al., 2002; Saini et al., 2009; Bertorelle et al., 2014). High levels of TA in tumour cells contribute to increased cell proliferation, cellular immortality, carcinogenesis and cancer progression, while the activity of telomerase and hTERT expression are usually suppressed in most human somatic tissues (Cong et al., 1999). The involvement of telomerase in cellular immortality is further highlighted by the fact that most cell lines, including the first ever ‘immortal cell line’, cervical HeLa cells express very high levels of TA (Pandita et al., 1997). The involvement and activation of telomerase and telomere maintenance during tumorigenesis has been intensely studied over the years. In HeLa cells, as in many cervical cancers, the mechanism of telomerase activation is regulated by specific proteins from human papillomavirus Types 16 and 18 (Kyo et al., 1996; Sakamoto et al., 2000; James et al., 2006). Other cancers that do not involve viral infection in the pathogenesis also demonstrate high TA but the exact mechanism of their telomerase activation is not fully understood. Telomerase suppressing mechanisms that are downstream of hTERT transcription and mRNA splicing are present in rapidly proliferating embryonic tissue (Ulaner et al., 2000), but these are lost during neoplastic transformation (Ulaner et al., 2000). Therefore, the tight physiological mechanisms of telomerase regulation do not exist in cancer cells where TA levels remain constitutively high.
There are reports of shorter or longer TLs in different cancer cells compared with their benign counterparts. Short TLs in cancer cells may result from excessive cell proliferation prior to telomere stabilization (with TA or an alternative telomere maintenance mechanism (ALT)). Telomere attrition can result in genomic instability which can subsequently initiate carcinogenesis. Absolute TL is therefore not relevant for cancer cells as long as telomeres are sufficiently maintained in a capped state in order to supply the cells with an indefinite proliferation capacity. There are at least four activating mutations reported in TERT, POT1, TPP1 and TERF2IP (RAP1) genes of the telomerase and telomere complexes which can result in longer TLs while several other telomere and telomerase associated gene mutations (including repressor mutations in POT1 and activating mutations of TRF1/2) result in short TL (reviewed in Mengual Gomez et al., 2016). Since either lengthening or shortening of telomeres can result in abnormal cell proliferation or genomic instability, they can be implicated in carcinogenesis (reviewed in Holysz et al., 2013; Mengual Gomez et al., 2016; Shay, 2016). In this review, we examine the available evidence for specific aberrations in telomeres/telomerase in the premalignant and malignant conditions of the endometrium.
Endometrial hyperplasia
EH is characterized by irregular proliferation of endometrial glands that may precede or co-exist with endometrial carcinoma (Hapangama and Bulmer 2016; Kamal et al., 2016). Hyperplastic glands show extremely high proliferative indices in comparison to either stromal cells or normal glands during the proliferative phase (Dallenbach-Hellweg and Dallenbach, 2010). EH is almost always due to exposure to high estrogen levels and typically accompanied by a chronic insufficiency of progesterone. Classical causes therefore include corpus luteum insufficiency/anovulatory cycles, PCOS, obesity with metabolic syndrome (extra-ovarian aromatization of androgens) and inappropriate postmenopausal hormone therapy (tamoxifen, insufficient dosage of progestagens) (Hapangama and Bulmer 2016; Kamal et al., 2016).
High TA levels have been detected in EH, including the simple, complex and complex with atypia subtypes (Shroyer et al., 1997). It was also suggested that TA could be a useful diagnostic tool to screen postmenopausal women with endometrial premalignant and malignant lesions (Maida et al., 2002). However, there is a considerable proportion of EH samples included in these studies that lack TA (Shroyer et al., 1997) and the absence of detectable TA did not have a specific negative predictive value (Shroyer et al., 1997). Considering the method used to sample the endometrium (for example, an outpatient endometrial biopsy typically samples approximately 4% of the uterine cavity), it is unlikely that a small area of EH is reliably sampled and detected with this approach. Furthermore, since TA is a feature of normal proliferating endometrial cells, including simple hyperplasia, the level of TA is unlikely to be a sufficient discriminator to detect malignant or premalignant conditions of the human endometrium.
Endometrial cancer
Most previous research on endometrial telomerase had been focussed on EC that is associated with high TA. EC is the commonest gynaecological malignancy and is an estrogen driven disease (Kamal et al., 2016). The risk factors for EC include advanced age, obesity and exposure to unopposed estrogen (or progesterone deficiency). It is of interest that despite the high estrogenic milieu associated with the premenopausal period where most vigorous proliferative and regenerative activity takes place in the endometrium, carcinogenesis commonly occurs in the relatively quiescent, hypo-estrogenic postmenopausal period (Hapangama and Bulmer 2016; Kamal et al., 2016).
Very low but detectable TA levels are reported in the postmenopausal endometrium (Tanaka et al., 1998). Estrogen induces telomerase in a dose dependent manner (Kyo et al., 1999). Various risk factors (such as obesity) for EC will marginally increase the weak estrogen (estrone) levels in the postmenopausal endometrium (reviewed in Kamal et al., 2016). The intermittent and low levels of estrogen associated with these conditions (Kamal et al., 2016) may be sufficient for inducing low TA levels to initiate epithelial proliferation in the postmenopausal endometrial cells. Furthermore, both obesity and ageing are chronic inflammatory conditions that are associated with oxidative stress, thus accelerating telomere shortening in proliferating cells (von Zglinicki et al., 1995; von Zglinicki 2000; Kamal et al., 2016). Therefore the most likely mechanism to explain EC associated telomere aberrations is the insufficient TA/short-TL/damaged telomeres theory. We propose that the hypo-estrogenic hormonal maelstrom in high risk postmenopausal endometrium may be associated with a potentially deficient amount of TA that is insufficient to maintain the short endometrial TLs and their integrity during cell division. Therefore, the ongoing proliferation in postmenopausal epithelial cells with short TLs may render them vulnerable to subsequent genetic instability and carcinogenic transformation similar to various other cancer types (Greider and Blackburn 1996; Bertorelle et al., 2014; Meena et al., 2015). In line with the above hypothesis, short TLs have been reported in most sporadic ECs.
Lynch syndrome is an autosomal dominant condition characterized by germ line mutation in DNA mismatch repair genes resulting in increased risk of developing a variety of cancers including EC. Long telomeres are associated with familial cancer syndromes such as familial melanoma (Akbay et al., 2008; Horn et al., 2013). However, short TLs and hTERT gene polymorphisms predict initiation of EC in Lynch patients (Segui et al., 2013).
High TA, hTERT and hTERC expression as well as high hTERT protein levels have been described by many groups in ECs (Kyo et al., 1996; Saito et al., 1997; Ebina et al., 1999; Maida et al., 2002). Some preliminary data in a very small patient population also suggests that hTERT mRNA in PBMCs can be used to diagnose early micro-metastases of EC (Liang et al., 2016). This observation needs further validation in an appropriately powered study. Frequent PTEN mutations and P53 loss known to occur in ECs could be associated with telomerase up-regulation but the exact mechanism through which these different events interact is not yet clear (Zhou et al., 2006; Akbay et al., 2013). Further recent work also showed hTERT to be involved in EMT, cell motility and metabolism, which are processes associated with cancer metastasis (reviewed in Saretzki, 2014). In a mouse model, simultaneous deletion of p53 and POT1 resulted in precursor lesions of endometrial epithelium and induced ECs with non-endometrioid, Type II phenotype, suggesting that telomeric instability has a critical role to play in Type II ECs (Akbay et al., 2013). TERT promoter mutations seem to be rare in ECs (Wu et al., 2014), except for the clear cell (Type II EC) subtype (Huang et al., 2015). Activating TERT promotor mutations are a feature of cancers derived from tissues with low relative cellular turnover such as the brain, therefore such mutations are not expected in the highly regenerative endometrial epithelium (Killela et al., 2013; Shay 2016).
There are limited reports suggesting that the ALT pathway may be active in some endometrial carcino-sarcomas or uterine sarcomas (Lee et al., 2012), but its prevalence in endometrioid ECs appears to be low (Heaphy et al., 2011). Further studies are required to dissect out the exact function high TA plays in ECs and how telomerase regulation in the postmenopausal endometrium may induce carcinogenesis, particularly, in the context where a high risk hormonal environment is present. In the light of many reported alterations in the components of the telomere complex, telomerase holoenzyme and their associated proteins in cancer (Greider and Blackburn, 1996; Shay, 2016), in addition to the complex interactions of these changes with numerous tumour suppressor proteins and oncogenes, more detailed studies are required to fully understand the role of alterations in the telomere maintenance pathways particular to all subtypes of EC.
Future directions and wider implications of interventions into telomerase biology
hTERT has been reported to be up-regulated by various pharmaceutical and metabolic agents such as angiotensin-converting enzyme inhibitors (Donnini et al., 2010), essential fatty acids (Das 2008), beta blockers (Wang et al., 2011) and calcium channel blockers (Hayashi et al., 2014) in a variety of tissue types. Telomerase inhibition has been reported as an off-target effect of various chemotherapeutic agents, however this it could be a secondary effect to apoptosis or senescence induction (Saretzki, 2010). Imetelstat is the only clinically applicable synthetic telomerase inhibitor, which is a lipid based conjugate of the oligonucleotide GRN163 that binds with high affinity to the hTERC component of telomerase (Roth et al., 2010; Salloum et al., 2016). Imetelstat had shown promising pre-clinical telomerase inhibitory activity across a wide range of cancer types (Roth et al., 2010). However, despite high expectations, all reported data on its clinical effectiveness in at least six different clinical cancer trials have been disappointing (Salloum et al., 2016), with treatment resulting in significant side effects such as myelo-suppression. Findings from a recent small clinical pilot study demonstrate a possible specific beneficial effect of Imetelstat in clinical outcomes in myelo-proliferative conditions that needs to be further confirmed in larger trials (Baerlocher et al., 2015). The side effect profile of this drug, however, precludes its use in benign endometrial conditions. The examination of different compounds with off-target inhibitory effects on TA and a more favourable side effect profile is required before clinical application in endometrial disease.
Our understanding of telomerase and telomere biology is rapidly expanding with many groups working towards developing this exciting field, as telomeres and telomerase pertain to a diverse range of cellular functions and processes. In addition, telomerase regulation and function in the human endometrium appears to be unique. The majority of treatments we have available at present for common benign and chronic diseases of the endometrium such as heavy menstrual bleeding, infertility, polyps, endometriosis and EH are directed towards terminally differentiated endometrial cells of the endometrial functionalis. The functionalis is shed each month with menstruation, thus requiring continuous therapy for each new functionalis layer that is regenerated. Most currently available therapeutic options therefore include ovarian or other hormonal analogues to manipulate the ovarian cycle resulting in considerable side effects. Since telomerase appears to be involved in almost all endometrial pathologies, it provides a distinctive route to formulate possible curative (involving stem cells), non-hormonal therapies to treat them. Furthermore, our current understanding of telomere and telomerase biology in ECs is far from comprehensive and the fact that high TA is present in normal proliferating endometrial cells makes it difficult to extrapolate the data from other cancers to ECs. TA in normal endometrium has strict physiological regulation, mainly by hormones. During tumourigenesis this regulation becomes dysfunctional, supplying cells with a constitutively high amount of TA, conferring a selective advantage and a high proliferative capacity. In addition, telomerase and telomere biology of the endometrial cells may be modified by the cellular interactions in a new environment (e.g. endometriotic lesions growing in the peritoneal cavity). A better understanding of these processes may facilitate formulating new interventions. Further studies in this field for both benign and malignant diseases of the endometrium should therefore focus on understanding the precise regulation mechanisms for telomerase which could potentially reveal novel targets for new treatment strategies based on telomerase in endometrial disease.
Conclusion
We are only beginning to understand the central role of telomeres and telomerase in the biology of the endometrium. Since telomerase is pivotal to endometrial regeneration, further studies elucidating the involvement of telomere and telomerase associated proteins and their regulation in normal endometrial regeneration, and in endometrial pathologies, will help in developing novel treatment strategies that are not only non-hormonal but also potentially curative.
Acknowledgements
The authors are grateful to Mr Anthony Valentijn (isolation of endometrial cells for the data included in Fig. 3), Dr Nicola Tempest (qFISH in Fig. 3), Dr David Mathew and Mr Druvi Edirisinghe (language editing) for their assistance with study and manuscript preparation.
Authors’ roles
D.H. conceived the manuscript design and prepared the first draft. G.S. revised the manuscript critically for important intellectual content. D.H./A.K. drafted the figures and tables. All authors revised and read the manuscript and approved the submitted final version.
Funding
We acknowledge the support by Wellbeing of Women project grant RG1073 (D.K.H./G.S.) and RG1487 (D.K.H./G.S.).
Conflict of interest
None declared.
References
- Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod 2013;88:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci 2008;121:1046–1053. [DOI] [PubMed] [Google Scholar]
- Aix E, Gutierrez-Gutierrez O, Sanchez-Ferrer C, Aguado T, Flores I. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J Cell Biol 2016;213:571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbay EA, Contreras CM, Perera SA, Sullivan JP, Broaddus RR, Schorge JO, Ashfaq R, Saboorian H, Wong KK, Castrillon DH. Differential roles of telomere attrition in type I and II endometrial carcinogenesis. Am J Pathol 2008;173:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbay EA, Pena CG, Ruder D, Michel JA, Nakada Y, Pathak S, Multani AS, Chang S, Castrillon DH. Cooperation between p53 and the telomere-protecting shelterin component Pot1a in endometrial carcinogenesis. Oncogene 2013;32:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akincilar SC, Unal B, Tergaonkar V. Reactivation of telomerase in cancer. Cell Mol Life Sci 2016;73:1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama M, Hideshima T, Hayashi T, Tai YT, Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC, Anderson KC. Cytokines modulate telomerase activity in a human multiple myeloma cell line. Cancer Res 2002;62:3876–3882. [PubMed] [Google Scholar]
- Angrisani A, Vicidomini R, Turano M, Furia M. Human dyskerin: beyond telomeres. Biol Chem 2014;395:593–610. [DOI] [PubMed] [Google Scholar]
- Baerlocher GM, Burington B, Snyder DS. Telomerase inhibitor imetelstat in essential thrombocythemia and myelofibrosis. N Engl J Med 2015;373:2580. [DOI] [PubMed] [Google Scholar]
- Barbier CS, Becker KA, Troester MA, Kaufman DG. Expression of exogenous human telomerase in cultures of endometrial stromal cells does not alter their hormone responsiveness. Biol Reprod 2005;73:106–114. [DOI] [PubMed] [Google Scholar]
- Barrett EL, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell 2011;10:913–921. [DOI] [PubMed] [Google Scholar]
- Batista LF. Telomere biology in stem cells and reprogramming. Prog Mol Biol Transl Sci 2014;125:67–88. [DOI] [PubMed] [Google Scholar]
- Baumann P, Price C. Pot1 and telomere maintenance. FEBS Lett 2010;584:3779–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Van Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Res 2005;25:3011–3021. [PubMed] [Google Scholar]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, von Bornemann Hjelmborg J et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 2013;12:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A, Kimura M, Labat C, Buchoff GM, Huber S, Labat L, Lu X, Aviv A. A model of canine leukocyte telomere dynamics. Aging Cell 2011;10:991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko AL, Olsen NJ, Kovacs WJ. Estrogen and telomerase in human peripheral blood mononuclear cells. Mol Cell Endocrinol 2012;364:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez Y, Yang H, Cheng JQ, Kruk PA. Pyk2/ERK 1/2 mediate Spl- and c-Myc-dependent induction of telomerase activity by epidermal growth factor. Growth Factors 2008;26:1–11. [DOI] [PubMed] [Google Scholar]
- Bertorelle R, Rampazzo E, Pucciarelli S, Nitti D, De Rossi A. Telomeres, telomerase and colorectal cancer. World J Gastroenterol 2014;20:1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R et al. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res 2016;118:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Davis H, Irshad S, Sandberg T, Worthley D, Leedham S. Microenvironmental control of stem cell fate in intestinal homeostasis and disease. J Pathol 2015;237:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres: do the ends justify the means. Cell 1984;37:7–8. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Walking the walk from genes through telomere maintenance to cancer risk. Cancer Prev Res (Phila) 2011;4:473–475. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Chan S, Chang J, Fulton TB, Krauskopf A, McEachern M, Prescott J, Roy J, Smith C, Wang H. Molecular manifestations and molecular determinants of telomere capping. Cold Spring Harb Symp Quant Biol 2000;65:253–263. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Collins K. Telomerase: an RNP enzyme synthesizes DNA. Cold Spring Harb Perspect Biol 2011;3(5). pii: a003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 2015;350:1193–1198. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol 1978;120:33–53. [DOI] [PubMed] [Google Scholar]
- Bodvarsdottir SK, Steinarsdottir M, Bjarnason H, Eyfjord JE. Dysfunctional telomeres in human BRCA2 mutated breast tumors and cell lines. Mutat Res 2012;729:90–99. [DOI] [PubMed] [Google Scholar]
- Boggess JF, Zhou CX, Bae-Jump VL, Gehrig PA, Whang YE. Estrogen-receptor-dependent regulation of telomerase activity in human endometrial cancer cell lines. Gynecol Oncol 2006;103:417–424. [DOI] [PubMed] [Google Scholar]
- Bojovic B, Booth RE, Jin Y, Zhou X, Crowe DL. Alternative lengthening of telomeres in cancer stem cells in vivo. Oncogene 2015;34:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatz G, Klapper W, Barthe A, Heidorn K, Jonat W, Krupp G, Parwaresch R. Analysis of telomerase expression and proliferative activity in the different layers of cyclic endometrium. Biochem Biophys Res Commun 1998;253:214–221. [DOI] [PubMed] [Google Scholar]
- Bono Y, Kyo S, Takakura M, Maida Y, Mizumoto Y, Nakamura M, Nomura K, Kiyono T, Inoue M. Creation of immortalised epithelial cells from ovarian endometrioma. Br J Cancer 2012;106:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien TP, Kallakury BV, Lowry CV, Ambros RA, Muraca PJ, Malfetano JH, Ross JS. Telomerase activity in benign endometrium and endometrial carcinoma. Cancer Res 1997;57:2760–2764. [PubMed] [Google Scholar]
- Bryan TM, Marusic L, Bacchetti S, Namba M, Reddel RR. The telomere lengthening mechanism in telomerase-negative immortal human cells does not involve the telomerase RNA subunit. Hum Mol Genet 1997;6:921–926. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 2006;248:94–103. [DOI] [PubMed] [Google Scholar]
- Bunch K, Tinnemore D, Huff S, Hoffer ZS, Burney RO, Stallings JD. Expression patterns of progesterone receptor membrane components 1 and 2 in endometria from women with and without endometriosis. Reprod Sci 2014;21:190–197. [DOI] [PubMed] [Google Scholar]
- Calado RT, Dumitriu B. Telomere dynamics in mice and humans. Semin Hematol 2013;50:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 2009;114:2236–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro MC, Henriques CM, Nabais J, Ferreira T, Carvalho T, Ferreira MG. Short telomeres in key tissues initiate local and systemic aging in zebrafish. PLoS Genet 2016;12:e1005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003;361:393–395. [DOI] [PubMed] [Google Scholar]
- Cen J, Zhang H, Liu Y, Deng M, Tang S, Liu W, Zhang Z. Anti-aging effect of estrogen on telomerase activity in ovariectomised rats—animal model for menopause. Gynecol Endocrinol 2015;31:582–585. [DOI] [PubMed] [Google Scholar]
- Cerrudo CS, Mengual Gomez DL, Gomez DE, Ghiringhelli PD. Novel insights into the evolution and structural characterization of dyskerin using comprehensive bioinformatics analysis. J Proteome Res 2015;14:874–887. [DOI] [PubMed] [Google Scholar]
- Cervello I, Gil-Sanchis C, Mas A, Faus A, Sanz J, Moscardo F, Higueras G, Sanz MA, Pellicer A, Simon C. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One 2012;7:e30260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervello I, Mas A, Gil-Sanchis C, Peris L, Faus A, Saunders PT, Critchley HO, Simon C. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One 2011;6:e21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare AJ, Karlseder J. A three-state model of telomere control over human proliferative boundaries. Curr Opin Cell Biol 2012;24:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y, Kwon SJ, Seol W, Park KS. Estrogen receptor-alpha mediates the effects of estradiol on telomerase activity in human mesenchymal stem cells. Mol Cells 2008;26:454–458. [PubMed] [Google Scholar]
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 2004;70:1738–1750. [DOI] [PubMed] [Google Scholar]
- Chao A, Lin CY, Tsai CL, Hsueh S, Lin YY, Lin CT, Chou HH, Wang TH, Lai CH, Wang HS. Estrogen stimulates the proliferation of human endometrial cancer cells by stabilizing nucleophosmin/B23 (NPM/B23). J Mol Med (Berl) 2013;91:249–259. [DOI] [PubMed] [Google Scholar]
- Chen LY, Zhang Y, Zhang Q, Li H, Luo Z, Fang H, Kim SH, Qin L, Yotnda P, Xu J et al. Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol Cell 2012;47:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YJ, Kim SH, Tessarollo L, Campisi J, Hodes RJ. Telomere-associated protein TIN2 is essential for early embryonic development through a telomerase-independent pathway. Mol Cell Biol 2004;24:6631–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinigarzadeh A, Muniandy S, Salleh N. Combinatorial effect of genistein and female sex-steroids on uterine fluid volume and secretion rate and aquaporin (AQP)-1, 2, 5, and 7 expression in the uterus in rats. Environ Toxicol 2016. DOI: 10.1002/tox.22283 [In press]. [DOI] [PubMed] [Google Scholar]
- Chronowska E. Regulation of telomerase activity in ovarian granulosa cells. Indian J Exp Biol 2012;50:595–601. [PubMed] [Google Scholar]
- Chu TW, D'Souza Y, Autexier C. The insertion in fingers domain in human telomerase can mediate enzyme processivity and telomerase recruitment to telomeres in a TPP1-dependent manner. Mol Cell Biol 2016;36:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Khadka P, Chung IK. Nuclear import of hTERT requires a bipartite nuclear localization signal and Akt-mediated phosphorylation. J Cell Sci 2012;125:2684–2697. [DOI] [PubMed] [Google Scholar]
- Cimino-Reale G, Pascale E, Battiloro E, Starace G, Verna R, D'Ambrosio E. The length of telomeric G-rich strand 3’-overhang measured by oligonucleotide ligation assay. Nucleic Acids Res 2001;29:E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NM, Podolski AJ, Brooks ED, Chizen DR, Pierson RA, Lehotay DC, Lujan ME. Prevalence of polycystic ovary syndrome phenotypes using updated criteria for polycystic ovarian morphology: an assessment of over 100 consecutive women self-reporting features of polycystic ovary syndrome. Reprod Sci 2014;21:1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science 2007;315:1850–1853. [DOI] [PubMed] [Google Scholar]
- Cong YS, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet 1999;8:137–142. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, Gupta P, Dawood F, Koot YE, Atik RB et al. PROMISE: first-trimester progesterone therapy in women with a history of unexplained recurrent miscarriages—a randomised, double-blind, placebo-controlled, international multicentre trial and economic evaluation. Health Technol Assess (Rockv) 2016;20:1–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J 1992;11:1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003;426:194–198. [DOI] [PubMed] [Google Scholar]
- D'Souza Y, Lauzon C, Chu TW, Autexier C. Regulation of telomere length and homeostasis by telomerase enzyme processivity. J Cell Sci 2013;126:676–687. [DOI] [PubMed] [Google Scholar]
- Dalgard C, Benetos A, Verhulst S, Labat C, Kark JD, Christensen K, Kimura M, Kyvik KO, Aviv A. Leukocyte telomere length dynamics in women and men: menopause vs age effects. Int J Epidemiol 2015;44:1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallenbach-Hellweg GSD, Dallenbach F. Normal endometrium In: Dallenbach-Hellweg G SD, Dallenbach F (eds).. Atlas of Endometrial Histopathology. Springer, Berlin, Germany, 2010;7–44. [Google Scholar]
- Das UN. Can essential fatty acids reduce the burden of disease(s). Lipids Health Dis 2008;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boeck G, Forsyth RG, Praet M, Hogendoorn PC. Telomere-associated proteins: cross-talk between telomere maintenance and telomere-lengthening mechanisms. J Pathol 2009;217:327–344. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005;19:2100–2110. [DOI] [PubMed] [Google Scholar]
- Deane JA, Ong YR, Cain JE, Jayasekara WS, Tiwari A, Carlone DL, Watkins DN, Breault DT, Gargett CE. The mouse endometrium contains epithelial, endothelial and leucocyte populations expressing the stem cell marker telomerase reverse transcriptase. Mol Hum Reprod 2016;22:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bufalo D, Rizzo A, Trisciuoglio D, Cardinali G, Torrisi MR, Zangemeister-Wittke U, Zupi G, Biroccio A. Involvement of hTERT in apoptosis induced by interference with Bcl-2 expression and function. Cell Death Differ 2005;12:1429–1438. [DOI] [PubMed] [Google Scholar]
- Ding DC, Chu TY, Chiou SH, Liu HW. Enhanced differentiation and clonogenicity of human endometrial polyp stem cells. Differentiation 2011;81:172–180. [DOI] [PubMed] [Google Scholar]
- Djojosubroto MW, Choi YS, Lee HW, Rudolph KL. Telomeres and telomerase in aging, regeneration and cancer. Mol Cells 2003;15:164–175. [PubMed] [Google Scholar]
- Donnini S, Terzuoli E, Ziche M, Morbidelli L. Sulfhydryl angiotensin-converting enzyme inhibitor promotes endothelial cell survival through nitric-oxide synthase, fibroblast growth factor-2, and telomerase cross-talk. J Pharmacol Exp Ther 2010;332:776–784. [DOI] [PubMed] [Google Scholar]
- Dracxler RC, Oh C, Kalmbach K, Wang F, Liu L, Kallas EG, Giret MTM, Seth-Smith ML, Antunes D, Keefe DL et al. Peripheral blood telomere content is greater in patients with endometriosis than in controls. Reprod Sci 2014;21:1465–1471. [DOI] [PubMed] [Google Scholar]
- Dudognon C, Pendino F, Hillion J, Saumet A, Lanotte M, Segal-Bendirdjian E. Death receptor signaling regulatory function for telomerase: hTERT abolishes TRAIL-induced apoptosis, independently of telomere maintenance. Oncogene 2004;23:7469–7474. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids 2013;78:782–785. [DOI] [PubMed] [Google Scholar]
- Ebina Y, Yamada H, Fujino T, Furuta I, Sakuragi N, Yamamoto R, Katoh M, Oshimura M, Fujimoto S. Telomerase activity correlates with histo-pathological factors in uterine endometrial carcinoma. Int J Cancer 1999;84:529–532. [DOI] [PubMed] [Google Scholar]
- Egan ED, Collins K. Specificity and stoichiometry of subunit interactions in the human telomerase holoenzyme assembled in vivo. Mol Cell Biol 2010;30:2775–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt M, Kumar R, Albanell J, Pettengell R, Han W, Moore MA. Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells. Blood 1997;90:182–193. [PubMed] [Google Scholar]
- Fakhoury J, Marie-Egyptienne DT, Londono-Vallejo JA, Autexier C. Telomeric function of mammalian telomerases at short telomeres. J Cell Sci 2010;123:1693–1704. [DOI] [PubMed] [Google Scholar]
- Farwell DG, Shera KA, Koop JI, Bonnet GA, Matthews CP, Reuther GW, Coltrera MD, McDougall JK, Klingelhutz AJ. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am J Pathol 2000;156:1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J et al. The RNA component of human telomerase. Science 1995;269:1236–1241. [DOI] [PubMed] [Google Scholar]
- Figueira PG, Abrao MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci 2011;1221:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores I, Canela A, Vera E, Tejera A, Cotsarelis G, Blasco MA. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev 2008;22:654–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojtova M, Fajkus J. Epigenetic regulation of telomere maintenance. Cytogenet Genome Res 2014;143:125–135. [DOI] [PubMed] [Google Scholar]
- Fu D, Collins K. Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol Cell 2003;11:1361–1372. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 2012;14:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo F, Chartrand P. Telomerase biogenesis: the long road before getting to the end. RNA Biol 2008;5:212–215. [DOI] [PubMed] [Google Scholar]
- Gardano L, Holland L, Oulton R, Le Bihan T, Harrington L. Native gel electrophoresis of human telomerase distinguishes active complexes with or without dyskerin. Nucleic Acids Res 2012;40:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Brosens JJ, Puttemans P, Benagiano G, Brosens I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol Hum Reprod 2014;20:591–598. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update 2016;22:137–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Wu S, Xue Y, Tao J, Li F, Chen Y, Liu H, Ma W, Huang J, Zhao Y. Preferential extension of short telomeres induced by low extracellular pH. Nucleic Acids Res 2016;44:8086–8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladych M, Wojtyla A, Rubis B. Human telomerase expression regulation. Biochem Cell Biol 2011;89:359–376. [DOI] [PubMed] [Google Scholar]
- Gonzalez OG, Assfalg R, Koch S, Schelling A, Meena JK, Kraus J, Lechel A, Katz SF, Benes V, Scharffetter-Kochanek K et al. Telomerase stimulates ribosomal DNA transcription under hyperproliferative conditions. Nat Commun 2014;5:4599. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Nguyen H, Shroyer N. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat Rev Mol Cell Biol 2015;16:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Cheung NKM, Yip BWP, Au DWT. Medaka fish exhibits longevity gender gap, a natural drop in estrogen and telomere shortening during aging: a unique model for studying sex-dependent longevity. Front Zool 2013;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M, Wolf M, Staebler A, Buchweitz O, Kelsch R, Schuring AN, Kiesel L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol 2008;215:317–329. [DOI] [PubMed] [Google Scholar]
- Grandi G, Toss A, Cortesi L, Botticelli L, Volpe A, Cagnacci A. The association between endometriomas and ovarian cancer: preventive effect of inhibiting ovulation and menstruation during reproductive life. Biomed Res Int 2015;2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Telomeres, telomerase and cancer. Sci Am 1996;274:92–97. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell 1999;97:503–514. [DOI] [PubMed] [Google Scholar]
- Hapangama DK. Mifepristone: the multi-faceted anti-hormone. J Drug Eval 2003;1:149–175. [Google Scholar]
- Hapangama DK, Bulmer JN. Pathophysiology of heavy menstrual bleeding. Women Health (Lond) 2016;12:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapangama DK, Kamal AM, Bulmer JN. Estrogen receptor beta: the guardian of the endometrium. Hum Reprod Update 2015;21:174–193. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Raju RS, Valentijn AJ, Barraclough D, Hart A, Turner MA, Platt-Higgins A, Barraclough R, Rudland PS. Aberrant expression of metastasis-inducing proteins in ectopic and matched eutopic endometrium of women with endometriosis: implications for the pathogenesis of endometriosis. Hum Reprod 2012;27:394–407. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Turner MA, Drury J, Heathcote L, Afshar Y, Mavrogianis PA, Fazleabas AT. Aberrant expression of regulators of cell-fate found in eutopic endometrium is found in matched ectopic endometrium among women and in a baboon model of endometriosis. Hum Reprod 2010;25:2840–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapangama DK, Turner MA, Drury JA, Martin-Ruiz C, Von Zglinicki T, Farquharson RG, Quenby S. Endometrial telomerase shows specific expression patterns in different types of reproductive failure. Reprod Biomed Online 2008;17:416–424. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Turner MA, Drury JA, Quenby S, Hart A, Maddick M, Martin-Ruiz C, von Zglinicki T. Sustained replication in endometrium of women with endometriosis occurs without evoking a DNA damage response. Hum Reprod 2009;24:687–696. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Turner MA, Drury JA, Quenby S, Saretzki G, Martin-Ruiz C, Von Zglinicki T. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum Reprod 2008;23:1511–1519. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamaguchi T, Sakakibara Y, Taguchi K, Maeda M, Kuzuya M, Hattori Y. eNOS-dependent antisenscence effect of a calcium channel blocker in human endothelial cells. PLoS One 2014;9:e88391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, Netto GJ, Epstein JI, Lotan TL, Westra WH et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol 2011;179:1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle ES, Han Z, Tang N, Rai P, Luo Y, Linn S. Sequence-specific DNA cleavage by Fe2+-mediated fenton reactions has possible biological implications. J Biol Chem 1999;274:962–971. [DOI] [PubMed] [Google Scholar]
- Henson JD, Neumann AA, Yeager TR, Reddel RR. Alternative lengthening of telomeres in mammalian cells. Oncogene 2002;21:598–610. [DOI] [PubMed] [Google Scholar]
- Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 2012;3:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer 2007;96:1020–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012;336:1549–1554. [DOI] [PubMed] [Google Scholar]
- Holysz H, Lipinska N, Paszel-Jaworska A, Rubis B. Telomerase as a useful target in cancer fighting-the breast cancer case. Tumor Biol 2013;34:1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach-Klonisch S, Kehlen A, Fowler PA, Huppertz B, Jugert JF, Bischoff G, Schluter E, Buchmann J, Klonisch T. Regulation of functional steroid receptors and ligand-induced responses in telomerase-immortalized human endometrial epithelial cells. J Mol Endocrinol 2005;34:517–534. [DOI] [PubMed] [Google Scholar]
- Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013;339:959–961. [DOI] [PubMed] [Google Scholar]
- Hrdlickova R, Nehyba J, Bose HR Jr. Alternatively spliced telomerase reverse transcriptase variants lacking telomerase activity stimulate cell proliferation. Mol Cell Biol 2012;32:4283–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JG, Yuan R. The expression levels of stem cell markers importin13, c-kit, CD146, and telomerase are decreased in endometrial polyps. Med Sci Monit 2011;17:Br221–Br227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HN, Chiang YC, Cheng WF, Chen CA, Lin MC, Kuo KT. Molecular alterations in endometrial and ovarian clear cell carcinomas: clinical impacts of telomerase reverse transcriptase promoter mutation. Mod Pathol 2015;28:303–311. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liang P, Liu D, Huang J, Songyang Z. Telomere regulation in pluripotent stem cells. Protein Cell 2014;5:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug N, Lingner J. Telomere length homeostasis. Chromosoma 2006;115:413–425. [DOI] [PubMed] [Google Scholar]
- Jacob NK, Lescasse R, Linger BR, Price CM. Tetrahymena POT1a regulates telomere length and prevents activation of a cell cycle checkpoint. Mol Cell Biol 2007;27:1592–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MA, Lee JH, Klingelhutz AJ. HPV16-E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300. Int J Cancer 2006;119:1878–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Tempest N, Parkes C, Alnafakh R, Makrydima S, Adishesh M, Hapangama DK. Hormones and endometrial carcinogenesis. Horm Mol Biol Clin Investig 2016;25:129–148. [DOI] [PubMed] [Google Scholar]
- Kamal AM, Bulmer JN, DeCruze SB, Stringfellow HF, Martin-Hirsch P, Hapangama DK. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br J Cancer 2016;114:688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzers G, Liu D, Rasmussen LJ. Exonuclease 1 and its versatile roles in DNA repair. Crit Rev Biochem Mol Biol 2016;1–12. [DOI] [PubMed] [Google Scholar]
- Kibe T, Osawa GA, Keegan CE, de Lange T. Telomere protection by TPP1 is mediated by POT1a and POT1b. Mol Cell Biol 2010;30:1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr., Friedman AH, Friedman H, Gallia GL, Giovanella BC et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA 2013;110:6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CM, Oh YJ, Cho SH, Chung DJ, Hwang JY, Park KH, Cho DJ, Choi YM, Lee BS. Increased telomerase activity and human telomerase reverse transcriptase mRNA expression in the endometrium of patients with endometriosis. Hum Reprod 2007;22:843–849. [DOI] [PubMed] [Google Scholar]
- Kimura A, Ohmichi M, Kawagoe J, Kyo S, Mabuchi S, Takahashi T, Ohshima C, Arimoto-Ishida E, Nishio Y, Inoue M et al. Induction of hTERT expression and phosphorylation by estrogen via Akt cascade in human ovarian cancer cell lines. Oncogene 2004;23:4505–4515. [DOI] [PubMed] [Google Scholar]
- King AE, Collins F, Klonisch T, Sallenave JM, Critchley HO, Saunders PT. An additive interaction between the NFkappaB and estrogen receptor signalling pathways in human endometrial epithelial cells. Hum Reprod 2010;25:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature 1990;347:400–402. [DOI] [PubMed] [Google Scholar]
- Kiss T, Fayet-Lebaron E, Jady BE. Box H/ACA small ribonucleoproteins. Mol Cell 2010;37:597–606. [DOI] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 1998;396:84–88. [DOI] [PubMed] [Google Scholar]
- Knight SW, Heiss NS, Vulliamy TJ, Greschner S, Stavrides G, Pai GS, Lestringant G, Varma N, Mason PJ, Dokal I et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Hum Genet 1999;65:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C, Spillman MA, Jackson TA, Jacobsen BM, Murphy SK, Lessey BA, Jordan VC, Bradford AP. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol 2012;127:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Mor G, Lockwood C. The immortalization of human endometrial cells. Methods Mol Med 2006;121:79–83. [DOI] [PubMed] [Google Scholar]
- Kuimov AN. Polypeptide components of telomere nucleoprotein complex. Biochemistry (Mosc) 2004;69:117–129. [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 1998;139:4708–4713. [DOI] [PubMed] [Google Scholar]
- Kusama K, Yoshie M, Tamura K, Daikoku T, Takarada T, Tachikawa E. Possible roles of the cAMP-mediators EPAC and RAP1 in decidualization of rat uterus. Reproduction 2014;147:897–906. [DOI] [PubMed] [Google Scholar]
- Kyo S, Kanaya T, Ishikawa H, Ueno H, Inoue M. Telomerase activity in gynecological tumors. Clin Cancer Res 1996;2:2023–2028. [PubMed] [Google Scholar]
- Kyo S, Nakamura M, Kiyono T, Maida Y, Kanaya T, Tanaka M, Yatabe N, Inoue M. Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. Am J Pathol 2003;163:2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. Estrogen activates telomerase. Cancer Res 1999;59:5917–5921. [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kohama T, Inoue M. Telomerase activity in human endometrium. Cancer Res 1997;57:610–614. [PubMed] [Google Scholar]
- Lange CA, Richer JK, Horwitz KB. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol 1999;13:829–836. [DOI] [PubMed] [Google Scholar]
- Lebeau J, Fouchet P, Ory K, Chevillard S. Down-regulation of telomerase activity after progesterone treatment of human breast cancer cells: essential role of the cell cycle status. Anticancer Res 2002;22:2161–2166. [PubMed] [Google Scholar]
- Lee H, Oh JY, Sung YA, Chung H, Kim HL, Kim GS, Cho YS, Kim JT. Genome-wide association study identified new susceptibility loci for polycystic ovary syndrome. Hum Reprod 2015;30:723–731. [DOI] [PubMed] [Google Scholar]
- Lee SS, Bohrson C, Pike AM, Wheelan SJ, Greider CW. ATM kinase is required for telomere elongation in mouse and human cells. Cell Rep 2015;13:1623–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Park NH, Lee H. Prognostic value of alternative lengthening of telomeres-associated biomarkers in uterine sarcoma and uterine carcinosarcoma. Int J Gynecol Cancer 2012;22:434–441. [DOI] [PubMed] [Google Scholar]
- Lehner R, Enomoto T, McGregor JA, Shroyer AL, Haugen BR, Pugazhenthi U, Shroyer KR. Quantitative analysis of telomerase hTERT mRNA and telomerase activity in endometrioid adenocarcinoma and in normal endometrium. Gynecol Oncol 2002;84:120–125. [DOI] [PubMed] [Google Scholar]
- Leon-Blanco MM, Guerrero JM, Reiter RJ, Pozo D. RNA expression of human telomerase subunits TR and TERT is differentially affected by melatonin receptor agonists in the MCF-7 tumor cell line. Cancer Lett 2004;216:73–80. [DOI] [PubMed] [Google Scholar]
- Leri A, Malhotra A, Liew CC, Kajstura J, Anversa P. Telomerase activity in rat cardiac myocytes is age and gender dependent. J Mol Cell Cardiol 2000;32:385–390. [DOI] [PubMed] [Google Scholar]
- Lewis KA, Tollefsbol TO. Regulation of the telomerase reverse transcriptase subunit through epigenetic mechanisms. Front Genet 2016;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Yin G, Chen M, Wu A. Detection of hTERT mRNA in peripheral blood and its implication for diagnosis of early stage postoperative endometrial cancer micrometastasis. Panminerva Med 2016;58:206–210. [PubMed] [Google Scholar]
- Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 1997;276:561–567. [DOI] [PubMed] [Google Scholar]
- Listerman I, Gazzaniga FS, Blackburn EH. An investigation of the effects of the core protein telomerase reverse transcriptase on Wnt signaling in breast cancer cells. Mol Cell Biol 2014;34:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Q, Li K, Chen L, Li W, Hou M, Liu T, Yang J, Lindvall C, Bjorkholm M et al. Telomerase reverse transcriptase promotes epithelial–mesenchymal transition and stem cell-like traits in cancer cells. Oncogene 2013;32:4203–4213. [DOI] [PubMed] [Google Scholar]
- Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 2016;106:6–15. [DOI] [PubMed] [Google Scholar]
- Long N, Liu N, Liu XL, Li J, Cai BY, Cai X. Endometrial expression of telomerase, progesterone, and estrogen receptors during the implantation window in patients with recurrent implantation failure. Genet Mol Res 2016;15. doi: 10.4238/gmr.15027849. [DOI] [PubMed] [Google Scholar]
- Lundblad V. Telomere end processing: unexpected complexity at the end game. Genes Dev 2012;26:1123–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafra FA, Christofolini DM, Cavalcanti V, Vilarino FL, Andre GM, Kato P, Bianco B, Barbosa CP. Aberrant telomerase expression in the endometrium of infertile women with deep endometriosis. Arch Med Res 2014;45:31–35. [DOI] [PubMed] [Google Scholar]
- Maida Y, Kyo S, Kanaya T, Wang Z, Tanaka M, Yatabe N, Nakamura M, Inoue M. Is the telomerase assay useful for screening of endometrial lesions. Int J Cancer 2002;100:714–718. [DOI] [PubMed] [Google Scholar]
- Martinez P, Gomez-Lopez G, Garcia F, Mercken E, Mitchell S, Flores JM, de Cabo R, Blasco MA. RAP1 protects from obesity through its extratelomeric role regulating gene expression. Cell Rep 2013;3:2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massard C, Zermati Y, Pauleau AL, Larochette N, Wetivier D, Sabatier L, Kroemer G, Soria JC. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene 2006;25:4505–4514. [DOI] [PubMed] [Google Scholar]
- Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One 2010;5:e10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Drury JA, Valentijn AJ, Vasieva O, Hapangama DK. In silico, in vitro and in vivo analysis identifies a potential role for steroid hormone regulation of FOXD3 in endometriosis-associated genes. Hum Reprod 2016;31:345–354. [DOI] [PubMed] [Google Scholar]
- Meena J, Rudolph KL, Gunes C. Telomere dysfunction, chromosomal instability and cancer. Recent Results Cancer Res 2015;200:61–79. [DOI] [PubMed] [Google Scholar]
- Mengual Gomez DL, Armando RG, Cerrudo CS, Ghiringhelli PD, Gomez DE. Telomerase as a cancer target. Development of new molecules. Curr Top Med Chem 2016;16:2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhelson VM, Gamaley IA. Telomere shortening is a sole mechanism of aging in mammals. Curr Aging Sci 2012;5:203–208. [DOI] [PubMed] [Google Scholar]
- Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen J, Hirte HW, Piaggio G, Sacchi A, Pontecorvi A, Bacchetti S et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol 2000;20:3764–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdy AD, Cech TR. Low abundance of telomerase in yeast: implications for telomerase haploinsufficiency. RNA 2006;12:1721–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 2013;12:509–519. [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997;277:955–959. [DOI] [PubMed] [Google Scholar]
- Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol 2013;14:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni S, Narducci M, Della Pietra L, Moretti F, Grasselli A, De Carli P, Sacchi A, Pontecorvi A, Farsetti A. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest 2002;110:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness R, Pearce C, Stram D, Berchuck A, Pike M, Pharoah P. Lifetime risk of ovarian cancer based on endometriosis and other risk factors. Int J Gynecol Cancer 2015;25:50–51. [DOI] [PubMed] [Google Scholar]
- Neumann AA, Watson CM, Noble JR, Pickett HA, Tam PP, Reddel RR. Alternative lengthening of telomeres in normal mammalian somatic cells. Genes Dev 2013;27:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourbakhsh M, Golestani A, Zahrai M, Modarressi MH, Malekpour Z, Karami-Tehrani F. Androgens stimulate telomerase expression, activity and phosphorylation in ovarian adenocarcinoma cells. Mol Cell Endocrinol 2010;330:10–16. [DOI] [PubMed] [Google Scholar]
- Novak P, Jensen TJ, Garbe JC, Stampfer MR, Futscher BW. Stepwise DNA methylation changes are linked to escape from defined proliferation barriers and mammary epithelial cell immortalization. Cancer Res 2009;69:5251–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura F, Wakao S, Kuroda Y, Tsuchiyama K, Bagheri M, Heneidi S, Chazenbalk G, Aiba S, Dezawa M. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev 2014;23:717–728. [DOI] [PubMed] [Google Scholar]
- Olovnikov AM. [Principle of marginotomy in template synthesis of polynucleotides]. Dokl Akad Nauk SSSR 1971;201:1496–1499. [PubMed] [Google Scholar]
- Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 1973;41:181–190. [DOI] [PubMed] [Google Scholar]
- Opresko PL, Shay JW. Telomere-associated aging disorders. Ageing Res Rev 2016; doi:10.1016/j.arr.2016.05.009 [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshita T, Nagai N, Mukai K, Shigemasa K, Hiura M, Ohama K. Telomerase activation in endometrial epithelial cells by paracrine effectors from stromal cells in primary cultured human endometrium. Int J Mol Med 2004;13:425–430. [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet 2008;42:301–334. [DOI] [PubMed] [Google Scholar]
- Pandita TK, Benvenuto JA, Shay JW, Pandita RK, Rakovitch E, Geard CR, Antman KH, Newman RA. Effect of penclomedine (NSC-338720) on telomere fusions, chromatin blebbing, and cell viability with and without telomerase activity and abrogated p53 function. Biochem Pharmacol 1997;53:409–415. [DOI] [PubMed] [Google Scholar]
- Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng ZJ, Cheung P, Ji H et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009;460:66–U77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson RJ, Boostanfar R, Saadat P, Mor E, Tourgeman DE, Slater CC, Francis MM, Jain JK. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. JAMA 2002;288:2320–2323. [DOI] [PubMed] [Google Scholar]
- Petersen S, Saretzki G, von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res 1998;239:152–160. [DOI] [PubMed] [Google Scholar]
- Pines A. Telomere length and telomerase activity in the context of menopause. Climacteric 2013;16:629–631. [DOI] [PubMed] [Google Scholar]
- Pirvulet V. Gastrointestinal stem cell up-to-date. J Med Life 2015;8:245–249. [PMC free article] [PubMed] [Google Scholar]
- Plaza-Parrochia F, Bacallao K, Poblete C, Gabler F, Carvajal R, Romero C, Valladares L, Vega M. The role of androst-5-ene-3beta,17beta-diol (androstenediol) in cell proliferation in endometrium of women with polycystic ovary syndrome. Steroids 2014;89:11–19. [DOI] [PubMed] [Google Scholar]
- Prianishnikov VA. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978;18:213–223. [DOI] [PubMed] [Google Scholar]
- Radan L, Hughes CS, Teichroeb JH, Vieira Zamora FM, Jewer M, Postovit LM, Betts DH. Microenvironmental regulation of telomerase isoforms in human embryonic stem cells. Stem Cells Dev 2014;23:2046–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanto YS, Jung JG, Wu RC, Kobayashi Y, Heaphy CM, Meeker AK, Wang TL, Shih IM. Inactivating ARID1A tumor suppressor enhances TERT transcription and maintains telomere length in cancer cells. J Biol Chem 2016;291:9690–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane JK, Greener S, Frame FM, Mann VM, Simms MS, Collins AT, Berney DM, Maitland NJ. Telomerase activity and telomere length in human benign prostatic hyperplasia stem-like cells and their progeny implies the existence of distinct basal and luminal cell lineages. Eur Urol 2016;69:551–554. [DOI] [PubMed] [Google Scholar]
- Robin JD, Ludlow AT, Batten K, Magdinier F, Stadler G, Wagner KR, Shay JW, Wright WE. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes Dev 2014;28:2464–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi L, Pacilli A, Sethi R, Penzo M, Schneider RJ, Trere D, Brigotti M, Montanaro L. Dyskerin depletion increases VEGF mRNA internal ribosome entry site-mediated translation. Nucleic Acids Res 2013;41:8308–8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Harley CB, Baerlocher GM. Imetelstat (GRN163L)—telomerase-based cancer therapy. Recent Results Cancer Res 2010;184:221–234. [DOI] [PubMed] [Google Scholar]
- Royale NJ. Telomeres, 2nd edn Cold Spring Harbor Laboratory Press: New York, 2006. [Google Scholar]
- Runge KW, Lustig AJ. Editorial: the evolving telomeres. Front Genet 2016;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini N, Srinivasan R, Chawla Y, Sharma S, Chakraborti A, Rajwanshi A. Telomerase activity, telomere length and human telomerase reverse transcriptase expression in hepatocellular carcinoma is independent of hepatitis virus status. Liver Int 2009;29:1162–1170. [DOI] [PubMed] [Google Scholar]
- Saito T, Schneider A, Martel N, Mizumoto H, Bulgay-Moerschel M, Kudo R, Nakazawa H. Proliferation-associated regulation of telomerase activity in human endometrium and its potential implication in early cancer diagnosis. Biochem Biophys Res Commun 1997;231:610–614. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Toyoizumi T, Kikuchi Y, Okamoto A, Nakayama H, Aoki D, Yamamoto K, Hata H, Sugishita T, Tenjin Y. Telomerase activity in gynecological tumors. Oncol Rep 2000;7:1003–1009. [DOI] [PubMed] [Google Scholar]
- Salloum R, Hummel TR, Kumar SS, Dorris K, Li S, Lin T, Daryani VM, Stewart CF, Miles L, Poussaint TY et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. J Neurooncol 2016;129:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samartzis EP, Noske A, Dedes KJ, Fink D, Imesch P. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int J Mol Sci 2013;14:18824–18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 2004;3:399–411. [DOI] [PubMed] [Google Scholar]
- Saretzki G. Cellular senescence in the development and treatment of cancer. Curr Pharm Des 2010;16:79–100. [DOI] [PubMed] [Google Scholar]
- Saretzki G. Extra-telomeric functions of human telomerase: cancer, mitochondria and oxidative stress. Curr Pharm Des 2014;20:6386–6403. [DOI] [PubMed] [Google Scholar]
- Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells 2008;26:455–464. [DOI] [PubMed] [Google Scholar]
- Sasano H, Suzuki T, Miki Y, Moriya T. Intracrinology of estrogens and androgens in breast carcinoma. J Steroid Biochem Mol Biol 2008;108:181–185. [DOI] [PubMed] [Google Scholar]
- Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J 2011;30:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JC, Cech TR. Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes Dev 2015;29:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguí N, Pineda M, Guinó E, Borràs E, Navarro M, Bellido F, Moreno V, Lázaro C, Blanco I, Capellá G, Valle L. Telomere length and genetic anticipation in Lynch syndrome. PLoS One. 2013;8(4):e61286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimiya H, Sawada H, Muramatsu Y, Shimizu M, Ohko K, Yamane K, Tsuruo T. Involvement of 14-3-3 proteins in nuclear localization of telomerase. EMBO J 2000;19:2652–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 2009;138:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao G, Balajee AS, Hei TK, Zhao Y. p16INK4a downregulation is involved in immortalization of primary human prostate epithelial cells induced by telomerase. Mol Carcinog 2008;47:775–783. [DOI] [PubMed] [Google Scholar]
- Shay JW. Telomerase as a Target for Cancer Therapeutics. In: Jack A. Roth (ed). Gene-Based Therapies for Cancer, Springer: New York, 2010;231–249. [Google Scholar]
- Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov 2016;6:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Takeuchi T, Mita S, Notsu T, Mizuguchi K, Kyo S. Kruppel-like factor 4 mediates anti-proliferative effects of progesterone with G(0)/G(1) arrest in human endometrial epithelial cells. J Endocrinol Invest 2010;33:745–750. [DOI] [PubMed] [Google Scholar]
- Shroyer KR, Stephens JK, Silverberg SG, Markham N, Shroyer AL, Wilson ML, Enomoto T. Telomerase expression in normal endometrium, endometrial hyperplasia, and endometrial adenocarcinoma. Int J Gynecol Pathol 1997;16:225–232. [DOI] [PubMed] [Google Scholar]
- Shukla S, Schmidt JC, Goldfarb KC, Cech TR, Parker R. Inhibition of telomerase RNA decay rescues telomerase deficiency caused by dyskerin or PARN defects. Nat Struct Mol Biol 2016;23:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhapol C, Pal D, Czapiewski R, Porika M, Nelson G, Saretzki GC. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS One 2013;8:e52989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smekalova EM, Shubernetskaya OS, Zvereva MI, Gromenko EV, Rubtsova MP, Dontsova OA. Telomerase RNA biosynthesis and processing. Biochemistry (Mosc) 2012;77:1120–1128. [DOI] [PubMed] [Google Scholar]
- Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol 2003;5:474–479. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem 2004;73:177–208. [DOI] [PubMed] [Google Scholar]
- Soda H, Raymond E, Sharma S, Lawrence R, Davidson K, Oka M, Kohno S, Izbicka E, Von Hoff DD. Effects of androgens on telomerase activity in normal and malignant prostate cells in vitro. Prostate 2000;43:161–168. [DOI] [PubMed] [Google Scholar]
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med 2014;2014:179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler G, Rahimov F, King OD, Chen JC, Robin JD, Wagner KR, Shay JW, Emerson CP Jr., Wright WE. Telomere position effect regulates DUX4 in human facioscapulohumeral muscular dystrophy. Nat Struct Mol Biol 2013;20:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol 2003;13:1549–1556. [DOI] [PubMed] [Google Scholar]
- Takai KK, Kibe T, Donigian JR, Frescas D, de Lange T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell 2011;44:647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kyo S, Takakura M, Kanaya T, Sagawa T, Yamashita K, Okada Y, Hiyama E, Inoue M. Expression of telomerase activity in human endometrium is localized to epithelial glandular cells and regulated in a menstrual phase-dependent manner correlated with cell proliferation. Am J Pathol 1998;153:1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Xie R, Qin Y, Xiao YF, Yong X, Zheng L, Dong H, Yang SM. Human telomerase reverse transcriptase (hTERT) promotes gastric cancer invasion through cooperating with c-Myc to upregulate heparanase expression. Oncotarget 2016;7:11364–11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terali K, Yilmazer A. New surprises from an old favourite: the emergence of telomerase as a key player in the regulation of cancer stemness. Biochimie 2016;121:170–178. [DOI] [PubMed] [Google Scholar]
- Theimer CA, Feigon J. Structure and function of telomerase RNA. Curr Opin Struct Biol 2006;16:307–318. [DOI] [PubMed] [Google Scholar]
- Thelen P, Wuttke W, Jarry H, Grzmil M, Ringert RH. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J Urol 2004;171:1934–1938. [DOI] [PubMed] [Google Scholar]
- Tichon A, Gowda BK, Slavin S, Gazit A, Priel E. Telomerase activity and expression in adult human mesenchymal stem cells derived from amyotrophic lateral sclerosis individuals. Cytotherapy 2009;11:837–848. [DOI] [PubMed] [Google Scholar]
- Toki T, Mori A, Shimizu M, Nikaido T, Fujii S. Localization of apoptotic cells within the human endometrium and correlation between apoptosis and p21 expression. Mol Hum Reprod 1998;4:1157–1164. [DOI] [PubMed] [Google Scholar]
- Tomlinson RL, Ziegler TD, Supakorndej T, Terns RM, Terns MP. Cell cycle-regulated trafficking of human telomerase to telomeres. Mol Biol Cell 2006;17:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AS, Stern JL, Sfeir A, Kartawinata M, de Lange T, Zhu XD, Bryan TM. ATM and ATRsignaling regulate the recruitment of human telomerase to telomeres . Cell Rep 2015;13:1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley DM, Dumitriu B, Liu D, Biancotto A, Weinstein B, Chen C, Hardy N, Mihalek AD, Lingala S, Kim YJ et al. Danazol treatment for telomere diseases. N Engl J Med 2016;374:1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaner GA, Giudice LC. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol Hum Reprod 1997;3:769–773. [DOI] [PubMed] [Google Scholar]
- Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res 1998;58:4168–4172. [PubMed] [Google Scholar]
- Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Tissue-specific alternate splicing of human telomerase reverse transcriptase (hTERT) influences telomere lengths during human development. Int J Cancer 2001;91:644–649. [PubMed] [Google Scholar]
- Ulaner GA, Hu JF, Vu TH, Oruganti H, Giudice LC, Hoffman AR. Regulation of telomerase by alternate splicing of human telomerase reverse transcriptase (hTERT) in normal and neoplastic ovary, endometrium and myometrium. Int J Cancer 2000;85:330–335. [PubMed] [Google Scholar]
- Valentijn AJ, Palial K, Al-lamee H, Tempest N, Drury J, Von Zglinicki T, Saretzki G, Murray P, Gargett CE, Hapangama DK. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod 2013;28:2695–2708. [DOI] [PubMed] [Google Scholar]
- Valentijn AJ, Saretzki G, Tempest N, Critchley HOD, Hapangama DK. Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Hum Reprod 2015;30:2816–2828. [DOI] [PubMed] [Google Scholar]
- Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, Terns MP, Artandi SE. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science 2009;323:644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JD, Register TC, Gupta M, Cline JM. Estrogen replacement therapy induces telomerase RNA expression in the macaque endometrium. Fertil Steril 2002;77:601–608. [DOI] [PubMed] [Google Scholar]
- Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett 2005;579:2541–2545. [DOI] [PubMed] [Google Scholar]
- Vogan JM, Collins K. Dynamics of human telomerase holoenzyme assembly and subunit exchange across the cell cycle. J Biol Chem 2015;290:21320–21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci 2000;908:99–110. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 2002;27:339–344. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence. Exp Cell Res 1995;220:186–193. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Serra V, Lorenz M, Saretzki G, Lenzen-Grossimlighaus R, Gessner R, Risch A, Steinhagen-Thiessen E. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor. Lab Invest 2000;80:1739–1747. [DOI] [PubMed] [Google Scholar]
- Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 2007;445:506–510. [DOI] [PubMed] [Google Scholar]
- Wang XB, Zhu L, Huang J, Yin YG, Kong XQ, Rong QF, Shi AW, Cao KJ. Resveratrol-induced augmentation of telomerase activity delays senescence of endothelial progenitor cells. Chin Med J (Engl) 2011;124:4310–4315. [PubMed] [Google Scholar]
- Wang Z, Kyo S, Takakura M, Tanaka M, Yatabe N, Maida Y, Fujiwara M, Hayakawa J, Ohmichi M, Koike K et al. Progesterone regulates human telomerase reverse transcriptase gene expression via activation of mitogen-activated protein kinase signaling pathway. Cancer Res 2000;60:5376–5381. [PubMed] [Google Scholar]
- Wang Z, Rhee DB, Lu J, Bohr CT, Zhou F, Vallabhaneni H, de Souza-Pinto NC, Liu Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet 2010;6:e1000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD. The regulation of DNA synthesis in eukaryotes. Adv Cell Biol 1971;2:1–46. [DOI] [PubMed] [Google Scholar]
- Watson JD. Origin of concatemeric T7 DNA. Nat New Biol 1972;239:197–201. [DOI] [PubMed] [Google Scholar]
- Webb CJ, Zakian VA. Telomerase RNA is more than a DNA template. RNA Biol 2016;13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet 1997;17:498–502. [DOI] [PubMed] [Google Scholar]
- Williams CD, Boggess JF, LaMarque LR, Meyer WR, Murray MJ, Fritz MA, Lessey BA. A prospective, randomized study of endometrial telomerase during the menstrual cycle. J Clin Endocrinol Metab 2001;86:3912–3917. [DOI] [PubMed] [Google Scholar]
- Wu RC, Ayhan A, Maeda D, Kim KR, Clarke BA, Shaw P, Chui MH, Rosen B, Shih IeM, Wang TL. Frequent somatic mutations of the telomerase reverse transcriptase promoter in ovarian clear cell carcinoma but not in other major types of gynaecological malignancy. J Pathol 2014;232:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi L, Cech TR. Inventory of telomerase components in human cells reveals multiple subpopulations of hTR and hTERT. Nucleic Acids Res 2014;42:8565–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WB, Awadallah A, Xin W. Loss of ARID1A/BAF250a expression in ovarian endometriosis and clear cell carcinoma. Int J Clin Exp Pathol 2012;5:642–650. [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Marvin ME, Ivanova IG, Lydall D, Louis EJ, Maringele L. Rif1 and Exo1 regulate the genomic instability following telomere losses. Aging Cell 2016;15:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Przyborski S, Cooke MJ, Zhang X, Stewart R, Anyfantis G, Atkinson SP, Saretzki G, Armstrong L, Lako M. A key role for telomerase reverse transcriptase unit in modulating human embryonic stem cell proliferation, cell cycle dynamics, and in vitro differentiation. Stem Cells 2008;26:850–863. [DOI] [PubMed] [Google Scholar]
- Yang J, Huang F. Stem cell and endometriosis: new knowledge may be producing novel therapies. Int J Clin Exp Med 2014;7:3853–3858. [PMC free article] [PubMed] [Google Scholar]
- Yang XWW, Wang Y, Chen W, Li X. Radiant decrease in telomerase and its RNA in progressive passage of human endometrial stromal stem cells. J Med Col PLA 2011;26:254–263. [Google Scholar]
- Yeap BB, Knuiman MW, Divitini ML, Hui J, Arscott GM, Handelsman DJ, McLennan SV, Twigg SM, McQuillan B, Hung J et al. Epidemiological and mendelian randomization studies of dihydrotestosterone and estradiol and leukocyte telomere length in men. J Clin Endocrinol Metab 2016;101:1299–1306. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Takahashi Y, Morishita S, Hashimoto M, Niwa K, Tamaya T. Telomerase activity in the human endometrium throughout the menstrual cycle. Mol Hum Reprod 1998;4:173–177. [DOI] [PubMed] [Google Scholar]
- Yoo HH, Chung IK. Requirement of DDX39 DEAD box RNA helicase for genome integrity and telomere protection. Aging Cell 2011;10:557–571. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Sumida T, Liu S, Onishi A, Shintani S, Desprez PY, Hamakawa H. Growth inhibition of human salivary gland tumor cells by introduction of progesterone (Pg) receptor and Pg treatment. Endocr Relat Cancer 2007;14:1107–1116. [DOI] [PubMed] [Google Scholar]
- Yuan R-H, Chang K-T, Chen Y-L, Hsu H-C, Lee P-H, Lai P-L, Jeng Y-M. S100P Expression Is a Novel Prognostic Factor in Hepatocellular Carcinoma and Predicts Survival in Patients with High Tumor Stage or Early Recurrent Tumors United States: Public Library of Science, 2013;e65501–e65501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafrakas M, Grimbizis G, Timologou A, Tarlatzis BC. Endometriosis and ovarian cancer risk: a systematic review of epidemiological studies. Front Surg 2014;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Guo L, Peng Y, Chen B. Expression of T-STAR gene is associated with regulation of telomerase activity in human colon cancer cell line HCT-116. World J Gastroenterol 2006;12:4056–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ST, Zuo C, Li WN, Fu XQ, Xing S, Zhang XP. Identification of key genes associated with the effect of estrogen on ovarian cancer using microarray analysis. Arch Gynecol Obstet 2016;293:421–427. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen LY, Han X, Xie W, Kim H, Yang D, Liu D, Songyang Z. Phosphorylation of TPP1 regulates cell cycle-dependent telomerase recruitment. Proc Natl Acad Sci U S A 2013;110:5457–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sfeir AJ, Zou Y, Buseman CM, Chow TT, Shay JW, Wright WE. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell 2009;138:463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Bae-Jump VL, Whang YE, Gehrig PA, Boggess JF. The PTEN tumor suppressor inhibits telomerase activity in endometrial cancer cells by decreasing hTERT mRNA levels. Gynecol Oncol 2006;101:305–310. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Voss M, Kaiser S, Kapp U, Waller CF, Martens UM. Lack of telomerase activity in human mesenchymal stem cells. Leukemia 2003;17:1146–1149. [DOI] [PubMed] [Google Scholar]