Abstract
STUDY QUESTION
Does developmental exposure to the combination of hyperandrogenemia and western-style diet (WSD) worsen adult metabolic function compared to either treatment alone?
SUMMARY ANSWER
Young female rhesus macaques treated for 3 years, beginning at menarche, with combined testosterone (T) and WSD have increased weight gain and insulin resistance compared to controls and animals treated with either T or WSD alone.
WHAT IS KNOWN ALREADY
Hyperandrogenemia is a well-established component of polycystic ovary syndrome (PCOS) and can be observed in peripubertal girls, indicating a potential pubertal onset of the disease. Obesity is often associated with hyperandrogenemia in peripubertal girls, and overweight girls appear to be at higher risk for the development of PCOS later in life.
STUDY DESIGN, SIZE, DURATION
Juvenile (2.5- year old) female rhesus macaques were divided into four groups (n = 10/group): control animals receiving cholesterol implants and a control diet with 15% of calories derived from fat (C), animals receiving T implants (mean serum levels: 1.35 ± 0.01 ng/ml) and a control diet (T), animals receiving a cholesterol implant and a WSD with 36% of calories derived from fat (WSD) and animals receiving a T implant and a WSD (T + WSD). Animals were maintained on the treatments for 36 months and were 5.5 years old at study completion.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Metabolic testing consisted of body measurements including weight, dual-energy X-ray absorptiometry scans, activity monitoring, and glucose tolerance testing at zero months and at least once every 12 months for the remainder of the study. Indirect calorimetry and serum hormone assays were performed following 36 months of treatment.
MAIN RESULTS AND THE ROLE OF CHANCE
Body weight and fat mass gain were significantly increased in T + WSD at 24 and 36 months of treatment compared to the other three groups. Log transformed fasting insulin and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) were significantly increased in T + WSD animals at 3 years of treatment compared to all other groups. T-treatment caused a greater rate of decline in activity after 18 months, while food intake and metabolic rate were largely unaffected by treatments.
LIMITATIONS REASONS FOR CAUTION
Variability was present in the metabolic parameters measured; however, this is similar to the heterogeneity observed in human populations.
WIDER IMPLICATIONS OF THE FINDINGS
Chronic hyperandrogenemia beginning at puberty may exacerbate metabolic dysfunction in women consuming a WSD and account for the increased rates of obesity and insulin resistance observed in PCOS patients. Counseling of female patient populations with elevated androgens about the potential benefit of consuming a lower fat diet could improve long-term metabolic health outcomes.
STUDY FUNDING/COMPETING INTEREST(S)
Eunice Kennedy Shriver National Institute of Child Health & Human Development P50HD071836 and Oregon National Primate Center Grant P51 OD011092. The authors have no competing conflict of interests to disclose.
Keywords: PCOS, hyperandrogenemia, macaque, western-style diet, obesity, hyperinsulinemia
Introduction
Polycystic ovary syndrome (PCOS) is an infertility disorder affecting ~5–15% of women in the USA, with a similar incidence reported around the world (Goodarzi and Azziz, 2006; Sirmans and Pate, 2013). Due to the heterogeneity within this patient population, the Rotterdam criteria for diagnosis requires only two of the three following symptoms (Rotterdam, 2004): oligoamennorhea, hyperandrogenemia and polycystic ovaries upon ultrasound. Basic science has provided considerable evidence that early developmental exposure to androgens in animal models, including monkeys and sheep, can reproduce many symptoms of PCOS (Eisner et al., 2002; Foecking et al., 2005; Steckler et al., 2005; Xita and Tsatsoulis, 2006; Abbott et al., 2009). Previous studies have largely relied on a model of in utero androgen exposure by delivering large doses of androgens to pregnant dams. While maternal elevation of androgens in animal models seems to recapitulate symptoms of PCOS, there is little evidence this pathology underlies the development of PCOS in humans. Hyperandrogenemia is observed during development, but much later during the peripubertal transition. Peripubertal girls with a 2- to 4-fold increase in total testosterone (T) have previously been described (McCartney et al., 2007) and, significantly, these levels of hyperandrogenemia are also associated with early signs of reproductive dysfunction (Apter et al., 1994). This includes increased LH pulse frequency during the night, which could precede the development of increased 24-h LH pulse frequency observed in PCOS patients. Studies using a nonhuman primate model demonstrated that administration of androgens to prepubertal monkeys also results in increased LH pulse frequency (McGee et al., 2012). When these same animals were switched to a high-fat western-style diet (WSD), evidence of ovarian dysfunction, as demonstrated by increases in the number of small antral follicles, was also present. These findings indicate a later onset of androgen elevation may also predispose toward PCOS-like phenotypes.
In addition to the initial three criteria used for diagnosis of PCOS, obesity and insulin resistance make up a fourth common feature of the disorder. Roughly 50–70% of PCOS patients have signs of insulin resistance and 50–80% are overweight or obese (Legro et al., 2001; Ovalle and Azziz, 2002). Both lean and obese PCOS women have increased rates of insulin resistance and type two diabetes compared with BMI matched controls (Dunaif et al., 1989; Legro et al., 2001; Norman et al., 2001). Although such metabolic dysregulation is not required as part of the diagnosis, there is evidence that it plays a potentially causative role in some PCOS patients. Metformin, an insulin sensitizer, can be prescribed for the treatment of PCOS, and was initially reported to improve reproductive outcomes, indicating that insulin insensitivity may underlie infertility symptoms in at least some PCOS patients (Velazquez et al., 1994; Jakubowicz et al., 2002). The efficacy of metformin for improving reproductive function has since been debated (Tang et al., 2006; Morin-Papunen et al., 2012); however, improvement of insulin sensitivity following weight loss also has been reported to improve reproductive outcomes in PCOS patients (Pasquali et al., 1989; Tang et al., 2006). Importantly, obesity in peripubertal girls is associated with higher androgen levels indicating an early link between metabolic dysfunction and hyperandrogenemia (McCartney et al., 2006, 2007).
A critical question in PCOS is parsing how potential causative factors like hyperandrogenemia and metabolic dysfunction interact. In our previous pilot study, combined T-treatment and exposure to an obesogenic WSD decreased insulin sensitivity and increased the numbers of small antral follicles in nonhuman primates (McGee et al., 2014). While this study provided critical evidence that the combination of T and WSD worsens symptoms associated with PCOS, it was limited in its capacity to provide the necessary controls to determine whether the effects of these treatments were additive or synergistic. To systematically investigate the combined roles of T and WSD on PCOS-like symptoms, a collaborative center project is being performed at the Oregon National Primate Research Center. Juvenile female rhesus macaques began treatment with either T, WSD or T + WSD around the time of menarche and results following three years of treatment are reported here. During this time, general metabolic health was investigated and is described in the current study. Additional projects on this model included investigations of adipocyte physiology (see companion paper by Varlamov et al., 2017) and reproductive health (manuscript in preparation).
Materials and Methods
Animals
All animal procedures were approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee and comply with the Animal Welfare Act and the APA Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research. Female rhesus macaques were selected for the study and randomly assigned to one of four treatment groups (n = 10/group) (Table I): control animals receiving cholesterol implants and a control diet (C), animals receiving T implants and a control diet (T), animals receiving cholesterol implants and a WSD and animals receiving T implants and a WSD (T + WSD). The average age at the beginning of the study, corresponding to the time 0 glucose tolerance test (GTT), was 2.38 ± 0.04 years of age. Animals were typically pair-housed in the same treatment group and maintained on a 07:00–19:00 light cycle with ad libitum access to water. Individual enrichment devices were provided on each cage and animals were given weekly access to additional enrichment activities (radio, TV etc.). All sedations for procedures were induced with ketamine HCl (5–15 mg/kg) (Ketamine™ Henry Schein Animal Health, Dublin, OH) or Tiletamine HCl and Zolazepam HCL mixture (3–5 mg/kg) (Telazol™ Zoetis Inc, Kalamazoo, MI) unless otherwise noted.
Table I.
Treatment paradigms and animal groups.
| Group (n = 10) | Treatment (subcutaneous silastic implant) | Diet | ||
|---|---|---|---|---|
| Fat (%) | Carbohydrate (%) | Protein (%) | ||
| Chow (Purina 5052) | ||||
| C | Cholesterol | 15 | 58 | 27 |
| T | Testosterone (serum: 1.35 ± 0.01 ng/ml) | |||
| WSD (Purina 5L0P) | ||||
| WSD | Cholesterol | 36 | 46 | 18 |
| T + WSD | Testosterone (serum: 1.35 ± 0.01 ng/ml) | |||
Testosterone treatment
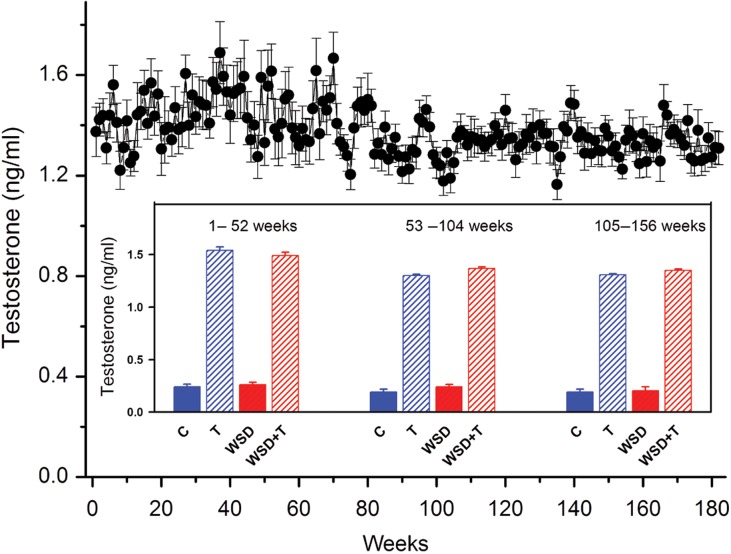
T-releasing capsules were prepared by packing medical grade Silastic tubing (0.34 cm i.d.; 0.64 cm o.d.; Dow Corning, Midland MI) with a mixture of cholesterol and T (Sigma) at a ratio of 9:1 (T and T + WSD groups). The capsule was implanted subcutaneously in the interscapular region of the monkeys. Weekly blood samples were assayed for serum T, and when levels dropped below 1.0 ng/ml, the capsules were replaced. A target range of ~1.4 ng/ml was maintained by changing the length of the T-releasing capsules from 1 to 5 cm over the study period. This range approximated the 3- to 4-fold increase in T levels observed in peripubertal girls at risk for PCOS (McCartney et al., 2006, 2007). Serum T was assayed on an automated clinical platform Roche Cobas e411 by the Oregon National Primate Research Center (ONPRC) Endocrine Technology Support Core Laboratory (Fig. 1). Average total T-values in all T-treated animals across the 36-month interval was 1.35 ± 0.01 ng/ml (T and T + WSD, n = 20) and was significantly higher than the average serum T levels in the cholesterol treated groups 0.27 ± 0.03 ng/ml (C and WSD, n = 20; Student's t-test P < 0.05). To validate the Roche assay, a subset of samples from T-treated monkeys was assayed by liquid chromatography mass spectrometry (LC-MS). That comparison revealed no significant difference (Student's t-test, P = 0.14) between levels assayed on LC-MS (1.19 ± 0.06 ng/ml) and Roche platforms (1.29 ± 0.05 ng/ml).
Figure 1.
Serum testosterone (T) levels in combined T and T + WSD treatment groups. Testosterone was measured weekly by Roche platform in both T-treated groups (mean 1.35 ± 0.01 ng/ml, n = 20). Implants were replaced when testosterone levels dropped below 1.0 ng/ml. Comparison of T-treated groups to groups receiving cholesterol implants (inset; mean levels in C and WSD treatment groups 0.27 ± 0.03) demonstrated a consistent elevation of T across the 36-month treatment period (P < 0.01). WSD, western-style diet.
Diet treatments
The control diet, in standard use within the ONPRC colony, contained 15% of calories derived from fat (Purina 5000/5052). The WSD, in standard use within the ONPRC's Obese Primate Resource, contained 36% of calories from fat (Purina 5L0P; Table I), and has been previously described (McCurdy et al., 2009). The increased fat content in WSD is due to increased animal-based fat and a decrease in both protein and carbohydrate content. All animals received diet supplements in the forms of daily fruits and vegetables. WSD treated animals also received an additional calorically dense treat composed of peanut butter, honey, banana, cornstarch and WSD pellets (~250 kcal).
Body composition measurements
Body weights were measured prior to treatment onset (time 0) and at 1, 3, 6, 12, 18, 24 and 36 months on treatment. Animals were sedated prior to GTT and weighed on a scale. To determine body composition, a dual-energy X-ray absorptiometry (DEXA) Scan (Horizon A QDR DXA System, Hologic Inc.) was performed at all the listed time points, except at 1 month. The adult scan setting was used and scans were typically performed on a separate day from GTTs following sedation with Telazol. Android and gynoid regions were defined manually as described previously (Kang et al., 2011). Animals also underwent measurements of crown-rump length for BMI calculations as well as abdominal circumference.
Indirect calorimetry for energy expenditure
Metabolic rate and respiratory quotient (RQ) were measured using indirect calorimetry following 36 months of treatment. Animals were placed in a stainless-steel metabolic chamber (dimensions: 2.7 × 2.7 × 2.5 ft.) with a computer-controlled indirect open-circuit calorimeter (Oxymax System, Columbus Instruments, Columbus, OH) to measure the amount of carbon dioxide produced and oxygen consumed using previously published methodology (Sullivan et al., 2006; Sullivan and Cameron, 2010). Animals were placed in the chamber the night before the test for an acclimation period of ~15 h, then metabolic measurements were collected for 24 h beginning at 07:00 h. Monkeys were fed a banana at 09:00 h and their usual diet (chow or WSD) at 14:00 h. Any remaining food was removed at 16:00 h and the chamber was washed and resealed. To limit social isolation during testing, a familiar monkey was housed across from the monkey undergoing metabolic testing. Oxymax software calculations of heat (kcal/h) and RQ (VC02/V02) were used for statistical analysis. Basal metabolic rate (BMR) was determined by averaging heat values from 01:00 to 05:00 h, a time period when monkeys were typically asleep. Daily kcal energy expenditure was calculated by summing the average heat values across the 24-h sampling period. RQ was used to determine metabolic substrate preference and daytime averages were taken between 08:00 and 18:00 h and nighttime averages were taken between 20:00 to 06:00 h.
Physical activity measurements
Physical activity was assessed continuously throughout this study, as described previously (Sullivan et al., 2006; Papailiou et al., 2008). All monkeys wore metal collars with a metal case housing an omnidirectional accelerometer (Actical; Philips, Bend, OR). The accelerometers were programmed to record total activity counts per minute, and data were downloaded approximately once every 5 weeks when monkeys were sedated. Whenever possible, acclerometers were downloaded and reprogrammed when the monkeys were sedated for collection of other experimental measures.
Glucose tolerance test
An intravenous GTT was performed at time 0 and following 1, 3, 6, 12, 18, 24 and 36 months of treatment as described previously (McCurdy et al., 2009). Briefly, animals were fasted overnight, sedated (with Telazol) and a glucose bolus (50% dextrose solution) administered at a dose of 0.6 g/kg via the saphenous vein. Baseline blood samples were obtained prior to the glucose injection, and 1-ml blood samples were taken at 1, 3, 5, 10, 20, 40 and 60 min later by venipuncture (typically saphenous vein). Glucose was measured immediately using a OneTouch Ultra Blood Glucose Monitor (LifeScan), and the remainder of the blood was placed in heparinized tubes on ice for measurements of insulin. Baseline GTT samples following 36 months of treatment were used for hormone assays (below). After i.v. GTT, samples were centrifuged at 2400 g for 20 min, and plasma was stored at −80°C until assayed. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was determined as described previously (Matthews et al., 1985; Bonora et al., 2000) and calculated as fasting serum insulin (μU/ml) × fasting plasma glucose (mg/dl)/405.
Hormone assays
Plasma concentrations of insulin, leptin, adiponectin, ghrelin and C-reactive protein (CRP) were measured by the Endocrine Technologies Support Core (ETSC) at the ONPRC. All quality controls and calibrations provided by the company, as well as ETSC monkey serum controls, were analyzed with test samples. Assay parameters and previous validation are provided in Supplementary Materials and Methods.
Statistics
Longitudinal data were analyzed by mixed model in SPSS to examine treatment effects (chow versus WSD, cholesterol versus T implant) over time. When sphericity was violated, Greenhouse–Geisser calculations were used. Single time-point data sets were analyzed by linear model in SPSS to examine treatment effects. Post hoc analysis consisted of Tukey's multiple comparison tests between the four treatment groups using Prism GraphPad 7.0. Individual AUC was calculated from zero in Prism GraphPad 7.0. Longitudinal data that was not normally distributed was log transformed, while single time point data sets were analyzed by Mann–Whitney nonparametric analysis in SPSS. For longitudinal data, x-axis ‘month’ values were adjusted ±1 month to allow for better visualiziation when datapoints from multiple groups overlapped.
Daily mean activity was calculated for each monkey for each day of the study. Activity during days on which monkeys were sedated or anesthetized for procedures was removed prior to analyses. For some procedures (e.g. fat biopsies) activity was depressed for more than 1 day. In these cases, all days of activity that were lower than the 10-day baseline activity prior to the procedure were removed prior to analyses. The daily mean activity levels were log base 10 transformed for normality and quarterly (3-month) means of these log transformed daily activity levels were then calculated for each monkey. A random effects repeated-measures regression of quarterly activity levels was calculated using a linear mixed model analysis (JMP, version 13), with an AR(1) repeated covariance type for the quarterly data and entering T, WSD and T + WSD interaction term as fixed effects. Because examination of the curves suggested that a polynomial would better describe them, a repeated-measures general linear model with a polynomial decomposition of the within-subject contrasts was also done to examine activity changes over time. Data are presented as mean ± SEM and all graphs were composed in Prism GraphPad 7.0.
Results
Body weight and composition
Longitudinal analysis found that body weight increased steadily in the control group over the 36-month period (Fig. 2A). T-treatment caused greater increases in body weight over time (T-treatment ∗ time: F = 7.80, P < 0.01), with a trend towards an interaction between T- and WSD-treatment to further increase weight over time (T-treatment ∗ diet ∗ time: F = 3.43, P = 0.06). Post hoc analysis revealed that T + WSD animals weighed significantly more than all other groups at both 24 and 36 months of treatment (P's < 0.05). Similarly, normalized weight gain revealed T-treatment caused a greater increase in the percentage of weight gained over time (Fig. 2B; T-treatment ∗ time: F = 11.11, P < 0.01) along with a significant interaction for T- and WSD-treatments to further increase weight gain over time (T-treatment ∗ diet ∗ time: F = 4.33, P = 0.03). Post hoc analysis again found T + WSD animals had significantly increased weight gain compared to all other groups at 18, 24 and 36 months of treatment (P's < 0.05). To determine whether animal size was a contributing factor, BMI was calculated (BW/(CR)2). In the control group, BMI increased over time but appeared to be leveling off towards the end of the 36-month period (Fig. 2C). Longitudinal analysis found effects of both T- and WSD-treatments to cause a greater increase in BMI over time (diet ∗ time: F = 3.21, P = 0.02; T-treatment ∗ time: F = 5.73, P < 0.01) as well as a significant interaction for T- and WSD-treatments to further increase BMI over time (T-treatment ∗ diet ∗ time: F = 2.76, P = 0.04). Post hoc analysis revealed significantly increased BMI in the T + WSD compared to the other three groups at 24 and 36 months of treatment (P's < 0.05).
Figure 2.
Testosterone (T) + WSD increases body weight and fat mass with no direct effect on food intake. Raw body weight (A), change in body weight (B), BMI measurements (C), change in fat mass (D), change in lean mass (E) and abdominal circumference (F) were evaluated in Control (C), T, WSD and T + WSD groups at time 0 and following 3, 6, 12, 18, 24 and 36 months of treatment. Values are the mean ± SEM with n = 10/group. Significant effects of diet, T-treatment or interactions over time as determined by a mixed models ANOVA are presented as text within the graphs. Asterisks denote significant difference between T + WSD group and C, T and WSD groups by Tukey's multiple comparison post hoc analysis.
Longitudinal analysis of body composition by DEXA scan revealed little change in fat mass in the control group over the first year of the treatment, but increased fat mass gain over the latter 24 months of the treatment period (Fig. 2D). There was a significant effect of T- and WSD-treatments to increase fat mass gain over time (diet ∗ time: F = 7.21, P < 0.01; T ∗ time: F = 5.00, P = 0.02), and a trend for T- and WSD-treatments to interact and cause a further gain in fat mass over time (T-treatment ∗ diet ∗ time: F = 3.42, P = 0.06). Post hoc analysis revealed a significant increase in fat mass gained in the T + WSD group compared to all other groups at 24 and 36 months of treatment (P's < 0.05). Unlike the change in fat mass, the control group showed large increases in lean mass during the first 12 months of treatment and then a slower rate of increase in the latter 24 months of treatment (Fig. 2E). Longitudinal analysis of the change in lean mass found an overall effect of T-treatment to further increase lean mass over time (T-treatment ∗ time: F = 8.77, P < 0.01), but there were no post hoc differences between the four groups. Of note, all groups showed a level of heterogeneity in measures of body weight and fat mass gain and specifically T + WSD treatment appeared to have a larger impact on some animals compared to others (Supplementary Fig. S1). Food intake was measured across the study, and caloric intake across pairs of animals found WSD-treatment decreased caloric intake over time (diet ∗ time: F = 5.55, P < 0.01; Supplementary Fig. S2). However, post hoc analysis did not reveal any significant differences in food intake between the four individual groups at any time point.
Body fat distribution
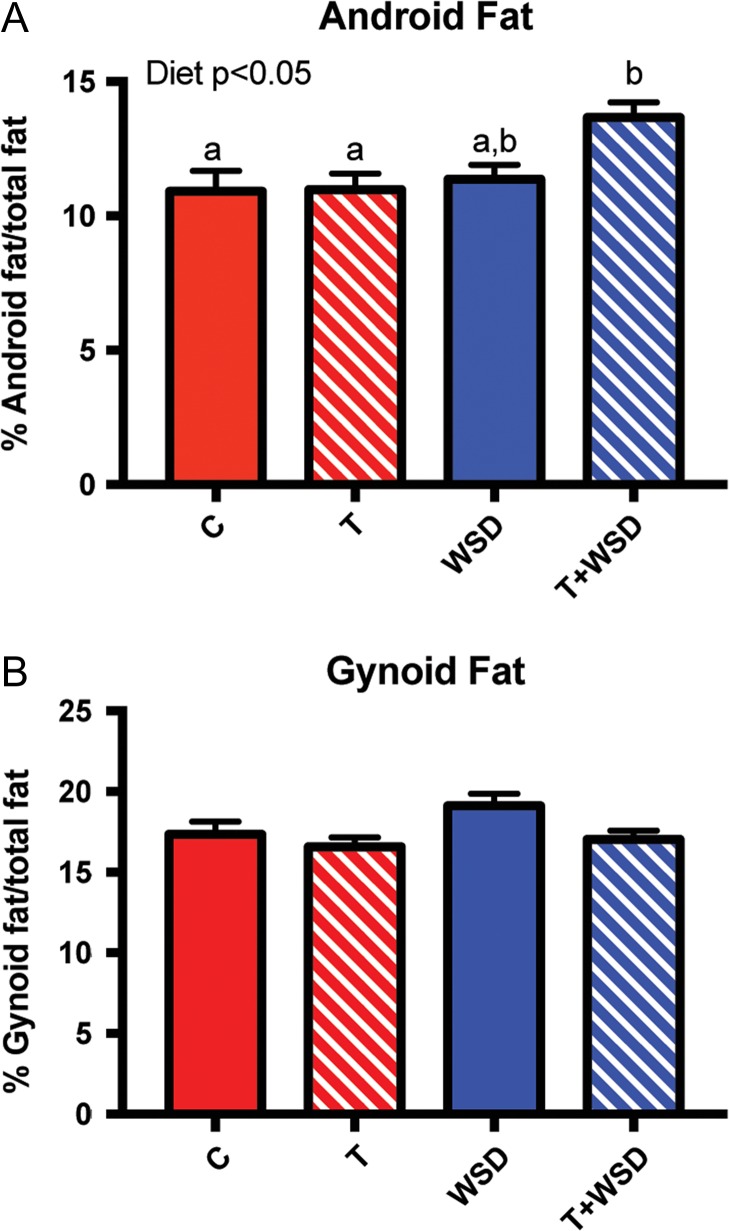
Abdominal circumference showed a gradual increase over the 36 months of treatment in the control group (Fig. 2F). Longitudinal analysis found that T-treatment caused a greater increase in abdominal circumference over time (T-treatment ∗ time: F = 3.25, P = 0.04) with a trend for WSD-treatment to also increase abdominal circumference (diet ∗ time: F = 2.75, P = 0.06) and an interaction for T- and WSD-treatments to cause further increases over time (T-treatment ∗ diet ∗ time: F = 2.56, P = 0.08). Post hoc analysis revealed a significant increase in abdominal circumference in the T + WSD group compared to the other three groups at 24 and 36 months of treatment (P's < 0.05). At 36 months of treatment, the percentage of fat that was in the android region was significantly greater with WSD-treatment (diet: F = 6.34, P = 0.02) but not by T-treatment (Fig. 3A). Post hoc analysis revealed a significant increase in android fat in the T + WSD group compared to C and T groups (P's < 0.05). Fat in the gynoid region was not affected by either T- or WSD-treatment (Fig. 3B).
Figure 3.
Testosterone (T) + WSD increases fat accumulation in the android region. DEXA analysis of fat in the android (A) and gynoid (B) regions was calculated as a percentage of the total fat. Significant effects of diet, T-treatment or interactions over time as determined by two-way ANOVA for android fat are presented as text within the graphs. Different letters denote significant differences between groups as determined by Tukey's multiple comparison post hoc analysis. DEXA, dual-energy X-ray absorptiometry.
Insulin sensitivity
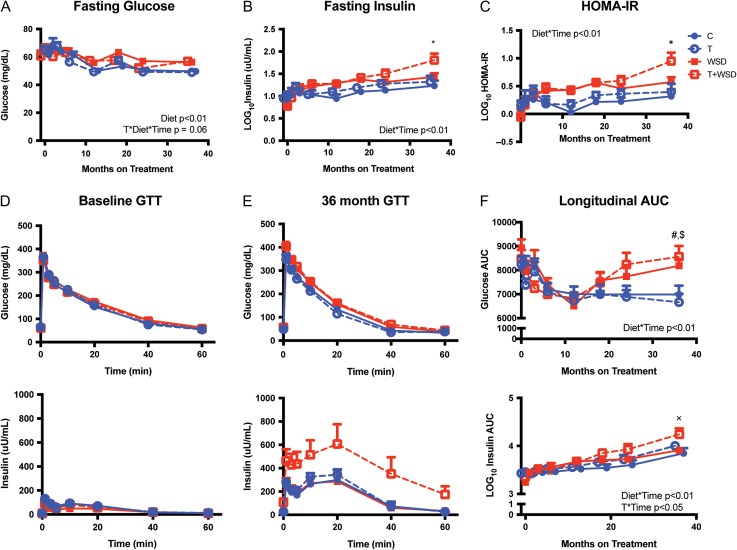
Fasting glucose showed a modest decline in the control group over the first 12 months, with relatively little change over the latter 24 months of treatment (Fig. 4A). Longitudinal analysis indicates that WSD-treatment increased fasting glucose over time (diet ∗ time: F = 3.59, P < 0.01) and there was a trend for an interaction of T- and WSD-treatments to further increase fasting glucose over time (T-treatment ∗ diet ∗ time: F = 1.98, P = 0.06). Post hoc analysis did not reveal individual group differences at any time point. Log transformed fasting insulin increased gradually over the 36 months in the control group (Fig. 4B). Longitudinal analysis found an overall effect of WSD-treatment to cause a greater increase in fasting insulin over time (diet ∗ time: F = 4.66, P < 0.01). Post hoc analysis found that log10 fasting insulin was significantly higher in the T + WSD group compared the other three groups at 36 months of treatment. Log transformed HOMA-IR was relatively static over the treatment period in the control group (Fig. 4C). Longitudinal analysis of log10 HOMA-IR indicated an increase in HOMA-IR with WSD-treatment over time (diet ∗ time: F = 5.31, P < 0.01). Post hoc analysis indicated that the T + WSD group had significantly elevated HOMA-IR compared to the other three groups at the 36-month time point (P's < 0.05).
Figure 4.
Testosterone (T) + WSD treated animals show decreased insulin sensitivity. Fasting glucose (A), insulin (B) and HOMA-IR (C) were evaluated at time 0 and following 1, 3, 6, 12, 18, 24 and 36 months of control (C), T, WSD and T + WSD treatment. Values are the mean ± SEM with n = 10/group. Glucose and insulin curves at time 0 (D) and following 36 months of treatment (E) following an intravenous GTT, with glucose injection immediately following the time = 0 blood collection. GTTs were performed at time 0 and following 1, 3, 6, 12, 18, 24 and 36 months of treatment and glucose and insulin AUC (F) was calculated for each. Significant effects of diet, T-treatment or interactions over time as determined by a mixed models ANOVA are presented as text within the graphs. Tukey's multiple comparison post hoc analysis: *T+WSD significantly different from C, T and WSD groups; #T + WSD significantly different from C and T groups; $WSD group significantly different from T group; xT + WSD significantly different from C and WSD groups.
GTTs performed prior to the onset of treatment revealed no group differences in glucose or insulin secretion (Fig. 4D). After the 36-month treatment interval, glucose clearance appeared relatively similar to time 0, but insulin secretion was increased in all groups (Fig. 4E). This increase in insulin secretion appeared largest in the T + WSD group. Glucose and insulin AUCs were calculated for each GTT performed during the 36-month interval. Glucose AUC dropped initially in the C group but then remained relatively static (Fig. 4F). However, longitudinal analysis of glucose AUC found WSD-treatment increased glucose AUC over time (diet ∗ time: F = 5.15, P < 0.01). Post hoc analysis indicated that T + WSD had significantly elevated glucose AUC compared to C and T groups after 36 months of treatment (P's < 0.05). Glucose AUC was also significantly elevated in WSD group compared to the T group at 36 months (P's < 0.05). Longitudinal analysis demonstrated a modest and steady increase in log transformed insulin AUC for the C group (Fig. 4F). WSD-treatment caused a greater increase in insulin AUC over time (diet ∗ time: F = 3.62, P < 0.01) and as did T-treatment (T-treatment ∗ time: F = 2.91, P = 0.02). Post hoc analysis found that the T + WSD had higher insulin secretion compared to C and WSD groups at 36 months of treatment (P's < 0.05). Similar to measures of body composition, there was significant heterogeneity in fasting insulin responses to the treatments particularly in the T + WSD group (Supplementary Fig. S1).
Hormonal profiles associated with metabolic dysfunction were investigated, and although ghrelin and adiponectin levels were unchanged, both leptin and the proinflammatory CRP levels were elevated in WSD and T + WSD groups (leptin, diet: F = 21.68, P < 0.01; CRP, diet: F = 8.36, P < 0.01; Supplementary Fig. S3).
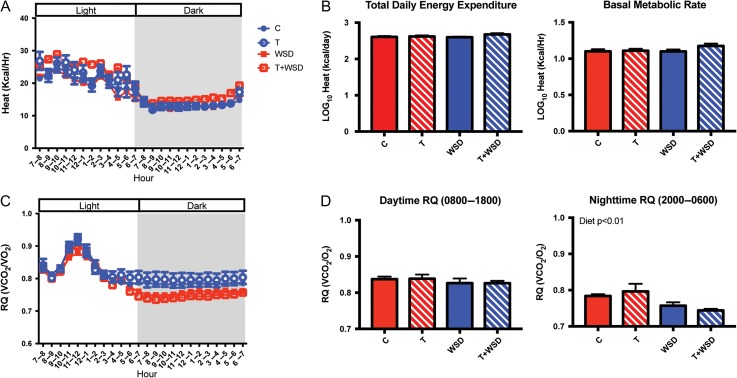
Metabolic rate
As expected, at 36 months of treatment all groups showed higher heat (kcal/h) values during lights on when animals were awake than during lights off when animals were asleep (Fig. 5A). Neither total daily energy expenditure or BMR showed any effects of T- or WSD-treatments and no post hoc differences were observed between the groups (Fig. 5B). The RQ is a measure of fuel source being oxidized and all four groups had RQ values between 0.7–1.0 indicating a mixture of carbohydrates and fat was being oxidized (Fig. 5C), with a spike at the 09:00 morning meal. The average daytime RQ was not significantly affected by T- or WSD-treatments; however, the average nighttime RQ was reduced by WSD-treatment (diet: Mann–Whitney P < 0.01), consistent with more fat oxidation in animals consuming a WSD compared to chow-fed animals (Fig. 5D).
Figure 5.
Indirect calorimetry at 36 months of treatment. Hourly averages of heat (kcal/h) are shown for a 24-h sampling period (A) in control (C), testosterone (T), WSD and T + WSD groups. Total daily energy expenditure was calculated as the sum of hourly averages of heat (kcal/h). BMR was the average of heat values between 01:00-05:00 am when animals are asleep (B). Both measures were log transformed to correct for normality. The hourly RQ is shown for the same 24-h sampling period (C) and average RQ for day and nighttime were analyzed (D). Values are the mean ± SEM with n = 10/group. Mann–Whitney significance finding for the nighttime RQ is presented as text within the graph. RQ, respiratory quotient.
Physical activity
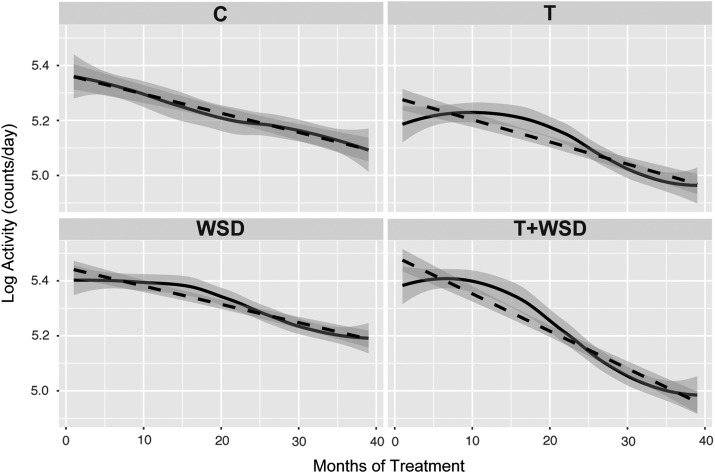
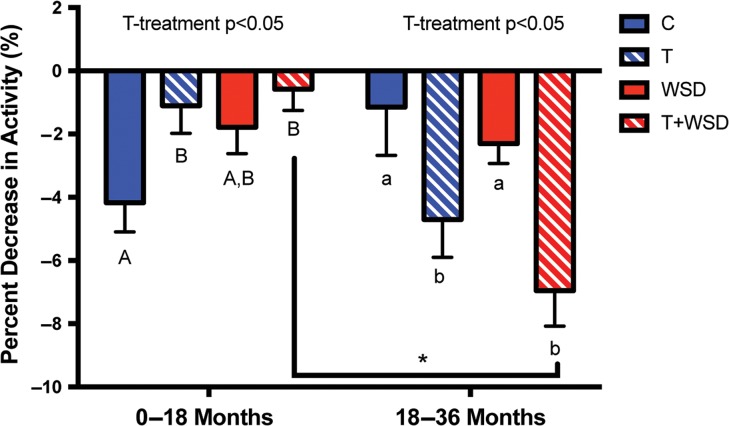
Activity decreased significantly (P < 0.01) over the 36-month treatment period as monkeys progressed through adolescence (Fig. 6). There was a significant effect of T- (P = 0.02) and WSD-treatments (P = 0.05) to alter the rate of decline in activity during adolescence (Fig. 6), but no significant interaction between T and WSD. A repeated-measures general linear model with a polynomial decomposition of the within-subject contrasts was done, and the quadratic factor was significant for T versus cholesterol implant (P = 0.03). Further analyses of the changes in activity in the first half versus the second half of the study, indicated that the altered rate of decline appeared due in part to an effect of T-treatment to delay the initial pubertal decline in activity followed by a more rapid decline in the second half of the treatment period (Figs 6 and 7). The control animals showed a similar percent decrease in activity for the first half of the treatment period (0–18 months) and the second half of the treatment period (18–36 months; Fig. 7). T-treatment had the effect to reduce the decline in activity in the first half of the study (0–18 months) but to increase the decline in activity in the second half of the study (18–36 months; Fig. 7). This effect was strongest in the T + WSD group, which showed a significantly greater percent decrease in activity in the second half of the study compared to the first half (paired t-test, P < 0.01).
Figure 6.
Pubertal-associated decline in activity across the 3-year treatment period. Daily activity was averaged for each month of treatment and values were log transformed to correct for normality. The mean monthly activity curves are presented as solid lines, and the dotted lines shows the linear regression. The shaded region associated with each represents the 95% CIs. C, control; T, testosterone; WSD, western-style diet.
Figure 7.
The percent decrease in activity between the first and second 18 months of the treatment period. Monthly average activity data was log transformed then the percent change in activity was calculated between time 0 and following 18 months of treatment (0–18 months) and between 18 and 36 months of treatment (18–36 months) for controls (C), testosterone (T), WSD and T + WSD treatments. Values presented are the mean ± SEM with n = 10/group. Two-way ANOVA significance findings are presented as text within the graph. Different uppercase letters denote significant differences between groups during the 0–18 month period, while different lowercase letters denote significant differences between groups during the 18–36-month period as determined by Tukey's multiple comparison post hoc analysis. Asterisk denotes significant difference in the T + WSD group between the 0–18 and 18–36 time points by paired t-test.
Discussion
The current study provides critical evidence that combined hyperandrogenemia and a WSD increases metabolic dysfunction in young adult female nonhuman primates compared to either treatment alone. These treatments seem to have combined effects to increase weight and fat mass gain over the three-year treatment interval. Interestingly, this increase in fat mass was associated with a significant increase in fat accumulation in the android region, which is highly associated with insulin resistance (Carey et al., 1996). Consistent with this finding, T + WSD treated animals maintain relative euglycemia but demonstrate hyperinsulinemia as well as an elevated HOMA-IR. These data indicate that the T + WSD group likely has increased insulin resistance, although hyperinsulinemic clamp studies are needed to further validate this assertion. Surprisingly, T and WSD treatments in isolation had minimal effects on parameters of body composition and insulin sensitivity, as demonstrated by a lack of significant differences between these groups and the control animals. The combined T + WSD treatment did not appear to alter food intake or BMR; however, T + WSD animals did show the greatest decline in activity in the latter half of the study. In particular, T-treatment appeared to delay the puberty-associated decline in activity for the first 18 months of treatment and then cause a more rapid decline in activity in the second 18 months. These findings are the first in a controlled animal model to assess how hyperandrogenemia in the peripubertal interval and beyond interacts with a WSD to affect metabolism in young adult female macaques.
The T dosing of the current study was chosen to mimic the 3- to 4-fold increase in total T observed in hyperandrogenemic peripubertal girls at risk for the development of PCOS (Apter et al., 1994; McCartney et al., 2006, 2007). However, it appears that control T-values were lower than anticipated in vehicle-treated animals, such that the achieved serum level of 1.35 ng/ml represents closer to a 5-fold increase in total T over control females. On average post-pubertal women with PCOS demonstrate more modest increases in testosterone, in the range of 2-fold increases (Hahn et al., 2005; Diamanti-Kandarakis and Panidis, 2007; Dumesic et al., 2016); therefore, the elevation in the current study is potentially greater than that observed clinically in adults. However, a study characterizing obese PCOS patients reported total T levels averaging 1.2 ng/ml, similar to values reported here (Eagleson et al., 2003). In addition, there remains controversy on the normal range of testosterone in women, since low testosterone levels are difficult to detect with conventional immunoassays (Boots et al., 1998), leading to the need to validate by more accurate LC-MS as done in the current study. It remains possible that normal women have lower T levels than previously estimated and thus PCOS represents greater than a 2-fold increase in total T levels. T-treatment did produce expected physiological effects such as an increase in lean mass consistent with the known anabolic role of T. Therefore, we believe T levels achieved in the current study were in the high pathophysiological range and provide a valid model to understand how hyperandrogenemia in young women affects metabolism.
Surprisingly, the group of female macaques treated with WSD alone had limited metabolic impairments during the 3-year treatment interval compared to the C group. A number of factors could have led to this finding. The first is that the WSD for nonhuman primates was originally designed to mimic a typical American diet and as such had significantly fewer calories from fat (36%) than high-fat diets typically used in rodents studies (up to 60% of calories from fat) (Wagner et al., 2009; Hariri and Thibault, 2010). Previous work utilizing the same WSD in adult macaques required animals to be maintained on the WSD for a minimum of 2–4 years to develop obesity in the majority of animals prior to study (McCurdy et al., 2009; Grant et al., 2012). This brings us to a second potential explanation, which is that the monkeys in the current study were examined during adolescence while they were still growing and thus likely to be less sensitive to the obesogenic effects of the diet. We hypothesize that once activity has reached lower adult levels and animals cease growing, the obesogenic effects of the WSD will become more prominent. Previous research demonstrates that a consistent proportion of monkeys remain resistant to the obesogenic effects of this WSD despite prolonged exposure (McCurdy et al., 2009) and this heterogeneity was present in T + WSD group (Supplementary Fig. S1). A subset of animals in this group do significantly worse metabolically, while others remain near control levels in terms of weight and measures of insulin sensitivity. This variability in phenotypes is not unlike that observed in PCOS patients, where obesity and insulin resistance are seen in the majority but not all patients. Last, the combined WSD and T + WSD groups did reveal overall effects of WSD on BMI, change in fat mass, fasting glucose, insulin and HOMA-IR. Although this appears largely driven by changes in the T + WSD group, as demonstrated by a lack of post hoc significance between C and WSD groups, we cannot exclude the possibility that small changes in the WSD group are also present and contributing to this finding.
Adolescent animals in the T alone group had relatively few differences from control animals in measures of body composition and insulin sensitivity. This is in contrast to previous work finding subtle but consistent metabolic impairments following prenatal androgen exposure in mice, sheep and primate models (Eisner et al., 2002; Manikkam et al., 2004; Recabarren et al., 2005; Roland et al., 2010). The lack of metabolic effects of T in the current study using a later peripubertal window of T exposure, could indicate that the prenatal developmental period is more sensitive to T effects on metabolism. Consistent with this hypothesis, administration of T beginning at 1 year of age in nonhuman primates was previously found to have little effect on insulin sensitivity (McGee et al., 2012). While the T group was largely unchanged compared to the C group, analysis of T-treatment as a factor, grouping both T and T + WSD groups together, frequently found detrimental effects of T-treatment on body composition measures. Similar to the WSD effects, it remains possible that continued hyperandrogenemia in isolation would result in metabolic impairments, but that these were simply not detectable in relatively young animals following only 3 years of treatment. However, given that post hoc analysis frequently revealed the T + WSD to be the only group different from controls, it is also possible that this group alone drove T-treatment effects. Significant synergistic effects of T and WSD were observed in gained weight and fat mass, indicating that weight gain was greater than the expected additive effects of T- and WSD-treatments in isolation. Regardless of whether statistics provided evidence of a synergistic or additive effects, the current results clearly demonstrate a worse metabolic outcome for T + WSD animals than animals exposed to either T or WSD alone.
One measure that did demonstrate a clear and strong effect by T-treatment was the altered pattern of the puberty-associated decline in activity. Throughout life, animals of all species experience a steady decline in physical activity (Sallis, 2000). Moreover, the sharpest decline in activity typically occurs during adolescence (Nelson et al., 2006; Dumith et al., 2011; Majid et al., 2016). Individuals who have more severe declines in physical activity during adolescence are reportedly at greater risk for obesity and diabetes (Davison and Birch, 2001; Kimm et al., 2002). Following this reasoning, monkeys who experienced a greater activity decline in this study would be at greater risk for obesity and metabolic dysregulation, as we found. Low levels of physical activity are closely associated with increases in body weight in both adolescent and adult human females (Ekelund et al., 2002; Janz et al., 2002; Abbott and Davies, 2004; Sternfeld et al., 2005) and animal models (Brownlow et al., 1996; Sullivan et al., 2006). In the current study, T-treatment caused an apparent delay in the puberty-associated decline in activity in the first 18 months of the study and a more dramatic decline in activity in the latter 18 months of the study. Notably, this altered pattern of activity appears most severe in the T + WSD group. This would argue that hyperandrogenemia itself may contribute to increased adiposity by decreasing activity and energy expenditure, but only in the presence of a WSD did this lead to significant increases in weight gain and signs of insulin resistance. Consistent with this hypothesis, we observed that the timing of weight gain in T + WSD animals appears to coincide with the accelerated decrease in activity. We propose a general timeline of rapid pubertal decline in activity followed by gains in body weight and fat mass followed eventually by early signs of insulin resistance in T + WSD animals.
The mechanism for the combined effects of T and WSD on weight gain is unclear. Although the decline in activity in the latter 18 months of the study was greatest in the T + WSD group, there were no synergistic effects of the T and WSD treatments on activity. Additionally, the T group was relatively normal metabolically but had a similar change in activity to the T + WSD group; therefore, it is unlikely changes in activity fully account for the worsened metabolic phenotype in the T + WSD group. Of note, the current results are consistent with previous findings of worsened metabolic outcomes in the presence of combined T and an obesogenic environment, including our pilot project preceding the current study (McGee et al., 2014). One hypothesis for the synergistic effect on weight gain is that androgens and WSD both influence adipose tissue physiology and potentially interact at this tissue to drive metabolic dysfunction. The hypothesis that T and WSD specifically interact at the level of adipocytes was explored in the current cohort of animals and reported in a companion paper by Varlamov et al. (2017).
The current paper along with the companion article (Varlamov et al., 2017) represents a comprehensive analysis of the effects of chronic hyperandrogenemia and/or WSD beginning at puberty to drive symptoms of PCOS. Importantly this model was also utilized to assess the impact of these treatments on ovarian and uterine function to categorize any reproductive dysfunction as well (manuscripts in preparation). The current model could be used to address multiple additional questions surrounding PCOS. For example, it has been hypothesized that puberty is a critical period for the programming of PCOS. Reversal of hyperandrogenemia, WSD or both in the current model could determine what if any defects persist after the removal of exogenous steroids and/or WSD. Understanding if puberty represents a critical period for the development of PCOS could significantly shift the therapeutic window for this disorder, particularly for high-risk populations such as obese hyperandrogenemic peripubertal girls. Finally, this model is currently being utilized to study fertility and examine how PCOS-like conditions affect implantation and gestation in mothers, as well as early metabolic function in the offspring and reproductive function later in life, illuminating the potential transgenerational transmission of PCOS.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Acknowledgements
We would like to acknowledge the Endocrine Technologies Support Core (ETSC) at the Oregon National Primate Research Center (ONPRC) which is supported by NIH Grant P51 OD011092 awarded to ONPRC. We would like to thank the additional collaborators on this group project that provided feedback at semi-annual update meetings including Cecily Bishop, Oleg Varlamov, Charles Roberts, Mary Zelinski, Diana Gordon, Jon Hennebold, Daniel Dumesic and Gregorio Chazenbalk.
Author's roles
C.A.T. prepared the manuscript, as well as participated in study design, execution, statistical analysis and discussion. D.L.T. participated in preparation of the manuscript, study design, execution and critical discussion. E.C.M., K.R.B., M.C.W., A.R.C., L.A.B. and T.A.D. participated in study design and execution. S.E.B. and N.D.R. participated in the statistical analysis, preparation of the manuscript and critical discussion. O.D.S., J.L.C. and R.L.S. participated in study design, statistical analysis, preparation of the manuscript and critical discussion.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number P50HD071836. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Oregon National Primate Research Center support grant P51 OD011092.
Conflict of interest
None delcared.
References
- Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol 2009;9:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott RA, Davies PS. Habitual physical activity and physical activity intensity: their relation to body composition in 5.0-10.5-y-old children. Eur J Clin Nutr 2004;2:285–291. [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab 1994;1:119–125. [DOI] [PubMed] [Google Scholar]
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;1:57–63. [DOI] [PubMed] [Google Scholar]
- Boots LR, Potter S, Potter D, Azziz R. Measurement of total serum testosterone levels using commercially available kits: high degree of between-kit variability. Fertil Steril 1998;2:286–292. [DOI] [PubMed] [Google Scholar]
- Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6 J mice. Physiol Behav 1996;1:37–41. [DOI] [PubMed] [Google Scholar]
- Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 1996;5:633–638. [DOI] [PubMed] [Google Scholar]
- Davison KK, Birch LL. Childhood overweight: a contextual model and recommendations for future research. Obes Rev 2001;3:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol (Oxf) 2007;5:735–742. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA et al. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab 2016;11:4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumith SC, Gigante DP, Domingues MR, Kohl HW III. Physical activity change during adolescence: a systematic review and a pooled analysis. Int J Epidemiol 2011;3:685–698. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;9:1165–1174. [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Bellows AB, Hu K, Gingrich MB, Marshall JC. Obese patients with polycystic ovary syndrome: evidence that metformin does not restore sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by ovarian steroids. J Clin Endocrinol Metab 2003;11:5158–5162. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril 2002;1:167–172. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Aman J, Yngve A, Renman C, Westerterp K, Sjostrom M. Physical activity but not energy expenditure is reduced in obese adolescents: a case-control study. Am J Clin Nutr 2002;5:935–941. [DOI] [PubMed] [Google Scholar]
- Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod 2005;6:1475–1483. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Azziz R. Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab 2006;2:193–205. [DOI] [PubMed] [Google Scholar]
- Grant WF, Nicol LE, Thorn SR, Grove KL, Friedman JE, Marks DL. Perinatal exposure to a high-fat diet is associated with reduced hepatic sympathetic innervation in one-year old male Japanese macaques. PLoS One 2012;10:e48119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Tan S, Elsenbruch S, Quadbeck B, Herrmann BL, Mann K, Janssen OE. Clinical and biochemical characterization of women with polycystic ovary syndrome in North Rhine-Westphalia. Horm Metab Res 2005;7:438–444. [DOI] [PubMed] [Google Scholar]
- Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev 2010;2:270–299. [DOI] [PubMed] [Google Scholar]
- Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab 2002;2:524–529. [DOI] [PubMed] [Google Scholar]
- Janz KF, Levy SM, Burns TL, Torner JC, Willing MC, Warren JJ. Fatness, physical activity, and television viewing in children during the adiposity rebound period: the Iowa Bone Development Study. Prev Med 2002;6:563–571. [DOI] [PubMed] [Google Scholar]
- Kang SM, Yoon JW, Ahn HY, Kim SY, Lee KH, Shin H, Choi SH, Park KS, Jang HC, Lim S. Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS One 2011;11:e27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm SY, Glynn NW, Kriska AM, Barton BA, Kronsberg SS, Daniels SR, Crawford PB, Sabry ZI, Liu K. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med 2002;10:709–715. [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 2001;8:607–613. [DOI] [PubMed] [Google Scholar]
- Majid HA, Amiri M, Mohd Azmi N, Su TT, Jalaludin MY, Al-Sadat N. Physical activity, body composition and lipids changes in adolescents: analysis from the MyHeART Study. Sci Rep 2016;6:30544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 2004;2:790–798. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;7:412–419. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007;2:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006;5:1714–1722. [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009;2:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod 2012;2:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee WK, Bishop CV, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. Am J Physiol Endocrinol Metab 2014;11:E1292–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Papunen L, Rantala AS, Unkila-Kallio L, Tiitinen A, Hippelainen M, Perheentupa A, Tinkanen H, Bloigu R, Puukka K, Ruokonen A et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab 2012;5:1492–1500. [DOI] [PubMed] [Google Scholar]
- Nelson MC, Neumark-Stzainer D, Hannan PJ, Sirard JR, Story M. Longitudinal and secular trends in physical activity and sedentary behavior during adolescence. Pediatrics 2006;6:e1627–1634. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod 2001;9:1995–1998. [DOI] [PubMed] [Google Scholar]
- Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril 2002;6:1095–1105. [DOI] [PubMed] [Google Scholar]
- Papailiou A, Sullivan E, Cameron JL. Behaviors in rhesus monkeys (Macaca mulatta) associated with activity counts measured by accelerometer. Am J Primatol 2008;2:185–190. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Antenucci D, Casimirri F, Venturoli S, Paradisi R, Fabbri R, Balestra V, Melchionda N, Barbara L. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab 1989;1:173–179. [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Duran C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab 2005;5:E801–E806. [DOI] [PubMed] [Google Scholar]
- Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol 2010;2:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;1:19–25. [DOI] [PubMed] [Google Scholar]
- Sallis JF. Age-related decline in physical activity: a synthesis of human and animal studies. Med Sci Sports Exerc 2000;9:1598–1600. [DOI] [PubMed] [Google Scholar]
- Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2013;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 2005;7:3185–3193. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP Jr. Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc 2005;7:1195–1202. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Cameron JL. A rapidly occurring compensatory decrease in physical activity counteracts diet-induced weight loss in female monkeys. Am J Physiol Regul Integr Comp Physiol 2010;4:R1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Koegler FH, Cameron JL. Individual differences in physical activity are closely associated with changes in body weight in adult female rhesus monkeys (Macaca mulatta). Am J Physiol Regul Integr Comp Physiol 2006;3:R633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod 2006;1:80–89. [DOI] [PubMed] [Google Scholar]
- Varlamov O, Bishop CV, Handu M, Takahashi D, Srinivasan S, White A, Roberts C. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Hum Reprod 2017;32:1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994;5:647–654. [DOI] [PubMed] [Google Scholar]
- Wagner JD, Jorgensen MJ, Cline JM, Lees CJ, Franke AA, Zhang L, Ayers MR, Schultz C, Kaplan JR. Effects of soy vs. casein protein on body weight and glycemic control in female monkeys and their offspring. Am J Primatol 2009;9:802–811. [DOI] [PubMed] [Google Scholar]
- Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab 2006;5:1660–1666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.