Abstract
MicroRNA-122 (miR-122) is an important host factor for the hepatitis C virus. Treatment with RG-101, a GalNAc conjugated anti-miR-122 oligonucleotide, resulted in a significant viral load reduction in patients with chronic hepatitis C (CHC) infection. Here, we analyzed the effects of RG-101 therapy on antiviral immunity. 32 CHC patients HCV genotype 1, 3 and 4 received a single subcutaneous administration with RG-101 at 2 mg/kg (n=14), 4 mg/kg (n=14) or placebo (n=2 per dosing group). Plasma and PBMCs were collected at multiple time points and comprehensive immunological analyses were performed. Following RG-101 administration, HCV RNA declined in all patients (mean decline at week 2: 3.27 log10 IU/mL). At week 8 HCV RNA was undetectable in 15/28 patients. Plasma IP-10 levels declined significantly upon dosing with RG-101. Furthermore, the frequency of NK cells increased, the proportion of NK cells expressing activating receptors normalized and NK cell IFN-γ production decreased after RG-101 dosing. By week 8 post RG-101 injection, functional HCV-specific IFN-γ-T cell responses declined significantly in patients who had undetectable HCV RNA. No increase in the magnitude of HCV-specific T cell responses was observed at later time points, including 3 patients who were HCV RNA negative 76 weeks post dosing.
Conclusions
Dosing with RG-101 is associated with a restoration of NK cell proportions and a decrease of NK cells expressing activation receptors. However, the magnitude and functionality of ex vivo HCV-specific T cell responses did not increase following RG-101 injection. Our data suggests that NK cells, but not HCV adaptive immunity may contribute to HCV viral control following RG-101 therapy.
Keywords: Hepatitis C virus, miR-122, anti-miR, ISG, TRAIL, virus-specific T cell
Chronic hepatitis C (CHC) virus infection is a global health problem. Patients with CHC infection are at increased risk of developing liver-related complications such as hepatocellular carcinoma and cirrhosis.(1) Successful HCV treatment reduces the risk of complications and improves survival.(2,3) Recently, direct acting antivirals (DAAs) have become available, which can directly interfere with the HCV viral machinery. These DAAs have drastically changed the field of chronic hepatitis C treatment, by achieving sustained virological response (SVR) in a high proportion of patients.(4) With such high cure rates for HCV, the next challenge of research needs to focus on vaccine development and optimizing current treatment regimens and delivering them cost effectively.(5) The cost of DAAs is considerable and combining DAAs with compounds that have a different mechanism of action may reduce both duration and cost of treatment. An alternative therapeutic option lies in inhibition of an important host factor utilised by HCV; microRNA-122 (miR-122).
miR-122 is a highly conserved, liver-specific micro-RNA which has important functions in the regulation of cholesterol and fatty acid synthesis.(6) In addition, miR-122 can bind to the HCV genome and thereby promote virus replication.(7,8) Targeting this host factor with an antisense oligonucleotide has been proven effective in inhibiting HCV across several genotypes.(9–11) A single subcutaneous dose of the GalNAc-conjugated anti-miR-122 oligonucleotide RG-101 resulted in substantial decreases in HCV RNA in all treated patients and HCV RNA negativity for at least 76 weeks in 3 patients.(9)
The exact mechanism of HCV inhibition by RG-101 is not yet known. As binding of miR-122 protects HCV RNA from degradation by exonucleases, blocking miR-122 could uncover the genome to these innate defence pathways.(8,12–14) Furthermore, HCV RNA replication could be disturbed by knocking down miR-122.(14,15) Inhibition of HCV replication and a reduction in viral antigen expression could restore natural killer (NK) cell and HCV-specific T cell responses, and thereby contribute to viral clearance.
Since former treatment options all included interferon-alfa, an immune modulator, the effects of new therapies on the immune system are substantially different. Exogenous interferon-alfa treatment of patients with CHC enhanced interferon stimulated gene (ISG) expression and activated NK cells, but did not lead to recovery of the T cell compartment.(16–18) The inhibition of host factor miR-122 by RG-101 acts through a different mechanism and could potentially restore antiviral immunity.
The aim of this study was to investigate whether treatment with RG-101 would change important immune effectors in CHC infection and whether restored antiviral immunity could play a role in long term virologic impact observed in patients treated with a single dose of RG-101.
Patients and Methods
Patients
We included 32 CHC patients at two sites in The Netherlands (Academic Medical Center Amsterdam and University Medical Center Groningen)(Table 1). Males and post-menopausal females with chronic HCV genotype 1, 3 or 4 infection were enrolled. Patients were treatment naïve or had previously experienced a virological relapse after interferon-based therapy. Patients with co-infection (hepatitis B virus or human immunodeficiency virus infection), evidence of decompensated liver disease, or a history of HCC were excluded. The study was approved by the regulatory authority and the independent ethics committee at each participating site. All patients gave written informed consent, and the study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements. All authors had access to the study data and reviewed and approved the final manuscript.
Table 1. Baseline characteristics9.
| RG-101 (n=28) |
Placebo (n=4) |
Total | ||
|---|---|---|---|---|
| 2 mg/kg | 4 mg/kg | |||
| N = | 14 | 14 | 4 | 32 |
| Male (n) | 13 | 7 | 4 | 24 (75) |
| Age (years) | 51 (46-53) | 54 (48-58) | 55 (52-57) | 52 (49-57) |
| Weight (kg) | 84 (71-98) | 83 (69-89) | 79 (74-83) | 83 (72-92) |
| Ethnicity (n) | ||||
| Caucasian | 13 | 12 | 4 | 29 (91) |
| Asian | 1 | 1 | 0 | 2 (6) |
| Other | 0 | 1 | 0 | 1 (3) |
| IFNL3 CC genotype (n) | 3 | 3 | 2 | 8 (25) |
| IFN naïvea (n) | 9 | 12 | 3 | 24 (75) |
| Baseline HCV RNA level (log10, IU/mL) | 6·12 (5·78-6·75) | 6·19 (5·78-6·64) | 6·38 (5·83-6·85) | 6·16 (5·80-6·68) |
| HCV genotype (n) | ||||
| 1a | 8 | 4 | 2 | 14 (44) |
| 1b | 1 | 1 | 0 | 2 (6) |
| 3 | 4 | 5 | 1 | 10 (31) |
| 4 | 1 | 4 | 1 | 6 (19) |
| ALT level (U/L) | 60 (41-139) | 52 (44-61) | 90 (35-232) | 52 (39-124) |
| Fibroscan (kPa) | 6·8 (5·1-10·8) | 7·7 (5·5-10·9) | 5·1 (3·4-11·7) | 7·5 (5·3-10·7) |
| HLA-A2+ (n) | 4 | 5 | 2 | 11 (34) |
Data are given as median (IQR) or as frequency (percentage).
naïve to IFN-alpha based therapy.
Study
In this randomised, double-blind, placebo-controlled, phase 1b trial,(9) patients received a single subcutaneous injection of RG-101, a GalNAc-conjugated oligonucleotide antagonizing miR-122 (EudraCT number 2013-002978-49). Dosage was 2 mg/kg (n=14) or 4 mg/kg (n=14), and 2 patients in each group received placebo. The initial follow-up was 8 weeks after dosing. At week 8, only patients with > 2 log 10 decrease from baseline, and < 1 log 10 increase in HCV RNA level from nadir were included in an extended follow-up study (n=22, including n=10 2 mg/kg and n= 12 4 mg/kg), of these, 15 patients (n=6 2 mg/kg and n= 9 4 mg/kg) had HCV RNA levels below the lower limit of quantification (< LLOQ) at week 8. Patients who did not meet above criteria were excluded from the study at week 8 (n=10, including placebo treated patients). The extended follow-up lasted until week 76 (Supplementary Figure 1). If patients had a virological rebound (defined as > 1 log 10 increase) during follow-up, a retest was performed, after which patients were excluded from the study. In the extended follow-up, rebounds occurred at week 12 (n=8), week 16 (n=2), week 20 (n=1), week 28 (n=2), week 36 (n=2) and week 52 (n=1) and 3 patients were lost to follow-up (Supplementary Figure 2). At week 76, 3 patients had undetectable HCV RNA levels after a single dose of RG-101. HCV RNA levels were measured using Roche COBAS AmpliPrep/COBAS Taqman HCV v2.0 assay, with a reported LLOQ of 15 IU/mL.
Sampling
Plasma samples for cytokine analyses were collected at baseline, day 3, weeks 1, 4 and 8. Healthy control plasma (n=6) was not matched for age, gender or ethnicity. Peripheral blood mononuclear cells (PBMCs) were collected at baseline, weeks 2, 8, 20, 28 and 52, at time of viral rebound or retest (between week 12 to 52) and at end of follow-up (week 76) (Supplementary Figure 1). PBMCs from healthy blood donors were used as heathy controls (n=13). PBMCs were separated by density gradient and cryopreserved for later analyses.
Luminex analyses
Plasma cytokine levels were measured using a Luminex 20-plex immunoassay (Affymetrix eBioscience, San Diego, CA, USA). This included IL-12, CCL2, CCL3, CCL4, CD54, Interferon gamma-induced protein 10 (IP-10 or CXCL10), GM-CSF, IFN-alpha, IFN-gamma, IL-1alpha, IL-1 beta, IL-10, IL-13, IL-17A, IL-4, IL-6, IL-8, sCD62E, sCD62P and TNF-alpha. Plasma samples from 6 healthy controls were included in the analyses.
Flow cytometry
PBMCs were thawed and stained for 30 minutes at 4°C with different combinations of fluorescent label-conjugated mouse anti-human monoclonal antibodies (mAbs); CD56 BUV-395, CD27 BUV-373, HLA-DR FITC, CD3 V500, CD16 BV786, CD16 BV421, CD14 PE-CF594, CD19 PE-CF594, CD38 PE-Cy7 (BD, San Jose, CA, USA) NKp46 PerCP-efluor710, CD45RA eFluor 605NC (eBioscience, San Diego, CA, USA) CXCR6 BV-421, CD8 BV-711, CD8 BV-785, NKp30 APC (Biolegend, San Diego, CA, USA) Live/Dead fixable dead cell stain RED (Invitrogen, Life Technologies, Carlsbad, CA, USA) NKG2A PE, (Beckman Coulter, Fullerton, CA, USA) TRAIL/CD253 APC (Miltenyi Biotec, Bergisch Gladbach, Germany).
For intracellular staining, cells were fixed after surface staining, permeabilized (FoxP3/Transcription Factor Staining buffer set, eBioscience, San Diego, CA, USA), and stained with mAbs; Perforin FITC (BD, San Jose, CA, USA) Eomes PerCP-efluor710, T-bet PE-Cy7 (eBioscience, San Diego, CA, USA) Ki67 BV711, (Biolegend, San Diego, CA, USA) Granzyme B PE (Sanquin, Amsterdam, The Netherlands).
Ex vivo CD8+ T cell frequencies were determined in HLA-A2 positive patients (n=11 patients, genoype 1a (n=6), 3a (n=3), 4a (n=1) and 4d (n=1)) by HLA-class I multimers. Multimers were loaded with dominant epitopes NS31073-1081 (CINGVCWTV) (APC) (Sanquin, Amsterdam, The Netherlands), NS41406-1415 (KLVALGINAV)(PE) and NS5B2594-2602 (ALYDVVTKL)(FITC) (Immudex, Copenhagen, Denmark). As control, we stained for CMV-pp65495–504 (NLVPMVATV) and EBV BMLF-1259-267 (GLCTLVAML) tetramers (Sanquin, Amsterdam, The Netherlands). All measurements were performed on an LSR Fortessa cytometer (BD Biosciences, San Jose, CA, USA) and analyzed by FlowJoMacV9.7.5 software.
NK cell function
Patients were selected when samples were available for day 1 as well as day 57 (n=13), and healthy blood donors were added as controls. PBMC were thawed and incubated overnight with IL-12 and IL-15 or no stimulus in the presence of CD107a FITC (eBioscience, San Diego, CA, USA). For the last 3 hours, monensin and brefeldin A were added, after which cells were stained with live/dead stain, CD3 V500 and CD56 BUV-395 as above. After fixing the cells, intracellular staining was performed (IFNγ BV421, TNFα AF700, MIP-1β Pe-Cy7 BD, San Jose, CA, USA) and measurements were done on an LSR Fortessa. The proportion of IL-12/IL-15 activated cells was calculated by subtracting the number of positive cells in the unstimulated condition.
IFN-γ-ELISpot assays
IFN-γ-ELISpot assays were performed ex vivo in duplicate at 2x105 PBMCs/well. Thawed PBMC were rested overnight (37 °C + CO2) and were stimulated with panels of 15 mer peptides that overlapped by 11 amino acids corresponding to HCV genotypes 1a, 1b, 3a or 4a (described in Barnes et al. 2012 and Kelly et al. (19,20)). Patient PBMCs were stimulated with the panel of peptides that matched their own viral genotype and subtype (where possible). The peptides were arranged into 10 pools corresponding to core, E1, E2, P7&NS2, NS3p, NS3h, NS4, NS5A, NS5B I, and NS5B II. Each peptide was used at a final concentration of 3µg/ml. Internal controls were: dimethyl sulfoxide (DMSO) (Sigma-Aldrich, UK) as a negative control and concanavalin A (Sigma-Aldrich, UK) as a positive control. Other antigens used were a pool of MHC class 1 restricted epitopes of influenza A, EBV and CMV (BEI Resources, Manassas, VA, USA), and a lysate of CMV infected cells (Virusys Corp, Taneytown, MD, USA). Spot forming units were calculated per 106 cells and background-subtracted data is shown.
Statistical analyses
For differences between groups, the T-test (normal distribution) or Mann–Whitney test (non-normal distribution) was used. For longitudinal analysis in individual patients the Wilcoxon signed rank test was used. P values < 0.05 were considered statistically significant. GraphPad Prism version 6.07 for Windows (GraphPad Software, La Jolla, CA, USA) was used for analyses.
Results
IP-10 levels decrease in patients dosed with RG-101
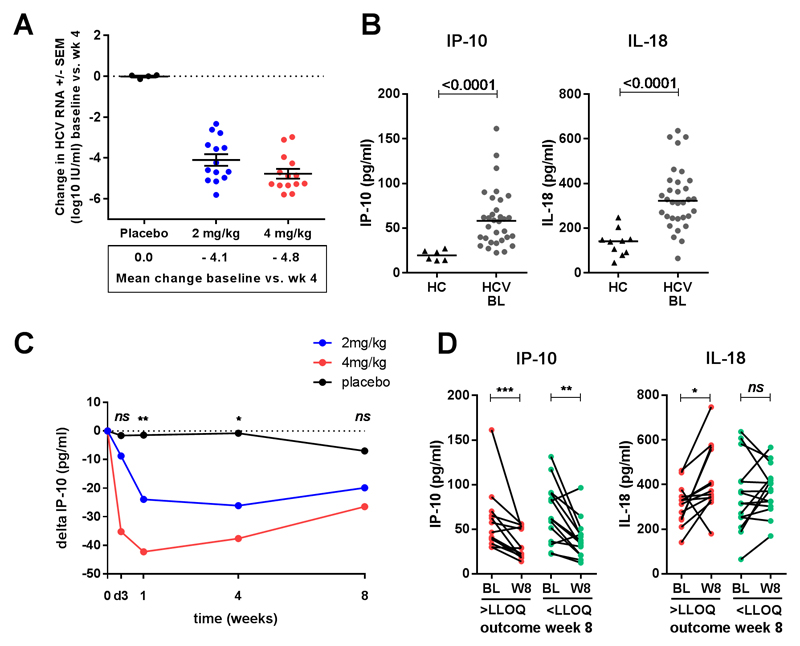
HCV RNA levels declined in all patients dosed with RG-101 (n=28). At week 4, the mean reduction was 4.1 log10 IU/ml in patients dosed with 2 mg/kg and 4.8 log10 IU/ml in patients dosed with 4 mg/kg, as compared to 0.0 log10 IU/ml in placebo treated patients (Figure 1A). Plasma IP-10 levels were significantly higher in CHC patients at baseline as compared to healthy controls (median 58.1 pg/ml and 19.3 pg/ml respectively, p<0.0001, Figure 1B) and no significant differences were observed within the 3 groups of CHC patients at baseline (placebo, 2 mg/kg and 4 mg/kg; data not shown).
Figure 1.
(A) Mean change in HCV RNA levels between baseline and week 4 in patients dosed with placebo (black dots), 2 mg/kg (red dots) and 4 mg/kg (blue dots) RG-101. (B) Plasma IP-10 levels in healthy controls (HC) and CHC patients (HCV) at baseline, bars indicate median. (C) Change in plasma IP-10 levels (median) in patients dosed with placebo (black dots), 2 mg/kg (red dots) and 4 mg/kg (blue dots) RG-101, level of significant difference indicated between RG-101 dosed (2 mg/kg and 4 mg/kg) and placebo dosed patients. (D) Change in plasma IP-10 levels between baseline and week 1 in patients with HCV RNA levels < LLOQ and > LLOQ at week 8, bars indicate median. Statistical testing; T-test (A) and Mann–Whitney test (B-D).
Upon dosing with RG-101 IP-10 levels decreased significantly in patients dosed with RG-101 (Figure 1C). At week 1, the median decline in IP-10 levels was 23.9 pg/mL in 2mg/kg dosed patients, 42.3 pg/mL in 4 mg/kg treated patients (p=0.02 and p=0.007 compared to placebo, respectively), and 1.4 pg/mL in placebo treated patients. 15 of 28 patients had undetectable HCV RNA 8 weeks post-dosing, however, the magnitude of the change in IP-10 post-dosing could not be used to predict outcome at 8 weeks (median decline from baseline to week 1, patients with HCV <LLOQ and >LLOQ; 37.7 and 26.3 pg/mL respectively, p=0.29, Figure 1D). None of the other measured cytokines or chemokines showed a significant change upon dosing with RG-101, including interferon-γ, interferon-α, tumor necrosis factor-α (Supplementary Figure 3).
TRAIL expression decreases on CD56bright NK cells
At baseline, CHC patients and healthy controls had comparable frequencies of NK cells in the blood (median 7.4% and 7.0% respectively, p=0.71, Figure 2A, full gating in Supplementary Figure 4A). In patients dosed with RG-101, the proportion of NK cells increased significantly from baseline 7.4% to median 10.1% (p=0.02) at week 8 and 10.3% at follow-up week 12-28 (which include patients with viral rebound defined as >1 log increase in viral load from nadir between week 12 and 28, and patients with HCV RNA < LLOQ at week 28 (n=5)), p=0.02. In placebo-treated patients, no changes were observed in NK cell proportions during follow-up (Figure 2A). Furthermore, no changes in lymphocyte count were observed in the blood (Supplementary figure 4B). Patients who had HCV RNA levels < LLOQ at week 8 had significantly lower proportions of NK cells as compared to patients who had HCV RNA > LLOQ at week 8 (8.4% and 13.1% respectively, p=0.01 Figure 2A). NK cells can be divided into two subsets, depending on their expression of CD56; these include CD56bright and CD56dim NK cells (Figure 2B). Upon dosing, CD56dim NK cells significantly increased from median 91.7% to 94.8 % at week 12-28 (p=0.0007), whereas CD56bright NK cells decreased (median 8.3% at baseline and 4.8% at follow-up week 12-28, p=0.001, Figure 2B). The expression of TNF-related apoptosis inducing ligand (TRAIL) on CD56bright NK cells was significantly upregulated in CHC patients at baseline as compared to healthy controls (median 13.3% and 7.3% of CD56bright NK cells respectively, p=0.001, Figure 2C). TRAIL expression significantly decreased as soon as week 2 in patients upon dosing with RG-101 (from median 13.3% at baseline to 6.4% of CD56bright NK cells at week 2, p<0.0001, Figure 2C,D). No changes in TRAIL expression were observed in placebo treated patients (not shown). No difference in TRAIL expression was observed in patients who had HCV RNA levels < LLOQ versus patients who had HCV RNA levels > LLOQ at week 8 (Figure 2C green and grey dots).
Figure 2.
(A) Proportion of NK cells as a percentage of total lymphocytes in healthy controls (HC) and CHC patients dosed with RG-101 at baseline (bl), week (wk) 2, 8 and 12-28 (including patients with viral rebound between week 12 and 28 and patients with HCV RNA < LLOQ at week 28). Data is shown grouped in patients who are HCV RNA positive or have HCV RNA levels < LLOQ, as well as in placebo dosed patients, bars indicate median. (B) Proportion of CD56bright and CD56dim cells as a percentage of total NK cells in RG-101 dosed patients at baseline(bl), week (wk) 2, 8, and 12-28, bars indicate median. Representative dot plot showing NK cell gating in total lymphocyte population. CD56bright and CD56dim NK cell gates are shown (C) Percentage of TRAIL+ cells within CD56bright NK cells in healthy controls and patients dosed with RG-101, bars indicate median. Patients who had HCV RNA levels < LLOQ are depicted in green dots, patients who had HCV RNA levels > LLOQ in red. (D) Representative dot plot showing TRAIL gating, percentages of TRAIL positive cells within the CD56bright NK cell population (black) are shown. CD56dim NK cells are shown in grey for comparison. Statistical testing; Mann–Whitney test (A,C) and Wilcoxon matched pairs test (B,C).
Decreased expression of NK cell activation receptors
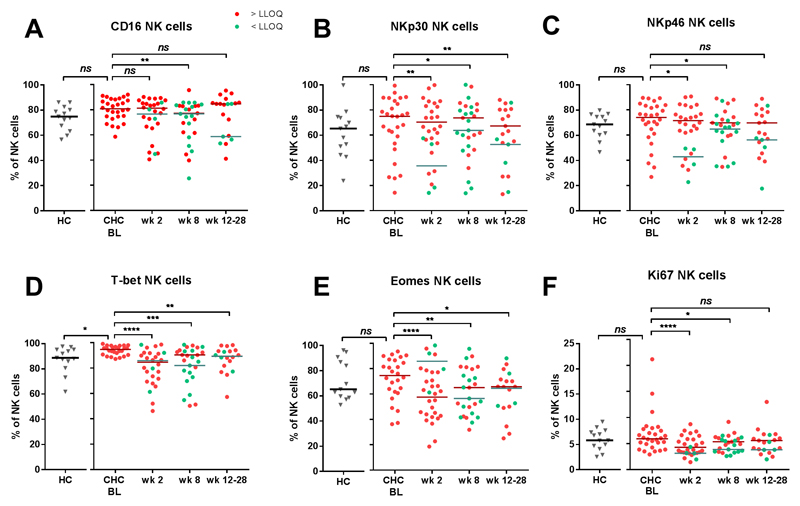
In CHC patients, the expression of Fcγ-receptor CD16 on NK cells was significantly higher as compared to healthy controls (median 80.8% and 74.9%, respectively, p=0.04), and this decreased after dosing with RG-101 (80.8% at baseline to 77.0% at week 8, p= 0.0019, Figure 3A). Furthermore, the expression of activating receptors NKp30 and NKp46 on NK cells decreased in patients who were dosed with RG-101 (NKp30; 75.0% at baseline to 62.8% at week 8, p=0.01 and NKp46; 74% at baseline and 67.2% at week 8, p= 0.01, Figure 3B,C). The expression of T-box transcription factors T-bet and Eomes decreased upon dosing with RG-101 (from 94.7% at baseline to 89.2% at week 8 for T-bet p=0.0003 and from 74.6% to 60.8% for Eomes, p=0.001, Figure 3D,E), whereas NK cell IFN-γ production significantly decreased (Supplementary Figure 5). The expression of the cell cycle marker Ki67 was 5.9% at baseline and significantly decreased at week 2 (3.8%, p<0.0001) and week 8 (4.9%, p=0.02)(Figure 3F). Other markers expressed on NK cells showed minor or no changes (Supplementary Figure 6). Changes in T-bet, Eomes and Ki67 expression were also observed in the CD8+ T cell and CD56+ NKT cell compartments (Supplementary Figure 7). No differences were observed between patients with HCV RNA levels < LLOQ and patients with HCV RNA levels > LLOQ at week 8 (Figure 3A-F).
Figure 3.
Proportion of (A) CD16, (B) NKp30, (C) NKp46, (D) T-bet, (E) Eomes and (F) Ki67 in healthy controls (HC) as well as in CHC patients dosed with RG-101 (n=28) at baseline (bl), week (wk) 2 and 8, bars indicate median. Statistical testing; Mann–Whitney test and Wilcoxon matched pairs test (A-F). Patients who have HCV RNA levels < LLOQ are shown in green dots, patients with HCV RNA levels > LLOQ are in red dots.
HCV-specific T cells responses did not change in patients dosed with RG-101
Next, we investigated whether HCV-specific T cells play a role in the (long term) viral load reduction after RG-101 dosing. The frequency of HCV-specific T cells was measured in all HLA-A2+ patients (n=11, including 2 placebo treated patients). Baseline ex vivo HCV-specific CD8+ T cell frequencies were low in CHC patients (median 0.009%, range 0% - 0.21%, Figure 4A) and did not change upon dosing with RG-101 (median 0.008%, range 0% - 0.19% at week 2 and median 0.007%, range 0% - 0.05% at week 8, p=ns), or in patients who received placebo (Supplementary Figure 8A). Similarly, CMV and EBV specific T cell proportions did not change upon dosing with RG-101 (Supplementary Figure 8B). The functional capacity of HCV-specific T cells, as measured by interferon-γ-ELISpot, did not change significantly in patients who were treated with RG-101 as compared to placebo (Figure 4B). In patients who had HCV RNA levels < LLOQ at week 8, HCV-specific T cells responses decreased significantly (from median 100 to 90 SFU, p=0.04) (Figure 4C). There was no difference in the change from baseline to week 8 in patients with and without HCV RNA levels < LLOQ at week 8 (median -45 SFU in patients < LLOQ ; and +10 SFU in patients > LLOQ, p=0.36, Figure 4D).
Figure 4.
(A) Ex vivo multimer positive cells as a proportion of total CD8+ T cells in RG-101 dosed patients at baseline (bl), week (wk) 2, wk 8 (including n=2 HCV RNA <LLOQ, excluding n=2 due to missed visit). Each dot represents one epitope, a positive response was defined as > 5 events with a lower threshold of 10,000 measured CD8+ T cells, bars indicate median. (B) ELISpot responses at baseline and week 8 in patients dosed with RG-101 (left) and placebo (right). Patients’ PBMCs were stimulated ex vivo with genotype specific HCV-peptide pools. (C) ELISpot responses at baseline and week 8 (and week 76) in patients dosed with RG-101 with HCV RNA levels > LLOQ (left) and < LLOQ (right) at week 8. (D) Change between baseline and week 8 ELISpot responses in patients dosed with RG-101 with HCV RNA levels > LLOQ (green dots) and < LLOQ (blue dots) at week 8 as well as placebo dosed patients, bars indicate median. Statistical testing; Mann–Whitney test (D) and Wilcoxon matched pairs test (A-C).
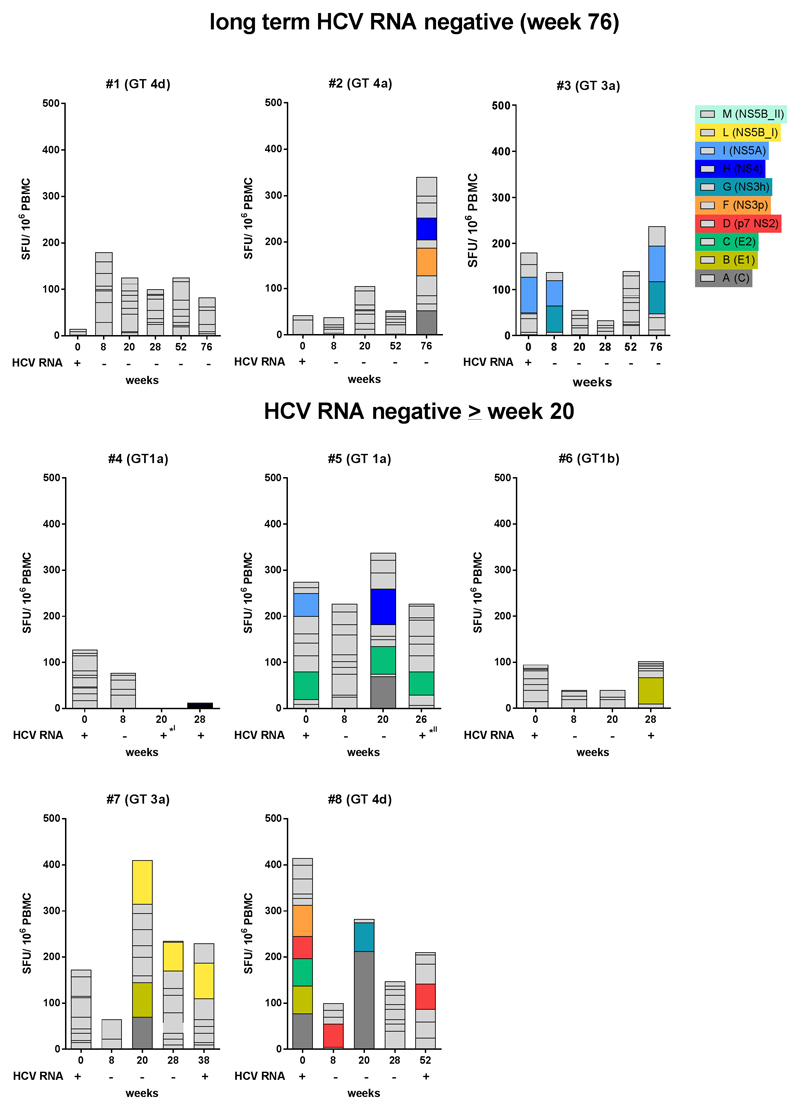
Three patients were tested HCV RNA negative up to 76 weeks after dosing with RG-101. In addition, 5 patients had a late viral rebound at week 28 (n=2), week 36 (n=2) and week 52 (n=1). In none of these patients, an increase in HCV-specific T cells responses was observed (Figure 5).
Figure 5.
ELISpot responses (left y-axis) and viral load (right y-axis) in patients who were HCV RNA negative for 76 weeks (upper graphs, patients #1-3) as well as in patients who had HVC RNA levels < LLOQ for 20 or more weeks (lower graphs, patients #4-8). (I): patient #4 had < 1 log10 increase in HCV RNA at week 20 (PBMC missing), and was negative again at week 24. Rebound was at week 28. (II): patient #5 had > 1 log10 increase in HCV RNA at week 24 and < 1 log10 increase in HCV RNA at retest (week 26, last available PBMCs), rebound was at week 36.
Discussion
In this unique phase 1b study we assessed the effects of dosing with the GalNAc conjugated anti-miR-122 oligonucleotide RG-101 on antiviral immunity in CHC patients for the first time in man. We show that, after a single monotherapy dose of RG-101, as a consequence of HCV RNA decline, IP-10 levels decrease, NK cell proportions increase, and the expression of NK cell activation receptors decreases. Furthermore, NK cell IFNγ production significantly decreased, and no restoration of ex vivo HCV-specific T cell functionality was observed after viral load decline, nor after long term HCV RNA negativity.
In CHC patients, continuous immune activation is demonstrated by elevated levels of ISGs, such as IP-10 (or CXCL10), and viral load suppression during DAA treatment is associated with a decrease in IP-10 levels.(21–23) In patients who have viral relapse, IP-10 levels have been shown to increase again.(21) In our study, IP-10 levels decreased in all patients who were dosed with RG-101, irrespective of their treatment outcome. We did not observe a subsequent increase in IP-10 levels in patients who had experienced a viral rebound; however, since patients were not followed after rebound, IP-10 levels may have increased at a later time. Alternatively, RG-101 could induce other mechanisms leading to IP-10 decrease, irrespective of viral load decline.(24) In in vitro studies, miR-122 has been shown to have pro-inflammatory effects which can be attenuated by inhibition with anti-miRNA-122.(25) No other measured cytokines or chemokines were altered in the plasma of patients dosed with RG-101, which is consistent with preclinical data showing RG-101 does not elicit an undesirable systemic immune responses.(26)
NK cells play an important role in HCV viral infection. NK cells significantly increased in frequency in the blood of patients after dosing with RG-101. Interestingly, at week 8, the frequency of NK cells was higher in patients who were HCV RNA positive as compared to the patients who had HCV RNA levels < LLOQ. Possibly, a rebound in viral load in these patients could have led to NK cell expansion. After RG-101 dosing, CD56bright NK cells decreased while CD56dim NK cells increased, similar to data from DAA treated patients.(22) TRAIL is an important ligand expressed by NK cells which can induce apoptosis in target cells via the TRAIL receptor (TRAIL-R1 or TRAIL-R2). Target cells include virally infected hepatocytes, which have increased TRAIL-receptor expression in CHC infection.(27) Furthermore, TRAIL expression on NK cells is upregulated in HCV infected patients(28,29) and has been shown to decrease upon viral load reduction by DAA treatment.(22,23) We observed a similar decrease in TRAIL expression on CD56bright NK cells after a single dose of RG-101. In addition, the expression of the Fc-gamma-receptor, CD16, as well as cell cycle marker Ki67 decreased in patients dosed with RG-101, suggesting a reduction of the activated NK cell phenotype. Other markers also indicated decreased NK cell activation upon RG-101 dosing, including NKp30 and NKp46 which have previously been shown to decrease upon DAA treatment(23), and T-bet.(30) As some of these changes in NK cell markers showed similar changes on bulk CD8+ T cells and CD56+ NK T cells, the observed changes in the NK cell compartment might be the result of overall decrease in immune activation upon a decline in HCV viral load. However, as miR-122 has been implicated in immune regulation, direct effects of miR-122 inhibition could also contribute to the changes in NK cells observed.(24,25,31,32)
Chronic CHC infection leads to dysfunctional (‘exhausted’) HCV-specific CD8+ T cells. In patients successfully treated with interferon-free therapy, a restoration of the proliferative capacity of these HCV-specific CD8+ T cells has been observed.(33) However, whether the function of these HCV-specific CD8+ T cells also improves is unknown, nor whether a restoration of HCV immunity could contribute to HCV viral control. We therefore analyzed HCV-specific CD8+ T cells in RG-101 dosed patients, and we did not observe an increase in the ex vivo magnitude of HCV-specific CD8+ T cells as measured by HLA-A2 restricted multimers. To overcome the limitation of only measuring three specificities with multimers, which could be not optimally genotype-matched(20) or could have undergone viral escape, we subsequently analysed T cell functionality by stimulating with overlapping peptide pools and assessing IFN-γ production in ELISpot assays. This also allowed us to measure the functionality of both CD8+ and CD4+ HCV-specific T cells. The magnitude of HCV-specific T cell responses decreased in patients who were HCV RNA negative at week 8, which could be the result of the loss of viral replication and antigenic stimuli as seen in HIV-specific T cells after HAART therapy.(34,35) Furthermore, this could suggest T cells do not contribute to HCV RNA decline after RG-101 dosing. In line with this, we observed no specific change in the HCV-specific T cell responses in patients who were long term HCV RNA negative, suggesting other mechanisms play a role in the long term viral load reduction after RG-101 dosing.
In conclusion, one dose of anti-miR-122 RG-101 leads to a decrease in HCV RNA levels in all patients and sustained virological response >76 weeks in 3 patients. Dosing with RG-101 does not elicit systemic immune activation. A decrease in IP-10 levels and normalization of NK cell phenotype is observed which are likely the result of HCV RNA decline. Lastly, our data suggests that HCV-specific T cell recovery does not play a role in the decline or long term negativity of HCV RNA in patients dosed with RG-101.
Supplementary Material
funding
This study was funded by Regulus Therapeutics, Inc. Paul Klenerman received financial support from Wellcome Trust (WT091663MA), the Medical Research Council (STOP HCV, MR/K01532X/1), the NIHR Biomedical Research Centre, Oxford, the Oxford Martin School, NIH (U19AI082630) and an NIHR Senior Fellowship. Eleanor Barnes is funded through an MRC Senior Fellowship award and the Oxford NIHR Biomedical Research Centre.
The funders had no involvement in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
abbreviations used in this paper
- EBV
Epstein Barr virus
- ELISpot
Enzyme-Linked ImmunoSpot
- CHC
chronic hepatitis C
- CMV
cytomegalovirus
- DAAs
direct acting antivirals
- DMSO
dimethyl sulfoxide
- GalNAc
N-Acetylgalactosamine
- HCC
hepatocellular carcinoma
- HCV
Hepatitis C
- HLA
human leukocyte antigen
- IFN-γ
interferon-γ
- IP-10
interferon gamma-induced protein 10
- ISG
interferon stimulated gene
- LLOQ
lower limit of quantification
- mAbs
monoclonal antibodies
- miR-122
microRNA-122
- NK
natural killer
- PBMCs
peripheral blood mononuclear cells
- SVR
sustained virological response
- TRAIL
TNF-related apoptosis inducing ligand.
Footnotes
conflict of interest: Sophie Willemse: served as a speaker, a consultant and an advisory board member for AbbVie, Bristol-Myers-Squibb, Gilead Sciences, Janssen Therapeutics and Roche. Marc van der Valk: served on a scientific advisory board for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Johnson and Johnson, MSD and a data safety monitoring board for ViiV healthcare; Through his institution he received non-financial support by MSD. Paul Grint and Steven Neben are employees at Regulus Therapeutics. Henk Reesink received grants and personal fees from Roche, Bristor Myers Squibb, Gilead Sciences, Abbvie, Janssen-Cilag, MSD, PRA-international, Regulus Therapeutics and Replicor, received personal fees from Alnylam, and received a grant from Boehringer Ingelheim. All other authors: none declared.
author contributions: FS, MR, JV, SW, MV, HR performed the clinical study and sample collection, FS, MR, LS, PK, EB, NK, HR were involved in study design, FS, MS, AB, LS performed experiments and data analyses, FS wrote the manuscript, MR, MS, AB, LS, JV, SW, MV, PG, SN, PK, EB, NK, HR critically revised the manuscript.
References
- 1.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–S68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 2.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association Between Sustained Virological and Advanced Hepatic Fibrosis. J Am Med Assoc. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 3.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-ytter Y, et al. Eradication of Hepatitis C Virus Infection and the Development of Hepatocellular Carcinoma. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Pawlotsky JM. New hepatitis C therapies: The toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176–1192. doi: 10.1053/j.gastro.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Lindenbach BD. What’s next for hepatitis C virus research? Hepatology. 2016;63:1408–1410. doi: 10.1002/hep.28494. [DOI] [PubMed] [Google Scholar]
- 6.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–81. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 8.Machlin ES, Sarnow P, Sagan SM. Masking the 5’ terminal nucleotides of the hepatitis C virus genome by an unconventional microRNA-target RNA complex. Proc Natl Acad Sci U S A. 2011;108:3193–3198. doi: 10.1073/pnas.1012464108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Ree MH, de Vree JML, Stelma F, Willemse SB, van der Valk M, Rietdijk S, et al. A Single Dose of RG-101, a GalNAc-conjugated Oligonucleotide Antagonizing miR-122, Results in Significant Viral Load Reductions in Chronic Hepatitis C Patients: a Randomised Controlled Trial. Lancet. (in press) [Google Scholar]
- 10.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 11.Li Y-P, Gottwein JM, Scheel TK, Jensen TB, Bukh J. MicroRNA-122 antagonism against hepatitis C virus genotypes 1-6 and reduced efficacy by host RNA insertion or mutations in the HCV 5’ UTR. Proc Natl Acad Sci U S A. 2011;108:4991–6. doi: 10.1073/pnas.1016606108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Yamane D, Lemon SM. Dissecting the roles of the 5’ exoribonucleases Xrn1 and Xrn2 in restricting hepatitis C virus replication. J Virol. 2015;89:4857–65. doi: 10.1128/JVI.03692-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Masaki T, Yamane D, McGivern DR, Lemon SM. Competing and noncompeting activities of miR-122 and the 5’ exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc Natl Acad Sci U S A. 2013;110:1881–6. doi: 10.1073/pnas.1213515110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thibault PA, Huys A, Amador-Cañizares Y, Gailius JE, Pinel DE, Wilson JA. Regulation of Hepatitis C Virus Genome Replication by Xrn1 and MicroRNA-122 Binding to Individual Sites in the 5’ Untranslated Region. J Virol. 2015;89:6294–311. doi: 10.1128/JVI.03631-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masaki T, Arend KC, Li Y, Yamane D, McGivern DR, Kato T, et al. MiR-122 stimulates hepatitis C virus RNA synthesis by altering the balance of viral RNAs engaged in replication versus translation. Cell Host Microbe. 2015;17:217–228. doi: 10.1016/j.chom.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feld JJ, Nanda S, Huang Y, Chen W, Cam M, Pusek SN, et al. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment response. Hepatology. 2007;46:1548–1563. doi: 10.1002/hep.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markova AA, Mihm U, Schlaphoff V, Lunemann S, Filmann N, Bremer B, et al. PEG-IFN alpha but not ribavirin alters NK cell phenotype and function in patients with chronic Hepatitis C. PLoS One. 2014;9:e94512. doi: 10.1371/journal.pone.0094512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes E, Harcourt G, Brown D, Lucas M, Phillips R, Dusheiko G, et al. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743–754. doi: 10.1053/jhep.2002.35344. [DOI] [PubMed] [Google Scholar]
- 19.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [Author names in bold designate shared co-first authorship] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly C, Swadling L, Brown A, Capone S, Folgori A, Salio M, et al. Cross-reactivity of hepatitis C virus specific vaccine-induced T cells at immunodominant epitopes. Eur J Immunol. 2015;45:309–316. doi: 10.1002/eji.201444686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV Treatment outcome. J Clin Invest. 2014;124:3352–3363. doi: 10.1172/JCI75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HLA, et al. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J Infect Dis. 2015;213:216–23. doi: 10.1093/infdis/jiv391. [DOI] [PubMed] [Google Scholar]
- 23.Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015:1–11. doi: 10.1053/j.gastro.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li A, Qian J, He J, Zhang Q, Zhai A, Song W, et al. Modulation of miR-122 expression affects the interferon response in human hepatoma cells. Mol Med Rep. 2013;7:585–590. doi: 10.3892/mmr.2012.1233. [DOI] [PubMed] [Google Scholar]
- 25.Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [Author names in bold designate shared co-first authorship] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neben S, Liu K, Berman C, Tay J, Esau C, Kaiser R, et al. Pharmacokinetics, pharmacodynamics, and toxicity profile of RG-101, a novel GalNAc-conjugated hepatocyte targeting inhibitor of micro-RNA-122, in rhodentsand cynomolgus monkeys. J Hepatol. 2015;62:S684–S685. [Google Scholar]
- 27.Mundt B, Kühnel F, Zender L, Paul Y, Tillmann H, Trautwein C, et al. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94–6. doi: 10.1096/fj.02-0537fje. [DOI] [PubMed] [Google Scholar]
- 28.Wandrer F, Falk CS, John K, Skawran B, Manns MP, Schulze-Osthoff K, et al. Interferon-mediated cytokine induction determines sustained virus control in chronic hepatitis C Virus Infection. J Infect Dis. 2016;213:746–754. doi: 10.1093/infdis/jiv505. [DOI] [PubMed] [Google Scholar]
- 29.Serti E, Park H, Keane M, O’Keefe AC, Rivera E, Liang TJ, et al. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNα. Gut. 2016;0:1–12. doi: 10.1136/gutjnl-2015-310033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burchill MA, Golden-Mason L, Wind-Rotolo M, Rosen HR. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat. 2015;22:983–991. doi: 10.1111/jvh.12465. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura M, Kanda T, Sasaki R, Haga Y, Jiang X, Wu S, et al. MicroRNA-122 inhibits the production of inflammatory cytokines by targeting the PKR activator PACT in human hepatic stellate cells. PLoS One. 2015;10:e0144295. doi: 10.1371/journal.pone.0144295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, et al. Restoration of HCV-specific CD8+ T-cell function by Interferon-free therapy. J Hepatol. 2014;61:538–43. doi: 10.1016/j.jhep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 34.Kelly C, Swadling L, Brown A, Capone S, Folgori A, Salio M, et al. Cross-reactivity of hepatitis C virus specific vaccine-induced T cells at immunodominant epitopes. Eur J Immunol. 2015;45:309–316. doi: 10.1002/eji.201444686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin X, Ogg G, Bonhoeffer S, Safrit J, Vesanen M, Bauer D, et al. An antigenic threshold for maintaining human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. Mol Med. 2000;6:803–809. [PMC free article] [PubMed] [Google Scholar]
- 36.Conrad JA, Ramalingam RK, Duncan CB, Smith RM, Wei J, Barnett L, et al. Antiretroviral therapy reduces the magnitude and T cell receptor repertoire diversity of HIV-specific T cell responses without changing T cell clonotype dominance. J Virol. 2012;86:4213–21. doi: 10.1128/JVI.06000-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.