Abstract
Human CAXII was initially identified as a cancer marker in different cancers and tumors. Expression of CAXII is regulated by hypoxia and estrogen receptors. CAXII expression has been also detected in several tissues, whereas in cancer and tumor tissues its expression is several fold higher. In brain tumors, an alternatively spliced form of CAXII is expressed. Higher expression of CAXII in breast cancer is indicative of lower grade disease. CAXII plays a key role in several physiological functions. Mutation in the CAXII gene causes cystic fibrosis-like syndrome and salt wasting disease. CAXII is also seen in nuclear pulposus cells of the vertebrae. Aging dependent stiffness or degeneration of backbone correlates with CAXII expression level. This finding suggests a possible implication of CAXII as a biomarker for chronic back pain and a pharmacological target for possible treatment of chronic back pain.
INTRODUCTION
Carbonic anhydrases (CAs) catalyze a reversible reaction of carbon dioxide hydration and dehydration (1; 2). Based on cellular and sub-cellular location, CAs are classified into four different groups: cytosolic (CA I, II, III, VII, XIII); mitochondrial (CA VA, VB); secretory (CAVI), and membrane associated (CA IV, IX, XII, XIV, XV) (1–6). These isoforms differ with respect to enzyme activity and extent of inhibition with CA-inhibitors (2; 3). All membrane-associated CAs are high activity enzymes and have all essential histidine residues for enzyme activity (3; 5). With the exception of human CAIV, all membrane associated CAs are glycoproteins (7–10). Oligosaccharide moieties of the enzyme do not affect structure and function of the protein (9). Membrane associated CAs contain one or two disulfide bonds that are essential for the native structure and optimal function of the enzyme (10). Reduction of the disulfide bridge with reducing agents results in loss of enzyme activity (10). CA-related proteins have been identified, which include structural homologues of the enzyme without anhydrase activity due to lack of essential histidine residues at the active site (4). Several CA-isoforms play important roles in different physiological functions (4; 5; 7–15).
Carbonic anhydrase IV was the first membrane associated, GPI-anchored carbonic anhydrase that has been extensively characterized for its structure and function (16). Mutations in the leader sequence or in the gene body cause retinitis pigmentosa-17 (RP-17), a protein folding disease in humans (17). Progressive renal injuries have also been observed in transgenic mice expressing folding mutants of CAIV (18). Recently, it has been reported that mouse CAIV is also a tumor suppresser protein as well (19), and it may have some role in wound healing (Altonen M., Barker H., Pan P., unpublished observations, 2016).
CAXII and CAIX, both membrane-associated enzymes, have been characterized as tumor markers in human cancers (20). Expression of both membrane-associated CAs was induced by hypoxia in breast tumors and in several cancer cell lines (21–23). In breast tumors, only CAXII expression, and not CAIX, is under ER regulation (24; 25). CAXII expression in breast tumors is indicative of lower grade disease, lower relapse rates, and better overall patient survival (26; 27). An alternatively spliced form of CAXII is expressed in brain tumors, where its expression is associated with poorer prognosis (28). Mutation in CAXII has also been associated with the cystic fibrosis-like syndrome, including hyponatremia (29–32).
CAXII was initially identified from human renal clear cell carcinoma using the serological expression screening method (21). Simultaneously, CAXII was also cloned from renal carcinoma cells as a target of the von Hippel Lindau (VHL) gene using the RNA differential display method (22). Overexpression of wild type VHL in cancer cell lines reduced the overexpression of the CAXII gene, while overexpression of mutant VHL enhanced the expression of CAXII protein (22). CAXII expression, like CAIX, is induced by hypoxia in different cell lines (22). Unlike CAIX, CAXII is expressed in breast and renal tissues (23). The expression of CAXII is under estrogen receptor regulation (25–27; 33) and expression in breast tumors is associated with positive ER alpha receptor status (26; 27).
To understand the role of CAXII in renal physiology (34), carcinogenesis (35; 36), astrocytic gliomas (28), and hyponatremia (29; 30), CAXII cDNA has been cloned from human and mouse kidney tissues (21; 22). The cDNAs were expressed in heterologous mammalian cell lines (21) and in E. coli to produce recombinant CAXII proteins (8; 9). Recombinant CAXII has been used to study the structure and function of the enzyme by crystallography (9) and enzyme kinetics (8). Affinity purified enzymes have been used to produce rabbit specific CAXII antibodies and the antibodies were characterized by immunological procedures and used for immunohistochemistry of CAXII (8; 9; 21–23; 28; 32; 35; 36).
Carbonic anhydrase XII regulates pH and CO2 homeostasis in different tissues and in cancer cells and tumors. CAXII is widely expressed (22; 23; 37).
GENOMIC ORGANIZATION AND CHROMOSOMAL LOCALIZATION OF CAXII
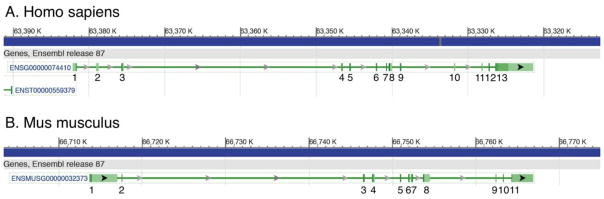
The mouse CAXII cDNA is 3,716 bp long and contains 11 coding exons that encode a protein of 354 amino acid residues (21; 35). The human CAXII gene locus has been mapped to 15q22, a band sensitive to amplification in some human cancers (21). The genomic organization of the CAXII gene is similar to other human carbonic anhydrase genes (2). Genomic organization of CAXII has been compared between humans and mice. The results are summarized in Figure 1. The amino acid sequences are depicted in Figure 2 (22).
Figure 1.
Genomic organization of CAXII gene. A. The Homo sapiens gene contains 13 exons whereas the mouse gene (B) has 11 exons (38). Other mouse CA genes contain different numbers of exons; however, the functional domains are highly conserved (2). The amino acid sequences for Homo sapiens (Gene ID 771) and Mus musculus (Gene ID 76459) shown below were obtained from the Gene database in NCBI using the Ensembl Genome Browser (release 87).
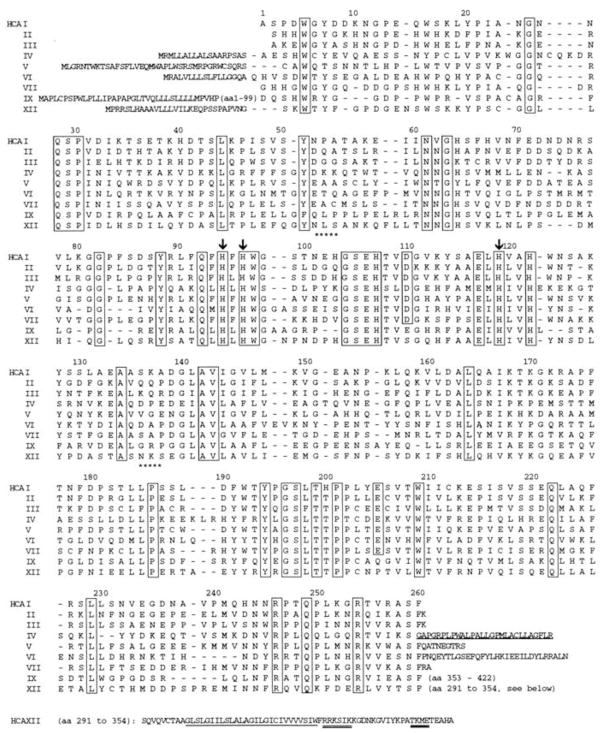
Figure 2.
Amino acid sequence homology of human carbonic anhydrases. Sequence homology alignment of human CAs show highly conserved residues among all enzymes. Conserved amino acids near the active site, including important histidine residues, are boxed. Metal binding histidine residues are marked by an arrow. Glycosylation sites are not conserved among all CAs and are indicated for CAXII by stars. Trans-membrane domains and phosphorylation sites are underlined (21), Copyright 1998.
GENOMIC CLONING OF CAXII
Full-length human CAXII cDNA was isolated from cDNA expression libraries developed from renal clear cell carcinoma (RCC) of a 69-year-old woman (21). Immunoreactive clones against autologous serum of RCC patients was isolated and characterized as described (21). Full-length cDNA was also cloned from human kidney (21). Initially, a 4.5-kb transcript that was overexpressed in RCC and not in surrounding tissues was characterized. Sequence analysis of the transcript suggested that it is a partial transcript of 1,670 bp. Therefore, a partial transcript was cloned into a 1,032-bp fragment using the technique of rapid amplification of cDNA ends. This analysis gave rise to a complete human CAXII cDNA clone encoding 7 bp of 5’ untranslated sequence containing a classical initiation codon, followed by a 1,062-bp open reading frame and 1,584 bp of 3’ untranslated sequence (21).
MECHANISM OF CAXII FUNCTION
CAXII, like many alpha-CAs, shows great homology of amino acid sequences near the active site, folding of polypeptides, and three-dimensional structure (9). The active site amino acids are occupied with a Zn+ ion that is coordinated with three histidine residues, His94, His96, and His119, and a water molecule or hydroxide ion (9). In summary, Zn+ with a hydroxyl ion binds to a CO2 molecule and catalyzes the formation of bicarbonate. The bicarbonate ion is displaced by a water molecule by releasing into water or buffer solution. This reaction can be described using the following equations:
| Equation (1) |
| Equation (2) |
The rate-limiting step is the proton transfer reaction that is carried by proton transfer histidine residue 64 (His64).
MOLECULAR CHARACTERIZATION OF CAXII
The open reading frame of CAXII predicted a protein of 354 amino acids with apparent molecular weight of 39.4 kDa (21). A homology search of CAXII showed homology to a conserved region of human carbonic anhydrases and CA-related proteins (21). Deduced amino acid residues from CAXII cDNA predicted 29 amino acids at the N-terminal as a signal sequence preceding the predicted cleavage site between Gly-1 and Ser-1. A 261-amino acid carbonic anhydrase homology domain of CAXII contains all conserved amino acid residues near the active site, a common feature among all carbonic anhydrases (21). CAXII also contains all critical histidine residues, His-94, His-96, and His-119, that are necessary for zinc binding, and also His-64, which contributes to the efficiency of the enzyme by acting as proton shuttle between the Zn-bound water molecule and surrounding buffer molecules (21). The extracellular CA-domain contains two potential asparagine residues for N-linked glycosylation. The N-linked glycosylation of the enzyme was confirmed by the treatment of CAXII with endoglycosydases.
The removal of oligosaccharides by Endo F from CAXII results in a decrease in the apparent molecular mass of the protein (21). The extracellular CA domain of the enzyme also contains four cystine residues, two of which, Cys-23 and Cys-203, might form an important disulfide bond that is conserved among other membrane associated carbonic anhydrases to provide structural stability and function to the enzyme molecule (21). The hydropathy plot of the CA domain suggests a 26-amino acid hydrophobic segment of the protein that serves as a transmembrane domain for anchoring the enzyme to the plasma membrane (9; 21). The transmembrane domain of CAXII contains two distinct motifs, GXXXG and GXXXS, which are involved in dimerization of the protein polypeptides (9; 21). Beyond the transmembrane domain, a 29-amino acid hydrophilic cytoplasmic domain at the C-terminus contains two potential sites for phosphorylation (9; 21). It has been suggested that phosphorylation of these sites might play a role in metabolic signaling by recruiting kinases to the cytosolic tail of the CAXII (9). The biological significance of the cytosolic tail was further suggested when an alternate spliced isoform of CAXII was identified in astrocytic gliomas (28). The mutant CAXII in astrocytomas was 11 amino acids shorter than the wild type enzyme. The shorter alternatively spliced isoform of CAXII lacks the unique GXXXG sequence, resulting in a loss of quaternary structure and metabolic signaling function (21; 28). Deletion of the hydrophobic, membrane spanning domain and cytosolic tail allows expression of a fully active secretory form of CAXII.
PURIFICATION OF RECOMBINANT CAXII
The secretory form of CAXII cDNA was cloned into a mammalian (21) or bacterial expression system (9) to produce and purify recombinant CAXII enzyme for specific antibody production (8; 9; 21; 23), enzyme kinetic measurements (8), and for crystallographic studies to determine the 3D structure of the CAXII enzyme (9). Using CA-inhibitor affinity chromatography (p-aminobenzene sulfonamide linked Sepharose resin), CAXII was purified from secretion medium of CHO cells or E. coli extracts expressing CAXII (8; 9; 21; 23).
STRUCTURE/FUNCTION OF CAXII
The affinity purified CAXII produced from E. coli extracts migrates as a 30-kDa protein; however, CAXII purified from CHO cell secretions showed a 35-kDa peptide on SDS-PAGE under reducing conditions, suggesting that CAXII is a glycoprotein (9). CAXII isolated from CHO cell secretions was also eluted as a dimeric protein on a sizing column. These results suggested that the secretory form of CAXII enzyme is a dimeric glycoprotein (9). This finding is a very striking difference between CAXII and all other soluble or membrane associated carbonic anhydrases (7; 10; 11). Purified human CAXII is a dimeric enzyme in solution as well as in crystal form (9). Both glycosylated and non-glycosylated CAXII showed similar crystal structures, suggesting that the oligosaccharide chain does not affect protein folding (9). The polypeptide fold of CAXII is similar to the beta-fold observed for other CA-isozymes (9). The amino acid sequence of CAXII derived from cDNA showed two glycosylation sites at Asn-52 and Asp-136 and a single disulfide bond between Cys-23 and Cys-203 (21). This disulfide bond is also present in CAIV, where it helps to stabilize the structure and function of the enzyme (8; 9; 21).
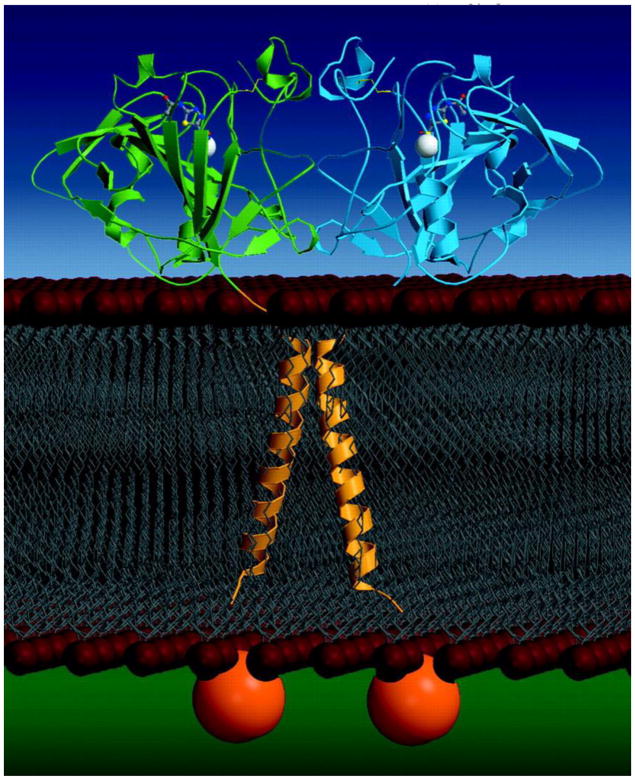
Further molecular characterization was carried out by determining the high resolution, 3D structure of the recombinant enzyme by x-ray crystallographic methods at 1.55 Å resolution. The structure of CAXII showed that two CAXII domains associate to form an isologous dimer that was also seen by gel-filtration studies of the enzyme in solution. The dimer interface is located such that the active site of each monomer is fully exposed for optimum function of the enzyme. A high-resolution structure of CAXII without and with CA-inhibitor provides a further tool for possible use in chemotherapy (9). The crystal structure is shown in Figure 3.
Figure 3.
Crystal structure of CAXII. Human carbonic anhydrase XII was crystallized at pH 7.5. Diffraction results of CAXII at 1.55 Å resolution suggested that the crystals belong to space group C2 with unit dimensions a=1.46.7 Å, b=44.6 Å, c=85.2 Å, and B=94.1 Å. One homodimer occupied the asymmetric unit. The native structure of CAXII consists of a dimer containing two zinc atoms, two Zn bound acetate ions, and 537 water molecules (9), Copyright 2001.
The active site structure of CAXII is nearly identical to that of CAII and CAIV (9). The catalysis of CO2 hydration by CAXII shows a maximum Kcat/Km value of 34 μM per second that is similar to membrane associated CAIV (16). The catalytic proton shuttle, His-64, is conserved in CAXII (8; 9; 16; 21). Substitution of His-64 suggested that it plays an important function in proton shuttling (8) Inhibition of CAXII by a 10 μM concentration of acetazolamide was found to inhibit invasion of renal carcinoma cells in vitro (39). In a separate study, CAXII knockdown has shown an effect on invasion, migration, and cell growth of breast cancer cells (40).
EXPRESSION OF CAXII IN DIFFERENT TISSUES
Expression of CAXII protein has been observed in different tissues (21; 22), including the basolateral membrane of epithelial cells of endometrium (41) and the basolateral surface of the epithelial cells in male efferent ducts (42). From these results, it has been concluded that CAXII may play an important role in bicarbonate and proton homeostasis in reproductive tissues (41; 42).
CAXII has been detected in the human gut, including all segments of the large intestine; however, it is absent from the small intestine (36). Immunostaining of CAXII has also been observed at the basolateral plasma membrane of enterocytes (36). Therefore, CAXII seems to play an important role in acidification and concentration of extracellular fluids in the human gut (36). CAXII is also weakly expressed in the gastric mucosa (43).
In human renal tissues, CAXII is expressed at the basolateral plasma membrane of epithelial cells in the thick ascending limb of the loop of Henle, distal convoluted tubules, and principal cells of the collecting ducts (35; 44). A weak signal of immunostaining of CAXII is also seen in the epithelium of the proximal convoluted tubules.
CAXII expression in the pancreatic epithelium at the basolateral plasma membrane of acinar and duct cells was seen (44).
In the human eye, CAXII is expressed in the non-pigmented ciliary epithelial cells, suggesting a role in humor production (45). Expression of CAXII in human eyes has been shown to increase in patients with glaucoma (45).
CAXII is also expressed in sweat glands and the enzyme might be responsible for salt homeostasis in sweat (29–32).
CAXII is expressed in different tissues, including mouse and rat kidney (46), gastrointestinal tract (47), and endometrium (48). Staining of CAXII has also been observed in the corpus and proximal caudal regions of the epididymis (49). The wide-spread expression of CAXII in mammalian tissues is a testament to its physiological significance (46–49).
EXPRESSION OF CAXII IN TUMOR AND CANCER CELLS
Following initial CAXII discovery as tumor marker (21; 22), expression of CAXII has been reported in different cancers (22) with higher expression in tumors than tissues (35). It has also been reported that the level of CAXII expression in cancer may be correlated with the outcome of the disease from poor to favorable prognosis (35).
In kidney cancer, CAXII expression has been found mostly in clear cell carcinomas and oncocytomas (35). In clear cell carcinomas, the level of CAXII expression correlates with the histological grade of the tumor (35).
In colorectal tumors, CAXII expression is very distinct from tissues (36). The extent of positive staining of CAXII was found to increase with a high grade of dysplasia (36).
Overexpression of CAXII has been detected in brain tumors, gliomas, hemangioblastomas, and meningiomas (50). CAXII expression has also been detected in diffuse astrocytomas (28).
CAXII expression has been detected in many other tumors and cancers, such as breast cancer (39), non-small cell lung cancer (51), and cervical cancer (52). In breast cancer, the degree of CAXII expression predicts a favorable outcome of the disease (27).
EXPRESSION OF CAXII DURING EMBRYONIC DEVELOPMENT
CAXII expression has been detected in a variety of mouse embryonic tissues, including the central nervous system, heart, lung, stomach, pancreas, liver, GI-tract, and urogenital tissues (53).
Expression of CAXII in human embryonic tissues is limited to cells involved in secretion and absorption of water (54).
MOLECULAR MECHANISM OF CAXII EXPRESSION
Expression of both CAXII and CAIX as tumor markers is regulated by hypoxia and they are known as hypoxia inducible genes (23; 37). Until recently, only CAXII, and not CAIX, showed a strong positive correlation with estrogen receptor (ER) expression in breast tumors (24; 25). It has been shown that CAXII is upregulated by estrogen using ER-α in breast cancer cells. This regulation involves ER binding to a distal estrogen-responsive enhancer region of CAXII (33). This study suggests an important role of ER in CAXII expression (33).
PHYSIOLOGICAL FUNCTIONS OF CAXII
RENAL FUNCTION
Approximately 95% of the total CA activity in the kidney comes from the cytosolic fraction of the enzyme and about 5% of the remaining activity is due to the membrane-associated enzyme that comprises CAIV, CAXII, and CAXIV (6; 7; 55; 56). CAIX is not expressed in kidney; however, its expression has been observed in renal cancer tissues (21; 22; 35; 46). In luminal membranes of the kidney, membrane associated CAs catalyze the dehydration of carbonic acid (H2CO3) to CO2 and water for removal of bicarbonate (57). CAXII is localized at the basolateral surface of the nephrons where efflux of bicarbonate is enhanced by CAXII (31; 32). CAXII also plays an important role for fluid and bicarbonate homeostasis in epithelial cells (31; 34), suggesting an important role of CAXII in kidney physiology (31; 34). CAXII may also function in the kidney as a metabolan of the membrane-associated CAXII and the chloride/bicarbonate exchanger (AE1) (31; 32) or sodium/bicarbonate co-transporter (NBCe) (32), and proton anti-porter (34; 58).
NEURONAL FUNCTION
Several observations have been reported for extracellular CA function in the neuronal activity of rat cerebellum (59). Initially, extracellular CA activity was identified as CAIV. CAIV was the only known CA isozyme that associated with the extracellular surface of neuronal endothelial and epithelial cells (60). Based on EST data, two more membrane associated CAs, CAXII and CAXIV, are also expressed in the brain (61; 62). Using CAIV and CAXIV knockout mice, the physiological function of both membrane associated CAs has been analyzed (38). The buffering efficiency of the extracellular space of the brain and response to light in mouse eyes have been studied, and both CAIV and CAXIV showed their respective roles (38). However, there has been no study to implicate CAXII in brain physiology.
EYE FUNCTION
Membrane associated carbonic anhydrases are important for eye function (63; 64). Mutations in the signal sequence of CAIV are associated with an autosomal dominant eye disease, retinitis pigmentosa or RP-17 (63; 64). CAIV and CAXIV double knockout mice showed abnormality in eye physiology (65). Expression of CAIX and CAXII transcripts in non-pigmented epithelial cells (NPE) has been reported (45), and the NPE from glaucoma patients showed increased CAXII gene expression. From these results, it has been concluded that CAXII is expressed in ciliary cells, and thus, may be involved in aqueous humor production (45).
SKELETAL AND CARDIAC MUSCLE FUNCTION
Both the sodium-proton exchanger (NHE1) and the chloride-bicarbonate exchanger are functionally linked to cytosolic CA and membrane associated CAs (66). CAXII in mouse heart has not been detected. However, membrane associated CAIV, CAIX, and CAXIV in mouse skeletal muscle play an important role (67; 68). It is uncertain whether human skeletal and cardiac muscle fibers may also require membrane associated CAXII for their function.
CAXII FUNCTIONS IN METABOLONS
After it was discovered that the association of CAII with AE proteins enhances the transport of bicarbonate ions (69), CAIV and CAIX (70) have also been shown to associate with AE proteins and enhance bicarbonate transport. Most recently, it was reported that CAXII regulates the function of the chloride-bicarbonate exchanger (AE2) by associating with the proteins (31; 34). Mutations in bicarbonate transporters such as CFTR, AE2, and NBCe1 cause defects in fluid homeostasis and bicarbonate secretion (71; 72), which results in pathological diseases like cystic fibrosis (73), pancreatitis (71; 74), and Sjogren’s syndrome (71; 72).
LOSS OF FUNCTION MUTATIONS IN CAXII RESULT IN CYSTIC FIBROSIS-LIKE DISEASE
Mutations in CAXII have been associated with an autosomal recessive form of salt wasting disease that results in hyponatremia in two consanguineous Bedouin kindred (29; 30). Clinical symptoms include high sweat chloride concentration, dehydration, and failure to thrive in infancy (30). In both pedigrees, similar missense mutations were observed. The effect of mutation on the enzyme activity was analyzed and a modest reduction (30%) in enzyme activity was observed. This result was unexpected for an autosomal recessive disorder where mutations generally result in complete loss of function. Thus, these authors suggested that the partial reduction in CAXII function results in a pathological phenotype limited to the sweat glands (29; 30).
In a separate study, Lee et al. (32) have shown that CAXII, with either a His121Gln or Glu143Lys mutation, localized to the basolateral membranes of MDCK cells, similar to wild type CAXII. However, the enzyme activity of the CAXII mutants was completely diminished at physiological concentrations of sodium chloride. Thus, loss of function of CAXII in sweat glands and lungs is the molecular basis in Cystic Fibrosis-like patients with normal CFTR. Since CAXII and AE2 are part of the metabolan (31; 34), mutant CAXII might compromise epithelial AE2 function, resulting in a decrease of fluid secretion and causing dry mouth syndrome in patients with a mutation in the CAXII enzyme (31; 32; 34).
CARBONIC ANHYDRASE XII IS A FUNCTIONAL BIO-MARKER OF NUCLEUS PULPOSUS
Degeneration of inter-vertebral discs (IVD) is associated with chronic neck and lower back pain (75). This degeneration results in a major disability causing many health issues. The IVD contains two distinct interdependent tissues: a gelatinous center, known as the nucleus pulposus (NP), and the surrounding coaxial lamellae that generate the inner and outer annulus fibrosus (AF). Due to natural aging, the gelatinous NP region of the disc becomes a more rigid and fibrous disc. This results in chronic back pain (76).
In order to develop specific therapeutic drugs, NP cell specific proteins were studied by DNA microarray analyses (77). Several hundred genes are upregulated in NP cells of which 90 contained trans-membrane domains. Twenty-eight were further characterized by RT-PCR. The most prominent signal was observed in CAXII genes of NP cells from young subjects and in degenerative tissues.
From these results, it was concluded that for the diagnosis of degenerative disc causing back pain, CAXII may be a suitable extracellular biomarker and may be applicable for therapeutic targets. The presence of CAXII in NP cells further suggests a role for CAXII in intracellular homeostasis of proton and bicarbonate ions. Thus, CAXII may function to normalize the cellular acidosis in the hypoxic NP cells under chronic back pain syndrome.
Highlights.
CAXII is a cancer marker
CAXII gene is a target of VHL gene
CAXII expression is under estrogen receptor regulation
CAXII expression in breast is indicative of lower grade disease and better patient survival
Alternatively spliced form of CAXII is expressed in brain tumors
Mutant form of CAXII is associated with cystic fibrosis-like syndrome
CAXII is a functional biomarker of nuclear pulposus
Acknowledgments
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series--a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The authors would like to thank Tracey Baird and Medina Hasanagic for editorial assistance in the preparation of this manuscript.
The corresponding Gene Wiki entry for this review can be found here: ≪https://en.wikipedia.org/wiki/CA12≫.
List of Abbreviations
- CA
carbonic anhydrase
- GPI
glycophoshatidylinositol lipid anchor
- VHL
von Hippel Lindau
- RCC
renal clear carcinoma
- CHO cells
Chinese hamster ovary cells
- E. coli
Escherichia coli
- GI-tract
gastrointestinal tract
- ER
estrogen receptor
- AE1
anion exchanger isoform 1
- NBCe
sodium bicarbonate cotransporter isoform e
- NPE
non-pigmented epithelial cells
- NHE1
sodium proton exchanger
- AE2
chloride-bicarbonate exchanger 2
- CFTR
cystic fibrosis transporter receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tashian RE. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989;10:186–92. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- 2.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 3.Nishimori I. Acatalytic CAs: Carbonic Anhydrase-Related Proteins. In: Supuran CT, Scozzafava A, Conway J, editors. Carbonic Anhydrase: Its Inhibitors and Activators. CRC Press; Boca Raton: 2004. pp. 25–42. [Google Scholar]
- 4.Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7:168–81. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 5.Imtaiyaz Hassan M, Shajee B, Waheed A, Ahmad F, Sly WS. Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem. 2013;21:1570–82. doi: 10.1016/j.bmc.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Waheed A, Zhu XL, Sly WS. Membrane-associated carbonic anhydrase from rat lung. Purification, characterization, tissue distribution, and comparison with carbonic anhydrase IVs of other mammals. J Biol Chem. 1992;267:3308–3311. [PubMed] [Google Scholar]
- 7.Zhu XL, Sly WS. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990;265:8795–8801. [PubMed] [Google Scholar]
- 8.Ulmasov B, Waheed A, Shah GN, Grubb JH, Sly WS, Tu C, Silverman DN. Purification and kinetic analysis of recombinant CA XII, a membrane carbonic anhydrase overexpressed in certain cancers. Proc Natl Acad Sci USA. 2000;97:14212–14217. doi: 10.1073/pnas.97.26.14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittington DA, Waheed A, Ulmasov B, Shah GN, Grubb JH, Sly WS, Christianson DW. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc Natl Acad Sci USA. 2001;98:9545–50. doi: 10.1073/pnas.161301298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waheed A, Okuyama T, Heyduk T, Sly WS. Carbonic anhydrase IV: purification of a secretory form of the recombinant human enzyme and identification of the positions and importance of its disulfide bonds. Arch Biochem Biophys. 1996;333:432–438. doi: 10.1006/abbi.1996.0412. [DOI] [PubMed] [Google Scholar]
- 11.Waheed A, Pham T, Won M, Okuyama T, Sly WS. Human carbonic anhydrase IV: in vitro activation and purification of disulfide-bonded enzyme following expression in Escherichia coli. Protein Expr Purif. 1997;9:279–287. doi: 10.1006/prep.1996.0691. [DOI] [PubMed] [Google Scholar]
- 12.Hilvo M, Tolvanen M, Clark A, Shen B, Shah GN, Waheed A, Halmi P, Hanninen M, Hamalainen JM, Vihinen M, Sly WS, Parkkila S. Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase. Biochem J. 2005;392:83–92. doi: 10.1042/BJ20051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esbaugh AJ, Tufts BL. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir Physiol Neurobiol. 2006;154:185–98. doi: 10.1016/j.resp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Dodgson SJ, Forster RE, 2nd, Schwed DA, Storey BT. Contribution of matrix carbonic anhydrase to citrulline synthesis in isolated guinea pig liver mitochondria. J Biol Chem. 1983;258:7696–701. [PubMed] [Google Scholar]
- 15.Supuran CT. Carbonic anhydrases--an overview. Curr Pharm Des. 2008;14:603–14. doi: 10.2174/138161208783877884. [DOI] [PubMed] [Google Scholar]
- 16.Waheed A, Sly WS. Carbonic Anhydrase IV. In: Supuran CT, De Simone G, editors. Carbonic Anhydrases as Biocatalysts: From Theory to Medical and Industrial Applications. Elsevier; Amsterdam: 2015. pp. 109–124. [Google Scholar]
- 17.Rebello G, Ramesar R, Vorster A, Roberts L, Ehrenreich L, Oppon E, Gama D, Bardien S, Greenberg J, Bonapace G, Waheed A, Shah GN, Sly WS. Apoptosis-inducing signal sequence mutation in carbonic anhydrase IV identified in patients with the RP17 form of retinitis pigmentosa. Proc Natl Acad Sci USA. 2004;101:6617–6622. doi: 10.1073/pnas.0401529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta R, Waheed A, Bonapace G, Shah GN, Sly WS. Pathogenesis of retinitis pigmentosa associated with apoptosis-inducing mutations in carbonic anhydrase IV. Proc Natl Acad Sci USA. 2009;106:3437–42. doi: 10.1073/pnas.0813178106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Tsoi H, Li X, Wang H, Gao J, Wang K, Go MY, Ng SC, Chan FK, Sung JJ, Yu J. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP-WT1-TBL1 axis. Gut. 2015 doi: 10.1136/gutjnl-2014-308614. Published Online First 12 June 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavada J, Zavadova Z, Pastorekova S, Ciampor F, Pastorek J, Zelnik V. Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer. 1993;54:268–74. doi: 10.1002/ijc.2910540218. [DOI] [PubMed] [Google Scholar]
- 21.Tureci O, Sahin U, Vollmar E, Siemer S, Gottert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA. 1998;95:7608–7613. doi: 10.1073/pnas.95.13.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA. 1998;95:12596–601. doi: 10.1073/pnas.95.21.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–19. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruvberger S, Ringner M, Chen Y, Panavally S, Saal LH, Borg A, Ferno M, Peterson C, Meltzer PS. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61:5979–84. [PubMed] [Google Scholar]
- 25.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der KK, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 26.Wykoff CC, Beasley N, Watson PH, Campo L, Chia SK, English R, Pastorek J, Sly WS, Ratcliffe P, Harris AL. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol. 2001;158:1011–1019. doi: 10.1016/S0002-9440(10)64048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson PH, Chia SK, Wykoff CC, Han C, Leek RD, Sly WS, Gatter KC, Ratcliffe P, Harris AL. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br J Cancer. 2003;88:1065–1070. doi: 10.1038/sj.bjc.6600796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haapasalo J, Hilvo M, Nordfors K, Haapasalo H, Parkkila S, Hyrskyluoto A, Rantala I, Waheed A, Sly WS, Pastorekova S, Pastorek J, Parkkila AK. Identification of an alternatively spliced isoform of carbonic anhydrase XII in diffusely infiltrating astrocytic gliomas. Neuro Oncol. 2008;10:131–8. doi: 10.1215/15228517-2007-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldshtein M, Elkrinawi S, Yerushalmi B, Marcus B, Vullo D, Romi H, Ofir R, Landau D, Sivan S, Supuran CT, Birk OS. Hyperchlorhidrosis caused by homozygous mutation in CA12, encoding carbonic anhydrase XII. Am J Hum Genet. 2010;87:713–20. doi: 10.1016/j.ajhg.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhammad E, Leventhal N, Parvari G, Hanukoglu A, Hanukoglu I, Chalifa-Caspi V, Feinstein Y, Weinbrand J, Jacoby H, Manor E, Nagar T, Beck JC, Sheffield VC, Hershkovitz E, Parvari R. Autosomal recessive hyponatremia due to isolated salt wasting in sweat associated with a mutation in the active site of Carbonic Anhydrase 12. Hum Genet. 2011;129:397–405. doi: 10.1007/s00439-010-0930-4. [DOI] [PubMed] [Google Scholar]
- 31.Hong JH, Muhammad E, Zheng C, Hershkovitz E, Alkrinawi S, Loewenthal N, Parvari R, Muallem S. Essential role of carbonic anhydrase XII in secretory gland fluid and HCO3 (−) secretion revealed by disease causing human mutation. J Physiol. 2015;593:5299–312. doi: 10.1113/JP271378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee M, Vecchio-Pagan B, Sharma N, Waheed A, Li X, Raraigh KS, Robbins S, Han ST, Franca AL, Pellicore MJ, Evans TA, Arcara KM, Nguyen H, Luan S, Belchis D, Hertecant J, Zabner J, Sly WS, Cutting GR. Loss of carbonic anhydrase XII function in individuals with elevated sweat chloride concentration and pulmonary airway disease. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw065. Published Online First 23 Feb 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnett DH, Sheng S, Charn TH, Waheed A, Sly WS, Lin CY, Liu ET, Katzenellenbogen BS. Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res. 2008;68:3505–3515. doi: 10.1158/0008-5472.CAN-07-6151. [DOI] [PubMed] [Google Scholar]
- 34.Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int. 2007;71:103–15. doi: 10.1038/sj.ki.5002020. [DOI] [PubMed] [Google Scholar]
- 35.Parkkila S, Parkkila AK, Saarnio J, Kivela J, Karttunen TJ, Kaunisto K, Waheed A, Sly WS, Tureci O, Virtanen I, Rajaniemi H. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem. 2000;48:1601–8. doi: 10.1177/002215540004801203. [DOI] [PubMed] [Google Scholar]
- 36.Kivela A, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Waheed A, Sly WS, Grubb JH, Shah G, Tureci O, Rajaniemi H. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol. 2000;156:577–584. doi: 10.1016/S0002-9440(10)64762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–83. [PubMed] [Google Scholar]
- 38.Shah GN, Ulmasov B, Waheed A, Becker T, Makani S, Svichar N, Chesler M, Sly WS. Carbonic anhydrase IV and XIV knockout mice: roles of the respective carbonic anhydrases in buffering the extracellular space in brain. Proc Natl Acad Sci USA. 2005;102:16771–6. doi: 10.1073/pnas.0508449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA. 2000;97:2220–4. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh MJ, Chen KS, Chiou HL, Hsieh YS. Carbonic anhydrase XII promotes invasion and migration ability of MDA-MB-231 breast cancer cells through the p38 MAPK signaling pathway. Eur J Cell Biol. 2010;89:598–606. doi: 10.1016/j.ejcb.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Karhumaa P, Parkkila S, Tureci O, Waheed A, Grubb JH, Shah G, Parkkila A, Kaunisto K, Tapanainen J, Sly WS, Rajaniemi H. Identification of carbonic anhydrase XII as the membrane isozyme expressed in the normal human endometrial epithelium. Mol Hum Reprod. 2000;6:68–74. doi: 10.1093/molehr/6.1.68. [DOI] [PubMed] [Google Scholar]
- 42.Karhumaa P, Kaunisto K, Parkkila S, Waheed A, Pastorekova S, Pastorek J, Sly WS, Rajaniemi H. Expression of the transmembrane carbonic anhydrases, CA IX and CA XII, in the human male excurrent ducts. Mol Hum Reprod. 2001;7:611–6. doi: 10.1093/molehr/7.7.611. [DOI] [PubMed] [Google Scholar]
- 43.Leppilampi M, Saarnio J, Karttunen TJ, Kivela J, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S. Carbonic anhydrase isozymes IX and XII in gastric tumors. World J Gastroenterol. 2003;9:1398–403. doi: 10.3748/wjg.v9.i7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kivela AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Rajaniemi H. Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol. 2000;114:197–204. doi: 10.1007/s004180000181. [DOI] [PubMed] [Google Scholar]
- 45.Liao SY, Ivanov S, Ivanova A, Ghosh S, Cote MA, Keefe K, Coca-Prados M, Stanbridge EJ, Lerman MI. Expression of cell surface transmembrane carbonic anhydrase genes CA9 and CA12 in the human eye: overexpression of CA12 (CAXII) in glaucoma. J Med Genet. 2003;40:257–61. doi: 10.1136/jmg.40.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyllonen MS, Parkkila S, Rajaniemi H, Waheed A, Grubb JH, Shah GN, Sly WS, Kaunisto K. Localization of carbonic anhydrase XII to the basolateral membrane of H+-secreting cells of mouse and rat kidney. J Histochem Cytochem. 2003;51:1217–1224. doi: 10.1177/002215540305100912. [DOI] [PubMed] [Google Scholar]
- 47.Halmi P, Lehtonen J, Waheed A, Sly WS, Parkkila S. Expression of hypoxia-inducible, membrane-bound carbonic anhydrase isozyme XII in mouse tissues. Anat Rec A Discov Mol Cell Evol Biol. 2004;277:171–7. doi: 10.1002/ar.a.20001. [DOI] [PubMed] [Google Scholar]
- 48.Hynninen P, Hamalainen JM, Pastorekova S, Pastorek J, Waheed A, Sly WS, Tomas E, Kirkinen P, Parkkila S. Transmembrane carbonic anhydrase isozymes IX and XII in the female mouse reproductive organs. Reprod Biol Endocrinol. 2004;2:73. doi: 10.1186/1477-7827-2-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermo L, Chong DL, Moffatt P, Sly WS, Waheed A, Smith CE. Region- and cell-specific differences in the distribution of carbonic anhydrases II, III, XII, and XIV in the adult rat epididymis. J Histochem Cytochem. 2005;53:699–713. doi: 10.1369/jhc.4A6575.2005. [DOI] [PubMed] [Google Scholar]
- 50.Proescholdt MA, Mayer C, Kubitza M, Schubert T, Liao SY, Stanbridge EJ, Ivanov S, Oldfield EH, Brawanski A, Merrill MJ. Expression of hypoxia-inducible carbonic anhydrases in brain tumors. Neuro Oncol. 2005;7:465–75. doi: 10.1215/S1152851705000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ilie MI, Hofman V, Ortholan C, Ammadi RE, Bonnetaud C, Havet K, Venissac N, Mouroux J, Mazure NM, Pouyssegur J, Hofman P. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int J Cancer. 2011;128:1614–23. doi: 10.1002/ijc.25491. [DOI] [PubMed] [Google Scholar]
- 52.Yoo CW, Nam BH, Kim JY, Shin HJ, Lim H, Lee S, Lee SK, Lim MC, Song YJ. Carbonic anhydrase XII expression is associated with histologic grade of cervical cancer and superior radiotherapy outcome. Radiat Oncol. 2010;5:101. doi: 10.1186/1748-717X-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kallio H, Pastorekova S, Pastorek J, Waheed A, Sly WS, Mannisto S, Heikinheimo M, Parkkila S. Expression of carbonic anhydrases IX and XII during mouse embryonic development. BMC Dev Biol. 2006;6:22. doi: 10.1186/1471-213X-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao SY, Lerman MI, Stanbridge EJ. Expression of transmembrane carbonic anhydrases, CAIX and CAXII, in human development. BMC Dev Biol. 2009;9:22. doi: 10.1186/1471-213X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wistrand PJ, Knuuttila KG. Renal membrane-bound carbonic anhydrase. Purification and properties. Kidney Int. 1989;35:851–9. doi: 10.1038/ki.1989.63. [DOI] [PubMed] [Google Scholar]
- 56.McKinley DN, Whitney PL. Particulate carbonic anhydrase in homogenates of human kidney. Biochim Biophys Acta. 1976;445:780–90. doi: 10.1016/0005-2744(76)90128-5. [DOI] [PubMed] [Google Scholar]
- 57.Brown D, Zhu XL, Sly WS. Localization of membrane-associated carbonic anhydrase type IV in kidney epithelial cells. Proc Natl Acad Sci USA. 1990;87:7457–7461. doi: 10.1073/pnas.87.19.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–68. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 59.Kraig RP, Ferreira-Filho CR, Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983;49:831–50. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- 60.Ghandour MS, Langley OK, Zhu XL, Waheed A, Sly WS. Carbonic anhydrase IV on brain capillary endothelial cells: a marker associated with the blood-brain barrier. Proc Natl Acad Sci USA. 1992;89:6823–6827. doi: 10.1073/pnas.89.15.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hewett-Emmett D. Evolution and Distribution of the Carbonic Anhydrase Gene Families. In: Chegwidden WR, Carter ND, Edwards YH, editors. The Carbonic Anhydrases: New Horizons. Birkhauser; Basel: 2000. pp. 29–76. [DOI] [PubMed] [Google Scholar]
- 62.Parkkila S, Parkkila AK, Rajaniemi H, Shah GN, Grubb JH, Waheed A, Sly WS. Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci USA. 2001;98:1918–1923. doi: 10.1073/pnas.98.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hageman GS, Zhu XL, Waheed A, Sly WS. Localization of carbonic anhydrase IV in a specific capillary bed of the human eye. Proc Natl Acad Sci USA. 1991;88:2716–2720. doi: 10.1073/pnas.88.7.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagelhus EA, Mathiisen TM, Bateman AC, Haug FM, Ottersen OP, Grubb JH, Waheed A, Sly WS. Carbonic anhydrase XIV is enriched in specific membrane domains of retinal pigment epithelium, Muller cells, and astrocytes. Proc Natl Acad Sci USA. 2005;102:8030–5. doi: 10.1073/pnas.0503021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogilvie JM, Ohlemiller KK, Shah GN, Ulmasov B, Becker TA, Waheed A, Hennig AK, Lukasiewicz PD, Sly WS. Carbonic anhydrase XIV deficiency produces a functional defect in the retinal light response. Proc Natl Acad Sci USA. 2007;104:8514–8519. doi: 10.1073/pnas.0702899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hallerdei J, Scheibe RJ, Parkkila S, Waheed A, Sly WS, Gros G, Wetzel P, Endeward V. T tubules and surface membranes provide equally effective pathways of carbonic anhydrase-facilitated lactic acid transport in skeletal muscle. PLoS One. 2010;5:e15137. doi: 10.1371/journal.pone.0015137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Alvarez BV, Johnson DE, Sowah D, Soliman D, Light PE, Xia Y, Karmazyn M, Casey JR. Carbonic anhydrase inhibition prevents and reverts cardiomyocyte hypertrophy. J Physiol. 2007;579:127–45. doi: 10.1113/jphysiol.2006.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villafuerte FC, Swietach P, Youm JB, Ford K, Cardenas R, Supuran CT, Cobden PM, Rohling M, Vaughan-Jones RD. Facilitation by intracellular carbonic anhydrase of Na+-HCO3-co-transport but not Na+ / H+ exchange activity in the mammalian ventricular myocyte. J Physiol. 2014;592:991–1007. doi: 10.1113/jphysiol.2013.265439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vince JW, Reithmeier RA. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J Biol Chem. 1998;273:28430–7. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 70.Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A, Zatovicova M, Barathova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem. 2012;287:3392–402. doi: 10.1074/jbc.M111.286062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 2012;92:39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almstahl A, Wikstrom M. Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins. Arch Oral Biol. 2003;48:337–44. doi: 10.1016/s0003-9969(02)00200-5. [DOI] [PubMed] [Google Scholar]
- 73.Quinton PM. Role of epithelial HCO3(−) transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010;299:C1222–33. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maleth J, Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell Calcium. 2014;55:337–45. doi: 10.1016/j.ceca.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 76.Johnson WE, Evans H, Menage J, Eisenstein SM, El Haj A, Roberts S. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976) 2001;26:2550–7. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- 77.Power KA, Grad S, Rutges JP, Creemers LB, van Rijen MH, O'Gaora P, Wall JG, Alini M, Pandit A, Gallagher WM. Identification of cell surface-specific markers to target human nucleus pulposus cells: expression of carbonic anhydrase XII varies with age and degeneration. Arthritis Rheum. 2011;63:3876–86. doi: 10.1002/art.30607. [DOI] [PubMed] [Google Scholar]