Abstract
Organic photoredox catalysts have been shown to operate organocatalyzed atom transfer radical polymerizations (O-ATRP) using visible light as the driving force. In this work, the effect of light intensity from white LEDs was evaluated as an influential factor in control over the polymerization and the production of well-defined polymers. We posit the irradiation conditions control the concentrations of various catalyst states necessary to mediate a controlled radical polymerization. Systematic dimming of white LEDs allowed for consideration of the role of light intensity on the polymerization performance. The general effects of decreased irradiation intensity in photoinduced O-ATRP were investigated through comparing two different organic photoredox catalysts: perylene and an 3,7-di(4-biphenyl) 1-naphthalene-10-phenoxazine. Previous computational efforts have investigated catalyst photophysical and electrochemical characteristics, but the broad and complex effects of varied irradiation intensity as an experimental variable on the mechanism of O-ATRP have not been explored. This work revealed that perylene requires more stringent irradiation conditions to achieve controlled polymer molecular weight growth and produce polymers with dispersities <1.50. In contrast, the 3,7-di(4-biphenyl) 1-naphthalene-10-phenoxazine is more robust, achieving linear polymer molecular weight growth under relative irradiation intensity as low as 25%, to produce polymers with dispersities <1.50. This finding is significant, as the discovery of highly robust catalysts is necessary to allow for the adoption of successful O-ATRP in a wide scope of conditions, including those which necessitate low light intensity irradiation.
Graphical abstract
1. INTRODUCTION
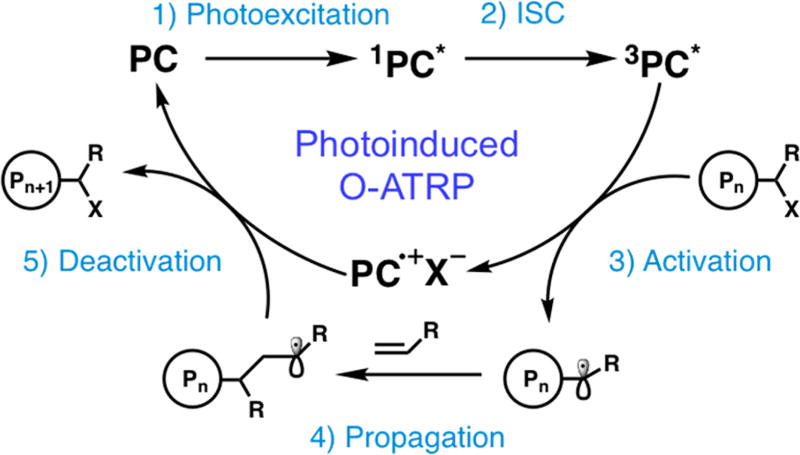
Controlled radical polymerization (CRP) methodologies have enabled the construction of well-defined and precise polymeric materials by providing synthetic approaches that produce polymers with well-defined molecular weight (MW), dispersity (Đ), and composition.1–5 Organocatalyzed atom transfer radical polymerization (O-ATRP) has recently emerged as a methodology to produce well-defined polymers without the use of metal catalysts.6 Since initial reports that perylene7 and phenylphenothiazine8 could operate O-ATRP, more organic photoredox catalysts (PCs) have been introduced, including several diaryl dihydrophenazine,9–11 carbazole,12 phenoxazine,13 anthracene/pyrene,14 and other phenothiazine15–18 derivatives. A proposed mechanism of O-ATRP proceeds through an oxidative quenching pathway which consists of five main processes: (1) photoexcitation of the ground state PC to a singlet excited state (1PC*); (2) intersystem crossing to a triplet excited state (3PC*);9,19 (3) direct reduction of an alkyl halide initiator or polymer chain end by 3PC*, generating an active radical for polymerization propagation and formation of a radical cation/halide anion “deactivator” complex (PC•+/X−); (4) polymer MW growth via polymerization propagation; and (5) oxidation of the active radical by PC•+/X− to reversibly deactivate the polymerization and regenerate PC (Figure 1).
Figure 1.
Proposed oxidative quenching cycle for O-ATRP mediated by photoredox catalysts.
Maintaining a low radical concentration by favoring dormant polymer chains through an efficient radical deactivation process is critical for CRPs to produce well-defined polymers. Minimizing bimolecular termination pathways allows for the synthesis of polymers with predictable MW and low Đ through a controlled polymerization, achieving a linear increase in polymer MW with monomer conversion. In an ideal polymerization, no exogenous additives would be necessary for the system to be controlled, but in practice CRPs can be greatly improved by the addition of deactivating species or reagents that regenerate active catalysts.20,21 For example, a strategy to maintain a low radical concentration in copper ATRP is the addition of excess or exclusively22,23 deactivator in the form of Cu(II) salts at the beginning of the polymerization.24 Further, if excess Cu(II) species are not added, it is proposed that a steady-state Cu(II) concentration must be met before control over the system can be realized.25 This approach influences the activation vs deactivation equilibrium to favor dormant polymer chains by increasing the concentration of the deactivating species that regenerates Cu(I) and the halide-capped polymer. If this condition is not met, deviation of the polymer MW from the theoretical values can be observed as a consequence of poor deactivation.26 For O-ATRP, this translates to the requirement for a sufficient buildup of PC•+/Br− to be met to enable control over the polymerization. Holistically, the oxidizing power of this deactivator complex is also of importance, as the efficiency of deactivation is a function of both the concentration and reactivity of PC•+/Br−.
In the O-ATRP mechanism, we hypothesize 3PC* is the predominant species responsible for reduction of the alkyl bromide and activation of the propagating radical for polymerization due to the longer excited state lifetime of the triplet state relative to the singlet state.27 Thereafter, the resulting PC•+Br− ion pair must be sufficiently oxidizing to deactivate the propagating radical and realize a CRP. Furthermore, as is the case in all CRPs, the rate of deactivation (kdeact) must be equal to or greater than the rate of activation (kact) to minimize undesirable termination pathways.28 An idealized scenario to realize this balance of rates would involve the oxidized PC, PC•+Br−, remaining associated with the same polymer chain after activating the radical and rapidly deactivating the same radical. However, the ion pair can likely diffuse away from the neutral propagating radical and potentially deactivate other radicals in solution. Such diffusion between deactivating species and propagating radicals in CRPs has been experimentally confirmed through crossover studies in both polymerizations and model small molecule systems.29–32 As such, a critical concentration of the deactivating species is required to achieve control over the polymerization, which can either be added at the onset of the polymerization or generated through the activation step.33,34 In photoredox mediated ATRP, the PC•+Br− deactivator is produced through the alkyl bromide reduction, which is dictated by the reduction potential and [3PC*]. In turn, [3PC*] is influenced by a combination of the light intensity, initial PC loading, and the triplet quantum yield of the PC. In short, the [PC•+Br−], through controlling intensity of irradiation, in concert with the photophysical and thermodynamic properties of the photocatalyst, must be optimized for kdeact to be sufficiently large to realize a controlled process through efficient deactivation.
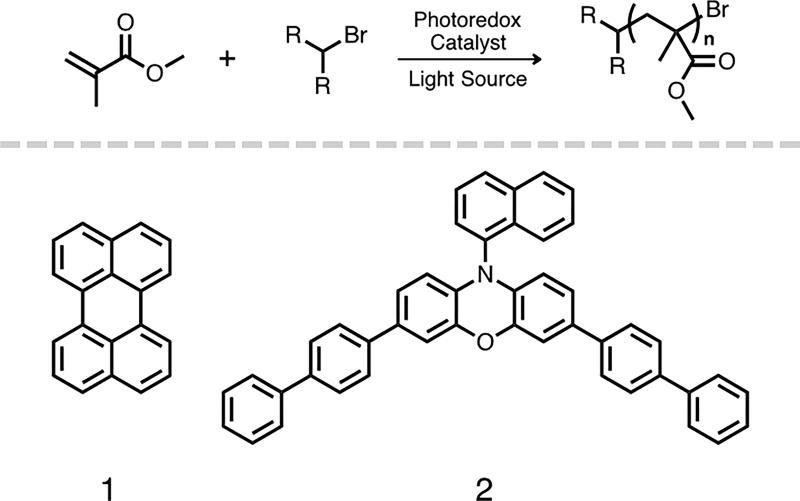
The two PCs used in this study of the effects of light intensity on the control over O-ATRP—perylene (PC 1) and an 3,7-di(4-biphenyl) 1-naphthalene-10-phenoxazine (PC 2)—represent a comparison between an early example of an organic photoredox catalyst7 and a more successful PC designed to be highly reducing and absorptive under visible irradiation,13 respectively. In regards to polymerization performance, PC 1 typically achieved low initiator efficiency (I*), while PC 2 commonly achieved nearly quantitative I* while producing polymers with low dispersity (Đ < 1.3). By evaluating PCs 1 and 2 directly under previously employed conditions, which have demonstrated different levels of success in the polymerization of methacrylate monomers, the impact of the light source on the control over O-ATRP can be evaluated more completely. This reaction parameter has not been systematically explored for O-ATRP systems, even though the photoexcitation event adds a rich level of complexity to the polymerization mechanism. Recently, irradiation conditions have been shown to be key optimization parameters for small molecule reactions mediated by a variety of photocatalysts.35 For photoinduced O-ATRP, the photoexcitation process controls the efficiency of activation directly and the efficiency of deactivation indirectly. The characteristics of absorption (PC → PC*), quantum yield, excited-state lifetime, and reduction potential of 3PC* are major factors in the efficiency of the polymerization catalytic cycle. Modulating the irradiation intensity influences the concentration of 3PC* in solution and the rate of initiation, with decreased light intensity lowering the rate of initiation. As such, the concentration of PC•+/Br− is decreased under lower irradiation, as this complex is generated through activation.36 Herein, the influence of light intensity and PC concentration are investigated as factors that affect the performance of OATRP by its modulation of [3PC*], using PCs 1 and 2 as catalysts for the polymerization of methyl methacrylate (MMA).
2. RESULTS AND DISCUSSION
Seminal work with perylene in batch conditions demonstrated the feasibility of O-ATRP but did not achieve the control over the polymerization that is expected from ATRP. More recently, O-ATRP under continuous flow conditions was developed, improving the control of MW growth with respect to monomer conversion using perylene.37 The photophysical properties of perylene are well studied and potentially provide insight into the poor performance of PC 1 as a PC for O-ATRP in batch conditions; the excited state reduction potential of 3PC*was computed to be −0.78 V (vs SCE) (see Supporting Information). This value is similar to the activated alkyl bromide initiators38,39 commonly employed in ATRP and thus just strong enough of a reductant for their activation; Marcus theory explains that a stronger reducing 3PC* (compared to the alkyl halide) will provide a more favorable electron transfer.40,41 Additionally, the quantum yield for fluorescence of perylene is ~0.98 with τ = 4.9 ns,42–45 which is most likely not sufficiently long for efficient bimolecular electron transfer and reduction of the alkyl bromide. Typically, excited state lifetimes exceeding 10 ns are required for productive electron transfer processes.46 The combination of the excited state reduction potential of 3PC* of perylene, just strong enough for the reduction of an alkyl bromide ATRP initiator, and the inefficient formation of 3PC* could impact the activation efficiency of the alkyl bromide, yielding a low concentration of PC•+Br−.
In contrast, phenoxazines are much stronger reductants in their photoexcited triplet states (E0* ~ −2.0 V vs SCE), and such classes of molecules are known to access long-lived 3PC* more efficiently.42–44 For instance, at 77 K, 10-phenylphenoxazine was reported to access a 3PC* with a lifetime up to 2.3 s and a phosphorescence quantum yield of 94%.47 Under otherwise similar reaction conditions, the visible light absorbing PC 2 produced polymer product with Đ as low as 1.13 and quantitative I*, as compared to perylene which synthesized polymers with Đ as low as 1.39, and I* < 50% for a variety of experimental conditions. As such, we questioned if the poor control over the previously reported visible light induced O-ATRP using perylene as the PC could be attributed to poor irradiation conditions or simply that perylene is a poor catalyst for O-ATRP.
We reasoned that increasing the concentration of 3PC* in the polymerization reaction would enhance the activation step and increase the concentration of PC•+Br−, and thus the efficiency of deactivation, improving control over the polymerization. The concentration of PC* can be increased through increasing the catalyst loading and/or modulating the irradiation intensity. Under irradiation by white LEDs, polymerizations were performed with catalyst loadings of 0.1 or 0.4 mol % (Table 1). Increasing the [1] decreased the Đ from 1.82 to 1.38 for the polymerization of MMA (runs 1 and 2). A general increase in the I* is observed as well, with most trials ~40%, representing a significant improvement for this system over previously reported results.7
Table 1.
Results of the O-ATRP of MMA Mediated by PC 1 with Varied PC Concentrations and Irradiation Intensitiesa
| run no. | hν (intensity)a (%) | [I]:[PC]b | time (h) | convc (%) | Mwd (kDa) | Mnd (kDa) | Đ (Mw/Mn)d | I*e (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 10:1 | 6 | 50.4 | 21.6 | 11.9 | 1.82 | 43.6 |
| 2 | 100 | 10:4 | 6 | 49.4 | 19.4 | 14.0 | 1.38 | 36.1 |
| 3 | 100 | 10:6 | 6 | 56.2 | 18.8 | 13.9 | 1.36 | 41.6 |
| 4 | 100 | 10:10 | 6 | 47.3 | 16.7 | 11.8 | 1.42 | 41.2 |
| 5 | 50 | 10:4 | 6 | 41.5 | 14.6 | 10.1 | 1.44 | 41.9 |
| 6 | 25 | 10:4 | 6 | 42.6 | 16.5 | 12.6 | 1.69 | 34.6 |
| 7 | 5 | 10:4 | 6 | 25.6 | 34.6 | 15.1 | 2.29 | 17.3 |
See Supporting Information for details, polymerizations performed with 1.00 mL of MMA (1.87 M).
Molar ratio of initiator ([I]) to photocatalyst ([PC]).
Measured by 1H NMR.
Measured by GPC coupled with light scattering.
I* = (theoretical number-average MW)/(experimentally measured number-average MW) × 100.
By increasing the amount of catalyst from 0.1 to 0.4 mol %, the Đ of the resulting polymer greatly decreased (from 1.82 to 1.38) while I* was similar (43.6% vs 36.1%). Further increasing the catalyst loading did not lead to significant enhancement, with the 0.6 and 1 mol % trials resulting in less controlled number-average molecular weight (Mn) growth than for 0.4 mol %, as evidenced by plots of Mn as a function of monomer conversion, with linear growth a key characteristic to define a polymerization as “controlled” (runs 3 and 4, Figure S7). As such, 0.4 mol % was chosen as the catalyst loading to systematically explore the effect of light intensity on the degree of control over the polymerization, performed with relative irradiation intensities of 100%, 50%, 25%, or 5%, by use of a LED dimer (Table 1, runs 5–7, Figures S3–S6). By lowering the relative intensity of irradiation from 100% to 50%, 25%, or 5%, the Đ increases from 1.38 to 1.44, 1.69, and 2.29, respectively. From these results, it is clear light intensity has a significant impact on the control over the polymerization.
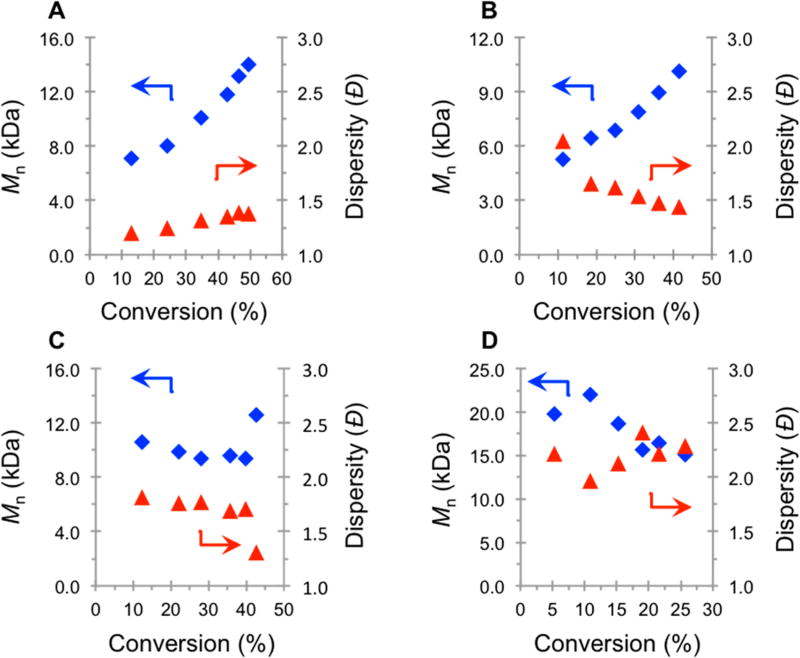
Investigating the polymerization of MMA and the growth in polymer MW in more detail, for the 100% relative intensity trial a linear increase in the Mn as a function of monomer conversion is observed (Figure 3A). The linear Mn growth as a function of monomer conversion had yet to be demonstrated for a perylene-mediated O-ATRP under batch reactor conditions. The y-intercept of the trendline for Mn vs monomer conversion in Figure 3A is ~4 kDa, deviating from the ideal value of 0.24 kDa, the mass of the alkyl bromide initiator. The y-intercept can be used to judge the efficiency at which a system achieves control in early periods of polymerization, with values closer to the mass of the initiator indicating more controlled and predictable MW growth. The 4 kDa y-intercept of this plot when using PC 1 suggests that there is an initial period of uncontrolled polymer growth at low monomer conversion. Additionally, a nonlinear growth in Mn vs monomer conversion holds mechanistic implications. This uncontrolled MW growth in early periods of polymerization is indicative of slow deactivation33,34,48 due to a low [PC•+Br−] during the early stages of polymerization. The polymerization irradiated at 50% relative intensity also resulted in a linear increase in polymer MW; however, the Đ is high for the early period of polymerization (Figure 3B). This overall trend in Đ supports the hypothesis that decreasing the intensity of light decreases the efficiency of deactivation.
Figure 3.
Plots of Mn (blue ◆) and Đ (red ▲) as a function of monomer conversion irradiated with 100% (A), 50% (B), 25% (C), and 5% (D) relative irradiation intensity for the polymerization of MMA using PC 1 (see Supporting Information for experimental details).
An interesting effect is observed in the evolution of Đ for the 100% and 50% relative intensity polymerizations. At 100% relative intensity, the trend in the evolution of Đ is increasing, while decreasing for the 50% trial. These trends highlight the importance of matching catalyst properties with the reaction conditions to achieve a controlled radical polymerization. Lowering the light intensity to 25% further results in an increase in Đ and a loss of controlled growth in Mn for the early period of polymerization (Figure 3C), as evidenced by the nonlinear growth in polymer MW. As the relative light intensity is lowered to 5%, control over the MW growth is lost, and polymer with high dispersity (Đ > 2.0) is obtained due to a complete loss of control over the polymerization. A possible explanation for the improvement in the performance of PC 1 in this study can be attributed to the increased concentration of excited state photocatalyst achieved through the combination of higher catalyst loading and increased irradiation intensity, as compared to the previous report.7
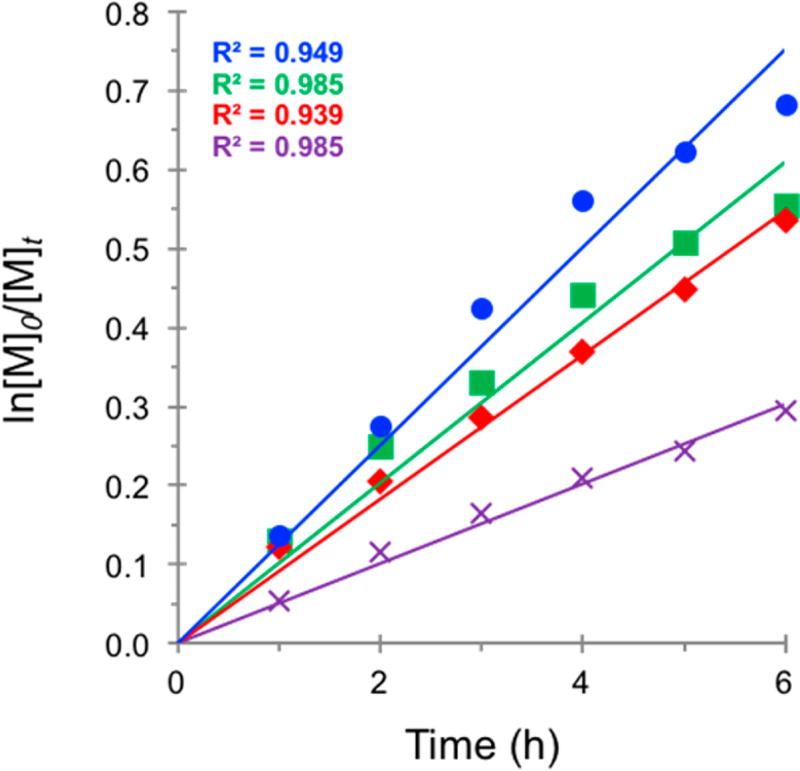
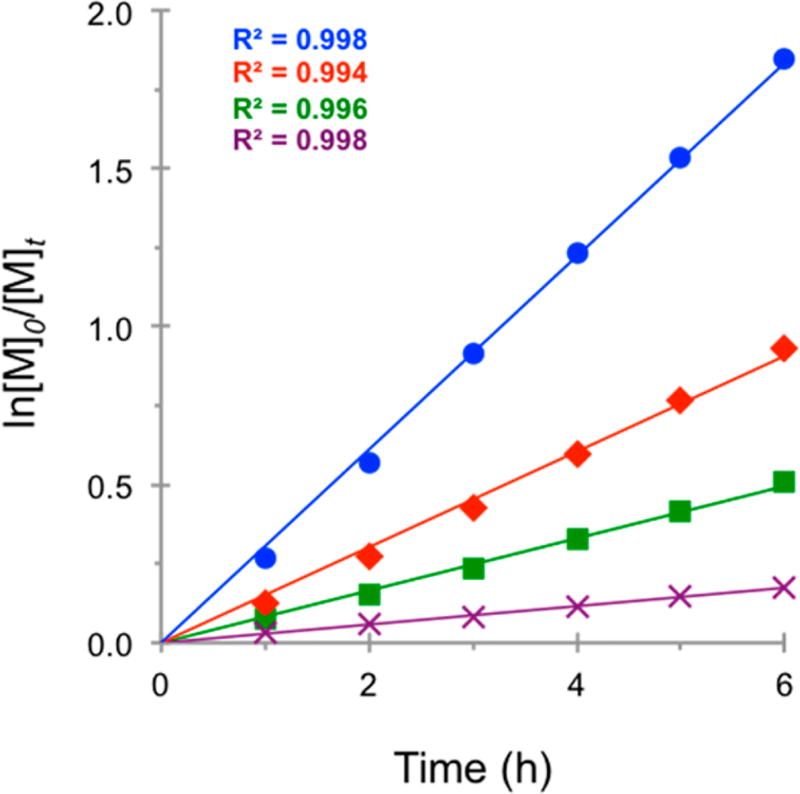
The kinetics of the polymerizations were also greatly affected by the relative intensity, with the rates of polymerization roughly correlating to the intensity of irradiation and decreasing with lower light intensities (Figure 4). However, the rate of polymerization for the 25% intensity trial exceeded that of the 50% intensity trial, presumably a consequence of the loss of control over the polymerization, which agrees with the nonlinear trend in MW growth (Figure 3C). As this was a surprising result, the polymerization under these conditions was repeated five times, and the averaged data were plotted with error bars (Figure S9). Together, these features imply that there is sufficient irradiation to activate radicals, although not sufficient reaction conditions to produce an acceptable [PC•+Br−] to enable a CRP. The rate of polymerization using 5% intensity was the lowest level evaluated, and control over the polymerization is entirely lost under these conditions. It is conceivable that 5% intensity is too low for a high concentration of either PC* or active radical in solution, resulting in both lower rate of polymerization and loss of control. These data support the hypothesis that improvement in control over the polymerization mediated by PC 1 can be realized by increasing the intensity of irradiation. Further, by systematically decreasing the intensity, the loss in control over the polymerization can be related to the concentration of key species in solution.
Figure 4.
First order kinetic plot of the O-ATRP of MMA using PC 1, irradiated at 100% (blue ●), 50% (red ◆), 25% (green ■), and 5% (violet ×) relative irradiation intensity (see Supporting Information for experimental details).
To further examine the trends observed from O-ATRP mediated by PC 1, the effects of varied light intensity on PC 2 were studied to investigate the activity of a PC that previously demonstrated superior performance in O-ATRP. PC 2 consistently produced polymer products with relatively low Đ (<1.30) and predictable Mn in a highly efficient manner (I* > 90%).11 In comparing the PCs, both PC 1 and PC 2 have high molar extinction coefficients (>20 000 L/(mol cm)) and absorption profiles in the visible region49 but differ in other photophysical characteristics. The more reducing triplet excited state reduction potential of PC 2 vs 1 (−1.93 V vs −0.78 V, respectively) likely results in a more efficient reduction of the alkyl bromide.
Overall, PC 2 is robust to broad changes in experimental conditions, as shown by maintaining a controlled O-ATRP in a wide range of irradiation intensities (100–25%) and catalyst concentrations (0.01–0.2 mol %). The catalyst loading of PC 2 could be significantly lowered and still effectively catalyze OATRP (Table 2). At 0.05 and 0.01 mol %, low Đ and I* > 80% are obtained, indicating maintained control over the polymerization across an order of magnitude of catalyst loading (runs 8–11). Increasing the amount of PC 2 to 0.2 mol % also provides successful results (I* = 91.6% and Đ = 1.22), although 0.2 mol % is beyond the solubility for this catalyst and the polymerization becomes heterogeneous in PC. A number of general trends are observed for polymerizations mediated by PC 2 under the different irradiation conditions. By decreasing the intensity of irradiation from 100% to 5%, a significant decrease in I* is observed from 96.3% to 31.0%. This lower I* is accompanied by an increase in Đ from 1.20 to 1.80 (Table 2, runs 12–14). Maintaining a controlled O-ATRP in a wide range of irradiation intensities (100–25%) and catalyst concentrations (0.01–0.2 mol %) makes PC 2 an attractive candidate for future applications of O-ATRP.
Table 2.
Results of the O-ATRP of MMA Mediated by PC 2 with Varied PC Concentrations and Irradiation Intensitiesa
| run no. | hν (intensity)a (%) | [I]:[PC]b | time (h) | convc (%) | Mwd (kDa) | Mnd (kDa) | Đ (Mw/Mn)d | I*e (%) |
|---|---|---|---|---|---|---|---|---|
| 8 | 100 | 10:0.1 | 6 | 83.3 | 14.4 | 10.2 | 1.42 | 84.1 |
| 9 | 100 | 10:0.5 | 6 | 88.6 | 12.9 | 10.3 | 1.25 | 87.7 |
| 10 | 100 | 10:1 | 6 | 83.9 | 10.7 | 8.9 | 1.20 | 96.3 |
| 11 | 100 | 10:2 | 6 | 75.9 | 10.3 | 8.5 | 1.22 | 91.6 |
| 12 | 50 | 10:1 | 6 | 60.6 | 9.3 | 7.8 | 1.20 | 80.1 |
| 13 | 25 | 10:1 | 6 | 39.9 | 5.8 | 7.5 | 1.28 | 69.7 |
| 14 | 5 | 10:1 | 6 | 16.2 | 9.7 | 5.4 | 1.80 | 31.0 |
See Supporting Information for details, polymerizations performed with 1.0 mL of MMA (4.67 M).
Molar ratio of initiator ([I]) to photocatalyst ([PC]).
Measured by 1H NMR.
Measured by GPC coupled with light scattering.
I* = (theoretical number-average MW)/(experimentally measured number-average MW) × 100.
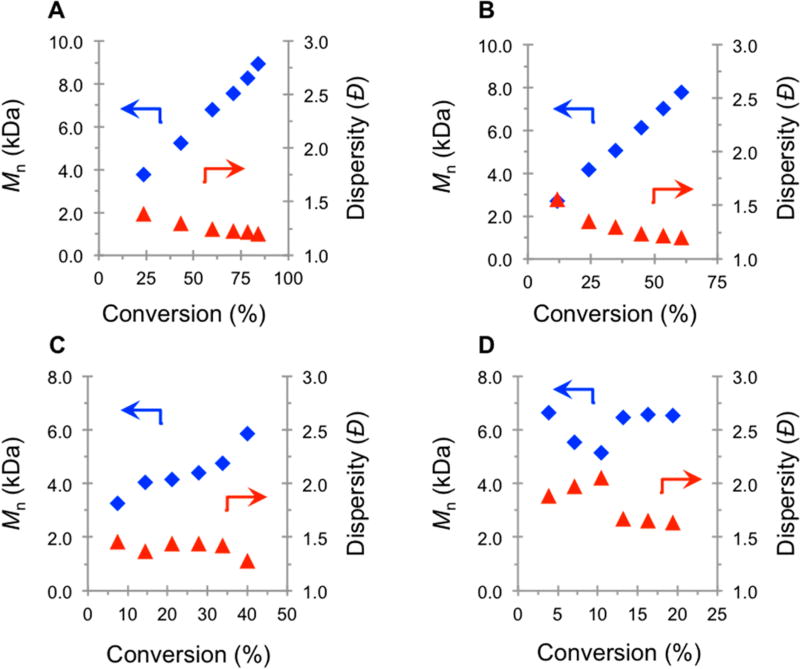
PC 2 exhibits successful performance in O-ATRP under a much wider range of irradiation conditions (Figure 5). For O-ATRP mediated by PC 2, Mn growth is present from 100%, 50%, and 25% relative irradiation intensity (Figure 5A–C). Unlike PC 1, the polymer Mn increases with monomer conversion for the 25% irradiation trial, although not in a completely linear fashion. When lowered to 5% relative intensity, there is a loss in control, as evidenced by a nonlinear growth in Mn with monomer conversion and high dispersity (Đ > 2.0) (Figure 5D). Further differentiating PCs 1 and 2 is the trend in the evolution of Đ for the 100% and 50% relative irradiation trials. Under both irradiation conditions, the Đ decreases with monomer conversion, as is expected for ATRP. These results indicate that unlike for PC 1, PC 2 maintains control over the polymerization over broader irradiation conditions. Additionally, lower light intensity leads to lower rates of polymerization, and the first-order kinetic plots for the four different intensity trials remained linear, even at 5% relative intensity (Figure 6). Further, the degree of control does not significantly differ for polymerization in a photoreactor cooled by a fan (33 °C) and not cooled (50 °C) (Figure S8). These kinetic and MW data prove that the highly performing PC 2 can mediate a successful O-ATRP across broad irradiation intensities.
Figure 5.
Plots of Mn (blue ◆) and Đ (red ▲) as a function of monomer conversion at 100% (A), 50% (B), 25% (C), and 5% (D) relative irradiation intensity for the polymerization of MMA mediated by PC 2 (see Supporting Information for experimental details).
Figure 6.
First-order kinetic plot for the polymerization of MMA using PC 2 at 100% (blue ●), 50% (red ◆), 25% (green ■), and 5% (violet ×) relative irradiation intensities (see Supporting Information for experimental details).
3. CONCLUSIONS
Light intensity has been shown to have a significant effect on the degree of control achieved for photoinduced O-ATRP. Through exploring the effect of decreased irradiation intensity on multiple polymerization metrics, changes in experimental conditions were connected to concentrations of key catalyst states and their ability to successfully operate O-ATRP. Deeper understanding of the role that light intensity plays in the modulation of excited state catalyst concentrations allowed for the realization of conditions in which perylene catalyzed a controlled and linear growth in polymer molecular weight as a function of monomer conversion. Additionally, by maintaining growth in polymer MW with monomer conversion and linear first-order kinetics under significantly decreased irradiation intensity, the N-1-naphthylphenoxazine catalyst proved to be robust to broad experimental conditions, highlighting its potential for success in a variety of future applications where low catalyst loading or low intensity irradiation is necessary.
Figure 2.
General reaction scheme for the O-ATRP of methyl methacrylate (top). Structures of the two organic photoredox catalysts used in this study (bottom).
Acknowledgments
This work was supported by the University of Colorado Boulder and the Advanced Research Projects Agency-Energy (DE-AR0000683). Acknowledgment is made to the donors of The American Chemical Society Petroleum Research Fund for partial support of this research. Research reported in this publication was supported by the National Institutes of Health under Award R35GM119702. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. M.D.R. is grateful for support from the U.S. Department of Education’s Graduate Assistance in Areas of National Need Program. We thank Dr. Chern-Hooi Lim for the computed reduction potential of perylene. We thank Steven Sartor for spectral measurements. We acknowledge the use of XSEDE supercomputing resources (NSF ACI-1053575).
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Materials and methods, detailed polymerization procedures, and supplemental data (PDF)
The authors declare no competing financial interest.
References
- 1.Pan X, Tasdelen MA, Laun J, Junkers T, Yagci Y, Matyjaszewski K. Photomediated Controlled Radical Polymerization. Prog. Polym. Sci. 2016;62:73–125. [Google Scholar]
- 2.Chen M, Zhong M, Johnson JA. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016;116(17):10167–10211. doi: 10.1021/acs.chemrev.5b00671. [DOI] [PubMed] [Google Scholar]
- 3.Matyjaszewski K, Tsarevsky NV. Macromolecular Engineering by Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2014;136(18):6513–6533. doi: 10.1021/ja408069v. [DOI] [PubMed] [Google Scholar]
- 4.Braunecker WA, Matyjaszewski K. Controlled/Living Radical Polymerization: Features, Developments, and Perspectives. Prog. Polym. Sci. 2007;32:93–146. [Google Scholar]
- 5.Kamigaito M, Ando T, Sawamoto M. Metal-Catalyzed Living Radical Polymerization. Chem. Rev. 2001;101(12):3689–3746. doi: 10.1021/cr9901182. [DOI] [PubMed] [Google Scholar]
- 6.Theriot JC, McCarthy BG, Lim C-H, Miyake GM. Organocatalyzed Atom Transfer Radical Polymerization: Perspectives on Catalyst Design and Performance. Macromol. Rapid Commun. 2017:1700040. doi: 10.1002/marc.201700040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyake GM, Theriot JC. Perylene as an Organic Photocatalyst for the Radical Polymerization of Functionalized Vinyl Monomers through Oxidative Quenching with Alkyl Bromides and Visible Light. Macromolecules. 2014;47(23):8255–8261. [Google Scholar]
- 8.Treat NJ, Sprafke H, Kramer JW, Clark PG, Barton BE, Read de Alaniz J, Fors BP, Hawker CJ. Metal-Free Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2014;136(45):16096–16101. doi: 10.1021/ja510389m. [DOI] [PubMed] [Google Scholar]
- 9.Theriot JC, Lim C-H, Yang H, Ryan MD, Musgrave CB, Miyake Organocatalyzed Atom Transfer Radical Polymerization Driven by Visible Light. Science. 2016;352:1082–1086. doi: 10.1126/science.aaf3935. [DOI] [PubMed] [Google Scholar]
- 10.Lim C-H, Ryan MD, McCarthy BG, Theriot JC, Sartor SM, Damrauer NH, Musgrave CB, Miyake GM. Intramolecular charge transfer and Ion pairing in N,N-Diaryl Dihydrophenazine Photoredox Catalysts for Efficient Organocatalyzed Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2017;139(1):348–355. doi: 10.1021/jacs.6b11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan MD, Theriot JC, Lim C-H, Yang H, Lockwood AG, Garrison NG, Lincoln SR, Musgrave CB, Miyake GM. Solvent Effects on the Intramolecular Charge Transfer Character of N,N-Diaryl Dihydrophenazine Catalysts for Organocatalyzed Atom Transfer Radical Polymerization. J. Polym. Sci., Part A: Polym. Chem. 2017 doi: 10.1002/pola.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z, Gu Y, Liu X, Zhang L, Cheng Z, Zhu X. Metal-Free Atom Transfer Radical Polymerization of Methyl Methacrylate with ppm Level of Organic Photocatalyst. Macromol. Rapid Commun. 2017;38:1600461. doi: 10.1002/marc.201600461. [DOI] [PubMed] [Google Scholar]
- 13.Pearson RM, Lim C-H, McCarthy BG, Musgrave CB, Miyake GM. Organocatalyzed Atom Transfer Radical Polymerization Using N-Aryl Phenoxazines as Photoredox Catalysts. J. Am. Chem. Soc. 2016;138(35):11399–11407. doi: 10.1021/jacs.6b08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allushi A, Jockusch S, Yilmaz G, Yagci Y. Photoinitiated Metal-Free Controlled/Living Radical Polymerization Using Polynuclear Aromatic Hydrocarbons. Macromolecules. 2016;49:7785–7792. [Google Scholar]
- 15.Pan X, Fang C, Fantin M, Malhotra N, So WY, Peteanu LA, Isse AA, Gennaro A, Matyjaszewski K. Mechanism of Photoinduced Metal-Free Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2016;138(7):2411–2425. doi: 10.1021/jacs.5b13455. [DOI] [PubMed] [Google Scholar]
- 16.Pan X, Lamson M, Yan J, Matyjaszewski K. Photoinduced Metal-Free Atom Transfer Radical Polymerization of Acrylonitrile. ACS Macro Lett. 2015;4(2):192–196. doi: 10.1021/mz500834g. [DOI] [PubMed] [Google Scholar]
- 17.Dadashi-Silab S, Pan X, Matyjaszewski K. Phenyl Benzo[b]-phenothiazine as a Visible Light Photoredox Catalyst for Metal-Free Atom Transfer Radical Polymerization. Chem. - Eur. J. 2017;23:5972. doi: 10.1002/chem.201605574. [DOI] [PubMed] [Google Scholar]
- 18.Yan J, Pan X, Schmitt M, Wang Z, Bockstaller MR, Matyjaszewski K. Enhancing Initiation Efficiency in Metal-Free Surface-Initiated Atom Transfer Radical Polymerization (SI-ATRP) ACS Macro Lett. 2016;5:661–665. doi: 10.1021/acsmacrolett.6b00295. [DOI] [PubMed] [Google Scholar]
- 19.Jockusch S, Yagci Y. The Active Role of Excited States of Phenothiazines in Photoinduced Metal Free Atom Transfer Radical Polymerization: Singlet or Triplet Excited States? Polym. Chem. 2016;7:6039–6043. [Google Scholar]
- 20.Williams VA, Ribelli TG, Chmielarz P, Park S, Matyjaszewski K. A Silver Bullet: Elemental Silver as an Efficient Reducing Agent for Atom Transfer Radical Polymerization of Acrylates. J. Am. Chem. Soc. 2015;137(4):1428–1431. doi: 10.1021/ja512519j. [DOI] [PubMed] [Google Scholar]
- 21.Jakubowski W, Matyjaszewski K. Activators Regenerated by Electron Transfer for Atom Transfer radical Polymerization of (Meth)acrylates and Related Block Copolymers. Angew. Chem., Int. Ed. 2006;45:4482–4486. doi: 10.1002/anie.200600272. [DOI] [PubMed] [Google Scholar]
- 22.Wang J-S, Matyjaszewski K. Living”/Controlled Radical Polymerization. Transition-Metal-Catalyzed Atom Transfer Radical Polymerization in the Presence of a Conventional Radical Initiator. Macromolecules. 1995;28(22):7572–7573. [Google Scholar]
- 23.Xia J, Matyjaszewski K. Controlled/”Living” Radical Polymerization. Homogenous Reverse Atom Transfer Radical Polymerization Using AIBN as the Initiator. Macromolecules. 1997;30(25):7692–7696. [Google Scholar]
- 24.Min K, Gao H, Matyjaszewski K. Preparation of Homopolymers and Block Copolymers in Miniemulsion by ATRP Using Activators Generated by Electron Transfer (AGET) J. Am. Chem. Soc. 2005;127(11):3825–2830. doi: 10.1021/ja0429364. [DOI] [PubMed] [Google Scholar]
- 25.Matyjaszewski K, Patten TE, Xia J. Controlled/“Living” Radical Polymerization. Kinetics of the Homogenous Atom Transfer Radical Polymerization of Styrene. J. Am. Chem. Soc. 1997;119(4):674–680. [Google Scholar]
- 26.Matyjaszewski K, Nakagawa Y, Jasieczek CB. Polymerization of n-Butyl Acrylate by Atom Transfer Radical Polymerizaiton. Remarkable Effect of Ethylene Carbonate and Other Solvents. Macromolecules. 1998;31:1535–1541. [Google Scholar]
- 27.Arias-Rotondo DM, McCusker JK. The Photophysics of Photoredox Catalysis: A Roadmap for Catalyst Design. Chem. Soc. Rev. 2016;45:5803–5820. doi: 10.1039/c6cs00526h. [DOI] [PubMed] [Google Scholar]
- 28.Matyjaszewski K, Paik HJ, Zhou P, Diamanti S. Determination of Activation and Deactivation Rate Constants in Atom Transfer Radical Polymerization. Macromolecules. 2001;34:5125–5131. [Google Scholar]
- 29.Wessely I, Mugnaini V, Bihlmeier A, Jeschke G, Brase S, Tsotsalas M. Radical Exchange Reaction of Multi-Spin Isoindoline Nitroxides Followed by EPR Spectroscopy. RSC Adv. 2016;6:55715–55719. [Google Scholar]
- 30.Yamaguchi G, Higaki Y, Otsuka H, Takahara A. Reversible Radical Ring-Crossover Polymerization of an Alkoxyamine-Containing Dynamic Covalent Macrocycle. Macromolecules. 2005;38:6316–6320. [Google Scholar]
- 31.Turro NJ, Lem G, Zavarine IS. A Living Free Radical Exchange Reaction for the Preparation of Photoactive End-Labeled Monodisperse Polymers. Macromolecules. 2000;33:9782–9785. [Google Scholar]
- 32.Hawker CJ, Barclay GG, Dao J. Radical Crossover in Nitroxide Mediated “Living” Free Radical Polymerizatinos. J. Am. Chem. Soc. 1996;118(46):11467–11471. [Google Scholar]
- 33.Lacroix-Desmazes P, Lutz J-F, Chauvin F, Severac R, Boutevin B. Living Radical Polymerization: Use of an Excess of Nitroxide as a Rate Moderator. Macromolecules. 2001;34(26):8866–8871. [Google Scholar]
- 34.Veregin RPN, Odell PG, Michalak LM, Georges MK. The Pivotal Role of Excess Nitroxide Radical in Living Free Radical Polymerizations with Narrow Polydispersity. Macromolecules. 1996;29(8):2746–2754. [Google Scholar]
- 35.Le C, Wismer MK, Shi Z-C, Zhang R, Conway DV, Li G, Vachal P, Davies IW, MacMillan DWC. A General Small-Scale Reactor to Enable Standardization and Acceleration of Photocatalytic Reactions. ACS Cent. Sci. 2017 doi: 10.1021/acscentsci.7b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalevee J, Morlet-Savary F, Dietlin C, Graff B, Fouassier J-P. Photochemistry and Radical Chemistry under Low Intensity Visible Light Sources: Application to Photopolymerization Reactions. Molecules. 2014;19:15026–15041. doi: 10.3390/molecules190915026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsey BL, Pearson RM, Beck LR, Miyake GM. Photoinduced Organocatalyzed Atom Transfer Radical Polymerization Using Continuous Flow. Macromolecules. 2017;50:2668–2674. doi: 10.1021/acs.macromol.6b02791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth HG, Romero NA, Nicewicz DA. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett. 2016;27:714–723. [Google Scholar]
- 39.Isse AA, Lin CY, Coote ML, Gennaro A. Estimation of Standard Reduction Potentials of Halogen Atoms and Alkyl Halides. J. Phys. Chem. B. 2011;115:678–684. doi: 10.1021/jp109613t. [DOI] [PubMed] [Google Scholar]
- 40.Saveant J-M. Dissociative Electron Transfer. New Tests of the Theory in the Electrochemical and Homogenous Reduction of Alkyl Halides. J. Am. Chem. Soc. 1992;114(26):10595–10602. [Google Scholar]
- 41.Eberson L. Electron Transfer Reactions in Organic Chemistry. II. An Analysis of Alkyl Halide Reduction by Electron Transfer Reagents on the Basis of the Marcus Theory. Acta Chem. Scand. B. 1982;36(8):533–543. [Google Scholar]
- 42.Gomez ML, Montejano HA, Previtali CM. Excited States Interaction of Polycyclic Aromatic Hydrocarbons with Diphenyliodonium Chloride: The Effective One Electron Reduction Potential of Diphenyliodonium Cation. J. Photochem. Photobiol., A. 2008;197:18–24. [Google Scholar]
- 43.Goldschmidt CR, Ottolenghi M. Laser Photolysis of Perylene Solutoins. J. Phys. Chem. 1971;75(25):3894–3897. [Google Scholar]
- 44.Dreeskamp H, Koch E, Zander M. Fluorescence of Bromoperylenes and the Requirements of Heavy-Atom Quenching. Chem. Phys. Lett. 1975;31(2):251–253. [Google Scholar]
- 45.Turro NJ, Ramamurthy V, Scaiano JC. Modern Molecular Photochemistry of Organic Molecules. University Science Books; Sausalito: 2010. [Google Scholar]
- 46.Kavarnos GJ, Turro NJ. Photosensitization by Reversible Electron Transfer: Theories, Experimental Evidence, and Examples. Chem. Rev. 1986;86:401–449. [Google Scholar]
- 47.Huber JR, Mantulin WW. Emission Properties of Aromatic Amines in Solution. Phenoxazine System. J. Am. Chem. Soc. 1972;94:3755–3760. [Google Scholar]
- 48.Chauvin F, Dufils P-E, Gigmes D, Guillaneuf Y, Marque SRA, Tordo P, Bertin D. Nitroxide-Mediated Polymerization: The Pivotal Role of the kd value of the Initiating Alkoxyamine and the Importance of the Experimental Conditions. Macromolecules. 2006;39(16):5238–5250. [Google Scholar]
- 49.George C, Strekowski RS, Kleffmann J, Stemmler K, Ammann M. Photoenhanced Uptake of Gaseous NO2 on Solid Organic Compounds: A Photochemical Source of HONO? Faraday Discuss. 2005;130:195–210. doi: 10.1039/b417888m. [DOI] [PubMed] [Google Scholar]