Abstract
BACKGROUND
Successfully treating illicit drug use has become paramount, yet elusive. Devising specialized treatment interventions could increase positive outcomes, but it is necessary to identify risk factors of poor long-term outcomes to develop specialized, efficacious treatments. We investigated whether functional network connectivity (FNC) measures were predictive of substance abuse treatment completion using machine learning pattern classification of functional magnetic resonance imaging data.
METHODS
Treatment-seeking stimulant- or heroin-dependent incarcerated participants (n = 139; 89 women) volunteered for a 12-week substance abuse treatment program. Participants performed a response inhibition Go/NoGo functional magnetic resonance imaging task prior to onset of the substance abuse treatment. We tested whether FNC related to the anterior cingulate cortex would be predictive of those who would or would not complete a 12-week substance abuse treatment program.
RESULTS
Machine learning pattern classification models using FNC between networks incorporating the anterior cingulate cortex, striatum, and insula predicted which individuals would (sensitivity: 81.31%) or would not (specificity: 78.13%) complete substance abuse treatment. FNC analyses predicted treatment completion above and beyond other clinical assessment measures, including age, sex, IQ, years of substance use, psychopathy, anxiety and depressive symptomatology, and motivation for change.
CONCLUSIONS
Aberrant neural network connections predicted substance abuse treatment outcomes, which could illuminate new targets for developing interventions designed to reduce or eliminate substance use while facilitating long-term outcomes. This work represents the first application of machine-learning models of FNC analyses of functional magnetic resonance imaging data to predict which substance abusers would or would not complete treatment.
Keywords: Drug treatment, Error processing, fMRI, ICA, Machine learning, Prediction
Tens of millions of Americans use illicit drugs, costing $193 billion annually in health care and lost productivity (1). Nearly 10% of Americans report lifetime drug dependence, 25% of whom seek treatment (2). Many who seek treatment do not successfully eliminate drug use behavior. Substance use disorders (SUDs) are highly prevalent in U.S. prisons, with 50% to 80% of incarcerated offenders meeting diagnostic criteria for a SUD (3) and only 40% to 49% participating in SUD treatment while incarcerated (4). Substance abuse treatment completion is one of the factors most associated with favorable patient outcomes (5). Although treatment discontinuation is not unique to substance abuse treatment, its consequences tend to be more severe and include substance use relapse, poor health, and legal and financial difficulties (6). This highlights the primary need to establish successful, potentially specialized treatment interventions for individuals with SUDs. Accomplishing this goal requires identifying risk factors for poor outcomes and potential treatment targets and, subsequently, the development of efficacious interventions.
Several cognitive and affective functions have been identified to differentiate individuals with and without SUDs. Broadly, individuals with SUDs, relative to those without, exhibit dys-regulation in attention, memory, reward processing, and executive control (7–9). Dopaminergic dysfunction is thought to be at the heart of many of these group differences (9,10). Specifically, dopamine (DA) released from the ventral tegmental area into the nucleus accumbens, prefrontal cortex, and amygdala has been linked to initiation and maintenance of addictive behaviors (11). Drug use increases DA release in the mesocorticolimbic (MCL)-DA system (12,13), which is thought to be an important element in learning, goal-directed behavior, and reward processing (14,15), which can lead to drug dependence. Many regions have been implicated in MCL-DA dysfunction in drug users such as the dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), inferior frontal gyrus, orbitofrontal cortex, striatum, hippocampus, basolateral amygdala, and insula [for review, see (7–9)]. Additionally, interconnections, or network connectivity, among these areas have been found to be deficient in drug users (16–19). Two networks and their interconnections within the MCL-DA system have emerged as primary networks of interest: the salience network (SN) and executive control network (ECN) (20). Activation in the SN is thought to reflect bottom-up processes and in the ECN is thought to reflect top-down executive control. Importantly, the SN includes dorsal ACC and insula with extensive connections to subcortical and limbic structures (20), and the ECN includes dorsolateral frontal and parietal cortices (20). Individuals with SUDs have exhibited deficiencies in both the ECN and SN as measured by processing drug-related cues (i.e., craving: SN) (21,22) and executive control (i.e., ECN) (23,24). A proposed biomarker for substance abuse is dysregulation between the SN and default mode network (DMN) (25).
Recent investigations have incorporated neuroimaging methodologies to identify individuals at greatest risk for poor substance abuse outcomes (e.g., prematurely discontinuing substance abuse treatment or relapse to drug use). Resting-state network connectivity has many clinical applications (26) including predicting relapse to drug use (27,28). Specifically, several regions related to the MCL-DA system have distinguished individuals who relapse to stimulants (29–33), nicotine (34), and alcohol (35). Additionally, cocaine users have dysregulation of network connectivity between reward circuitry and nodes of the SN (36). Such results support a recent theory that substance users have difficulty switching between DMN and SN processing (25,36). This theory suggests that the insula, a node in the SN network (20), is an important switch modulating allocation of resources between the DMN and SN because of its connections with other SN nodes (i.e., the ACC) and DMN nodes (i.e., the posterior cingulate cortex). Previous task-based probes of the SN using event-related potentials (ERPs) have predicted substance use outcomes (37,38); however, localization of the underlying brain regions generating the neural signal proves difficult (39). The ERP measures could be generated from caudal and rostral regions of the ACC (40) or the posterior cingulate cortex (41), suggesting that either the SN or DMN is predictive of the outcomes measured.
To date, a handful of studies have used functional magnetic resonance imaging (fMRI) to predict individuals who discontinue treatment, identifying regions including the amygdala and parahippocampal gyrus (42), thalamus (43), and dlPFC (44). The strongest studies to predict relapse with fMRI used network connectivity measures and implicated the SN (27,28). Network connectivity may also predict individuals who will discontinue treatment prematurely. Here, we recruited a large sample of treatment-seeking incarcerated individuals with SUDs enrolled in a cognitive behavioral substance abuse treatment program. This sample overcomes many limitations of previous studies such as small sample sizes or failure to account for confounding variables (e.g., age, IQ) that limit generalizability. Participants completed several clinical assessments and a response inhibition Go/NoGo fMRI task (45) to probe the SN.
We investigated neural activity elicited by erroneous responses (i.e., false alarms) compared with correct responses (i.e., hits). Functional network connectivity (FNC) (46) measures were calculated and used in models predicting who would and would not complete drug treatment. Machine learning prediction models were developed to identify FNC measures predictive of substance abuse treatment outcomes. Based on previous research implicating the MCL-DA system, specifically the ACC and networks connected to it (30–33,37), we hypothesized network connectivity measures would be predictive of treatment outcomes. To our knowledge, this is the first implementation of prediction models of substance abuse treatment outcomes with FNC, while also identifying targets for developing new efficacious treatments.
METHODS AND MATERIALS
Participants
Participants were 139 (89 women) treatment-seeking incarcerated individuals who identified their drug of choice as cocaine (n = 58; 14 discontinued), methamphetamine (n = 60; 13 discontinued), heroin (n = 20; 4 discontinued), or polydrug (n = 1; 1 discontinued) and were recruited from two medium-security prisons in the state of New Mexico. The mean age of participants was 34.00 years (SD = 7.97) at the time of the baseline assessment, when the fMRI data were collected. ERPs were also collected from some of these participants and previously published [52.52% of participants overlap with Steele et al. (37); 53.24% with experiments 1 and 2 in Fink et al. (47); and 39.57% with experiment 3 in Fink et al. (47)]. Participants were randomized into one of three 12-week manualized interventions; because each treatment type (addictions counseling [AC], n = 47; relapse prevention [RP], n = 46; substance expectations therapy [SET], n = 39; 7 participants discontinued treatment before treatment group assignment) and the completion proportion of each group (completion group: AC, n = 39; RP, n = 34; SET, n = 34; discontinuation group: AC, n = 8; RP, n = 12; SET, n = 5; unassigned, n = 6) were well represented, we collapsed across treatment types. Approximately 12% of participants were left-hand dominant, 57% of the sample self-identified as Hispanic/Latino, 30% as white, 6% as black/African American, 4% as American Indian, and 3% selected more than one category, Native American, or other. One-hundred seven (75 women) participants completed the therapy protocol [i.e., at least nine sessions of the 12-session protocol (48)], and 32 (14 women) participants discontinued treatment before completing the therapy protocol, receiving eight or fewer sessions. Individuals who did not complete 9 weeks of treatment for reasons other than voluntary discontinuation (e.g., early release from prison or paroled, transferred out of general population or another facility, enrolled in another drug treatment program, or absconded) were not included in the analyses (see the Supplement for a detailed description and ethical considerations).
Inclusion Criteria
Participants included in the current study met the following inclusion criteria: 1) currently incarcerated; 2) cocaine, methamphetamine, or heroin dependent at time of incarceration; 3) no history of head injury resulting in significant loss of consciousness; 4) no history of psychosis or first-degree relative with psychosis; 5) at least a sixth-grade English reading level; and 6) an estimated IQ greater than 70.
Assessment Measures
Trained researchers administered several assessments, including the Psychopathy Checklist–Revised (49); Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (50); self-report measures of anxiety (51), depression (52), motivation for change (53); and the Addiction Severity Index (54). These measures did not differ between the treatment completion and treatment discontinuation groups, ts < 0.90 (Supplemental Table S1). Full descriptions of assessments are included in the Supplement.
Experimental Go/NoGo Task
Participants performed a difficult, previously published, Go/NoGo paradigm (45) described in detail in the Supplement prior to initiation of treatment. Briefly, participants were instructed to respond as “quickly and accurately as possible” with their right index finger every time the target (Go) stimulus (a white X) appeared, and to withhold a response when the distracter NoGo stimuli (a white K) appeared. Targets appeared with higher frequency (84%, 412 trials) than distractors (16%, 78 trials) to establish a strong stimulus-response mapping on Go trials. The task has been optimized for fMRI data collection.
fMRI Data Collection and Analysis
Imaging data were collected on a Siemens Avanto 1.5T (Siemens Corp., Erlangen, Germany) mobile scanner with advanced SQ gradients (max slew rate 200 T/m/s, 346 T/m/s vector summation, rise time 200 ms) equipped with a 12-element head coil. The echo-planar imaging gradient-echo pulse sequence (repetition time/echo time 2000/39 ms, flip angle 75°, field of view 24 × 24 cm, 64 × 64 matrix, 3.4- × 3.4-mm in-plane resolution, 5-mm slice thickness, 30 slices) effectively covered the entire brain (150 mm) in 2000 ms. Head motion was limited using padding and restraint. Functional images were reconstructed offline at 16-bit resolution and manually reoriented to the anterior commissure/posterior commissure plane. Functional image runs were motion corrected using an algorithm based on the principle of M-estimation, which reduces the influence of large local intensity changes (INRIAlign) (55,56) as implemented in the SPM software (Wellcome Trust Centre for Neuroimaging - UCL [www.fil.ion.ucl.ac.uk]).
Group independent components analysis was performed on the preprocessed fMRI time series data (57) in the MATLAB 2014a (The MathWorks, Inc., Natick, MA) toolbox for independent components analysis of fMRI (GIFT; http://mialab.mrn.org/software/gift), detailed previously (57,58). Thirty-four task-related and nonartifactual components were selected for FNC analysis (Supplemental Figures S1–5). The FNC (46) among independent components analysis time courses across the entire task was computed using Pearson correlation coefficients. These coefficients were used in support vector machine (SVM) models predicting which individuals would or would not complete treatment. These data reduction steps and permutation analyses are discussed further in the Supplement.
SVM models with a radial basis function kernel were used for classification. A nested, twofold cross-validation technique was implemented (Supplemental Figure S6), similar to previous SVM models from our group (37,47,59–61). First, 10-fold cross-validation was used to estimate the performance of the classifier. In each run, 90% of the data were used for training and 10% for testing. Second, fivefold cross-validation was used nested inside the training set to estimate the parameters of SVM via grid search. The grid search used a logarithmic grid search with basis of 2. The range for C (the soft margin hyperparameter) and s (the radial basis function kernel hyperparameter) were [0.25 to 512] and [0.125 to 1024], respectively. This method ensures complete independence between selecting and testing features thus eliminating potential cross-contamination and inflation of predictions. The permutation analyses assess stability in the models to suggest reliability of the findings.
Three SVM models were calculated: 1) FNC features only, 2) clinical assessment variables (sex, age, IQ, years of drug use, psychopathy, anxiety and depressive symptomatology, and motivation for change), and 3) FNC features with clinical assessment variables. Mean replacement was implemented for any missing clinical assessment data (177 of 1807 total). All features were normalized by z-scoring. These models were designed to identify the best set of FNC, clinical assessments, or combination thereof to predict substance abuse treatment outcomes.
RESULTS
Behavioral Results
Independent samples t tests were conducted for response time (RT) and error rates to Go and NoGo stimuli for the discontinuation group versus completion group. Go stimuli did not elicit group differences in RT (completion group: mean = 471 ms, SD = 56 ms; discontinuation group: mean = 461 ms, SD = 58 ms; t137 = −0.84, p = .403) or error rates (completion group: mean = 12.92 trials, SD = 29.52 trials; discontinuation group: mean = 13.69 trials, SD = 22.82; t137 = 0.14, p = .892). Similarly, incorrect NoGo stimuli did not elicit group differences in RT (completion group: mean = 403 ms, SD = 46 ms; discontinuation group: mean = 401 ms, SD = 44 ms; t137 = −0.24, p = .81) or error rates (completion group: mean = 17.30 trials, SD = 9.93 trials; discontinuation group: mean = 18.00 trials, SD = 10.69 trials; t137 = 0.34, p = .731). Overall, significant post-error slowing occurred (t138 = −5.93, p < .001), with slower RTs to hits preceded by false alarms (mean = 488 ms, SD = 132 ms) than RTs to hits preceded by correct rejections (mean = 435 ms, SD = 60 ms). The discontinuation group (mean = 12 ms, SD = 85 ms) slowed less post-error than the completion group (mean = 65 ms, SD = 109 ms; t137 = 2.52, p = .013).
Classification With SVM
The best overall models included FNC measures to predict treatment outcomes. Models 1 (FNC measures alone; permutation-generated p < .001) and 3 (FNC and clinical assessment variables; permutation-generated p = .026) were nearly identical, with overall accuracy of 80.58% and 81.29%, respectively (Table 1). Model 2 (permutation-generated p value = .117), including only clinical assessments as features, produced lower overall accuracy (67.63%) than models 1 and 3. It was possible, however, to predict who would and would not complete treatment with 80.58% accuracy with only FNC measures in model 1, which is substantiated as a stable finding with permutation analyses. This corroborates previous findings (61,62) suggesting that FNC measures are sufficient to predict outcomes because covariates such as age and sex contribute similarly to prediction models as brain connectivity features. Model 1 misclassified 27 participants with little pattern related to assigned treatment type (AC = 9; RP = 11; SET = 5; discontinued prior to treatment assignment = 2) or drug of choice (drug of choice: cocaine = 12; heroin = 5; methamphetamine = 10).
Table 1.
Support Vector Machine Models Predicting Treatment Completion
| FNC Model 1 |
Clinical Assessments Model 2 |
FNC and Clinical Assessments Model 3 |
|
|---|---|---|---|
| Overall Accuracy | 80.58% | 67.63% | 81.29% |
| Sensitivity | 81.31% | 70.09% | 82.24% |
| Specificity | 78.13% | 59.38% | 78.13% |
| Positive Predictive Value | 92.55% | 85.23% | 92.63% |
| Negative Predictive Value | 55.56% | 37.25% | 56.82% |
Three support vector machine models predicting substance treatment completion were computed. The first model used only functional network connectivity (FNC) measures, the second model used clinical assessments alone, and the third model used both FNC measures and clinical assessment measures. Sensitivity is the measure of how well the model identified who would complete drug treatment, and specificity is the measure of how well the model identified who would discontinue drug treatment. Positive predictive value represents the ratio of individuals who completed treatment to combined individuals identified correctly and incorrectly to be in the completion group. Negative predictive value represents the ratio of individuals who discontinued treatment to combined individuals identified correctly and incorrectly to be in the discontinued group. Permutation p values related to the overall classification accuracy were calculated for each model: model 1, p < .001; model 2, p = .117; model 3, p = .026.
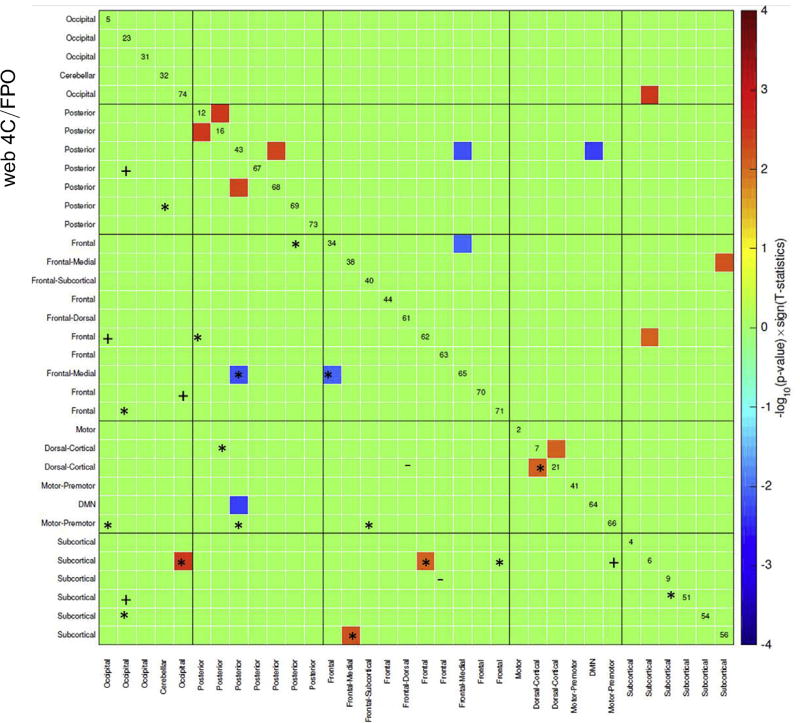
Several FNC measures were significant between groups (Figure 1; Table 2), corrected for multiple comparisons with false discovery rate correction (63,64), or feature selected in at least 80% of the models with the double-input symmetric relevance method during cross-validation (see Supplemental Methods). In both models 2 and 3, all clinical assessment variables were selected in the feature selection step for all iterations. Six FNCs were both significant between groups and selected in the double-input symmetric relevance step at least 80% of the time. All six were selected in both models 1 and 3 (Figures 1 and 2). Four of these FNCs were increased for the individuals who completed treatment, relative to those who prematurely discontinued treatment, and two had the opposite relationship.
Figure 1.
Functional network connectivity (FNC) measures between extracted independent components. The matrix is thresholded to only show the FNC measures significantly different between groups and selected in at least 80% of the prediction models. Red and blue squares signify group differences, with the completion group having greater connectivity than the discontinued group in red, with the opposite effect in blue (false discovery rate corrected for multiple comparisons). FNC measures selected only in model 1 (−), only in model 3 (+), and in both models 1 and 3 (*) are highlighted in the lower, left half of this mirrored matrix. The six FNCs shown to be significantly different between groups and selected in prediction models are of most interest. DMN, default mode network.
Table 2.
Summary of Independent Component Regions of Interest
| Location | x | y | z | Voxels | Brodmann Area |
|---|---|---|---|---|---|
| Component 6 | |||||
| Thalamus | 18 | −18 | 3 | 1993 | N/A |
| Lentiform nucleus | −30 | −6 | −3 | 230 | N/A |
| Component 7 | |||||
| Middle frontal gyrus | 0 | −30 | 63 | 3645 | 6 |
| Component 21 | |||||
| Superior frontal gyrus | −12 | 3 | 66 | 4196 | 6 |
| Component 34 | |||||
| Superior temporal gyrus | −45 | 3 | −12 | 4241 | 38 |
| Culmen | −9 | −36 | −18 | 430 | N/A |
| Insula | 27 | 24 | 18 | 223 | 13 |
| Superior frontal gyrus | 3 | 30 | 51 | 157 | 8 |
| Component 38 | |||||
| Medial frontal gyrus | 0 | 45 | 18 | 3226 | 9 |
| Inferior frontal gyrus | −30 | 15 | −18 | 214 | 47 |
| Component 43 | |||||
| Precuneus | 12 | −60 | 57 | 4253 | 8 |
| Middle frontal gyrus | −33 | 45 | 30 | 205 | 10 |
| Middle frontal gyrus | 36 | 42 | 33 | 153 | 9 |
| Caudate | 0 | 15 | 12 | 278 | N/A |
| Insula | −36 | −45 | 15 | 152 | 13 |
| Middle frontal gyrus | −30 | −6 | 60 | 273 | 6 |
| Middle frontal gyrus | 27 | 0 | 66 | 161 | 6 |
| Component 56 | |||||
| Parahippocampal gyrus | −27 | −6 | −18 | 4282 | N/A |
| Culmen | 0 | −42 | 3 | 505 | N/A |
| Caudate | −21 | −9 | 24 | 231 | N/A |
| Component 62 | |||||
| Insula | −42 | −3 | 15 | 3708 | 13 |
| Parahippocampal gyrus | 42 | −45 | −3 | 188 | 19 |
| Hypothalamus | −6 | −6 | −3 | 308 | N/A |
| Cingulate gyrus | 18 | −39 | 45 | 255 | 31 |
| Component 65 | |||||
| Superior frontal gyrus | 6 | 9 | 54 | 4189 | 6 |
| Precentral gyrus | −39 | −18 | 51 | 625 | 4 |
| Middle frontal gyrus | −30 | 42 | 27 | 150 | 10 |
| Insula | 36 | 15 | 6 | 205 | 13 |
| Insula | −33 | 15 | 9 | 246 | 13 |
| Component 74 | |||||
| Inferior temporal gyrus | −51 | −66 | −6 | 12,381 | 19 |
Location names, Montreal Neurological Institute coordinates, extant voxels, and Brodmann areas are presented for each of the 10 independent components with functional network connections predictive of substance abuse treatment outcomes. Only areas with 150 or more extant voxels are presented.
N/A, not available.
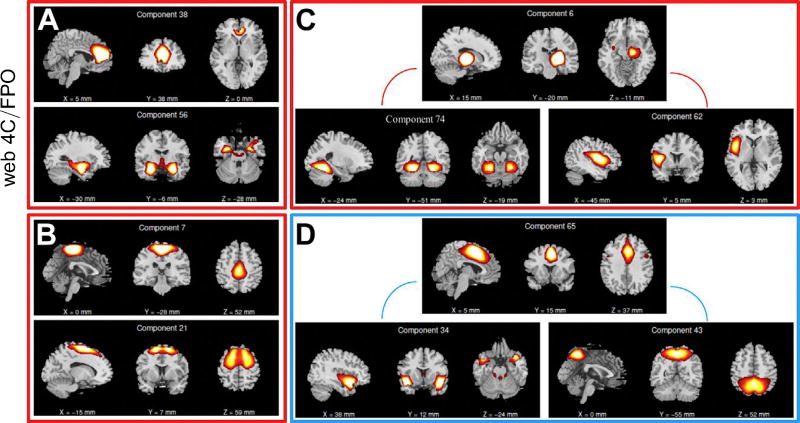
Figure 2.
Functional network connectivity (FNC) measures that were significantly different between groups and predictive of substance abuse treatment outcomes. Stronger FNC in the completion group relative to the discontinued group (bounded by red boxes) was found between (A) component 38 (rostral anterior cingulate cortex) and component 56 (comprising the amygdala, hippocampus, and striatum); (B) component 7 (middle frontal gyrus) and component 21 (superior frontal gyrus); (C) component 6 (right thalamus and putamen) and component 74 (inferior temporal gyrus); and (C) component 6 (right thalamus and putamen) and component 62 (left insula, hypothalamus, cingulate gyrus, and parahippocampal gyrus). Stronger FNC in the discontinued group relative to the completion group (bounded by a blue box) was found in (D) component 65 (caudal anterior cingulate cortex) and component 34 (superior temporal gyrus, culmen, and insula); and (D) component 65 (caudal anterior cingulate cortex) and component 43 (precuneus). These FNC measures extend previous event-related potential findings (37) and are substantially useful in predicting treatment outcomes.
Stronger FNC was found in the completion group relative to the discontinued group among 1) the frontal-medial component 38 (rostral ACC) and the subcortical component 56 (comprising the amygdala, hippocampus, and striatum), 2) the dorsomedial component 7 (middle frontal gyrus) and the dorsomedial component 21 (superior frontal gyrus), 3) the subcortical component 6 (right thalamus and putamen) and the temporal component 74 (inferior temporal gyrus), and 4) the subcortical component 6 (right thalamus and putamen) and the insular component 62 (left insula, hypothalamus, cingulate gyrus, and parahippocampal gyrus; see Figure 2). Stronger FNC in the discontinued group relative to the completion group was found in 1) the frontal-medial component 65 (caudal ACC) and the frontal/temporal component 34 (superior temporal gyrus, culmen, and insula) and 2) the frontal-medial component 65 (caudal ACC) and the posterior component 43 (precuneus; see Figure 2). The time course for each of these components was regressed against the task design matrix to identify those components whose time series were most strongly related to task conditions. As seen in Supplemental Table S2, each component was related to at least one task condition. Table 3 shows that multiple components had significant relationships in beta weights and false alarms (components 34 and 38), when a response was made (component 6 and 62), Go trials (components 21 and 74), all conditions (components 7 and 65), and mixed relationship (components 43 and 56). Results presented in Table 3 and Supplemental Table S2 largely replicate previous findings relating several distinct regions to processing and responding to Go/NoGo stimuli (65,66).
Table 3.
Summary of Independent Component Relationship to the Design Matrix
| Component | Hits (t Value) |
Correct Rejects (t Value) |
False Alarms (t Value) |
|---|---|---|---|
| 6 | 6.66a | 1.76 | 4.49a |
| 7 | −13.07a | −11.69a | −9.62a |
| 21 | 5.25a | 8.09a | −1.51 |
| 34 | −9.63a | −2.09 | 9.28a |
| 38 | −5.02a | −7.33a | 12.42a |
| 43 | −16.74a | 2.74b | −4.04a |
| 56 | 0.75 | −7.38a | −4.21a |
| 62 | 5.17a | 2.07 | 5.77a |
| 65 | 7.67a | 14.51a | 20.32a |
| 74 | 5.53a | 15.82a | 0.05 |
Relationship between time courses of independent components and the design matrix of experimental conditions hits, correct rejections, and false alarms are presented. The functional network connectivities among these 10 components are presented in Figure 2 and were most useful in predicting substance abuse treatment completion. A false discovery rate correction for multiple comparisons was applied (63,64).
p < .001.
p < .01.
DISCUSSION
In the current study, FNC measures were incorporated within SVM learning models to predict substance abuse treatment outcomes with high accuracy (80.58%). Importantly, high accuracy was achieved for both individuals who would (81.31%) and individuals who would not (78.13%) complete treatment. Robust twofold cross-validated SVM models with permutation tests were implemented, bolstering generalizability of these findings. Further, the FNC measures implicate abnormalities in the MCL-DA system within individuals who prematurely discontinue treatment. The ACC, a node in the dysregulated MCL-DA system (7–9) and SN of SUDs (25,27,36), and its related functional connections were important contributors to these prediction models. Interestingly, participants who prematurely discontinued treatment did not exhibit post-error slowing to the same degree as participants who completed treatment. This could reflect differences between groups in learning from experiences and planning for the future that ultimately affected whether or not an individual completed treatment.
Presented here are task-based fMRI findings extending previous resting-state fMRI findings of SN dysregulations in SUDs (25,27,36), which could inform specialized treatment interventions. Bridging task-based and resting-state measures of the SN was accomplished by probing the ACC node of the SN with a Go/NoGo task known to elicit such activation (65,66). The insula, another SN node, implicated in interception (67), contributes to both the prediction models and the SUDs (68,69). Incorporating task-based measures allows for moving beyond exploring the DMN (70,71) to mapping cognitive functions onto SN FNC measures predictive of treatment outcomes. Specifically, individuals who prematurely discontinue substance abuse treatment have aberrant FNCs exhibited during error monitoring in this Go/NoGo task. Individuals who discontinue prematurely do not exhibit universal modulations of FNCs (Figure 1). Therefore, interventions could target specific FNCs to affect behavioral outcomes. For example, the prevalence of mindfulness interventions has increased with initial evidence, suggesting benefits to specifically targeting error processing (72). Also, despite its nascence, there is evidence to suggest working memory training improves concurrent cognitive processing (73,74), which could be beneficial when attempting to normalize deficiencies in error processing. Error processing elicited by false alarms in the current task is both a biomarker for poor outcomes (i.e., premature discontinuation) and a prime target for intervention to reduce SUDs.
Noninvasive brain stimulation (NIBS) is yet another potential avenue for treating SUDs. Repetitive transcranial magnetic stimulation, a type of NIBS, over the left dlPFC is Food and Drug Administration–approved to treat depression (75,76) but has also been explored in other disorders (77), with substantial evidence for reducing depression symptoms (78). Network connectivity between the dlPFC and ACC is normalized with this treatment (79) and increases in dopamine release in the caudate nucleus (80,81), suggesting network malleability with NIBS. Stimulation of the left dlPFC induces broad activity changes (79) leading to a potential intervention tool in disorders of the DA system, such as addiction (82,83). In fact, NIBS has successfully reduced craving for cocaine (84–87) and nicotine (88). The cognitive dysregulations identified here related to addiction and neural activity in the ACC, insula, or striatum could be modulated by left dlPFC NIBS to facilitate long-term positive outcomes.
Overall, these findings have significant implications for substance abuse treatment retention by identifying individuals at greatest risk for poor outcomes (i.e., premature discontinuation). Treatment retention is essential in reducing the long-term negative impact of substance use, regardless of incarceration status, because more treatment is related to better long-term outcomes (89–91). The prediction models based on task-based FNCs identified SN dysregulations and targets for SUD treatment interventions.
Future Directions and Conclusions
Future studies could improve and extend our findings by specifically targeting certain aspects not previously addressed. For example, after individuals discontinued treatment, no further contact with participants was possible. Future explorations of this topic should investigate specific reasons for withdrawal (e.g., incompatibility with the treatment) and be used to develop treatment techniques targeting these reasons. Also, as apparent in Table 1, the negative predictive value is lower than desired and was likely caused by disparate group sizes. This value represents the ratio of individuals who discontinued treatment to combined individuals identified correctly and incorrectly to be in the discontinued group. Future large samples with similar group sizes may be necessary to increase the negative predictive value. However, misidentifying an individual to be at higher risk for discontinuation and thus allowing more treatment designed to reduce poor outcomes may have a positive rather than a negative impact. Adding more features into the prediction models may increase accuracy overall. Large-scale efforts to include several modalities (e.g., structure, volumetric) to best identify individualized medical treatment are impressive (92). The field is certainly moving toward including more features in such prediction models, and we applaud those efforts provided care is taken to not overfit models with too many variables. The goal of the current experiment was to identify the underlying cognitive functions predictive of treatment outcome. With that in mind, adding more features (e.g., structure or volumetric measures) may increase accuracy but would limit interpretability in relation to cognitive functions and increase complexity of the models. Similarly, implementing a task allowing for back-to-back commission errors may help differentiate groups in a meaningful way.
In the current report, we identified SN dysregulations within FNC measures of task-based fMRI data as accurate predictors of substance abuse treatment outcomes overall (80.58%) by implementing machine learning pattern classifiers. Importantly, high rates of predicting both completion (81.31%) and discontinuation (78.13%) were achieved. We extended previous findings of SN dysregulations identified in resting-state fMRI (25,27,36) with FNCs related to error processing that could be used specifically as biomarkers for poor outcomes and targets future SUD interventions. Targeted treatments, whether they are specialized cognitive behavioral treatment, mindfulness interventions, psychopharmacological interventions, NIBS, or combinations of interventions, should integrate these findings to facilitate positive long-term outcomes for individuals with SUDs. We highlight that error processing may be an essential biomarker of MCL-DA and SN dysregulation that should not be overlooked as a risk factor or a target for intervention.
Supplementary Material
Acknowledgments
This work was supported by National Institute on Drug Abuse Grant Nos. 1 R01 DA020870 and R01DA026964 (to KAK); National Institute of Child Health and Human Development Grant No. 1R01HD082257 (to KAK); the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health (Baltimore, Maryland) (to VRS); National Center for Advancing Translational Science Grant Nos. KL2 TR000089 and UL1 TR000041 (to BCF); National Institute on Alcohol Abuse and Alcoholism and National Institute on Drug Abuse Grant Nos. R21AA020594, R21AA021201, and R21DA037546 (to EDC); and National Institutes of Health Grants Nos. P20GM103472, R01DA040487, and R01EB020407 (to VDC).
KAK led the National Institutes of Health project that collected the data and conceived of the study approach. All authors contributed to devising and implementing the analytical approach, performing data analysis, and developing interpretations. VRS and JMM drafted the manuscript with contributions from all the other coauthors. All authors provided critical revisions and approved the final version for submission.
Footnotes
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available at http://dx.doi.org/10.1016/j.bpsc.2017.07.003.
References
- 1.National Drug Intelligence Center. National Drug Threat Assessment. [Accessed October 25, 2015];2010 Available at: https://www.justice.gov/archive/ndic/pubs38/38661/38661p.pdf.
- 2.Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, et al. Epidemiology of DSM-5 drug use disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2016;73:39–47. doi: 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute on Drug Abuse. Treating offenders with drug problems: Integrating public health and public safety. [Accessed June 22, 2013];2011 Available at: http://www.drugabuse.gov/sites/default/files/drugs_crime.pdf.
- 4.Mumola CJ, Karberg JC. Drug use and dependence, state and federal prisoners. Bureau of Justice Statistics Special Report. [Accessed March 27, 2013];2006 Available at: https://www.bjs.gov/content/pub/pdf/dudsfp04.pdf.
- 5.Dalsbø TK, Hamerstrom K, Vist GE, Gjermo H, Smedslund G, Steiro, et al. Psychosocial interventions for retention in druge abuse treatment. Cochrane Database Syst Rev. 2010;2010:CD008220. [Google Scholar]
- 6.Brorson HH, Amevik EA, Rand-Hendriksen K, Dickert F. Dropout from addiction treatment: A systematic review of risk factors. Clin Psychol Rev. 2013;33:1010–1024. doi: 10.1016/j.cpr.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci Biobehav Rev. 2013;37:1838–1859. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele VR, Pariyadath V, Goldstein RZ, Stein EA. Reward circuitry and drug addiction. In: Charney DS, Nestler EJ, Buxbaum J, Sklar P, editors. Neurobiology of Mental Illness. 5. Oxford, UK: Oxford University Press; (in press) [Google Scholar]
- 10.Volkow ND, Morales M. The brain on drugs: From reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley AE. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 14.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 15.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 16.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A. 2014;111:16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, et al. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol Addict Behav. 2013;27:477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 22.Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypo-activity in cocaine users during a Go-Nogo task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hester R, Garavan H. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: Implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse. 2013;39:424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- 28.Pariyadath V, Stein EA, Ross TJ. Machine learning classification of resting state functional connectivity predicts smoking status. Front Hum Neurosci. 2014;8:425. doi: 10.3389/fnhum.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark VP, Beatty G, Anderson RE, Kodituwakku P, Phillips J, Lane TDR, et al. Reduced fMRI activity predicts relapse in patients recovering from stimulant dependence. Hum Brain Mapp. 2014;35:414–428. doi: 10.1002/hbm.22184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adinoff B, Gu H, Merrick C, McHugh M, Jeon-Slaughter H, Lu H, et al. Basal hippocampal activity and its functional connectivity predicts cocaine relapse. Biol Psychiatry. 2015;78:496–504. doi: 10.1016/j.biopsych.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 32.Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, et al. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136:1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marhe R, Luijten M, Van de Wetering BJ, Smits M, Franken IHA. Individual differences in anterior cingulate activation associated with attentional bias predict cocaine use after treatment. Neuropsychopharmacology. 2013;38:1085–1093. doi: 10.1038/npp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- 35.Wilson SJ, Myles J, Grasby PM, Nutt DJ. Imaging alcohol cue exposure in alcohol dependence using a PET 15 O-H 2 O paradigm: Results from a pilot study. Addict Biol. 2006;11:107–115. doi: 10.1111/j.1369-1600.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 37.Steele VR, Fink BC, Maurer JM, Arbabshirani MR, Wilber CH, Jaffe AJ, et al. Brain potentials measured during a Go/NoGo task predict completion of substance abuse treatment. Biol Psychiatry. 2014;76:75–83. doi: 10.1016/j.biopsych.2013.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marhe R, van de Wetering BJ, Franken IH. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. Biol Psychiatry. 2013;73:782–788. doi: 10.1016/j.biopsych.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Luck SJ. An Introduction to the Event-Related Potential Technique. 2. Cambridge, MA: MIT Press; 2014. [Google Scholar]
- 40.Edwards BG, Calhoun VD, Kiehl KA. Joint ICA of ERP and fMRI during error-monitoring. Neuroimage. 2012;59:1896–1903. doi: 10.1016/j.neuroimage.2011.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agam Y, Hämäläinen MS, Lee AKC, Dyckman KA, Friedman JS, Isom M, et al. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proc Natl Acad Sci U S A. 2011;108:17556–17561. doi: 10.1073/pnas.1103475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, et al. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, et al. Working memory fMRI activation in cocaine-dependent subjects: Association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potensa MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- 46.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fink BC, Steele VR, Maurer JM, Fede SJ, Calhoun VD, Kiehl KA. Brain potentials predict substance abuse treatment completion in a prison sample. Brain Behav. 2016;6:e00501. doi: 10.1002/brb3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaffe AJ, Wilber CH. A Treatment Manual for Substance Expectation Therapy (SET): Expectations, Motivation, and Personal Meaning in the Treatment of Substance Abuse. New Haven, CT: Substance Abuse Treatment Unit, Connecticut Mental Health Center, Yale University School of Medicine; 2001. [Google Scholar]
- 49.Hare RD. Manual for the Hare Psychopathy Checklist-Revised. 2. Toronto, Canada: Multi-Health Systems; 2003. [Google Scholar]
- 50.Wechsler D. Wechsler Adult Intelligence Scale. New York, NY: Psychological Corporation; 1997. [Google Scholar]
- 51.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 52.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II (BDI-II) San Antonio, TX: Harcourt Assessment, Inc.; 1996. [Google Scholar]
- 53.McConaughy EA, Prochaska JO, Velicer WG. Stages of change in psychotherapy: Measuremnt and sample profiles. Psychotherapy. 1983;20:368–375. [Google Scholar]
- 54.McLellan AT, Kushner HI, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 55.Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- 56.Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- 57.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steele VR, Claus ED, Aharoni E, Vincent GM, Calhoun VD, Kiehl KA. Multimodal imaging measures predict rearrest. Front Hum Neurosci. 2015;9:425. doi: 10.3389/fnhum.2015.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cope LM, Ermer E, Gaudet LM, Steele VR, Eckhardt AL, Arbabshirani MR, et al. Abnormal brain structure in youth who commit homicide. Neuromage Clin. 2014;4:800–807. doi: 10.1016/j.nicl.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steele VR, Rao V, Calhoun VD, Kiehl KA. Machine learning of structural magnetic resonance imaging predicts psychopathic traits in adolescent offenders. Neuroimage. 2017;145:265–273. doi: 10.1016/j.neuroimage.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:1–23. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benjamini Y, Hockberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 64.Yekutieli D, Benjamini Y. Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. J Stat Plan Inference. 1997;82:171–196. [Google Scholar]
- 65.Steele VR, Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, Stevens MC, et al. A large scale (N = 102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav Brain Res. 2013;256:529–536. doi: 10.1016/j.bbr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steele VR, Claus ED, Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, et al. A large scale (N = 102) functional neuroimaging study of error-processing in an Go/NoGo task. Behav Brain Res. 2014;268:127–138. doi: 10.1016/j.bbr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Namkung H, Kim SH, Sawa A. The insula: An underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 2017;40:200–207. doi: 10.1016/j.tins.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74:538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fedota JR, Matous AL, Salmeron BJ, Gu H, Ross TJ, Stein EA. Insula demonstrates a non-linear response to varying demand for cognitive control and weaker resting connectivity with the executive control network in smokers. Neuropsychopharmacology. 2016;41:2557–2565. doi: 10.1038/npp.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Larson MJ, Steffen PR, Primosch M. The impact of a brief mindfulness meditation intervention on cognitive control and error-related performance monitoring. Front Hum Neurosci. 2013;7:308. doi: 10.3389/fnhum.2013.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 75.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 76.Pascual-leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 77.Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: Therapeutic promises and scientific gaps. Pharmacol Ther. 2012;133:98–107. doi: 10.1016/j.pharmthera.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: A systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–239. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- 79.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the sub-genual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:1–4. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keck ME, Welt T, Muller MB, Erhardt A, Ohl H, Toschi N, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and meostriatal system. Neuropharmacology. 2002;43:101–109. doi: 10.1016/s0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 82.Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev. 2010;34:559–574. doi: 10.1016/j.neubiorev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Jansen JM, Daams JG, Koeter MWJ, Veltman DJ, van den Brink W, Groudiaan AE. Effects of non-invasive neurostimulation on craving: A meta-analysis. Neurosci Biobehav Rev. 2013;37:2472–2480. doi: 10.1016/j.neubiorev.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 84.Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih M-C, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 2016;26:37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict. 2008;17:345–346. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- 87.Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, et al. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015;1628:199–209. doi: 10.1016/j.brainres.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013;73:714–720. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hubbard RL, Craddock SG, Anderson J. Overview of 5-year followup outcomes in the drug abuse treatment outcome studies (DATOS) J Subst Abuse Treat. 2003;25:125–134. doi: 10.1016/s0740-5472(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 90.Sayre SL, Schmitz JM, Stotts AL, Averill PM, Rhoades HM, Grabowski JJ. Determining predictors of attrition in an outpatient substance abuse program. Am J Drug Alcohol Abuse. 2002;28:55–72. doi: 10.1081/ada-120001281. [DOI] [PubMed] [Google Scholar]
- 91.Harrell A, Roman J. Reducing drug use and crime among offenders: The impact of graduated sanctions. J Drug Issues. 2001;31:207–231. [Google Scholar]
- 92.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.