Abstract
Background
Research on the neural correlates associated with risk for suicidal ideation (SI) has been limited, particularly in one increasingly at risk group—adolescents. Previous research with adolescents indicates that poor emotion regulation skills are linked with SI, but these studies have not previously examined neural activation in service of emotion regulation between those with and without SI histories.
Methods
Here we examine whether SI is associated with neural responses during an emotion regulation fMRI task in a group of adolescents (N=49) ages 13 to 20 years old (M = 16.95).
Results
While there were no differences between youth with and without SI in self-reported emotional responses to negative pictures, youth with SI activated the dorsolateral prefrontal cortex (dlPFC) more than youth without SI on trials where they attempted to regulate their emotional responses compared to trials where they passively viewed negative pictures. In contrast, during passive viewing of negative stimuli, youth with SI activated the dlPFC, temporoparietal junction, and cerebellum less than same age controls.
Conclusions
These findings were robust to controls for depression and adversity exposure and are consistent with the idea that youth with SI have disrupted emotion regulation, potentially related to differences in recruitment of top-down control regions. In contrast, youth without SI activated regions implicated in emotion regulation even when not directed to effortfully control their emotional response. This is the first study to examine neural function during emotion regulation as a potential neural correlate of risk for SI in adolescents.
Keywords: suicidal ideation, top down control, emotion regulation, dlpfc, cognitive reappraisal, adolescent suicide
Suicide is currently the second leading cause of death worldwide for American youth between 10 and 24 years of age (1, 2). Rates of suicidal ideation (SI; defined as thoughts about death, dying, plans for suicide, or desire for death) are remarkably high in this age range (3). This is because SI increases dramatically during the transition to adolescence (4) with between 12.1–18% of high school students seriously considering suicide (4, 5). SI is frequently associated with suicidal behavior and suicide death ((6, 7) and places adolescents at serious risk for premature death. To prevent adolescent suicide, we require greater understanding of factors that identify which adolescents with SI are most at risk for future suicidal behavior. Unfortunately, despite years of research on risk factors for suicide, few reliable within person predictors or successful interventions have been identified (8). At least one possibility for the stagnation in the adolescent suicide literature is a lack of studies examining neural processes underlying commonly purported risk factors for SI, such as emotion regulation (9, 10). Examining neural correlates of processes underlying SI may help identify targets for prevention and intervention. However, few studies to date have linked neural structure or function with SI, leaving this potentially informative avenue virtually unexplored in adolescents.
Some research has examined neural correlates of suicidal ideation in adults. Although an extensive review of these studies is beyond the scope of this paper given our focus on adolescent suicidal ideation, results generally suggest that dysfunction of frontal control regions is commonly associated with histories of suicidal ideation across samples of adults with recent onset schizophrenia (11–13), recent onset major depressive disorder with psychotic features (14, 15), and major depressive disorder (16–20). Surprisingly little research has examined neural correlates of suicidal ideation or behavior in youth. Johnston and colleagues (21) found that youth with a history of suicide attempts have decreased grey matter volume in the orbitofrontal cortex, hippocampus, and cerebellum as well as decreased functional connectivity between the amygdala and left ventral and right rostral PFC when viewing emotional faces. Another study found that youth with suicide attempts relative to peers with a history of depression but no suicide attempt exhibited increased activation in the right anterior cingulate gyrus, left dorsolateral prefrontal cortex (dlPFC), and the right middle temporal gyrus while viewing negative relative to positive facial expressions during an emotion perception task (22). In this same sample, activation of the right thalamus during high risk trials and the left caudate during low risk trials of the Iowa Gambling Task differentiated adolescents with a suicide attempt history from healthy and depressed peers (23). Together the available research suggests that activation of the PFC, temporal lobe, and limbic regions such as the amygdala, caudate, and thalamus during emotionally evocative tasks may distinguish adolescents with versus without suicidal behavior. However, the available data is limited to only suicidal behavior, and results vary across studies.
One reason that previous findings may vary is that past studies of neural function associated with suicidality in youth have relied on tasks which are only loosely related to risk for SI (e.g., passive viewing of emotional faces). Unmanageable emotional distress is a common antecedent to SI in youth (24–26) and individuals who report more emotion regulation difficulties are at increased risk for SI even after accounting for depression symptoms (27). Yet to date, the neural correlates of emotion regulation have not been measured in association with SI; this is surprising given that the neural correlates of emotion regulation have been well studied across a wide age range (28–30).
Effortful emotion regulation, an intentional attempt to decrease one’s negative response to specific stimuli, is typically accomplished by employing cognitive strategies such as reappraisal (28). A recent meta-analysis of studies employing a cognitive reappraisal emotion regulation task (31) concludes that effortful regulation via cognitive reappraisal consistently activates prefrontal control regions including the dorsal medial PFC (dmPFC), dlPFC and ventral lateral PFC (vlPFC) (28). Thus, available research suggests that emotion regulation is easily studied using existing, well-understood paradigms and that emotional reactivity is likely modulated by activation of the lateral and dorsomedial PFC in healthy samples of adolescents. In addition, current evidence points to the possibility that emotion regulation deficits underlie risk for SI, yet no study to date has examined neural activation in response to an emotion regulation task in youth with and without SI. We address this gap in the current study.
Current Study
Our goal was to examine whether neural regions recruited in support of emotion reactivity and regulation distinguish adolescents with and without SI. Given evidence that adolescents with SI exhibit greater self-reported emotional reactivity (9), we hypothesized that adolescents with versus without SI histories would show increased activity in limbic regions when viewing negative stimuli relative to neutral stimuli. Additionally, adolescents with versus without SI histories self-report greater emotion dysregulation (10). Thus, we hypothesized that adolescents with versus without SI histories would differentially recruit frontal control regions (dmPFC, dlPFC, and vlPFC) frequently implicated in cognitive reappraisal (28) during attempts to effortfully regulate emotional responses to negative stimuli relative to passive viewing of negative stimuli. Together, this study will allow us to examine both reactivity and regulation among youth with and without SI histories as an initial step to better understanding the mechanisms which put youth at risk for suicide.
Methods
Participants
Participants included 49 adolescents (ages 13–201; M = 16.95, SD = 1.54; 59% female) recruited in the context of a larger study examining the effects of childhood maltreatment on development (32, 33). The original sample included 51 youth who participated in the scanning procedures described below. Of these 51, 49 individuals provided valid data for the main outcome variable, SI. The current sample included individuals with and without exposure to childhood maltreatment, which we control for in all analyses. Exclusion criteria included psychiatric medications that could not be discontinued for 24 hours prior to scan (all but stimulant medications), braces, claustrophobia, left handedness, active substance dependence or use on the day of the scan, pervasive developmental disorders, lack of ability to speak/read English, and presence of active safety concerns (acute suicidal crisis). All procedures were approved by the hospital affiliated institutional review board.
Measures
Suicidal Ideation
SI was assessed using data from well-validated self-report and structured clinical interviews. We created a composite present (1) or absent (0) score for lifetime SI in the current study. SI was considered present (1) for any of the following: a score greater than zero on the Scale for Suicidal Ideation (SSI; self-report for past two weeks; (34), a response of “yes” to the SI question on the Diagnostic Interview Schedule for Children Version IV (DISC; past year questions(35), or a response of “yes” to the SI question on the Self-Injurious Thoughts and Behaviors Interview (SITBI; clinical interview covering SI age of onset and age of most recent SI; (36). All three measures have strong psychometric properties and have been used in numerous previous studies with adolescent SI;(7, 37). Prior research has noted that multiple methods of assessment of SI (i.e., clinical interview and self-report instruments) are preferable to either method alone (e.g., (38). Thus, combining information across these three instruments represents a strength for the current manuscript. Further, we elected to use a dichotomous indicator of SI history because the presence or absence (and not severity) of SI is one of the most consistent and strongest predictors of future SI and suicidal behavior (7).
In the current sample, nine individuals were included in the SI group for endorsing “yes” to the SITBI question “Have you ever had thoughts of killing yourself?” Four individuals were included in the SI group for endorsing “sometimes” or “often” on the SSI questions “my reasons for living or dying or about equal” or “I have made some preparations for committing suicide.” One individual was included in the SI group for endorsing “yes” to the DISC question “in the past year, did you think about killing yourself?” A total of 14 individuals (28% of total sample) were included in the SI group. Of those with SI, four endorsed a previous suicide attempt. These rates are similar to other work which includes youth with elevated clinical symptoms (e.g., depression) and exposure to child adversity (39, 40).
Depressive symptoms
Past-year depressive symptoms were assessed using the DISC-IV (35). Trained graduate students administered the DISC, which is a structured clinical interview that assesses the presence of DSM-IV symptoms and disorders. Total symptom count for the prior 12-months was derived from all questions in the major depressive disorder module.
Childhood maltreatment
Participants completed the Childhood Trauma Questionnaire (CTQ; (41) and were considered to have experienced lifetime maltreatment (1) or not (0) based on their total score using a validated threshold (42).
Functional Magnetic Resonance Imaging (fMRI) Task
During the fMRI scan, participants completed an event-related task assessing neural markers of emotion regulation (31), which has been used with children (30, 32). Design and contrasts of this task were based on previous literature (30, 31). Participants viewed neutral, negative, and positive images from the International Affective Picture System (43). Before each negative picture, participants saw a cue to “look” or “decrease;” before each positive picture participants saw a cue to “look” or “increase.” Neutral pictures were preceded by a “look” cue. Positive and negative images were presented in a quasi-blocked design; in each run participants viewed mixed negative and neutral images in randomized order for half the run and mixed positive and neutral images in randomized order for half the run (32). As our interest was in the regulation of negative emotion as a potential marker of risk for SI, we did not analyze positive trials. During look trials, participants were instructed to simply look at the image and allow their emotions to unfold naturally without altering their emotional reaction. During decrease trials, participants were instructed to use specific cognitive reappraisal strategies to reduce their emotional reaction to the negative stimuli. After each stimulus, participants rated the strength of their emotional reaction on a 5-point Likert scale.
Prior to scanning, participants were trained with specific cognitive reappraisal strategies to use in the scanner for “decrease” trials. They observed examples completed out loud by the experimenter and completed practice trials using stimuli different than those used in the scanner. Participants were instructed to think about the image as more psychologically distant by either imagining the scene as further away, not involving them, or simply involving actors. These strategies have been used in previous studies (30, 31, 44).
Stimuli were presented in 4 runs each lasting 9 minutes each. Average valence (M = 2.64, Range = 1.76 – 4.69), arousal (M = 5.82, Range = 4.47 – 7.09), and number of faces within each image were equivalent for look negative and decrease trials (all p’s > .45). The task included 26 trials of each type, and the emotional stimulus and inter-trial interval were jittered (see 32).
Image Acquisition
Scanning was performed on a 3T Siemens Trio Scanner at the Harvard Center for Brain Science, using a 32-channel head coil (see Supplemental Materials for full acquisition parameters and image processing information). Blood oxygen level dependent (BOLD) signal during functional runs was acquired using a gradient-echo T2*-weighted EPI sequence. Thirty-nine 3-mm-thick slices were acquired parallel to the AC-PC line (TR = 2,500 ms, TE = 30 ms, flip angle = 90°, bandwidth 2,240 Hz/Px, echo spacing = 0.51 ms, FOV = 216×216 mm, matrix size = 72×72 mm). Prior to each scan, four images were acquired and discarded to allow for longitudinal magnetization to reach equilibrium. An online prospective motion correction algorithm (PACE) was used to reduce motion artifacts.
Preprocessing and statistical analysis of fMRI data was performed in Nipype (45). Preprocessing included spatial realignment, slice-time correction, and spatial smoothing (6-mm full width at half maximum [FWHM]), implemented in FSL. Data were inspected for artifacts and single point outlier regressors were modeled to account for any motion exceeding 1.5mm. Additionally, 6 rigid-body motion regressors were included in person-level models.
fMRI Analysis
Regressors were created for each phase of the task: instructional cue, stimulus, and rating periods separately for look and decrease trials for neutral and negative stimuli. A general linear model (GLM) was used to estimate the association between BOLD signal and task demands across time for each subject, prior to normalization (see Supplementary Materials for more information). As has been done in previous studies (28), we measured emotional reactivity as the contrast of look negative > neutral trials and emotion regulation as the contrast of decrease > look trials for negative stimuli (i.e., isolating neural response during emotion regulation independent of viewing negative images). We examined differences in BOLD response during contrasts of interest as a function of SI in whole-brain analyses. We applied cluster-level correction in FSL (z > 2.3, p <.05). To aid in interpretation of our results, we also include within-group comparisons during look versus baseline (fixation cross) and decrease versus baseline in the supplemental materials (see Supplemental Figure 2).
Because we felt it was important to determine whether our results held when controlling for well-known correlates of SI, including depression symptoms (46), age (4), and child maltreatment (47) we control for these variables. Results below are presented with all covariates included; however, when including depression changed the findings, we additionally report that finding. Results for the main effects of task and child maltreatment status are reported in McLaughlin et al. (32). Associations between the emotion regulation task and psychopathology are available in McLaughlin, et al. (48).
Behavioral analysis
We compared self-reported emotional intensity for any trial between those with and without SI using independent samples t-tests.
Results
Descriptive Statistics
Table 1 presents comparisons between those with and without SI histories including sex, race, parental education, age, scores on the CTQ, and major depression symptoms.
Table 1.
Demographic information and comparisons between those with and without suicidal ideation histories
| Individuals With Suicidal Ideation Histories (n=14) | Individuals without Suicidal Ideation Histories (n=32) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| % | (n) | % | (n) | χ2 | p-value | |
| Female | 71.4 | 10 | 54.3 | 19 | 1.22 | .34 |
| Race | 5.33 | .26 | ||||
| White | 21.4 | 3 | 31.4 | 11 | ||
| Black | 28.6 | 4 | 34.3 | 12 | ||
| Hispanic/Latino | 35.7 | 5 | 11.4 | 4 | ||
| Asian | 0.0 | 0 | 11.4 | 4 | ||
| Other/Biracial | 14.3 | 2 | 11.4 | 4 | ||
| Parent Education | 5.12 | .16 | ||||
| High School or Less | 14.3 | 2 | 19.4 | 6 | ||
| Some College | 35.7 | 5 | 12.9 | 4 | ||
| College Degree | 14.3 | 2 | 41.9 | 13 | ||
| Graduate School | 35.7 | 5 | 25.8 | 8 | ||
| Mean | (SD) | Mean | (SD) | t-value | p-value (d) | |
|
|
||||||
| Age | 16.70 | (1.73) | 17.06 | (1.47) | .73 | .47 (.22) |
| CTQ Abuse Subscale | 17.57 | (7.84) | 11.28 | (2.02) | −4.45 | <.001 (1.3) |
| Major Depression Symptoms | 9.93 | (3.60) | 5.09 | (3.73) | −4.448 | <.001 (1.3) |
Note. CTQ = Childhood Trauma Questionnaire
Self-report of Emotion Intensity
There were no differences in self-reported emotional intensity for any trial for those with (vs. without) SI (t(47)= −1.38 – −0.27, p’s = .17-.79, d’s = .10 – .37), and the change in self-reported emotional intensity from look negative to decrease trials did not vary by SI (t(47)=.46, p = .65, d = .14).
Neural Response to Passive Viewing of Negative Emotional Stimuli
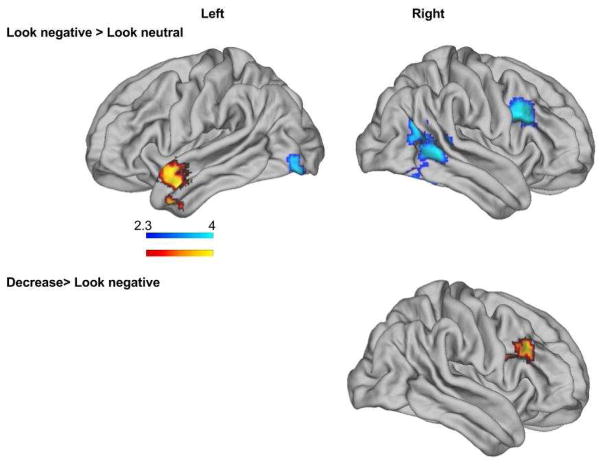
In the look negative > neutral contrast, youth with SI, relative to youth without SI, exhibited significantly less activation in four separate clusters with peaks in the thalamus, dlPFC, temporoparietal junction (TPJ), and the cerebellum, respectively (see Figure 1 and Table 1). Youth with versus without SI histories exhibited greater activation in the temporal pole for this same contrast (See Table 1). In analyses that did not control for depression, the TPJ activation was not significantly different between groups (See Supplemental Figure 1). All other results remained the same.
Figure 1.
Neural responses to passive viewing and effortful regulation of negative emotional stimuli in individuals with suicidal ideation histories versus those without suicidal ideation histories. Regions with significant blood-oxygen level-dependent (BOLD) activation depicted. Control variables included age, maltreatment status, and depression severity.
Neural Response to Effortful Emotion Regulation towards Negative Stimuli
In the decrease > look negative trials, youth with SI exhibited significantly greater activation in the dlPFC relative to youth without SI histories (See Table 2). Results were unchanged in analyses with and without covariates included (See Supplemental Figure 1). There were no areas with significantly greater activation for youth without SI compared to youth with SI for this contrast.
Table 2.
Regions of peak activation
| Trial Type | Region of Peak Activation | Broadman’s Area | Cluster Size | x | y | z | z Value |
|---|---|---|---|---|---|---|---|
| Look negative > look neutral | |||||||
| Youth with SI < Youth without SI | dlPFC (R) | 44 | 715 | 44 | 18 | 30 | 4.03 |
| Thalamus (R) | - | 809 | 14 | −10 | 10 | 3.99 | |
| Cerebellum/Lateral Occipital (L) | - | 768 | −8 | −74 | −22 | 3.67 | |
| Temporoparietal Junction (R) | 39 | 1782 | 40 | −58 | 22 | 3.94 | |
| Youth with SI > Youth without SI | Temporal Pole (L) | 38 | 647 | −50 | 2 | −20 | 3.48 |
| Decrease > look negative | |||||||
| Youth with SI > Youth without SI | dlPFC (R) | 9 | 463 | 50 | 22 | 30 | 3.71 |
Note Exact age, child maltreatment history, and depression symptom severity were included as nuisance repressors in all analyses. dlPFC = dorsolateral prefrontal cortex; SI = suicidal ideation
Discussion
The purpose of the present study was to examine neural processes underlying emotion regulation that may differ between adolescents with versus without SI histories. This is the first study to examine patterns of neural activation during an emotion regulation task between those with and without SI histories representing an important first step in better understanding the mechanisms driving this significant public health concern. Our results partially supported our hypotheses, demonstrating that adolescents with SI histories show differential patterns of activation compared to adolescents without SI when passively viewing negative stimuli and when attempting to effortfully regulate their responses to negative stimuli. For both kinds of trials, we observed significant differences between youth with and without SI in frontal control regions, but no differences in limbic system reactivity for either trial type. These findings highlight the importance of future work on the relative importance of emotional control and prefrontal cortex function in the pathophysiology of SI.
Our first hypothesis that adolescents with SI histories would exhibit greater activation in limbic regions compared to adolescents without SI histories during passive viewing trials was not supported. However, results from whole brain analyses demonstrated that youth with versus without SI showed greater activation in the left temporal pole when passively viewing negative versus neutral stimuli. Accumulating evidence suggests that the temporal pole plays a role in emotional control by modulating emotional reactions to evocative visual stimuli, particularly when perceiving or imaging emotions as in the present study (49). Whether recruitment of the left temporal pole when viewing negative stimuli is further implicated in adolescent suicide remains an intriguing question for future research.
In contrast to youth with SI, during passive viewing relative to neutral trials, youth without SI activated the dlPFC, and TPJ to a greater extent. Historically, in this task the dlPFC is implicated in active emotion regulation (28), and in other studies activation of the dlPFC is associated with working memory (50). In the context of emotion regulation, it is thought that activation in this region may reflect holding potential reappraisals in mind while examining stimuli (28, 29). Thus, it is possible that youth without SI histories were more likely to engage this region during passive viewing compared to neutral trials to downregulate emotional reactions even without prompting. A robust body of literature has linked the TPJ with deciphering others’ mental states or “theory of mind” (51). In the context of this emotion reactivity task for which we specifically selected pictures from the IAPS which included human figures and often those depicting interpersonal violence, it is possible that control youth were activating this region to imagine others’ mental states. Again, it may be that this enhanced representation was in the service of unprompted emotion regulation for control participants. Finally, the cerebellum and thalamus were significantly more active for control youth relative to youth with SI. Increased activation of these regions may indicate enhanced salience or perceptual processing of these stimuli for control youth relative to youth with SI. Interestingly, a recent study found increased resting state connectivity between cerebellum and lingual gyrus of depressed youth with suicide attempt histories (52). Further exploration of this area with regard to adolescent suicidal ideation and behavior is warranted.
Because we and many others observe differences in PFC activation for regulate versus look trials and a decrease in self-reported emotion for regulate relative to look trials across all subjects (28, 32), it is unlikely that the observation that control youth activated the dlPFC and TPJ during look trials means that youth without SI were not properly engaged with the task most of the time. However, it may be that relative to controls, youth with SI were less likely to spontaneously bring to mind ways of reappraising negative stimuli and this may be linked with their self-reported and observed emotion regulation deficits elsewhere (10). Importantly, our interpretation of these findings is limited; we did not observe behavioral differences in self-reported emotion between youth with and without SI. In the context of a null behavioral result, it is difficult to interpret these findings as firm evidence of enhanced spontaneous emotion regulation in youth without SI. Future research with larger samples may be able to confirm our findings and extend them by linking self-reported emotion on this task with real world behavior for adolescents with SI.
Our second hypothesis that youth with SI would differentially recruit frontal control regions during effortful regulation trials compared to passive viewing of negative images was supported. During trials where youth were told to effortfully downregulate their emotional responses to negative stimuli compared to passively viewing negative stimuli, youth with SI histories demonstrated greater activation in the dlPFC compared youth without SI histories. This indicates that differences in activation of this dlPFC region between regulation and passive viewing trials was greater for youth with versus without SI. Thus, it appears that youth with SI can successfully recruit this region in the service of emotion regulation, but unlike youth without SI, they only do so only when explicitly instructed. This observation, coupled with the lack of behavioral difference in self-reported emotion between controls and youth with SI for regulate trials, is consistent with the possibility that youth with SI can respond to explicit instruction to successfully recruit the dlPFC in the service of emotion regulation. In the contrast where regulation trials were compared to reactivity trial, youth with SI evidence increased in activation in the dlPFC compared to youth without SI. An alternative explanation to the one we provide above is that individuals with SI inefficiently recruit the dlPFC during regulation trials. This offers an intriguing alternative comparison to be explored in future, larger studies.
Taken together, results demonstrate that youth without SI histories may activate regions implicated in emotion regulation, including the dlPFC, even when instructed to passively view emotional stimuli. In contrast, youth with SI do not engage these regions during passive viewing trials. However, youth with SI are able to engage the dlPFC in the service of cognitive reappraisal (28, 32) but only when explicitly instructed. Generally, these results are consistent with studies with adults, which suggest specific frontal control region dysfunction among individuals with (versus without) suicidal ideation histories; e.g., 14). Although much more research is necessary to further untangle these relationships, it is possible that the pattern observed during this task relates to experiences of youth with SI in real world settings. Indeed, research from the behavioral literature suggests that during emotional crises, youth with SI demonstrate limited access to emotion regulation (10) and problem solving (53, 54) strategies, but that explicit instruction in emotion regulation, in the form of dialectical behavioral therapy, can mitigate these deficits (55). Given that the transition from thinking about suicide to acting on suicidal thoughts tends to be quite rapid (56), treatment targeting access to automatic emotion regulation strategies could be beneficial in preventing suicidal behavior.
The current study represents an important first step in examining neural underpinnings of adolescent SI. However, future work should address limitations from this study. The present study was cross-sectional. Future studies with a longitudinal design can determine whether neural processes observed in the current study are prospective risk factors for, or consequences of SI. While one-quarter of the current sample had SI, future studies should make efforts to recruit larger samples with a greater incidence of SI and a wider range of suicidal behavior to replicate the current study’s findings and provide more precise testing of neural differences between those without any kind of SI or suicidal behaviors, those with SI alone, and those that have made a suicide attempt. Further, a larger sample of youth with SI will allow examination of differences between passive and active SI (see 57). The emotion regulation strategies in this study were tested in a controlled setting, however, it is possible that youth with SI may exhibit more difficulty regulating emotions in the context of real world experiences or even with more naturalistic stimuli. Future research should address this possibility.
The current study is among the first to investigate neural mechanisms underlying emotion regulation—a commonly purported risk factor for SI in adolescence. Results suggest that youth with and without SI differentially recruit regions implicated in emotion regulation when viewing negative stimuli and regulating responses to negative stimuli. These findings build on a growing body of work suggesting a critical role of disruptions in emotion regulation as a process underlying SI in adolescents and extend previous findings by examining neural markers of emotion regulation rather than relying on self report.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institute of Mental Health awarded to ABM (F32MH108238) and KAM (R01-MH103291, R01-MH106482, and K01-MH092526) and a grant from the Charles H. Hood Foundation Child Health Research Award (KAM).
Footnotes
Only one individual was 20 years old.
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam Bryant Miller, University of North Carolina at Chapel Hill.
Katie A. McLaughlin, University of Washington.
Daniel S. Busso, Harvard Graduate School of Education.
Stephanie Brueck, Boston College School of Social Work.
Matthew Peverill, University of Washington.
Margaret A. Sheridan, University of North Carolina at Chapel Hill
References
- 1.Curtin S, Warner M, Hedegaard H. NCHS Data Brief. Hyattsville, MD: National Center for Health Statistics; 2016. Increase in suicide in the United States, 1999–2014. [Google Scholar]
- 2.Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, Bcheraoui CE, Moradi-Lakeh M, et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2016;387:2383–2401. doi: 10.1016/S0140-6736(16)00648-6. [DOI] [PubMed] [Google Scholar]
- 3.Grinshteyn E, Hemenway D. Violent Death Rates: The US Compared with Other High-income OECD Countries, 2010. Am J Med. 2016;129:266–273. doi: 10.1016/j.amjmed.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, Kessler RC. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013;70:300–310. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kann L. Youth Risk Behavior Surveillance — United States, 2015. MMWR Surveill Summ. 2016:65. doi: 10.15585/mmwr.ss6506a1. [DOI] [PubMed] [Google Scholar]
- 6.Prinstein MJ, Nock MK, Simon V, Aikins JW, Cheah CS, Spirito A. Longitudinal trajectories and predictors of adolescent suicidal ideation and attempts following inpatient hospitalization. J Consult Clin Psychol. 2008;76:92. doi: 10.1037/0022-006X.76.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro JD, Franklin JC, Fox KR, Bentley KH, Kleiman EM, Chang BP, Nock MK. Self-injurious thoughts and behaviors as risk factors for future suicide ideation, attempts, and death: a meta-analysis of longitudinal studies. Psychol Med. 2016;46:225–236. doi: 10.1017/S0033291715001804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017;143:187–232. doi: 10.1037/bul0000084. [DOI] [PubMed] [Google Scholar]
- 9.Nock MK, Wedig MM, Holmberg EB, Hooley JM. The emotion reactivity scale: development, evaluation, and relation to self-injurious thoughts and behaviors. Behav Ther. 2008;39:107–116. doi: 10.1016/j.beth.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Rajappa K, Gallagher M, Miranda R. Emotion Dysregulation and Vulnerability to Suicidal Ideation and Attempts. Cogn Ther Res. 2012;36:833–839. [Google Scholar]
- 11.Minzenberg MJ, Lesh T, Niendam T, Yoon JH, Cheng Y, Rhoades R, Carter CS. Conflict-related anterior cingulate functional connectivity is associated with past suicidal ideation and behavior in recent-onset schizophrenia. J Psychiatr Res. 2015;65:95–101. doi: 10.1016/j.jpsychires.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Minzenberg MJ, Lesh TA, Niendam TA, Yoon JH, Rhoades RN, Carter CS. Frontal cortex control dysfunction related to long-term suicide risk in recent-onset schizophrenia. Schizophr Res. 2014;157:19–25. doi: 10.1016/j.schres.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Minzenberg MJ, Lesh T, Niendam T, Yoon JH, Cheng Y, Rhoades RN, Carter CS. Frontal motor cortex activity during reactive control is associated with past suicidal behavior in recent-onset schizophrenia. Crisis. 2015 doi: 10.1027/0227-5910/a000335. Retrieved from http://econtent.hogrefe.com/doi/full/10.1027/0227-5910/a000335. [DOI] [PubMed]
- 14.Minzenberg MJ, Lesh TA, Niendam TA, Yoon JH, Cheng Y, Rhoades RN, Carter CS. Control-related frontal-striatal function is associated with past suicidal ideation and behavior in patients with recent-onset psychotic major mood disorders. J Affect Disord. 2015;188:202–209. doi: 10.1016/j.jad.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Minzenberg MJ, Lesh TA, Niendam TA, Cheng Y, Carter CS. Conflict-Related Anterior Cingulate Functional Connectivity Is Associated With Past Suicidal Ideation and Behavior in Recent-Onset Psychotic Major Mood Disorders. J Neuropsychiatry Clin Neurosci. 2016;28:299–305. doi: 10.1176/appi.neuropsych.15120422. [DOI] [PubMed] [Google Scholar]
- 16.Marchand WR. Self-Referential Thinking, Suicide, and Function of the Cortical Midline Structures and Striatum in Mood Disorders: Possible Implications for Treatment Studies of Mindfulness-Based Interventions for Bipolar Depression. Depress Res Treat. 2011;2012:e246725. doi: 10.1155/2012/246725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pu S, Nakagome K, Yamada T, Yokoyama K, Matsumura H, Yamada S, et al. Suicidal ideation is associated with reduced prefrontal activation during a verbal fluency task in patients with major depressive disorder. J Affect Disord. 2015;181:9–17. doi: 10.1016/j.jad.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Myung W, Han CE, Fava M, Mischoulon D, Papakostas GI, Heo J-Y, et al. Reduced frontal-subcortical white matter connectivity in association with suicidal ideation in major depressive disorder. Transl Psychiatry. 2016;6:e835. doi: 10.1038/tp.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du L, Zeng J, Liu H, Tang D, Meng H, Li Y, Fu Y. Fronto-limbic disconnection in depressed patients with suicidal ideation: A resting-state functional connectivity study. J Affect Disord. 2017;215:213–217. doi: 10.1016/j.jad.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Chase HW, Segreti AM, Keller TA, Cherkassky VL, Just MA, Pan LA, Brent DA. Alterations of functional connectivity and intrinsic activity within the cingulate cortex of suicidal ideators. J Affect Disord. 2017;212:78–85. doi: 10.1016/j.jad.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, et al. Multimodal Neuroimaging of Frontolimbic Structure and Function Associated With Suicide Attempts in Adolescents and Young Adults With Bipolar Disorder. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2016.15050652. appi.ajp.2016.15050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan LA, Hassel S, Segreti AM, Nau SA, Brent DA, Phillips ML. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol Med. 2013;43:2129–2142. doi: 10.1017/S0033291712002966. [DOI] [PubMed] [Google Scholar]
- 23.Pan LA, Segreti A, Almeida J, Jollant F, Lawrence N, Brent D, Phillips M. Preserved hippocampal function during learning in the context of risk in adolescent suicide attempt. Psychiatry Res Neuroimaging. 2013;211:112–118. doi: 10.1016/j.pscychresns.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisani AR, Wyman PA, Petrova M, Schmeelk-Cone K, Goldston DB, Xia Y, Gould MS. Emotion Regulation Difficulties, Youth–Adult Relationships, and Suicide Attempts Among High School Students in Underserved Communities. J Youth Adolesc. 2013;42:807–820. doi: 10.1007/s10964-012-9884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shneidman ES. Suicide as psychache: A clinical approach to self-destructive behavior. Jason Aronson; 1993. Retrieved July 18, 2012, from http://books.google.com/books?hl=en&lr=&id=lnBCjKkwW-YC&oi=fnd&pg=PR9&dq=Shneidman,+E.+S.+(1993).+Suicide+As+Psychache&ots=p09k3alK64&sig=zt_p-ltz6BnoUDMWVHWVkQLjvnU. [Google Scholar]
- 26.Wyman PA, Gaudieri PA, Schmeelk-Cone K, Cross W, Brown CH, Sworts L, et al. Emotional Triggers and Psychopathology Associated with Suicidal Ideation in Urban Children with Elevated Aggressive-Disruptive Behavior. J Abnorm Child Psychol. 2009;37:917–928. doi: 10.1007/s10802-009-9330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khurana A, Romer D. Modeling the Distinct Pathways of Influence of Coping Strategies on Youth Suicidal Ideation: A National Longitudinal Study. Prev Sci. 2012;13:644–654. doi: 10.1007/s11121-012-0292-3. [DOI] [PubMed] [Google Scholar]
- 28.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silvers JA, McRae K, E D, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12:1235–1247. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child maltreatment and neural systems underlying emotion regulation. J Am Acad Child Adolesc Psychiatry. 2015;54:753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin KA, Sheridan MA, Alves S, Mendes WB. Child maltreatment and autonomic nervous system reactivity: identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat. Psychosom Med. 2014;76:538–546. doi: 10.1097/PSY.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 35.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Nock MK, Holmberg EB, Photos VI, Michel BD. Self-Injurious Thoughts and Behaviors Interview: Development, reliability, and validity in an adolescent sample. Psychol Assess. 2007;19:309–317. doi: 10.1037/1040-3590.19.3.309. [DOI] [PubMed] [Google Scholar]
- 37.Miller AB, Esposito-Smythers C, Weismoore JT, Renshaw KD. The relation between child maltreatment and adolescent suicidal behavior: A systematic review and critical examination of the literature. Clin Child Fam Psychol Rev. 2013;16:146–172. doi: 10.1007/s10567-013-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan ML, Asnis GM, Sanderson WC, Keswani L, de Lecuona JM, Joseph S. Suicide assessment: Clinical interview vs. self-report. J Clin Psychol. 1994;50:294–298. doi: 10.1002/1097-4679(199403)50:2<294::aid-jclp2270500224>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 39.Brezo J, Paris J, Vitaro F, Hébert M, Tremblay RE, Turecki G. Predicting suicide attempts in young adults with histories of childhood abuse. Br J Psychiatry. 2008;193:134–139. doi: 10.1192/bjp.bp.107.037994. [DOI] [PubMed] [Google Scholar]
- 40.Giletta M, Hastings PD, Rudolph KD, Bauer DJ, Nock MK, Prinstein MJ. Suicide ideation among high-risk adolescent females: Examining the interplay between parasympathetic regulation and friendship support. Dev Psychopathol. 2016:1–15. doi: 10.1017/S0954579416001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, et al. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatry. 1999;56:609–613. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- 43.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. Retrieved November 15, 2014, from http://www.hsp.epm.br/dpsicobio/Nova_versao_pagina_psicobio/adap/instructions.pdf. [Google Scholar]
- 44.McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinformatics. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Connor RC, Smyth R, Ferguson E, Ryan C, Mark J. Psychological processes and repeat suicidal behavior: A four-year prospective study. J Consult Clin Psychol. 2013;81:1137–1143. doi: 10.1037/a0033751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King CA, Merchant CR. Social and interpersonal factors relating to adolescent suicidality: a review of the literature. Arch Suicide Res. 2008;12:181–196. doi: 10.1080/13811110802101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA. Amygdala Response to Negative Stimuli Predicts Ptsd Symptom Onset Following a Terrorist Attack. Depress Anxiety. 2014;31:834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 50.D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 51.Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Chen J, Kuang L, Cao J, Zhang H, Ai M, et al. Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry. 2016;16:337. doi: 10.1186/s12888-016-1047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esposito CL, Clum GA. Social support and problem-solving as moderators of the relationship between childhood abuse and suicidality: Applications to a delinquent population. J Trauma Stress. 2002;15:137–146. doi: 10.1023/A:1014860024980. [DOI] [PubMed] [Google Scholar]
- 54.Grover KE, Green KL, Pettit JW, Monteith LL, Garza MJ, Venta A. Problem solving moderates the effects of life event stress and chronic stress on suicidal behaviors in adolescence. J Clin Psychol. 2009;65:1281–1290. doi: 10.1002/jclp.20632. [DOI] [PubMed] [Google Scholar]
- 55.Mehlum L, Tørmoen AJ, Ramberg M, Haga E, Diep LM, Laberg S, et al. Dialectical Behavior Therapy for Adolescents With Repeated Suicidal and Self-harming Behavior: A Randomized Trial. J Am Acad Child Adolesc Psychiatry. 2014;53:1082–1091. doi: 10.1016/j.jaac.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Millner AJ, Ursano RJ, Hwang I, King AJ, Naifeh JA, Sampson NA, et al. Lifetime Suicidal Behaviors and Career Characteristics Among U.S. Army Soldiers: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) Suicide Life Threat Behav. n.d doi: 10.1111/sltb.12363. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Millner AJ, Lee MD, Nock MK. Single-Item Measurement of Suicidal Behaviors: Validity and Consequences of Misclassification. PLOS ONE. 2015;10:e0141606. doi: 10.1371/journal.pone.0141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.