Abstract
Cell-based cartilage repair procedures are becoming more widely available and have shown promising potential to treat a wide range of cartilage lesion types and sizes, particularly in the knee joint. More recently, techniques have evolved from 2-step techniques that use autologous chondrocyte expansion to 1-step techniques that make use of mesenchymal stem cells (MSCs) embedded onto biocompatible scaffolding. Our 1-step technique has been further developed to provide cell-based cartilage repair using MSCs that have the potential to be used in an off-the-shelf manner, without the need for autologous tissue harvest. Precursor MSCs can be isolated in abundance from the Wharton's jelly of umbilical cord tissue. These cells have been shown to have the desired capacity for proliferation, differentiation, and release of trophic factors that make them an excellent candidate for use in the clinical setting to provide cell-based restoration of hyaline-like cartilage. Although allogeneic in nature, these cells stimulate little or no host immune response and can be stored for long periods while maintaining viability. We present a technique of cartilage repair in the knee using Wharton's jelly–derived MSCs embedded onto scaffolding and implanted in a minimally invasive fashion using dry arthroscopy.
Injury to articular cartilage is often associated with progressive cartilage wear that may result in osteoarthritic changes to the joint and worsening pain and dysfunction. There is limited inherent capacity for self-regeneration of cartilage lesions, and this has been a prominent focus in the development of treatment strategies.
Cell-based cartilage repair techniques such as autologous chondrocyte implantation have shown good to excellent clinical outcomes and hyaline-like cartilage restoration; however, these are 2-step techniques that require the patient to undergo multiple surgical procedures. Cell-based repair using scaffolding embedded with mesenchymal stem cells (MSCs) sourced from bone marrow aspirate concentrate, such as the technique of hyaluronic acid-based scaffold embedded with bone marrow aspirate concentrate (HA-BMAC), is receiving increasing attention by clinicians because of the encouraging medium-term clinical outcomes reported and the capacity to restore hyaline-like cartilage.1, 2
One-stage cell-based cartilage repair techniques using stem cells are highly advantageous, given the potential for achieving clinical success in the setting of a cost-controlled method of treatment that avoids exposing the patient to a second surgical procedure. In addition to a source of precursor cells for cartilage restoration, MSCs provide numerous trophic and anti-inflammatory factors that provide a favorable environment for chondrogenesis. Although these cells may be obtained from an autologous source such as bone marrow or adipose tissue, precursor cells may also be isolated in abundance from allogeneic sources, such as the Wharton's jelly of human umbilical cords.
Wharton's jelly is a tissue that surrounds umbilical cord blood vessels and contains high concentrations of precursor MSCs that have increased proliferation and differentiation capabilities compared with adult sources of stem cells.3 Use of such allogeneic cells may be performed in a clinical setting without eliciting an immune response from the host,4, 5 and they do not undergo malignant transformation.6 Furthermore, suspensions of MSCs sourced from Wharton's jelly may be stored for long periods while maintaining cell viability, allowing for off-the-shelf use.
Recent developments in cell-based cartilage repair techniques that use dry arthroscopic methods have further advanced this field, given the advantages of a minimally invasive technique that reduces morbidity and optimizes postoperative rehabilitation progression.7, 8 This Technical Note describes a method of cell-based cartilage repair using allogeneic MSCs sourced from Wharton's jelly (WJ-MSCs) that are embedded onto a type I/III collagen scaffold and implanted under dry arthroscopy (Video 1).
Surgical Procedure
Cell Culture and Preparation of WJ-MSC Isolate
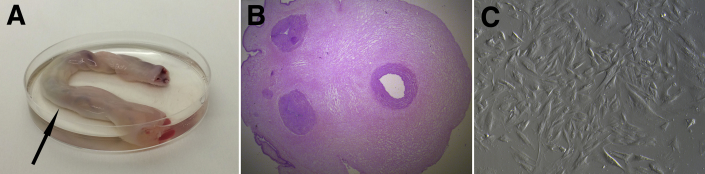
Umbilical cord sections are collected after informed consent is obtained from the donor mother in cases of either cesarean or natural delivery (Fig 1A). The samples of umbilical cord tissue are maintained in a temperature-controlled environment and are processed within 48 hours of procurement. The umbilical cord segment is washed in a sterile solution of saline and antibiotic-antimycotic fluid and then portioned into 2-cm-long pieces, followed by removal of blood vessels to isolate the Wharton's jelly. The isolated Wharton's jelly is portioned into 2-cm3 fragments, which are then cultured in xeno-free medium supplemented with antibiotics. After 2 to 3 weeks of culture incubation at 37°C, stem cells are collected after reaching 90% confluence (Fig 1C), and the remaining tissue is discarded. These WJ-MSCs are then reseeded at a concentration of 1.2 × 104 cells/cm2 for further expansion. Trypan blue exclusion in a hemocytometer is used to verify the WJ-MSC viability of the expanded cell lines, and the phenotype is confirmed by an immunophenotyping technique to show that cells are positive for surface markers CD73, CD90, and CD105 and negative for markers CD14, CD19, CD34, CD45, and human leukocyte antigen (HLA) DR. Isolated WJ-MSCs are then placed into freezing bags, suspended in a mixture containing human albumin and 10% dimethylsulfoxide, and placed into cell containers. These containers are cooled in a controlled-rate freezer and subsequently stored at a temperature of −195°C in liquid nitrogen.
Fig 1.
Wharton's jelly–derived mesenchymal stem cell preparation. (A) Human umbilical cord measuring 10 cm in length (arrow). (B) Histologic slide of sectioned umbilical cord with hematoxylin and eosin staining (magnification ×20). (C) Contrast image of Wharton's jelly–derived mesenchymal stem cells after expansion in xeno-free medium.
The freezing bag containing the WJ-MSC suspension is removed from liquid nitrogen storage approximately 30 minutes before the expected time of implantation and warmed in a water bath at 37°C. Dimethylsulfoxide is removed from the WJ-MSC suspension by a dilutional saline wash using a 2-step centrifugation process. The suspension is transferred to a sterile conical tube containing 50 mL of saline solution and then centrifuged at 300g for 7 minutes at 22°C. After removal of the supernatant, the remaining pellet of cells is resuspended in 50 mL of saline solution and again centrifuged. Finally, after supernatant removal, the pellet of WJ-MSCs is suspended in 1 mL of saline solution and transferred to a sterile syringe.
Patient Positioning and Arthroscopic Examination
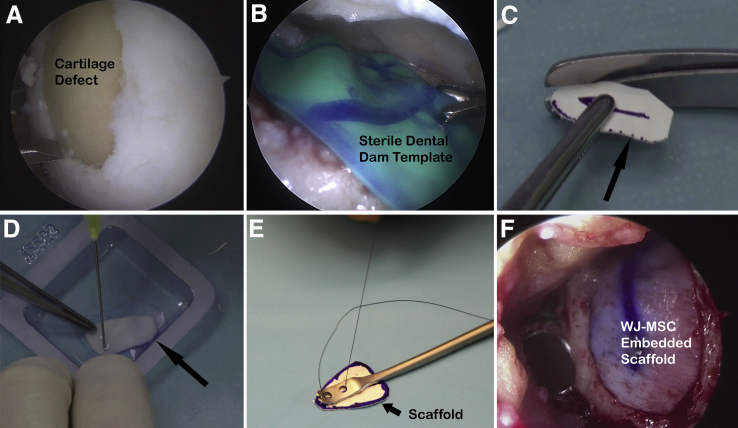
The patient is positioned as for standard knee arthroscopy and given general or spinal anesthesia. A diagnostic arthroscopy is performed, and a complete assessment of the articular cartilage confirms the location and size of the cartilage lesion or lesions. Complete visual appreciation of the extent of cartilage injury is necessary to confirm that arthroscopic cartilage repair treatment is appropriate. Loose cartilage is removed, and the cartilage lesion is prepared with careful attention paid to creating vertical peripheral walls about the defect (Fig 2A). The base of the lesion is prepared to the depth of subchondral bone, ensuring removal of the calcified cartilage layer, without significantly damaging the subchondral plate. To obtain improved access to the defect periphery and to assist with creation of vertical walls about the lesion, specialized arthroscopic instruments (Chondrectomes Set; ATMED-Z Rafalski, Katowice, Poland) may be used, as lesion preparation before cartilage repair is a crucial component of the procedure that will optimize regeneration of articular cartilage.
Fig 2.
(A) Preparation of cartilage lesion located at medial trochlea and lateral aspect of medial femoral condyle, with patient supine and arthroscope positioned in anterolateral portal. (B) Sterile dental dam used for templating of lesion. (C) Size matching of type I/III collagen scaffold (arrow) to prepared defect. (D) Type I/III collagen scaffold (arrow) saturated with Wharton's jelly–derived mesenchymal stem cell suspension. (E) Specialized graft-inserting instrument used to simplify arthroscopic implant delivery through anteromedial portal. (F) Dry arthroscopic application of type I/III collagen scaffold embedded with Wharton's jelly–derived mesenchymal stem cells (WJ-MSCs) onto chondral defect of medial trochlea and lateral aspect of medial femoral condyle, with visualization through anterolateral portal.
WJ-MSC–Embedded Scaffold Preparation and Dry Arthroscopic Implantation
Accurate sizing of the prepared cartilage defect is performed using an arthroscopic measuring device, and a template of the lesion is created using a sterile latex dental dam, aluminum foil, or other appropriate material on a sterile table. The template may be applied arthroscopically to the defect multiple times and resized as necessary (Fig 2B). A size-matched type I/III collagen scaffold (Chondro-Gide; Geistlich, Wolhusen, Switzerland) is then created that conforms to the template and defect dimensions (Fig 2C). The side of the bilayer scaffold containing the more densely arranged collagen fibers should be marked to ensure the porous side of the scaffold is placed in direct opposition to the subchondral plate at the time of graft application. The scaffold is gently moistened with saline solution and then immersed in the WJ-MSC suspension for a period of 5 minutes to create the final WJ-MSC–embedded scaffold graft (Fig 2D).
Thorough arthroscopic visualization confirms access to the entire cartilage lesion. Fluid within the joint space is drained to prepare for implantation of the graft under dry arthroscopy. To optimize access to the prepared defect under dry arthroscopy, a specialized retraction system (Arthroscopic Retracting System; ATMED-Z Rafalski) may be used, which is particularly useful for the treatment of lesions within the patellofemoral compartment. To minimize collapse of soft tissues that may occur under negative pressure when draining the arthroscopic fluid, a valveless cannula or skid is placed into the working portal to equalize pressure. The WJ-MSC–embedded scaffold is then introduced into the relevant knee compartment through the skid or valveless cannula and placed into the prepared cartilage defect using a specialized graft-inserting instrument (Scaffold Inserter; ATMED-Z Rafalski) (Fig 2E). A grasper or nontoothed forceps may be used for implanting the graft if a similar tool is unavailable. An arthroscopic probe is used to gently seat and secure the WJ-MSC graft, using previously applied markings to ensure the porous side of the scaffold is facing the defect base (Fig 2F). Fibrin glue is typically applied around the periphery of the graft to improve security of fixation. The knee is cycled through flexion and extension to confirm secure seating of the implant within the prepared cartilage defect. The surgical incisions are closed appropriately, a sterile dressing is applied, and a brace is positioned to immobilize the knee in extension. Pearls and pitfalls of the technique are presented in Table 1 and advantages and limitations in Table 2. A step-by-step technique summary is presented in Table 3.
Table 1.
Pearls and Pitfalls of Dry Arthroscopic WJ-MSC–Embedded Scaffold Cartilage Repair
| Pearls |
| Complete visualization of the cartilage lesion and access of instrumentation to the entire defect periphery are necessary for proper lesion preparation. |
| The surgeon should ensure cartilage defect preparation achieves perpendicularity of the cartilage walls about the lesion and prepare the base of the defect by removing the calcified cartilage layer. |
| The long axis of the compacted collagen layer of the bilayer type I/III collagen scaffold should be marked to ensure the porous collagen layer is positioned facing the subchondral plate and in the correct orientation at the time of graft implantation. |
| Obliquely oriented access to the lesion from the working portal is recommended for ease of graft implantation. |
| During dry arthroscopy, minor fogging of the lens can be addressed by touching the camera to fatty tissue; when there is extensive fogging, a swab can be introduced from the working portal to clean the lens. |
| Pitfalls |
| When there is inadequate arthroscopic access to the entirety of the cartilage lesion (or lesions), additional retraction plates may be introduced or a mini-open surgical approach should be performed. |
| The surgeon should avoid excessive handling and manipulation of the WJ-MSC–embedded scaffold to maximize cell viability at the time of graft implantation. |
| Security of graft fixation should be confirmed with direct arthroscopic visualization to minimize the risk of graft delamination in the early postoperative period. |
WJ-MSC, Wharton's jelly–derived mesenchymal stem cell.
Table 2.
Advantages and Limitations of Dry Arthroscopic WJ-MSC–Embedded Scaffold Cartilage Repair
| Advantages |
| The technique allows 1-stage cell-based cartilage repair that has the potential to be used in an off-the-shelf manner. |
| The technique uses a minimally invasive approach associated with lower morbidity and accelerated early rehabilitation. |
| The allogeneic source of the stem cells avoids potential donor-site morbidity. |
| Limitations |
| Medium- and long-term outcome data are not yet available. |
| Accessibility of mesenchymal stem cell isolates obtained from umbilical cord Wharton's jelly is limited at present. |
| The liquid nitrogen storage vessel containing the WJ-MSC isolate can be cumbersome to transport. |
WJ-MSC, Wharton's jelly–derived mesenchymal stem cell.
Table 3.
Step-by-Step Technique of Cartilage Repair in Knee Using Umbilical Cord WJ-MSCs Embedded Onto Collagen Scaffolding and Implanted Under Dry Arthroscopy
| 1. Place the freezing bag containing the WJ-MSC suspension in a 37°C water bath 30 minutes before the expected time of surgical implantation. |
| 2. When thawed, transfer the WJ-MSC suspension into a sterile conical tube containing 50 mL of saline solution and centrifuge at 300g for 7 minutes at 22°C. |
| 3. Remove the supernatant from the tube, and resuspend the pellet of cells in a second sterile tube containing 50 mL and repeat centrifugation. |
| 4. Remove the supernatant, and suspend the WJ-MSC pellet in 1 mL of saline solution and transfer it to a sterile syringe to create the final suspension of cells that is cleared of DMSO. |
| 5. Position the patient as for standard knee arthroscopy and administer general or spinal anesthesia. |
| 6. Examine the knee with the patient under anesthesia to confirm or identify associated pathology. |
| 7. Perform diagnostic arthroscopy and identify any coexisting conditions that require treatment. |
| 8. Visualize cartilage lesions in their entirety to ensure adequate access for arthroscopic treatment. Traction sutures or specialized soft-tissue retraction instruments may increase the working space and improve visualization. |
| 9. Treat associated pathology and perform appropriate osteotomy as indicated. |
| 10. Prepare the cartilage lesions by removing unstable or loose tissue, and prepare vertical cartilage walls about the periphery to create a well-shouldered lesion using ring curettes or Chondrectomes. |
| 11. Remove the calcified cartilage layer at the base of the defect without significant damage to the subchondral plate. |
| 12. Use an arthroscopic measuring tool to determine the dimensions of the defect and create a size-matched template using a sterile dental dam or aluminum foil. Insert the template into the defect, and modify the size as needed to accurately re-create the dimensions of the defect. |
| 13. Use the template to perform appropriate size matching of a bilayer type I/III collagen scaffold to the defect. Mark the scaffold surface composed of densely compacted collagen to ensure the porous layer will be appropriately placed against the subchondral bone at the time of graft insertion. |
| 14. Moisten the collagen scaffold with saline solution, and immerse this into the suspension of WJ-MSCs for a period of 5 min. |
| 15. Drain fluid from the articular space, and place a skid or valveless cannula through the working portal in preparation for dry arthroscopy. |
| 16. Confirm complete visualization of the prepared cartilage defects, and reassess adequate access to the entire base and periphery. |
| 17. Gently insert the WJ-MSC–embedded scaffold into the relevant knee compartment through the valveless cannula using a specialized graft inserter, grasper, or nontoothed forceps. Place the porous layer of the scaffold against the subchondral plate, with the marked layer visible and facing outward. |
| 18. Press fit the WJ-MSC–embedded scaffold graft into the defect. Apply fibrin glue to the periphery of the graft to improve stability of fixation. |
| 19. Cycle the knee under arthroscopic visualization to confirm stability of the implanted graft. |
| 20. Close the surgical wounds appropriately, apply a dressing, and place the brace into a knee brace locked in extension. |
DMSO, dimethylsulfoxide; WJ-MSC, Wharton's jelly–derived mesenchymal stem cell.
Rehabilitation
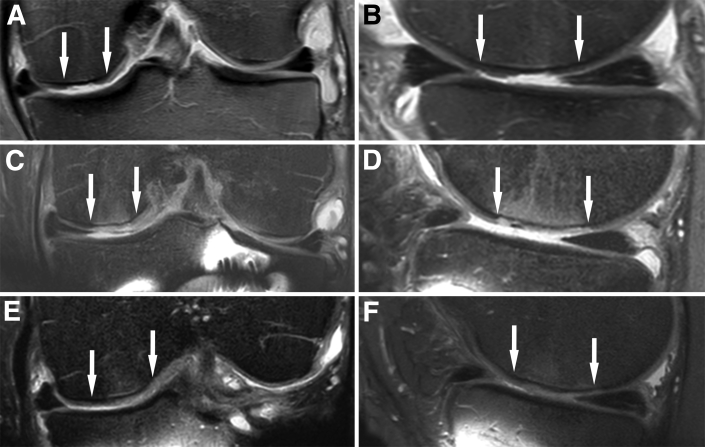
The operative knee is immobilized in a brace initially, typically for a period of 5 days, to maintain stability of the graft construct and to encourage maximal seeding of WJ-MSCs onto the subchondral surface. After the initial immobilization period, continuous passive motion is applied daily until 90° of flexion is restored. Weight bearing is restricted for the first 4 weeks in cases of medial- or lateral-compartment cartilage repair, with partial weight bearing commencing at week 4 and unrestricted weight bearing at week 6. Weight bearing is not typically restricted in the case of lesions treated within the patellofemoral compartment. At 6 weeks, proprioceptive exercises begin, and therapy will focus on muscle conditioning and restoring normal gait. At 3 months, aerobic training begins and there is progression of strength training. Magnetic resonance imaging is used routinely at 6 weeks, 12 weeks, and 6 months postoperatively to monitor the repaired lesion (Fig 3), and rehabilitation progression may be modified depending on graft integration. Sport-specific training will typically commence at 6 to 8 months postoperatively, when muscle strength and conditioning have been adequately restored. Depending on the sport and patient-specific therapeutic progress, return to sport is allowed between 8 and 12 months postoperatively.
Fig 3.
Proton density–weighted magnetic resonance images (coronal and sagittal views) showing a grade III to IV cartilage lesion of the medial femoral condyle (arrows) preoperatively (A, B); Wharton's jelly–derived mesenchymal stem cell–embedded scaffold (arrows) 6 months after dry arthroscopic implantation (C, D); and Wharton's jelly–derived mesenchymal stem cell–embedded scaffold (arrows) 9 months after implantation, depicting integration of regenerative tissue with surrounding cartilage and subchondral lamina (E, F).
Discussion
Early treatment of cartilage injury has the potential to prevent or limit the progression of articular cartilage wear that often results in great functional disability. Cell-based cartilage repair procedures have been developed to address this pathology and are increasingly being provided as a first-line treatment worldwide. The presented technique describes a method for cell-based cartilage repair using allogeneic stem cells derived from umbilical cord Wharton's jelly. The procedure can be performed arthroscopically to treat cartilage lesions affecting any of the knee compartments, and the potential for off-the-shelf use of this scaffold-embedded stem cell therapy provides a further advantage.
Cell-based repair techniques that involve implantation of autologous chondrocytes after off-site expansion of cell lines have been extensively studied, and good to excellent outcomes are expected, even in cases of large areas of chondral injury. There are notable disadvantages to autologous chondrocyte implantation or matrix-assisted autologous chondrocyte implantation techniques, such as the need to expose patients to increased surgical risk with multiple procedures, as well as the high cost of cell culture and expansion. Furthermore, although these chondrocytes may be stored for later use, a period of several weeks is needed for cell culture, and the expanded cells are required to be used promptly after culture.
The regenerative capacity of Wharton's jelly–derived stem cells has been shown to be superior to that of autologous sources of adult MSCs, and these cells maintain their phenotype and immunomodulatory characteristics after ex vivo cellular expansion.6, 9 Furthermore, these stem cells do not undergo malignant transformation, which is an important feature for safe clinical use.6 WJ-MSC suspensions provide a high concentration of precursor cells that maintain an excellent capacity to regenerate articular cartilage and may be stored for long periods, making them readily available for clinical use at the surgeon's discretion.
Dry arthroscopic implantation of WJ-MSC–embedded scaffolds can be performed to treat cartilage injury affecting any knee compartment for properly indicated lesions. This minimally invasive approach is highly advantageous, given the potential implications to accelerate early rehabilitation while minimizing morbidity. Although suture may be used to stabilize the graft during open techniques, fibrin glue has been shown to increase the stability of fixation of type I/III collagen patches,10 and we have used fibrin glue routinely to secure scaffolds after arthroscopic implantation with excellent results.7, 8
Although WJ-MSCs are allogeneically sourced, they are considered weakly immunogenic or non-immunogenic because of the low expression of HLA class I. The ability of these cells to promote chondrogenesis, without eliciting an immunogenic response, makes them an excellent candidate for providing cell-based cartilage repair in an off-the-shelf fashion. Moreover, use of WJ-MSCs for cartilage repair in older patients will address concerns related to MSC number and immunomodulatory capacity with autologous harvest in aging patients, making this technique a promising advancement in the treatment of cartilage injury for this demographic.
Ideal cartilage repair procedures should provide restoration of hyaline-like articular cartilage and be performed in a minimally invasive, single-stage manner to minimize patient morbidity. The use of MSCs sourced from autologous tissue in combination with biocompatible scaffolding has shown encouraging clinical results at medium term, comparable with more traditional methods of cell-based cartilage repair using autologous chondrocytes. The use of WJ-MSC–embedded scaffolds for cartilage repair procedures provides the additional potential advantages of off-the-shelf use and avoidance of morbidity related to autologous tissue harvest. It is important to note that this technique may be used in older patients to provide a source of stem cells for cartilage repair that do not have limited regenerative and trophic properties. Although initial results are encouraging, further clinical follow-up will be necessary to compare the success of this procedure with other methods of cell-based cartilage repair.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Cartilage repair in a knee using Wharton's jelly–derived mesenchymal stem cells (WJ-MSCs) embedded onto type I/III collagen scaffolding and implanted in a minimally invasive fashion using dry arthroscopy. The patient is supine, and an arthroscopic view of the cartilage lesion at the medial trochlea that extends to the medial femoral condyle is visualized from the anterolateral portal, with instrumentation through the anteromedial portal.
References
- 1.Gobbi A., Whyte G.P. One-stage cartilage repair using a hyaluronic acid-based scaffold with activated bone marrow-derived mesenchymal stem cells compared with microfracture: Five-year follow-up. Am J Sports Med. 2016;44:2846–2854. doi: 10.1177/0363546516656179. [DOI] [PubMed] [Google Scholar]
- 2.Gobbi A., Scotti C., Karnatzikos G., Mudhigere A., Castro M., Peretti G.M. One-step surgery with multipotent stem cells and hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc. 2017;25:2494–2501. doi: 10.1007/s00167-016-3984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H.-S., Hung S.-C., Peng S.-T. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 4.Law S., Chaudhuri S. Mesenchymal stem cell and regenerative medicine: Regeneration versus immunomodulatory challenges. Am J Stem Cells. 2013;2:22–38. [PMC free article] [PubMed] [Google Scholar]
- 5.Pigott J.H., Ishihara A., Wellman M.L., Russell D.S., Bertone A.L. Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immunopathol. 2013;156:99–106. doi: 10.1016/j.vetimm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Chen G., Yue A., Ruan Z. Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS One. 2014;9:1–9. doi: 10.1371/journal.pone.0098565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whyte G.P., Gobbi A., Sadlik B. Dry arthroscopic single-stage cartilage repair of the knee using a hyaluronic acid-based scaffold with activated bone marrow-derived mesenchymal stem cells. Arthrosc Tech. 2016;5:e913–e918. doi: 10.1016/j.eats.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadlik B., Gobbi A., Puszkarz M., Klon W., Whyte G.P. Biologic inlay osteochondral reconstruction: Arthroscopic one-step osteochondral lesion repair in the knee Using morselized bone grafting and hyaluronic acid-based scaffold embedded with bone marrow aspirate concentrate. Arthrosc Tech. 2017;6:e383–e389. doi: 10.1016/j.eats.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Rocca G., Lo Iacono M., Corsello T., Corrao S., Farina F., Anzalone R. Human Wharton's jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: New perspectives for cellular therapy. Curr Stem Cell Res Ther. 2013;8:100–113. doi: 10.2174/1574888x11308010012. [DOI] [PubMed] [Google Scholar]
- 10.Whyte G.P., McGee A., Jazrawi L., Meislin R. Comparison of collagen graft fixation methods in the porcine knee: Implications for matrix-assisted chondrocyte implantation and second-generation autologous chondrocyte implantation. Arthroscopy. 2016;32:820–827. doi: 10.1016/j.arthro.2015.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cartilage repair in a knee using Wharton's jelly–derived mesenchymal stem cells (WJ-MSCs) embedded onto type I/III collagen scaffolding and implanted in a minimally invasive fashion using dry arthroscopy. The patient is supine, and an arthroscopic view of the cartilage lesion at the medial trochlea that extends to the medial femoral condyle is visualized from the anterolateral portal, with instrumentation through the anteromedial portal.