Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a common condition that is becoming increasingly prevalent. It affects 13.2% of the population over age 40 in Germany. In 2020, it will be the third most common cause of morbidity and mortality around the world. It markedly impairs the quality of life of those who suffer from it and presents a major economic challenge to the health-care system.
Methods
This review is based on pertinent publications retrieved by a selective literature search and on the authors’ clinical experience.
Results
Pulmonary rehabilitation (PR) for patients with COPD is supported by evidence on the highest level. It is associated with statistically significant (p <0.001) and clinically relevant improvement in physical performance (6-minute walk distance: + 44 m; 95% confidence interval [33; 55]), shortness of breath (Chronic Respiratory Disease Questionnaire: +0.79 points [0.56; 1.03]), and the quality of life (Saint George´s Respiratory Questionnaire: –6.9 points [–9.3; –4.5]). The benefits of PR are especially evident after an acute exacerbation of COPD: it significantly lowers the rate of readmission to the hospital (odds ratio 0.22 [0.08; 0.58], p = 0.002) and improves physical performance ability (6-minute walk distance: + 62 m [38; 86] and the quality of life (Saint George´s Respiratory Questionnaire: –7.8 points [–12.1; –3.5]; p <0.001 for both).
Conclusion
PR is an effective and cost-effective therapeutic intervention that improves physical performance ability, shortness of breath, and the quality of life in patients with COPD, but it has not yet been fully implemented as recommended in the relevant guidelines. There is a need for targeted, problem-oriented referral to a range of PR programs with problem-specific content. The necessary outpatient PR structures still need to be established in Germany.
According to the 2017 report of the Global Initiative for Chronic Obstructive Lung Disease (GOLD), pulmonary rehabilitation (PR) is the most effective therapeutic intervention for reducing dyspnea and improving physical performance and quality of life (1). Furthermore, PR is one of the most cost effective treatments in chronic obstructive pulmonary disease (COPD) (2).
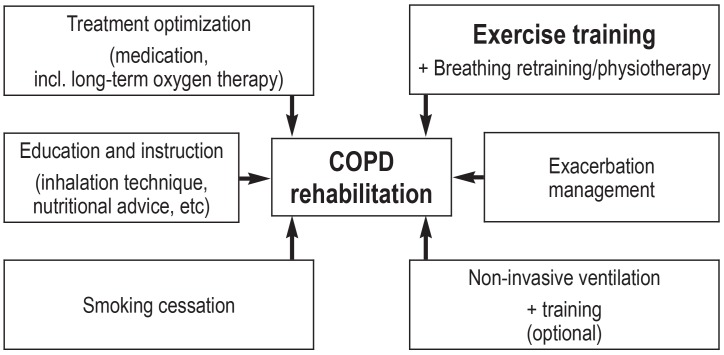
The American Thoracic Society (ATS) and the European Respiratory Society (ERS) define PR as “an evidence-based, multidisciplinary, and comprehensive intervention for patients with chronic respiratory diseases who are symptomatic and often have decreased daily life activities” (3). Furthermore, it is an intervention that is delivered by a multidisciplinary rehabilitation team, in which comprehensive diagnostic evaluation forms the basis of an individual, patient centered therapeutic program. Obligatory components of such a program—which is, however, not restricted to just these— include physical exercise, patient education, and behavioral change, all of which aim to improve the physical and mental state of persons with chronic respiratory diseases and to effect sustained health promoting behaviors (3). In this review article, we present the importance of PR as well as its elements (figure 1) within the German health care system.
Figure 1.
Components of a complex, multimodal rehabilitation program in COPD (chronic obstructive pulmonary disease)
Method
This review article is based on a selective literature search in PubMed—using the search terms “pulmonary rehabilitation”, “chronic obstructive pulmonary disease”, and “COPD”—and on the authors’ own clinical experience.
Results
Evidence in support of PR
The effectiveness of PR is supported by highest-level evidence (table 1) (4, 5). All patients will benefit, independently of the severity of their illness, even though the confirmed evidence is best for patients with moderate to severe COPD (6). The international PR guidelines show that even patients with low-grade flow limitation will benefit if their symptoms are severe. Initiating PR after a hospital stay for an acute exacerbation has been found to be safe and effective. A Cochrane meta-analysis found that PR in this phase of the disease can improve physical performance and quality of life in a particularly effective way. This did not only reduce hospital readmissions (odds ratio 0.22; 95% confidence interval [0.08; 0.58]; P=0.002) but also—significantly—mortality (0.28; [0.10; 0.84.]; P=0.02) (7). Such an effect has so far not been shown for any medication treatment for COPD (8). A subgroup analysis showed that the effects of PR are dependent on the quality of the PR—that is, higher quality rehabilitation will yield better results (9, 10).
Table 1. Evidence for pulmonary rehabilitation in COPD*.
| Effects |
Evidence level |
| Improved quality of life | A |
| Improved general physical performance ability | A |
| Improved strength and leg muscle mass | A |
| Improved strength and arm muscle mass | A |
| Reduction of resting and exertional dyspnea | A |
| Reduction in hospital admissions and days spent in hospital | B |
| Decrease in COPD-associated anxiety and depression symptoms | B |
* After Ries et al. 2007 (4); COPD, chronic obstructive pulmonary disease
Prescribing practice
In Germany, the responsibility for PR prescriptions lies with the statutory health insurers, pension schemes, and employer‘s liability insurance associations. Current therapeutic guidelines regard PR as indicated from stage II or B according to GOLD (1) (disease severity stages I–IV or A–D) (3). In Germany, PR is delivered almost exclusively on an inpatient basis in rehabilitation clinics over 3 weeks. By contrast, data from the international literature relate practically exclusively to outpatient PR or domiciliary rehabilitation.
The components of PR
In order to obtain an objective picture of a patient’s current health status and changes after PR, it is first of all essential that valid testing methods are used (table 2).
Table 2. Selected testing methods in pulmonary rehabilitation.
| Test (result parameter) | |
| Physical performance | ● 6-minute walk distance (meter) ● Shuttle walk tests (meter) ● Incremental cycle test (peak work rate) ● “constant work rate cycling“-test (time) ● Spiroergometry (VO 2max, among others) ● “Sit to stand“ tests (number of repetitions or time) ● Isometric/isokinetic maximum strength measurement (e.g., Newton) |
| Lung function | ● Bodyplethysmography ● Blood gas analysis |
| Dyspnea | ● Borg scale ● Visual analogue scale |
| Quality of life | ● Chronic Respiratory Disease Questionnaire (CRQ) ● St. George‘s Respiratory Questionnaire (SGRQ) ● COPD-Assessment Test (CAT) ● Short-Form 36 Questionnaire (SF-36) ● Hospital Anxiety and Depression Scale (HADS) |
COPD chronic obstructive pulmonary disease
Medical management
The complexity of differentiated PR has increased substantially, and appropriate management by medical professionals is becoming increasingly important. This includes subtle diagnostic evaluation to capture the medical status, performance ability, and relevant comorbidities. Additionally, medication therapy and inhalation techniques need to be checked.
Where indicated, it is crucial that guideline conform long-term oxygen therapy is guaranteed (11). To this end, the required flow rates at rest, at exertion, and at night need to be individually titrated. In very advanced disease, non-invasive ventilation (NIV) can have a role in a differentiated PR program (12). This requires the necessary technical expertise in guiding individualized NIV during the process of PR. In individual cases it is even possible to train patients having concomitant NIV on the exercise bike or treadmill and to help them gain a greater stimulus to exercise in this way.
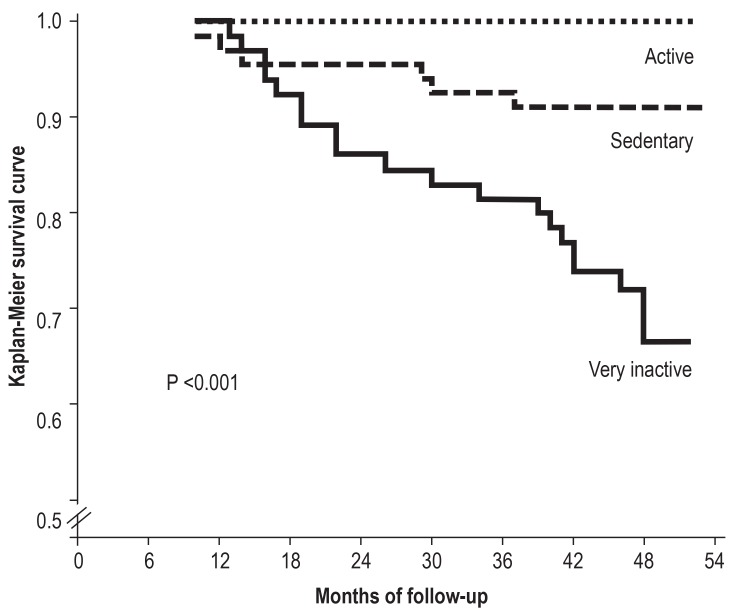
It is important to define realistic objectives for patients. Furthermore, it is of central importance to train and motivate patients to adopt a more active and health oriented lifestyle for the long-term, because the extent of physical activity in daily life is crucial for their prognosis (figure 2) (13). Only by continuing what was learnt in PR will patients achieve the best possible disease course.
Figure 2.
Relevance of an active lifestyle in chronic obstructive pulmonary disease (COPD)—data adjusted for age and sex. (from: Waschki B, et al.: Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140: 331–42. [13]; reproduced with permission from Elsevier)
Exercise therapy
Endurance training
Endurance training is the classic form of training for the upper and lower limbs and as such popular. Most endurance training programs are based on the continuous method, with uninterrupted training for a lengthy period of time and mostly at a constant level of intensity. More recent data from a randomized controlled study have shown that especially in advanced COPD, patients may benefit from interval training (14). Compared with the continuous method, intensive interval training leads to a lesser degree of dynamic pulmonary hyperinflation, and this is one of the reasons why it enables a notably longer tolerated training period and, simultaneously, a lower degree of exertional dyspnea (dyspnea on a 0–10 point Borg scale: interval training 6.2 versus continuous method 7.2; P=0.012) (14). In addition to training on a stationary bicycle, endurance training can also be performed as a walking exercise (on the treadmill or floor). To adjust the intensity, aiming for a subjectively experienced exertion of 4–6 on the modified Borg scale (15) has been found to be viable, effective, and safe.
Strength training
Endurance training in combination with strength training has become an established basic component of training therapy in chronic pulmonary disorders, and a meta-analysis including data from 750 COPD patients showed that it resulted in a significantly greater improvement in quality of life (St George’s Respiratory Questionnaire: –7.44 points; P=0.005, with –4 points being defined as clinically relevant) and strength ability (leg strength +12 kg; P<0.001) compared with endurance training alone (16).
In the context of strength training, the idea is to strengthen major muscle groups, with the emphasis on the lower limb, because this is where disease-related muscular atrophy is usually most pronounced. Targeted strength training can be done by using strength training equipment, free weights, or one’s own body weight—at a level that is tailored to the individual patient. According to evidence-based recommendations, it is of crucial importance for building strength or muscular hypertrophy to achieve momentary muscular failure of the exercised muscles durin a training unit. (17).
Respiratory muscle training
Specific training for the respiratory muscles, using inspiratory muscle training equipment, can increase the capacity of the inspiratory muscles, which in turn means that these will be able better to compensate the acute increase in exertion as a result of physical activity. Pooled data from a meta-analysis of 32 studies showed the following: if inspiratory muscle training is the sole intervention, respiratory muscle strength (PImax: +13 cm H20; P <0.001), physical performance ability (6-minute walk distance +32 meter; P<0.001), and exertional dyspnea (transitional dyspnea index: +20 points; P<0.001) can improve significantly (18). The additive effect of respiratory muscle training alongside multimodal PR including general exercise training is comparatively small.
Neuromuscular electrical stimulation
During electrical muscle stimulation, electrical impulses are being delivered through electrodes affixed to the skin, which trigger intense contraction of the muscles underneath. This training method is used especially in patients who are severely impaired or immobile; according to a recent meta-analysis it can improve the 6-minute walk distance significantly in this subgroup, by +37m (P<0.001) (19).
Whole body vibration training
Vibration training is characterized by external stimulation by means of an oscillating vibration stimulus, usually by standing on a vibration platform. Above a specific vibration frequency, the stretch stimulus triggers a reflex that leads to an involuntary muscle contraction. A recent randomized controlled study found a significant improvement in COPD patients’ ability to balance after vibration training (+36%, effect size 1.04; P<0.001) (20). An earlier randomized controlled study in patients with very severe COPD found in addition that vibration training on top of general training improved physical performance ability in the 6-minute walk test significantly (P<0.05) more, by 27m, than traditional endurance and strength training alone (21).
Patient education
A primary objective of instructing and educating patients during PR is to bring about an increase in self efficiency. Training courses have developed from a merely didactic tool to adaptive and lifelong behavioral changes and changes in attitudes and awareness, including patients’ own self competence (3). Positive examples for changed behaviors include:
Improved adherence to medication
Continuation of the exercise and dietary modifications
Increase in physical activity
Smoking cessation
Use of energy saving strategies during activities of daily life.
For this reason, in addition to merely imparting knowledge and information, the primary objectives are practicing practical, realistic skills (for example, learning the correct inhalation techniques) and developing an active way of coping with illness.
Further essential components of successful instruction and training for patients are the early identification of exacerbations and emergency situations, and the subsequent implementation of individualized emergency plans. All these approaches aim to bring about sustained and continued changes in patients’ attitudes and awareness levels, as well as their behaviors, that then should become part of their daily lives. The greatest possible amount of physical activity is a crucial prerequisite to facilitating the best possible disease course (figure 2).
Elements that should be taught are, for example, included in the established German COBRA training program (COBRA: chronic obstructive bronchitis with or without emphysema), which can be delivered on an outpatient basis but also in the setting of inpatient PR (22). Especially for COPD patients who have not completely given up smoking, smoking cessation is a primary objective as a central component of PR (1). Inpatient PR, which is completely detached from a patient’s everyday life, may possibly provide a particularly beneficial setting for this purpose. (23).
Respiratory physiotherapy
Compared with the confirmed evidence for training therapy, the data for respiratory physiotherapy are rather less robust. Still, the recommendation is to acquire breathing strategies that may provide immediate relief in acute dyspnea (4). Most patients develop increasing dynamic pulmonary hyperinflation while conducting activities of daily life even in the early stages of COPD; this is accompanied by exertional dyspnea (24). For this reason, it makes sense to to teach positive expiratory pressure breathing techniques to prolong the expiratory interval and trigger pulmonary “de-flation.” Exertional dyspnea can be improved by using the pursed lips breathing technique (25).
Coughing and impaired elimination of mucus are common characteristics of COPD. In respiratory tract instability, which is common, it is often difficult to improve mucus expectoration—which is mostly experienced as a torment. Special coughing techniques (huffing, autogenic drainage), combined with oscillating expiratory breathing devices, may be effective in this setting (25).
Extrapulmonary manifestations of COPD/comorbidities
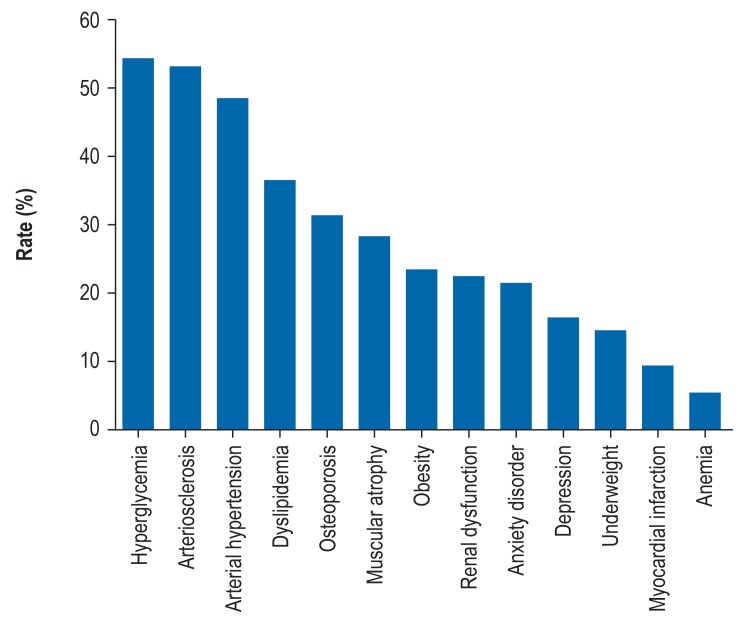
In COPD, comorbidities that require treatment are very often found in the context of PR (Figure 3,[e1]). In addition to the symptoms directly associated with COPD, they affect a patient’s entire health status in crucial ways. Although numerically they may not be in first place, for PR in COPD, psychological comorbidities in the form of depression, anxiety, and panic play the most important part.
Figure 3.
Rates of objectively measured comorbidities in chronic obstructive pulmonary disease (COPD) (after Vanfleteren et al. [e1])
At the start of PR, for example, prevalence rates of anxiety and depression of 32% and 27% have been reported (26). The rates are even higher in advanced stages of the disease or in patients using long-term oxygen therapy (47–66%) (27). Depression in this context does not necessarily mean major depression but, rather, depressive symptoms of resignation. The physical performance ability of such patients is often reduced because of fear avoidance behavior (28). PR alone, without involving a special psychotherapeutic intervention, can reduce anxiety symptoms by –14% and symptoms of depression by –41% (29).
Since fear and panic have an adverse effect on breathing patterns and often contribute to pulmonary hyperinflation, specialized breathing training and acquisition of suitable coping strategies are among the components of PR (3). There is some evidence that supervised physical training of affected patients in combination with training in coping with stress improves their strategies for dealing with COPD (30).
Nutritional counseling
In patients with COPD, a loss in fat-free body mass has been found to be a poor prognostic parameter (31). The loss of fat free mass, which can be evaluated by using bioelectrical impedance analysis, is accompanied by muscle weakness and reduced quality of life. Targeted calorific food supplementation can have a beneficial effect on fat free mass, especially in malnourished patients, and can furthermore improve physical performance ability, quality of life, and even lung function (32).
In patients who cannot eat large main meals because of dyspnea, more frequent, small meals are recommended. Adding vitamins or minerals is not considered necessary if the patient’s diet is balanced (33). Individual nutritional therapy is an effective and underrated intervention in dealing with COPD, especially in malnourished patients and when combined with physical training.
Social care needs assessment, careers advice/social services counseling
Another purpose of PR is a social care needs assessment and social services counseling or careers advice. These include in particular the individually tailored initiation of services to enable the patient to participate in working life, such as redeployments within a company or re-training. For older patients in particular, what is crucial is general counseling about available social care services. These include, for instance, possible home care or services for severe disability, the provision of therapeutic appliances/assistive technologies, and advice on social services and institutions of social care. For patients having long-term oxygen therapy, applying for the passport for severely disabled persons for use on local public transport (for example, so they can access parking spaces for disabled people) can provide practical relief and relieve the financial burden.
Discussion
Successful rehabilitation need to be sustained and supported by a transfer of more physical activity and self efficiency into patients’ everyday lives (according to the motto: “Use it or lose it”). Unfortunately, improvements in muscle function and physical performance ability achieved during PR do not automatically trigger an increase in activity behaviors in patients’ everyday lives (34). This underlines once more the importance of patients’ own motivation. Since special motivational programs to increase activity in the long-term and thereby to embed permanently the beneficial effects achieved during PR have delivered contradictory results to date (35), further research is needed. It is probably important for everyone participating in the treatment process to continue to encourage patients repeatedly to take up physical activity.
Furthermore, activities should be identified that patients like doing. After a Nordic Walking program, for example, the improved physical activity level on COPD patients was still measurable 6 months after the end of the study (36), since most of the patients continued with the training in their everyday lives. PR is therefore a good opportunity for patients to find a repertoire of activity options that is suitable for them.
A possible effect of new technologies—for example, activity monitors in the shape of fitness wristbands, apps, or telemonitoring—on physical activities in pulmonary patients remains to be seen. Such devices can feed back on physical activity objectively and can provide a motivational aid for COPD patients through direct feedback.
Furthermore, it is desirable for outpatient and inpatient PR programs with long-term measures—for example, ambulatory exercise training programs for patients with chronic lung diseases (www.lungensport.org) (37)—to become part of a network.
Existing data from the literature provide evidence for multimodal and multidisciplinary PR that is beyond doubt. In 2016, Deutsches Ärzteblatt published “Choosing Wisely” recommendations for pneumology. The positive recommendations included among others that patients who had been admitted to the hospital for an acute exacerbation should subsequently be referred to PR (38). This recommendation cannot be implemented completely at the present time because of a lack of capacity. The aim should be to identify patients with frequent exacerbations and let them participate in multimodal PR, as this can improve their prognosis.
The report of the German Institute of Medical Documentation and Information (Deutsches Institut für Medizinische Dokumentation und Information, DIMDI) (39) on outpatient PR has been available since 2010 and was rated positively by the Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, IQWiG). This report should prompt a nationwide expansion of specialized outpatient PR services, which are currently not available in Germany. In order to ensure that, once these PR services have been established, they really do deliver the positive effects known from the literature, a quality-based and result oriented measuring system should be set up, as has become established with success in the Netherlands (40). Only then can multimodal, multidisciplinary PR become established as a crucial component in the overall treatment process for COPD.
Key messages.
Pulmonary rehabilitation (PR) improves—among others—physical performance, quality of life, and dyspnea in patients with chronic obstructive pulmonary disease (COPD).
The effectiveness of PR in COPD is backed by highest-level evidence (level of evidence A).
COPD patients should be referred to multimodal PR, especially after acute exacerbations requiring hospital admission.
The objectives of PR are to improve patients’ self efficiency and to promote behavioral changes towards a greater amount of physical activity in everyday life
In Germany, PR is almost exclusively provided in inpatient settings—outpatient structures are lacking.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
Dr Rainer Gloeckl has received conference delegate fees and travel expenses as well as lecture honoraria from Boehringer Ingelheim and Roche. He has received study funding (third party funding) from AstraZeneca, Böhringer Ingelheim, CSL Behring, Resmed, Novartis, Linde, Heinen & Löwenstein, and Weinmann.
Tessa Schneeberger, MSc PT, has received conference delegate fees and travel expenses from Resmed. She has received study funding (third party funding) from AstraZeneca, Böhringer Ingelheim, CSL Behring, Resmed, Novartis, Linde, Heinen & Löwenstein, and Weinmann.
Inga Jarosch, sports scientist, has study funding (third party funding) from AstraZeneca, Böhringer Ingelheim, CSL Behring, Resmed, Novartis, Linde, Heinen & Löwenstein, and Weinmann.
Prof. Klaus Kenn has received consultancy fees from CSL-Behring, GSK, and AstraZeneca. He has received conference delegate fees and travel expenses as well as lecturer honoraria from Böhringer Ingelheim, Grifols und Resmed. For (co)authorship in the context of a publication that touches on the subject of the article he has received honoraria from AstraZeneca, Berlin-Chemie und Böhringer Ingelheim. He has received study funding (third party funding) from AstraZeneca, Böhringer Ingelheim, CSL Behring, Resmed, Novartis, Linde, Heinen & Löwenstein, and Weinmann.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report. Global strategy of the diagnosis, management, and prevention of chronic obstructive pulmonary disease. www.goldcopd.org (last accessed on 15 January 2018) 2017 [Google Scholar]
- 2.Rochester CL, Vogiatzis I, Holland AE, et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192:1373–1386. doi: 10.1164/rccm.201510-1966ST. [DOI] [PubMed] [Google Scholar]
- 3.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 4.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD003793.pub3. CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenn K, Gloeckl R, Soennichsen A, et al. Predictors of success for pulmonary rehabilitation in patients awaiting lung transplantation. Transplantation. 2015;99:1072–1077. doi: 10.1097/TP.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 7.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005305.pub3. CD005305. [DOI] [PubMed] [Google Scholar]
- 8.Gillissen A, Haidl P, Kohlhäufl M, Kroegel K, Voshaar T, Gessner C. The pharmacological treatment of chronic obstructive pulmonary disease. Dtsch Arztebl Int. 2016;113:311–316. doi: 10.3238/arztebl.2016.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12 doi: 10.1002/14651858.CD005305.pub4. CD005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puhan MA. Managing an acute exacerbation of COPD: is pulmonary rehabilitation the solution? Abstract. Denver, Colorado (USA) Annual Meeting of the American Thoracic Society. 2015 [Google Scholar]
- 11.Magnussen H, Kirsten AM, Kohler D, Morr H, Sitter H, Worth H. Guidelines for long-term oxygen therapy German Society for Pneumology and Respiratory Medicine. Pneumologie. 2008;62:748–756. doi: 10.1055/s-2008-1038290. [DOI] [PubMed] [Google Scholar]
- 12.Koehnlein T, Schonheit-Kenn U, Winterkamp S, Welte T, Kenn K. Noninvasive ventilation in pulmonary rehabilitation of COPD patients. Respir Med. 2009;103:1329–1336. doi: 10.1016/j.rmed.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 14.Gloeckl R, Halle M, Kenn K. Interval versus continuous training in lung transplant candidates: arandomized trial. J Heart Lung Transplant. 2012;31:934–941. doi: 10.1016/j.healun.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Gloeckl R, Marinov B, Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respir Rev. 2013;22:178–186. doi: 10.1183/09059180.00000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao WH, Chen JW, Chen X, et al. Impact of resistance training in subjects with COPD: a systematic review and meta-analysis. Respir Care. 2015;60:1130–1145. doi: 10.4187/respcare.03598. [DOI] [PubMed] [Google Scholar]
- 17.Fisher J, Steele J, Bruce-Low S, Smith D. Evidence-based resistance training recommendations. Medicina Sportiva. 2011;15:147–162. [Google Scholar]
- 18.Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 19.Chen RC, Li XY, Guan LL, et al. Effectiveness of neuromuscular electrical stimulation for the rehabilitation of moderate-to-severe COPD: a meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:2965–2975. doi: 10.2147/COPD.S120555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gloeckl R, Jarosch I, Bengsch U, et al. What‘s the secret behind the benefits of whole-body vibration training in patients with COPD? A randomized, controlled trial. Respir Med. 2017;126:17–24. doi: 10.1016/j.rmed.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Gloeckl R, Heinzelmann I, Baeuerle S, et al. Effects of whole body vibration in patients with chronic obstructive pulmonary disease–a randomized controlled trial. Respir Med. 2012;106:75–83. doi: 10.1016/j.rmed.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Dhein Y, Munks-Lederer C, Worth H. Evaluation eines ambulanten strukturierten Schulungsprogramms fur Patienten mit COPD - eine Pilotstudie. Pneumologie. 2003;57:591–597. doi: 10.1055/s-2003-43019. [DOI] [PubMed] [Google Scholar]
- 23.Paone G, Serpilli M, Girardi E, et al. The combination of a smoking cessation programme with rehabilitation increases stop-smoking rate. J Rehabil Med. 2008;40:672–677. doi: 10.2340/16501977-0234. [DOI] [PubMed] [Google Scholar]
- 24.van Helvoort HA, Willems LM, Dekhuijzen PR, van Hees HW, Heijdra YF. Respiratory constraints during activities in daily life and the impact on health status in patients with early-stage COPD: a cross-sectional study. NPJ Prim Care Respir Med. 2016;26 doi: 10.1038/npjpcrm.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bott J, Blumenthal S, Buxton M, et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax. 2009;64(1):i1–i51. doi: 10.1136/thx.2008.110726. [DOI] [PubMed] [Google Scholar]
- 26.Janssen DJ, Spruit MA, Leue C, et al. Symptoms of anxiety and depression in COPD patients entering pulmonary rehabilitation. Chron Respir Dis. 2010;7:147–157. doi: 10.1177/1479972310369285. [DOI] [PubMed] [Google Scholar]
- 27.Lacasse Y, Rousseau L, Maltais F. Prevalence of depressive symptoms and depression in patients with severe oxygen-dependent chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2001;21:80–86. doi: 10.1097/00008483-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Keil DC, Stenzel NM, Kuhl K, et al. The impact of chronic obstructive pulmonary disease-related fears on disease-specific disability. Chron Respir Dis. 2014;11:31–40. doi: 10.1177/1479972313516881. [DOI] [PubMed] [Google Scholar]
- 29.Tselebis A, Bratis D, Pachi A, et al. A pulmonary rehabilitation program reduces levels of anxiety and depression in COPD patients. Multidiscip Respir Med. 2013;8 doi: 10.1186/2049-6958-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison SL, Greening NJ, Williams JE, Morgan MD, Steiner MC, Singh SJ. Have we underestimated the efficacy of pulmonary rehabilitation in improving mood? Respir Med. 2012;106:838–844. doi: 10.1016/j.rmed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD000998.pub3. 12CD000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abholz H, Gillissen A, Magnussen H, et al. Nationale Versorgungsleitlinie COPD Version 19. www.leitlinien.de/nvl/copd last accessed on 3 May 2017 [Google Scholar]
- 34.Cindy Ng LW, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis. 2012;9:17–26. doi: 10.1177/1479972311430335. [DOI] [PubMed] [Google Scholar]
- 35.Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2016;11:3121–3136. doi: 10.2147/COPD.S121263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breyer MK, Breyer-Kohansal R, Funk GC, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res. 2010;11 doi: 10.1186/1465-9921-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glöckl R, Göhl O, Spielmanns M, et al. Stellenwert ambulanter, gerätegestützter Trainingstherapie bei Atemwegs- und Lungenkrankheiten. Pneumologie. 2016;70:446–453. doi: 10.1055/s-0042-105898. [DOI] [PubMed] [Google Scholar]
- 38.Jany B. Klug entscheiden in der Pneumologie. Dtsch Arztebl. 2016;113 A-930. [Google Scholar]
- 39.Korczak D, Huber B, Steinhauser G, Dietl M. Health Technology Assessment. Köln: 2010. Versorgungssituation und Wirksamkeit der ambulanten im Vergleich mit der stationären pneumologischen Rehabilitation DIMDI Schriftenreihe. [Google Scholar]
- 40.Spruit MA, Augustin IM, Vanfleteren LE, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J. 2015;46:1625–1635. doi: 10.1183/13993003.00350-2015. [DOI] [PubMed] [Google Scholar]
- E1.Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;1877:28–35. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]