Abstract
The development of immune system modulating agents, such as immune checkpoint inhibitors (ICIs), has revolutionized cancer treatment. Nivolumab, a human monoclonal antibody against PD-1, has emerged as an efficient treatment for various malignancies, including non-small cell lung cancer (NSCLC); however, it is associated with important immune related side-effects, attributed to organ-specific inflammation, such as immune-mediated pneumonitis, a relatively uncommon, albeit potentially fatal adverse event. We herein present the unique case of severe interstitial pneumonitis with concomitant detection of Human Herpes Virus 6 (HHV-6) in a nivolumab treated patient with NSCLC. Potential pathogenetic mechanisms are discussed.
Keywords: Human Herpesvirus 6, Nivolumab, Immunotherapy, Lung cancer
Introduction
Immune checkpoint inhibitors (ICIs) have recently dramatically changed cancer treatment. Among them, nivolumab, a human IgG4 monoclonal antibody against PD-1, is approved for treatment of advanced melanoma, non-small cell lung carcimoma (NSCLC), renal cell carcinoma and classical Hodgkin’s lymphoma [1]; it is nevertheless associated with a wide range of side effects termed immune-related adverse events (irAEs), among which pneumonitis may occur in 2–5% of patients under nivolumab [2]. The underlying pathogenetic mechanisms have not yet been fully elucidated, although it is postulated that dysregulated effector T cells accumulate in lung interstitium, leading to increased inflammatory response [3]. We herein report the unusual case of a severe interstitial pneumonitis with concomitant detection of Human Herpes Virus 6 (HHV-6) in a patient with NSCLC being treated with nivolumab and discuss potential mechanisms and clinical implications.
Presentation of case
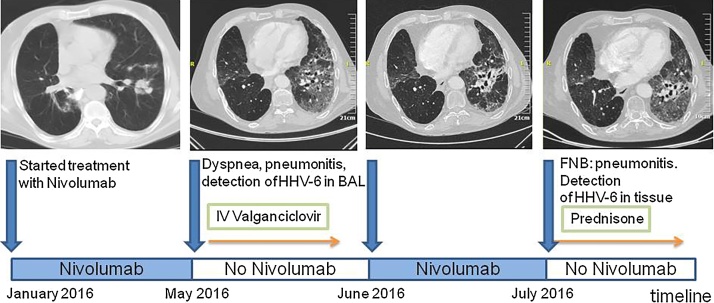
A 58-year-old male was first seen in March 2009, following right lower lobectomy for a stage pT3N2M0 (stage IIIA) bronchogenic squamous cell carcinoma. Following various chemotherapeutic schemes and palliative radiotherapy, progressive disease persisted until February 2016(Fig. 1), when he was started on nivolumab at 3 mg/kg every 2 weeks. He was admitted in May 2016, due to emerging dyspnea on exercise; chest CT angiography excluded pulmonary embolism and was suggestive of pneumonitis (infectious or otherwise). Nivolumab was discontinued and he was started on intravenous broad-spectrum antimicrobials and trimethoprim/sulfamethoxazole. PCR was performed in bronchoalveolar lavage (BAL) fluid by means of two commercial real-time PCR kits (Pneumocystis jirovecii Real-TM and CMV/EBV/HHV6 Quant Real-TM, Sacace, Italy) on DNA extracted using the QiAmp DNA mini kit: it was negative for Pneumocystis jiroveci, cytomegalovirus (CMV) and Epstein-Barr virus (EBV) but positive for HHV-6, whereas PCR for HHV-6 DNA was negative in a blood specimen. Trimethoprim/sulfamethoxazole was discontinued and he was started on oral valganciclovir 900 mg bid based on previously published data [4]. Clinical and radiological improvement was seen 4 days later, whereby he was discharged with instructions for a 2 week course of valganciclovir.
Fig. 1.
Nivolumab treatment timeline.
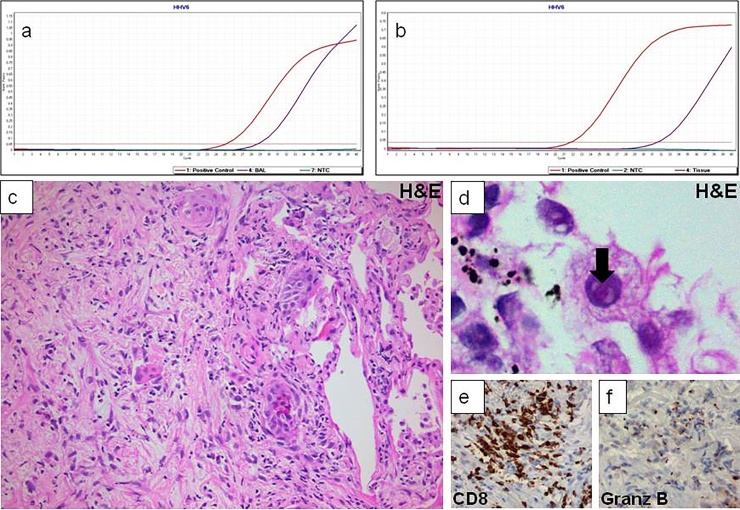
Nivolumab treatment was reinstituted in June 2016, together with valganciclovir prophylaxis once a day. Three weeks later, the patient was readmitted due to worsening dyspnea, with bilateral lung infiltrates on chest CT (Fig. 1); he was immediately started on intravenous prednisolone at a dose of 3 mg/kg/day upon the assumption of pneumonitis. A CT-guided lung fine needle biopsy (FNB), performed five days later, disclosed pulmonary fibrosis with focal lymphoplasmacytic chronic inflammation, suggestive of nivolumab-related pneumonitis (Fig. 2); moreover, a few cells with enlarged nuclei were seen, one containing an intranuclear eosinophilic inclusion. The aforementioned PCR assay was performed on DNA extracted from the tissue sample and was again positive for HHV-6. Furthermore, immunostaining disclosed many CD8+/Granzyme B+ cytotoxic T cells.
Fig. 2.
Pulmonary fibrosis with focal lymphoplasmacytic chronic inflammation, suggestive of nivolumab-related pneumonitis.
Because of gradual improvement, tapering of steroids was initiated, whereas nivolumab was permanently discontinued. Six months later, cutaneous metastases of the pulmonary carcinoma developed; despite re-introduction of chemotherapy in combination with valganciclovir prophylaxis, there was no clinical response and the patient died within one month. Autopsy permission was not granted.
Discussion
Infectious complications have been previously reported in patients on immune checkpoint inhibitor treatment. We herewith report the first (to our best knowledge) case of severe interstitial pneumonitis with concomitant detection of HHV-6 in a patient under nivolumab. Although HHV-6 has been detected in the lung of healthy individuals, detection of viral DNA both in BAL and tissue specimen supports viral pneumonitis rather than simple pulmonary viral shedding [5]; an assumption further corroborated by identification of cells with enlarged nuclei (probably residual alveolar epithelium), one of them with an intranuclear inclusion (Fig. 2d), a feature previously described in HHV-6-related infections [6]. On the other hand, we should bear in mind that because of the high prevalence of the primary HHV-6 infection in hospitalized patients with various debilitating conditions [7], HHV-6 could represent an innocent bystander rather than a cause of pneumonitis. Furthermore, in such cases the physician needs to exclude the possibility of chromosomal integration usually suspected when high levels of HHV-6 DNA are detected by PCR performed on whole blood or serum [8]. In contrast, cases with latent but not integrated HHV-6, viral DNA is detected at much lower levels [9]. Fluorescent in situ hybridization has been used to exclude chromosomal integration of HHV-6 [10].
Recent data have shown that immune checkpoint inhibitors may reverse the functional exhaustion status of virus-specific T cells and mount appropriate T cell responses and virus clearance [11]. In AIDS or other immunosuppressive conditions, recovery of the immune system is known to induce tissue damage due to inappropriate inflammatory response against an infectious agent, the immune reconstitution inflammatory syndrome (IRIS) [12]. However, reports from HHV-6 reactivation in bone marrow transplant recipients do not support a role for immune reconstitution in relation to long term viral reactivation [13]. On the other hand, lung tumors are known to repress both innate and adaptive immune system modulation, including overexpression of membrane complement regulators, such as CD46, a well known cellular receptor for HHV-6 [14]. A complex interplay between drug-specific immune response, herpes viruses reactivation (including HHV6) and antiviral immune response has been proposed to underlie drug-induced hypersensitivity syndromes; this could also hold true in our case, the order of events being compatible with such an interplay between immune re-activation boosted by anti-PD1 treatment and a subclinical HHV-6 infection facilitated by tumor micro-environment. Moreover, HHV6 infection itself could have led to further dysregulation of the immune system due to superantigen expression [15] or by IL-15 activation-dependent enhancement of natural killer (NK) cell activity [16]; this last point could underlie the large number of CD8+/Granzyme B+ cytotoxic T cells detected in our case. Clinical improvement following ganciclovir and prednisone treatment further supports a virus-related inflammatory pathology during nivolumab treatment.
Conclusion
Assessment of the potential role of HHV-6 in nivolumab-related pneumonitis and of the effectiveness of the boosted immune system by means of longitudinal studies could provide the basis for preventive anti-virus treatment, to further reduce pulmonary side effects of PD1-PD-L1 axis blockade. On the other hand, failure of the immune system to mount an effective anti-viral response could be used as a surrogate marker for a defective anti-tumor immunity in the lung microenvironment.
Conflict of interest
None.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Martin-Liberal J., Ochoa de Olza M., Hierro C., Gros A., Rodon J., Tabernero J. The expanding role of immunotherapy. Cancer Treat Rev. 2017;54:74–86. doi: 10.1016/j.ctrv.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Leventakos K., Mansfield A.S. Advances in the treatment of non-small cell lung cancer: focus on nivolumab, pembrolizumab, and atezolizumab. BioDrugs. 2016;30:397–405. doi: 10.1007/s40259-016-0187-0. [DOI] [PubMed] [Google Scholar]
- 3.Tabchi S., Messier C., Blais N. Immune-mediated respiratory adverse events of checkpoint inhibitors. Curr Opin Oncol. 2016;28:269–277. doi: 10.1097/CCO.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 4.Isaacson E., Glaser C.A., Forghani B., Amad Z., Wallace M., Armstrong R.W. Evidence of human herpesvirus 6 infection in 4 immunocompetent patients with encephalitis. Clin Infect Dis. 2005;40:890–893. doi: 10.1086/427944. [DOI] [PubMed] [Google Scholar]
- 5.Cone R.W. Human herpesvirus 6 as a possible cause of pneumonia. Semin Respir Infect. 1995;10:254–258. [PubMed] [Google Scholar]
- 6.Maric I., Bryant R., Abu-Asab M., Cohen J.I., Vivero A., Jaffe E.S. Human herpesvirus-6-associated acute lymphadenitis in immunocompetent adults. Mod Pathol. 2004;17:1427–1433. doi: 10.1038/modpathol.3800179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D.Y., Boitnott J.B., Schwarz K.B. Human herpes virus-6 (HHV-6): a cause for fulminant hepatic failure or an innocent bystander? J Pediatr Gastroenterol Nutr. 2003;37:95. doi: 10.1097/00005176-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Ward K.N., Leong H.N., Nacheva E.P., Howard J., Atkinson C.E., Davies N.W. Human herpesvirus 6 chromosomal integration in immunocompetent patients results in high levels of viral DNA in blood, sera, and hair follicles. J Clin Microbiol. 2006;44:1571–1574. doi: 10.1128/JCM.44.4.1571-1574.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark D.A., Ait-Khaled M., Wheeler A.C., Kidd I.M., McLaughlin J.E., Johnson M.A. Quantification of human herpesvirus 6 in immunocompetent persons and post-mortem tissues from AIDS patients by PCR. J Gen Virol. 1996;77(Pt. 9):2271–2275. doi: 10.1099/0022-1317-77-9-2271. [DOI] [PubMed] [Google Scholar]
- 10.Clark D.A., Nacheva E.P., Leong H.N., Brazma D., Li Y.T., Tsao E.H. Transmission of integrated human herpesvirus 6 through stem cell transplantation: implications for laboratory diagnosis. J Infect Dis. 2006;193:912–916. doi: 10.1086/500838. [DOI] [PubMed] [Google Scholar]
- 11.Rutigliano J.A., Sharma S., Morris M.Y., Oguin T.H., 3rd, McClaren J.L. Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells. J Virol. 2014;88:1636–1651. doi: 10.1128/JVI.02851-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopal R., Rapaka R.R., Kolls J.K. Immune reconstitution inflammatory syndrome associated with pulmonary pathogens. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0042-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greco R., Crucitti L., Noviello M., Racca S., Mannina D., Forcina A. Human herpesvirus 6 infection following haploidentical transplantation: immune recovery and outcome. Biol Blood Marrow Transplant. 2016;22:2250–2255. doi: 10.1016/j.bbmt.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Santoro F., Kennedy P.E., Locatelli G., Malnati M.S., Berger E.A., Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 15.Tai A.K., Luka J., Ablashi D., Huber B.T. HHV-6A infection induces expression of HERV-K18-encoded superantigen. J Clin Virol. 2009;46:47–48. doi: 10.1016/j.jcv.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Flamand L., Stefanescu I., Menezes J. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J Clin Invest. 1996;97:1373–1381. doi: 10.1172/JCI118557. [DOI] [PMC free article] [PubMed] [Google Scholar]