Abstract
Sponges (Porifera) are recognized as aquatic multicellular organisms which developed an effective biochemical pathway over millions of years of evolution to produce both biologically active secondary metabolites and biopolymer-based skeletal structures. Among marine demosponges, only representatives of the Verongiida order are known to synthetize biologically active substances as well as skeletons made of structural polysaccharide chitin. The unique three-dimensional (3D) architecture of such chitinous skeletons opens the widow for their recent applications as adsorbents, as well as scaffolds for tissue engineering and biomimetics. This study has the ambitious goal of monitoring other orders beyond Verongiida demosponges and finding alternative sources of naturally prestructured chitinous scaffolds; especially in those demosponge species which can be cultivated at large scales using marine farming conditions. Special attention has been paid to the demosponge Mycale euplectellioides (Heteroscleromorpha: Poecilosclerida: Mycalidae) collected in the Red Sea. For the first time, we present here a detailed study of the isolation of chitin from the skeleton of this sponge, as well as its identification using diverse bioanalytical tools. Calcofluor white staining, Fourier-transform Infrared Spcetcroscopy (FTIR), electrospray ionization mass spectrometry (ESI-MS), scanning electron microscopy (SEM), and fluorescence microscopy, as well as a chitinase digestion assay were applied in order to confirm with strong evidence the finding of a-chitin in the skeleton of M. euplectellioides. We suggest that the discovery of chitin within representatives of the Mycale genus is a promising step in their evaluation of these globally distributed sponges as new renewable sources for both biologically active metabolites and chitin, which are of prospective use for pharmacology and biomaterials oriented biomedicine, respectively.
Keywords: Porifera, Demosponges, Mycale, chitin, sponge skeleton
1. Introduction
The structural polysaccharide chitin exists as a dominant component in the skeletal structures of diverse fungi [1,2,3], diatoms [4], sponges [5,6,7,8,9], corals [10], mollusks [11,12], annelids [13] and arthropods (see for review [14]). This very ancient biopolymer generally occurs in association with different kinds of organic biomacromolecules (pigments, lipids, other polysaccharides and proteins), as well as with calcium- and silica-based biominerals [15]. Interaction between chitin and the other organic and inorganic components listed above provides rigidification to a broad variety of invertebrate skeletal constructs. Mechanical stiffness of skeletons are also of crucial importance in sponges (Porifera), where chitin was recently reported in both marine (see for review [16,17]) and fresh water [18,19] species representing the class Demospongiae. In some sponges, chitin has been suggested as a template for formation of biomineralized structures in form of aragonite-silica-chitin [20], or silica-chitin [6,21], composites.
Analysis of the structural and physicochemical properties of this amino polysaccharide can be performed using a range of modern instrumentation [22]; and several review articles covering this biopolymer have been published recently [23,24,25]. There are no doubts that chitin (even without its derivative chitosan) is still of interest for applications as an adsorbent [23,26] and biomaterial for biomedical aims; for example, reconstruction of peripheral nerves or wound management [24,25,27]. It is worth noting here that thus far, only chitin of fungal and arthropod origins can be isolated on industrial scales. However, only sponges produce tube-like, fibrous three-dimensional (3D) chitinous scaffolds which are originally macroporous due to their basic role in the skeletons of these filter-feeder organisms. Chitin of demosponge origin in particular resembles the shape of the living sponges [9]. This phenomenon was also observed in the Cambrian fossil demosponges, Vauxia gracilenta [28]. The mechanical and thermal stability of chitinous scaffolds are key to their successful applications in modern biomimetics [29,30,31,32,33,34] and tissue engineering [8,35,36]. However, these achievements have been based exclusively on chitin isolated from diverse representatives of only one order of marine demosponges, the order Verongiida. We even suggested that the presence of chitin within skeletons of demosponges is unique for the order Verongiida only, and, consequently, the proposal to use this feature as diagnostic tool for systematics of all sponges related to the order Verongiida arose. Our single doubt was based on the finding of chitin in fresh water non-verongiid demosponges [18,19] (Spongillida) and, definitively, derived from marine sponges in ancient times.
Consequently, two years ago we started an intensive study of diverse non-verongiid marine demosponges with the aim to purify and identify chitin from other taxa of marine sponges. Especially, we have taken advantage of the worldwide distribution of the members of the genus Mycale [37] (Demospongiae: Heteroscleromorpha: Poecilosclerida: Mycalidae) living in a considerable depth range from intertidal to abyssal depth [38,39,40,41,42,43,44,45,46,47,48,49,50]. About 251 valid species belong to the genus Mycale are currently accepted from a pool of more than 500 nominal names [49,51,52]. These sponges are known as fast-growing species [53] with partially investigated life cycles [54,55]. Some species of Mycale have been adapted for laboratory cultivation [8,56], as well as marine farming [57]. For example, the development of aquaculture of M. hentscheli over seven years and through three generations of cultivars has been conducted in the New Zealand [58,59]. Furthermore, aquaculture methods based on the sexual reproduction of the demosponge M. phyllophila have been established in the Dongshan Bay (Fujian, China) [60] where the reproductive activity of the sponge lasted for 5–6 months and peaked when the average water temperature was above 25 °C.
Thus, after preliminary experiments with respect to identification of chitin, we focused our attention on the sponge Mycale euplectellioides [61] reported only from the northern part of the Red Sea [62] (Figure 1).
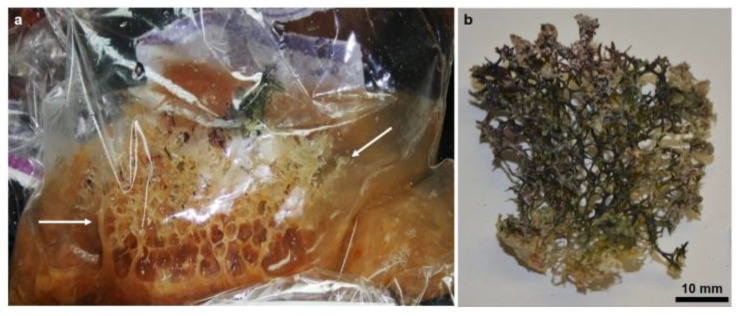
Figure 1.
Specimen of Mycale euplectellioides as collected by scuba diving. After cutting the sponge from the basal part underwater, it starts to lose the outer soft mucous tissue from the hard internal skeleton. As a result, a very mucous and viscous mass appears at the bottom of the collection bag leaving the hard internal skeleton (arrows, a). Finally, only greenish-brown skeletal fragments can be isolated in the laboratory from the collection bags (b).
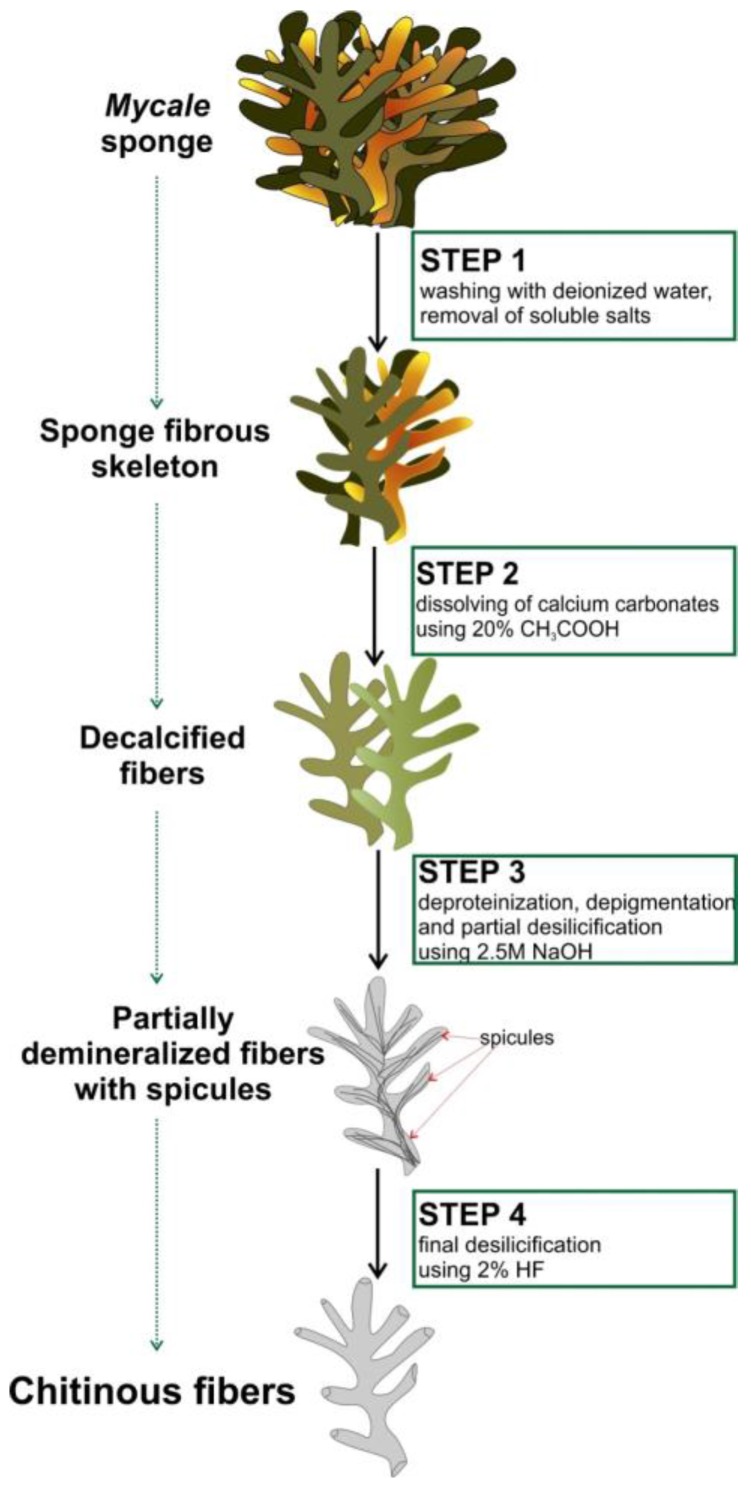
Here, we represent the first study on isolation and purification of chitin from the skeleton of M. euplectellioides demosponge according to the stepwise procedure (Figure 2) and identification of this polysaccharide using corresponding bioanalytical methods.
Figure 2.
Step-by-step isolation scheme of chitinous fibers from the skeleton of the marine demosponge M. euplectellioides.
2. Results
2.1. Morphology and Structural Peculiarities of Organic Scaffold Isolated from M. euplectellioides Skeleton
Chitin, in contrast to diverse pigments, lipids and proteins remain stable after treatment in both alkali-based solution (i.e., 5% NaOH) and hydrofluoric acid (HF) up to concentration of 40% at temperatures between 25 °C and 40 °C. Alternatively to spicule-free chitin-based skeletons of marine demosponges related to the order Verongiida, representatives of the genus Mycale (order Poecilosclerida) are rich on siliceous spicules. They also contain structural scleroprotein spongin which, however, is quickly dissolved in 2.5 M NaOH solutions. Consequently, the step-by-step treatment procedure shown in Figure 2 lead to isolation of protein- and spicules-free scaffold with 3D architecture (Figure 3) remaining to be structurally stable.
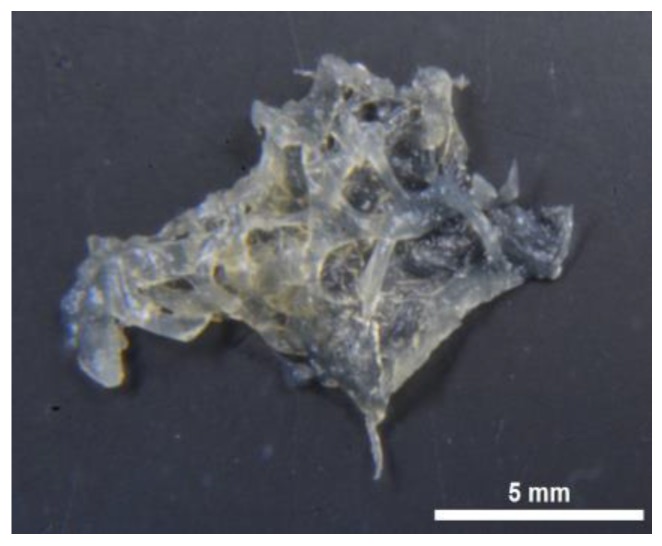
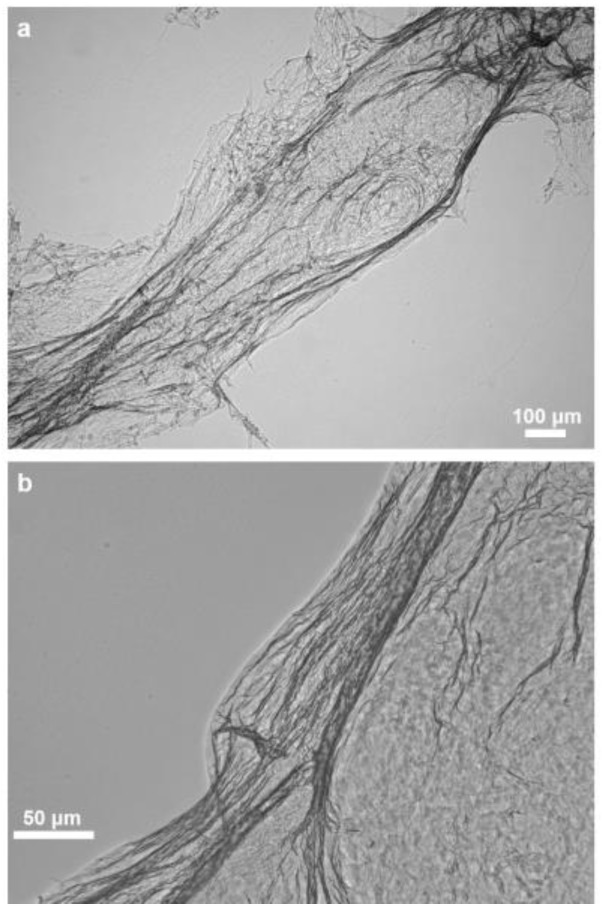
Figure 3.
Spicule-free, colorless 3D scaffold obtained from M. euplectellioides according to the isolation procedure represented in Figure 2. Microstructural features of selected fibers are well visible in Figures 5 and 7b,d,e.
The isolated scaffold proves that the applied chemical treatment steps lead to the isolation of morphologically defined three-dimensional tubular construct composed of microfibers which exhibit a hollow, pipe-like, and translucent structure (Figure 3). The presented image also shows that the overall shape and morphology of the extracted skeletons closely resemble the original shape and morphology of the M. euplectellioides sponge (see Figure 1b). This means the extraction procedure does not lead to a breakdown of the—sometimes very fragile—demosponge structures even after supporting spicules (Figure 4) have been dissolved after treatment of the scaffold with HF-based solution (Figure 5).
Figure 4.
Remaining spicules (arrows in a,b) within partially demineralized skeletal fragments of M. euplectellioides after treatment with acetic acid and alkali. For details see Section 4.
Figure 5.
Skeletal fibers of M. euplectellioides after hydrofluoric acid (HF)-based treatment showing no evidence for the presence of siliceous spicules.
Structural integrity of the isolated scaffold is well visible under light and fluorescence microscopy (Figure 6), as well as using scanning electron microscopy (SEM). However, the microfibers are not so densely packed as in the case of multilayered chitin microfibers observed in diverse verongiid sponges [4,5,7,8,63]. The morphology of M. euplectellioides fibers is similar to that observed in chitinous fibers of the fresh water demosponge Spongilla lacustris [19]. This spicule-producing sponge belongs to the Spongillida and not to the order Verongiida.
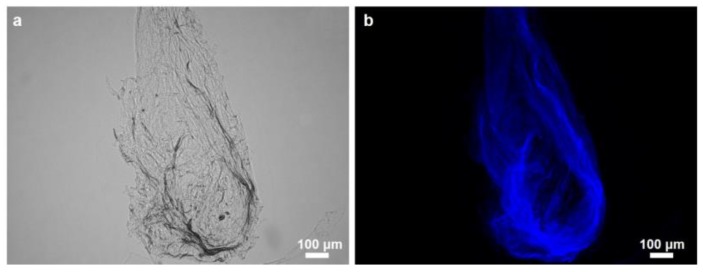
Figure 6.
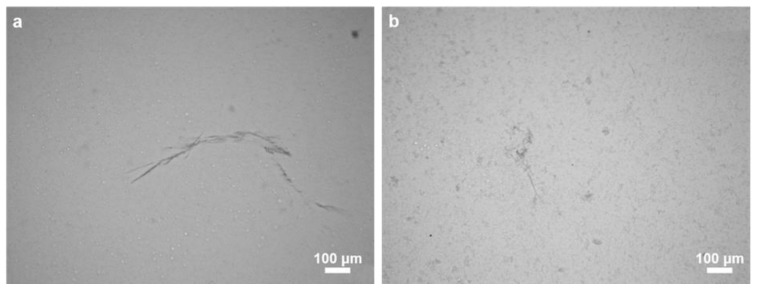
Purified skeletal fibers of M. euplectellioides in light (a) and fluorescence (b) microscopy modus. Very intensive fluorescence (light exposure time1/4800 s) (b) after Calcofluor White (CFW) staining for chitin confirms the chitinous nature of the sponge skeleton.
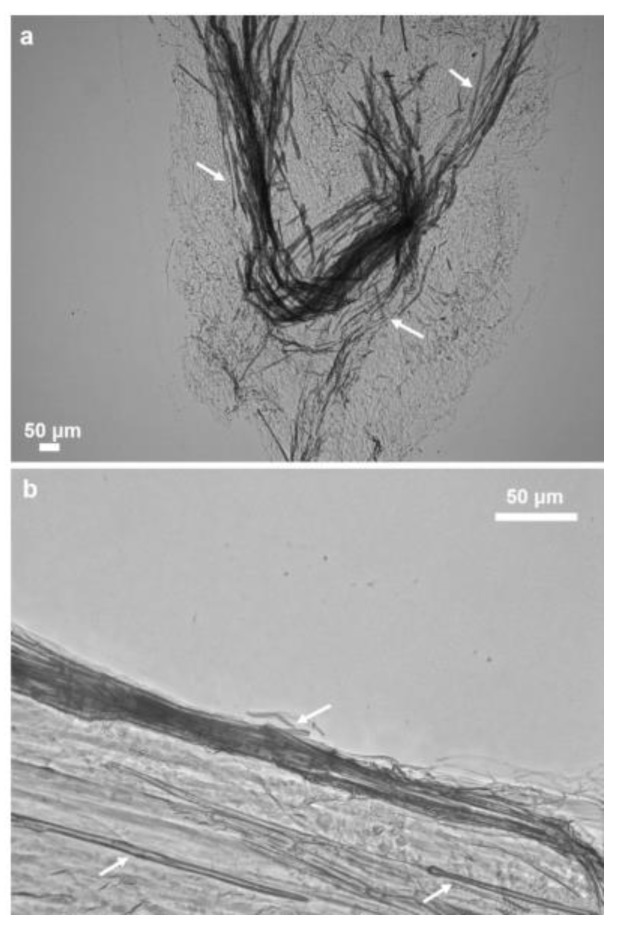
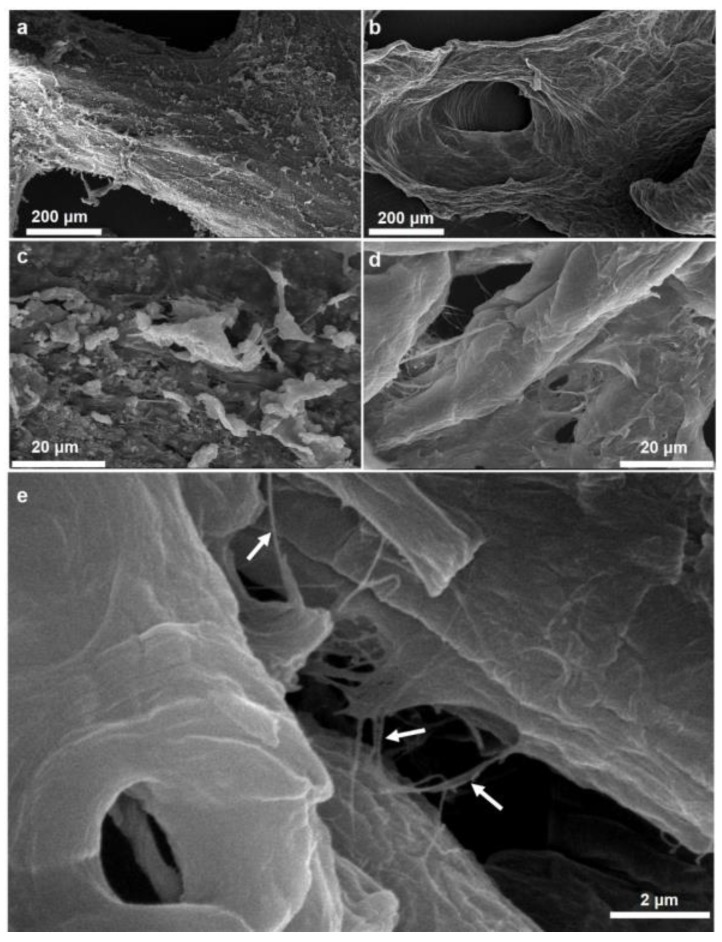
Investigations using SEM confirm with strong evidence the nanofibrillar structure of the isolated scaffold (Figure 7). Additionally, performed energy-dispersive X-ray spectroscopy (EDX) analysis (Supplementary Material) shown that isolated material is free from inorganic impurities (e.g., Ca, Mg, P, Si) and it confirms effectiveness of proposed isolation method.
Figure 7.
Scanning electron microscopy (SEM) imagery of the purified M. euplectellioides skeleton prior (a,c) and after demineralization procedure (b,d,e). The demineralized sample showed the nanofibrillar organization (arrows) of the fibers.
2.2. Identification of Chitin
Traditionally, CFW staining of demineralized skeletons of sponges which are still preserved after alkali-based treatment is the first step in the series of analytical methods used for chitin identification. Fluorescence microscopy analysis of the M. euplectellioides scaffold displays very strong fluorescence even under light exposure time of 1/4800 s (Figure 6). Similar results have been obtained previously for all chitin structures isolated from both demosponges and glass sponges [5,6,7,8,28,64]. To obtain more information about what kind of chitin isomorph is present in the sponge under our study, we carried out spectroscopic investigations using FTIR.
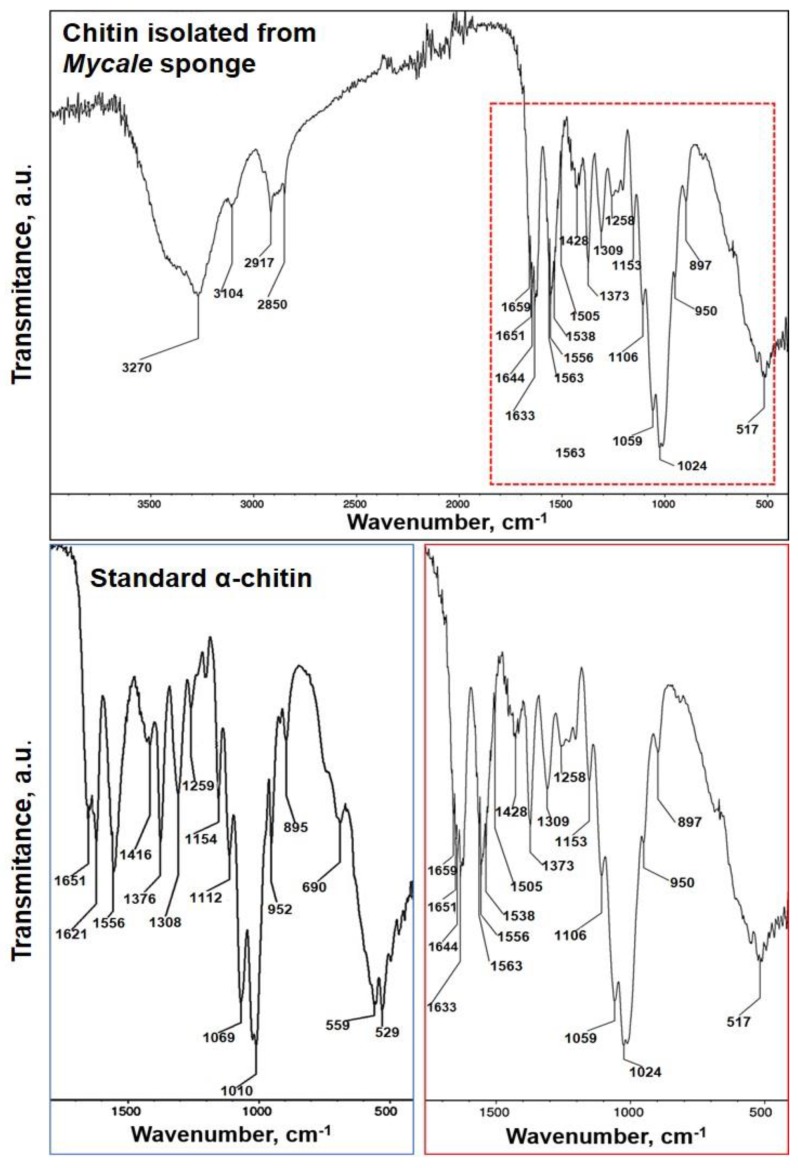
FTIR spectra of the purified chitinous scaffold of M. euplectellioides (Figure 8) were compared with that of α-chitin standard. The region of the amide moiety, between 1700 and 1500 cm−1, yields different signatures for chitin polymorphs [65]. In this region, the spectrum of the matrix isolated from M. euplectellioides shows a strong adsorption band associated with the stretching vibrations of C=O group characteristic for the amide band I. The characteristic for α-chitin stretching vibrations arise from the intermolecular C=O⋯H–N and C=O⋯HO–CH2 hydrogen bonds, which split the amide band I split two peaks at 1659 cm−1 and 1633 cm−1, respectively [66]. Another feature, the characteristic intense band at νmax 950 cm−1 assigned to γCHx is observed in both α-chitin standard and chitin isolated from M. euplectelloides. Additionally, the α-chitin indicative band assigned to a β-glycosidic bond is observed at νmax 897 cm−1 in the spectrum of the scaffold isolated from M. euplectelloides. It is worth to highlight that in registered spectrum of M. euplectelloides, the characteristic bands for CaCO3 (855–876 cm−1) and SiO2 (720 cm−1) were not observed, confirming high purity of isolated chitin.
Figure 8.
FTIR spectra of chitin isolated from M. euplectellioides demosponge in comparison with that of α-chitin standard.
Chitinases possess the ability to degrade chitin directly to low molecular weight chitooligomers including N-acetyl-d-glucosamine (GlcNAc). Consequently, this kind of enzymatic treatment resulted in the loss of chitin integrity and the release of residual chitin microfibers of steadily decreasing size [17]. The activity of chitinase is clearly visible using an optical microscope (Figure 9). This result from the chitinase digestion test confirms the chitinuous nature of the isolated M. euplectellioides scaffolds.
Figure 9.
Chitinase digestion of purified and completely demineralized skeletal fiber isolated from M. euplectellioides. Initial stage (a) and the same fragment after 5 h treatment with chitinase (b).
The Morgan-Elson assay was used as a precise method to quantify the GlcNAc released after chitinase treatment. Determination of GlcNAc in chitin-based scaffolds of M. euplectellioides showed 700 ± 1.5 µg N-acetyl-glucosamine per mg of alkali-resistant skeleton residues of this sponge. This result is similar to that reported for S. lacustris chitin [19]. This corresponds to approximately 70% of chitin in the dry weight of the whole sponge skeleton.
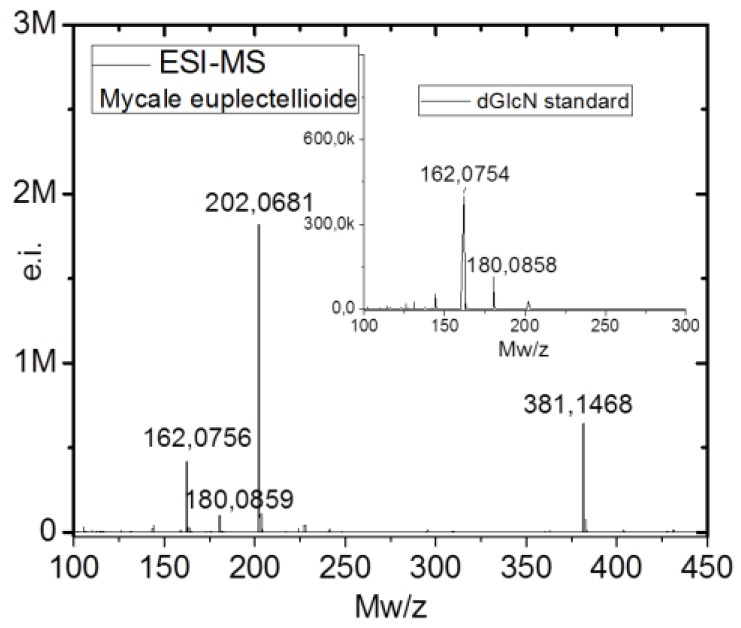
Additionally, electrospray-ionization mass spectroscopy (ESI-MS) measurements were used to identify the presence of chitin. Acetic hydrolysis of chitin resulted in the formation of d-glucosamine (dGlcN), which can be easily identified by the ESI-MS spectroscopy. This method is a standard for chitin identification and was used for chitin visualization in complex organisms [21,64] and even in 505-million-year-old chitin-containing fossil remains [28]. The ESI-MS spectrum of the M. euplectellioides hydrolyzed skeletal scaffold revealed four main ion peaks at m/z 162.08, 180.09, 202.07 and 381,15 (Figure 10). The ion peaks at m/z 162.08, and 180.09 are identical to the peaks in the ESI-MS spectrum of the commercial (dGlcN) standard (Figure 10 insert). The ion peak at m/z 180.9 is equivalent to the [M + H]+ of dGlcN molecule, while the ion peak at 161.85 is equivalent to dGlcN after loss of one molecule of H2O [M − H2O + H]+. The week ion peaks at m/z 202.07 and 381.15 are corresponding to [M + K]+ and [2M + K]+ species, respectively, which represent the K-bound-dGlcN monomer and noncovalent dimer correspondingly.
Figure 10.
Electrospray-ionization mass spectroscopy (ESI-MS) investigation of the chitin isolated from the skeletal scaffold of M. euplectellioides. Insertion is the ESI-MS spectra of commercial (dGlcN) standard for comparison.
3. Discussion
The obtained results showed that in contrast to non-spiculated demosponges of the Verongiida order, the chitin isolation procedure (see Figure 2) is more complex in the case of M. eplectelloides. The morphology of isolated chitinous fibers differs from tube-like, multilayered skeletal architecture known in verongiid sponges [5,7,8]. It is well recognized that secondary metabolites of verongiids as bromotyrosines are inhibitors of microbial chitinases [7,8]. Thus, a biological function of these compounds for survival of verongiid sponges can be suggested. What is the situation with secondary metabolites within the genus Mycale and their relationship with the skeletal chitin?
Sponges of the genus Mycale are, probably, among the richest sources of pharmacologically active compounds isolated from marine organisms [67,68,69]. Such secondary metabolites as pateamines [70], mycalolides [71] as well as mycalamide A and D [72,73] are known to be extremely cytotoxic [74,75,76]. Mycalamide A and B also showed antiviral, antitumor [77,78] and antibacterial [79] features. Dihydroxymycalolide A isolated from M. izuensis was cytotoxic against HeLa cells with an IC50 value of 2.6 ng/mL [80]. Interestingly, the same sponge species are able to synthetize so-called azumamides, which are related to cyclic tetrapeptides with histone deacetylase inhibitory activity [81,82]. Secomycalolide A has been described as a proteasome inhibitor [83]. Peloruside A, from, M. hentscheli, possesses anti-mitotic properties with paclitaxel-like microtubule-stabilizing activity [70,84,85,86], and has shown potent antiproliferative activity in cancer cell lines in addition to its inhibitory effects on tumor growth in mouse models [87,88]. Peloruside B, have been reported as a potent antitumor macrolide [89]. Lipophilic 2,5-disubstituted pyrroles isolated from a Mycale sp. inhibited hypoxia-induced factors HIF with moderate potency (IC50 values < 10 μM) [90]. These compounds appear to disrupt mitochondrial reactive oxygen species (mROS) regulated HIF-1 signaling under hypoxic conditions. The antidiabetic activities of some octapyrroles from M. mytilorum [91] and 5-alkylpyrrole-2-carboxaldehyde derivatives from the South China Sea sponge M. lissochela [92] are reported. Data about synthesis of diverse steroids by Mycale are reported in the literature [93,94]. New steroidal lactone named mycalone has been isolated from an Australian Mycale species [95]. New steroidal oligoglycosides, mycalosides, have been isolated from the polar extract of the Caribbean sponge M. laxissima [96]. These compounds inhibited the fertilization of eggs by sperm of the sea urchin Strongylocentrotus nudus preincubated with these mycalosides.
To our best knowledge, there are only few reports on the chemistry of the Red Sea sponge M. euplectellioides. New fatty acids related to hexacosa-(6Z,10Z)-dienoic acid methyl ester and hexacosa-(6Z,10Z)-dienoic acid with weak activity against A549 non-small cell lung cancer, the U373 glioblastoma and the PC-3 prostate cancer cell lines have been described recently in the research group of Youssef [97]. In addition, new ceramides have been isolated from the methanolic extracts of this demosponge. These compounds were proposed as promising lead ceramides for the discovery and design of potent anti-choline esterase drug candidates, which would be used for Alzheimer disease eradication [98]. The possible inhibitory activity of the compounds produced by diverse Mycale species as listed above against chitinases of microbial origin is unknown.
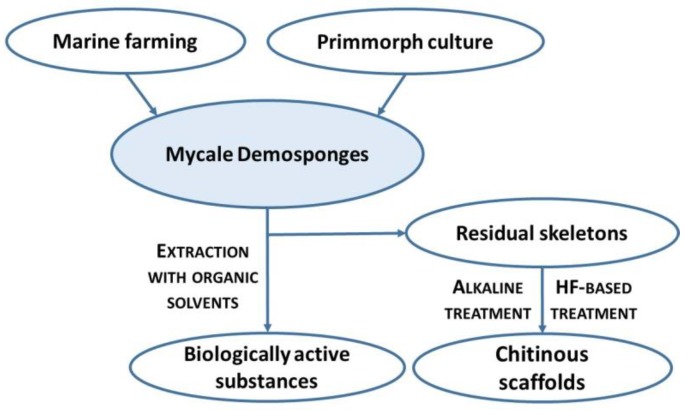
Until now, diverse secondary metabolites from members of the genus Mycale have been purified using traditional organic solvent-based extraction approaches. There are no reports on isolation methods for these metabolites which are based on treatment with alkaline solutions, as well as about structural stability of such biomacromolecules at alkaline pH levels. Experiments with bromotyrosine- and chitin-producing demosponges represented by the order Verongiida showed that bromotyrosines and chitin-based scaffolds could be isolated from the sponge skeletons using a stepwise extraction procedure mainly based on the use of NaOH [6]. Recently, a patented method for isolation of both bromotyrosines and chitinous skeletal frameworks from selected sponges without disruption of the skeletons in the mortar (this being the traditional procedure for extraction of bromotyrosines) has been proposed [99]. Here, we propose the schematic view of the principal steps which can be now applied for purification of secondary metabolites and chitin from the Mycale sponges (Figure 11).
Figure 11.
Schematic view of the possible uses of Mycale sponges.
There are no doubts about the necessity of the development of novel, more effective methodologies for extraction of biologically active compounds together with chitinous scaffolds from Mycale demosponges. In particular, Mycale species, which are already adapted for cultivation under marine ranching conditions [58,60], showed high potential in this case.
We suggest that the discovery of chitin within other representatives of the genus Mycale would be the next step in the evaluation of the possibility to accept these worldwide distributed sponges as novel renewable source for both biologically active metabolites and chitin which are perspective for pharmacology and biomaterials oriented biomedicine, respectively.
4. Materials and Methods
4.1. Collection of the Samples
The specimens of Mycale euplectellioides (Row, 1911) (Demospongiae: Poecilosclerida: Mycalidae) were collected by scuba diving at depths of 7–10 m in Red Sea 20 km south of Hurghada, Egypt (N 27°02’46.8’’ E 33°54’21.4’’), in July 2017. Originally, the sponge composed of 15–30 cm hollow reddish tubes―connected together at a basal part attached to a hard substrate (rock). The tubes were hollow and measured about 7–10 cm at the apical part. When the sponge cut with a knife underwater, it exposes reddish mucous-like material (Figure 1).
4.2. Isolation of Chitin from M. euplectellioides
Isolation of the chitin-based skeletal fibers from M. euplectellioides was achieved in several steps (Figure 2). Freeze-dried skeletons of the sponge M. euplectellioides (Figure 1b) were washed three times with demineralized water for removal of various water-soluble impurities including salts. The washed samples were placed into glassy Petri dishes and cut into 1.5 × 2 cm large fragments. Highly visible greenish-colored and mechanically rigid skeletal fragments have been decalcified at room temperature using 20% acetic acid during 4 h and rinsed in distilled water up to pH 6.8 (Figure 2, Step 2). This procedure is necessary to remove possible calcium and magnesium carbonate containing contaminations (i.e., debris of crustaceans, or mollusks) within the sponge skeltone. After decalcification, the skeletal fragments were treated with 2.5 M NaOH (Sigma-Aldrich, Taufkirchen, Germany) at 37 °C for 72 h (Memmert Incubator, Schwabach, Germany) to achieve depigmentation, deproteinization, as well as partial desilicification. After the removal of residual pigmentation using multiple washing in demineralized water, colorless scaffolds were obtained (Figure 2, Step 3). Observation using stereo and light microscopy showed that glassy spicules were still present within skeletal scaffolds (Figure 4). Subsequently, the colorless skeletal fibers were placed in plastic boxes and treated with 2% HF (Sigma-Aldrich, Taufkirchen, Germany) during 24 h at room temperature for complete desilicification (Figure 2, step 4). Afterwards, the samples were isolated from the plastic boxes and rinsed with demineralized water up to pH 6.8. Obtained fibrous scaffolds (Figure 3) were placed into 50 mL glass bottles and stored in demineralized water at 4 °C till their use for analytical investigations with respect to further chitin identification.
4.3. Light and Fluorescent Microscopy Analysis and Imaging
Collected sponge samples and isolated skeletons as well as purified chitinous scaffolds of M. euplectellioides have been studied using stereomicroscope Di-Li (Kaiserslautern, Germany), BZ-9000 microscope (Keyence, Osaka, Japan) in light and in fluorescent microscopy modus. Photos and macroscopic close-up pictures were made using camera Nikon D-7100 with objective lenses Nikon AF-S DX 18–105 mm f/3.5–5.6 G or Nikon AF-S VR Micro-Nikkor 105 mm f/2.8G IF-ED, Tokyo, Japan). Figures were prepared using freeware software (GNU Image Manipulation Program “GIMP 2.8”).
4.4. Calcofluor White Staining Test
Calcofluor white (CFW) (Fluorescent Brightener M2R, Sigma-Aldrich, Taufkirchen, Germany), which shows enhanced fluorescence when it binds to polysaccharides, especially chitin, was used. The fragments of natural and demineralized sponge skeleton samples were placed in 0.1 M Tris–HCl buffer (pH 8.5) for 30 min. Afterwards, the samples were stained using 0.1% CFW solution for 2 h in darkness, rinsed several times with demineralized water, dried at room temperature during 5 h and analyzed using fluorescent microscopy.
4.5. Scanning Electron Microscopy Analysis
The surface morphology and microstructure of the isolated M. euplectellioides skeletal fragments (Figure 1b) as well as isolated scaffolds (Figure 3) were analyzed using ESEM XL 30 Scanning Electron Microscope (Philips, Eindhoven, The Netherlands). Prior the examination, samples were fixed in a sample holder and covered with a carbon layer for 1 min using an Edwards S150B sputter coater.
4.6. Chitinase Digestion Test
Yatalase® from culture supernatants of Corynebacterium sp. OZ-21 (Cosmo Bio, Tokyo, Japan) was used for this test. Yatalase is a complex enzyme, consists mainly of chitinase, chitobiase and β-1,3-glucanase. One unit of this enzyme released 1 μmol of N-acetylglucosamine from 0.5% chitin solution and 1 μmol of p-nitrophenol from p-nitrophenyl-N-acetyl-β-D-glucosaminide solution in 1 min at 37 °C and pH 6.0. The selected, completely demineralized scaffolds of M. euplectellioides (Figure 3) were incubated in enzyme solution containing 10 mg/mL Yatalase dissolved in citrate phosphate buffer at pH 5.0 for 5 h. The progress of digestion was monitored under light microscopy using BZ-9000 microscope (Keyence, Osaka, Japan).
4.7. Estimation of N-acetyl-d-glucosamine (NAG) Contents and Electrospray Ionization Mass Spectrometry
The Morgan-Elson assay was used to quantify the N-acetyl-d-glucosamine released after chitinase treatment. Purified and dried M. eplecteoides chitin (6 mg) was pulverized to a fine powder in an agate mortar. The samples were suspended in 400 mL of 0.2 M phosphate buffer at pH 6.5. A positive control was prepared by solubilizing 0.3% colloidal chitin in the same buffer. Equal amounts (1 mg/mL) of three chitinases (EC 3.2.1.14 and EC 3.2.1.30): N-acetyl-d-glucosaminidase from Trichoderma viride (Sigma-Aldrich, No. C-8241), and two poly (1,4-a-(2-acetamido-2-deoxy-d-glucoside)) glycanohydrolases from Serratia marcescens (Sigma-Aldrich, No. C-7809), and Streptomyces griseus (Sigma-Aldrich, No. C-6137), respectively, were suspended in 100 mM sodium phosphate buffer at pH 6.0. Digestion was started by mixing 400 mL of the samples and 400 mL of the chitinase mix. Incubation was performed at 37 °C. The reaction was stopped after 114 h by adding 400 mL of 1% NaOH, followed by boiling for 5 min. The vessels were centrifuged at 7000 rpm for 5 min and the products analyzed using the 3,5-dinitrosalicylic acid assay (DNS). For this purpose, 250 ml of the supernatants and 250 mL of 1% DNS were dissolved in a solution containing 30% sodium potassium tartrate in 0.4 M NaOH, mixed and incubated for 5 min in a boiling water bath. Thereafter, the absorbance at 540 nm was recorded using a Tecan Spectrafluor Plus Instrument (Mannedorf/Zurich, Switzerland). Data were interpolated into a standard curve via the serial dilution (0–3.0 mM) of N-acetyl-d-glucosamine (Sigma-Aldrich, No. A-8625) and DNS. A sample which contained chitinase solution without substrate was used as control.
Sample preparation for ESI-MS: specimens obtained after HF treatment (Figure 3 and Figure 5) were hydrolyzed in 6 M HCl for 24 h at 50 °C. After the HCl hydrolysis the samples were filtrated with 0.4 µm filter and freeze-dried in order to remove excess HCl. The remaining solid was dissolved in water for ESI-MS analysis. Standard d-glucosamine was purchased from Sigma-Aldrich (Taufkirchen, Germany). All ESI-MS measurements were performed on Waters TQ Detector ACQUITYuplc mass spectrometer (Waters, Wilmslow, UK) equipped with ACQUITYuplc pump (Waters, Wilmslow, UK) and BEHC18 1.7 mm 2.1 × 50 mm UPLC column. Nitrogen was used as nebulizing and desolvation gas. Graphs were generated using Origin 8.5 for PC.
4.8. FTIR Spectroscopy
Transmission spectra for isolated scaffolds and α-chitin (as reference sample) were measured with the spectral resolution of 4 cm−1 using a FTIR spectrometer TENSOR 27 (Bruker, Mannheim, Germany). α-Chitin standard was obtained from INTIB GmbH (Freiberg, Germany).
5. Conclusions
Marine demosponges of the genus Mycale seem to represent a gold mine for marine pharmacology, marine biotechnology, as well as for marine-bioinspired materials science. Their high potential for many applications is due to their ability to grow under marine farming conditions and to synthetize a broad variety of secondary metabolites with antiviral, antibiotic, antidiabetic, cytotoxic and antitumor activities, as well as chitin. Here, we showed for the first time that chitin is present as a structural component in skeletons of the Red Sea demosponge M. euplectelloides. The question of chitin synthesis among members of the genus Mycale should gain importance as a result of our findings. Consequently, the evolution, localization and functions of chitin within the demosponge M. euplectelloides as well as in other representatives of the family Mycalidae should now be examined. Further investigations on detailed structural features of chitin from M. euplectelloides using solid state NMR, X-ray diffraction (XRD), high resolution transmission electron microscopy (HRTEM) and electron diffraction are in progress now and will be presented in a separate bioanalytical publication. Additionally, separate studies should be carried out to identify chitin synthase genes within the genomes of diverse representatives of the Mycale genus. Also, additional investigations are necessary to obtain understanding of the nature and origin of spicules-containing skeletons of these demosponges. It is still not clear how much spongin and chitin domains are present in them. Novel approaches must be proposed which will bring together modern bioanalytical and molecular biology methods for better understanding of the poriferan chitins synthesis in diverse taxa on the molecular level. The best way to solve this challenging task is a coherent synergetic collaboration of spongologists together with experts in marine chemistry, pharmacology, marine biology, marine biotechnology and biomaterials science using their multidisciplinary knowledge and experiences.
Acknowledgments
This study was supported by DFG Project HE 394/3-2 and PUT Research Grant no. 03/32/DSPB/0806. M.W. is grateful for financial support from Foundation for Polish Science―START 097.2017, and S.Ż.A. for support from DAAD as well as Erasmus Plus programs. Research of V.N.I was supported by the Russian Foundation for Basic Research (grant no. 15-29-02601). Literature and taxonomical data analysis were supported by the Russian Science Foundation (grant no. 14-50-00029). We thank the EEAA and the Red Sea Protectorate of Egypt permission to make collection of the Red Sea sponge Mycale euplectellioides.
Supplementary Materials
The following is available online: http://www.mdpi.com/1660-3397/16/2/68/s1. Figure S1. EDX spectrum of chitin isolated from M. euplectellioides demosponge. Spectrum was registered with use of a XL 30 ESEM Philips-Scanning Electron Microscope (Netherlands). The uncoated samples were investigated at an accelerating voltage of 20 kV.
Author Contributions
H.E., T.J., Y.J and S.Z.A. designed the study protocol and wrote the manuscript; D.Y. collected the sponge materials; L.S., S.S.E, D.Y., K.T., V.N.I. and I.P. prepared samples and performed chemical characterization of chitin from M. euplectelloides; M.T., S.Z.A., N.B., H.M and M.W. conducted SEM and other microscopy investigations and analyzed the data. All the authors critically reviewed and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Merzendorfer H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011;90:759–769. doi: 10.1016/j.ejcb.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Deguchi S., Tsujii K., Horikoshi K. In situ microscopic observation of chitin and fungal cells with chitinous cell walls in hydrothermal conditions. Sci. Rep. 2015;5:11907. doi: 10.1038/srep11907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gow N.A.R., Latge J., Munro C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017;5:1–25. doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PubMed] [Google Scholar]
- 4.Brunner E., Ehrlich H., Schupp P., Hedrich R., Hunoldt S., Kammer M., Machill S., Paasch S., Bazhenov V.V., Kurek D.V., et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009;168:539–547. doi: 10.1016/j.jsb.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrlich H., Maldonado M., Spindler K., Eckert C., Hanke T., Born R., Simon P., Heinemann S., Worch H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera) J. Exp. Zool. Part B. 2007;356:347–356. doi: 10.1002/jez.b.21156. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich H., Krautter M., Hanke T., Simon P., Knieb C., Heinemann S., Worch H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera) J. Exp. Zool. Part B. 2007;308B:473–483. doi: 10.1002/jez.b.21174. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich H., Ilan M., Maldonado M., Muricy G., Bavestrello G., Kljajic Z., Carballo J.L., Schiaparelli S., Ereskovsky A., Schupp P., et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Bol. Macromol. 2010;47:132–140. doi: 10.1016/j.ijbiomac.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich H., Steck E., Ilan M., Maldonado M., Muricy G., Bavestrello G., Kljajic Z., Carballo J.L., Schiaparelli S., Ereskovsky A., et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010;47:141–145. doi: 10.1016/j.ijbiomac.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Brunner E., Richthammer P., Ehrlich H., Paasch S., Simon P., Ueberlein S., van Pée K.-H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. 2009;48:9724–9727. doi: 10.1002/anie.200905028. [DOI] [PubMed] [Google Scholar]
- 10.Bo M., Bavestrello G., Kurek D., Paasch S., Brunner E., Born R., Galli R., Stelling A.L., Sivkov V.N., Petrova O.V., et al. Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa: Cnidaria) Int. J. Bol. Macromol. 2012;51:129–137. doi: 10.1016/j.ijbiomac.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Connors M.J., Ehrlich H., Hog M., Godeffroy C., Araya S., Kallai I., Gazit D., Boyce M., Ortiz C. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. 2012;177:314–328. doi: 10.1016/j.jsb.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Cuong H.N., Minh N.C., Van Hoa N., Trung T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis) Int. J. Biol. Macromol. 2016;93:442–447. doi: 10.1016/j.ijbiomac.2016.08.085. [DOI] [PubMed] [Google Scholar]
- 13.Guggolz T., Henne S., Politi Y., Schütz R., Mašić A., Müller C.H.G., Meißner K. Histochemical evidence of β-chitin in parapodial glandular organs and tubes of Spiophanes (Annelida, Sedentaria: Spionidae), and first studies on selected Annelida. J. Morphol. 2015;276:1433–1447. doi: 10.1002/jmor.20432. [DOI] [PubMed] [Google Scholar]
- 14.Kaya M., Mujtaba M., Ehrlich H., Salaberria A.M., Baran T., Amemiya C.T., Galli R., Akyuz L., Sargin I., Labidi J. On chemistry of γ-chitin. Carbohydr. Polym. 2017;176:177–186. doi: 10.1016/j.carbpol.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010;52:661–699. doi: 10.1080/00206811003679521. [DOI] [Google Scholar]
- 16.Wysokowski M., Petrenko I., Stelling A., Stawski D., Jesionowski T., Ehrlich H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers. 2015;7:235–265. doi: 10.3390/polym7020235. [DOI] [Google Scholar]
- 17.Wysokowski M., Bazhenov V.V., Tsurkan M.V., Galli R., Stelling A.L., Stöcker H., Kaiser S., Niederschlag E., Gärtner G., Behm T., et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013;62:94–100. doi: 10.1016/j.ijbiomac.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich H., Kaluzhnaya O.V., Tsurkan M.V., Ereskovsky A., Tabachnick K.R., Ilan M., Stelling A., Galli R., Petrova O.V., Nekipelov S.V., et al. First report on chitinous holdfast in sponges (Porifera) Proc. R. Soc. B. 2013;280:20130339. doi: 10.1098/rspb.2013.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrlich H., Kaluzhnaya O.V., Brunner E., Tsurkan M.V., Ereskovsky A., Ilan M., Tabachnick K.R., Bazhenov V.V., Paasch S., Kammer M., et al. Identification and first insights into the structure and biosynthesis of chitin from the freshwater sponge Spongilla lacustris. J. Struct. Biol. 2013;183:474–483. doi: 10.1016/j.jsb.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich H., Simon P., Carrillo-Cabrera W., Bazhenov V.V., Botting J.P., Ilan M., Ereskovsky A.V., Muricy G., Worch H., Mensch A., et al. Insights into chemistry of biological materials: Newly discovered silica-aragonite-chitin biocomposites in Demosponges. Chem. Mater. 2010;22:1462–1471. doi: 10.1021/cm9026607. [DOI] [Google Scholar]
- 21.Ehrlich H., Maldonado M., Parker A.R., Kulchin Y.N., Schilling J., Köhler B., Skrzypczak U., Simon P., Reiswig H.M., Tsurkan M.V., et al. Supercontinuum Generation in Naturally Occurring Glass Sponges Spicules. Adv. Opt. Mater. 2016;4:1608–1613. doi: 10.1002/adom.201600454. [DOI] [Google Scholar]
- 22.Philibert T., Lee B.H., Fabien N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017;181:1314–1337. doi: 10.1007/s12010-016-2286-2. [DOI] [PubMed] [Google Scholar]
- 23.Anastopoulos I., Bhatnagar A., Bikiaris D., Kyzas G. Chitin Adsorbents for Toxic Metals: A Review. Int. J. Mol. Sci. 2017;18:114. doi: 10.3390/ijms18010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bąk M., Gutlowska O., Wagner E., Gosk J. The role of chitin and chitosan in peripheral nerve reconstruction. Polym. Med. 2017;47:43–47. doi: 10.17219/pim/75653. [DOI] [PubMed] [Google Scholar]
- 25.Singh R., Shitiz K., Singh A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017;14:1276–1289. doi: 10.1111/iwj.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleuter D., Günther A., Paasch S., Ehrlich H., Kljajić Z., Hanke T., Bernhard G., Brunner E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013;92:712–718. doi: 10.1016/j.carbpol.2012.08.090. [DOI] [PubMed] [Google Scholar]
- 27.Anitha A., Sowmya S., Kumar P., Deepthi S., Chennazhi K.P., Ehrlich H., Tsurkan M., Jayakumar R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014;39:1644–1667. doi: 10.1016/j.progpolymsci.2014.02.008. [DOI] [Google Scholar]
- 28.Ehrlich H., Rigby J.K., Botting J.P., Tsurkan M., Werner C., Schwille P., Petrasek Z., Pisera A., Simon P., Sivkov V., et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013;3:3497. doi: 10.1038/srep03497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrlich H. Biomimetic potential of chitin-based composite biomaterials of poriferan origin. In: Ruys A.J., editor. Biomimetic Biomaterials: Structure and Applications. Woodhead Publishing; Cambridge, UK: 2013. pp. 46–66. [Google Scholar]
- 30.Wysokowski M., Motylenko M., Rafaja D., Koltsov I., Stöcker H., Szalaty T.J., Bazhenov V.V., Stelling A.L., Beyer J., Heitmann J., et al. Extreme biomimetic approach for synthesis of nanocrystalline chitin-(Ti,Zr)O2 multiphase composites. Mater. Chem. Phys. 2017;188:115–124. doi: 10.1016/j.matchemphys.2016.12.038. [DOI] [Google Scholar]
- 31.Wysokowski M., Motylenko M., Bazhenov V.V., Stawski D., Petrenko I., Ehrlich A., Behm T., Kljajic Z., Stelling A.L., Jesionowski T., et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013;7:248–260. doi: 10.1007/s11706-013-0212-x. [DOI] [Google Scholar]
- 32.Wysokowski M., Motylenko M., Beyer J., Makarova A., Stöcker H., Walter J., Galli R., Kaiser S., Vyalikh D., Bazhenov V.V., et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015;8:2288–2301. doi: 10.1007/s12274-015-0739-5. [DOI] [Google Scholar]
- 33.Stepniak I., Galinski M., Nowacki K., Wysokowski M., Jakubowska P., Bazhenov V.V., Leisegang T., Ehrlich H., Jesionowski T. A novel chitosan/sponge chitin origin material as a membrane for supercapacitors – preparation and characterization. RSC Adv. 2016;6:4007–4013. doi: 10.1039/C5RA22047E. [DOI] [Google Scholar]
- 34.Petrenko I., Bazhenov V.V., Galli R., Wysokowski M., Fromont J., Schupp P.J., Stelling A.L., Niederschlag E., Stöker H., Kutsova V.Z., et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017;104:1626–1632. doi: 10.1016/j.ijbiomac.2017.01.084. [DOI] [PubMed] [Google Scholar]
- 35.Mutsenko V.V., Bazhenov V.V., Rogulska O., Tarusin D.N., Schütz K., Brüggemeier S., Gossla E., Akkineni A.R., Meißner H., Lode A., et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017;104:1966–1974. doi: 10.1016/j.ijbiomac.2017.03.116. [DOI] [PubMed] [Google Scholar]
- 36.Mutsenko V.V., Gryshkov O., Lauterboeck L., Rogulska O., Tarusin D.N., Bazhenov V.V., Schütz K., Brüggemeier S., Gossla E., Akkineni A.R., et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017;104:1955–1965. doi: 10.1016/j.ijbiomac.2017.03.161. [DOI] [PubMed] [Google Scholar]
- 37.Gray J.E. Notes on the arrangement of sponges, with the descriptions of some new genera. Proc. Zool. Soc. London. 1867;2:492–558. [Google Scholar]
- 38.Topsent E. Révision des Mycale de l’Eéurope occidentale. Annales de l’Institut océanographique. Ann. l’Institut Océanographique. 1924;1:77–118. [Google Scholar]
- 39.Doumenc D., Levi C. Anisochelae analysis and taxonomy of the genus Mycale Gray (Demospongiae) In: Vacelet J., Boury-Esnault N., editors. Taxonomy of Porifera from the Northeast Atlantic and Mediterranean Sea. Springer-Verlag; Berlin, Germany: 1987. pp. 73–92. [Google Scholar]
- 40.Bergquist P.R., Fromont P.J. The Marine Fauna of New Zealand: Porifera, Demospongiae. Part 4 (Poecilosclerida) New Zealand Oceanographic Institute; Wellington, New Zealand: 1988. [Google Scholar]
- 41.Reiswig H.M., Kaiser H. Description of Mycale bamfieldense n. sp. (Porifera, Demospongiae, Poecilosclerida) from Vancouver Island, British Columbia. Can. J. Zool. 1989;67:674–677. doi: 10.1139/z89-097. [DOI] [Google Scholar]
- 42.Carballo J.L., García-Gómez J.C. The Northeastern Atlantic species Mycale micracanthoxea Buizer Van Soest, 1977 (Porifera, Poecilosclerida) in the Strait of Gibraltar (Southern Spain) Beaufortia. 1994;44:11–16. [Google Scholar]
- 43.Hajdu E., Desquevroux-Faundez R. A synopsis of South American Mycale (Mycale) (Poecilosclerida, Demospongiae), with description of three new species and a cladistic analysis of Mycalidae. Rev. Suisse Zool. 1994;101:563–600. doi: 10.5962/bhl.part.79918. [DOI] [Google Scholar]
- 44.Hajdu E., Zea S., Kielman M., Peixinho S. Mycale escarlatei n. sp. and Mycale unguifera n. sp. (Demospongiae) from the tropical-western Atlantic. Beaufortia. 1995;45:1–16. [Google Scholar]
- 45.Carballo J.L., Hajdu E. Micromorphology in Mycale taxonomy (Mycalidae, Poecilosclerida, Demospongiae), with the description of two new micracanthoxea-bearing species. Contrib. to Zool. 1998;67:187–195. [Google Scholar]
- 46.Coles S.L., Bolick H. Assessment of Invasiveness of the Orange Keyhole Sponge Mycale armata in Kaneohe Bay, Oahu, Hawaii. Final Report Year 1 Prepared for Hawaii Coral Reef Initiative. Bishop Museum; Honolulu, HI, USA: 2006. [Google Scholar]
- 47.Coles S.L., Bolick H. Invasive introduced sponge Mycale grandis overgrows reef corals in Kāne’ohe Bay, O’ahu, Hawai’i. Coral Reefs. 2007;26:911. doi: 10.1007/s00338-007-0295-x. [DOI] [Google Scholar]
- 48.Carballo J.L., Cruz-Barraza J.A. A revision of the genus Mycale (Poecilosclerida: Mycalidae) from the Mexican Pacific Ocean. Contrib. Zool. 2010;79:165–194. [Google Scholar]
- 49.Van Soest R.W.M., Beglinger E.J., De Voogd N.J. Mycale species (Porifera: Poecilosclerida) of Northwest Africa and the Macaronesian Islands. Zool. Med. Leiden. 2014;88:59–109. [Google Scholar]
- 50.Riesgo A., Taboada S., Sánchez-Vila L., Solà J., Bertran A., Avila C. Some like it fat: Comparative ultrastructure of the embryo in two demosponges of the genus Mycale (order poecilosclerida) from Antarctica and the Caribbean. PLoS ONE. 2015;10:e0118805. doi: 10.1371/journal.pone.0118805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Soest R.W.M., Hajdu E. Family Mycalidae Lundbeck, 1905. In: Hooper J.N.A., Van Soest R.W.M., Willenz P., editors. Systema Porifera: A Guide to the Classification of Sponges. Springer; Boston, MA, USA: 2002. pp. 669–690. [Google Scholar]
- 52.Van Soest R.W.M., Boury-Esnault N., Hooper J.N.A., Rützler K., de Voogd N.J., Alvarez B., Hajdu E., Pisera A.B., Manconi R., Schönberg C., et al. World Porifera Database. Mycale grandis Gray, 1867. [(accessed on 20 February 2018)]; Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=192533.
- 53.Vincente J., Silbiger N.J., Beckley B.A., Raczkowski C.W., Hill R.T. Impact of high pCO2 and warmer temperatures on the process of silica biomineralization in the sponge Mycale grandis. ICES J. Mar. Sci. 2016;73:704–714. doi: 10.1093/icesjms/fsv235. [DOI] [Google Scholar]
- 54.Qiu F., Ding S., Ou H., Wang D., Chen J., Miyamoto M.M. Transcriptome changes during the life cycle of the red sponge, Mycale phyllophila (Porifera, Demospongiae, Poecilosclerida) Genes. 2015;6:1023–1052. doi: 10.3390/genes6041023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corriero G., Scalera Liaci L., Nonnis Marzano C., Gaino E. Reproductive strategies of Mycale contarenii (Porifera: Demospongiae) Mar. Biol. 1998;131:319–327. doi: 10.1007/s002270050325. [DOI] [Google Scholar]
- 56.Custódio M.R., Hajdu E. In vivo study of microsclere formation in sponges of the genus Mycale (Demospongiae, Poecilosclerida) Zoomorphology. 2002;121:203–211. doi: 10.1007/s00435-002-0057-9. [DOI] [Google Scholar]
- 57.Mohamed N.M., Enticknap J.J., Lohr J.E., McIntosh S.M., Hill R.T. Changes in bacterial communities of the marine sponge Mycale laxissima on transfer into aquaculture. Appl. Environ. Microbiol. 2008;74:1209–1222. doi: 10.1128/AEM.02047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Page M.J., Northcote P.T., Webb V.L., Mackey S., Handley S.J. Aquaculture trials for the production of biologically active metabolites in the New Zealand sponge Mycale hentscheli (Demospongiae: Poecilosclerida) Aquaculture. 2005;250:256–269. doi: 10.1016/j.aquaculture.2005.04.069. [DOI] [Google Scholar]
- 59.Page M.J., Handley S.J., Northcote P.T., Cairney D., Willan R.C. Successes and pitfalls of the aquaculture of the sponge Mycale hentscheli. Aquaculture. 2011;312:52–61. doi: 10.1016/j.aquaculture.2010.12.006. [DOI] [Google Scholar]
- 60.Huang D., Ou H., Wang D., Chen J., Ding S. Sexual reproduction of the potentially cultivable sponge Mycale phyllophila (Porifera, Demospongiae) J. Mar. Biol. Assoc. U. K. 2016;96:1073–1081. doi: 10.1017/S0025315415001708. [DOI] [Google Scholar]
- 61.Row R.W.H. Reports on the Marine Biology of the Sudanese Red Sea, from Collections made by Cyril Crossland, M.A., B.Sc., F.Z.S. XIX. Report on the Sponges collected by Mr. Cyril Crossland in 1904-5. Part II. Non-Calcarea. J. Linn. Soc. Zool. 1911;31:35–41. doi: 10.1111/j.1096-3642.1911.tb00461.x. [DOI] [Google Scholar]
- 62.Van Soest R.W.M., Boury-Esnault N., Hooper J.N.A., Rützler K., de Voogd N.J., Alvarez B., Hajdu E., Pisera A.B., Manconi R., Schönberg C., et al. World Porifera Database. Mycale euplectellioides (Row, 1911) [(accessed on 20 February 2018)]; Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=194743.
- 63.Wysokowski M., Behm T., Born R., Bazhenov V.V., Meiβner H., Richter G., Szwarc-Rzepka K., Makarova A., Vyalikh D., Schupp P., et al. Preparation of chitin-silica composites by in vitro silicification of two-dimensional Ianthella basta demosponge chitinous scaffolds under modified Stöber conditions. Mater. Sci. Eng. C. 2013;33:3935–3941. doi: 10.1016/j.msec.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 64.Ehrlich H., Bazhenov V.V., Debitus C., de Voogd N., Galli R., Tsurkan M.V., Wysokowski M., Meissner H., Bulut E., Kaya M., et al. Isolation and identification of chitin from heavy mineralized skeleton of Suberea clavata (Verongida: Demospongiae: Porifera) marine demosponge. Int. J. Biol. Macromol. 2017;104:1706–1712. doi: 10.1016/j.ijbiomac.2017.01.141. [DOI] [PubMed] [Google Scholar]
- 65.Kumirska J., Czerwicka M., Kaczyński Z., Bychowska A., Brzozowski K., Thöming J., Stepnowski P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs. 2010;8:1567–1636. doi: 10.3390/md8051567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lavall R.L., Assis O.B.G., Campana-Filho S.P. Beta-chitin from the pens of Loligo sp.: Extraction and characterization. Bioresour. Technol. 2007;98:2465–2472. doi: 10.1016/j.biortech.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Zeng X., Xu S., Yang K., He H. Study on chemical constituents from Mycale parishi. Zhong Yao Cai. 2003;26:715–718. [PubMed] [Google Scholar]
- 68.Wang R.P., Lin H.W., Li L.Z., Gao P.Y., Xu Y., Song S.J. Monoindole alkaloids from a marine sponge Mycale fibrexilis. Biochem. Syst. Ecol. 2012;43:210–213. doi: 10.1016/j.bse.2012.03.016. [DOI] [Google Scholar]
- 69.Zhou X., Lin X., Guo X., Yang B., Yang X.W., Liu Y. Chemical constituents of the sponge Mycale species from South China Sea. Rec. Nat. Prod. 2013;7:119–123. [Google Scholar]
- 70.Northcote P.T., Blunt J.W., Munro M.H.G. Pateamine: A potent cytotoxin from the New Zealand Marine sponge, Mycale sp. Tetrahedron Lett. 1991;32:6411–6414. doi: 10.1016/0040-4039(91)80182-6. [DOI] [Google Scholar]
- 71.Matsunaga S., Sugawara T., Fusetani N. New mycalolides from the marine sponge Mycale magellanica and their interconversion. J. Nat. Prod. 1998;61:1164–1167. doi: 10.1021/np980102r. [DOI] [PubMed] [Google Scholar]
- 72.Thompson A.M., Blunt J.W., Munro M.H.G., Perry N.B. Chemistry of the mycalamides, antiviral and antitumour compounds from a marine sponge. Part 5. Acid-catalysed hydrolysis and acetal exchange, double bond additions and oxidation reactions. J. Chem. Soc. Perkin Trans. 1. 1995 doi: 10.1039/p19950001233. [DOI] [Google Scholar]
- 73.Thompson A.M., Blunt J.W., Murray H.G., Munro S.M.H.G., Perry N.B., Pannell L.K. Chemistry of the Mycalamides, antiviral and antitumour compounds from a marine sponge. Part 3. Acyl, Alkyl and Silyl derivatives. J. Chem. Soc. Perikin. Trans. 1992;1:1335–1342. doi: 10.1039/p19920001335. [DOI] [Google Scholar]
- 74.West L.M., Northcote P.T., Hood K.A., Miller J.H., Page M.J. Mycalamide D, a new cytotoxic amide from the New Zealand marine sponge Mycale species. J. Nat. Prod. 2000;63:707–709. doi: 10.1021/np9904511. [DOI] [PubMed] [Google Scholar]
- 75.Hood K.A., West L.M., Northcote P.T., Berridge M.V., Miller J.H. Induction of apoptosis by the marine sponge (Mycale) metabolites, mycalamide A and pateamine. Apoptosis. 2001;6:207–219. doi: 10.1023/A:1011340827558. [DOI] [PubMed] [Google Scholar]
- 76.Dyshlovoy S.A., Fedorov S.N., Kalinovsky A.I., Shubina L.K., Bokemeyer C., Stonik V.A., Honecker F. Mycalamide A shows cytotoxic properties and prevents EGF-induced neoplastic transformation through inhibition of nuclear factors. Mar. Drugs. 2012;10:1212–1224. doi: 10.3390/md10061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perry N.B., Blunt J.W., Munro M.H.G., Thompson A.M. Antiviral and Antitumor Agents from a New Zealand Sponge, Mycale sp. 2. Structures and Solution Conformations of Mycalamides A and B. J. Org. Chem. 1990;55:223–227. doi: 10.1021/jo00288a037. [DOI] [Google Scholar]
- 78.Perry N.B., Blunt J.W., Munro M.H.G. Mycalamide A, an antiviral compound from a New Zeland sponge of the genus Mycale. J. Am. Chem. Soc. 1988;110:4850–4851. doi: 10.1021/ja00222a067. [DOI] [Google Scholar]
- 79.Gürel G., Blaha G., Steitz T.A., Moore P.B. Structures of triacetyloleandomycin and mycalamide A bind to the large ribosomal subunit of Haloarcula marismortui. Antimicrob. Agents Chemother. 2009;53:5010–5014. doi: 10.1128/AAC.00817-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phuwapraisirisan P., Matsunaga S., Van Soest R.W.M., Fusetani N. Isolation of a new mycalolide from the marine sponge Mycale izuensis. J. Nat. Prod. 2002;65:942–943. doi: 10.1021/np010663+. [DOI] [PubMed] [Google Scholar]
- 81.Nakao Y., Yoshida S., Matsunaga S., Shindoh N., Terada Y., Nagai K., Yamashita J.K., Ganesan A., Van Soest R.W.M., Fusetani N. Azumamides A–E: Histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew. Chemie Int. Ed. 2006;45:7553–7557. doi: 10.1002/anie.200602047. [DOI] [PubMed] [Google Scholar]
- 82.Maulucci N., Chini M.G., Di Micco S., Izzo I., Cafaro E., Russo A., Gallinari P., Paolini C., Nardi M.C., Casapullo A., et al. Molecular insights into azumamide E histone deacetylases inhibitory activity. J. Am. Chem. Soc. 2007;129:3007–3012. doi: 10.1021/ja0686256. [DOI] [PubMed] [Google Scholar]
- 83.Tsukamoto S., Koimaru K., Ohta T. Secomycalolide A: A new proteasome inhibitor isolated from a marine sponge of the genus Mycale. Mar. Drugs. 2005;3:29–35. doi: 10.3390/md302029. [DOI] [Google Scholar]
- 84.Hood K.A., West L.M., Rouwé B., Northcote P.T., Berridge M.V., Wakefield S.J., Miller J.H. Peloruside A, a novel antimitotic agent with paclitaxel-like microtubule-stabilizing activity. Cancer Res. 2002;62:3356–3360. [PubMed] [Google Scholar]
- 85.Wilmes A., Bargh K., Kelly C., Northcote P.T., Miller J.H. Peloruside A synergizes with other microtubule stabilizing agents in cultured cancer cell lines. Mol. Pharm. 2007;4:269–280. doi: 10.1021/mp060101p. [DOI] [PubMed] [Google Scholar]
- 86.Miller J.H., Singh A.J., Northcote P.T. Microtubule-stabilizing drugs from marine sponges: Focus on peloruside A and zampanolide. Mar. Drugs. 2010;8:1059–1079. doi: 10.3390/md8041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller J.H., Rouwé B., Gaitanos T.N., Hood K.A., Crume K.P., Bäckström B.T., La Flamme A.C., Berridge M.V., Northcote P.T. Peloruside A enhances apoptosis in H-ras-transformed cells and is cytotoxic to proliferating T cells. Apoptosis. 2004;9:785–796. doi: 10.1023/B:APPT.0000045789.54694.cf. [DOI] [PubMed] [Google Scholar]
- 88.Kanakkanthara A. Peloruside A: A lead non-taxoid-site microtubule-stabilizing agent with potential activity against cancer, neurodegeneration, and autoimmune disease. Nat. Prod. Rep. 2016;33:549–561. doi: 10.1039/C5NP00146C. [DOI] [PubMed] [Google Scholar]
- 89.Singh A.J., Xu C.X., Xu X., West L.M., Wilmes A., Chan A., Hamel E., Miller J.H., Northcote P.T., Ghosh A.K. Peloruside B, a potent antitumor macrolide from the New Zealand marine sponge Mycale hentscheli: Isolation, structure, total synthesis, and bioactivity. J. Org. Chem. 2010;75:2–10. doi: 10.1021/jo9021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mao S.C., Liu Y., Morgan J.B., Jekabsons M.B., Zhou Y.D., Nagle D.G. Lipophilic 2,5-disubstituted pyrroles from the marine sponge Mycale sp. inhibit mitochondrial respiration and HIF-1 activation. J. Nat. Prod. 2009;72:1927–1936. doi: 10.1021/np900444m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reddy S.G., Dhananjaya N. Chemical investigation of Mycale mytilorum and a study on toxicity and antidiabetic activity of 5-octadecylpyrrole-2-carboxaldehyde. Bioorganic Med. Chem. 2000;8:27–36. doi: 10.1016/S0968-0896(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 92.Xue D.Q., Liu H.L., Chen S.H., Mollo E., Gavagnin M., Li J., Li X.W., Guo Y.W. 5-Alkylpyrrole-2-carboxaldehyde derivatives from the Chinese sponge Mycale lissochela and their PTP1B inhibitory activities. Chinese Chem. Lett. 2017;28:1190–1193. doi: 10.1016/j.cclet.2017.03.040. [DOI] [Google Scholar]
- 93.Carballeira N.M., Negron V., Reyes E.D. Novel Monounsaturated Fatty Acids From the Sponges Amphimedon compressa and Mycale laevis. J. Nat. Prod. 1992;55:333–339. doi: 10.1021/np50081a009. [DOI] [Google Scholar]
- 94.Valle H.A.Z., Santafé G.G.P. Free Sterols from the Marine Sponge Mycale laevis. Vitae. 2009;16:103–109. [Google Scholar]
- 95.Rochfort S.J., Gable R.W., Capon R.J. Mycalone: A new steroidal lactone from a southern Australian marine sponge, Mycale sp. Aust. J. Chem. 1996;49:715–718. [Google Scholar]
- 96.Antonov A.S., Afiyatullov S.S., Kalinovsky A.I., Ponomarenko L.P., Dmitrenok P.S., Aminin D.L., Agafonova I.G., Stonik V.A. Mycalosides B–I, eight new spermostatic steroid oligoglycosides from the sponge Mycale laxissima. J. Nat. Prod. 2003;66:1082–1088. doi: 10.1021/np0300030. [DOI] [PubMed] [Google Scholar]
- 97.Mohamed G.A., Abd-Elrazek A.E.E., Hassanean H.A., Alahdal A.M., Almohammadi A., Youssef D.T.A. New fatty acids from the Red Sea sponge Mycale euplectellioides. Nat. Prod. Res. 2014;28:1082–1090. doi: 10.1080/14786419.2014.907286. [DOI] [PubMed] [Google Scholar]
- 98.Abdelhameed R., Elgawish M.S., Mira A., Ibrahim A.K., Ahmed S.A., Shimizu K., Yamada K. Anti-choline esterase activity of ceramides from the Red Sea marine sponge Mycale euplectellioides. RSC Adv. 2016;6:20422–20430. doi: 10.1039/C5RA26424C. [DOI] [Google Scholar]
- 99.Ehrlich H., Brunner E., Richter W., Ilan M., Schupp P. Two or Three-Dimensional Cleaned Chitin Skeleton of Dictyoceratid Sponges. Method for the Production and Use Thereof. WO2011023531A3. 2011 Jun 23
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.