Abstract
BACKGROUND
Monoclonal gammopathy of undetermined significance (MGUS) occurs in approximately 3% of persons 50 years of age or older.
METHODS
We studied 1384 patients who were residing in southeastern Minnesota and in whom MGUS was diagnosed at the Mayo Clinic in the period from 1960 through 1994; the median follow-up was 34.1 years (range, 0.0 to 43.6). The primary end point was progression to multiple myeloma or another plasma-cell or lymphoid disorder.
RESULTS
During 14,130 person-years of follow-up, MGUS progressed in 147 patients (11%), a rate that was 6.5 times (95% confidence interval [CI], 5.5 to 7.7) as high as the rate in the control population. The risk of progression without accounting for death due to competing causes was 10% at 10 years, 18% at 20 years, 28% at 30 years, 36% at 35 years, and 36% at 40 years. Among patients with IgM MGUS, the presence of two adverse risk factors — namely, an abnormal serum free light-chain ratio (ratio of kappa to lambda free light chains) and a high serum monoclonal protein (M protein) level (≥1.5 g per deciliter) — was associated with a risk of progression at 20 years of 55%, as compared with 41% among patients who had one adverse risk factor and 19% among patients who had neither risk factor. Among patients with non-IgM MGUS, the risk of progression at 20 years was 30% among those who had the two risk factors, 20% among those who had one risk factor, and 7% among those who had neither risk factor. Patients with MGUS had shorter survival than was expected in the control population of Minnesota residents of matched age and sex (median, 8.1 vs. 12.4 years; P<0.001).
CONCLUSIONS
Significant differences were noted in the risk of progression between patients with IgM MGUS and those with non-IgM MGUS. Overall survival was shorter among patients with MGUS than was expected in a matched control population. (Funded by the National Cancer Institute.)
Monoclonal gammopathy of undetermined significance (MGUS) occurs in 3.2% of persons 50 years of age or older and in 5.3% of those 70 years of age or older.1 MGUS is defined as the presence of a serum monoclonal protein (M protein) at a concentration of 3.0 g per deciliter or less, no monoclonal protein or only modest amounts of monoclonal light chains in the urine, the absence of CRAB features (i.e., hypercalcemia, renal insufficiency, anemia, and bone lesions) that are related to the M protein,2,3 and 10% or fewer monoclonal plasma cells in the bone marrow.
In previous, smaller series of patients with MGUS, malignant transformation occurred in 7 to 19% of the patients at 5 to 10 years.4–6 However, the small numbers of patients or the short followup in these studies limit the reliability of these results. Furthermore, the two major biologic subtypes of MGUS, IgM and non-IgM, have different modes of progression, but data regarding the prognosis and risk stratification associated with these entities have not been well characterized.7 Information regarding overall survival after diagnosis is also limited. We present the results of a study of the rates of progression and survival associated with IgM and non-IgM MGUS in a cohort of 1384 patients with MGUS who were identified in a well-defined geographic area and followed for a median of 34.1 years.
METHODS
PATIENTS
The details of this cohort and their natural history have been described previously.8 In brief, we identified 1395 persons with MGUS who resided in the 11 counties of southeastern Minnesota and who had a serum M protein concentration of 3 g per deciliter or less and 10% or fewer plasma cells in the bone marrow (if assessed). Patients with light-chain MGUS were not included, since this entity was defined after the establishment of the cohort at the inception of the study.9 In accordance with our clinical practice, bone marrow examination was deemed unnecessary unless the patient had unexplained anemia, renal insufficiency, or bone pain. The patients were evaluated at the Mayo Clinic from January 1, 1960, through December 31, 1994. A total of 11 patients who had previously signed a form prohibiting review of their medical records for any type of research at the Mayo Clinic were excluded.8,10 Of the remaining 1384 patients, 514 (37%) resided in Olmsted County, which had a population of 92,006 persons in 1980, and the remaining 870 patients resided in the other counties of southeastern Minnesota (1980 population, 312,559 persons). The medical-records-linkage system of the Rochester Epidemiology Project11 makes it possible to obtain complete case ascertainment among the residents of Olmsted County.
Follow-up included the review of each patient’s inpatient and outpatient medical records at the Mayo Clinic and the review of death certificates for patients who had died. Death certificates can be currently obtained from only 10 states; however, in almost all other patients we were able to ascertain survival status by contacting the patient’s family or primary care physician. For the purposes of this study, the follow-up of the original cohort was extended by more than 15 years to December 31, 2015; this change yielded an increase by a factor of 1.3 in the number of person-years of follow-up and in the number of observed progressions.
The M proteins were identified and quantitated by means of cellulose acetate or agarose-gel electrophoresis.12 If there was an abnormal band or equivocal pattern, immunoelectrophoresis or immunofixation was performed to confirm the presence of M protein and to ascertain the type. Patients were advised to undergo serum protein electrophoresis annually.
There was no commercial funding for this study. All the authors participated in the study concept, study design, and data collection; two of the authors conducted the data analysis. The authors vouch for the accuracy of the data and analyses presented and for the fidelity of the study to the protocol.
END POINTS
The primary end point of the study was progression to multiple myeloma or plasma-cell or lymphoid disorders. Analyses were performed with respect to progression to multiple myeloma or related disorders according to MGUS subtype (IgM or non-IgM). Patients with biclonal gammopathy were excluded from all the analyses that were performed according to subtype. Overall survival among patients with IgM MGUS and those with non-IgM MGUS was also determined.
PROGNOSTIC FACTORS
The effect of the following factors on the risk of progression was studied: age; sex; levels of hemoglobin, serum creatinine, and serum albumin; the level and type of the serum M protein; presence, type, and amount of monoclonal urinary light chain; low concentration of uninvolved immunoglobulins (as compared with the reference range); and the serum free light-chain ratio (the ratio of kappa to lambda free light chains, with an abnormal ratio defined as a value <0.26 or >1.65). These values were derived from the time of the first diagnosis in 1366 patients (99%); in 16 patients, these data were derived from the first presentation at the Mayo Clinic that occurred more than 30 days after the initial diagnosis date, and data are unclear in 2 patients to establish a date of first diagnosis. The cutoff points for the serum M protein level, low concentration of uninvolved immunoglobulins, and serum free light-chain ratio were based on established levels in the literature or were based on the normal range; all other numerical markers were analyzed as continuous variables.
STATISTICAL ANALYSIS
The end points with respect to progression were calculated in terms of both the cumulative probability and the cumulative incidence of progression, accounting for competing causes of death. The cumulative probability was calculated with the use of a Kaplan–Meier estimate13 in which data from patients who died were censored; curves were compared by means of the log-rank test.14 However, the cumulative incidence of progression explicitly accounted for other causes of death and was computed by the method of Putter et al.15 The effect of previously established risk factors on progression rates was examined in Cox proportional-hazards models.16
The risk of progression to each disease studied, as compared with the risk in the general population, was determined by applying age-specific and sex-specific incidence rates for these conditions in the cohort of white participants from the Iowa Surveillance, Epidemiology, and End Results (SEER) program17 to the age-, sex-, and calendar year–specific number of person-years of follow-up in our study cohort. The age-specific and sex-specific incidence rates of immunoglobulin light-chain (AL) amyloidosis were based on data from Olmsted County, Minnesota, because these rates are not included in the SEER program.18 The confidence intervals for the relative risks were based on a Poisson approach.19 The follow-up time was estimated with the use of the median time to censoring, which was obtained from a Kaplan–Meier estimate in which data from patients who died were censored at the time of death and data from patients who were alive at last follow-up were uncensored at that time point.20
All the statistical tests were two-sided. Analyses were performed with the use of SAS software, version 9.3 (SAS Institute), and R software, version 3.2.0 (R Core Team, R Foundation for Statistical Computing).
RESULTS
CHARACTERISTICS OF THE PATIENTS AT BASELINE
Of the 1384 patients included in the study, 753 (54%) were men and 631 (46%) were women (Table 1). The median age of the patients at the diagnosis of MGUS was 72 years. The immunoglobulin type was IgG in 70% of the patients, IgA in 12%, and IgM in 15%; a biclonal gammopathy was found in 3% of the patients. The light-chain type was kappa in 61% of the patients and lambda in 39%. The concentration of uninvolved immunoglobulins was low in 38% of the 840 patients in whom levels were measured at the time of diagnosis of MGUS. The ratio of kappa to lambda free light chains was abnormal in 33% of the 1148 patients in whom the test was performed. Electrophoresis and immunoelectrophoresis or immunofixation were performed on urine samples obtained from 418 of the patients with MGUS: 21% of the patients had a monoclonal kappa light chain, 10% had a lambda light chain, and 69% had negative results; only 17% of the patients had a urinary M protein value of more than 150 mg per 24 hours.
Table 1.
Characteristics of the Patients.*
| Characteristic | Entire Cohort (N=1384) |
IgM MGUS (N=210) |
Non-IgM MGUS (N=1129) |
|---|---|---|---|
| Follow-up (person-yr) | 14,130 | 1893 | 11,883 |
| Median (yr) | 34.1 | 29.3 | 34.1 |
| Range (yr) | 0.0–43.6 | 0.0–37.2 | 0.0–43.6 |
| Male sex (%) | 54 | 58 | 54 |
| Age at MGUS diagnosis | |||
| Median (yr) | 72 | 74 | 72 |
| <40 yr (%) | 2 | 1 | 2 |
| Median M protein level (g/dl) | 1.2 | 1.1 | 1.2 |
| Abnormal free light-chain ratio (%)† | 33 | 34 | 33 |
The numbers of patients in the type-specific groups do not sum to 1384 owing to the exclusion of 45 patients with biclonal gammopathy. MGUS denotes monoclonal gammopathy of undetermined significance.
An abnormal free light-chain ratio of kappa to lambda light chains was defined as a value less than 0.26 or greater than 1.65.
The results of bone marrow examination at diagnosis were available for 160 patients (12%). The median percentage of plasma cells in bone marrow was 3% (range, 0 to 10). The initial hemoglobin value was less than 12 g per deciliter in 23% of the patients. In each case, anemia was due to causes other than plasma-cell proliferation, such as iron deficiency, renal insufficiency, or myelodysplasia. The serum creatinine value was at least 2 mg per deciliter (180 μmol per liter) in 6% of the patients, but in each patient, the elevation could be attributed to causes that were unrelated to the plasma-cell proliferative disorder, such as diabetes, hypertension, or glomerulonephropathy.
PROGRESSION TO CANCER OR PLASMA-CELL OR LYMPHOID DISORDER
The 1384 patients in this study have now been followed for 14,130 person-years (median follow-up, 34.1 years; range, 0 to 43.6) (Table 1). During this time, 1300 patients (94%) have died. Of the 84 patients who are still alive, 5 have had disease progression and 79 have not had progression, which leaves them at risk for progression. During follow-up, multiple myeloma, lymphoma with an IgM serum M protein, AL amyloidosis, macroglobulinemia, chronic lymphocytic leukemia, or plasmacytoma developed in 147 patients (11%), which represented a risk of these disorders that was 6.5 times (95% confidence interval [CI], 5.5 to 7.7) as high as the risk in the age- and sex-matched background population (Table 2). As compared with the background SEER population (except for the population of patients with AL amyloidosis, for which data were from Olmsted County, Minnesota18), the risk of progression among patients with IgM MGUS (relative risk, 10.8; 95% CI, 7.5 to 15.0) was higher than the risk among those with non-IgM MGUS (relative risk, 5.7; 95% CI, 4.7 to 6.9) (Table 2). Of the 147 patients who had disease progression, 5 are still alive.
Table 2.
Risk and Type of Progression.*
| End Point, in Entire Cohort and According to MGUS Type | Observed No. of Patients | Expected No. of Patients | Relative Risk (95% CI) |
|---|---|---|---|
| Entire cohort | |||
| Any progression | 147 | 22.5 | 6.5 (5.5–7.7) |
| Multiple myeloma | 97 | 4.1 | 23.8 (19.3–29.1) |
| Non-Hodgkin’s lymphoma | 19 | 11.6 | 1.6 (1.0–2.6) |
| AL amyloidosis | 14 | 1.6 | 8.8 (4.8–14.7) |
| Waldenström’s macroglobulinemia | 13 | 0.3 | 47.5 (25.3–81.3) |
| Chronic lymphocytic leukemia | 3 | 4.9 | 0.6 (0.1–1.8) |
| Plasmacytoma | 1 | 0.1 | 12.6 (0.3–70.2) |
| IgM MGUS | |||
| Any progression | 34 | 3.2 | 10.8 (7.5–15.0) |
| Multiple myeloma | 0 | 0.6 | 0.0 (0.0–6.5) |
| Non-Hodgkin’s lymphoma | 17 | 1.6 | 10.6 (6.2–17.0) |
| AL amyloidosis | 3 | 0.2 | 13.1 (2.7–38.1) |
| Waldenström’s macroglobulinemia | 11 | <0.1 | 287.7 (143.6–514.7) |
| Chronic lymphocytic leukemia | 3 | 0.7 | 4.3 (0.9–12.6) |
| Plasmacytoma | 0 | <0.1 | 0.0 (0.0–342.6) |
| Non-IgM MGUS | |||
| Any progression | 107 | 18.7 | 5.7 (4.7–6.9) |
| Multiple myeloma | 93 | 3.4 | 27.5 (22.2–33.7) |
| Non-Hodgkin’s lymphoma | 2 | 9.6 | 0.2 (0.0–0.7) |
| AL amyloidosis | 11 | 1.3 | 8.3 (4.2–14.9) |
| Waldenström’s macroglobulinemia | 0 | 0.2 | 0.0 (0.0–16.2) |
| Chronic lymphocytic leukemia | 0 | 4.1 | 0.0 (0.0–0.9) |
| Plasmacytoma | 1 | 0.1 | 15.0 (0.4–83.7) |
There were 1384 patients in the entire cohort, of whom 210 had IgM MGUS and 1129 had non-IgM MGUS. A total of 45 patients had biclonal gammopathy, of whom 4 had progression to multiple myeloma and 2 had progression to Waldenström’s macroglobulinemia. The risk of events in the study cohort was compared with the risk in an age-, sex-, and calendar year–specific control population from the Iowa Surveillance, Epidemiology, and End Results (SEER) program, with the exception of amyloid immunoglobulin light-chain (AL) amyloidosis, which is from Olmsted County, Minnesota.18
The cumulative risk of progression to one of these disorders (not accounting for death due to competing risk) was 10% at 10 years, 18% at 20 years, 28% at 30 years, 36% at 35 years, and 36% at 40 years. The risk of progression was 1.1 events per 100 person-years among patients with IgM MGUS, as compared with 0.8 events per 100 person-years among those with non-IgM MGUS (P<0.001).
The risk factors for progression in the overall cohort are shown in Figure 1. The risk factors according to subtype of MGUS are shown in Figures S1 and S2 in the Supplementary Appendix, available with the full text of this article at NEJM.org. The risk of progression among patients with IgM MGUS was 2% per year in the first 10 years after diagnosis and 1% per year thereafter (Fig. S1 in the Supplementary Appendix). In contrast, the risk of progression remained unchanged according to duration of follow-up among patients with non-IgM MGUS (Fig. S2 in the Supplementary Appendix). The initial concentration of the serum M protein and the serum free light-chain ratio were the most important univariate risk factors for progression to a plasma-cell disorder among patients with IgM or non-IgM MGUS. Combined, these two variables provided prognostic value in both IgM MGUS and non-IgM types of MGUS. These two variables were also confirmed to be of independent significance on multivariable analysis (data not shown). Among patients with IgM MGUS, the presence of two adverse risk factors — namely, an abnormal serum free light-chain ratio and high serum M protein level (≥1.5 g per deciliter) — was associated with a risk of progression at 20 years of 55%, as compared with 41% among patients who had one adverse risk factor and 19% among patients who had no risk factors. Among patients with non-IgM MGUS, the risk of progression was 30% among those who had the two risk factors, 20% among those who had one risk factor, and 7% among those who had neither risk factor. There were 3.6 events (95% CI, 1.8 to 7.2) per 100 person-years, as compared with 1.1 events (95% CI, 0.6 to 2.0) per 100 person-years among patients with a normal serum free light-chain ratio and a low M protein level (<1.5 g per deciliter) (Fig. S1 in the Supplementary Appendix). The corresponding rates among patients with non-IgM MGUS were 1.8 events (95% CI, 1.2 to 2.8) per 100 person-years among patients with the two risk factors and 0.4 events (95% CI, 0.3 to 0.6) per 100 person-years among patients with neither risk factor (Fig. S2 in the Supplementary Appendix). The age of the patient at diagnosis and the duration of follow-up were not risk factors for progression (Fig. 1). When the age of the patient at follow-up (the current age) was considered, there was an increasing annual risk of progression with older age (Fig. 1).
Figure 1. Rate of Progression of Monoclonal Gammopathy of Undetermined Significance (MGUS) per 100 Person-Years in the Entire Cohort.
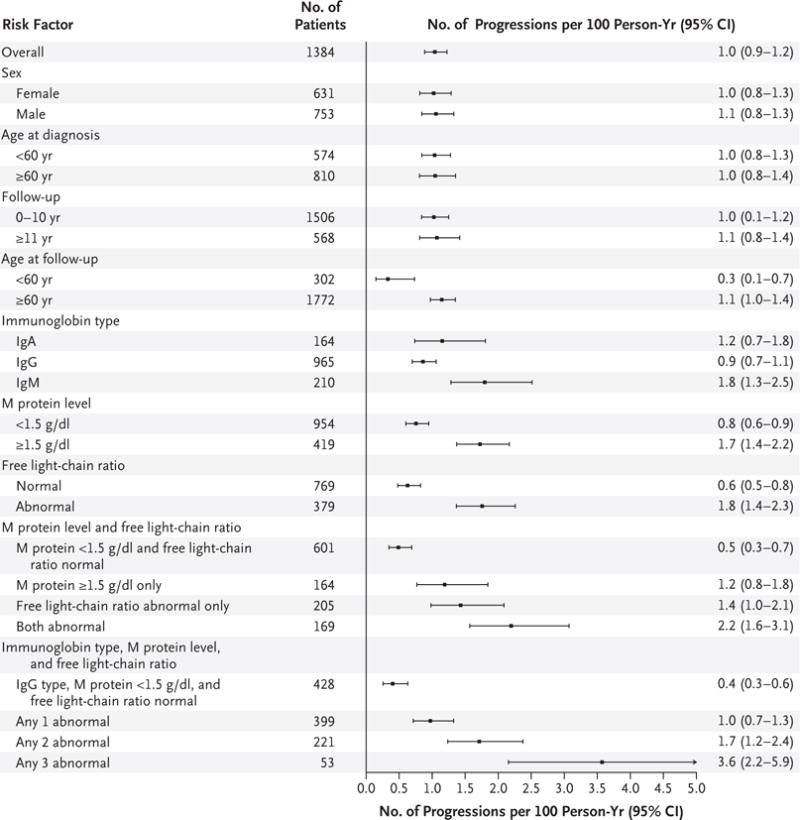
The primary end point of the study was progression to multiple myeloma or other plasma-cell or lymphoid disorders among patients with MGUS in a cohort of 1384 patients. Subgroups involving follow-up were analyzed with the use of a person-year approach, so patients may have been counted in both categories. Data on immunoglobulin type were excluded for 45 patients with biclonal status, data on the monoclonal protein (M protein) level were missing for 11, and data on the free light-chain ratio were missing for 236. The normal immunoglobulin type was IgG, with types other than IgG considered to be abnormal; the normal M protein level was less than 1.5 g per deciliter, with higher values considered to be abnormal.
Among other risk factors studied, the risk of progression was higher when there was a low concentration of two uninvolved immunoglobulins (hazard ratio vs. normal concentration, 2.0; 95% CI, 1.1 to 3.7; P = 0.03). However, a low concentration of just one uninvolved immunoglobulin did not have a significant effect on the risk of progression (hazard ratio vs. normal concentration, 1.3; 95% CI, 0.8 to 2.1; P = 0.22).
The M protein level became undetectable during follow-up in 75 of 1384 patients (5%); this finding was due to therapy for unrelated disorders in 43 patients. In 32 of 1384 patients (2%), the M protein level was undetectable without a known cause, but the majority of these patients had small unmeasurable M protein levels at diagnosis.
OVERALL SURVIVAL
Figure 2 shows the cumulative risk of progression and death without progression (a competing risk) among patients with IgM MGUS, as compared with those with non-IgM MGUS. At 40 years, the death rates among patients with MGUS were 11% owing to plasma-cell disorders and 87% owing to non–plasma-cell disorders, such as cardiovascular and cerebrovascular diseases and non–plasma-cell cancers. A total of 2% of the patients are still alive at the time of this analysis and are at risk for progression or death. Patients with MGUS had a shorter median survival than was expected in the control population of Minnesota residents of matched age and sex (8.1 vs. 12.4 years, P<0.001) (Fig. 3). There were 474 excess deaths in the cohort and 142 persons who had progression to multiple myeloma or a related disorder — findings that indicate that many additional deaths cannot be attributed to disease progression. The overall survival rate at 30 years was 4% (95% CI, 2 to 9) among patients with IgM MGUS and 7% (95% CI, 6 to 9) among those with non-IgM MGUS (P = 0.12).
Figure 2. Cumulative Incidence of Progression of MGUS, with Death Accounted for as a Competing Risk.
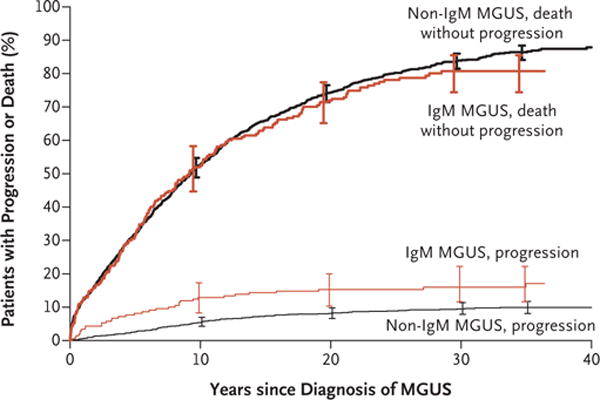
I bars indicate 95% confidence intervals.
Figure 3. Observed Survival Rate in the Cohort versus the Expected Survival Rate in the Control Population.
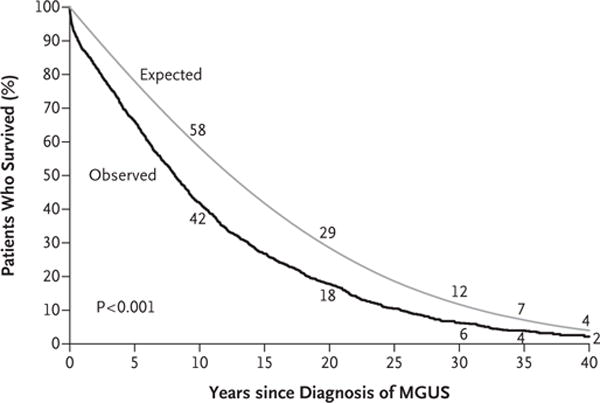
The numerical values are the observed rates of survival at 10, 20, 30, 35, and 40 years among the patients in the study and the expected rates in the age-and sex-matched control population.
DISCUSSION
We have previously reported on the natural history of MGUS in this cohort of 1384 patients from southeastern Minnesota.8 We extended the follow-up of this cohort by more than 15 years (a total of 14,130 person-years of follow-up) and found that there was a persistent risk of progression of 1% per year. Furthermore, MGUS has now been reclassified into two major subtypes, IgM and non-IgM, because the clonal cell that is involved and the nature of progression differ between these two types.7 IgM MGUS typically arises from a CD20+ lymphoplasmacytic cell that has not undergone switch recombination, and this disease type is associated with a risk of progression to lymphoma or Waldenström’s macroglobulinemia. In contrast, non-IgM MGUS typically arises from mature plasma cells that have undergone switch recombination and is associated with a risk of progression to multiple myeloma. Both disease types can progress to AL amyloidosis. Our study showed significant differences in the mode and risk of progression between patients with IgM MGUS and those with non-IgM MGUS (Fig. 2). The data that are provided in Table 2 regarding the risk of progression according to MGUS subtype and specific associated risk factors may allow clinicians to provide more accurate prognostic information to patients on the basis of MGUS type and may help to refine the nature and type of monitoring that is needed for each patient. We also found that the current risk-stratification model according to the M protein level and serum free light-chain assay was valid in these two forms of MGUS.21
Another key finding is that the risk of progression may vary according to the duration of follow-up among patients with IgM MGUS. This finding is analogous to what we have previously reported regarding patients with smoldering multiple myeloma.22 In contrast, the risk of progression among patients with non-IgM MGUS was fixed at approximately 1% per year regardless of the duration of follow-up, a finding that suggests a random multiple-factor (so-called multi-hit) model of malignant progression.
Few risk factors add meaningful additional value to the prognoses that are associated with MGUS type, M protein level, and serum free light-chain ratio. Studies suggest that the suppression of uninvolved immunoglobulins, especially of the same immunoglobulin type, may be associated with a greater risk of progression.23,24 A study that involved 728 patients with MGUS in Sweden showed that the suppression of one or two immunoglobulins was associated with a greater risk of progression than those without suppression of one or two immunoglobulins, but the overall risk of progression in that study was 0.5% per year.25 We found that the reduction in the level of one immunoglobulin had no effect on the risk of progression, but a reduction in the levels of two normal immunoglobulins was a significant risk factor. Although the risk of progression appeared to increase with age, more studies are needed in order to determine the significance of this finding, since we did not have serial follow-up M protein values to determine whether the effect of age was independent of any possible change in M protein levels that may have occurred during that time period. A bone marrow biopsy was not routinely performed in this study, and thus it could be argued that some patients may have had more than 10% plasma cells. However, this situation is unlikely since the inclusion of such patients would have led to a greater risk of progression than we observed during the first few years of the study, which is not evident in our data. The International Myeloma Working Group does not currently recommend routine bone marrow examination in patients with low-risk MGUS.26 A study showed that the test is of low yield in such patients,27 and we along with other investigators who are not at the Mayo Clinic concur with this assessment.28
Finally, we describe the overall survival among patients with MGUS. We found that patients with MGUS had shorter survival than did an age- and sex-matched control population. In addition to the risk of malignant progression, this finding may be related to potentially serious disorders that led to the initial unexpected diagnosis of MGUS. Our data reflect that the risk of progression to myeloma or a related disorder is much less than the competing risk of death due to other causes (Fig. 2).
Despite MGUS being a prevalent disorder that is associated with a modest but persistent lifetime risk of progression to incurable cancer, there are limited data at present to indicate that screening for MGUS or monitoring improves outcomes in patients. A randomized trial of screening and intervention involving patients with MGUS has been initiated in Iceland (www.blodskimun.is).
Supplementary Material
Acknowledgments
Supported in part by research grants (CA107476, CA168762, and CA186781) from the National Cancer Institute.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA. Monoclonal gammopathy of undetermined significance: natural history in 241 cases. Am J Med. 1978;64:814–26. doi: 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., III Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original Mayo Clinic series 25 years later. Mayo Clin Proc. 2004;79:859–66. doi: 10.4065/79.7.859. [DOI] [PubMed] [Google Scholar]
- 4.Blade J, Lopez-Guillermo A, Rozman C, et al. Malignant transformation and life expectancy in monoclonal gammopathy of undetermined significance. Br J Haematol. 1992;81:391–4. doi: 10.1111/j.1365-2141.1992.tb08245.x. [DOI] [PubMed] [Google Scholar]
- 5.van de Poel MH, Coebergh JW, Hillen HF. Malignant transformation of monoclonal gammopathy of undetermined significance among out-patients of a community hospital in southeastern Netherlands. Br J Haematol. 1995;91:121–5. doi: 10.1111/j.1365-2141.1995.tb05256.x. [DOI] [PubMed] [Google Scholar]
- 6.Baldini L, Guffanti A, Cesana BM, et al. Role of different hematologic variables in defining the risk of malignant transfor mation in monoclonal gammopathy. Blood. 1996;87:912–8. [PubMed] [Google Scholar]
- 7.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 8.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–9. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 9.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton LJ., III The threat to medical-records research. N Engl J Med. 1997;337:1466–70. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.Kyle R, Katzmann JA. Immunochemical characterization of immunoglobulins. In: Rose NR, Conway de Macario E, Folds JD, Lane HC, Nakamura RM, editors. Manual of clinical laboratory immunology. 5th. Washington, DC: ASM Press; 1997. pp. 156–76. [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 14.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc [Ser A] 1972;135:185–207. [Google Scholar]
- 15.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 17.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence — SEER 9 Regs Research Data. 2014 Nov; Sub (1973-2012) <Rita Population Adjustment> — Linked To County Attributes – Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission ( https://www.seer.cancer.gov).
- 18.Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817–22. [PubMed] [Google Scholar]
- 19.Berry G. The analysis of mortality by the subject-years method. Biometrics. 1983;39:173–84. [PubMed] [Google Scholar]
- 20.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–7. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–90. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 23.Katzmann JA, Clark R, Kyle RA, et al. Suppression of uninvolved immunoglobulins defined by heavy/light chain pair suppression is a risk factor for progression of MGUS. Leukemia. 2013;27:208–12. doi: 10.1038/leu.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesana C, Klersy C, Barbarano L, et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol. 2002;20:1625–34. doi: 10.1200/JCO.2002.20.6.1625. [DOI] [PubMed] [Google Scholar]
- 25.Turesson I, Kovalchik SA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014;123:338–45. doi: 10.1182/blood-2013-05-505487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyle RA, Durie BG, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangiacavalli S, Cocito F, Pochintesta L, et al. Monoclonal gammopathy of undetermined significance: a new proposal of workup. Eur J Haematol. 2013;91:356–60. doi: 10.1111/ejh.12172. [DOI] [PubMed] [Google Scholar]
- 28.Kyle RA, San-Miguel JF, Mateos MV, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Hematol Oncol Clin North Am. 2014;28:775–90. doi: 10.1016/j.hoc.2014.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


