Abstract
IMPORTANCE
In the United States, national associations of individual dietary factors with specific cardiometabolic diseases are not well established.
OBJECTIVE
To estimate associations of intake of 10 specific dietary factors with mortality due to heart disease, stroke, and type 2 diabetes (cardiometabolic mortality) among US adults.
DESIGN, SETTING, AND PARTICIPANTS
A comparative risk assessment model incorporated data and corresponding uncertainty on population demographics and dietary habits from National Health and Nutrition Examination Surveys (1999–2002: n = 8104; 2009–2012: n = 8516); estimated associations of diet and disease from meta-analyses of prospective studies and clinical trials with validity analyses to assess potential bias; and estimated disease-specific national mortality from the National Center for Health Statistics.
EXPOSURES
Consumption of 10 foods/nutrients associated with cardiometabolic diseases: fruits, vegetables, nuts/seeds, whole grains, unprocessed red meats, processed meats, sugar-sweetened beverages (SSBs), polyunsaturated fats, seafood omega-3 fats, and sodium.
MAIN OUTCOMES AND MEASURES
Estimated absolute and percentage mortality due to heart disease, stroke, and type 2 diabetes in 2012. Disease-specific and demographic-specific (age, sex, race, and education) mortality and trends between 2002 and 2012 were also evaluated.
RESULTS
In 2012, 702 308 cardiometabolic deaths occurred in US adults, including 506 100 from heart disease (371 266 coronary heart disease, 35 019 hypertensive heart disease, and 99 815 other cardiovascular disease), 128 294 from stroke (16 125 ischemic, 32 591 hemorrhagic, and 79 578 other), and 67 914 from type 2 diabetes. Of these, an estimated 318 656 (95% uncertainty interval [UI], 306 064–329 755; 45.4%) cardiometabolic deaths per year were associated with suboptimal intakes—48.6% (95% UI, 46.2%–50.9%) of cardiometabolic deaths in men and 41.8% (95% UI, 39.3%–44.2%) in women; 64.2% (95% UI, 60.6%–67.9%) at younger ages (25–34 years) and 35.7% (95% UI, 33.1%–38.1%) at older ages (≥75 years); 53.1% (95% UI, 51.6%–54.8%) among blacks, 50.0% (95% UI, 48.2%–51.8%) among Hispanics, and 42.8% (95% UI, 40.9%–44.5%) among whites; and 46.8% (95% UI, 44.9%–48.7%) among lower-, 45.7% (95% UI, 44.2%–47.4%) among medium-, and 39.1% (95% UI, 37.2%–41.2%) among higher-educated individuals. The largest numbers of estimated diet-related cardiometabolic deaths were related to high sodium (66 508 deaths in 2012; 9.5% of all cardiometabolic deaths), low nuts/seeds (59 374; 8.5%), high processed meats (57 766; 8.2%), low seafood omega-3 fats (54 626; 7.8%), low vegetables (53 410; 7.6%), low fruits (52 547; 7.5%), and high SSBs (51 694; 7.4%). Between 2002 and 2012, population-adjusted US cardiometabolic deaths per year decreased by 26.5%. The greatest decline was associated with insufficient polyunsaturated fats (−20.8% relative change [95% UI, −18.5% to −22.8%]), nuts/seeds (−18.0% [95% UI, −14.6% to −21.0%]), and excess SSBs (−14.5% [95% UI, −12.0% to −16.9%]). The greatest increase was associated with unprocessed red meats (+14.4% [95% UI, 9.1%–19.5%]).
CONCLUSIONS AND RELEVANCE
Dietary factors were estimated to be associated with a substantial proportion of deaths from heart disease, stroke, and type 2 diabetes. These results should help identify priorities, guide public health planning, and inform strategies to alter dietary habits and improve health.
Dietary habits influence many risk factors for cardiometabolic health, including heart disease, stroke, and type 2 diabetes, which collectively pose substantial health and economic burdens.1 In both global2,3 and national4 modeling studies, the associations of suboptimal diet with overall health have been estimated. Understanding the relations of individual dietary components with cardiometabolic disease at the population level is essential to identify priorities, guide public health planning, and inform strategies to alter these dietary habits and improve health. In addition, the differences in these estimated health burdens by underlying personal characteristics, such as age, sex, race/ethnicity, and education, are relevant to consider more targeted approaches to reducing disparities.
For the United States, prior analyses have estimated the associations of suboptimal dietary habits with cardiometabolic health overall4 or for a limited number of dietary factors (eg, sodium, sugar-sweetened beverages).5 The results for other individual dietary components, as well as differences by age, sex, race/ethnicity, and socioeconomic status, are not well established. The current investigation used a comparative risk assessment modeling design2,6,7 to estimate the cardiometabolic mortality related to suboptimal intakes of 10 dietary factors, individually and jointly, among US adults in 2012; to assess diet-associated mortality by disease subtypes (heart disease and subtypes, stroke and subtypes, and type 2 diabetes) and population subgroups (age, sex, race, and education); and to evaluate trends between 2002 and 2012.
Methods
Study Design
A comparative risk assessment model was used to estimate the numbers and proportions of cardiometabolic deaths associated with suboptimal intakes of 10 dietary factors in the United States, both individually and in combination (eAppendix 1 in the Supplement). The model incorporated separately derived data and corresponding uncertainty on (1) population demographics and dietary habits by sex, age, race, and education from the National Health and Nutrition Examination Survey (NHANES); (2) the estimated relationships of 10 foods and nutrients with heart disease, stroke, or type 2 diabetes mortality, by age, from meta-analyses of prospective cohorts and randomized clinical trials, further evaluated by several validity analyses; (3) the optimal population intake distributions of these dietary factors based on observed intakes associated with lowest risk in observational studies; and (4) observed US disease-specific cardiometabolic deaths by sex, age, race, and education from the National Center for Health Statistics (NCHS). This modeling investigation was exempt from human subjects review because it was based on published data and nationally representative, deidentified data sets that included no personally identifiable information.
Identification of Relevant Dietary Factors
The methods and results for review, identification, and assessment of evidence for etiologic diet-disease relationships have been described (eAppendix 2 in the Supplement).8,9 Using Bradford-Hill criteria and considering consistency with other criteria for assessing potential causality of diet-disease relationships,10–12 probable or convincing evidence was identified for associations of 17 dietary factors with coronary heart disease (CHD), stroke, type 2 diabetes, body mass index (BMI), or systolic blood pressure (SBP) (eTables 1–5 in the Supplement). Of these, 10 were included in the present analysis (Table 1), excluding others with major overlap for estimating joint effects (eg, dietary fiber overlaps with whole grains, fish overlaps with omega-3 fats). Several other dietary factors were evaluated and not included because of insufficient evidence for casual relationships, including monounsaturated fats, vitamin D, magnesium, calcium, antioxidant vitamins, dairy products, cocoa, coffee, and tea. Evidence for potential associations of diet with other conditions such as cancer, osteoporosis, gallstones, inflammatory diseases, depression, cognitive function, or micronutrient deficiency diseases was not evaluated.
Table 1.
Dietary Factors in the Analysis, Consumption Levels Among Adults Aged ≥ 25 Years in 1999–2002 and 2009–2012, Optimal Consumption Levels, Related Cardiometabolic Disease Outcomes, and Relative Risks at Ages 50 and 70 Years
| Dietary Targetsa | Mean (SD)/Median (IQR) | Optimal Consumption, Meanc | Cardiometabolic Outcome | Unit of RRd | RR (95% Cl) | ||
|---|---|---|---|---|---|---|---|
| Consumption in 1999–2002b | Consumption in 2009–2012b | Age 50 ye | Age 70 ye | ||||
| Fruits excluding fruit juices, g/d | 96.4 (89.1)/14.2 (0–141.8) | 115.0 (107.5)/71.2 (3.5–174.9) | 300 | Decreased CHD | Per 100 g/d | 0.93 (0.89–0.97) | 0.95 (0.92–0.98) |
| Decreased ischemic stroke | 0.86 (0.80–0.92) | 0.90 (0.86–0.94) | |||||
| Decreased hemorrhagic stroke | 0.69 (0.56–0.84) | 0.77 (0.67–0.89) | |||||
| Vegetables including legumes, g/d | 179.8 (98.4)/136.5 (50.1–259.7) | 182.5 (106.2)/154.0 (83.6–243.2) | 400 | Decreased CHD | Per 100 g/d | 0.94 (0.91–0.97) | 0.96 (0.94–0.98) |
| Decreased ischemic stroke | 0.80 (0.70–0.92) | 0.86 (0.78–0.94) | |||||
| Decreased hemorrhagic stroke | 0.80 (0.67–0.96) | 0.86 (0.76–0.97) | |||||
| Nuts/seeds, g/d | 7.3 (11.8)/0 (0–4.1) | 11.7 (21.1)/1.5 (0–12.6) | 20.2 (5 1–oz servings/wk) | Decreased CHD | Per 1 oz/wk | 0.91 (0.87–0.94) | 0.93 (0.91–0.96) |
| Decreased diabetes | 0.96 (0.94–0.98) | 0.97 (0.96–0.99) | |||||
| Whole grains, g/d | 15.3 (15.6)/0.7 (0–19.5) | 21.2 (18.7)/12.4 (0–32.1) | 125 (2.5 50–g servings/d) | Decreased CHD | Per 50 g/d | 0.96 (0.93–0.99) | 0.97 (0.95–0.99) |
| Decreased ischemic stroke | 0.90 (0.83–0.97) | 0.93 (0.88–0.98) | |||||
| Decreased hemorrhagic stroke | 0.90 (0.83–0.97) | 0.93 (0.88–0.98) | |||||
| Decreased diabetes | 0.86 (0.80–0.92) | 0.90 (0.86–0.94) | |||||
| Red meats, unprocessed, g/d | 50.5 (22.6)/8.7 (0–84.2) | 47.4 (23.6)/34.4 (0–74) | 14.3 (1 100–g serving/wk) | Increased diabetes | Per 100 g/d | 1.24 (1.04–1.47) | 1.16 (1.03–1.30) |
| Processed meats, g/d | 29.9 (17.8)/0 (0–43.5) | 30.8 (19.0)/17.6 (0–47.6) | No intake | Increased CHD | Per 50 g/d | 1.47 (1.14–1.88) | 1.30 (1.09–1.54) |
| Increased diabetes | 1.65 (1.30–2.08) | 1.41 (1.20–1.65) | |||||
| SSBs, 8-oz servings/d | 1.52 (1.53)/0.6 (0–2.4) | 1.14 (1.44)/0.4 (0–1.7) | No intake | Increased BMI (baseline BMI <25)f | Per 8 oz/d | 0.10 (0.05–0.15) | 0.10 (0.05–0.15) |
| Increased BMI (baseline BMI ≥25)f | 0.23 (0.14–0.32) | 0.23 (0.14–0.32) | |||||
| Increased CHD (BMI adjusted)f | 1.26 (1.15–1.37) | 1.17 (1.10–1.24) | |||||
| Increased diabetes (BMI adjusted)f | 1.27 (1.11–1.46) | 1.18 (1.07–1.29) | |||||
| PUFAs, % energy replacing carbohydrates or saturated fatsg | 7.0 (1.4)/6.4 (4.7–8.6) | 7.7 (1.5)/7.4 (5.9–9.2) | 11 | Decreased CHD | Per 5% energy/d | 0.88 (0.83–0.94) | 0.92 (0.88–0.96) |
| Seafood omega-3 fats, mg/d | 117 (85)/27.5 (0–72) | 100 (69)/37.9 (15.1–88.5) | 250h | Decreased CHD | Per 100 mg/d | 0.82 (0.75–0.90) | 0.87 (0.82–0.93) |
| Sodium, mg/d | 3400 (552)/3261 (2636–3963) | 3480 (605)/3355 (2874–3933) | 2000 | Increased SBP, white, normotensivei | Per 2300 mg/d | 3.74 mm Hg (2.30–5.17) | 5.84 mm Hg (4.01–7.66) |
| Increased SBP, additional effect among black patientsi | 2.49 mm Hg (0.13–4.85) | 2.49 mm Hg (0.13–4.85) | |||||
| Increased SBP, additional effect among patients with hypertensioni | 1.87 mm Hg (0.12–3.63) | 1.87 mm Hg (0.12–3.63) | |||||
Abbreviations: IQR, interquartile range; PUFA, polyunsaturated fat; RR, relative risk; SBP, systolic blood pressure; SSB, sugar-sweetened beverage.
From 17 dietary factors with probable or convincing evidence, based on criteria for assessing causality,10–12 for etiologic relationships with cardiometabolic outcomes including coronary heart disease (CHD), stroke, type 2 diabetes, body mass index (BMI), or SBP (eTable 1 in the Supplement).9 Of these, beans/legumes were summed with vegetables; dietary fiber, glycemic load, and potassium were not included because of overlap with major food sources (eg, fruits, vegetables, legumes, whole grains), fish/seafood because of overlap with seafood omega-3 fats, yogurt because of lack of data on generalized least-squares dose-response relationships, and trans fats because of limited national data on intakes as well as rapidly declining US levels due to policy interventions.
Based on the National Health and Nutrition Examination Survey (see Methods and eTables 7–10 in the Supplement).
Based on observed levels at which the lowest disease risk occurs, with further considerations of feasibility and consistency with major guidelines (eTable 3 in the Supplement).13
Based on published or de novo dose-response meta-analyses of prospective cohorts or randomized clinical trials (eAppendix 2 and eTables 2 and 5 in the Supplement).
For SSBs and BMI, data are change in BMI calculated as weight in kilograms divided by height in meters squared; for sodium and SBP,change in SBP in mm Hg. Effect sizes correspond to associations between increased consumption of each dietary factor and cardio metabolic risk. Analyses used age-specific RRs (eAppendix 2 and eTable 5 in the Supplement).5,14
Evidence suggests that SSBs are associated with increased cardiometabolic risk through relationships with BMI plus additional BMI-independent relationships of SSBs with CHD and type 2 diabetes (eAppendix 1 and eTable 5 in the Supplement).15
Estimated RRs are similar for PUFAs replacing carbohydrates (49.4% of US calories in 2012) or saturated fats (10.7% of US calories in 2012); the former is reported herein. For comparison, in sensitivity analyses we also evaluated estimated mortality associated with excess saturated fats in place of PUFAs.
A linear reduction in risk was modeled until 250 mg/d, with no additional benefit thereafter.
Evidence suggests that sodium is associated with increased mortality due to heart disease and stroke through effects on SBP.7,14 Effects of sodium on SBP, including evidence for larger effects among older adults, blacks, and hypertensiveindividuals,weredeterminedfromrandomizedclinicaltrials(eAppendix1andeTable5intheSupplement).7
National Distributions of Dietary Intake and Demographics
Dietary intakes were estimated using nationally representative data from multiple NHANES cycles, accounting for complex survey design and sampling weights,16 to be representative of the US population aged 25 years or older (eTable 6 in the Supplement). As previously described,17 intakes were assessed from up to 2 standardized 24-hour dietary recalls per person, accounting for within-person variation (eTables 7–10 in the Supplement).18 Optimal metrics and units for each dietary factor were characterized to be consistent with studies providing evidence on etiologic diet-disease relationships (Table 1; eTable 3 in the Supplement).8 All dietary factors were adjusted for energy intake (using the residual method18 or, for polyunsaturated fats, as percentage energy) to reduce measurement error and account for potential differences in body size, lean mass, metabolic efficiency, and physical activity.
The means and standard deviations of intake of each dietary factor were estimated in population strata by age (25–34, 35–44, 45–54, 55–64, 65–74, or ≥75 years), sex (male or female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American/other Hispanic, or other race/mixed race), and education (less than high school diploma, high school diploma/equivalent or some college, or 4-year college degree or greater). These demographic characteristics were classified in NHANES based on self-report. Dietary factors were modeled based on the mean and standard deviations using gamma (rather than normal) distributions, allowing for and incorporating skewed distributions. To maximize power for subgroups, 2002 intakes were estimated by combining 1999–2000 and 2001–2002 cycles (the earliest with nationally representative 24-hour recalls; n = 8104; 48.2% men) and 2012 intakes by combining 2009–2010 and 2011–2012 cycles (n = 8516; 47.6% men).
Estimated Diet-Disease Relationships
Methods for reviewing and synthesizing evidence to estimate effect sizes (relative risks) for associations between dietary factors and cardiometabolic end points have been described (eAppendix 2 in the Supplement).8,9 The present analysis incorporated evidence from published or de novo meta-analyses of prospective cohorts or randomized clinical trials evaluating direct associations of dietary factors with CHD, stroke, or type 2 diabetes by age (Table 1; eTable 2 in the Supplement). We included additional BMI-mediated associations of sugar-sweetened beverages (SSBs) by age and overweight/obesity status on deaths due to CHD, hypertensive heart disease, stroke, and type 2 diabetes and SBP-mediated associations of dietary sodium by age, race, and hypertensive status on deaths due to heart disease, stroke, and type 2 diabetes (eTable 5 in the Supplement).
These estimated effects can be used to model associations with cardiometabolic diseases if bias from confounding (which might overestimate effects) or measurement error (which might underestimate effects) is limited. To reduce bias from confounding, all identified observational studies in these meta-analyses used multivariable adjustment for other risk factors. Measurement error was generally not addressed, although some studies used serial measures of diet. In addition, associations of individual dietary factors with health may be different from joint associations when consumed as diet patterns; eg, healthful dietary factors such as fruits, vegetables, and whole grains tend to positively correlate in diets while inversely correlating with unhealthful dietary factors such as SSBs or processed meats. To determine the extent to which the estimated multivariable-adjusted effect sizes might be biased because of these limitations, 3 separate validity analyses were performed comparing the estimated effect sizes for individual dietary components to (1) observed associations of overall dietary patterns with clinical end points in long-term observational studies; (2) effects of dietary patterns on cardiovascular risk factors (low-density lipoprotein cholesterol, SBP) in randomized clinical feeding trials; and (3) effects of dietary patterns on hard end points in a large randomized clinical trial (eAppendix 2 and eTable 4 in the Supplement).9,19,20 Each of these validity analyses demonstrated that estimated effect sizes for individual dietary components were very similar to what would be expected based on these other lines of evidence.
Characterization of Optimal Intakes
Optimal consumption levels for each dietary factor were characterized (Table 1) based on observed levels associated with lowest disease risk in meta-analyses of clinical end points, while further considering feasibility (observed national consumption levels in at least 2 to 3 countries around the world) and consistency with major dietary guidelines (eTable 3 in the Supplement).8 The population distribution (ie, standard deviation) around each optimal population mean was estimated from the optimal distributions of diet-related metabolic risk factors in the Global Burden of Diseases study (10% of the mean).2 For each dietary factor, the modeling assumed no additional health benefits beyond the optimal intake distribution within each sex, age, race, and education stratum.
National Mortality, BMI, and SBP Distributions by Sex, Age, Race, and Education
National disease-specific deaths in each stratum for 2002 and 2012 were obtained from the NCHS, which includes the entire US population (https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm). Deaths were excluded for foreign residents (individuals dying in the United States but whose place of residence is outside the United States), ages 25 years or younger, missing age information (2012: 0.005%; 2002: 0.006%), or, in education-stratified analyses, missing education information (2012: 2.1%; 2002: 6.2%). Diet-related cardiometabolic diseases were defined using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, including heart disease (the sum of CHD, hypertensive heart disease, and other cardiovascular disease), stroke (the sum of ischemic, hemorrhagic, and other stroke), and type 2 diabetes (Table 2; eTables 11–12 in the Supplement). Events were characterized by age, sex, race/ethnicity, and education as described above to match dietary strata. For associations mediated by BMI (SSBs), including with CHD, hypertensive heart disease, stroke, and type 2 diabetes, and by blood pressure (sodium), including with CHD, hypertensive heart disease, other cardiovascular disease, and stroke, the stratum-specific distributions (means and standard deviations) of BMI (based on measured heights and weights) and SBP (from certified examiners, using the mean of 3 measurements or 4 if necessary) in 2002 and 2012 were estimated from the 1999–2002 and 2009–2012 NHANES cycles, respectively. Hypertension was defined as systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or use of antihypertensive drugs.23
Table 2.
Cardiometabolic Deaths Among US Adults Aged ≥25 Years Associated With Suboptimal Dietary Habits in 2012
| Cardiometabolic Disease by Dietary Factor (Suboptimal Intake Level)a | Associated Deaths/y, No. (95% UI)b | Total Disease-Specific Deaths/y, %b |
|---|---|---|
| Overall Suboptimal Dietc | ||
| Heart disease | 223 960 (211 689–234 444) | 44.3 (41.8–46.3) |
| CHD | 197 981 (187 580–207 070) | 53.3 (50.5–55.8) |
| Hypertensive heart disease | 7958 (7158–8792) | 22.7 (20.4–25.1) |
| Other CVDd | 7449 (6858–8109) | 7.5 (6.9–8.1) |
| Stroke | 66 547 (62 799–69 915) | 51.9 (48.9–54.5) |
| Ischemic | 7170 (6412–7779) | 44.5 (39.8–48.2) |
| Hemorrhagic | 19 863 (18 301–21 265) | 60.9 (56.2–65.2) |
| Other | 39 289 (35 902–42 399) | 49.4 (45.1–53.3) |
| Diabetes | 32 732 (30 803–34 568) | 48.2 (45.4–50.9) |
| Total cardiometabolic disease | 318 656 (306 064–329 755) | 45.4 (43.6–47) |
| Fruits (<300 g/d) | ||
| CHD | 23 865 (18 658–28 884) | 6.4 (5.0–7.8) |
| Stroke | 28 741 (25 609–31 682) | 22.4 (20–24.7) |
| Ischemic | 1920 (1602–2267) | 11.9 (9.9–14.1) |
| Hemorrhagic | 10 317 (8661–11 823) | 31.7 (26.6–36.3) |
| Other | 16 470 (13 671–19 119) | 20.7 (17.2–24) |
| Total cardiometabolic disease | 52 547 (46 557–58 706) | 7.5 (6.6–8.4) |
| Vegetables (<400 g/d) | ||
| CHD | 25 443 (20 252–30 895) | 6.9 (5.5–8.3) |
| Stroke | 28 039 (23 525–31 941) | 21.9 (18.3–24.9) |
| Ischemic | 3466 (2567–4208) | 21.5 (15.9–26.1) |
| Hemorrhagic | 8041 (5987–9897) | 24.7 (18.4–30.4) |
| Other | 16 584 (12 721–20 123) | 20.8 (16–25.3) |
| Total cardiometabolic disease | 53 410 (46 290–60 398) | 7.6 (6.6–8.6) |
| Nuts/Seeds (<20.2 g/d) | ||
| CHD | 54 591 (46 447–63 554) | 14.7 (12.5–17.1) |
| Diabetes | 4732 (3763–5715) | 7.0 (5.5–8.4) |
| Total cardiometabolic disease | 59 374 (51 211–68 422) | 8.5 (7.3–9.7) |
| Whole Grains (<125 g/d) | ||
| CHD | 16 169 (11 749–20 833) | 4.4 (3.2–5.6) |
| Stroke | 13 449 (11 539–15 160) | 10.5 (9–11.8) |
| Ischemic | 1618 (1205–2072) | 10 (7.5–12.8) |
| Hemorrhagic | 4024 (3172–4810) | 12.3 (9.7–14.8) |
| Other | 7774 (6211–9233) | 9.8 (7.8–11.6) |
| Diabetes | 11 639 (10 102–13 143) | 17.1 (14.9–19.4) |
| Total cardiometabolic disease | 41 311 (36 141–46 360) | 5.9 (5.1–6.6) |
| Red Meats, Unprocessed (>14.3 g/d) | ||
| Diabetes | 2869 (2091–3694) | 4.2 (3.1–5.4) |
| Total cardiometabolic disease | 2869 (2091–3694) | 0.4 (0.3–0.5) |
| Processed Meats (>0 g/d) | ||
| CHD | 45 637 (35 048–56 391) | 12.3 (9.4–15.2) |
| Diabetes | 11 900 (10 070–13 833) | 17.5 (14.8–20.4) |
| Total cardiometabolic disease | 57 766 (47 220–68 866) | 8.2 (6.7–9.8) |
| Sugar-Sweetened Beverages (>0 g/d) | ||
| Heart disease | 40 552 (35 643–45 841) | 8.0 (7.0–9.1) |
| CHD | 39 937 (34 992–45 204) | 10.8 (9.4–12.2) |
| Hypertensive heart disease | 616 (433–830) | 1.8 (1.2–2.4) |
| Stroke | 916 (809–1028) | 0.7 (0.6–0.8) |
| Ischemic | 82 (69–98) | 0.5 (0.4–0.6) |
| Hemorrhagic | 405 (342–479) | 1.2 (1.1–1.5) |
| Other | 426 (345–512) | 0.5 (0.4–0.6) |
| Diabetes | 10 043 (8419–11 979) | 14.8 (12.4–17.6) |
| Total cardiometabolic disease | 51 694 (46 363–57 156) | 7.4 (6.6–8.1) |
| PUFAs Replacing Carbohydrates or Saturated Fats (<11% Energy/d)e | ||
| CHD | 16 025 (13 280–18 925) | 4.3 (3.6–5.1) |
| Total cardiometabolic disease | 16 025 (13 280–18 925) | 2.3 (1.9–2.7) |
| Seafood omega-3 Fats (<250 mg/d) | ||
| CHD | 54 626 (45 541–65 053) | 14.7 (12.3–17.5) |
| Total cardiometabolic disease | 54 626 (45 541–65 053) | 7.8 (6.5–9.3) |
| Sodium (>2000 mg/d)f | ||
| Heart disease | 52 711 (44 681–60 826) | 10.4 (8.8–12) |
| CHD | 37 744 (29 879–45 697) | 10.2 (8.0–12.3) |
| Hypertensive heart disease | 7505 (6627–8325) | 21.4 (18.9–23.8) |
| Other CVDd | 7439 (6859–8105) | 7.5 (6.9–8.1) |
| Stroke | 13 787 (12 018–15 870) | 10.7 (9.4–12.4) |
| Ischemic | 1629 (1349–1928) | 10.1 (8.4–12) |
| Hemorrhagic | 4011 (3306–4780) | 12.3 (10.1–14.7) |
| Other | 8131 (6580–10 023) | 10.2 (8.3–12.6) |
| Total cardiometabolic disease | 66 508 (58 500–74 840) | 9.5 (8.3–10.7) |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; PUFA, polyunsaturated fat; UI, uncertainty interval.
Based on the National Center for Health Statistics, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (eTable 11 in the Supplement). Cardiometabolic diseases included heart disease (the sum of CHD, hypertensive heart disease, and other CVD, including rheumatic heart disease, cardiomyopathy and myocarditis, atrial fibrillation and flutter, aortic aneurysm, peripheral vascular disease, endocarditis, and other cardiovascular and circulatory diseases), stroke (including ischemic, hemorrhagic, and other stroke [unclassified stroke or sequelae of stroke not specified as hemorrhage or infarction]), and type 2 diabetes. Diabetes deaths are those coded as proximally due to diabetes; diabetes is also separately a risk factor for CVD deaths along with other risk factors such as smoking, high blood pressure, high cholesterol, obesity, physical inactivity, etc.
Calculated from a total of 702 308 total US cardiometabolic deaths in 2012. Because each value represents central estimates from 1000 Monte Carlo simulations (see Methods section of text and eAppendix 1 in the Supplement), the subtype estimates may not sum perfectly to the total number. Deaths due to hypertensive heart disease were estimated from relationships with blood pressure (sodium) or body mass index (sugar-sweetened beverages) and deaths due to other CVD from relationships with blood pressure (sodium) (eTable 5 in the Supplement).
Based on the joint (multiplicative) population attributable fraction21 for the factors in this Table. For this, we used PUFAs replacing saturated fats rather than carbohydrates to be more conservative when estimating joint associations and excluded CHD estimates for processed meat because this relationships may be mainly driven by sodium content,22 already separately included.
For findings for subtypes of other CVD deaths, see eTable 14 in the Supplement.
Associated mortality was similar for insufficient PUFAs in place of saturated fats alone: 14 382 CHD deaths (95% UI, 11 732-17 079), representing 3.9% of CHD deaths (95% UI, 3.2%–4.6%) and 2.0% of total cardiometabolic disease deaths (95% UI, 1.7%–2.4%). In sensitivity analyses, we also modeled mortality associated with excess saturated fats (>10%) in place of PUFAs: 4244 CHD deaths (95% UI, 3366-5278), representing 1.1% of CHD deaths (95% UI, 0.9%–1.4%) and 0.6% of total cardiometabolic disease deaths (95% UI, 0.5%–0.8%).
Based on effects of sodium on systolic blood pressure in randomized trials, including by age, race, and hypertension status (eAppendix 1 and eTable 5 in the Supplement) and associations of blood pressure with heart disease and stroke by age (eTable 5 in the Supplement).7,14
Cardiometabolic Disease Burdens Attributable to Key Dietary Targets
All data inputs were combined in a comparative risk assessment model to estimate the absolute number and percentage of overall cardiometabolic deaths associated with suboptimal intake of each dietary factor. This framework21 incorporated each stratum-specific input and its uncertainty (except for uncertainty in baseline number of deaths, not reported by the NCHS) to estimate associated mortality by age and sex; by age, sex, and race; and by age, sex, and education. Stratification by all 4 demographic factors was not performed because of low sample size and unstable estimates in some strata. The main outcomes were the estimated absolute number and percentage of cardiometabolic mortality related to suboptimal intakes of 10 dietary factors, individually and jointly, in 2012. We also evaluated disease-specific and demographic-specific (age, sex, race, and education) mortality and trends between 2002 and 2012.
For each stratum, the model calculated the percentage of disease-specific mortality associated with each dietary factor by comparing the present distribution of consumption with the optimal distribution using the continuous population-attributable fraction (PAF) formula (eAppendix 1 in the Supplement).21 This PAF was multiplied by the actual number of disease-specific deaths in that stratum of the US population to estimate the absolute number of disease-specific deaths in that stratum related to the dietary factor. The joint associations of all 10 dietary factors was estimated by proportional multiplication of each stratum-specific PAF (eAppendix 1). For comparing trends between 2002 and 2012, the estimated absolute (2012–2002) and relative (2012–2002/2002×100) associated mortality rates in 2002 were age- and sex-standardized to 2012 age-sex distributions.
Uncertainty was quantified using multiway probabilistic Monte Carlo simulations, jointly incorporating stratum-specific uncertainties in dietary exposure distributions, diet-disease relative risk estimates, and, for sodium, prevalence of hypertension and proportion of non-Hispanic blacks. Corresponding 95% uncertainty intervals (UIs) were derived from the 2.5th and 97.5th percentiles of 1000 estimated models. Different outcomes were evaluated without adjustment for multiple comparisons, so the UI bounds for each finding should be interpreted in that context. These analyses represent the estimated total cardiometabolic mortality associated with each dietary factor, including any mediated relationships through major cardiovascular risk factors (eg, the estimated mortality from low fruit or vegetable consumption would include any association mediated by their effects on lowering of blood pressure and blood cholesterol). Except for SSBs, additional potential relationships of dietary habits with obesity were not considered, which could underestimate total diet-related cardiometabolic mortality. All analyses were performed using R statistical software, version 3.1.0.
Results
In both 2002 and 2012, national intakes of each dietary factor were suboptimal (Table 1; eTables 7–10 in the Supplement). In 2012, a total of 702 308 cardiometabolic deaths occurred in US adults, including 506 100 due to heart disease (including 371 266 due to CHD, 35 019 due to hypertensive heart disease, and 99 815 due to other cardiovascular disease), 128 294 from stroke (16 125 ischemic, 32 591 hemorrhagic, and 79 578 other), and 67 914 from type 2 diabetes (eTable 11 in the Supplement).
Estimated Cardiometabolic Mortality Attributed to Diet
When all 10 dietary factors were evaluated in combination, they were associated with 318 656 estimated cardiometabolic deaths, or nearly 1 in 2 (45.4%) of all US cardiometabolic deaths in 2012. Among individual factors, largest numbers of estimated diet-related cardiometabolic deaths were related to high sodium (66 508 estimated cardiometabolic deaths [9.5% of all cardiometabolic deaths]), low nuts/seeds (59 374 [8.5%]), high processed meats (57 766 [8.2%]), low seafood omega-3 fats (54 626 [7.8%]), low vegetables (53 410 [7.6%]), low fruits (52 547 [7.5%]), and high SSBs (51 694 [7.4%]) compared with optimal consumption levels (Table 2; eFigure 1 in the Supplement). Lowest estimated mortality burdens were associated with low polyunsaturated fats (16 025 [2.3%]) and high unprocessed red meats (2869 [0.4%]).
Among cardiometabolic diseases, the largest numbers of deaths due to CHD were associated with low nuts/seeds (54 591 [14.7% of CHD deaths]), low seafood omega-3 fats (54 626 [14.7%]), high processed meats (45 637 [12.3%]), high SSBs (39 937 [10.8%]), and high sodium (37 744 [10.2%]); due to total stroke, to low vegetables (28 039 [21.9%]), low fruits (28 741 [22.4%]), and high sodium (13 787 [10.7%]); due to hypertensive heart disease, to high sodium (7505 [21.4%]); and due to type 2 diabetes, to high processed meats (11 900 [17.5%]), low whole grains (11 639 [17.1%]), and high SSBs (10 043 [14.8%]) (Table 2).
Findings by Sex, Age, Race, and Education
Estimated cardiometabolic mortality associated with each dietary factor was modestly higher in men than in women, primarily because of generally unhealthier dietary habits in men (Figure 1; eFigure 5 and eTable 14 in the Supplement). The largest sex differences were seen for processed meats (10.8% of all cardiometabolic deaths in men and 5.4% in women; difference, +5.4%; 95% UI, 2.3%–8.3%) and SSBs (9.3% vs 5.3%; difference, +3.9%; 95% UI, 2.3%–5.4%). In men, the top 5 estimated dietary factors associated with cardiometabolic deaths were excess processed meats (38 632 deaths [10.8% of all cardiometabolic deaths]), sodium (35 777 [10.0%]), SSBs (33 314 [9.3%]); and insufficient nuts/seeds (31 587 [8.8%]) and seafood omega-3 fats (31 545 [8.8%]). In women, these were excess sodium (30 281 [8.8%]) and insufficient nuts/seeds (27 721 [8.1%]), vegetables (25 592 [7.4%]), fruits (24 449 [7.1%]), and omega-3 fats (23 032 [6.7%]). Jointly, suboptimal diet was related to 48.6% of estimated cardiometabolic deaths in men and 41.8% in women in 2012 (absolute difference, +6.9%; 95% UI, 3.3%–10.1%) (Figure 2).
Figure 1. Absolute and Proportional Cardiometabolic Disease Mortality Associated With Suboptimal Dietary Habits Among US Men and Women in 2012.
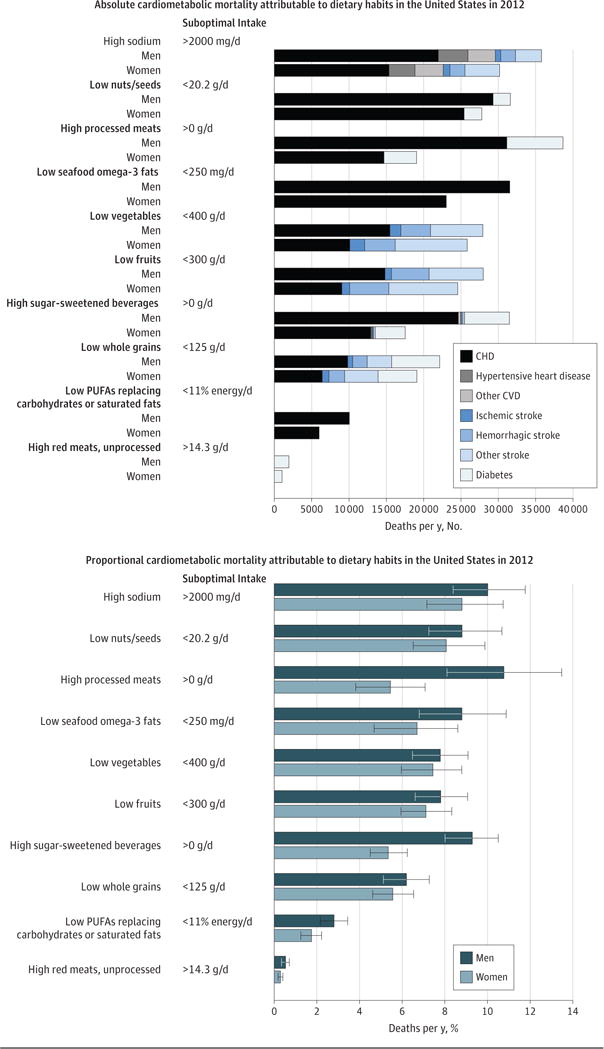
The bars represent the estimated absolute number (top panel) and percentage (bottom panel) of cardiometabolic deaths related to 10 dietary factors compared with optimal intakes. The dietary factors are listed in rank order of total mortality in men and women combined. Error bars indicate 95% uncertainty intervals. CHD indicates coronary heart disease; CVD, cardiovascular disease; PUFA, polyunsaturated fat.
Figure 2. Absolute and Proportional Cardiometabolic Disease Mortality Associated With Overall Suboptimal Diet in the United States in 2012 by Population Subgroups.
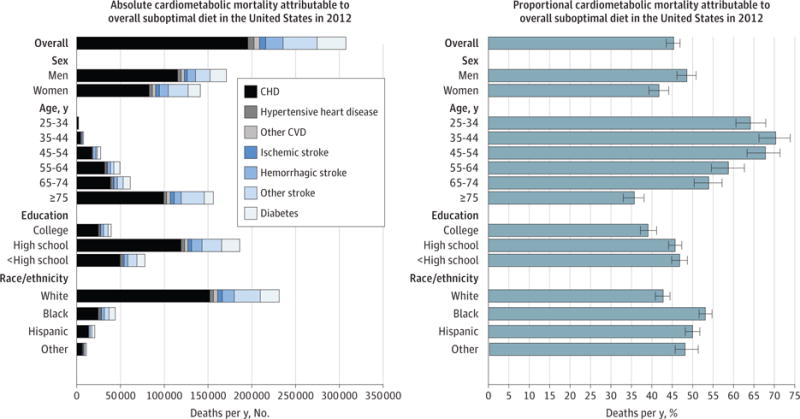
The bars represent the estimated absolute number (left panel) and percentage (right panel) of cardiometabolic deaths jointly related to suboptimal intakes of 10 dietary factors. The 10 factors were low intakes of fruits, vegetables, nuts/seeds, whole grains, seafood omega-3 fats, and polyunsaturated fats (replacing saturated fats) and high intakes of sodium, unprocessed red meats, processed meats, and sugar-sweetened beverages (see Table 1 for details). Error bars indicate 95% uncertainty intervals. CHD indicates coronary heart disease; CVD, cardiovascular disease.
By age, in 25- to 64-year-olds, excess SSBs and processed meats were the top estimated diet factors associated with cardiometabolic mortality; in 65-year-olds and older, these were excess sodium and insufficient nuts/seeds and vegetables (eFigure 2, eFigure 5, and eTable 14 in the Supplement). Overall, suboptimal diet was associated with 64.2% of all estimated cardiometabolic deaths in 25- to 34-year-olds and 35.7% in 75-year-olds and older (absolute difference, −28.6%; 95% UI, −32.9% to −24.0%) (Figure 2). The highest estimated proportional deaths at youngest ages (<44 years) were associated with SSBs followed by processed meat, fruits, nuts/seeds, and vegetables; at middle age (45–54 years), with SSBs, processed meat, nuts/seeds, and seafood omega-3 fats; and at oldest age (≥65 years), with sodium. For example, estimated proportions of SSB-related deaths were much higher at age 25–34 years (26.8%) and 35–44 years (28.9%) than at age ≥75 years (3.5%). Estimated proportions of deaths related to processed meat and nuts/seeds were higher at age 45–54 years (16.8% and 15.7%, respectively) than at age ≥75 years (4.9% and 6.8%).
By race/ethnicity, estimated proportional diet-related mortality was higher among blacks or Hispanics for most dietary factors assessed (Figure 2; eFigure 3, eFigure 5, eFigure 6, and eTable 14 in the Supplement). For example, estimated cardiometabolic mortality associated with SSBs was nearly twice as high in blacks (12.6%; the leading factor) vs whites (6.4%), and from low nuts/seeds, higher in Hispanics (11.7%; the leading factor) vs whites (7.9%). One exception was omega-3 fat–associated proportional mortality, which was higher in whites (8.0%). Relative rankings of cardiometabolic mortality related to different dietary factors were otherwise generally similar by race/ethnicity. Overall, suboptimal diet was associated with 53.1% of total estimated cardiometabolic deaths among blacks, 50.0% among Hispanics, and 42.8% among whites (absolute differences, +10.5% [95% UI, 8.0%–12.7%] for blacks vs whites and +7.2% [95% UI, 4.8%–9.8%] for Hispanics vs whites).
Estimated proportional diet-related cardiometabolic mortality was generally higher among individuals with low or medium education compared with high education (Figure 2; eFigure 4, eFigure 5, eFigure 7, and eTable 14 in the Supplement). This was most notable for nuts/seeds (in low vs high education, 10.7% vs 6.2% of cardiometabolic deaths), SSBs (8.4% vs 4.5%), and fruits (8.5% vs 6.4%). Overall, suboptimal diet was associated with 46.8% of cardiometabolic deaths for lower-, 45.7% for medium-, and 39.1% for higher-educated adults (absolute differences, +7.7% [95% UI, 4.9%–10.4%] for low vs high and +6.7% [95% UI, 4.1%–9.0%] for medium vs high).
Trends Between 2002 and 2012
Between 2002 and 2012, the total number of population-adjusted US cardiometabolic deaths per year decreased by 26.5%. Improvements were seen in national intakes of some factors, including polyunsaturated fats, nuts/seeds, SSBs, whole grains, and fruits (eFigure 8 in the Supplement). Thus, absolute numbers of diet-related cardiometabolic deaths decreased for all dietary factors (eTable 13 in the Supplement). As a percentage of annual cardiometabolic deaths, which accounts for underlying trends in absolute death rates, estimated diet-associated mortality declined for polyunsaturated fats (−20.8% smaller proportion of deaths; 95% UI, −18.5% to −22.8%), nuts/seeds (−18.0%; 95% UI, −14.6% to −21.0%), and SSBs (−14.5%; 95% UI, −12.0% to −16.9%); remained relatively stable for whole grains, fruits, vegetables, seafood omega-3 fats, and processed meats; and increased for sodium (+5.8%; 95% UI, 2.9%–8.8%) and unprocessed red meats (+14.4%; 95% UI, 9.1%–19.5%) (Figure 3). In 2002, excess SSB intake was the third leading risk factor for diet-associated cardiometabolic death among these 10 dietary factors, with an estimated 73 162 associated deaths, or 8.6% of all cardiometabolic deaths (see eTables 5, 8, and 12 for 2002 inputs and eFigures 9–16 in the Supplement for 2002 results overall and by population subgroups). In comparison, by 2012, SSBs had declined to the seventh cause of diet-associated deaths.
Figure 3. Change in Proportional Cardiometabolic Disease Mortality in the United States Between 2002 and 2012.
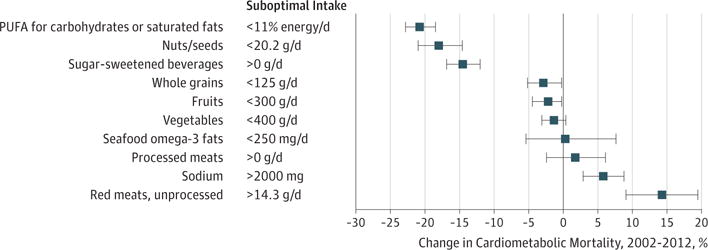
The bars represent the estimated relative changes in percentage of cardiometabolic deaths associated with 10 dietary factors between 2002 and 2012 compared with optimal intakes. These percentage changes correspond to (2012mortality−2002mortality)/2002mortality × 100. Error bars indicate 95% uncertainty intervals.
Proportional trends in cardiometabolic mortality associated with dietary factors were generally similar by sex and age (eFigures 5, 14, and 17 in the Supplement). Trends by race were also consistent with overall results, with some exceptions. For instance, the percentage of cardiometabolic deaths associated with insufficient nuts/seeds declined in whites (from 10.0% to 7.9%; −21.8% [95% UI, −35.8% to −3.4%]) but not in blacks or Hispanics, while the percentage of cardiometabolic deaths associated with insufficient whole grains declined in Hispanics (from 12.9% to 7.6%; −41.2% [95% UI, −49.8% to −28.8%]) but not in whites or blacks, yet Hispanics started at higher levels and declined to more similar associated burdens by 2012. Trends in diet-associated cardiometabolic deaths were also generally similar by education, except that the percentage of cardiometabolic deaths associated with low nuts/seeds declined in adults with high (8.7% to 6.2%; −29.7% [95% UI, −36.0% to −23.3%]) but not low (10.9% to 10.7%; −3.0% [95% UI, −8.4% to 6.3%]) education; and with SSBs declined more among adults with high (5.9% vs 4.5%; −23.9% [95% UI, −29.5% to −17.9%]) compared with low (9.2% vs 8.4%; −8.3% [95% UI, −12.6% to −4.0%]) education.
Discussion
Based on a comparative risk assessment model and nationally representative data, an estimated 45.4% of all cardiometabolic deaths (n=318 656 due to heart disease, stroke, and type 2 diabetes) were associated with suboptimal intakes of 10 dietary factors in 2012. By sex, larger diet-related proportional mortality was estimated in men than in women, consistent with generally unhealthier dietary habits in men. Suboptimal diet was also associated with larger proportional mortality at younger vs older ages, among blacks and Hispanics vs whites, and among individuals with low and medium education vs high education.
Among individual dietary components, the largest estimated mortality was associated with suboptimal sodium (9.5%) followed by nuts/seeds, processed meats, seafood omega-3 fats, vegetables, fruits, SSBs, and whole grains (each between 5.9%–8.5%), and, last, polyunsaturated fats (2.3%) and unprocessed red meats (0.4%). Estimated deaths related to processed meats and SSBs were higher among men than women. By age, SSBs were the leading estimated factor associated with cardiometabolic mortality between ages 25 and 64 years and sodium at age 65 years or older. Disparities were evident by race, especially for excess SSBs among blacks and insufficient nuts/seeds among Hispanics, and by education, especially for low nuts/seeds and fruits and excess SSBs among less-educated adults. Income-related disparities in current levels and trends over time of national consumption of nuts/seeds, fruits, and SSBs have been reported,17 which likely contribute to the disparities in diet-associated mortality by race and education identified in the present investigation.
Between 2002 and 2012, several improvements were identified. Even accounting for underlying declines in total cardiometabolic mortality, fewer diet-associated proportional deaths were related to excess SSBs and insufficient polyunsaturated fats and nuts/seeds. Improvements were not uniform. For example, less-educated individuals experienced no significant declines in cardiometabolic deaths associated with low nuts/seeds and smaller declines in cardiometabolic deaths associated with SSBs.
Nationally, estimated cardiometabolic deaths related to insufficient healthier foods/nutrients remained at least as substantial as those related to excess unhealthful foods/nutrients. These results inform strategies for prevention to reduce the health and economic burdens of cardiometabolic diseases in the United States. For example, positive messaging to patients, the public, and industry can emphasize maximizing the good (rather than simply reducing the harmful) food choices and products. Within the health system, changes to clinician education, multidisciplinary care teams, electronic health records, quality guidelines, and reimbursement standards can each facilitate lifestyle counseling and behavior change.1,23 At local or national levels, strategies with evidence for effectiveness include multicomponent school and workplace programs focused on healthier eating, economic incentives (eg, subsidies) for more healthful foods or taxation of less healthful foods, incentivized or mandated product reformulation (eg, to reduce additives such as sodium and trans fats), and restrictions on advertising of unhealthy foods to children.24,25 For example, the US Food and Drug Administration recently announced voluntary sodium reduction targets for the food industry,26 while in the 2016 elections, SSB taxes were passed in all 4 cities with this measure on the ballot.27 Compared with education alone, such “upstream” strategies could also reduce disparities. For example, disparities in diet-related cardiometabolic deaths identified in our investigation might be partly addressed by the Supplemental Nutrition Assistance Program (SNAP), which serves 44 million low-income individuals in the United States; for example, by expanding the SNAP Food Insecurity Nutrition Incentive program28 to provide wider incentives for purchasing fruits and vegetables as well as nuts/seeds and adding restrictions or disincentives for unhealthier products such as SSBs, processed meats, and high-sodium foods.29,30 Gaps in knowledge remain regarding cost-effectiveness, equity assurance, and political feasibility of dietary policies in different settings and within different subgroups; our results highlight the need for government and other stakeholders to prioritize implementation and evaluation of such strategies.
Among unhealthful foods/nutrients, the present findings suggest that sodium is a key target. Population-wide salt reduction policies that include a strong government role to educate the public and engage industry to gradually reduce salt content in processed foods (for example, as implemented in the United Kingdom and Turkey) appear to be effective, equitable, and highly cost-effective or even cost-saving.31,32 Such population approaches can also minimize challenges of public taste preferences, placing all companies on a level playing field and allowing the population’s taste receptors for salt to gradually upregulate, preventing any major perception of changes in taste.33 Functional benefits of salt in foods, such as for texture or food safety, must also be further addressed by advances in food processing.33
The decline in SSB-associated proportional mortality between 2002 and 2012 is promising. The current results suggest that continuing programs to reduce SSBs are important, especially among younger adults, blacks, Hispanics, and individuals in the United States with lower educational attainment. The price responsivity of SSBs34–36 makes tax strategies, already implemented in Mexico, the United Kingdom, and several US cities, an effective option. For example, evidence from Mexico suggests that a national SSB tax reduces overall consumption and with greatest benefits among those of lower socioeconomic status,35 reducing disparities. Whether these taxes ultimately improve health outcomes remains unknown.
This study extends and is consistent with a number of prior US analyses of diet-related cardiometabolic mortality have been performed. In an analysis from the Global Burden of Diseases Study, 26% of US deaths from all causes in 2010 were estimated to be related to joint suboptimal intakes of 14 dietary factors4; with approximately 40% of these deaths due to cardiometabolic diseases.4 In earlier analysis5 evaluating 5 dietary factors in 2005, excess sodium was estimated to be associated with 102 000 cardiovascular deaths, followed by low omega-3 fats (84 000 deaths), high trans fats (82 000 deaths), low fruits and vegetables (58 000 deaths), and low polyunsaturated fats (15 000 deaths). In global analyses of mortality related to excess sodium7 and SSBs15 in 2010, about 58 000 US cardiovascular deaths were estimated to be associated with sodium and 24 000 US cardiometabolic deaths with SSBs. The present investigation builds on and expands these prior reports by using direct national data on dietary intakes and mortality from NHANES and the NCHS rather than values imputed from these sources plus other global data4,5,7,15; gamma distributions for dietary factors, particularly relevant for skewed intakes such as for SSBs, nuts/seeds, and processed meats; and updated estimates of relative risks of dietary factors on disease risk.
Our investigation has several strengths. The modeling study design incorporated separately derived measures of demographics, dietary habits, optimal dietary intakes, disease rates, and estimated diet-disease relationships. This approach, which derived and estimated cardiometabolic mortality associated with dietary intakes based on external evidence, should be differentiated from an ecologic study design, which would assess cross-sectional correlations within a national data set. Relative risks were based on multivariable-adjusted meta-analyses of different dietary factors and cardiometabolic end points, with supportive validity analyses from both long-term cohorts and randomized clinical trials of dietary patterns. The modeling framework incorporated stratum-specific data, including by sex, age, race, and education, wherever relevant and available, increasing validity of our estimates and ability to evaluate disparities. Uncertainty was incorporated and quantified in the model inputs using probabilistic sensitivity analyses, allowing estimation of the bounds of plausible effects. Nationally representative data sets on dietary habits, cardiovascular risk factors, and death rates provide generalizability to the US adult population.
Potential limitations should be considered. Although every effort was made to maximize validity, minimize bias, and incorporate heterogeneity and uncertainty, the study’s comparative risk assessment model does not prove that changes in these dietary habits reduce disease risk. Causality is different from identifying associations. Estimated relative risks of individual dietary components could be limited by measurement error (typically causing underestimation of effects) and residual confounding (typically causing overestimation of effects). For any person, the contribution to health of each dietary component may be modified by other factors such as other dietary habits as well as age, sex, activity, adiposity, and genetics. Thus, as with any medical or public health intervention, our findings should be considered as estimates of the average population relationships. Dietary habits are intercorrelated, increasing complexity of estimating associations. Yet separate validity analyses of dietary pattern studies, including from interventional studies, suggested that the estimated relative risks for both individual components and their joint associations were reasonable. We limited our investigation to dietary factors with the strongest evidence, not including many other dietary factors that may influence cardiometabolic health, and except for SSBs did not incorporate additional potential effects of these dietary factors on obesity, which could underestimate the full health associations of poor diet.
Dietary habits were based on self-reported 24-hour recalls, which have known measurement errors for individual people; and sodium intake is best assessed by multiple 24-hour urine collections, not available in NHANES. Yet our analysis used stratum-specific mean intakes, which are measured reasonably well by 24-hour recalls; potential measurement error was further reduced by energy adjustment and distributions were corrected for within-person variation. Optimal intake levels for each dietary factor are not conclusively established and could be modestly lower or higher. If benefits continue beyond our optimal levels, the reported associated mortality may be underestimated. National cause-of-death data are prone to error; these findings should be considered the best available national data rather than perfect clinical determination of mortality burdens. National Center for Health Statistics data do not differentiate by type of diabetes, so our estimates may include a small number of deaths due to type 1 diabetes. Like most disease models, comparative risk assessment may underestimate mortality for risk factors with prolonged lag effects. For example, SSB intake is much higher in children and young adults, in whom measurable mortality would be low compared with older adults. Therefore, the full lifetime disease associations of suboptimal dietary habits at younger ages may be underestimated. Model estimates do not make assumptions about feasibility of interventions to improve diet, which must consider cost, production, distribution, cultural preferences, disparities, potential industry cooperation or opposition, and political feasibility.37 Thus, these findings should be considered estimates of national cardiometabolic mortality related to suboptimal intakes of these 10 dietary factors, and potential effects of specific interventions should be evaluated in future studies.
Conclusions
Dietary factors were estimated to be associated with a substantial proportion of deaths from heart disease, stroke, and type 2 diabetes. These results should help identify priorities, guide public health planning, and inform strategies to alter dietary habits and improve health.
Supplementary Material
Key Points.
Question
What is the estimated mortality due to heart disease, stroke, or type 2 diabetes (cardiometabolic deaths) associated with suboptimal intakes of 10 dietary factors in the United States?
Findings
In 2012, suboptimal intake of dietary factors was associated with an estimated 318 656 cardiometabolic deaths, representing 45.4% of cardiometabolic deaths. The highest proportions of cardiometabolic deaths were estimated to be related to excess sodium intake, insufficient intake of nuts/seeds, high intake of processed meats, and low intake of seafood omega-3 fats.
Meaning
Suboptimal intake of specific foods and nutrients was associated with a substantial proportion of deaths due to heart disease, stroke, or type 2 diabetes.
Acknowledgments
Funding/Support: This research was supported by the National Institutes of Health and the National Heart, Lung, and Blood Institute (grant R01 HL130735 to Dr Micha and grant R01 HL115189 to Dr Mozaffarian). Dr Peñalvo was partly supported by a Bunge Fellowship in Global Nutrition.
Role of the Funder/Sponsor: The funding agencies did not contribute to the design or conduct of the study; collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
CME Quiz at jamanetworkcme.com
Author Contributions: Drs Micha and Mozaffarian had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Micha, Mozaffarian. Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Micha, Peñalvo, Cudhea, Rehm, Mozaffarian.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Micha, Peñalvo, Cudhea, Rehm.
Obtained funding: Micha, Mozaffarian.
Administrative, technical, or material support: Micha, Rehm.
Supervision: Micha, Mozaffarian.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs Micha, Peñalvo, Cudhea, Rehm, and Mozaffarian report receipt of grants from the National Institutes of Health during the conduct of the study. Dr Imamura reports receipt of support from the Medical Research Council Epidemiology Unit Core Support. Dr Mozaffarian reports personal fees from Boston Heart Diagnostics, Haas Avocado board, AstraZeneca, GOED, DSM, Life Sciences Research Organization, and UpToDate.
References
- 1.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forouzanfar MH, Alexander L, Anderson HR, et al. GBD 2013 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ, Atkinson C, Bhalla K, et al. US Burden of Disease Collaborators The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: a multi-investigator collaboration for global comparative descriptive epidemiology. Lancet. 2012;380(9859):2055–2058. doi: 10.1016/S0140-6736(12)62134-5. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Fahimi S, Singh GM, et al. Global Burden of Diseases, Nutrition and Chronic Diseases Expert Group Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 8.Micha R, Kalantarian S, Wirojratana P, et al. Global Burden of Diseases, Nutrition and Chronic Disease Expert Group Estimating the global and regional burden of suboptimal nutrition on chronic disease: methods and inputs to the analysis. Eur J Clin Nutr. 2012;66(1):119–129. doi: 10.1038/ejcn.2011.147. [DOI] [PubMed] [Google Scholar]
- 9.Shulkin ML, Micha R, Rao M, Singh GM, Mozaffarian D. Major dietary risk factors for cardiometabolic disease: current evidence for causal effects and effect sizes from the Global Burden of Diseases (GBD) 2015 study. Circulation. 2016;133:AP279. [Google Scholar]
- 10.World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. Vol. 916. Geneva, Switzerland: World Health Organization; 2003. pp. i–viii. [PubMed] [Google Scholar]
- 11.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 12.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Agriculture, US Department of Health and Human Services. 2015-2020 Dietary Guidelines For Americans. 2015 https://health.gov/dietaryguidelines/2015/guidelines/. Accessed February 9, 2017.
- 14.Singh GM, Danaei G, Farzadfar F, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group; Asia-Pacific Cohort Studies Collaboration; Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in Europe; Emerging Risk Factor Collaboration; Prospective Studies Collaboration The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8(7):e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh GM, Micha R, Khatibzadeh S, Lim S, Ezzati M, Mozaffarian D; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group Estimated global, regional, and national disease burdens related to sugar-sweetened beverage consumption in 2010. Circulation. 2015;132(8):639–666. doi: 10.1161/CIRCULATIONAHA.114.010636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/. Accessed December 15, 2016.
- 17.Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999-2012. JAMA. 2016;315(23):2542–2553. doi: 10.1001/jama.2016.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett WC. Nutritional Epidemiology. 3rd. Oxford, England: Oxford University Press; 2013. Correction for the effects of measurement error; pp. 287–304. [Google Scholar]
- 19.Mozaffarian D. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med. 2013;369(7):673–674. doi: 10.1056/NEJMc1306659. [DOI] [PubMed] [Google Scholar]
- 20.Estruch R, Ros E, Salas-Salvadó J, et al. PREDIMED Study Investigators Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 21.Ezzati M, Lopez AD, Rodgers A, Murray CJ. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. 1/2. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 22.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodenheimer T. Helping patients improve their health-related behaviors: what system changes do we need? Dis Manag. 2005;8(5):319–330. doi: 10.1089/dis.2005.8.319. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Afshin A, Benowitz NL, et al. American Heart Association Council on Epidemiology and Prevention, Council on Nutrition, Physical Activity and Metabolism, Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on the Kidney in Cardiovascular Disease, Council on Peripheral Vascular Disease, and the American Heart Association Advocacy Coordinating Committee Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126(12):1514–1563. doi: 10.1161/CIR.0b013e318260a20b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afshin A, Penalvo J, Del Gobbo L, et al. CVD prevention through policy: a review of mass media, food/menu labeling, taxation/subsidies, built environment, school procurement, worksite wellness, and marketing standards to improve diet. Curr Cardiol Rep. 2015;17(11):98. doi: 10.1007/s11886-015-0658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. Draft Guidance for Industry: Voluntary Sodium Reduction Goals: Target Mean and Upper Bound Concentrations for Sodium in Commercially Processed, Packaged, and Prepared Foods. 2016 http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm494732.htm. Accessed November 25, 2016.
- 27.Belluz J. In a devastating blow to the beverage industry, 4 cities passed soda taxes. 2016 Nov 9; http://www.vox.com/2016/11/9/13571902/soda-taxes-vote-san-francisco-oakland-boulder-albany. Accessed November 25, 2016.
- 28.US Department of Agriculture. Food Insecurity Nutrition Incentive (FINI) grant program. 2016 https://nifa.usda.gov/program/food-insecurity-nutrition-incentive-fini-grant-program. Accessed November 25, 2016.
- 29.Harnack L, Oakes JM, Elbel B, Beatty T, Rydell S, French S. Effects of subsidies and prohibitions on nutrition in a food benefit program: a randomized clinical trial. JAMA Intern Med. 2016;176(11):1610–1618. doi: 10.1001/jamainternmed.2016.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Commision on Hunger. Recommendations of the National Commission on Hunger to Congress and the Secretary of the Department of Agriculture. Washington, DC: National Commission on Hunger; 2015. Freedom From Hunger: An Achievable Goal for the United States of America. [Google Scholar]
- 31.Wyness LA, Butriss JL, Stanner SA. Reducing the population’s sodium intake: the UK Food Standards Agency’s salt reduction programme. Public Health Nutr. 2012;15(2):254–261. doi: 10.1017/S1368980011000966. [DOI] [PubMed] [Google Scholar]
- 32.Webb M, Fahimi S, Singh GM, et al. Cost effectiveness of a government supported policy strategy to decrease sodium intake: global analysis across 183 nations. BMJ. 2017;356:i6699. doi: 10.1136/bmj.i6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henney JE, Taylor CL, Boon CS, editors. Strategies to Reduce Sodium Intake in the United States. Washington, DC: National Academies Press; 2010. Institute of Medicine Committee on Strategies to Reduce Sodium Intake Reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 34.Andreyeva T, Long MW, Brownell KD. The impact of food prices on consumption: a systematic review of research on the price elasticity of demand for food. Am J Public Health. 2010;100(2):216–222. doi: 10.2105/AJPH.2008.151415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704. doi: 10.1136/bmj.h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falbe J, Thompson HR, Becker CM, Rojas N, McCulloch CE, Madsen KA. Impact of the Berkeley excise tax on sugar-sweetened beverage consumption. Am J Public Health. 2016;106(10):1865–1871. doi: 10.2105/AJPH.2016.303362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afshin A, Micha R, Khatibzadeh S, Schmidt L, Mozaffarian D. Dietary policies to reduce noncommunicable diseases. In: Brown G, Yamey G, Wamala S, editors. The Handbook of Global Health Policy. Chichester, England: Wiley-Blackwell; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


