Abstract
Background
Nociceptive sensitization is an increase in pain perception in response to stimulus. Following brief irradiation of Drosophila larvae with UV, nociceptive sensitization occurs in class IV multiple dendritic (mdIV) neurons, which are polymodal sensory nociceptors. Diverse signaling pathways have been identified that mediate nociceptive sensitization in mdIV neurons, including TNF, Hedgehog, BMP, and Tachykinin, yet the underlying mechanisms are not completely understood.
Results
Here we report that duox heterozygous mutant larvae, which have normal basal nociception, exhibit an attenuated hypersensitivity response to heat and mechanical force following UV irradiation. Employing the ppk-Gal4 line, which is exclusively expressed in mdIV neurons, we further show that silencing duox in mdIV neurons attenuates UV-induced sensitization.
Conclusions
Our findings reveal a novel role for duox in nociceptive sensitization of Drosophila larvae, and will enhance our understanding of the mechanisms underlying this process in Drosophila sensory neurons.
Electronic supplementary material
The online version of this article (10.1186/s13041-018-0358-7) contains supplementary material, which is available to authorized users.
Keywords: Duox, ROS, Nociception, Drosophila
Background
Animals perceive noxious stimuli as pain. Peripheral sensory nociceptive neurons are activated upon nociceptive stimuli and transmit electric signals to central pain pathways, giving rise to pain perception and inducing escape behavior [1]. Nociceptive sensory neurons are ‘sensitized’ when nearby tissues are damaged, giving rise to pain hypersensitivity, which is manifested as hyperalgesia (pain amplification by painful stimuli) and allodynia (pain creation by non-painful stimuli) [2]. This pain sensitization is beneficial to animal survival since it helps to avoid touching damaged tissues until they are healed. However, in certain pathological conditions, persistent nociceptive sensitization generates chronic pain [3]. The molecular mechanisms underlying nociceptive sensitization are not fully understood.
In Drosophila larvae, class IV multiple dendritic (mdIV) neurons are polymodal nociceptive sensory neurons that induce arborization of dendrites underneath the larval skin [1, 4]. mdIV neurons acutely respond to diverse noxious stimuli including heat, mechanical force, noxious chemicals and reactive oxygen species (ROS) [1, 5–7]. Diverse ion channels are expressed in mdIV neurons to evoke depolarization in response to corresponding noxious stimuli [1, 5, 8–10]. Next to their acute nociceptive response, mdIV neurons accomplish nociceptive sensitization in response to brief ultraviolet-induced tissue damage in the larval skin [11]. Sensitized mdIV neurons give rise to hyperalgesia and allodynia in Drosophila larvae [11]. Like in mammals, tumor necrosis factor (TNF) signaling was shown to operate in mdIV neurons for nociceptive sensitization [11]. Recently, additional signalings including Hedgehog (hh) signaling, Bone Morphogenetic Protein (BMP) signaling and Tachykinin-like signaling have been shown to mediate nociceptive sensitization in mdIV neurons [12–14]. However, the underlying mechanisms are incompletely understood. Here we report the genetic analysis of ROS-generating Dual Oxidase (Duox) enzymes and find that Duox is required to mediate nociceptive sensitization in mdIV neurons.
Results
To examine whether Duox is involved in pain processing, we undertook a genetic analysis of duox mutants in D. melanogaster. The MiMiC element line MI11825 features an insertion into the 2nd intron of the duox gene (Fig. 1a). Duox [MI11825] homozygotes die as embryos, and are thus not available in the 3rd-instar larval stage for nociception analysis. We therefore used duox [MI11825] heterozygous mutant larvae, in which the transcript level of duox is greatly reduced (Fig. 1b). Duox heterozygous mutant larvae are normal in appearance, larval locomotion, and gentle touch response [15].
Fig. 1.
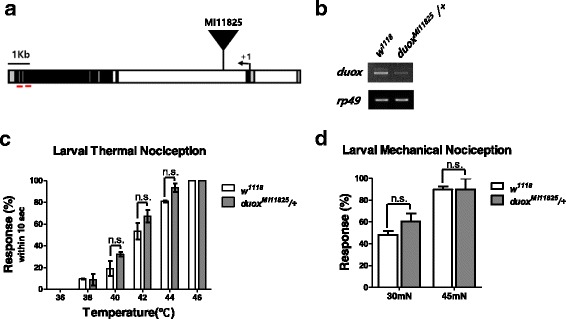
Basal nociception assay. a The Duox (CG3131) locus. The translation start site is marked with an angled arrow. The MiMiC element (Duox [MI11825]) was inserted into the 2nd intron. The UTRs, coding region, and introns are denoted in gray, black, and white, respectively. b RT-PCR of wild-type (w1118) and Duox [MI11825] heterozygous larvae. Binding sites of the primers used for PCR are indicated by short red lines in (a). The ribosomal protein Rp49 was used as a loading control. c Larval thermal nociception assay. Larval nociceptive response was counted if it occurred within 10 s of heat exposure (n = 60 per time section). d Larval mechanical nociception. Larval mechanical response to a mechanical force (30 and 45 mN) was counted (n = 75 per each mechanical force). Error bars denote +/− SEM. One-way ANOVA with Tukey post-test was used to analyze the differences. n.s., non-significant
Acute nociceptive response is not impaired in duox heterozygous mutant larvae
Wild-type larvae perceive heat and harsh mechanical force as nociceptive, and thus sensing these stimuli on the skin elicits a characteristic nociceptive response [1] (Additional files 1 and 2). Duox heterozygous mutant larvae exhibit a normal acute nociceptive response to heat and mechanical force, comparable to wild-type larvae (Fig. 1c-d), suggesting that their acute response to nociceptive stimuli is not impaired.
Additional file 1: Video 1. Typical larval behavior upon exposure to non-nociceptive substance. Heat probe, 35 °C. (MP4 4170 kb)
Additional file 2: Video 2. Typical larval nociceptive rolling behavior upon heat exposure. Heat probe, 45 °C. (MP4 4658 kb)
Nociceptive sensitization is impaired in duox heterozygous mutant larvae
Irradiating wild-type larvae briefly (5 s) with UV induces tissue damage that gives rise to nociceptive sensitization in mdIV neurons [11]. Accordingly, UV-irradiated wild-type larvae exhibited an increased nociceptive response to heat over time (Fig. 2a). Specifically, 20% of larvae demonstrated nociceptive response to a 40 °C heat probe, increasing to 40% of larvae at six hours post-irradiation; thus, this nociceptive sensitization is hyperalgesic (amplifies pain). In contrast, homozygous mutant larvae of the transient receptor potential ankyrin 1 (TrpA1) channel, a heat and chemical irritant sensor, exhibited no increased nociceptive response following UV irradiation (Fig. 2b), which is consistent with a published report [11]. Likewise, duox heterozygous mutant larvae exhibited no increased nociceptive response to heat (Fig. 2c), suggesting that duox is required for nociceptive sensitization, and hyperalgesia in particular.
Fig. 2.
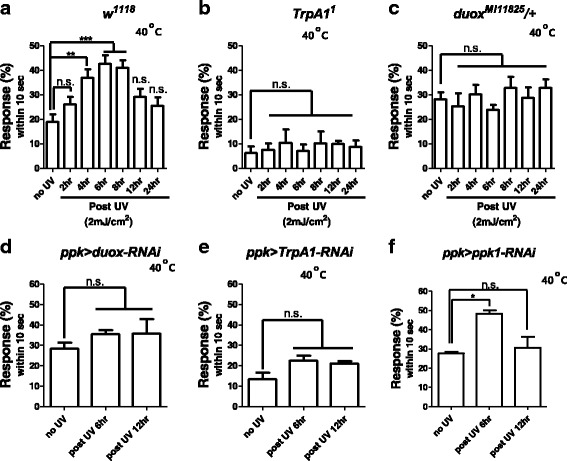
Thermal nociceptive sensitization assay. Larvae were irradiated with UV at 2 mJ/cm2. Larval response within 10 s to heat exposure (40 °C) was counted as nociceptive response. a Wild-type larvae (n = 90 per time section). b TrpA1 homozygous mutant larvae (n = 75 per time section). c Duox heterozygous mutant larvae (n = 90 per time section). d Duox silencing in mdIV neurons. Ppk > duox-RNAi denotes ppk-Gal4 > UAS-Duox-RNAi (38907) (n = 60 per time section). e TrpA1 silencing in mdIV neurons. Ppk > TRPA1-RNAi denotes ppk-Gal4 > UAS-TrpA1-RNAi (n = 45 per time section). f Ppk1 silencing in mdIV neurons. Ppk > ppk1-RNAi denotes ppk-Gal4 > UAS-ppk1-RNAi (n = 45 per time section). Error bars denote +/− SEM. One-way ANOVA with Tukey post-test was used to analyze the differences. *, ** and *** indicate p < 0.05, 0.01, and 0.001 respectively. n.s., non-significant
To examine whether duox functions in mdIV neurons, we silenced duox in mdIV neurons employing the pickpocket (ppk)-Gal4 line, which directs expression of Gal4 to mdIV neurons ([16]), and two duox RNAi lines (38,907 and 32,903). When driving expression of duox RNAi with ppk-Gal4 (ppk-Gal4 > UAS-Duox RNAi 38,907, 32,903), Duox transcript levels were reduced to 64% for duox RNAi 38,907 and to 78% for duox RNAi 32,903 (Additional file 3: Figure S1A-B). Importantly, nociceptive sensitization following UV treatment was attenuated for both duox RNAi 38,907 (Fig. 2d) and duox RNAi 32,903 (Additional file 3: Figure S1C). Likewise, silencing of TrpA1 in mdIV neurons (ppk-Gal4 > UAS-TrpA1 RNAi) attenuated nociceptive sensitization following UV treatment (Fig. 2e), consistent with a previous report. However, silencing of ppk1, a mechanosensitive channel, in mdIV neurons (ppk-Gal4 > UAS-ppk1 RNAi) did not abrogate heat nociceptive sensitization following UV irradiation (Fig. 2f), suggesting that Ppk1 is not involved in nociceptive sensitization.
It is of note that basal nociception was similar between ppk-Gal4 > UAS-duox (Fig. 2d) and ppk-Gal4 > UAS-ppk1 (Fig. 2f): ~ 28% of larvae from either line exhibited nociceptive response against 40 °C temperatures in the absence of UV irradiation. In contrast, basal nociception was reduced in ppk-Gal4 > UAS-TrpA1 (Fig. 2e), where ~ 12% of larvae demonstrated nociceptive response against 40 °C temperatures. These indicate that Duox and Ppk1 are not involved in basal nociception against heat while TrpA1 is, which was previously reported [17, 18].
We were additionally curious to learn whether UV-irradiated wild-type larvae (w1118) would exhibit an increased nociceptive response to mechanical force. Similar to the heat response, the increased nociceptive response of wild-type larvae (w1118) to mechanical force peaks at six hours after UV irradiation (Fig. 3a). In contrast, UV-irradiated TrpA1 homozygous mutant larvae exhibited no increased response to mechanical force (Fig. 3a). Similarly, UV-irradiated duox heterozygous mutant larvae exhibited no increased response to mechanical force (Fig. 3a). In agreement with the mutant analysis, duox and TrpA1 RNAi expression in mdIV neurons (ppk-Gal4 > UAS-Duox RNAi, ppk-Gal4 > UAS-TrpA1 RNAi) attenuated nociceptive sensitization (Fig. 3b-c).
Fig. 3.
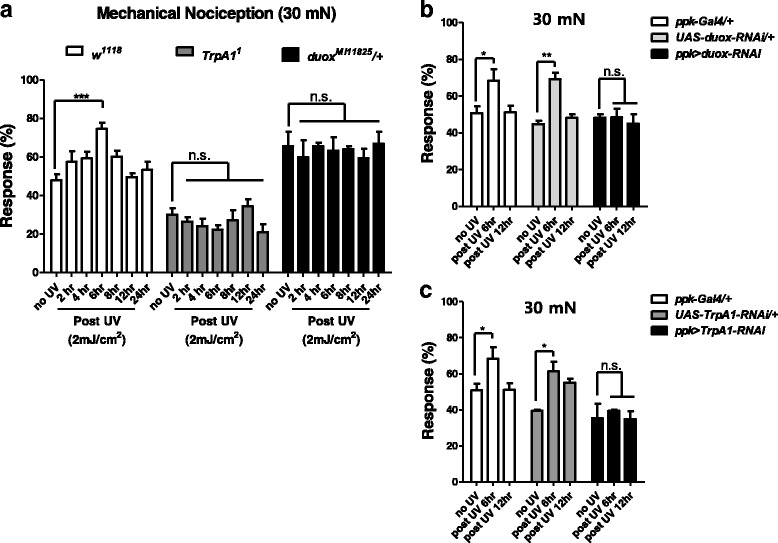
Mechanical nociceptive sensitization assay. Larvae were irradiated with UV at 2 mJ/cm2 . Larval nociceptive response to mechanical force (30 mN) was evaluated. a Wild-type (n = 120 per time section), TRPA1 homozygous mutant (n = 45 per time section), and duox heterozygous mutant larvae (n = 75 per time section). b Duox silencing in mdIV neurons. Ppk > duox-RNAi indicates ppk-Gal4 > UAS-duox RNAi (n = 60 per time section). c TrpA1 silencing in mdIV neurons. Ppk > TRPA1-RNAi indicates ppk-Gal4 > UAS-TRPA1 RNAi (n = 45 per time section). Error bars denote +/− SEM. One-way ANOVA with Tukey post-test was used to analyze the differences. *, ** and *** indicate p < 0.05, 0.01, and 0.001 respectively. n.s., non-significant
Discussion
We describe a novel role of duox in nociceptive sensitization in mdIV neurons. Firstly, our data show that duox heterozygous mutant larvae, which exhibit basal nociception, display defective hyperalgesia (pain amplification) to heat and mechanical force following UV irradiation. Secondly, duox silencing in mdIV neurons impairs induced hypersensitivity. Altogether, these genetic studies suggest that Duox is required in mdIV neurons to mediate UV irradiation-derived nociceptive sensitization.
It is of note that ~ 28% of larvae expressing either duox or ppk1 RNAi in mdIV neurons (ppk > Duox-RNAi and ppk > ppk1-RNAi) exhibited nociceptive response to 40 °C heat, as opposed to ~ 12% of larvae expressing TrpA1 RNAi. This suggests that silencing of duox or ppk1 in mdIV neurons does not affect basal nociception against 40 °C heat, while TrpA1 silencing reduces it. This makes sense in that Duox and Ppk1 are not heat sensors, while TrpA1 is [5, 17, 18]. Notably, duox silencing abrogated heat hypersensitivity while ppk1 silencing did not, highlighting the role of Duox in nociceptive sensitization.
We have shown that basal nociception against heat and harsh mechanical force is not affected by duox reduction, suggesting that mdIV neurons with reduced duox expression retain normal function in sensing nociceptive stimuli and in depolarization. To further confirm this notion, we determined whether structural defects were present in duox heterozygous mutant larvae. We examined the dendrites of duox heterozygotes using ppk-td-GFP lines that specifically expressed td-GFP in mdIV neurons [19]. Confocal images showed that the dendrites of mdIV neurons in duox heterozygous mutant larvae were not reduced in comparison to those of control larvae (Additional file 4: Figure S2); thus, the nociceptive sensitization defects in duox mutants are not due to a reduction of dendrites.
We propose that UV irradiation either directly or indirectly activates Duox expression and/or Duox activation in mdIV neurons. Diverse signaling pathways including TNF, Hedgehog, BMP, and Tachykinin have been shown to mediate UV irradiation-induced nociceptive sensitization in mdIV neurons [11–14]. These signaling pathways could induce the expression and/or activity of Duox [13, 20], and further research should be done to determine whether they do so in mdIV neurons.
The genetic knockdown of heat sensors painless and TrpA1 abolishes not only basal nociception but also UV-induced nociceptive sensitization [12]. This suggests that Painless and TrpA1 mediate nociceptive sensitization following UV irradiation. Duox is a member of the NADPH oxidase family, which produces reactive oxygen species (ROS) in a regulated manner [21]. We speculate that ROS produced by Duox following UV irradiation increase the gating of Painless and TrpA1 through direct oxidation.
Conclusions
Duox has been shown to catalyze dityrosine cross-links in epithelial cuticles, hormone synthesis, and mucosal immunity in Caenorhabditis elegans, D. melanogaster, and mammals. However, the role of Duox in pain signaling has not been addressed in any animal models. Our data uncover a novel role for Duox in the nociceptive sensitization of sensory nociceptors in Drosophila. Intriguingly, mammalian nociceptors employ a different member of the NADPH oxidase family in nociceptive sensitization [22]. Thus, our findings support the notion that the underlying mechanisms of nociceptive sensitization are evolutionarily conserved from insects to mammals.
Methods
Drosophila strains
Flies were reared on standard yeast/cornmeal agar medium at 25 °C. The ppk-GAL4 (#32078, #32079), ppk1-RNAi (#29571), TrpA1-RNAi (#31504), duox-RNAi (#32903, #38907), duox mutant line (duoxMI11825, #59037) and Ppk-td-GFP (35843) were from Bloomington Drosophila Stock Center.
RT-PCR
Larvae were collected under CO2 and frozen rapidly in liquid nitrogen. Total RNA from larvae was extracted using TRIzol (MRC) according to the manufacturer’s instructions. Reverse transcription (RT) was performed using AccuPower™ RT Premix (Bioneer K-2041) with 2 μg of total RNA in a 20-μl reaction. PCRs were performed on an AccuPower PCR Premix (Bioneer K-2016) with duox primers 5′- CTGCCCATCGCACAAGCACT-3′ and 5′- CTATCCAAAGTTCTCGAAGT-3′ and Rp49 primers 5’-AGATCGTGAAGAAGCGCACC-3′ and 5′-CACCAGGAACTTCTTGAATCCGG-3′.
UV treatment
Lightly ice-anesthetized early third-instar larvae were deposited on a 2% agarose plate and placed in a CL-1000 UV crosslinker (UVP). We used 0 mJ/cm2 (control) and 2 mJ/cm2 at a wavelength of 254 nm. After UV treatment, larvae were returned to the rearing medium at 25 °C before nociceptive sensitivity was assessed at various times after UV exposure.
Behavioral assays
Larval thermal nociception assays were performed as described previously [1]. Briefly, 3rd instar larvae were placed on 2% agarose medium in plastic petri dishes, and were laterally touched with a soldering iron with a 0.6-mm-wide chisel; its temperature was calibrated with a fine thermocouple. The behavioral responses of the larvae were recorded using a digital camera (Kenox, Samsung) and analyzed.
Larval mechanical nociception assays were performed as described earlier [5]. Briefly, 3rd instar larvae were stimulated at 45 mN with a calibrated Sulon monofilament fishing line (6-lb test, diameter 0.23 mm, length 18 mm) that was attached to a pipette. Noxious mechanical stimuli were delivered by rapidly depressing the larvae with the fiber on the dorsal side. Each larva was tested only once.
Additional files
Figure S1. A. (left) RT-PCR of 3rd instar larvae from lines Ppk-Gal4/+ (1), Ppk-Gal4 > UAS-duox-RNAi (38907) (2), and Ppk-Gal4 > UAS-Duox-RNAi (32903) (3). The primers used for duox PCR are the same as in Fig. 1. Rp49 was used as a loading control. (Right) Quantification of RT-PCR band areas by Image-J. (NIH). B. The band intensity of duox normalized to that of Rp49, and set to one for Ppk-Gal4/+. C. Larval thermal nociception assay. Rolling within 10 s of a 40 °C touch was counted as response (n = 30 per time section). Error bars denote +/− SEM. One-way ANOVA with Tukey post-test was used to analyze the differences. * and *** indicate p < 0.05 and 0.001 respectively. n.s., non-significant. (PPTX 148 kb)
Figure S2. Confocal microscopy reveals dendrites of mdIV neurons for Ppk-td-GFP/+ (left) and Ppk-td-GFP/duox [MI11852] larvae (right). These larvae specifically express td-GFP in mdIV neurons. (PPTX 755 kb)
Acknowledgments
We thank the Bloomington Drosophila Stock Center, Vienna Drosophila RNAi Center, and Exelixis Drosophila Collection for flies; and the Drosophila Genetic Resource Center for cDNA.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (Grant No. 2015R1A2A2A01004803).
Availability of data and materials
The datasets are included within the article.
Authors’ contributions
WJ conducted most of the genetic experiments, analyzed the results, and wrote most of the paper. MB conducted initial UV experiments. YSH provided materials. CK conceived the idea and wrote the paper with WJ. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interest with the contents of this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13041-018-0358-7) contains supplementary material, which is available to authorized users.
Contributor Information
Wijeong Jang, Email: kimoe@naver.com.
Minwoo Baek, Email: minwoo.baek@gmail.com.
Yeon Soo Han, Email: hanys@jnu.ac.kr.
Changsoo Kim, Email: changgk2001@hanmail.net.
References
- 1.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. Painless, a Drosophila gene essential for nociception. Cell. 2003;113(2):261–273. doi: 10.1016/S0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 2.Im SH, Galko MJ. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev Dyn. 2012;241(1):16–26. doi: 10.1002/dvdy.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17(24):2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20(5):429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468(7326):921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim MJ, Ainsley JA, Carder JW, Johnson WA. Hyperoxia-triggered aversion behavior in Drosophila foraging larvae is mediated by sensory detection of hydrogen peroxide. J Neurogenet. 2013;27(4):151–162. doi: 10.3109/01677063.2013.804920. [DOI] [PubMed] [Google Scholar]
- 8.Hwang RY, Stearns NA, Tracey WD. The ankyrin repeat domain of the TRPA protein painless is important for thermal nociception but not mechanical nociception. PLoS One. 2012;7(1):e30090. doi: 10.1371/journal.pone.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila piezo in mechanical nociception. Nature. 2012;483(7388):209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang W, Kim JY, Cui S, Jo J, Lee BC, Lee Y, Kwon KS, Park CS, Kim C. The anoctamin family channel subdued mediates thermal nociception in Drosophila. J Biol Chem. 2015;290(4):2521–2528. doi: 10.1074/jbc.M114.592758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19(10):799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babcock DT, Shi S, Jo J, Shaw M, Gutstein HB, Galko MJ. Hedgehog signaling regulates nociceptive sensitization. Curr Biol. 2011;21(18):1525–1533. doi: 10.1016/j.cub.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Im SH, Takle K, Jo J, Babcock DT, Ma Z, Xiang Y, Galko MJ. Tachykinin acts upstream of autocrine hedgehog signaling during nociceptive sensitization in Drosophila. elife. 2015;4:e10735. doi: 10.7554/eLife.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Follansbee TL, Gjelsvik KJ, Brann CL, McParland AL, Longhurst CA, Galko MJ, Ganter GK. Drosophila nociceptive sensitization requires BMP signaling via the canonical SMAD pathway. J Neurosci. 2017;37(35):8524–8533. doi: 10.1523/JNEUROSCI.3458-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12(6):1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 16.Grueber WB, Ye B, Yang CH, Younger S, Borden K, Jan LY, Jan YN. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134(1):55–64. doi: 10.1242/dev.02666. [DOI] [PubMed] [Google Scholar]
- 17.Neely GG, Keene AC, Duchek P, Chang EC, Wang QP, Aksoy YA, Rosenzweig M, Costigan M, Woolf CJ, Garrity PA, et al. TrpA1 regulates thermal nociception in Drosophila. PLoS One. 2011;6(8):e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong L, Bellemer A, Yan H, Ken H, Jessica R, Hwang RY, Pitt GS, Tracey WD. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP channel. Cell Rep. 2012;1(1):43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc Natl Acad Sci U S A. 2011;108(23):9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Lee WJ. Role of DUOX in gut inflammation: lessons from Drosophila model of gut-microbiota interactions. Front Cell Infect Microbiol. 2014;3:116. doi: 10.3389/fcimb.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, Kang D, Lee WJ. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009;16(3):386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Kallenborn-Gerhardt W, Schroder K, Geisslinger G, Schmidtko A. NOXious signaling in pain processing. Pharmacol Ther. 2013;137(3):309–317. doi: 10.1016/j.pharmthera.2012.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A. (left) RT-PCR of 3rd instar larvae from lines Ppk-Gal4/+ (1), Ppk-Gal4 > UAS-duox-RNAi (38907) (2), and Ppk-Gal4 > UAS-Duox-RNAi (32903) (3). The primers used for duox PCR are the same as in Fig. 1. Rp49 was used as a loading control. (Right) Quantification of RT-PCR band areas by Image-J. (NIH). B. The band intensity of duox normalized to that of Rp49, and set to one for Ppk-Gal4/+. C. Larval thermal nociception assay. Rolling within 10 s of a 40 °C touch was counted as response (n = 30 per time section). Error bars denote +/− SEM. One-way ANOVA with Tukey post-test was used to analyze the differences. * and *** indicate p < 0.05 and 0.001 respectively. n.s., non-significant. (PPTX 148 kb)
Figure S2. Confocal microscopy reveals dendrites of mdIV neurons for Ppk-td-GFP/+ (left) and Ppk-td-GFP/duox [MI11852] larvae (right). These larvae specifically express td-GFP in mdIV neurons. (PPTX 755 kb)
Data Availability Statement
The datasets are included within the article.


