Regulatory T-cell (Treg) depletion during chronic human immunodeficiency virus type 1 (HIV-1) infection increases immune activation and viral replication. During combination antiretroviral therapy, Treg depletion reactivates viral replication mainly in HIV-1–infected memory CD4+ T cells in lymphoid tissues, leading to a significant reduction of HIV-1 reservoirs upon resuppression.
Keywords: HIV-1, Tregs, chronic infection, antiviral treatment, cART-resistant reservoir
Abstract
Background
Regulatory T cells (Tregs) suppress T-cell immune activation and human immunodeficiency virus type 1 (HIV-1) replication, but the role of Tregs in HIV-1 reservoir persistence is poorly defined.
Methods
Tregs were depleted by denileukin diftitox in humanized mice with chronic HIV-1 infection. Viral replication in lineage cells was determined by p24 expression. Levels of HIV-1 RNA and DNA in human cells, as well as replication-competent-virus–producing cells, were measured to quantified viral replication and reservoirs.
Results
Treg depletion resulted in a blip of HIV-1 replication in T cells but not in myeloid cells. The major activated reservoir cells were memory CD4+ T cells in vivo. Interestingly, the transient activation of viral replication led to HIV-1 reservoir reduction after viremia resuppression, as indicated by the quantity of HIV-1 DNA and replication-competent-virus–producing cells. Furthermore, we demonstrated that Tregs use cyclic adenosine monophosphate (cAMP)–dependent protein kinase A pathway to inhibit HIV-1 activation and replication in resting conventional T cells in vitro.
Conclusion
Tregs suppress HIV-1 replication in T cells and contribute to HIV-1 reservoir persistence. cAMP produced in Tregs is involved in their suppression of viral gene activation and expression. Treg depletion combined with combination antiretroviral therapy provides a novel strategy for HIV-1 cure.
Human immunodeficiency virus type 1 (HIV-1) reservoirs, which harbor replication competent proviruses and persist during combination antiretroviral therapy (cART), compose one of the major barriers to curing HIV-1 infection [1–3]. Activation of the latent and/or low-replicating reservoir and subsequent killing of HIV-infected cells is required for cure [4, 5]. Studies suggest that pharmacological reagents such as histone deacetylase inhibitors can activate latent HIV-1 infection [6, 7]. However, these reagents alone are unlikely to efficiently activate and eliminate HIV-1 reservoirs in vivo. New approaches are needed to efficiently reactivate and kill HIV-1 reservoirs [8].
Humanized mice, which are created by transplantation of human hematopoietic stem cells or lymphoid tissue in immune-deficient mice, have been used to study human infectious diseases [9–11]. We have reported that NOD-rag1-/-Il2rgnull (NRG) humanized (hu-NRG) mice with functional human immune cells support persistent HIV-1 infection during suppressive cART [12]. Therefore, the hu-NRG mouse is a validated small-animal model for studying HIV-1 infection, immunopathogenesis, and therapy in vivo.
Regulatory T cells (Tregs) suppress T-cell activation and HIV-1 replication but are susceptible to HIV-1 infection [13–15]. In early infection, Tregs help establish viral infection in the acute phase of infection via Vpr-dependent mechanisms [14, 16]. In chronic infection, Tregs suppress hyperimmune activation, which may favor HIV-1 reservoir persistence [13]. Study demonstrated that Tregs inhibit HIV-1 replication in activated CD4+ T cells via a cyclic adenosine monophosphate (cAMP)–dependent mechanism [17], suggesting that Tregs may contribute to HIV-1 latency or low replication in T cells. The precise role of Tregs in HIV-1 replication during chronic infection, as well as in cART-resistant reservoirs, remains unknown. Here, we defined the role of Tregs in viral replication during chronic HIV-1 infection and its reactivation during cART in hu-NRG mice in vivo. Characterization of Treg-mediated suppression of HIV-1 replication and its reactivation during cART will shed light on the development of unique therapeutic approaches to cure HIV-1 infection.
MATERIALS AND METHODS
Construction of Humanized Mice
Human fetal liver tissue specimens were obtained from elective or medically indicated termination of pregnancy through a nonprofit intermediary working with outpatient clinics (Advanced Bioscience Resources). Approval for animal work was obtained from University of North Carolina (UNC) Institutional Animal Care and Use Committee. We constructed hu-NRG mice as previously reported [14, 15, 18–20].
Virus Production and Infection
HIV-1JR-CSF or vesicular stomatitis virus G protein (VSV-G)–pseudotyped HIV-1 expressing enhanced green fluorescent protein (EGFP) in nef were generated by transfection of pYK-JRCSF or R7GEmC/VSV-G plasmids, respectively, in 293T cells, using the calcium phosphate method. All plasmids were obtained from the National Institutes of Health (NIH) AIDS Reagent Program. Virus titers were measured by a p24 enzyme-linked immunosorbent assay (Cell Biolabs) and a MAGI-CCR5 assay [21, 22]. Animals were infected with HIV-1JR-CSF at a dose of 10 ng of p24 per mouse, through retro-orbital injection.
cART Regimens
Protease inhibitor (Brecanavir; 100 mg/kg) [23, 24], reverse transcriptase inhibitor (GW695634 [clinical trials identifier NCT00090077]; 100 mg/kg), and integrase inhibitor (Cabotegravir; 0.5 mg/kg) [25] were tested, formulated, and offered by GlaxoSmithKline as a preclinical test. All drugs were formulated as a wet-bead-milled suspension (2% Tween20, 2% PEG3350, and 4.5% mannitol) and administered subcutaneously every 5 days.
Denileukin Diftitox
Denileukin diftitox (Ontak; 150 μg/mL in citrate buffer) was provided by Ligand Pharmaceuticals. Humanized mice were injected intraperitoneally (100 μg/kg) with denileukin diftitox for 3 consecutive days. The percentage of CD25high CD4+ T cells in blood was used to assess Treg depletion over time in vivo.
Plasma Viral Load
HIV-1 RNA was purified from plasma, using the QIAamp Viral RNA Mini Kit. RNA was reverse transcribed and quantitatively detected by real-time polymerase chain reaction (PCR) analysis, using the TaqMan Fast Virus 1-Step PCR kit (ThermoFisher Scientific) and the QuantStudio 6 Flex PCR system (Applied Biosystems; detection limit, approximately 400 copies/mL) [12].
Flow Cytometry
Leukocytes were isolated from mouse lymphoid organs. Cells were stained and analyzed with either the CyAn ADP flow cytometer, using Summit 4.3 software (Dako), or a BD LSR Fortessa flow cytometer, using FACSDiva software (BD Biosciences). Data analysis was performed using Summit 4.3 or FlowJo software (FlowJo).
Cells were first stained with different combination of surface antibodies containing cell viability dye (anti-human HLA-DR-FITC, CD38-PE, CD4-PE/Cy5, CD3-PE/Cy7, CD3-Pacific Blue, CD8-PE/Cy7, CD25-PE, CD127-PerCP/Cy5.5, CD14-Pacific Blue, CD123-APC, CD11c-PE, and CD45-APC/Cy7 [all from Biolegend]; and anti-mouse CD45-Pacific Orange, anti-human CD4-PE/Texas Red or CD8-PE/Texas Red, and Live/Dead Fixable Aqua Dead Cell Stain Kit [all from Invitrogen]) and then stained intracellularly with anti-HIV p24-FITC (Beckman Coulter) or anti-human Foxp3 eFluor 660 (Affymetrix eBioscience) according to the manufactures’ instructions, as previously described [12, 14, 18, 20].
Immunofluorescence Staining
Antigen was retrieved in Diva Decloaker solution (Biocare Medical) for 30 minutes at 95°C, followed by cooling down for 1 hour. Tissue sections were blocked with Background Sniper (Biocare Medical), stained with the primary antibodies rabbit anti-human CD3 (Life Span Bio Sciences; 1:100 dilution) and mouse anti-HIV-1 p24 (Dako, 1:5 dilution) diluted in blocking buffer phosphate-buffered saline, 0.05% Tween 20, and 5% goat serum) overnight, and stained with the secondary antibodies Alexa Fluor 594 donkey anti-mouse immunoglobulin G (IgG; Life Technologies; 1:200 dilution) and Alexa Fluor 488 donkey anti-rabbit IgG (Life Technologies, 1:200 dilution). Finally, after DAPI staining, sections were mounted with antifade mounting medium (Abcam, Cambridge, MA). Sections were analyzed by confocal microscopy (Zeiss LSM 700).
Cell-Associated HIV-1 DNA
Total nucleic acid was extracted from spleen and bone marrow cells, using the DNeasy Mini Kit (Qiagen). HIV-1 DNA was quantified by real-time PCR analysis. Genomic DNA of ACH2, which contains 1 copy of HIV-1 genome in each cell, was serially diluted in mouse leukocyte DNA to generate a standard curve [12].
Cell-Associated HIV-1 RNA
Total RNA was extracted from spleen or bone marrow cells, using the RNeasy Plus Mini Kit (Qiagen). HIV-1 RNA was detected as described above. HIV-1 gag RNA expression were normalized to that of human CD4 messenger RNA, and relative levels of HIV-1 gene expression were calculated according to the 2-ΔΔCT method [12, 26].
Viral Outgrowth Assay
Splenocytes from humanized mice (1 × 106, 2 × 105, and 4 × 104 human cells in single, duplicates, or triplicates) were stimulated with phytohemagglutinin (2 μg/mL) and interleukin 2 (IL-2; 100 units/mL) for 24 hours. MOLT4/CCR5 cells were added on day 2 to enhance the survival of leukocytes and to support HIV-1 replication. Culture medium containing IL-2 and T-cell growth factor [12] was replaced on days 5 and 9. After 7 and 14 days of culture, supernatant was harvested individually, and HIV-1 real-time quantitative PCR was performed to score viral outgrowth. Estimated frequencies of cells with replication-competent HIV-1 were calculated using limiting dilution analysis.
Cell Isolation and Culture
Peripheral blood mononuclear cells were isolated from human blood buffy coats (Gulf Coast Regional Blood Center), using Ficoll (GE Healthcare). Resting CD4+ T cells were purified first by use of the CD4+ T Cell Isolation Kit (Miltenyi Biotec) and subsequently by negative selection for CD25− cells, using CD25 beads. CD4+CD25+CD127dim/− Tregs were purified by the Regulatory T Cell Isolation Kit II (Miltenyi Biotec). All isolation was done according to the manufacturer’s instructions. The purity of both conventional CD4+ T cells and Tregs was >90%.
In Vitro Culture Assay
Tregs or CD4+ T cells were first cultured with anti-CD3/CD28 antibodies (2 µg/mL) for 24 hours and then washed thoroughly before coculture. All cell cultures were incubated at 37°C and 5% CO2 in the presence of 20 U/mL IL-2 in Roswell Park Memorial Institute 1640 medium supplemented with 100 U/mL penicillin, 10 µg/mL streptomycin, 2 mM glutamine, 10 mM HEPES, and 10% fetal calf serum.
HIV-1-EGFP–transduced CD4+ T cells were cultured for 24 hours and then subjected to the following assays. Cells were treated with the cAMP agonist Sp-8-Br-CAMPS (125 µM; Biolog Life Science Institute), protein kinase A (PKA) inhibitor H89 (10 µM; Tocris Bioscience), or dimethyl sulfoxide individually. Forty-eight hours later, 50% of the supernatant was replaced with fresh medium containing the same amount of each compound, and culturing continued for another 48 hours before analysis.
HIV-1-EGFP–transduced CD4+ T cells were cocultured with activated Tregs or CD4+ T cells at ratio of 2:1 or were treated with the cAMP antagonist Rp-8-Br-CAMPS (500 µM; Biolog Life Science Institute) for 48 hours, and 50% of the supernatant was replaced with fresh medium. In some assay, Tregs were previously treated with 2′,5′-dideoxyadenosine (ddADA; 200 µM; Sigma-Aldrich), an inhibitor of adenyl cyclase, for 24 hours before coculture. For the cAMP antagonist assay, the same amount of drug was complemented in fresh medium. All cells were cultured for another 48 hours before analysis. All medium contained 20 U/mL human IL-2.
Statistical Analysis
A paired 2-tailed Student t test was used for analysis of all in vitro assay data. A P value of < .05 was considered statistically significant. An unpaired t test or Mann-Whitney test was performed to analyze animal data; a P value of < .05 was considered statistically significant. Data were analyzed using GraphPad Prism software, version 6.0 [15]. All data are reported as mean values ± standard deviations.
RESULTS
Persistent HIV-1 Infection and cART-Resistant Reservoirs in hu-NRG Mice
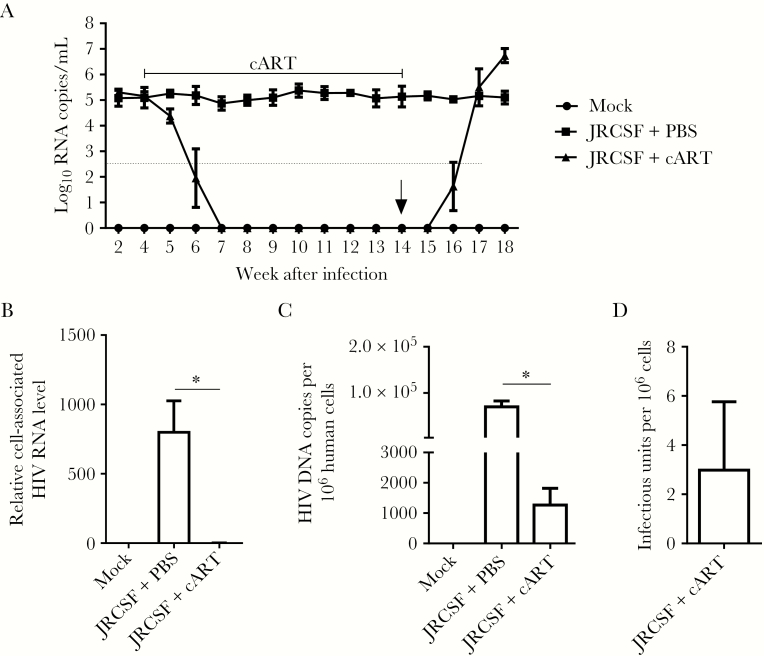
Blood samples were collected from the tail vein of hu-NRG mice infected with HIV-1JR-CSF for plasma viral load detection. HIV-1 viremia persisted stably for >18 weeks after infection (Figure 1A and Supplementary Figure 1A). The CD4+ T-cell count decreased progressively over time (Supplementary Figure 1B–E), whereas CD8+ T cells showed a persistently higher level of immune activation in infected animals (Supplementary Figure 1F and 1G). To model HIV-1 therapy, we administered cART regimens to hu-NRG mice to stably suppress viral replication, as quantified either by the viremia level or by immunohistochemical staining of lymphoid tissues. cART was started 4 weeks after infection, when chronic infection had been established. As shown in Figure 1A, viremia became undetectable 2–3 weeks after cART initiation and remained undetectable during cART. We euthanized several animals 14 weeks after infection but before cART withdrawal, when viral replication was significantly suppressed in cells (Figure 1B). Similar to findings in cART recipients with undetectable viremia [27], cell-associated viral DNA could be frequently detected in spleens (Figure 1C), while replication-competent HIV-1 reservoirs remained (Figure 1D) in animals despite their abolished viremia and markedly reduced cell-associated viral RNA load as compared to cART-naive animals. In addition, HIV-1 replication rebounded rapidly upon cART cessation (Figure 1A and 1B) [28–31]. Therefore, HIV-1 infection in hu-NRG mice is a relevant and robust model for studying persistent HIV-1 infection, immunopathogenesis, and cART-resistant reservoirs in vivo.
Figure 1.
Humanized mice support persistent human immunodeficiency virus type 1 (HIV-1) infection and respond to combination antiretroviral therapy (cART) with cART-resistant HIV-1 reservoirs. Humanized mice were infected with HIV-1JRCSF and started cART 4 weeks after infection. cART was stopped 14 weeks after infection (6 underwent mock infection [mock], 8 underwent HIV-1JRCSF infection and received phosphate-buffered saline [PBS] placebo [JRCSF+PBS], and 8 underwent HIV-1JRCSF infection and received cART [JRCSF+cART]). Several animals (3 in the mock group, 4 in the JRCSF+placebo group, and 4 in the JRCSF+cART group) were euthanized 14 weeks after infection. A, Plasma viral load measured over time. The dotted line indicates the detection limit (400 copies/mL), and “0” on the y-axis indicates that virus was undetected. B, Relative levels of cell-associated HIV-1 RNA in human cells from spleens were quantified by real-time polymerase chain reaction (PCR). C, Cell-associated HIV-1 DNA copies per million human cells in spleen, quantified by real-time PCR. D, Splenic cells producing replication-competent virus were detected by the quantitative virus outgrowth assay. Experiments were repeated twice. Data from each experiment were pooled and summarized. Error bars display standard deviations. *P < .05.
Tregs Suppress Viral Replication During Chronic HIV-1 Infection In Vivo
To confirm that denileukin diftitox, an IL-2 receptor binding domain fused to diphtheria toxin, could specifically deplete Tregs, we analyzed the frequency of Tregs or CD25+ T cells after denileukin diftitox treatment. We found that denileukin diftitox specifically depleted CD4+CD127−CD25highFoxP3+ Tregs (Supplementary Figure 2A and 2B) but not CD4+CD127+CD25+ (Supplementary Figure 2C and 2D) or CD4+CD127+FoxP3+ (Supplementary Figure 2E and 2F) T cells in lymphoid organs. CD8+CD25+ T cells were not significantly affected by denileukin diftitox in hu-NRG mice (Supplementary Figure 2G and 2H).
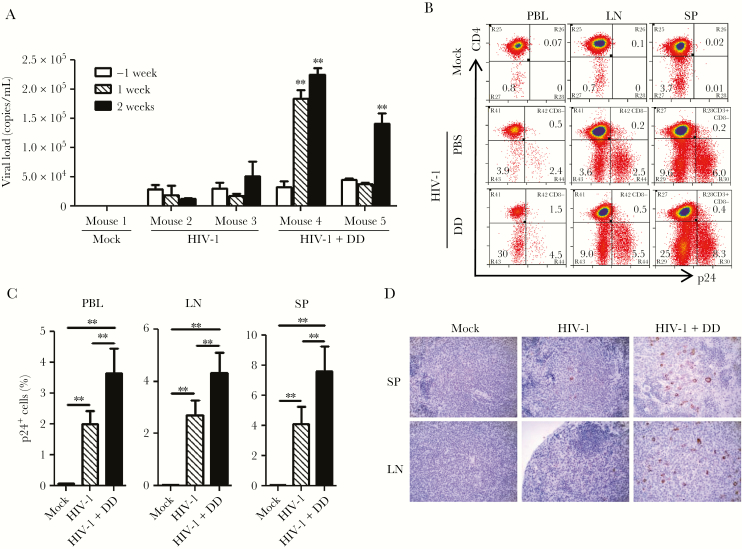
To determine the role of Tregs in chronic HIV-1 infection, we treated HIV-1–infected animals with denileukin diftitox 11 weeks after infection with single injections on 3 consecutive days, and all animals were euthanized 13 weeks after infection. As expected, denileukin diftitox increased the frequency of HLA-DR+CD38+CD8+ T cells in hu-NRG mice (Supplementary Figure 3) [14, 15] and significantly increased the plasma viral load 2 weeks after denileukin diftitox treatment (Figure 2A). At the time mice were euthanized, HIV-1 gag p24 expression in CD4+ T cells in peripheral blood specimens, mesenteric lymphoid nodes, and spleen specimens was mostly expressed in CD3+CD8−CD4+ T cells (Figure 2B), and viral replication was significantly enhanced by denileukin diftitox (Figure 2C), which was confirmed by p24 immunohistochemical staining of spleen specimens (Figure 2D). These data suggest that Tregs suppress host immune activation and HIV-1 replication during chronic HIV-1 infection.
Figure 2.
Regulatory T-cell (Treg) depletion increases human immunodeficiency virus type 1 (HIV-1) replication in chronically infected humanized mice. Phosphate-buffered saline (PBS; mock) or denileukin diftitox (DD; Ontak) was injected 11 weeks after infection to humanized mice infected with HIV-1JRCSF, and all mice were euthanized 2 weeks after DD treatment (13 weeks after infection). A, Plasma viral load measured before or after first DD injection. The week −1 time point indicates 1 week before DD treatment. B, Representative plots of HIV-1 p24 expression in CD3+CD8− human cells in peripheral blood (PBL), lymph node (LN), and spleen (SP) specimens are shown. C, The frequency of p24+ cells in CD3+CD8− cells from PBL, LN, and SP specimens from 3 mice in the mock group, 6 in the HIV-1 group, and 7 in the HIV-1+DD group. D, Immunohistochemical staining of HIV-1 p24 (brown) in SP and LN specimens from the mock, HIV-1, and HIV-1+DD groups. The experiments were repeated twice. The data from each experiment were merged and summarized. Error bars display standard deviations. *P < .05.
Treg Depletion Induces HIV-1 Activation During Suppressive cART in hu-NRG Mice
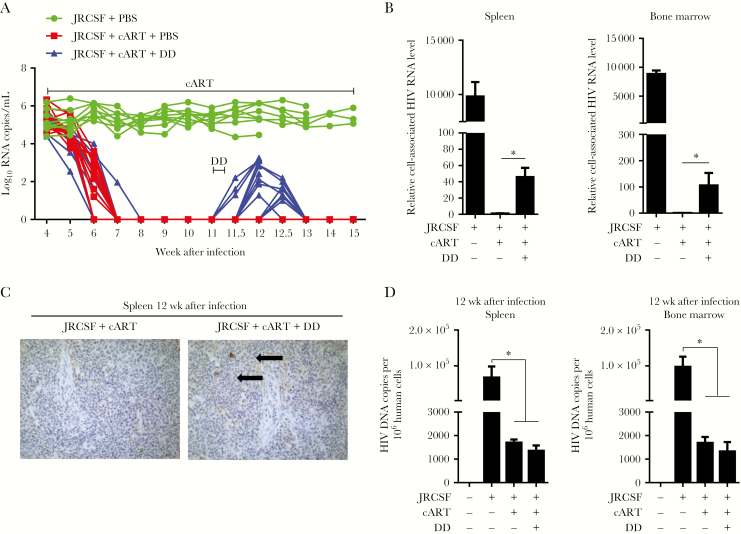
We hypothesized that Tregs contribute to the establishment and/or persistence of HIV-1 reservoirs during cART because of their suppression of T-cell activation and viral replication. To investigate the role of Tregs in HIV-1 reservoir maintenance, we started to deplete Tregs when viremia was completely suppressed by cART (Figure 3A). Interestingly, Treg depletion induced a blip of HIV-1 replication accompanied by a significant increase in the levels of cell-associated RNA in the spleen and bone marrow 12 weeks after infection (Figure 3A and 3B and Supplementary Figure 4). Immunohistochemical staining confirmed that a significant number of cells became p24 positive in the spleens of denileukin diftitox–treated mice, indicating that activation of HIV-1 replication was mediated by denileukin diftitox treatment (Figure 3C). However, there was no significant change in cell-associated viral DNA levels in lymphoid tissues 12 weeks after infection in denileukin diftitox–treated mice, compared with mice that received cART only (Figure 3D), nor was the level of cells with replication-competent virus affected by denileukin diftitox treatment (Supplementary Figure 5). The lack of increase in the number of HIV-1–infected cells indicates that the elevated HIV-1 replication induced by Treg depletion was not due to HIV-1 infection of new cells or to cART failure. We analyzed HIV-1 pol gene sequences from viruses associated with the rebound in viral load and found no mutations associated with cART resistance (data not shown), indicating that cART-resistant mutants or newly infecting virus is not responsible for the viral load rebound. Thus, these results suggest that HIV-1 replication was reactivated from the cellular reservoir (harboring latent or low-level-replicating virus) by Treg depletion.
Figure 3.
Regulatory T-cell (Treg) depletion induces human immunodeficiency virus type 1 (HIV-1) reactivation during combination antiretroviral therapy (cART) in humanized mice. HIVJRCSF-infected humanized mice were administered cART in their diet from 4 to 15 weeks after infection. Denileukin diftitox (DD; Ontak) was injected intraperitoneally for 3 consecutive days, beginning 11 weeks after infection (7 mice underwent mock infection [mock], 9 were infected with HIVJRCSF and received phosphate-buffered saline [PBS] placebo [JRCSF+PBS], 14 were infected with HIVJRCSF and received cART and PBS [JRCSF+cART+PBS], and 15 were infected with HIVJRCSF and received cART and DD [JRCSF+cART+DD]). Several mice (2 in the mock group, 3 in the JRCSF+PBS group, 6 in the JRCSF+cART+PBS group, and 6 in the JRCSF+cART+DD group) were euthanized 12 weeks after infection. A, Plasma viral load measured over time. B, Relative levels of cell-associated HIV-1 RNA in human cells from spleen and bone marrow. C, Immunohistochemical (IHC) staining of HIV-1 p24 (brown) in spleen specimens from the mock and HIV-1+cART+DD groups. D, Cell-associated HIV-1 DNA copies per million human cells in spleen and bone marrow specimens. The experiments were repeated three times. Twelve weeks after infection, data from 2 experiments were combined. Error bars display standard deviations. *P < .05.
HIV-1 Is Reactivated From Memory CD4+ T Cells but Not Myeloid Cells Upon Treg Depletion
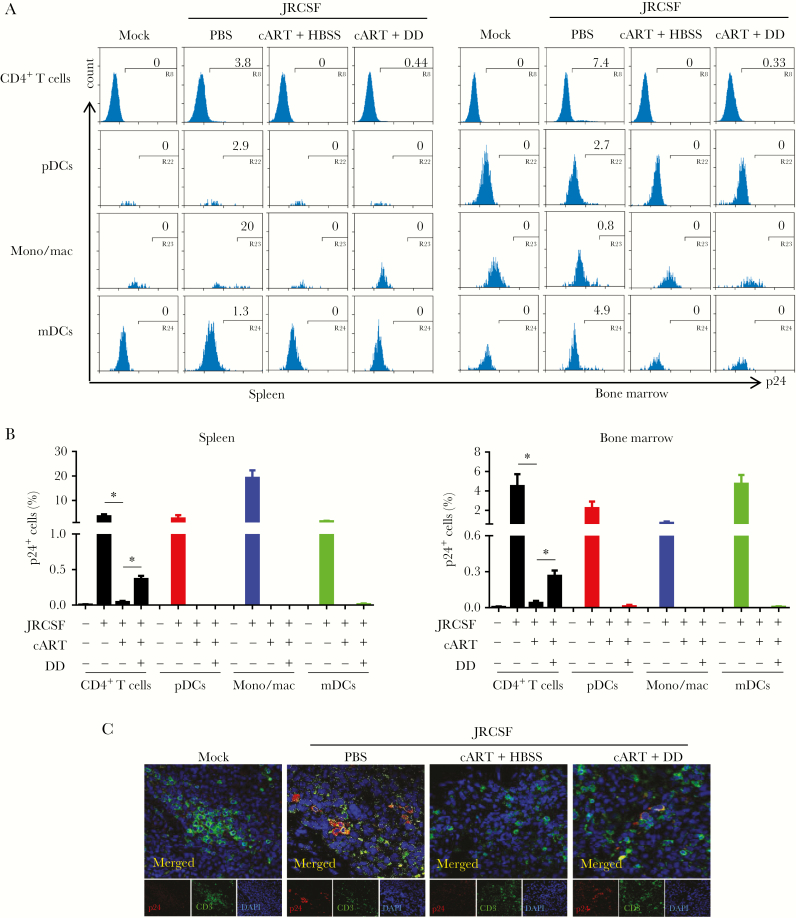
We used flow cytometry to understand what cell types respond to denileukin diftitox treatment and discovered that CD4+ T cells were the major cell type in spleen and bone marrow that showed activated viruses (Figure 4A and 4B). Other CD4+ cells, including plasmacytoid dendritic cells (CD3−CD4+CD123+), monocytes/macrophages (CD3−CD4+CD14+), and myeloid dendritic cells (CD3−CD14− CD4+CD11c+), did not exhibit HIV-1 replication (Figure 4A). Pooled data confirmed the preferential activation of HIV-1 gene expression in CD4+ T cells induced by Treg depletion (Figure 4B and Supplementary Figure 6A). We also performed immunofluorescence staining to detect both HIV-1–positive myeloid cells (p24+/CD3−) and T cells (p24+/CD3+) in spleen. However, all p24+ cells in Treg-depleted, cART-recipient mice were CD3+ cells, whereas no significant p24 expression was detected in mice that received cART only (Figure 4C), indicating that T cells infected with HIV-1 were the major reservoir cells responding to Treg depletion. Further analysis revealed that memory CD4+ T cells (CD3+CD8−CD45RA−) but not naive T cells (CD3+CD8−CCR7+CD45RA+) were the responding cells in spleen and bone marrow and that both effector memory CD4+ T cells (CD3+CD8−CCR7−CD45RA−) and central memory T cells (CD3+CD8−CCR7+CD45RA−) appeared to harbor activated viruses (Figure 5A and 5B). Pooled data clearly demonstrated the activation of HIV-1 in memory cells in vivo (Figure 5C and 5D and Supplementary Figure 6B and 6C). Notably, there was no dramatic increase in general immune activation in mice that received denileukin diftitox comparing to cART alone group (Supplementary Figure 7), as revealed by HLA-DR+CD38+ T-cell levels. Therefore, a mechanism other than suppression of T-cell activation contributes to suppression of HIV-1 replication by Tregs during suppressive cART.
Figure 4.
Depletion of regulatory T cells (Tregs) reactivates human immunodeficiency virus type 1 (HIV-1) replication in T cells but not in myeloid cells in humanized mice. Humanized mice were treated as described in Figure 1A and euthanized 12 weeks after infection. A, Representative histogram shows the percentage of HIVJRCSF gag p24+ cells in CD3+CD8− cells (CD4+ T cells), CD3−CD11c−CD4+CD123+ cells (plasmacytoid dendritic cells [pDCs]), CD3−CD4+CD14+ cells (monocytes/macrophages [mono/mac]), or CD3−CD14−CD4+CD11c+ cells (myeloid dendritic cells [mDCs]) in spleen and bone marrow. B, Summarized frequencies of HIVJRCSF gag p24+ cells in CD4+ T cells, pDCs, mono/mac, or mDCs in spleen and bone marrow specimens. C, HIVJRCSF p24 in spleen specimens was detected by immunofluorescence staining with anti-human CD3 (green), anti-HIV-1 p24 (red), and DAPI (blue). Data are representative results for merged (top) and individual (bottom) stains. Data are pooled from 2 experiments. Error bars display standard deviations. cART, combination antiretroviral therapy; DD, denileukin diftitox; HBSS, Hank’s balanced salt solution; PBS, phosphate-buffered saline. *P < .05.
Figure 5.
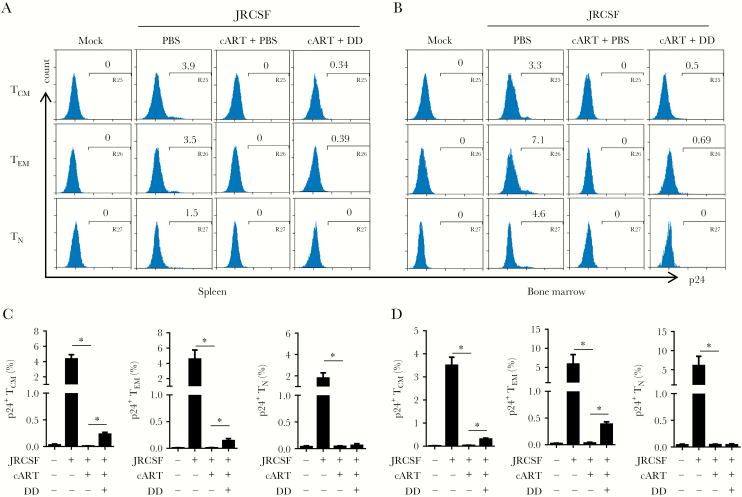
Regulatory T-cell (Treg) depletion reactivates human immunodeficiency virus type 1 (HIV-1) replication in memory T cells in vivo. Humanized mice were treated as described in Figure 1A and euthanized 12 weeks after infection (2 underwent mock infection [mock], 3 underwent HIVJRCSF infection and received phosphate-buffered saline [PBS] placebo [JRCSF+PBS], 6 underwent HIVJRCSF infection and received combination antiretroviral therapy [cART] and PBS [JRCSF+cART+PBS], and 6 underwent HIVJRCSF infection and received cART and denileukin diftitox [DD; JRCSF+cART+DD]). Naive CD4+ T cells (TN; CD3+CD8−CD45RA+CCR7+), central memory CD4+ T cells (TCM; CD3+CD8−CD45RA−CCR7+), and effector memory CD4+ T cells (TEM; CD3+CD8−CD45RA−CCR7−) were analyzed for p24 expression. A and B, Representative histograms of HIVJRCSF p24+ cells in TCM, TEM, and TN in spleen (A) and bone marrow (B) specimens. C and D, Summarized frequencies of HIVJRCSF gag p24+ cells in TCM, TEM, and TN in spleen (C) and bone marrow (D) specimens. Data from 2 experiments are pooled. Error bars display standard deviations. cART, combination antiretroviral therapy; DD, denileukin diftitox; PBS, phosphate-buffered saline. *P < .05.
Treg Depletion Leads to a Reduction of HIV-1 Reservoirs In Vivo
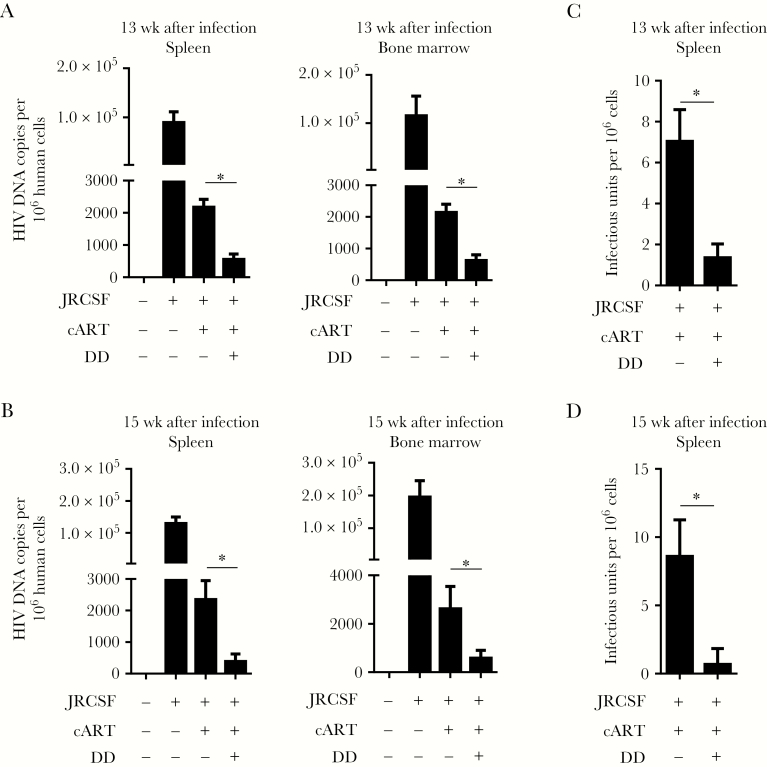
Viral replication was resuppressed by cART to an undetectable level 13 weeks after infection, and suppression continued until 15 weeks after infection, which again suggested cART-escape mutants or newly infecting viruses were not responsible for the rebound in HIV-1 load mediated by Treg depletion (Figure 3A). We analyzed the Treg status 12, 13, or 15 weeks after infection and found a transient depletion in Tregs at about 1 week after denileukin diftitox treatment (Supplementary Figures 4 and 8). The question is whether this transient activation and subsequent resuppression of viral replication could substantially reduce HIV-1 reservoirs. Therefore, we detected cell-associated viral DNA and cells with replication-competent virus at either 13 or 15 weeks after infection. Strikingly, along with the resuppression of viral replication, cell-associated viral DNA levels were significantly decreased in lymphoid tissue specimens from Treg-depleted animals at both 13 and 15 weeks after infection, compared with controls (Figure 6A and 6B). Accordingly, cells with replication-competent virus were also reduced significantly by denileukin diftitox treatment (Figure 6C and 6D). However, we did not observe a significant change in both total human CD45+ (Supplementary Figure 9) or human CD4+ (Supplementary Figure 10) T-cell numbers in spleen and bone marrow during 12–15 weeks after infection. Therefore, Treg depletion combined with suppressive cART could activate the HIV-1 reservoir in memory CD4+ T cells and subsequently reduce cART-resistant HIV-1 reservoirs, suggesting that Tregs contribute to the persistence of cART-resistant reservoir cells in vivo.
Figure 6.
Regulatory T-cell (Treg) depletion combined with suppressive combination antiretroviral therapy (cART) leads to a reduction of human immunodeficiency virus type 1 (HIV-1) reservoirs in humanized mice. Humanized mice were infected and treated as described in Figure 1A. Mice were euthanized 13 weeks after infection (3 mice underwent mock infection [mock], 3 underwent HIVJRCSF infection and received phosphate-buffered saline [PBS] placebo [JRCSF+PBS], 5 underwent HIVJRCSF infection and received combination antiretroviral therapy [cART] and PBS [JRCSF+cART+PBS], and 6 underwent HIVJRCSF infection and received cART and denileukin diftitox [DD; JRCSF+cART+DD]) or 15 weeks after infection (2 in the mock group, 3 in the JRCSF+PBS group, 3 in the JRCSF+cART+PBS group, and 3 in the JRCSF+cART+DD group). A, Cell-associated HIVJRCSF DNA copies per million human cells detected by real-time polymerase chain reaction (PCR) in spleen and bone marrow specimens 13 weeks after infection. B, Cell-associated HIVJRCSF DNA copies per million human cells detected by real-time PCR in spleen and bone marrow specimens 15 weeks after infection. C, Replication-competent virus producing cells in spleens detected by the quantitative virus outgrowth assay at 13 weeks after infection. D, Replication-competent HIVJRCSF in spleens detected by the quantitative virus outgrowth assay 15 weeks after infection. Data from 2 experiments are combined. Error bars display standard deviations. *P < .05.
HIV-1 Gene Activation in Resting CD4+ T Cells Is Suppressed via Adenylyl Cyclase–Dependent cAMP in Tregs
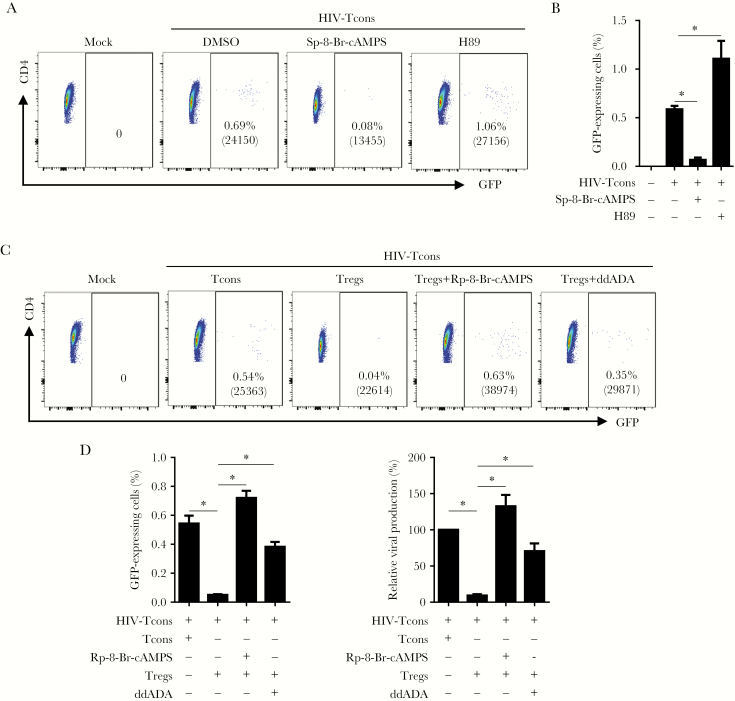
Previous reports suggested that Tregs suppress HIV-1 replication in conventional T cells through a cAMP-dependent mechanism [17, 32–35]. To determine whether cAMP is involved in suppression of HIV-1 activation in conventional T cells, we performed experiments on purified CD25− resting conventional CD4+ T cells, using the VSV-G HIV-1/eGFP reporter virus. Conventional T cells were treated with either the cAMP agonist Sp-8-Br-cAMPS (a PKA activator) or H89 (a PKA inhibitor) 24 hours after reporter virus inoculation (Supplementary Figure 11). As demonstrated in Figure 7A and 7B, HIV-1 gene expression (as determined by GFP expression) in conventional T cells was abolished by Sp-8-Br-cAMPS but significantly increased in HIV-1-infected cells treated with H89. Meanwhile, the GFP mean fluorescence intensity in GFP-expressing CD4+ T cells was also significantly reduced and enhanced, respectively. Notably, we found that a portion of HIV-1-infected cells did not express viral genes (ie, as determined by a lack of GFP expression) in resting cells but could be induced to express viral genes by activation with CD3/CD28 antibody (Supplementary Figure 12). Thus, the increased percentage of cells expressing GFP, as well as the mean fluorescence intensity of GFP, represents activation or enhancement of HIV replication. Furthermore, we treated purified Tregs with ddADA (an adenyl cyclase inhibitor) to decrease intracellular levels of cAMP in Tregs, and then we cocultured them with HIV-1-infected conventional T cells or cocultured infected resting CD4+ T cells with Tregs in the presence of the cAMP antagonist Rp-8-Br-cAMPS. We found that ddADA significantly decreased the suppressive activity of Tregs, whereas the cAMP antagonist increased HIV-1 gene expression as compared to the control (Figure 7C and 7D). In parallel, we labeled HIV-1–infected resting T cells with BrdU after cAMP agonist or PKA inhibitor treatment and detected no difference in cell proliferation between treatment and control (Supplementary Figure 13). Moreover, no CD25 upregulation was observed in cells during an in vitro assay (data not shown). These results suggest that Treg-produced cAMP-dependent PKA activation plays a pivotal role in Treg-mediated inhibition of HIV-1 activation or replication in resting CD4+ T cells.
Figure 7.
Human immunodeficiency virus type 1 (HIV-1) activation and gene expression in CD4+ T cells is suppressed via adenylyl cyclase–dependent cyclic adenosine monophosphate (cAMP) in regulatory T cells (Tregs). Resting conventional T cells (Tcons) were inoculated with HIV-1 expressing enhanced green fluorescent protein (GFP) and cultured for 24 hours. A and B, The cAMP agonist Sp-8-Br-CAMPS (125 µM), the protein kinase A (PKA) inhibitor H89 (10 µM), and activated Tcons or activated regulatory T cells (Tregs) were added into the culture individually. Forty-eight hours later, half of the medium was replaced with fresh medium containing the same amount of each compound and cultured for another 48 hours to analyze GFP-expressing CD4+ T cells. A, Representative plots show percentages of GFP-expressing conventional T cells. B, Summarized data show percentages of GFP-expressing conventional T cells from panel A. C and D, Coculture of HIV-Tcons with activated Tregs (2:1) with or without pretreatment with 2′,5′-dideoxyadenosine (ddADA) or in the presence of the cAMP antagonist Rp-8-Br-cAMPS. Activated Tcons were used as a control. C, Representative plots show percentages of GFP-expressing cells. D, Summarized GFP-expressing cells from Figure 7C are shown in either absolute (left panel) or relative (normalized to HIV-Tcons+Tcons; right panel) percentages. Mean fluorescence values for GFP are specified in parentheses in each plot. Bars represent standard deviations. DMSO, dimethyl sulfoxide. *P < .05.
DISCUSSION
Two of the most critical questions in the effort to cure HIV-1 infection are identification and activation of cART-resistant HIV-1–reservoir cells [2]. Humanized mouse models supporting a level of HIV DNA persistence in vivo comparable to that in HIV patients during suppressive cART [27] have been reported to be robust small-animal models for studying HIV-1 infection, persistence, and therapy [9, 20, 36–38]. Upon cART cessation, HIV-1 rapidly rebounds to pretreatment levels, and the cART-resistant HIV-1 reservoir is associated with latently infected memory T cells, as well as with a residual reservoir involving low-level replication in lymphoid organs (Figure 1) [29, 30]. We demonstrate that depletion of Tregs activates HIV-1 gene expression or replication in cART-resistant long-lived CD4+ memory T cells and subsequently reduces HIV-1 reservoirs during cART in vivo (Figures 3–6 and Supplementary Table 1). These findings significantly contribute to the understanding of the establishment and persistence of the HIV-1 reservoir in vivo and suggest that modulating Treg activity could be a novel strategy to activate the HIV-1 reservoir and cure infection [39].
A recent study demonstrated that depletion of Tregs in simian immunodeficiency virus (SIV)–controlling macaques activates latent SIV infection, increases immune activation, and boosts the SIV-specific T-cell immune response in the absence of cART [40]. It is possible that Treg depletion led to de novo SIV infection and increased immune activation, contributing to the rebound in virus load, as reported. With suppressive cART in this study, the increased cell-associated viral RNA and protein expression represents virus replication in reservoir cells mediated by Treg depletion in vivo, whereas we did not observe a marked increase in immune activation when combining Treg depletion with cART. More importantly, activation of HIV-1 reservoirs by treatment with denileukin diftitox reduced levels of HIV-1–reservoir cells in vivo. There are several potential mechanisms for the reduction of HIV-1 reservoirs. First, Tregs could be HIV-1–reservoir cells themselves, because Tregs are susceptible to HIV-1 infection, especially R5 viruses [15, 17], and support efficient HIV-1 replication, compared with resting conventional CD4+ T cells [32, 33, 41]. Second, Tregs suppress immune activation and the anti–HIV-1 immune response, which favors HIV-1 persistence [40, 42]. Therefore, Treg depletion could enhance the anti–HIV-1 immune response and kill HIV-1–positive cells during the rebound of viral replication. Third, CD4+ T cells are sensitive to cytopathic effects induced by HIV-1 replication [43]. The mechanisms by which Treg depletion induce significant activation and reduction in the level of HIV-1–reservoir cells should be further investigated.
Other reports suggest that proliferation of HIV-1–reservoir cells, due to HIV-1 integration in cancer-related genes [44, 45] or latency in long-lived memory T stem cells [46], may contribute to HIV-1 reservoir formation and maintenance. A main function of Tregs is to suppress naive and memory T-cell activation and proliferation [47]. Thus, Treg depletion possibly contributes to both the activation of HIV-1 and the expansion of HIV-1–reservoir cells. However, our data indicated no significant increase in cell-associated viral DNA during the peak level of virus rebound after denileukin diftitox treatment. Therefore, the increase in HIV replication is unlikely due to the expansion of HIV-1–reservoir cells.
The molecular mechanisms by which Tregs suppress HIV-1 activation during cART may be multifactorial. Recent reports have shown that the CD39/adenosine pathway in Tregs can suppress HIV-1 replication in T cells, as well as the activation and function of effector T cells [17, 33, 48], which requires cell-cell contact–dependent transfer of cAMP. Using an in vitro system, we showed that the suppression of HIV-1 activation and replication in resting conventional CD4+ T cells is dependent on PKA activation and cAMP produced by Tregs. Further research into the mechanisms of Treg-mediated HIV-1 persistence will shed light on the development of novel strategies to cure HIV-1 infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Anthony T. Curtis, Liqun Chi, and Qiong He, for technical assistance; Qi Jiang and Grisha Kovalev, for early assistance of the project.; the University of North Carolina (UNC) Division of Laboratory Medicine, for animal care; the UNC Center for AIDS Research, Flow Cytometry Core Facility, and Animal Histopathology Core Facility; and members of the Su laboratory, for critical reading and/or discussion of the manuscript and for their input and assistance.
G. L., J. N., L. C., and J. J. planned, designed, and performed the experiments and wrote the manuscript. N. R. B. performed tissue immunofluorescence staining. L. T. performed assays to detect HIV-1 RNA and DNA in tissue specimens. L. S. conceived the research project and wrote the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grants R01AI077454, AI080432, and R01AI095097 to L. S.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Immunology 2017, Washington, D. C., 12–16 May 2017.
References
- 1. Siliciano JD, Kajdas J, Finzi D et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–8. [DOI] [PubMed] [Google Scholar]
- 2. Siliciano RF, Greene WC. HIV latency. Cold Spring Harb Perspect Med 2011; 1:a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finzi D, Hermankova M, Pierson T et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 4. Choudhary SK, Margolis DM. Curing HIV: Pharmacologic approaches to target HIV-1 latency. Annu Rev Pharmacol Toxicol 2011; 51:397–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorenzo-Redondo R, Fryer HR, Bedford T et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Archin NM, Liberty AL, Kashuba AD et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012; 487:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shirakawa K, Chavez L, Hakre S, Calvanese V, Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol 2013; 21:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014; 20:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Su L. HIV-1 immunopathogenesis in humanized mouse models. Cell Mol Immunol 2012; 9:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bility MT, Li F, Cheng L, Su L. Liver immune-pathogenesis and therapy of human liver tropic virus infection in humanized mouse models. J Gastroenterol Hepatol 2013; 28(Suppl 1):120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung C, Chijioke O, Gujer C et al. Infectious diseases in humanized mice. Eur J Immunol 2013; 43:2246–54. [DOI] [PubMed] [Google Scholar]
- 12. Cheng L, Ma J, Li J et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J Clin Invest 2017; 127:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmes D, Jiang Q, Zhang L, Su L. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunol Res 2008; 41:248–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang Q, Zhang L, Wang R et al. FoxP3+CD4+ regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2-/-gammaC-/- mice in vivo. Blood 2008; 112:2858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oswald-Richter K, Grill SM, Shariat N et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol 2004; 2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med 2009; 15:696–700. [DOI] [PubMed] [Google Scholar]
- 17. Antons AK, Wang R, Oswald-Richter K et al. Naive precursors of human regulatory T cells require FoxP3 for suppression and are susceptible to HIV infection. J Immunol 2008; 180:764–73. [DOI] [PubMed] [Google Scholar]
- 18. Li G, Cheng M, Nunoya J et al. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS Pathog 2014; 10:e1004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L, Jiang Q, Li G, Jeffrey J, Kovalev GI, Su L. Efficient infection, activation, and impairment of pDCs in the BM and peripheral lymphoid organs during early HIV-1 infection in humanized rag2⁻/⁻γ C⁻/⁻ mice in vivo. Blood 2011; 117:6184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood 2007; 109:2978–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol 1992; 66:2232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pirounaki M, Heyden NA, Arens M, Ratner L. Rapid phenotypic drug susceptibility assay for HIV-1 with a CCR5 expressing indicator cell line. J Virol Methods 2000; 85:151–61. [DOI] [PubMed] [Google Scholar]
- 23. Lalezari JP, Ward DJ, Tomkins SA, Garges HP. Preliminary safety and efficacy data of brecanavir, a novel HIV-1 protease inhibitor: 24 week data from study HPR10006. J Antimicrob Chemother 2007; 60:170–4. [DOI] [PubMed] [Google Scholar]
- 24. Hazen R, Harvey R, Ferris R et al. In vitro antiviral activity of the novel, tyrosyl-based human immunodeficiency virus (HIV) type 1 protease inhibitor brecanavir (GW640385) in combination with other antiretrovirals and against a panel of protease inhibitor-resistant HIV. Antimicrob Agents Chemother 2007; 51:3147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrews CD, Spreen WR, Mohri H et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 2014; 343:1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–8. [DOI] [PubMed] [Google Scholar]
- 27. Lamers SL, Rose R, Maidji E et al. HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy-treated patients with undetectable viral loads. J Virol 2016; 90:8968–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choudhary SK, Archin NM, Cheema M, Dahl NP, Garcia JV, Margolis DM. Latent HIV-1 infection of resting CD4⁺ T cells in the humanized Rag2⁻/⁻ γc⁻/⁻ mouse. J Virol 2012; 86:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choudhary SK, Rezk NL, Ince WL et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2-/-{gamma}c-/- mouse. J Virol 2009; 83:8254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Denton PW, Olesen R, Choudhary SK et al. Generation of HIV latency in humanized BLT mice. J Virol 2012; 86:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marsden MD, Kovochich M, Suree N et al. HIV latency in the humanized BLT mouse. J Virol 2012; 86:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holmes D, Knudsen G, Mackey-Cushman S, Su L. FoxP3 enhances HIV-1 gene expression by modulating NFkappaB occupancy at the long terminal repeat in human T cells. J Biol Chem 2007; 282:15973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dunham RM, Cervasi B, Brenchley JM et al. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J Immunol 2008; 180:5582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moreno-Fernandez ME, Rueda CM, Rusie LK, Chougnet CA. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood 2011; 117:5372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jenabian MA, Seddiki N, Yatim A et al. Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV infection. PLoS Pathog 2013; 9:e1003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watanabe S, Terashima K, Ohta S et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 2007; 109:212–8. [DOI] [PubMed] [Google Scholar]
- 37. Sun Z, Denton PW, Estes JD et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med 2007; 204:705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hofer U, Schlaepfer E, Baenziger S et al. Inadequate clearance of translocated bacterial products in HIV-infected humanized mice. PLoS Pathog 2010; 6:e1000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katlama C, Deeks SG, Autran B et al. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet 2013; 381:2109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thorborn G, Pomeroy L, Isohanni H, Perry M, Peters B, Vyakarnam A. Increased sensitivity of CD4+ T-effector cells to CD4+CD25+ Treg suppression compensates for reduced Treg number in asymptomatic HIV-1 infection. PLoS One 2010; 5:e9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moreno-Fernandez ME, Zapata W, Blackard JT, Franchini G, Chougnet CA. Human regulatory T cells are targets for human immunodeficiency Virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J Virol 2009; 83:12925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nilsson J, Boasso A, Velilla PA et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 2006; 108:3808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Février M, Dorgham K, Rebollo A. CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses 2011; 3:586–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maldarelli F, Wu X, Su L et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014; 345:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wagner TA, McLaughlin S, Garg K et al. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014; 345:570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buzon MJ, Sun H, Li C et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 2014; 20:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med 2001; 193:1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bopp T, Becker C, Klein M et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med 2007; 204:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.