Summary
The reemergence of Zika virus (ZIKV) warrants the need to develop complementary diagnostic measures. In this study, flow cytometry was used to determine the presence of ZIKV NS3 antigen in blood monocytes from ZIKV-infected patients
Keywords: Zika virus, monocytes, detection, diagnosis
Abstract
Background
Epidemics caused by the reemergence of Zika virus (ZIKV) warrant the need to develop new diagnostic measures to complement currently used detection methods. In this study, we explored the detection of ZIKV antigen in a defined leukocyte subset from patients’ whole-blood specimens.
Methods
Whole-blood samples were obtained at the acute and early convalescent phases from ZIKV-infected patients during the Singapore outbreak in August–September 2016. Presence of ZIKV antigen was determined by flow cytometry staining for intracellular ZIKV NS3, using a ZIKV-specific polyclonal antibody. The presence of ZIKV antigen was determined in CD45+CD14+ monocytes.
Results
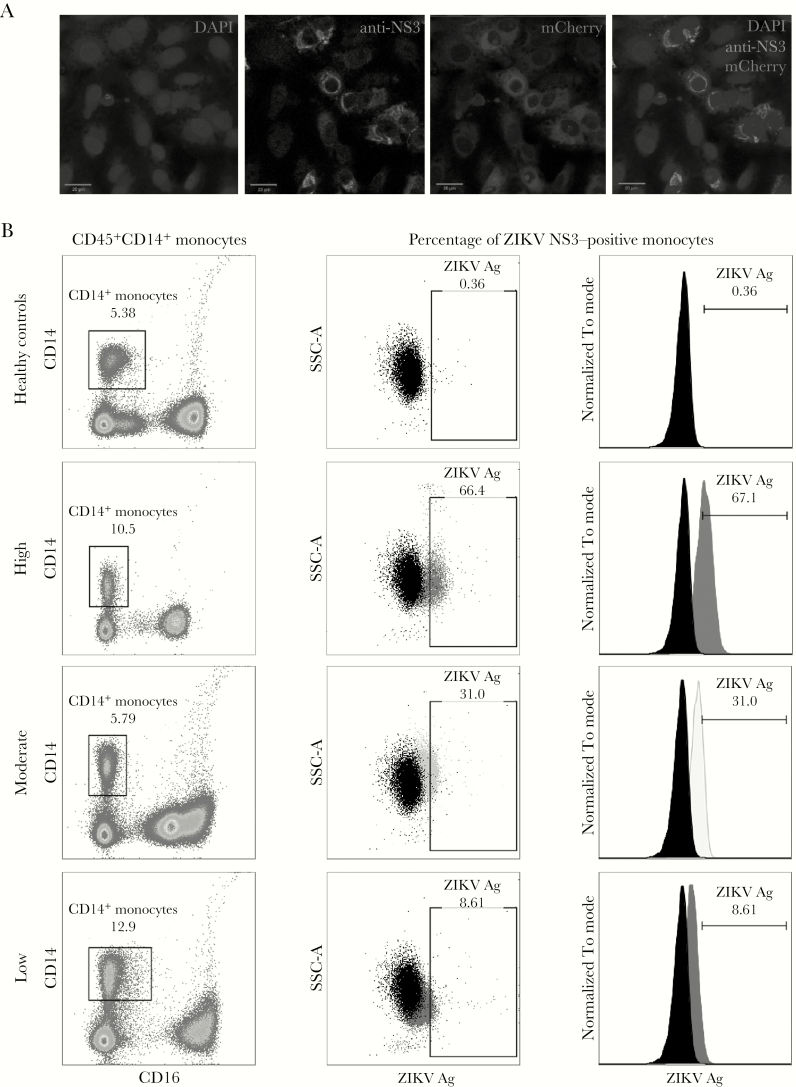
Data showed that ZIKV NS3 antigen could be detected in CD45+CD14+ monocytes. The levels of detection were further categorized into 3 groups: high (positivity among >40% of monocytes), moderate (positivity among 10%–40%), and low (positivity among <10%). While a majority of patients showed a decrease in the amount of ZIKV antigen detected at later time points, some patients displayed higher levels as the disease progressed.
Conclusions
Our data highlights an alternative approach in using flow cytometry as a sensitive method for detecting ZIKV antigen in whole blood. Importantly, it further confirms the role of CD14+ monocytes as an important cellular target for ZIKV infection during the viremic phase.
First identified in sentinel monkeys in Uganda [1], Zika virus (ZIKV) is an arthropod-borne flavivirus responsible for the ZIKV epidemics since 2007. This virus typically causes a self-limiting infection that can present as a mild febrile illness or have no apparent symptoms [2]. However, in extreme cases, ZIKV infection has been linked to neurological complications and congenital anomalies such as microcephaly in babies born to ZIKV-infected mothers [3].
ZIKV emerged as a global threat for the first time in 2007, in the Yap Island of the Federated States of Micronesia [2], and went on to cause numerous outbreaks around the world [4–7]. The impact of the ZIKV outbreaks is such that >1 million cases have been reported in Brazil alone [8]. As of October 2016, ZIKV transmission has now reemerged in countries in Southeast Asia, including Vietnam, Thailand, Malaysia, and Singapore [9]. In Singapore, approximately 500 cases of ZIKV infection have been identified since late August 2016 [10].
While investigation into ZIKV-related pathogenesis is important, efforts toward ZIKV diagnostic methods remain critical. Reverse-transcription polymerase chain reaction (RT-PCR) is the method of choice for most laboratories, and a positive test result is definitive indicator of acute ZIKV infection. In addition to cell-free serum, urine has been recommended as a suitable specimen because of prolonged shedding of the virus in urine [11]. However, as the disease symptoms are mild, with a significant proportion of patients being asymptomatic [2], the viremic phase may be over when the patients seek medical attention. On the other hand, the use of serological diagnostic methods remains limited by the frequent cross-reactivity of antibodies among other endemic flaviviruses [12–14]. Currently, there is also a lack of sensitive antigenic-detection procedures [11, 15]. Therefore, it is necessary to establish an alternative and complementary detection procedure that identifies active viral infection, such as one that detects ZIKV nonstructural proteins.
While ZIKV infection and tropism in human whole blood has not been extensively defined, it has been demonstrated that the myeloid cells remain a viable cellular target [16, 17]. In this study, we investigated the detection of ZIKV antigen in blood monocytes. This was achieved by staining for the presence of ZIKV NS3 protein in small volumes of whole-blood samples obtained from ZIKV-infected patients. This new method of ZIKV detection would be a nice complement to the clinical diagnosis of ZIKV infection and the screening of blood donations.
METHODS
Study Approval
Whole-blood samples were collected with ethylenediaminetetraacetic acid–lined Vacutainer tubes (Becton Dickinson) from patients referred to the Communicable Disease Centre, Tan Tock Seng Hospital, Singapore. Blood specimens were obtained from patients consenting to the study. All patients gave separate written informed consent. The study protocols were approved by the SingHealth Centralized Institutional Review Board (reference 2016/2219) and by the National Healthcare Group Domain Specific Review Board (reference 2015/00528). Blood samples collected from healthy donors were done with written consent in accordance with guidelines from the Health Sciences Authority of Singapore (National University of Singapore Institutional Review Board study approval number 10-250).
Patients and Whole-Blood Samples
This study included whole-blood samples obtained from 47 patients admitted to the Communicable Disease Centre at Tan Tock Seng Hospital from 27 August through 18 October 2016. Samples were collected at 2 phases: acute (1–7 days after illness onset) and early convalescent (8–14 days after illness onset). Of these patients, 23 (48.93%) were females, and 24 (51.07%) were males. The majority of the recruited patients were Chinese (39 [82.98%]), with 2 Indians (4.26%), 3 Malays (6.38%), and 3 with unknown ethnic group (6.38%) making up the cohort. The median age of the patients was 33 years (range, 16–63 years). These patients were confirmed to be infected with ZIKV [18], according to RT-PCR performed on serum and urine samples obtained during their first visit to the Communicable Disease Centre. All patients were screened to be negative for dengue virus (DENV) infection. Fever (temperature, >37°C) was determined during admission to the hospital (Table 1). Whole-blood samples were obtained from healthy volunteers as controls and were screened to be negative for both ZIKV and DENV RNA.
Table 1.
Infection and Viral Load Data From Zika Virus (ZIKV)–Infected Patients
| Patient | Viral Loada | Acute Phaseb | Early Convalescent Phaseb | Feverd | |||
|---|---|---|---|---|---|---|---|
| Serum | Urine | Time After Illness Onset, d | Infectionc | Time After Illness Onset, d | Infectionc | ||
| Patient 1 | + | + | … | … | 12 | Low | No |
| Patient 2 | – | + | … | … | 12 | Low | No |
| Patient 3 | – | + | … | … | 12 | Low | No |
| Patient 4 | + | + | 7 | Low | 14 | Moderate | Yes |
| Patient 5 | – | + | 6 | Moderate | 10 | Moderate | No |
| Patient 6 | + | + | 3 | Low | 12 | Moderate | No |
| Patient 7 | + | + | 1 | Low | … | … | No |
| Patient 8 | + | + | 5 | Low | 14 | Low | No |
| Patient 9 | + | + | 3 | Low | … | … | No |
| Patient 10 | – | + | 5 | Low | 12 | Low | No |
| Patient 11 | + | + | 2 | Low | … | … | No |
| Patient 12 | – | + | 4 | Low | … | … | No |
| Patient 13 | + | + | 7 | Moderate | 10 | Low | Yes |
| Patient 14 | + | + | 3 | High | … | … | No |
| Patient 15 | + | + | 3 | Low | 12 | High | No |
| Patient 16 | + | + | 4 | Moderate | 11 | Low | No |
| Patient 17 | + | + | 4 | High | 12 | Moderate | Yes |
| Patient 18 | – | + | 3 | High | … | … | Yes |
| Patient 19e | – | – | 4 | Moderate | 12 | Low | No |
| Patient 20 | + | + | 3 | Moderate | … | … | Yes |
| Patient 21 | + | + | 5 | High | 13 | Low | No |
| Patient 22 | + | + | 2 | High | 10 | Low | No |
| Patient 23 | + | + | 4 | Moderate | … | … | No |
| Patient 24 | + | + | 4 | Low | 12 | High | No |
| Patient 25 | + | + | 6 | Low | 12 | Low | No |
| Patient 26 | + | + | 6 | Low | 12 | Low | Yes |
| Patient 27 | + | + | 5 | Low | 11 | Moderate | No |
| Patient 28 | + | + | 6 | Moderate | 11 | Low | No |
| Patient 29 | + | + | 4 | Low | 12 | Moderate | Yes |
| Patient 30 | + | + | 4 | Low | 9 | Low | Yes |
| Patient 31 | + | + | 4 | Low | 11 | Low | Yes |
| Patient 32 | + | + | 2 | Low | … | … | Yes |
| Patient 33 | + | + | 5 | Low | 11 | Low | No |
| Patient 34 | + | + | 5 | Low | 11 | Moderate | No |
| Patient 35 | + | – | 3 | Low | 13 | Moderate | No |
| Patient 36 | + | + | 4 | Low | … | … | No |
| Patient 37 | + | + | 4 | Low | 12 | Moderate | Yes |
| Patient 38 | + | + | 4 | High | 12 | Moderate | No |
| Patient 39 | + | + | … | … | 8 | Moderate | No |
| Patient 40 | + | + | 3 | High | 11 | Moderate | No |
| Patient 41 | + | + | 2 | High | 12 | High | No |
| Patient 42 | – | + | 4 | Low | 12 | Low | NA |
| Patient 43 | – | + | … | … | 10 | Low | No |
| Patient 44 | – | + | … | … | 14 | Moderate | Yes |
| Patient 45 | NA | + | … | … | 8 | Low | No |
| Patient 46 | NA | + | … | … | 11 | Low | No |
| Patient 47 | NA | + | 7 | Low | … | … | No |
Abbreviations: NA, not available; –, negative; +, positive.
aDetermined via the detection of ZIKV RNA [18] in serum and urine samples collected during the patient’s first visit to the clinic.
bPatient samples were obtained during the acute phase (1–7 days after illness onset) and the convalescent phase (8–14 days after illness onset).
cThe amount of ZIKV NS3 antigen detected in patients’ monocytes were categorized into 3 groups: high (positivity among >40% of monocytes), moderate (positivity among 10%–40%), and low (positivity among <10%). Not all patients had paired data from the acute and early convalescent phases.
dFever (temperature, >37°C) was determined upon hospital.
eThis patient was a confirmed case, based on detection of ZIKV-specific immunoglobulin M.
Generation of ZIKV NS3–Specific Antibodies
Antibodies were generated against the RNA helicase region of the NS3 protein of ZIKV (Asian genotype, isolate BeH819016, from Brazil). Sequence encoding for amino acid residues 1683–2123 of ZIKV BeH819016 polyprotein (GI:975885966) was codon optimized for Escherichia coli expression, obtained as synthetic DNA (Genscript) and cloned into pET28(a) expression vector. Expression was performed in E. coli BL21(DE3) cells (Merck Millipore), using an autoinduction protocol. Recombinant soluble NS3 protein was purified to homogeneity by using Ni-affinity, ion-exchange, and size-exclusion chromatography as described previously [19]. Protein obtained was used for rabbit immunization and affinity purification of ZIKV NS3–specific antibodies (LabAs; Supplementary Figure 1).
Whole-Blood Staining and Flow Cytometry
A 100-µL aliquot of each patient’s whole-blood specimen was used for staining. Briefly, surface staining was first performed with mouse anti-human CD45 (Biolegend) and mouse anti-human CD14 (Biolegend) antibodies. Subsequently, cells were fixed, and lysis of red blood cells was performed with 1× fluorescence-activated cell-sorting (FACS) lysing solution (BD Biosciences). Permeabilization was achieved with 1× FACS Permeabilizing Solution 2 (BD Biosciences) before staining with the ZIKV NS3–specific rabbit polyclonal antibody. Stained cells were counterstained with a fluorophore-tagged secondary goat anti-rabbit immunoglobulin G (heavy plus light chains) antibody (Invitrogen). The threshold for ZIKV NS3–positive cells was determined from the gating results for 20 healthy volunteers (Supplementary Figure 2A). Cells were subsequently acquired with a Fortessa flow cytometer (BD Biosciences) with BD FACSDiva software (BD Biosciences). Duplets were excluded in all forward-scatter/side-scatter gating. The percentage of ZIKV NS3–positive cells was determined in total monocytes, defined as CD45+CD14+ cells. All analyses were performed using FlowJo software, version 9.3.2 (Tree Star).
Cross-reactivity Studies
For cross-reactive experiments involving DENV-infected patients, a 100-µL aliquot of a whole-blood specimen from each patient was stained in exactly the same manner as stated above. The same threshold as that for ZIKV NS3 staining was used to determine the percentage of DENV antigen–positive monocytes. For analysis of in vitro cross-reactivity against DENV, Vero cells were infected with either ZIKV or DENV (serotype 1) at a multiplicity of infection of 10 for 2 hours in a serum-free setting. Subsequently, the virus mixes were removed and replaced with complete cell culture medium. Infected cells were harvested 96 hours after infection. The presence of ZIKV or DENV NS3 antigen was subsequently determined via intracellular staining as described above. Vero cells were cultured and passaged in Dulbecco’s modified Eagle’s medium (HyClone) supplemented with 10% fetal bovine serum (HyClone).
Statistical Analyses
All statistical analyses were performed using GraphPad Prism 6. Analyses between groups were performed using a nonparametric Mann-Whitney U test (2 tails), assuming equal variance. A P value of <.05 is considered to be statistically significant.
RESULTS
Detection of ZIKV NS3 in Peripheral Blood Monocytes of Patients
It was recently reported that analysis of whole-blood specimens was more sensitive than analysis of serum for detection of ZIKV RNA [20]. Therefore, we explored the detection of ZIKV antigen in blood monocytes, using a whole-blood staining procedure. The presence of ZIKV antigen was determined using an affinity-purified rabbit polyclonal antibody targeting specifically the ZIKV NS3 antigen (Figure 1A). The specificity of this antibody was evaluated for the level of cross-reactivity against other clinically important flaviviruses, such as yellow fever virus and DENV. As we expected, some low levels of cross-reactivity were observed (Supplementary Figure 1B and 1C).
Figure 1.
Detection of Zika virus (ZIKV) antigens (Ags). A, Immunofluorescence imaging of Vero cells infected with mCherry-tagged ZIKV infectious clones and counterstained with a rabbit polyclonal anti-ZIKV NS3 antibody. B, Detection of ZIKV NS3 Ag in patients’ whole-blood specimens by anti-ZIKV NS3 antibody. Gating was performed on CD45+ singlet cells, and monocytes were identified as CD14 positive. Amount of ZIKV NS3 Ag detected was then determined and further categorized into 3 groups: high (positivity among >40% of monocytes), moderate (positivity among 10%–40%), and low (positivity among <10%). The threshold for positive ZIKV detection was determined from a number of healthy controls. Values in each box refer to the percentage of the gated population. Side scatter A (SSC-A) refers to the side scatter of light, which is proportional to the cells’ granularity.
Detection with this antibody was further assessed in blood specimens from healthy donors. The threshold of signal detected in healthy donor samples was set to be ≤0.5%, with minimal variations in the detected signals observed between the 20 healthy controls (Supplementary Figure 2A). A total of 47 patients were analyzed in this study (Table 1). These patients were confirmed to be infected with ZIKV because ZIKV RNA was detected by RT-PCR performed on urine and/or serum samples collected during their first visit to the clinic. The percentage of ZIKV NS3–positive monocytes detected varied widely between patients. Upon further analysis, they could be categorized into 3 groups: high (positivity among >40% of monocytes), moderate (positivity among 10%–40%), and low (positivity among <10%; Figure 1B). Whole-blood samples from DENV-infected patients were stained with this antibody, with low percentages of DENV NS3 positive cells (Supplementary Figure 2B), verifying that this antibody could possibly differentially detect ZIKV against DENV (Supplementary Figure 2C).
Detection of ZIKV NS3 Is Highest During the Acute Phase of Disease
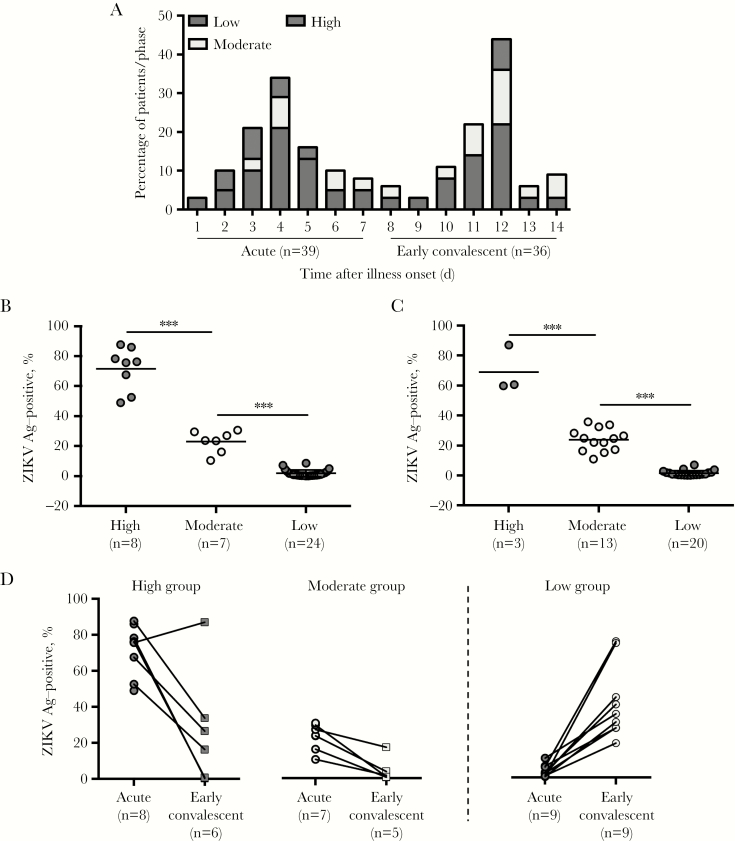
The majority of these patients provided whole-blood samples across the 2 time points of collection: the acute phase (1–7 days after illness onset) and the early convalescent phase (8–14 days after illness onset; Figure 2A). Within the 2 phases, a percentage (38%–44%) of patients had moderate-to-high levels of ZIKV antigen detected (Figure 2A–2C). It is also worthy to note that during the acute phase, samples from all patients within the high-detection group were obtained within 2–5 days after illness onset, perhaps indicative that this window is the ideal time to detect highly infected patients (Figure 2A). In the early convalescent phase, only 3 patients were in the high-detection group (Figure 2A and 2C), while the majority of the patients belonged to the low-detection group. In fact, across both phases, a significant portion of patients (56%–62%) had low levels of detected ZIKV NS3, with a majority (50%–60%) of these patients displaying detection levels comparable to those for the healthy controls.
Figure 2.
Profile and distribution of Zika virus (ZIKV) antigens (Ags) detected in patients. A, Demographic characteristics of ZIKV-infected patients’ ZIKV NS3 detection across acute (1–7 days after illness onset) and early convalescent (8–14 days after illness onset) phases. B and C, Dot plots showing the levels of ZIKV NS3 detected in CD14+ monocytes during the acute (B) and early convalescent (C) phases. Data are presented as mean ± standard error of the mean. D, A paired-line graph showing the transition of ZIKV NS3 detection in patients’ CD14+ monocytes from the acute to the early convalescent phase in the high and moderate groups, defined as groups in which the percentages of ZIKV NS3–positive monocytes were >40% and 10%–40%, respectively. E, Similar paired-line graph illustrating the amount of ZIKV NS3 detected in select individuals from the low-detection group (defined as positivity among <10% of monocytes) from the acute phase through to the early convalescent phase. The amount of ZIKV NS3 detected was determined with a ZIKV-specific rabbit polyclonal antibody. ***P < .001, by a 2-tailed nonparametric Mann-Whitney test.
As we expected, the percentage of ZIKV NS3–positive monocytes decreased in the early convalescent phase for patients in the high-detection groups during the acute phase (Figure 2D). However, in 1 patient, the level of ZIKV antigen remained high. Meanwhile, for patients with moderate levels of ZIKV antigen, the percentage of blood monocytes positive for ZIKV antigen decreased, falling into the low-level cluster in the early convalescence phase (Figure 2D). This was coupled with a lower absolute amount of ZIKV antigen in the blood monocytes (Figure 2D). Interestingly, higher levels of ZIKV antigen were detected during the early convalescent phase in a small group of patients who were previously categorized in the low-detection group (Figure 2D).
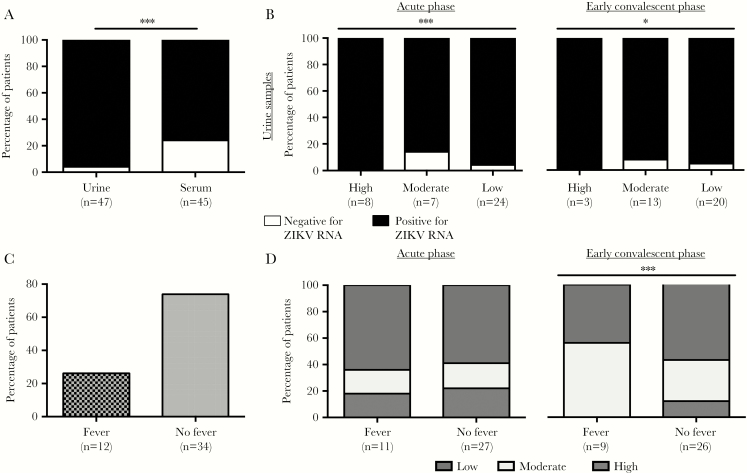
Analysis of Whole-Blood Specimens Is More Sensitive for Detecting ZIKV
In this cohort, ZIKV RNA was detected in both urine and serum samples collected during admission to the hospital (Figure 3A). These findings showed that analysis of urine specimens was more sensitive than analysis of serum samples for detection of ZIKV RNA. Almost 96% of patients were positive for ZIKV, based on analysis of urine samples, while only 76% of patients had ZIKV RNA detected in serum samples. However, the sensitivity of FACS for detecting ZIKV NS3 in whole-blood specimens was superior to that for serum specimens but not for urine specimens, as all patients in the high-detection group had detectable ZIKV RNA only in urine samples. As we expected, a low percentage of patients in the moderate and low-detection groups had urine specimens that were negative for ZIKV RNA (P < .001, by a 2-tailed χ2 test; Figure 3B). Interestingly, further grouping of the patients (Table 1) revealed that approximately 25% had fever upon hospital admission (Figure 3C). Notably, even though differences in patients with or without fever were comparable in all 3 ZIKV NS3 detection groups (ie, low, moderate, and high) during the acute phase (Figure 3D), 12% without fever still had high levels of detectable ZIKV NS3 during the early convalescent phase (Figure 3D). This observation was unexpected as none of the patients with fevers were in the high-detection group (Figure 3D).
Figure 3.
Association of qualitative viral load data with detectable levels of Zika virus (ZIKV) NS3 antigen. A, The percentage of patients with detectable ZIKV RNA in urine or serum samples. ***P < .001 by a 2-tailed Fisher exact test. B, The percentage of patients from both acute and early convalescent phases with measurable levels of ZIKV RNA in urine samples. All urine and serum samples were obtained during the patients’ first visit to the clinic. C, The percentages of patients with or without fever during admission. D, Segregation of ZIKV antigen detection during the acute and early convalescent phases among patients with or without fever. *P < .05 and ***P < .001, by a 2-tailed χ2 test.
DISCUSSION
While detection of ZIKV RNA remains the current gold standard to diagnose ZIKV infection, its presence alone might not indicate the existence of active ZIKV infection. To further demonstrate active infection, either viral proteins or viable infectious viral particles would need to be recovered from a patient’s samples. Current diagnostic methods are performed mainly by using patients’ serum or plasma, as well as urine and saliva [11, 15, 21], which are known to exhibit different sensitivities and reliabilities [11]. The findings in this report further validate earlier observations that urine is a better sample than serum for the detection of ZIKV RNA [21]. However, these samples are typically cell free, and analysis of them does not precisely reveal the infection status at the cellular level (eg, in the blood cells). Moreover, viruses are known to exhibit a certain level of stickiness that determines their efficiency to attach and detach [22]. While the stickiness of ZIKV to blood cells has not been thoroughly studied, there remains a possibility that ZIKV adheres to blood cells. Thus, the assessment of ZIKV antigens in whole-blood samples presents an attractive alternative in ZIKV detection. Moreover, there was a recent report on the detection of ZIKV RNA in patients’ whole-blood specimens [20, 23], accentuating the potential of patients’ whole blood as a valuable and important sample for diagnostic purposes.
The direct detection of ZIKV NS3 protein in a patient’s whole-blood specimen is a relatively cheap and fast alternative to detect active ZIKV infection. Likewise, the detection of DENV NS1 antigen has been reported as a method for early diagnosis of DENV infection [24]. Given that monocytes are targets for flaviviruses [25, 26], this immune cellular population therefore remains the main target in our approach. Furthermore, ZIKV has been shown to efficiently infect primary human monocytes and monocyte-derived macrophages [17]. However, the possibility of ZIKV infecting other hematopoietic populations remains to be determined. It is not surprising that the ZIKV NS3 antibody used in this study was able to cross-react with other flaviviruses, as the NS3 is fairly conserved [12]. Moreover, it is well reported that antibodies raised against flaviviruses are usually cross-reactive with other members within the genus [13]. Nonetheless, differential detection among different flaviviruses could still be achieved, and the use of ZIKV-specific antibodies for detection of ZIKV antigens holds promise.
Importantly, the results in this report showed that most patients fell into the low-detection group, which is a true reflection of ZIKV infection in humans, in whom it has a high prevalence rate of asymptomatic infections [2, 7]. This phenomenon could indicate that while many of these patients might have tested positive for ZIKV RNA during their visit to the clinic, the infection was so mild that the virus had already started to clear. Interestingly, in this patient cohort, in a small number of patients, ZIKV antigen was detected only during the early convalescent phase. It is known that variability among individuals can affect disease progression and severity [27]. This is because infection involves a complex interaction of multiple factors, such as host genetics, virus interactions with the host immune response, and the capability of the virus to infect and gain access to immunoprivileged sites [28]. As a result, follow-up studies on these patients are ongoing because it is crucial to further understand the relationship between delayed ZIKV infection and any long-term clinical complications [4].
Although a proportion of patients had high or moderate levels of ZIKV NS3 antigen, none of these patients had severe disease. This suggests that the amount of ZIKV antigen detected in blood monocytes does not determine disease severity during the acute phase. This finding corroborates results of an earlier study in Martinique suggesting that the viremia level is not a clear indication of disease severity [29]. In this study, it was observed that a modest percentage of patients with moderate levels of ZIKV NS3 had no measurable amounts ZIKV RNA in both urine and serum samples. These inverse relationships highlight the complex nature of the disease.
The detection of ZIKV antigen in blood monocytes would aid patient management, as this method allows for continual monitoring of patients for whom serum and urine samples are negative for ZIKV RNA but blood specimens are positive for ZIKV antigen. This is critical because virus-harboring blood monocytes could serve as a reservoir for latent ZIKV infection and for dissemination of ZIKV into other parts of the body, even though there is no evidence for this at this time. In recent studies performed in ZIKV-infected women of childbearing age, ZIKV RNA was detected in both vaginal secretions [23, 30] and blood [23]. Likewise, in men, ZIKV has been reported to persist in semen for extended periods [31]. A recent study in male mice showed that ZIKV infection could result in damaged testes, resulting in reduced testosterone levels and reproductive capabilities [32]. Together, this highlights the possibility of ZIKV using blood monocytes as a cellular vehicle in gaining entry into various immunoprivileged sites, such as the brain. This is especially so, given that microglial cells are a target of ZIKV and that the trafficking of ZIKV to the brain would lead to the infection of these cells and the inflammatory response that ensues [17], resulting in possible neurological insults. The findings in this report also bring caution to the screening of blood donations from donors previously infected with ZIKV. This is especially so for patients with no detectable fever, among whom we showed that approximately 12% still had high levels of ZIKV NS3 antigen detected during the early convalescent phase.
Currently, the understanding of ZIKV infection in human monocytes and its associated risks with long-term complications is still in its infancy. The use of whole-blood staining to detect ZIKV antigen in blood monocytes is a cost-effective method with a short turnaround time that should be explored in the future as an alternative to aid in the diagnosis of active ZIKV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Siti Naqiah Amrun, Jeslin J. L. Tan, Cheryl Y. P. Lee, Tze-Kwang Chua, Yiu-Wing Kam, Jonathan Cox, Yi-Hao Chan, Guillaume Carissimo, Farhana Abu Bakar, Nicholas Q. R. Kng, Kia-Joo Puan, and Nurhashikin Binte Yusof (Singapore Immunology Network [SIgN]), for their help in the processing of patient samples; Kaval Kaur and Katja Fink (Laboratory of Dengue Immunity) and Ivy Low, Seri Mustafah, and Anis Larbi (SIgN flow cytometry team), for their assistance; and all study participants and healthy volunteers, for their participation in the study.
Financial support. This work was supported by the Biomedical Research Council (BMRC; core research grants to the Singapore Immunology Network), the National Public Health Laboratory of Singapore, the University of Tartu in Estonia, the BMRC Agency for Science, Technology, and Research, Singapore (A*STAR)–led Zika Virus Consortium Fund (project 15/1/82/27/001), and A*STAR.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 2. Duffy MR, Chen TH, Hancock WT et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 3. Brasil P, Pereira JP Jr, Raja Gabaglia C et al. Zika virus infection in pregnant women in Rio de Janeiro–preliminary report. N Engl J Med 2016; 375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao-Lormeau VM, Blake A, Mons S et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016; 387:1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao-Lormeau VM, Musso D. Emerging arboviruses in the Pacific. Lancet 2014; 384:1571–2. [DOI] [PubMed] [Google Scholar]
- 6. Gyawali N, Bradbury RS, Taylor-Robinson AW. The global spread of Zika virus: is public and media concern justified in regions currently unaffected? Infect Dis Poverty 2016; 5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Korzeniewski K, Juszczak D, Zwolińska E. Zika–another threat on the epidemiological map of the world. Int Marit Health 2016; 67:31–7. [DOI] [PubMed] [Google Scholar]
- 8. Paixão ES, Barreto F, Teixeira Mda G, Costa Mda C, Rodrigues LC. History, epidemiology, and clinical manifestations of Zika: a systematic review. Am J Public Health 2016; 106:606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duong V, Dussart P, Buchy P. Zika virus in Asia. Int J Infect Dis 2017; 54:121–8. [DOI] [PubMed] [Google Scholar]
- 10. National Environment Agency Singapore. Zika cases and clusters http://www.nea.gov.sg/public-health/vector-control/overview/zika-cases-clusters. Accessed 9 April 2017.
- 11. Shukla S, Hong SY, Chung SH, Kim M. Rapid detection strategies for the global threat of Zika virus: current state, new hypotheses, and limitations. Front Microbiol 2016; 7:1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jain R, Coloma J, García-Sastre A, Aggarwal AK. Structure of the NS3 helicase from Zika virus. Nat Struct Mol Biol 2016; 23:752–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keasey SL, Pugh CL, Jensen SM et al. Antibody responses to Zika virus infections in environments of flavivirus endemicity. Clin Vaccine Immunol 2017; 24:e00036–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Priyamvada L, Quicke KM, Hudson WH et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 2016; 113:7852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol 2015; 68:53–5. [DOI] [PubMed] [Google Scholar]
- 16. Hamel R, Dejarnac O, Wichit S et al. Biology of Zika virus infection in human skin cells. J Virol 2015; 89:8880–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lum FM, Low DK, Fan Y et al. Zika virus infects human fetal brain microglia and induces inflammation. Clin Infect Dis 2017; 64:914–20. [DOI] [PubMed] [Google Scholar]
- 18. Lanciotti RS, Kosoy OL, Laven JJ et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das PK, Merits A, Lulla A. Functional cross-talk between distant domains of chikungunya virus non-structural protein 2 is decisive for its RNA-modulating activity. J Biol Chem 2014; 289:5635–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lustig Y, Mendelson E, Paran N, Melamed S, Schwartz E. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Euro Surveill 2016; 21:30269. [DOI] [PubMed] [Google Scholar]
- 21. Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis 2015; 21:84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Handel A, Akin V, Pilyugin SS, Zarnitsyna V, Antia R. How sticky should a virus be? The impact of virus binding and release on transmission fitness using influenza as an example. J R Soc Interface 2014; 11:20131083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray KO, Gorchakov R, Carlson AR et al. Prolonged detection of Zika Virus in vaginal secretions and whole blood. Emerg Infect Dis 2017; 23:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kassim FM, Izati MN, TgRogayah TA, Apandi YM, Saat Z. Use of dengue NS1 antigen for early diagnosis of dengue virus infection. Southeast Asian J Trop Med Public Health 2011; 42:562–9. [PubMed] [Google Scholar]
- 25. Kou Z, Quinn M, Chen H et al. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J Med Virol 2008; 80:134–46. [DOI] [PubMed] [Google Scholar]
- 26. Rios M, Zhang MJ, Grinev A et al. Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion. Transfusion 2006; 46:659–67. [DOI] [PubMed] [Google Scholar]
- 27. Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe 2014; 15:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ludlow M, Kortekaas J, Herden C et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol 2016; 131:159–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gallian P, Cabié A, Richard P et al. Zika virus in asymptomatic blood donors in Martinique. Blood 2017; 129:263–6. [DOI] [PubMed] [Google Scholar]
- 30. Prisant N, Breurec S, Moriniere C, Bujan L, Joguet G. Zika virus genital tract shedding in infected women of childbearing age. Clin Infect Dis 2017; 64:107–9. [DOI] [PubMed] [Google Scholar]
- 31. Matheron S, d’Ortenzio E, Leparc-Goffart I, Hubert B, de Lamballerie X, Yazdanpanah Y. Long-lasting persistence of Zika virus in semen. Clin Infect Dis 2016; 63:1264. [DOI] [PubMed] [Google Scholar]
- 32. Govero J, Esakky P, Scheaffer SM et al. Zika virus infection damages the testes in mice. Nature 2016; 540:438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.