Abstract
Zika virus was discovered in East Africa in 1947 by the Rockefeller Foundation during investigations on the ecology of yellow fever. Although it was subsequently shown to have widespread distribution in Africa and Asia, it was not known to cause epidemics until 2007. This paper describes the history of the virus discovery, emergence and evolution as an epidemic virus, and the its evolving clinical spectrum.
Keywords: Aedes aegypti, GBS, microcephaly, mosquito, Zika
DISCOVERY AND EMERGENCE
The confirmation that yellow fever (YF) was caused by a virus and was transmitted to humans by a mosquito resulted indirectly in the discovery of Zika and many other arboviruses by the Rockefeller Foundation YF program (reviewed in detail by Schwartz [1]). In brief, Zika virus (ZIKV) was first isolated in 1947 from a febrile sentinel rhesus monkey (no. 766) held in a cage on a platform in the canopy of the Zika Forest in Uganda during studies to identify the vector of sylvatic YF [2]. A blood sample from this monkey was collected on day 3 of fever and was inoculated intracerebrally into Swiss mice and into another rhesus monkey (no. 771). All mice showed signs of illness on day 10 postinoculation, and a filterable transmissible agent was isolated from the brains of these sick mice. Monkey 766 showed no other clinical signs or symptoms, and monkey 771 remained asymptomatic. The convalescent serum from both monkeys (766 and 771) neutralized the virus isolated from monkey 766 in mice, which was designated ZIKV 766. Preinfection sera from these monkeys did not neutralize ZIKV 766.
A few months later, another strain of ZIKV was isolated by inoculating mice with the homogenate from a pool of Aedes africanus mosquitoes collected in the same area of the Zika Forest [2, 3]. That virus strain was designated ZIKV E/1. The E/1 virus was also inoculated into a rhesus monkey (no. 758), which remained asymptomatic, but convalescent serum from this monkey neutralized ZIKV E/1. Both ZIKV 766 and ZIKV E/1 were neutralized by the convalescent serum from monkeys 766 and 758 showing that the 2 viruses were the same.
There is some controversy surrounding the first ZIKV isolate from humans. The first report was from serum of a 10-year-old Nigerian female in 1954 [4]. The patient was clearly jaundiced, but interpretation of the clinical presentation was complicated by coinfection with malaria. In cross-neutralization tests with convalescent sera from monkeys infected with Bunyamwera, Bwamba, Mengo, Ntaya, Semliki Forest, Uganda S, West Nile, YF, and Zika viruses, only the serum from the monkey infected with ZIKV neutralized the virus isolated from the patient, strongly suggesting the girl was infected with ZIKV. It was subsequently reported that the Nigerian isolate was more closely related to Spondweni virus (also called CHUKU) [5–7]. These events were recently summarized by Wikan and Smith [8] who suggest that the first case of confirmed human ZIKV infection occurred in Uganda in 1962–1963 [9]. Whether the Nigerian virus was Zika or Spondweni will have to await further study using new molecular technology. However, subsequent studies have documented that ZIKV does occur in humans and mosquitoes widely in West Africa [10–12].
Outside of Africa, ZIKV was isolated for the first time from mosquitoes (Aedes aegypti) in 1966 in Malaysia [13], but human infections in Asia were not reported until 1977 in Central Java, Indonesia [14]. However, serosurveys conducted in the 1950s, 1960s, and 1970s strongly suggested that ZIKV had a widespread geographic distribution in both tropical Africa and Asia [3]. Unfortunately, the extensive cross-reactivity among the antibodies produced by infection with closely related flaviviruses [3, 15–18] makes interpretation of serological results difficult, but more specific neutralization tests and virologic studies on humans, nonhuman primates, and mosquitoes have confirmed widespread circulation of ZIKV in these regions [3, 19–22]. Collectively, the data suggest that silent ZIKV transmission among humans, animals, and mosquitoes has occurred throughout tropical Africa and Asia for more than 70 years. Significant events in ZIKV history are summarized in Figure 1.
Figure 1.
Significant events in the history of Zika virus (ZIKV). Abbreviations: DNA, deoxyribonucleic acid; GBS, Guillain-Barré syndrome; RNA, ribonucleic acid; WHO, World Health Organization.
During the first 60 years of its known existence, epidemic ZIKV was never reported, and fewer than 20 human infections were recorded during this extended period of silent transmission [3]. Assuming the earlier serological data were correct, it is likely that sporadic human cases of ZIKV infection have occurred for decades but were unrecognized or misdiagnosed as dengue, Japanese encephalitis, West Nile, or one of the many other flaviviruses, other viruses, bacteria, and parasites that are enzootic or endemic in these regions. That being the case, then why has epidemic ZIKV emerged in recent years? The definitive answer to that question will have to await more detailed epidemiologic, ecologic, and virologic studies, but current understanding of factors responsible for emergence of other Aedes-transmitted viral diseases that have similar epidemiology or ecology suggests that, similar to dengue and chikungunya, genetic changes in the virus likely resulted in emergence of a virus strain with increased transmissibility leading to greater epidemic potential and perhaps virulence [23–31]. As with dengue and chikungunya, emergence and spread of ZIKV was probably facilitated by the global demographic, social and technological trends of population growth, unprecedented urbanization and globalization, combined with lack of effective mosquito control in urban areas, which provided conditions for the “perfect storm”, leading to increased transmission and spread of the viruses and their mosquito vectors [32, 33].
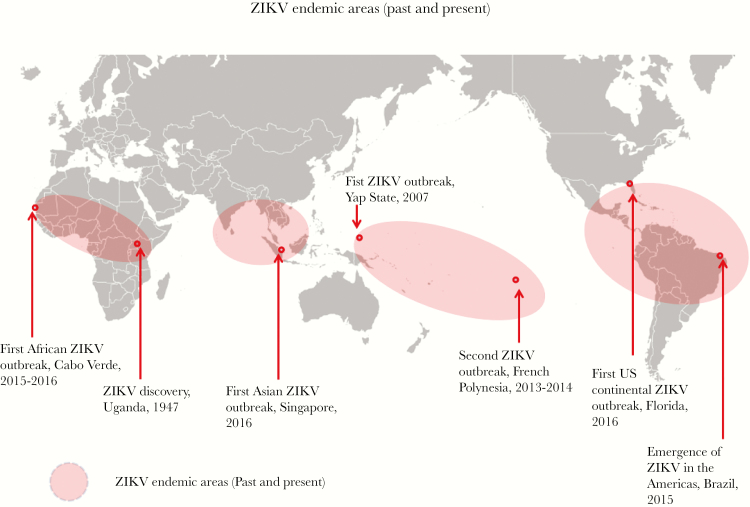
The first known ZIKV epidemic occurred in the isolated islands of Yap, Federated States of Micronesia, located in the Western Pacific [34] (Figure 2), when an epidemic of dengue-like illness was reported in April–May 2007. Although dengue had occurred there earlier [35, 36], local physicians suspected a different etiology because of atypical clinical presentation in some patients. Ross River virus was suspected, but this was ruled out at the University of Hawaii (D. J. G., unpublished data, 2007). Samples were sent to the Centers for Disease Control and Prevention (CDC) Arbovirus Diagnosis and Reference Laboratory (Fort Collins, CO) where ZIKV infection was confirmed [37]. The outbreak was relatively small (approximately 5000 infections, approximately 75% of the population), and all reported illness was mild [34].
Figure 2.
Geographic regions where Zika virus (ZIKV) is enzootic/endemic and has caused epidemics.
No further epidemic ZIKV transmission was reported until October 2013 when cases of ZIKV were reported in French Polynesia, a South Pacific territory [38], while in Southeast Asia only sporadic transmission was occurring [39]. A major epidemic occurred in 2013/2014 involving all French Polynesian islands with more than 30 000 cases, some with neurologic complications [40–42] (see below). The virus spread to New Caledonia, the Cook Islands, Easter Island, and to the rest of the South Pacific [43]. Zika virus is still circulating in the Pacific in 2017 (CDC web site: https://wwwnc.cdc.gov/travel/page/world-map-areas-with-zika). The latest data suggest the virus was introduced into Brazil as early as 2013 or 2014 [44, 45] from the Pacific, but the disease was not recognized until November 2015, when a major epidemic of neurologic disease in new born babies occurred, with a second peak in April 2016 [3, 46–49]. From Brazil, ZIKV spread rapidly throughout the American region [49].
In Asia, 2 outbreaks have been reported in Singapore [49, 50] and Vietnam [51], but widespread transmission has occurred in Thailand as well (Thailand Ministry of Public Health, unpublished data, 2017), and sporadic cases of ZIKV infection have occurred in 10 countries since 2007 [50, 51]. The first epidemic in Africa occurred in Cabo Verde in 2015–16, apparently the result of introduction from Brazil [52]; from 2007, over 80 countries have reported active transmission of ZIKV [49, 52].
EVOLUTION OF ZIKA VIRUS
The rapid geographic radiation of ZIKV in the past 3 years is consistent with a pattern of intense diversification (“boom and bust” period) (Figure 3), fueled, as noted above, by the explosive and rapid human movement by modern transportation, urbanization, poor water management, and vector control, and facilitated by large immunologically naive human populations and the global spread of the anthropophilic mosquito vector, Ae aegypti. The fundamental basis of ZIKV genetic diversity can be attributed to its error-prone ribonucleic acid (RNA)-dependent RNA polymerase, which does not have proof-reading capacity and is thought to produce approximately 1 mutation per round of genome replication. Analyses based on selective pressures, represented as the ratio of nonsynonymous to synonymous substitutions (dN/dS) per site, suggest that the majority of DENV mutations are deleterious and subject to strong purifying selection (dN/dS << 1) [53]. However, genetic variation and population diversity probably played a role for ZIKV to occupy and adapt to new and changing ecological niches and selective pressures.
Figure 3.
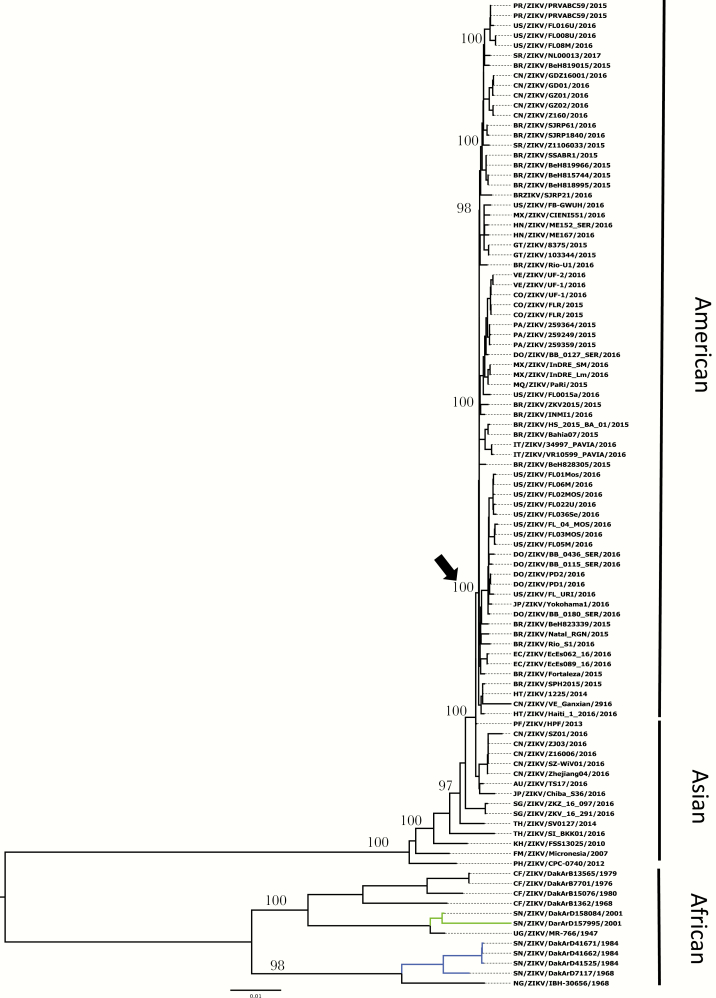
Phylogenetic tree of Zika virus based on 93 complete open reading frame sequences inferred using maximum likelihood methods.
Current analyses based on 93 complete genome sequences reinforce the hypothesis that ZIKV originated in Africa and diverged into 3 major lineages: African, Asian, and American (Figure 3). The African strains fall into 2 distinct groups: (1) the Uganda cluster, which is anchored by the prototype strain MR766 and includes isolates from Senegal and Central African Republic sampled from 1947 to 2001; and (2) the Nigeria cluster, which includes strains isolated in Nigeria and Senegal from 1968 to 1997. It is interesting to note that at least 2 distinct lineages are circulating in Senegal (blue and green lines, Figure 3), suggesting multiple introductions, likely fueled by trade routes [54]. It is worth mentioning that most of the known African lineage strains to date were isolated from enzootic vectors, reflecting continuous surveillance efforts in Senegal [54]. The Asian cluster is anchored by the P6-740 strain isolated in Malaysia in 1966 from Ae aegypti and includes strains isolated in Cambodia, Thailand, Micronesia, French Polynesia, and the recent introductions in Japan, China, Australia, and Singapore, supporting the presence of the Asian lineage throughout Southeast Asia. Within this cluster, a new American lineage has emerged and includes strains from Brazil, Colombia, Ecuador, Panama, Mexico, Honduras, Venezuela, the Dominican Republic, Puerto Rico, Haiti, Guatemala, United States, and Suriname, as well as Italy and China, reinforcing the role of modern transportation in the rapid spread of these viruses on a global scale.
Recent reports have suggested ZIKV recombination between field isolates [55] or with Spondweni virus [56]. Evidence for recombination among members of the genus Flavivirus has been reported mainly in dengue virus ([DENV] reviewed in reference [56]). Despite concerted efforts, however, recombination has not been achieved experimentally, and thus caution should be exercised when inferring conclusions about these putative recombination events based solely on coalescent bioinformatic tools. For natural recombination to be definitive, the following prerequisites should be met: (1) the recombinant crossover should be demonstrated in a single polymerase chain reaction amplicon after cloning to ensure it occurs in a single deoxyribonucleic acid molecule, (2) the recombination should be demonstrated repeatedly in clonal populations of viable virus (eg, a plaque harvest or limited endpoint dilution), and (3) the recombinant should maintain adequate sequence conservation during post-recombination evolution [56].
To explain the rapid global spread of ZIKV, 3 hypotheses have been put forward: (1) ZIKV underwent adaptive evolution facilitating more efficient urban transmission in Ae aegypti mosquitoes or in humans, (2) stochastic factors, and (3) a combination of genetic change and stochastic factors [3, 57]. Although a number of phylogenetic studies [57–59] may support the hypothesis that adaptive evolution may have occurred in southeast Asia where the virus has been circulating since at least the 1960s, to date experimental studies with laboratory colonies or feral populations of mosquitoes (reviewed in [58] and [59–63]) have failed to support this hypothesis, suggesting that the intense ZIKV transmission in the Americas was also influenced by other factors, including immunologically naive human populations. It is possible that the Asian ZIKV lineage may have adapted to generate higher viremia levels in humans, which would lead to more efficient mosquito infection, transmission, and spread. Higher viremia was also suggested to enhance transplacental transmission in humans, which could explain the dramatic emergence of microcephaly in the Americas and remains to be experimentally confirmed. Some studies based on bioinformatic analyses of ZIKV sequences suggested an increase in the use of human codons by the virus, which may support this hypothesis [54, 64, 65]. Nonetheless, the potential link between adaptive evolution and enhanced human infection will require comprehensive longitudinal studies in humans and/or animal models. Ultimately, this hypothesis will be difficult to test because various animal models may not respond to ZIKV infection in the same manner as humans. To date, however, evidence suggests that a combination of stochastic factors and selective evolution may have fueled the spectacular emergence and spread of ZIKV. The initial chance introduction of the virus into naive populations in the South Pacific likely facilitated sufficient amplification by competent mosquito vectors and raised the risk of transport to the Americas. Similar to the spread of dengue in the aftermath of World War II, increased air travel undoubtedly increased the risk of spreading other viruses with increased epidemic potential in recent decades [23–26]. In the case of ZIKV, the origin of introduction in Brazil is unknown, but athletic competitions in Brazil (Soccer Confederation Cup in June 2013, soccer World Cup in June 2014, Va’a World Sprint Championship canoe race in August 2014) are believed to have brought travelers from the South Pacific around the time that ZIKV circulation was discovered there [66–69]; however, molecular clock analyses suggested that introduction may have occurred between May and November 2013 [44, 45, 70]. One hypothesis cannot be favored over another, because introduction by a single traveler coming from the Pacific is also possible. The area of first introduction of ZIKV in Brazil is also a matter of debate The recent epidemics in Singapore and widespread introduced cases and/or sporadic transmission in other Asian countries support the notion that stochastic events have played a role in the global spread of ZIKV. Unanswered is the question of whether emergence and spread were facilitated by new strains of virus with greater epidemic potential that took advantage of the global trends of the 21st century [3, 49].
EVOLUTION OF THE CLINICAL PRESENTATION AND NONVECTOR-BORNE TRANSMISSION
Because of the small number of human cases reported before 2007, the clinical presentation associated with ZIKV infection was ill defined. When the virus emerged in Yap State in 2007, the majority of patients presented with rash, low-grade fever, conjunctivitis, arthralgia, and myalgia [3, 34]. The same clinical presentation was observed when ZIKV emerged in French Polynesia in 2013/2014 and in Brazil in 2015 [3, 38, 47, 71]. Serosurvey studies conducted after the outbreaks in Yap State and French Polynesia suggested that most of the infections were asymptomatic [34, 72]. The first description of severe neurological complications in adults and the potential for nonvector-borne transmission of ZIKV were reported during the French Polynesia outbreak [3]. These new data were subsequently confirmed with the emergence of ZIKV in the Americas and additionally the first description of severe central nervous system malformation in fetuses/neonates [49].
Neurological complications have been reported for arbovirus infections, especially those caused by the Flavivirus [73] and Alphavirus genera [74] (Table 1). Although Guillain-Barré syndrome (GBS) had been associated with other flaviviruses, the 20-fold increase in GBS observed during the ZIKV epidemic in French Polynesia was unexpected [3, 41, 75]. The incidence of GBS in French Polynesia was 1 in 6500 inhabitants, which is approximately the population of Yap State. Because it is a rare complication, it is not possible to know whether the potential for GBS was present in Yap State. For the same reason, microcephaly in newborns was reported only retrospectively in French Polynesia because of the small number of cases [76]. The link between ZIKV and GBS was demonstrated in a retrospective case-control study [41, 77, 78], and the association was subsequently confirmed in the Americas [79]. Similar findings were observed when West Nile virus (WNV) emerged in the Americas causing increased numbers of meningoencephalitis cases [80]. The potential for maternofetal transmission of ZIKV was suspected in French Polynesia [81]. During the 2015 ZIKV epidemic in Brazil, a 20-fold increase in the incidence of neonates with microcephaly coincided with reports of cases of a febrile rash illness compatible with ZIKV infection in pregnant women [82]. The link between ZIKV and severe central nervous system malformations (especially microcephaly) was confirmed in numerous studies [83–86] in Brazil and retrospectively reported in French Polynesia [87]. Other malformations in fetuses/neonates have been reported, but the “congenital ZIKV syndrome” is not yet fully described [88]. Although maternofetal transmission of arboviruses was reported for DENV [89] and chikungunya virus [90], fetus/neonate malformations were not. On the other hand, severe complications caused by the closely related DENV (plasma leakage, bleeding, and severe organ involvement) [91] have not been reported in ZIKV infections.
Table 1.
Clinical Features of ZIKV Compared With Other Arboviruses
| Flavivirus | Alphavirus | |||||
|---|---|---|---|---|---|---|
| ZIKV | DENV | WNV | YFV | JEV | CHIKV | |
| Nonvector borne transmission | ||||||
| Materno fetal | Yes | Yes | No | Yesa | No | Yes |
| Sexual | Yes | No | No | No | No | No |
| Transfusion | Yes | Yes | Yes | Yesa | No | No |
| Main complication in newborn | ||||||
| Microcephaly | Yes | No | No | No | No | No |
| Other CNS malformation | Yes | No | No | No | No | No |
| Main complications in infants and adults | ||||||
| Bleeding | No | Yes | Yes | Yes | No | Yes |
| Plasma leakage | No | Yes | No | Yes | No | No |
| Severe organ involvement | No | Yes | Yes | Yes | No | No |
| Chronic arthralgia | No | No | No | No | No | Yes |
| Main complications in adults | ||||||
| Guillain-Barré syndrome | Yes | Yes | Yes | Yesa | Yes | Yes |
| Meningoencephalitis | Yes | Yes | Yes | Yesa | Yes | Yes |
| Myelitis | Yes | Yes | No | Yesa | Yes | Yes |
| Meningitis | No | No | Yes | Yesa | Yes | No |
| Prevention and treatment | ||||||
| Vaccine | No | Yes | No | Yes | Yes | No |
| Specific treatment | No | No | No | No | No | No |
Abbreviations: CHIKV, chikungunya virus; CNS, central nervous system; DENV, dengue virus; JEV, Japanese encephalitis virus; WNV, West Nile virus; YVF, yellow virus fever; ZIKV, Zika virus.
aWith yellow fever vaccine virus.
Arbovirus transfusion-transmission (TT) has been described for WNV [92] and DENV [93], so the potential for ZIKV TT was suspected in French Polynesia [94] and demonstrated in Brazil [95]. Although predictable, the percentage of positive blood donations was higher than reported for DENV and WNV.
Also new for an arbovirus was sexual transmission, first suspected in a single case in a US citizen returning from Senegal [96]. Infectious ZIKV was then detected in the semen of a French Polynesian patient, and sexual transmission of ZIKV was confirmed after its emergence in the Americas [97, 98]. The duration of infectivity of semen and vaginal fluids, the impact of ZIKV infection on fertility, and the impact of nonvector-borne transmission on the burden of ZIKV disease remain to be determined [49].
As noted above, it is uncertain whether the unusual clinical pattern observed in ZIKV infections from its emergence in French Polynesia was the result of observing larger numbers of patients during the epidemics or whether genetic changes in the virus resulted in greater virulence [31] or, most likely, a combination of both [3, 50]. Zika virus shares common clinical features with other arboviruses, especially flaviviruses, including the high rate of asymptomatic infections and the neurotropism of the virus in adults. However, congenital central nervous system malformations and sexual transmission make ZIKV unique among the arboviruses.
CONCLUSIONS
Zika virus has spread rapidly throughout the Pacific and Americas in the past 10 years, but critical gaps remain in our knowledge of the epidemiology and biology of this virus. In the near term, the following concerns must be of the highest priority: (1) development and application of preventive measures, including use of repellants, insecticide-impregnated clothing, elimination of household breeding habitats, and sustainable vector control at the community, state, and federal level to decrease contact between people and Ae aegypti mosquitoes; (2) establishment of outreach and awareness programs targeted especially to sexually active individuals and pregnant women to avoid contact with the vectors and practice safe sex; (3) establishment of prospective cohorts to determine the risk of congenital Zika syndrome, GBS, and human-to-human transmission; and (4) determine whether ZIKV can establish an enzootic transmission cycle in the Americas. This prospect will certainly render future eradication efforts practically impossible, and it also might inhibit our ability to control the ongoing outbreak of congenital Zika syndrome. In the longer term, we need to develop the following: (1) more effective prevention tools, eg, vaccines, therapeutics, and mosquito control measures; (2) better diagnostics that are accurate, inexpensive, and user friendly for use at point-of-care, as well as for sustainable, laboratory-based surveillance—this may be challenging once the current epidemics subside with growing herd immunity and diagnostics that cannot distinguish ZIKV infections from DENV and other flaviviruses; and (3) more effective surveillance for arboviral diseases in general, especially in tropical areas where the potential risk of epidemic emergence is greatest.
Notes
Financial support. This work was partially funded by Duke-NUS Medical School (Singapore) and by National Institutes of Health Grants R24AI120942 and U01AI115577 (to University of Texas).
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
Potential conflicts of interest. N. V. serves as a consultant for NewLink/Bioprotection Systems. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schwartz DA. The origins and emergence of Zika virus, the newest TORCH infection: what’s old is new again. Arch Pathol Lab Med 2017; 141:18–25. [DOI] [PubMed] [Google Scholar]
- 2. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 3. Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev 2016; 29:487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954; 48:139–45. [DOI] [PubMed] [Google Scholar]
- 5. Boorman JP, Draper CC. Isolations of arboviruses in the Lagos area of Nigeria, and a survey of antibodies to them in man and animals. Trans R Soc Trop Med Hyg 1968; 62:269–77. [DOI] [PubMed] [Google Scholar]
- 6. Moore DL, Causey OR, Carey DE et al. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol 1975; 69:49–64. [DOI] [PubMed] [Google Scholar]
- 7. Karabatsos N. International Catalogue of Arboviruses Including Certain Other Viruses of Vertebrates, 3rd ed San Antonio: American Society of Tropical Medicine and Hygiene; 1985. [DOI] [PubMed] [Google Scholar]
- 8. Wikan N, Smith DR. First published report of Zika virus infection in people: Simpson, not MacNamara. Lancet Infect Dis 2017; 17:15–7. [DOI] [PubMed] [Google Scholar]
- 9. Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg 1964; 58:335–8. [PubMed] [Google Scholar]
- 10. Monlun E, Zeller H, Le Guenno B et al. Arbovirus affecting humans in southeast Senegal: surveillance in humans and mosquitoes (1988-1991). Bull Soc Pathol Exot 1993; 86:21–8. [PubMed] [Google Scholar]
- 11. Diallo D, Sall AA, Diagne CT et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014; 9:e109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grard G, Caron M, Mombo IM et al. Zika virus in Gabon (centralAfrica) 2007: a new threat from Aedes albopictus?PLoS Negl Trop Dis 2014; 8:e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg 1969; 18:411–5. [DOI] [PubMed] [Google Scholar]
- 14. Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981; 75:389–93. [DOI] [PubMed] [Google Scholar]
- 15. Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg 1958; 7:561–73. [DOI] [PubMed] [Google Scholar]
- 16. Mettler NE, Clarke DH, Casals J. Hemagglutination inhibition with arboviruses: relationship between titers and source of erythrocytes. Appl Microbiol 1971; 22:377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calisher CH, Karabatsos N, Dalrymple JM et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 1989; 70 (Pt 1):37–43. [DOI] [PubMed] [Google Scholar]
- 18. Kuno G. Serodiagnosis of flaviviral infections and vaccinations in humans. Adv Virus Res 2003; 61:3–65. [DOI] [PubMed] [Google Scholar]
- 19. Smith CE. The distribution of antibodies to Japanese encephalitis, dengue and yellow fever viruses in five rural communities in Malaysia. Tran R Soc Trop Med Hyg 1958; 52: 237–52. [DOI] [PubMed] [Google Scholar]
- 20. Lee VH, Nalim S, Olson JG et al. A survey of adult mosquitoes on Lombok Island, Republic of Indonesia. Mosquito News 1984; 44: 184–91. [Google Scholar]
- 21. Rudnick A. Dengue virus ecology in Malaysia, In: Rudnick A, Lim TW, eds. Dengue Fever Studies in Malaysia, Kuala Lumpur, Malaysia: Bull 23 from Inst Med Res Malaysia, 1986:PP51–154. [Google Scholar]
- 22. Herrera BB, Chang CA, Hamel DJ. et al. Continued transmission of Zika virus in humans in West Africa, 1992–2016. J Infect Dis 2017; jix182. doi https://doi.org/10.1093/infdis/jix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bennett SN, Holmes EC, Chirivella M et al. Selection-driven evolution of emergent dengue virus. Mol Biol Evol 2003; 20:1650–8. [DOI] [PubMed] [Google Scholar]
- 24. Messer WB, Gubler DJ, Harris E et al. Emergence and global spread of a dengue serotype 3, subtype III virus. Emerg Infect Dis 2003; 9:800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bennett SN, Drummond AJ, Kapan DD et al. Epidemic dynamics revealed in dengue evolution. Mol Biol Evol 2010; 27:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steel A, Gubler DJ, Bennett SN. Natural attenuation of dengue virus type-2 after a series of island outbreaks: a retrospective phylogenetic study of events in the South Pacific three decades ago. Virology 2010; 405:505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 2007; 3:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vazeille M, Moutailler S, Coudrier D et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One 2007; 2:e1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsetsarkin KA, Weaver SC. Sequential adaptive mutations enhance efficient vector switching by Chikungunya virus and its epidemic emergence. PLoS Pathog 2011; 7:e1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsetsarkin KA, Chen R, Yun R et al. Multi-peaked adaptive landscape for Chikungunya virus evolution predicts continued fitness optimization in Aedes albopictus mosquitoes. Nat Commun 2014; 5:4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu J, Du S, Shan C et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature 2017; 545:482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st Century. Trop Med Health 2011; 39: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Russell PK. The Zika pandemic-a perfect storm?PLoS Negl Trop Dis 2016; 10:e0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duffy MR, Chen TH, Hancock WT et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 35. Savage HM, Fritz CL, Rutstein D et al. Epidemic of dengue-4 virus in Yap State, Federated States of Micronesia, and implication of Aedes hensilli as an epidemic vector. Am J Trop Med Hyg 1998; 58:519–24. [DOI] [PubMed] [Google Scholar]
- 36. Durand MA, Bel M, Ruwey I et al. An outbreak of dengue fever in Yap State. Pac Health Dialog 2005; 12:99–102. [PubMed] [Google Scholar]
- 37. Lanciotti RS, Kosoy OL, Laven JJ et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao-Lormeau VM, Roche C, Teissier A et al. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wikan N, Smith DR. Zika virus from a Southeast Asian perspective. Asian Pac J Trop Med 2017; 10:1–5. [DOI] [PubMed] [Google Scholar]
- 40. Oehler E, Watrin L, Larre 1et al. Zika virus infection complicated by Guillain--Barre syndrome-case report, French Polynesia, December 2013. Euro Surveill 2014; 19: pii: 20720. [DOI] [PubMed] [Google Scholar]
- 41. Cao-Lormeau V, Blake A, Mons S et al. Guillain-Barré syndrome outbreak caused by Zika virus infection in French Polynesia: a case control study. Lancet 2016; 387:1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Musso D, Bossin H, Mallet HP et al. Zika virus in French Polynesia 2013–14: anatomy of a completed outbreak. Lancet Infect Dis 2017, in press. [DOI] [PubMed] [Google Scholar]
- 43. Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 2014; 20:O595–6. [DOI] [PubMed] [Google Scholar]
- 44. Ayllón T, Campos RM, Brasil P et al. Early evidence for Zika virus circulation among Aedes aegypti mosquitoes, Rio de Janeiro, Brazil. Emerg Infect Dis 2017; 23:1411–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Faria NR, Quick J, Claro IM et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017; 546:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Brito CA, Cordeiro MT. One year after the Zika virus outbreak in Brazil: from hypotheses to evidence. Rev Soc Bras Med Trop 2016; 49: 537–43. [DOI] [PubMed] [Google Scholar]
- 47. Brasil P, Calvet GA, Siqueira AM et al. Zika virus outbreak in Rio de Janeiro, Brazil: clinical characterization, epidemiological and virological aspects. PLoS Negl Trop Dis 2016; 10: e0004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Oliveira WK, de França GV, Carmo EH et al. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet 2017; 390:861–70. [DOI] [PubMed] [Google Scholar]
- 49. Baud D, Gubler DJ, Schaub B et al. An update on Zika virus infection. The Lancet 2017; pii: S0140-6736(17)31450–2. [DOI] [PubMed] [Google Scholar]
- 50. Musso D, Lanteri MC. Emergence of Zika virus: where does it come from and where is it going to?Lancet Infect Dis 2017; 17:255. [DOI] [PubMed] [Google Scholar]
- 51. Chu DT, Ngoc VT, Tao Y. Zika virus infection in Vietnam: current epidemic, strain origin, spreading risk, and perspective. Eur J Clin Microbiol Infect Dis 2017; doi: 10.1007/s10096-017-3038-8. [DOI] [PubMed] [Google Scholar]
- 52. World Health Organization. Situation Report. Zika Virus Microcephaly Guillain-Barré Syndorme. 10 March 2017. World Health Organization; 2017. [Google Scholar]
- 53. Ramaiah A, Dai L, Contreras D et al. Comparative analysis of protein evolution in the genome of pre-epidemic and epidemic Zika virus. Infect Genet Evol 2017; 51:74–85. [DOI] [PubMed] [Google Scholar]
- 54. Faye O, Freire CC, Iamarino A et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014; 8:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu Z, Chan JF, Tee KM et al. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg Microbes Infect 2016; 5:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen R, Vasilakis N [Dengue–quo tu et quo vadis?] Viruses 2011; 3:1562–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weaver SC, Costa F, Garcia-Blanco MA et al. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res 2016; 130:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vasilakis N, Weaver SC. Flavivirus transmission focusing on Zika. Curr Opin Virol 2017; 22:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Richard V, Paoaafaite T, Cao-Lormeau VM. Vector competence of French Polynesian Aedes aegypti and Aedes polynesiensis for Zika virus. PLoS Negl Trop Dis 2016; 10:e0005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weger-Lucarelli J, Rückert C, Chotiwan N et al. Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl Trop Dis 2016; 10:e0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chouin-Carneiro T, Vega-Rua A, Vazeille M et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis 2016; 10:e0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roundy CM, Azar SR, Rossi SL et al. Variation in Aedes aegypti Mosquito competence for Zika virus transmission. Emerg Infect Dis 2017; 23:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Azar SR, Roundy CM, Rossi SL et al. Differential vector competency of Aedes albopictus populations from the Americas for Zika virus. Am J Trop Med Hyg 2017; 97:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang H, Liu S, Zhang B, Wei W. Analysis of synonymous codon usage bias of Zika virus and its adaption to the hosts. PLoS One 2016; 11:e0166260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Butt AM, Nasrullah I, Qamar R, Tong Y. Evolution of codon usage in Zika virus genomes is host and vector specific. Emerg Microbes Infect 2016; 5:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zanluca C, Melo VC, Mosimann AL et al. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Q, Sun K, Chinazzi M et al. Spread of Zika virus in the Americas. Proc Natl Acad Sci 2017; 114: E4334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg Infect Dis 2015; 21:1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Petersen E, Wilson ME, Touch S et al. Unexpected and rapid spread of Zika virus in the Americas - implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic games. Int J Infect Dis 2016; 44:11–5. [DOI] [PubMed] [Google Scholar]
- 70. Faria NR, Azevedo RD, Kraemer MU et al. Zika virus in the Americas: early epidemiological and genetic findings. Science 2016; 352:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mallet HP, Vial AL, Musso D [Bilan de l’épidémie à virus Zika survenue en Polynésie Française entre Octobre 2013 et Mars 2014.] Bull Hebd Epidemiol 2016; 20–21:367–73. [Google Scholar]
- 72. Aubry M, Teissier A, Huart M et al. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg Infect Dis 2017; 23:669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Araujo AQ, Silva MT, Araujo AP. Zika virus-associated neurological disorders: a review. Brain 2016; 139:2122–30. [DOI] [PubMed] [Google Scholar]
- 74. Brizzi K. Neurologic manifestation of Chikungunya virus. Curr Infect Dis Rep 2017; 19:6. [DOI] [PubMed] [Google Scholar]
- 75. Oehler E, Watrin L, Larre P et al. Zika virus infection complicated by Guillain--Barre syndrome-case report, French Polynesia, December 2013. Euro Surveill 2014; 19:pii:20720. [DOI] [PubMed] [Google Scholar]
- 76. Besnard M, Eyrolle-Guignot D, Guillemette-Artur P et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill 2016; 21: doi: 10.2807/1560–7917.ES.2016.21.13.30181. [DOI] [PubMed] [Google Scholar]
- 77. Bautista LE, Sethi AK. Association between Guillain-Barré syndrome and Zika virus infection. Lancet 2016; 387:2599–600. [DOI] [PubMed] [Google Scholar]
- 78. Fontanet A, Cao-Lormeau VM, Dub T et al. Association between Guillain-Barré syndrome and Zika virus infection - Authors’ reply. Lancet 2016; 387:2600. [DOI] [PubMed] [Google Scholar]
- 79. Dos Santos T, Rodriguez A, Almiron M et al. Zika virus and the Guillain-Barré syndrome - case series from seven countries. N Engl J Med 2016; 375:1598–601. [DOI] [PubMed] [Google Scholar]
- 80. Nash D, Mostashari F, Fine A et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 2001; 344:1807–14. [DOI] [PubMed] [Google Scholar]
- 81. Besnard M, Lastère S, Teissier A et al. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19: pii:20751. [PubMed] [Google Scholar]
- 82. Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT et al. Increase in reported prevalence of micro+smission during the first trimester of pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:242–7. [DOI] [PubMed] [Google Scholar]
- 83. Driggers RW, Ho CY, Korhonen EM et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 2016; 374:2142–51. [DOI] [PubMed] [Google Scholar]
- 84. Mlakar J, Korva M, Tul N et al. Zika virus associated with microcephaly. N Engl J Med 2016; 374:951–8. [DOI] [PubMed] [Google Scholar]
- 85. Brasil P, Pereira JP, Raja Gabaglia C et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016; 375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects--reviewing the evidence for causality. N Engl J Med 2016; 374:1981–7. [DOI] [PubMed] [Google Scholar]
- 87. Cauchemez S, Besnard M, Bompard P et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet 2016; 387:2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. del Campo M, Feitosa IM, Ribeiro EM et al. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet Part A 2017; 173:841–57. [DOI] [PubMed] [Google Scholar]
- 89. Tan PC, Rajasingam G, Devi S, Omar SZ. Dengue infection in pregnancy: prevalence, vertical transmission, and pregnancy outcome. Obstet Gynecol 2008; 111:1111–7. [DOI] [PubMed] [Google Scholar]
- 90. Gérardin P, Barau G, Michault A et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the Island of La Réunion. PLoS Med 2008; 5:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control, 2009. Available at: http://www.who.int/rpc/guidelines/9789241547871/en. Accessed 18 September 2017. [PubMed] [Google Scholar]
- 92. Stramer SL, Fang CT, Foster GA et al. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med 2005; 353:451–9. [DOI] [PubMed] [Google Scholar]
- 93. Levi JE. Dengue virus and blood transfusion. J Infect Dis 2016; 213:689–90. [DOI] [PubMed] [Google Scholar]
- 94. Musso D, Nhan T, Robin E et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19: pii:20771. [DOI] [PubMed] [Google Scholar]
- 95. Motta IJ, Spencer BR, Cordeiro da Silva SG et al. Evidence for transmission of Zika virus by platelet transfusion. N Engl J Med 2016; 375:1101–3. [DOI] [PubMed] [Google Scholar]
- 96. Foy BD, Kobylinski KC, Chilson Foy JL et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Musso D, Roche C, Robin E et al. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. D’Ortenzio E, Matheron S, de Lamballerie X et al. Evidence of sexual transmission of Zika virus. N Engl J Med 2016; 374:2195–8. [DOI] [PubMed] [Google Scholar]
- 99. Guerbois M, Fernandez-Salas I, Azar SR et al. Outbreak of Zika virus infection, Chiapas State, Mexico, 2015, and first confirmed transmission by Aedes aegypti mosquitoes in the Americas. J Infect Dis 2016; 214:1349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]