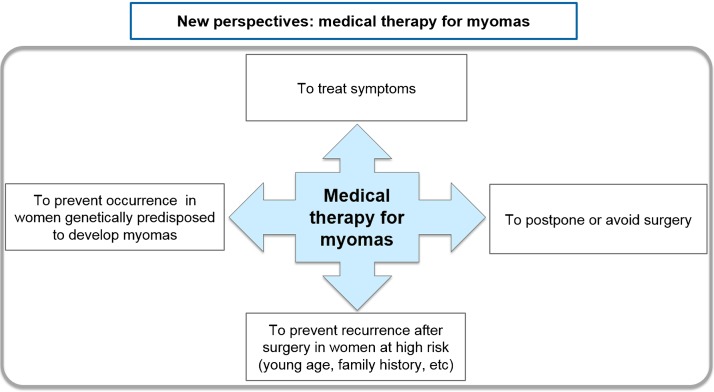
Abstract
Uterine fibroids (also known as leiomyomas or myomas) are the most common form of benign uterine tumors. Clinical presentations include abnormal bleeding, pelvic masses, pelvic pain, infertility, bulk symptoms and obstetric complications.
Almost a third of women with leiomyomas will request treatment due to symptoms. Current management strategies mainly involve surgical interventions, but the choice of treatment is guided by patient's age and desire to preserve fertility or avoid ‘radical’ surgery such as hysterectomy. The management of uterine fibroids also depends on the number, size and location of the fibroids. Other surgical and non-surgical approaches include myomectomy by hysteroscopy, myomectomy by laparotomy or laparoscopy, uterine artery embolization and interventions performed under radiologic or ultrasound guidance to induce thermal ablation of the uterine fibroids.
There are only a few randomized trials comparing various therapies for fibroids. Further investigations are required as there is a lack of concrete evidence of effectiveness and areas of uncertainty surrounding correct management according to symptoms. The economic impact of uterine fibroid management is significant and it is imperative that new treatments be developed to provide alternatives to surgical intervention.
There is growing evidence of the crucial role of progesterone pathways in the pathophysiology of uterine fibroids due to the use of selective progesterone receptor modulators (SPRMs) such as ulipristal acetate (UPA). The efficacy of long-term intermittent use of UPA was recently demonstrated by randomized controlled studies.
The need for alternatives to surgical intervention is very real, especially for women seeking to preserve their fertility. These options now exist, with SPRMs which are proven to treat fibroid symptoms effectively. Gynecologists now have new tools in their armamentarium, opening up novel strategies for the management of uterine fibroids.
Keywords: uterine fibroids, leiomyomas, selective progesterone receptor modulators, ulipristal acetate, surgery, medical therapy, myomectomy
Introduction
Uterine fibroids (also known as leiomyomas or myomas) are the most common form of benign uterine tumors (Stewart, 2001; Donnez and Jadoul, 2002; Bulun, 2013; Islam et al., 2013; Drayer and Catherino, 2015). They are monoclonal tumors of uterine smooth muscle, thus originating from the myometrium (Kim and Sefton, 2012; Bulun, 2013; Islam et al., 2013). They are composed of large amounts of extracellular matrix (ECM) containing collagen, fibronectin and proteoglycans (Parker, 2007; Sankaran and Manyonda, 2008; Kim and Safton, 2012). Leiomyomas occur in 50–60% of women, rising to 70% by the age of 50 (Baird et al., 2003), and, in 30% of cases, cause morbidity due to abnormal uterine bleeding (heavy menstrual bleeding inducing anemia) and pelvic pressure (urinary symptoms, constipation and tenesmus) (Donnez and Jadoul, 2002; Donnez et al., 2014a,b). Clinical presentations of uterine leiomyomas include pelvic masses, pelvic pain, infertility and obstetric complications (Donnez and Jadoul, 2002).
Risk factors
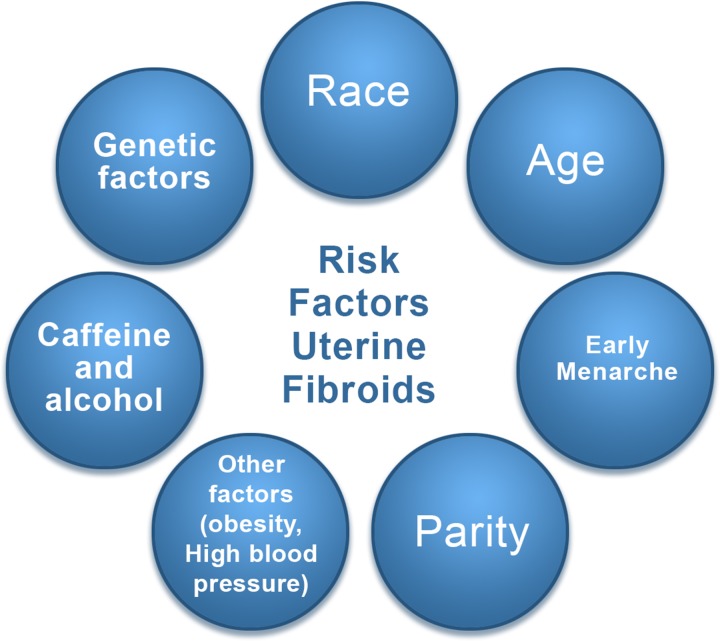
The risk factors for uterine fibroids are illustrated in Fig. 1.
Figure 1.
Risk factors for uterine fibroid. These include race, age, delayed pregnancy, early menarche, parity (protective effect), caffeine, genetic alterations, and others, such as obesity and a diet rich in red meat.
Race
Race constitutes an important risk factor for leiomyoma development (Marshall et al., 1997; Wise et al., 2004; Stewart et al., 2013; El Toukhi et al., 2014). An US study found that the incidence of uterine fibroids was 60% by age 35 among African-American women, increasing to >80% by age 50, while Caucasian women showed a rate of 40% by age 35, increasing to 70% by age 50 (Baird et al., 2003). Differences in gene expression in uterine fibroids between these two groups may influence these growth rates (Davis et al., 2013). Nevertheless, it is clear that African-American women have a greater chance of being affected by uterine fibroids, particularly at an earlier age (Wise et al., 2004, 2005; Wise and Laughlin-Tommaso, 2016). Among women of African origin living in Europe, a similar trend has been observed, with more severe symptoms and surgery required at a younger age. Moreover, recurrence rates after surgery (myomectomy) may be as high as 59% after an interval of 4–5 years (Malone, 1969; Donnez et al., 2014a,b) for women of African origin.
Age
Peddada et al. (2008) followed the size of 262 leiomyomas from 72 women for up to 12 months using magnetic resonance imaging (MRI). The average growth rate was 9% over 6 months, but growth rates differed between races when age was taken into account. White women under 35 years of age had faster-growing tumors than white women over 45, who exhibited a comparatively slow growth rate. On the other hand, women of African origin did not show any decrease in myoma growth rates with age.
Moreover, delaying the first pregnancy until the third decade of life also places women at higher risk of uterine fibroids (Petraglia et al., 2013).
Early menarche
Menarche at an early age increases the risk of developing fibroids and is also considered a risk factor for other hormonally mediated diseases, such as endometrial and breast cancers (Kim and Sefton, 2012; Khan et al., 2014).
Parity
Pregnancy has been found to have a protective effect on the development of uterine fibroids, but the mechanism remains unclear. It has been suggested that during post-partum uterine remodeling, small lesions may be subject to selective apoptosis. Furthermore fibroid tissue may be highly susceptible to ischemia during both uterine remodeling and parturition (Baird and Dunson, 2003; Laughlin et al., 2010).
Caffeine and alcohol
An association has been reported between alcohol and caffeine intake and an increased risk of developing uterine fibroids in a study concerning the health of women of African origin (Wise et al., 2004; Wise and Laughlin-Tommaso, 2016).
Genetic factors
Some specific genetic alterations are linked to fibroid growth (Mäkinen et al., 2011; Eggert et al., 2012; Islam et al., 2013; Mittal et al., 2015; Styer and Rueda, 2015). Mehine et al. (2013) performed whole genome sequencing and gene expression profiling of 38 uterine leiomyomas and corresponding myometrium. The common occurrence of chromothripsis in uterine fibroids suggests that it also plays a role in their genesis and progression (Mehine et al., 2013, 2014).
Other factors
General health status may also be predictive of leiomyoma growth, with factors such as obesity and high blood pressure playing a role. A diet rich in red meat appears to increase the risk of developing leiomyomas, while smoking decreases the risk, for unknown reasons (Kim and Sefton, 2012; Islam et al., 2013).
Classifications
Numerous classifications of myomas can be found in the literature (Lasmar et al., 2005; Stamatellos and Bontis, 2007). All of them take into account the degree of intramural extension and/or uterine cavity distortion. The fibroid classification adopted by the ESGE (European Society for Gynecological Endoscopy) has the advantage of being very simple (G0 is a pedunculated intrauterine myoma, G1 has its largest part (>50%) in the uterine cavity, and G2 has its largest part (>50%) in the myometrium).
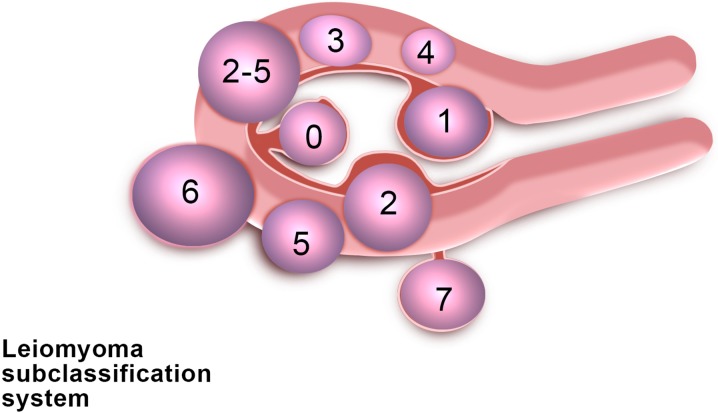
More recently, the FIGO classification was published (Munro et al., 2011), describing eight types of fibroids as well as a hybrid class (association of two types of myomas) (Fig. 2). As different types of fibroids are often present at the same time (depending on the site), this classification offers a more representative ‘map’ of fibroid distribution and will be used further for the establishment of new algorithms.
Figure 2.
FIGO classification of uterine fibroids according to Munro et al. (2011). Fibroid types range from 0 to 8. 0 = Pedunculated, intracavitary; 1 = Submucosal, <50% intramural; 2 = Submucosal, ≥50% intramural; 3 = Contact with endometrium, 100% intramural; 4 = Intramural; 5 = Subserosal, ≥50% intramural; 6 = Subserosal, <50% intramural; 7 = Subserosal, pedunculated; 8 = Other (e.g. cervical, parasitic). Where two numbers are given (e.g. 2–5), the first number refers to the relationship with the endometrium, while the second number refers to the relationship with the serosa; e.g. 2–5 = Submucosal and subserosal, each with less than half the diameter in the endometrial and peritoneal cavities respectively. Fibroid classification cartoon republished with permission from Munro et al. (2011).
Symptoms
Many fibroids are asymptomatic, but in 30–40% of cases, they show a variety of symptoms, depending on the location and size. Fibroids can cause heavy menstrual bleeding with subsequent anemia, which could be life-threatening (Parker, 2007; Nelson and Ritchie, 2015). African-American women have more severe symptoms in terms of heavy bleeding and anemia compared to white women (Stewart et al., 2013). Large fibroids can also result in pressure symptoms (bulk symptoms) that may be responsible for bowel and bladder dysfunction, including urgency, increased daytime urinary frequency and urinary incontinence (Gupta et al., 2008). Abdominal distention or distortion and pelvic pressure on the ureters (causing hydronephrosis) and pelvic blood vessels (particularly pelvic veins) could also interfere with quality of life (QoL) (Spies et al., 2002; Donnez et al., 2014a,b).
Dysmenorrhea and pelvic pain are frequently encountered, impacting on QoL and impairing daily activities (Spies et al., 2002). Infertility and recurrent miscarriage may also be symptoms of fibroids, depending on their location and size, especially for submucous and intramural myomas distorting the uterine cavity (Pritts et al., 2009; Sunkara et al., 2010, Yan et al., 2014; Zepiridis et al., 2015).
Fibroids can impair fertility through several possible mechanisms, including: (1) alteration of the local anatomy (anatomic distortion of the uterine cavity), with subsequent alterations to endometrial function (Somigliana et al., 2007); (2) functional changes, such as increased uterine contractility and impairment of the endometrial and myometrial blood supply (Donnez and Jadoul, 2002) and (3) changes to the local hormone milieu and paracrine molecular changes induced by fibroids, which could impair gamete transport and/or reduce blastocyst implantation (Sinclair et al., 2011; Galliano et al., 2015).
Moreover, fibroids can affect obstetric outcomes. Inflammatory pathways, associated or not with other reproductive disorders, may impair pregnancy outcomes (Vannuccini et al., 2016). Fibroids are significantly associated with preterm delivery (<37 weeks), primary cesarean section, breech presentation and lower birthweight infants (Shavell et al., 2012; Lam et al., 2014, Parazzini et al., 2015; Blitz et al., 2016). Very recently, a higher incidence of short cervix was also observed during pregnancy in women with fibroids (Blitz et al., 2016).
Diagnosis
Pelvic examination
Examination of the pelvis may reveal an enlarged uterus or mass. If fibroids are suspected and a woman reports heavy menstrual bleeding, a hemoglobin evaluation will allow detection of iron deficiency anemia.
Ultrasonography
An ultrasound is the gold standard test for uterine fibroids. Its widespread availability enables easy and inexpensive confirmation in almost all instances. Moreover, ultrasonography after infusion of saline into the uterine cavity can delineate submucous myomas and indicate the proximity of intramural myomas to the endometrial cavity (Seshadri et al., 2015). The advent of 3D imaging technology has seen 3D ultrasound establishes itself as a useful tool for the investigation of myometrial pathology due to its ability to reconstruct the coronal plane of the uterus (Andreotti and Fleischer, 2014; Wong et al., 2015).
Hysteroscopy
A hysteroscopy may be required to differentiate intracavitary myomas and large endometrial polyps (Bettocchi et al., 2003; Di Spiezio Sardo et al., 2010; Parazzini et al., 2015). Hysteroscopy is usually performed in an outpatient setting and does not require any anesthesia (Bettocchi et al., 2003). Ultrasonography with saline infusion and diagnostic hysteroscopy should be considered more as complementary examinations when hysteroscopic myomectomy is indicated. Of course, in case of irregular bleeding or if the patient has risk factors for endometrial hyperplasia (obesity, chronic anovulation), hysteroscopy may be combined with an endometrial biopsy.
Magnetic Resonance Imaging
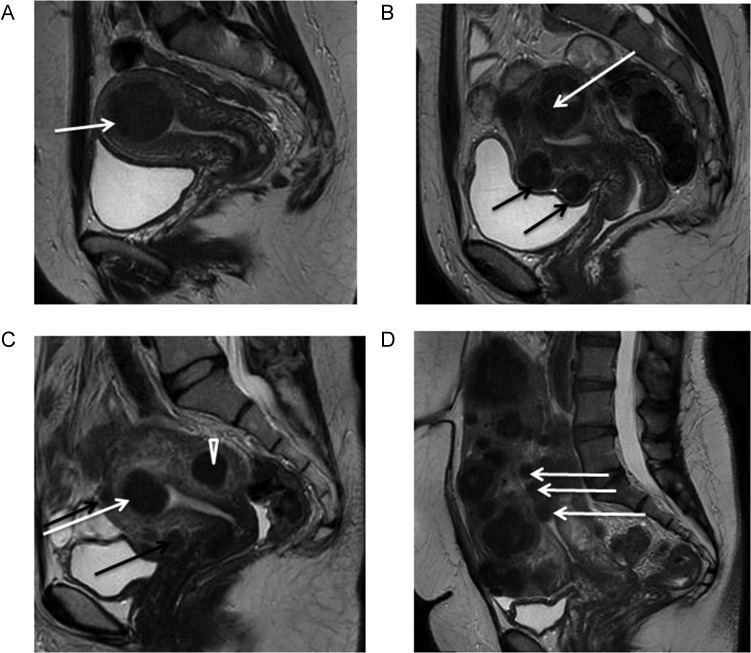
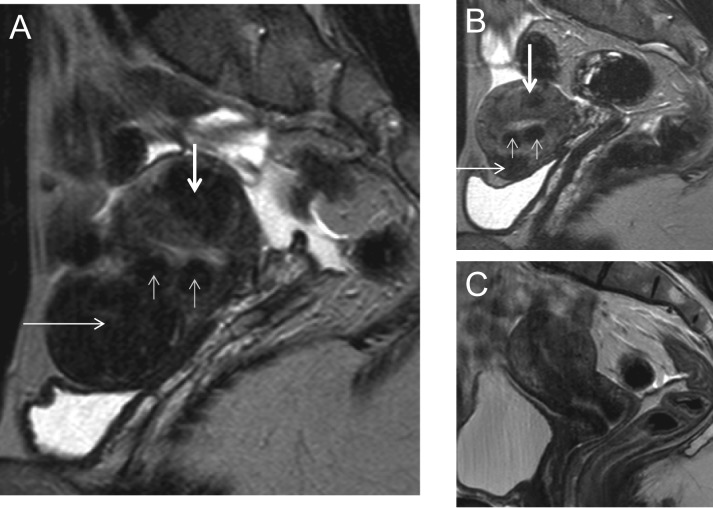
MRI can provide information on the number of fibroids, their size, vascularization, relationship with the endometrial cavity and serosal surface, and boundaries with normal myometrium (Fig. 3). It should nevertheless be stressed that like ultrasonography, MRI cannot diagnose malignancy with any certainty (Lumsden et al., 2015; Stewart, 2015). While MRI findings can suggest a diagnosis of sarcoma, there is currently no form of preoperative testing which can definitively rule it out (Lin et al., 2015). Possibly in the future, new types of imaging will improve the accuracy of detecting sarcoma, which remains a very infrequent condition (1/1500 in women aged <40 years and 1/1100 in women aged 40–44) (Wright et al., 2014).
Figure 3.
Magnetic resonance imaging (MRI) of fibroids. Midline sagittal T2-weighted images show different types of myomas according to the FIGO classification (Munro et al., 2011). Fibroids vary in size, number and site in the uterus. (A) Submucosal type 2 myoma. (B) Large type 2–5 myoma (white arrow): submucosal and subserosal, each with less than half the diameter in the endometrial and peritoneal cavities respectively. Subserosal type 5 myomas (subserosal, >50% intramural) (black arrows). (C) Submucosal type 2 myoma (>50% intramural) (white arrow). Intramural type 4 myoma (arrowhead). Small type 5 myomas (black arrows). (D) Multiple myomas, three of which are type 0 (intracavitary) (white arrows).
Current surgical management strategies
As stressed by Stewart (2015), there are areas of uncertainty surrounding the management of myomas, as only a few randomized trials have compared different therapies for fibroids. Moreover, data on their comparative effectiveness in terms of future fertility are lacking. There are also inadequate data on long-term outcomes in women who have undergone hysterectomy according to indication (Stewart, 2015). Prospective data and studies are essential to compare different options and evaluate long-term outcomes with regard to QoL, recurrence of symptoms (bleeding, bulk symptoms), fertility and even complications.
Indeed, in a cohort study of 30 117 Nurse's Health Study participants undergoing hysterectomy for benign disease, bilateral oophorectomy was found to be associated with increased mortality in patients under 50 years of age who had never used estrogen therapy (Parker et al., 2013).
While guidelines exist in the literature (ACOG, 2008; ASRM, 2008; Marret et al., 2012; Stewart, 2015), the risks and benefits of each option should be discussed with the patient. It should also be stressed that many other factors need to be taken into account, including the skill of the surgeons involved, as well as the experience of different centers in the available techniques.
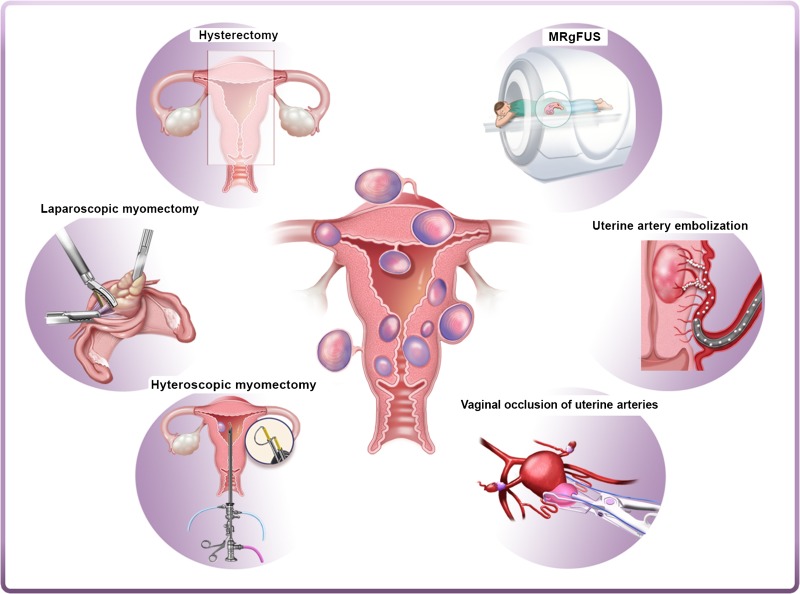
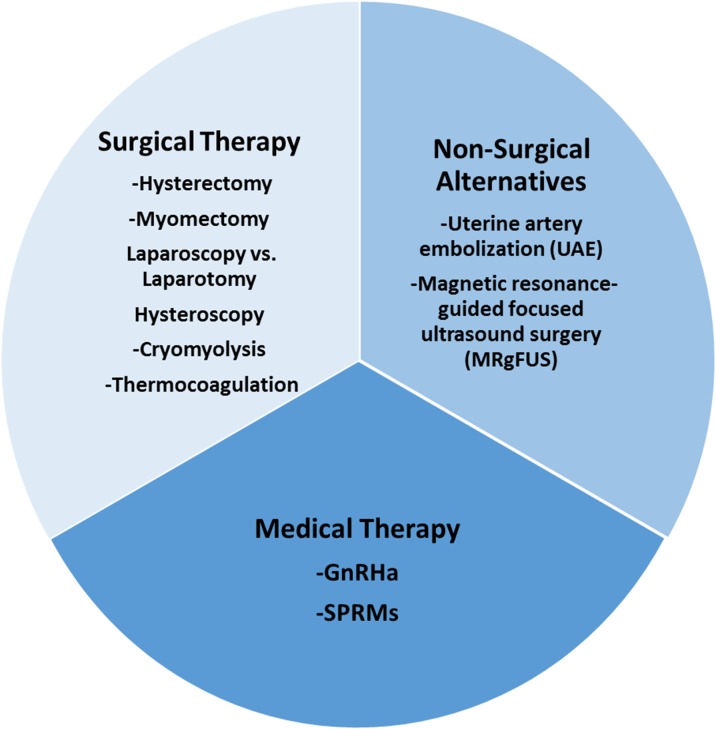
Current management strategies involve mainly surgical interventions, but the choice of treatment is guided by the patient's age and desired to preserve fertility or avoid ‘radical’ surgery such as hysterectomy (Donnez and Jadoul, 2002; Practice Committee of the American Society for Reproductive Medicine, 2008; Lumsden et al., 2015). Other surgical and non-surgical approaches include myomectomy by hysteroscopy, myomectomy by laparotomy or laparoscopy, uterine artery embolization (UAE) and other interventions performed under radiologic or ultrasound guidance (Fig. 4) (Donnez and Jadoul, 2002; Practice Committee of the American Society for Reproductive Medicine, 2008; Lumsden et al., 2015; Stewart, 2015; Zupi et al., 2015).
Figure 4.
Current surgical and non-surgical management strategies of myomas. Left panel: hysterectomy, laparoscopic myomectomy and hysteroscopic myomectomy are the most widely used surgical interventions for myomas. Right panel: alternatives to surgical intervention include uterine artery embolization (UAE), high-frequency magnetic resonance-guided focused ultrasound surgery (MRgFUS) and vaginal occlusion of uterine arteries.
Hysteroscopic myomectomy
Over the last 30 years, advances in instruments and techniques have promoted hysteroscopic myomectomy to the rank of a standard minimally invasive surgical procedure for submucous myomas. Small fibroids (<2 cm) are now routinely removed in an outpatient setting according to the technique described by Bettocchi (Bettocchi et al., 2003, 2004; Di Spiezio Sardo et al., 2010; Casadio et al., 2011; Mazzon et al., 2015; Vilos et al., 2015).
Depending on personal experience and available equipment, the gynecologist has a choice of several alternative procedures.
The first involves cutting the base of pedunculated fibroids with either the resectoscopic loop or laser fiber (Stamatellos and Bontis, 2007; Bettocchi et al., 2004; Di Spiezio Sardo et al., 2008; Tan and Lethaby, 2013). The base of the pedicle is cut and the fibroid is extracted by forceps or may be left in place.
The second alternative is a complete excision of fibroids by a one-step procedure (Di Spiezio Sardo et al., 2008, 2015). The most commonly used approach is the slicing technique. Repeated and progressive passage of the cutting loop allows the surgeon to cut the myoma into small chips. The operation is considered complete when the fasciculate fibers of the myometrium are visualized (Donnez et al., 1990; Bettocchi et al., 2004; Di Spiezio Sardo et al., 2015; Mazzon et al., 2015; Saridogan, 2016). Hysteroscopic resection is effective and safe and should be considered the technique of choice for type 1 myomas. The development of intrauterine morcellators has facilitated the implementation of hysteroscopic myomectomy (Lee and Matsuzono, 2016; Munro, 2016). If the myoma is large (>3 cm in diameter), there is an increased risk of operative complications (perforation, bleeding and fluid intravasation) and damage to surrounding myometrium due to use of electrosurgery. Interestingly, Casadio et al., (2011) demonstrated that during surgery, myometrial thickness increases when myoma slices are removed, leading to protrusion of the intramural component into the uterine cavity.
The third alternative is myomectomy by a two-step procedure (for large type 1–3 myomas of according to the FIGO classification, Munro et al., 2011). After resection or ablation of the protruded portion of the myoma during first-step hysteroscopy, the residual intramural component rapidly migrates to the uterine cavity, with a parallel increase in myometrial thickness, allowing complete and safe myoma excision during second-step hysteroscopy (Donnez et al., 1990; Bettocchi et al., 2004; Stamatellos and Bontis, 2007; Tan and Lethaby, 2013; Di Spiezio Sardo et al., 2008, 2015).
With all the techniques described here, there is risk of fluid (glycine) absorption while using monopolar energy. This risk is avoided by use of bipolar or laser energy with saline solution.
Hysteroscopic myomectomy is effective for control of bleeding, but failures are reported and are often related to growth of fibroids in other sites, association of fibroids with adenomyosis, and incomplete treatment of large intramural (partially submucous) myomas (Pritts, 2001; Pritts et al., 2009; Donnez and Jadoul, 2002; Donnez et al., 2014a; Parazzini et al., 2015).
In terms of reproductive outcomes, most studies are retrospective (Bosteels et al., 2010,a,b). They report post-surgery pregnancy rates ranging from 16.7% to 76.9%, with a mean of 45% (Donnez et al., 2014a). Their robustness could be criticized (Metwally et al., 2011, 2012), but the authors of a recent review (Bosteels et al., 2015) acknowledge that the benefits of hysteroscopic removal of submucous myomas for improving the chances of pregnancy ‘cannot be excluded’. In addition, one prospective randomized study (Casini et al., 2006) has provided good-quality evidence that surgical therapy (hysteroscopic myomectomy) yields higher pregnancy rates than alternative treatments in women with submucous myomas.
Laparoscopic myomectomy
Laparoscopic myomectomy is perceived by many gynecological surgeons to be more difficult, but the advantages are real: less severe post-operative morbidity, faster recovery with laparoscopic procedures and no significant difference between reproductive outcomes after laparoscopic or abdominal myomectomy (by minilaparotomy) (Donnez et al., 2014a,b; Bhave Chittawar et al., 2014; Segars et al., 2014). However, there have been reports of uterine rupture after laparoscopic myomectomy, thus emphasizing the importance of adequate closure of the myometrial defect (Dubuisson et al., 2000; Parker et al., 2010; Thomas et al., 2010). In a review of nine trials including 808 patients (Bhave Chittawar et al., 2014), there was no evidence of any difference in recurrence risk between laparoscopy and open myomectomy.
Usually, a 10 mm scope and two or three ancillary ports are used, although some gynecologists prefer a 5-mm scope. Depending on the site of the myoma, either a vertical (longitudinal) or a transversal incision is made. In the majority of cases, a unipolar hook probe is utilized, but CO2 laser myomectomy is also performed in some departments. In certain cases, uterine artery ligation may be beneficial to reduce intraoperative bleeding (Hald et al., 2004; Liu et al., 2007; Alborzi et al., 2009; Thomas et al., 2010; Bae et al., 2011; Donnez et al., 2014a). Robotic laparoscopic myomectomy has been evaluated in a few retrospective series (Gargiulo et al., 2012; Pitter et al., 2013; Lewis et al., 2015) but no prospective study has been published yet and considerable scepticism remains about the real advantages of the technique (Carbonnel et al., 2014).
Leiomyomas are usually removed with a morcellator, although some gynecologists propose vaginal removal through the cul-de-sac of Douglas or minilaparotomy to avoid the risk of dispersing tissue fragments during sarcoma morcellation. The risk of uterine fragment dispersion with the subsequent appearance of pelvic adenomyotic masses and parasitic leiomyomas was described in 2006 (Donnez et al., 2006, 2007) and remains a concern. This complication can be avoided by extensive peritoneal lavage and careful removal of all the fragments (Donnez et al., 2007), even if some authors still have misgivings (Pereira et al., 2015). Indeed, since their first publication, Donnez et al. no longer encountered this complication in a subsequent series of 400 laparoscopic hysterectomies (LHs), when caution was exercised and attention was paid to examine all areas of the abdominal cavity by placing the patient in the Trendelenburg and anti-Trendelenburg position and by extensive lavage (Donnez and Donnez, 2010). The risk of morcellation of uterine leiomyosarcomas has recently become a ‘hot’ topic, since the Food and Drug Administration (FDA) warned about the use of electromechanical power morcellation (Ton et al., 2015; Parker et al., 2015, 2016). It should nevertheless be stressed that the prevalence of sarcoma in leiomyomas is <0.3% and the debate on the use of electric morcellation has probably been overstated, not only because of the fear of medicolegal issues but also due to emotional reasons (Donnez et al., 2014a,b; Parker et al., 2016). In a recent study (Bojahr et al., 2015), the prevalence of sarcoma was just 0.06% in a series of 10 731 uteri morcellated for myomas during LH. Of course, we should do all we can to improve the diagnosis of sarcoma, but a similar low incidence (1/2000) was observed in a very recent meta-analysis by Pritts et al., (2015) and in a retrospective study including 4791 women in Norway (Lieng et al., 2015). Another meta-analysis by Brohl et al. (2015) concluded that leiomyosarcomas are diagnosed unexpectedly after surgery for what are presumed to be benign fibroids in 1 in 340 women, and that risks increase with age from less than one case per 500 women aged under 30 years to 1 in 98 women aged 75–79 years.
The technique of power morcellation in a bag was recently suggested to minimize the risk of inadvertent tissue spread (Kanade et al., 2014; Kho and Brown, 2015; Cholkeri-Singh and Miller, 2015), but there is no evidence that this technique will not increase the rate of post-operative complications (Donnez et al., 2014a,b).
Contraindications to laparoscopic myomectomy usually include the presence of an intramural myoma >10–12 cm in size or multiple myomas (≥4) in different sites of the uterus, requiring numerous incisions.
The dimensions and localization of the main myoma are the principal criteria for choosing the laparoscopic approach (Dubuisson et al., 2000; Alessandri et al., 2006; Palomba et al., 2007; Nezhat et al., 2009; Malzoni et al., 2010; Thomas et al., 2010; Donnez et al., 2014a,b; Segars et al., 2014; Parazzini et al., 2015). Thus, depending on the skill of the surgeon and his/her ability to suture the myometrial defect without delay, either laparoscopy or minilaparotomy may be selected.
In terms of infertility, several non-controlled studies have suggested that myomectomy yields a decrease in the miscarriage rate in women with myomas distorting the uterine cavity (Saravelos et al., 2011; Bernardi et al., 2014; Parazzini et al., 2015).In a review of prospective and retrospective studies, Donnez and Jadoul reported a pooled pregnancy rate of 49% (95% CI 46–52) in patients who underwent laparoscopic myomectomy (Donnez and Jadoul, 2002). In another review by Somigliana et al. (2007), the post-operative pregnancy rate was 57%. These post-myomectomy pregnancy rates have been confirmed by other studies, but the lack of randomized trials represents a serious drawback (Galliano et al., 2015). However, it should be pointed out that there are no significant differences in cumulative pregnancy rates or obstetric or perinatal outcomes when laparoscopic and abdominal myomectomy are compared (Metwally et al., 2011; Fukuda et al., 2013; Shen et al., 2015; Tian et al., 2015).
Laparoscopic hysterectomy
Hysterectomy has long been considered standard surgical treatment for symptomatic intramural and submucous fibroids, particularly for women not wishing to conceive or those of premenopausal age (40–50 years). In the US, more than 600 000 hysterectomies are performed each year (Flynn et al., 2006). In Denmark, the overall hysterectomy rate was around 180/100 000 women during the period 1977–2011 (Lykke et al., 2013).
Fibroids are the main indication for hysterectomy and, in the last decade, LH has become the ideal surgical approach to replace laparotomy. In some departments, the rate of LH exceeds 90% (Donnez et al., 2009). Vaginal hysterectomy (VH) still remains indicated in some conditions, depending on the skill and habits of the surgeon (Aarts et al., 2015).
Some studies have reported an increased risk of complications after LH (Johnson et al., 2005; Aarts et al., 2015), but in a very large series, Donnez et al., (2009) found a similar complication rate after LH, VH and abdominal hysterectomy (0.44% of major complications). In a personal prospective series of 400 cases, no major complications were encountered (Donnez and Donnez, 2010). Of course, as stressed by the authors, uterine volume of ≥13–14 weeks represents a relative contraindication. A very recent study demonstrated that in some conditions, hospitalization for LH could be less than 5 h (Donnez et al., 2015c).
Some ‘in bag’ morcellation techniques, one of them called the Sydney technique, were developed to address the concerns of morcellating large myomatous uteri after total or subtotal hysterectomy (Mc Kenna et al., 2014) but, as stressed earlier, no large studies have demonstrated any real benefit in terms of general safety. Moreover, the risk of leiomyosarcoma morcellation during LH must be weighed against procedure-related complications associated with laparotomy, including mortality (Siedhoff et al., 2015).
Laparoscopic cryomyolysis and thermo-coagulation
Both laparoscopic cryomyolysis and thermo-coagulation have the same goal: reduction or suppression of the primary blood supply and induction of myoma shrinkage by causing sclerohyaline degeneration (by very low or very high temperatures).
For cryomyolysis, a cryoprobe is inserted into the myoma and cooled to a temperature of <90°C (Zupi et al., 2004; Exacoustos et al., 2005). For laparoscopic thermocoagulation, either a monopolar or bipolar probe is inserted into the myoma before delivering the electrical current. In some studies, laser fibers (YAG) have also been used (Donnez et al., 2000). The limitation of all of these techniques is the lack of histological evaluation of the fibroids (Zupi et al., 2015).
Laparoscopic occlusion of the uterine arteries
Laparoscopic occlusion of the uterine arteries appears to have no specific advantage over vaginal occlusion, as it requires a laparoscopic approach. Moreover, when compared to UAE, the outcomes were found to be inferior in terms of myoma size reduction and devascularization (Hald et al., 2004).
Alternatives to surgical intervention
The alternatives to surgical interventions are illustrated on the right-hand side of Fig. 4.
Uterine artery embolization
Although rapidly adopted by enthusiasts, the introduction of UAE has varied widely across the globe (Moss and Christies, 2016). This technique was first used in 1995 (Ravina et al., 1995) to treat uterine fibroids in women wishing to preserve their uterus.
UAE constitutes complete uterine therapy, as most fibroids are supplied by the uterine arteries. In UAE, percutaneous ablation of the fibromatous uterus is applied to induce ischemic necrosis of the fibroids, while the myometrium revascularizes. Most fibroids are targeted simultaneously. UAE has been shown, in randomized trials, to result in QoL similar to that achieved after surgery, but with a shorter hospital stay and earlier resumption of normal activities (Gupta et al., 2012, 2014).
Although UAE is highly effective for treating symptoms (reduction in bleeding and fibroid size), the risk of reoperation is a reality: 15–20% after successful embolization and up to 50% in cases of incomplete infarction (Kroencke et al., 2010; Gupta et al., 2014; Mara and Kubinova, 2014; Vilos et al., 2015, Spies, 2016). Among possible complications, abdominal pain due to ischemic necrosis of fibroids and risk of infection should not be overlooked (Goodwin and Spies, 2009). The impact of UAE on the ovarian reserve is another concern (Gupta et al., 2014), but a systematic review of 15 randomized trials and prospective cohort studies demonstrated that loss of ovarian function occurred primarily in women over 45 years of age (Kaump et al., 2013). In a very recent review, Zupi et al. (2015) clearly detailed the results and complications of UAE. It was emphasized that a desire for future pregnancy is a relative contraindication, as the lack of data in the literature cannot ensure a good pregnancy outcome. In a randomized controlled trial (RCT) comparing UAE and myomectomy, surgical removal had a more favorable outcome than UAE in terms of pregnancy rate (78% vs 50%), delivery rate (48% vs 19%), and abortion rate (23% vs 64%) (Mara et al., 2008).
Gupta's article in the Cochrane Database of Systematic Reviews (Gupta et al., 2014) evaluated seven RCTs comparing UAE and surgery (abdominal hysterectomy or myomectomy). The authors clearly stated that there were limitations in the evidence. The main limitations of the studies were a serious lack of precision due to wide confidence intervals, failure to clearly report methods, and the absence of blinding for subjective outcomes. They also estimated that between 15% and 32% of subjects would require further surgery within two years of UAE.
Several trials comparing UAE and myomectomy and UAE with focused ultrasound are currently ongoing, whose outcomes are awaited (Stewart, 2015).
High-frequency magnetic resonance-guided focused ultrasound surgery
High frequency magnetic resonance-guided focused ultrasound surgery (MRgFUS) is thermal ablation using MRI to visualize the myoma and define the target. Ultrasonic energy is directed to a point inside the fibroid and coagulation tissue necrosis is induced in the myoma. In theory, damage to surrounding tissue is minimal (Clark et al., 2014; Park et al., 2014) but, in fact, the impact on critical neighboring structures cannot be excluded (Fischer et al., 2015; Kim et al., 2015).
As stressed by Zupi et al. in their review (2015), hyperintensive MRI images are associated with reduced treatment success compared with hypointensive images of fibroids. The principal limitations to the use of MRgFUS are that (i) only a fraction of patients with fibroids meet the inclusion criteria, (ii) future fertility may be compromised and (iii) the financial burden may be too heavy.
The literature is still scarce on this topic and additional studies are needed to evaluate safety profiles (Zupi et al., 2015). In a recent study, 30% of women underwent further fibroid surgery or procedures two years after MRgFUS (Jacoby et al., 2015). Screening and MRI-based prediction models for assessing therapeutic responses may reduce the risk of treatment failure (Kim et al., 2016). Several non-controlled clinical trials (Rabinovici et al., 2010; Berman et al., 2015) have reported pregnancies after MRgFUS, but a recent review by Clark et al. (2014) found a high rate of complications in the 34 documented pregnancies.
Vaginal occlusion of the uterine arteries
Occlusion of the uterine arteries with a clamp-like device that remains in place for 6 h leads to myoma ischemia by interfering with the blood supply to the uterus (Hald et al., 2004). However, this technique is not recommended for women wishing to conceive in the future.
In a study by Vilos et al. (2006), dominant fibroid volume decreased by 24% and heavy bleeding symptoms decreased by 51%. Here too, research with larger populations is needed to prove the efficacy of the technique.
Why we need new options
Fibroids are highly prevalent and represent a high health burden. Indeed, about 30% of women with leiomyomas will request treatment due to morbidities such as heavy menstrual bleeding, abdominal pain, pressure symptoms and/or infertility. Current treatments are mainly surgical and expensive. Among 600 000 hysterectomies performed each year in the USA, 200 000 are for fibroids (Flynn et al., 2006). In a study by Flynn et al. (2006), health care costs for the management of leiomyomas were estimated to be over $2 billion per year. There is no doubt that fibroids have a significant economic impact (Cardozo et al., 2012; Soliman et al., 2015), but the cost of therapy both to the health care system and women with fibroids must be balanced against the cost of untreated disease conditions, as well as the cost of ongoing or repeated investigations and treatment modalities (Vilos et al., 2015). Despite the lack of relevant medico-economic evaluations of the different therapeutics, it is likely that reducing the number of hysterectomies and other surgical procedures will reduce costs and morbidity. It is therefore necessary to develop and evaluate alternatives to surgical procedures especially when fertility preservation is the goal (Donnez et al., 2014,a,b).
Current medical therapy
Two recent Cochrane Reviews on the use of herbal preparations (Liu et al., 2013) and aromatase inhibitors (Song et al., 2013) concluded that there was no evidence to support the use of herbal preparations or aromatase inhibitors as medical therapy for treating myomas. However, as reviewed by Islam et al. (2013, 2014), there is evidence to suggest that certain dietary or alternative treatments like phytochemical herbal preparations may be effective. In addition, some synthetic and natural compounds as well as growth factors are now under laboratory investigation (Islam et al., 2013), while observational data suggest that increased consumption of fruits, vegetables and low-fat dietary products are associated with a reduced risk of developing fibroids (Wise et al., 2011). Nevertheless, some uncertainty remains due to insufficient high-quality studies with large enough sample sizes.
GnRH agonists
By inducing a state of hypoestrogenism and temporary menopause with amenorrhea, GnRH agonists have been used to shrink fibroids and restore hemoglobin levels in symptomatic women (Donnez et al., 1989, 1990; Carr et al., 1993; Lethaby et al., 2001) (Fig. 5). They cannot be used for long periods of time because of their side effects, such as hot flushes and bone loss. A very recent review demonstrated that there is modest evidence that add-back therapy (tibolone, raloxifene, estriol and ipriflavone) can help reduce bone loss and that medroxyprogesterone acetate (MPA) and tibolone may moderate vasomotor symptoms (Moroni et al., 2015).
Figure 5.
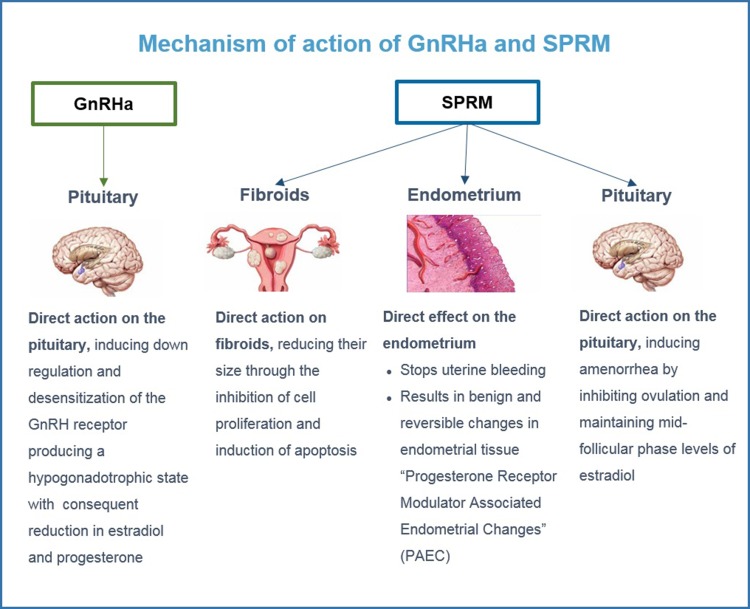
Mode of action of GnRH agonists and SPRMs (Selective Progesterone Receptor Modulators). GnRH agonists have a direct impact on the pituitary. SPRMs have a direct impact on fibroids, endometrium and the pituitary.
Use of GnRH agonist before surgery is still a matter of debate, but a review by Gutmann and Corson (Gutmann and Corson, 2005) reports that ‘preoperative use of GnRH agonist appears to be relevant and beneficial in patients with submucous fibroids’. Benefits include a resolution of preoperative anemia (Donnez et al., 1989; Lethaby et al., 2001; Stamatellos and Bontis, 2007; Doherty et al., 2014); a decrease in fibroid size (Donnez et al., 1989; Lethaby et al., 2001); a reduction of endometrial thickness and vascularization with subsequently improved visibility and reduced fluid absorption (Donnez and Jadoul, 2002; Metwally et al., 2011; Doherty et al., 2014) and the possibility of surgical scheduling (Donnez et al., 1990; Donnez and Jadoul, 2002; Pritts, 2001; Pritts et al., 2009). Conversely, this preoperative treatment is associated with post-injection endometrial bleeding due to the flare-up effect.
The future of medical therapy
Evidence of the crucial role of progesterone pathways in the pathophysiology of uterine fibroids by use of selective progesterone receptor modulators
To date, genetic and epigenetic factors, sex steroids, growth factors, cytokines, chemokines and ECM components have been identified as being implicated in the pathogenesis of leiomyomas (Bulun, 2013; Islam et al., 2013; Marsh et al., 2015; Protic et al., 2015; Yin et al., 2015). Many growth factors and also activin and myostatin play a role in the mechanisms involved in the development of leiomyomas (Ciarmela et al., 2011a,b; Protic et al., 2015). Of course, estrogen and progesterone and their respective receptors also have a very significant impact on leiomyoma growth (Kim and Sefton, 2012). Recently, Wong et al. (2016) demonstrated that testosterone was additionally implicated in the growth of uterine fibroids. In vitro studies have also shown that fibroid development depends on miRNA regulation of gene targets which impact cellular processes (Karmon et al., 2014). The initial event that triggers the first stages of tumorigenesis nevertheless involves somatic mutations (Kim and Sefton, 2012).
In the past, estrogen was considered to be the major growth factor in myoma development. However, already in the 1990s, a number of studies reported increased the expression of both progesterone receptor A (PR-A) and progesterone receptor B (PR-B) in leiomyoma tissue (Englund et al., 1998; Nisolle et al., 1999) compared with adjacent normal myometrium. Very recently, Tsigkou et al. showed that PR-B mRNA and PR-A and PR-B proteins were more concentrated in leiomyomas than in matched myometrium (Tsigkou et al., 2015). Levels of PR-B mRNA in leiomyoma tissue were directly associated with the number of myomas, but inversely correlated with the intensity of symptoms. Moreover, higher proliferative activity, demonstrated by proliferating cell nuclear antigen (PCNA) and the mitotic index, was observed in leiomyomas during the luteal (secretory) phase (Nisolle et al., 1999). There is evidence from preclinical and clinical trials, as well as from histological and pharmacological studies, that progesterone and its receptors play a key role in uterine fibroid growth (Bouchard et al., 2011; Bouchard, 2014; Chabbert-Buffet et al., 2011, 2012, 2014; Kim and Sefton, 2012; Bestel and Donnez, 2014; Moravek et al., 2015). In a review, Kim and Sefton (2012) described, in detail, the activation of signaling pathways in leiomyomas by both estrogen and progesterone. Progesterone is able to cause rapid, membrane-initiated effects, independent of gene transcription, that alter the production of second messengers involved in cell signaling transduction pathways. The PI3K/AKT pathway is mediated by progesterone which, through its receptors, can quickly activate this pathway, which is increasingly considered to be a potential promoter of leiomyoma growth. PTEN, on the other hand, should be considered as a negative regulator of AKT (Kim and Sefton, 2012). Progesterone and growth factor signaling pathways are interconnected and govern numerous physiological processes such as proliferation, apoptosis and differentiation.
Progesterone can modulate the expression of growth factor signaling proteins and is implicated in the regulation of genes associated with proliferation and apoptosis, but these genes have not yet been fully identified or studied in detail (Islam et al., 2013; Kim and Sefton, 2012; Moravek et al., 2015). There is therefore evidence that progesterone plays a crucial role, but the mechanism by which it promotes proliferation, the repertoire of genes involved, and how it crosstalks with growth factor signaling pathways all need to be investigated in greater depth. The recent discovery of stem cells and their paracrine interactions with more differentiated cell populations within leiomyoma tissue may lead to the development of therapeutics that temper leiomyoma growth as well as those that eradicate them (Moravek et al., 2015).
Having established the crucial role of progesterone in the growth and development of myomas, we can modulate the progesterone pathway by use of selective progesterone receptor modulators (SPRMs) (Chabbert-Buffet et al, 2005, 2011, 2015; Bouchard et al. 2011; Bouchard, 2014; Kim and Sefton, 2012; Bestel and Donnez 2014; Donnez et al., 2012a,b). SPRMs are synthetic compounds that exert either an agonistic or antagonistic effect on PRs (Fig. 5). Their binding allows these receptors to interact with coactivators and/or corepressors, and this is further impacted by the presence of coregulators in a particular cell type, which will dictate whether an SPRM acts more as an agonist or antagonist (Chabbert-Buffet et al., 2005, 2011). Hence, the mechanism of action of SPRMs on PRs depends on their structure and how they alter the PR conformation, resulting in exposure or inactivation of particular binding domains. Their activity is also mitigated by tissue types and physiological contexts (Kim and Sefton, 2012; Bouchard, 2014; Moravek et al., 2015).
SPRMs and fibroids: what we know so far
Four members of the family of compound SPRMs have been investigated in phase II clinical trials: mifepristone, asoprisnil, ulipristal acetate (UPA) and telapristone acetate (Spitz, 2009; Bouchard et al., 2011; Bouchard, 2014; Chabbert-Buffet et al., 2011; Nieman et al., 2011; Shen et al., 2013; Whitaker et al., 2014). All were shown to decrease leiomyoma size and reduce uterine bleeding in a dose-dependent manner. However, although three studies (Fiscella et al., 2006; Engman et al., 2009; Bagaria et al., 2009) showed a myoma volume reduction of ±30%, a review of the literature by Tristan et al. (2012) (Cochrane Review) found no clear evidence of this. Some follow-up studies have also raised concerns about unopposed estrogenic activity and liver toxicity (Williams et al., 2007; Spitz, 2009; Bouchard et al., 2011; Chabbert-Buffet et al., 2011; Tristan et al., 2012).
The latest antiprogestin to be studied in large clinical trials, UPA, has shown promising results in terms of efficiency and safety. UPA was compared to a placebo and to leuprolide acetate (a GnRH agonist) in two randomized trials (Donnez et al., 2012a,b). In these first clinical studies, uterine bleeding was controlled in more than 90% of patients receiving a three-month course of UPA, and the median times to control bleeding were shorter in the UPA group (5–7 days) than in the GnRH agonist group (21 days). The control of bleeding and subsequent correction of anemia were clinically relevant (Barlow et al., 2014; Donnez et al., 2012a,b). Indeed, it has been well documented that preoperative anemia, even to a mild degree, is associated with an increased risk of morbidity and mortality in patients undergoing surgery (Mussalam et al., 2011; Richards et al., 2015). UPA was also found to have a sustained effect (up to six months) in women who did not undergo surgery after the three-month study period. By contrast, those treated with GnRH agonist experienced rapid regrowth of their fibroids, whose size reached pre-therapy dimensions by six months post-treatment (Donnez et al., 2012a,b).
Importantly, the induced effects of SPRMs on the endometrium, now described as progesterone receptor modulator (PRM)-associated endometrial changes (PAECs) (Mutter et al., 2008) (Fig. 5), present in almost 70% of patients at the end of treatment, have proved to be benign and reversible, as they disappeared two months after the end of therapy (Williams et al., 2012; Donnez et al., 2012a,b). Safety has also been well documented in pharmacokinetic studies following multiple doses (Pohl et al., 2013, 2015).
The mechanism of action by which SPRMs reduce menstrual blood loss in women with fibroids remains unknown (Wilkens et al., 2013), although a number of possible factors have been proposed by Williams et al. (2007, 2012). Wilkens et al. (2013) reported that uterine NK cells regulate endometrial bleeding and were suppressed by asoprisnil.
Long-term intermittent administration of SPRMs, opening up new treatment perspectives
Because of the sustained effect observed in the first two trials (Donnez et al., 2012a,b), additional intermittent (12-week) courses of SPRMs with off-treatment intervals may be an alternative for long-term medical management of fibroids. The results of the first long-term intermittent administration study suggested that more than one course of SPRMs can maximize its potential benefits in terms of bleeding control and fibroid volume reduction (Donnez et al., 2014b).
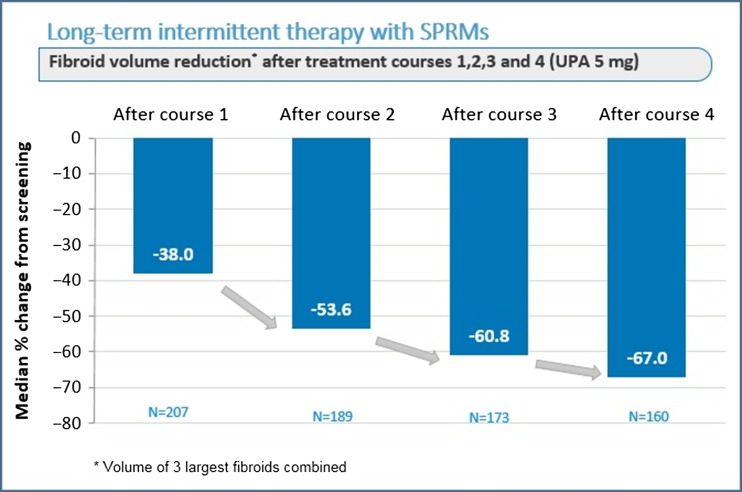
The latest clinical trial was initiated to investigate the efficacy and safety of four repeated 12-week courses of either 5 or 10 mg UPA daily for intermittent treatment of symptomatic uterine fibroids (Donnez et al., 2015,a,b,c). This study demonstrated a similar degree of response in both treatment groups. We will therefore focus on the results in terms of efficacy and safety of this trial using the approved dose of 5 mg UPA in a repeated intermittent therapy setting (four courses) (Donnez et al., 2015,b,c). The percentages of subjects identified as being in amenorrhea after individual treatment courses (1, 2, 3 and 4 in the study) were 75.8%, 84.1%, 86.4% and 87.5% in the 5 mg group (Donnez et al., 2015,a,c). The pictorial blood assessment chart (PBAC) (Higham et al., 1990) score was measured at initial screening and after 1, 2 and 4 courses to assess the level of menstrual bleeding during the off-treatment period. In the 5 mg group, (median) levels at screening were 224.0, dropping significantly with each subsequent course and finally reaching 77.5 after course 4 (Donnez et al., 2015,a, c). The percentage of subjects with a clinically significant volume reduction of ≥25% increased from course 1 to course 4 (from 62.3% to 78.1%), and those with a volume reduction of ≥50% also increased from course 1 to course 4, proving that repeated courses considerably maximize the impact of treatment. This was also proved by the volume reduction of the three largest fibroids which was increased from course 1 to course 4 (Fig. 6). The findings of this study therefore demonstrate the efficacy of 5 mg UPA treatment and further confirm the safety of repeated intermittent administration of UPA for symptomatic myomas (Donnez et al., 2015,a,b,c).
Figure 6.
Effect on fibroid volume reduction after four courses of three months of ulipristal acetate (UPA) 5 mg daily. The off-period between two courses was two natural cycles.
Adapted from Donnez et al. (2015a, 2016).
The safety profile of UPA during multiple treatment courses was well documented in this study (Donnez et al., 2015,a,b,c). Safety assessments, including vital signs, physical examinations and laboratory analyses, as well as reported adverse events (AEs) both on and off treatment, showed repeated intermittent administration of UPA to be well tolerated. The vast majority of AEs (97.6%) were of mild or moderate severity. Headaches and hot flushes were the most frequently reported AEs (less than 11% of subjects in any treatment course), but the frequency of these events decreased with each additional treatment course. Breast pain or discomfort was observed in 3% of subjects. In this series of 451 women (Donnez et al., 2015b, c), serious AEs related to medication included five cases of menorrhagia, one bipolar disorder, one spontaneous myoma expulsion, one abdominal pain and one back pain. No safety concerns were identified from physical examination, vital signs, ovarian ultrasound or electrocardiogram (ECG).
Based on the available data related to endometrial safety after up to four treatment courses, no increased occurrence of more serious conditions of the endometrium, such as hyperplasia with atypia or endometrial carcinoma, was noted. The frequency of SPRM-associated non-physiological endometrial changes (PAEC) did not appear to increase with repeated treatment courses, reaching 13.3% after a fourth treatment course, and returning to pretreatment levels within three months of completion of treatment. These data further confirm the rapid reversibility of PAEC following completion of treatment and subsequent menstruation. It is reassuring that median endometrial thickness (7–8 mm) was similar to screening levels after single and multiple treatment courses and remained stable during post-treatment follow-up (three months after treatment cessation).
A recent study by Courtoy et al. suggested an important role of UPA in collagen degradation induced by matrix metalloproteinase 2 (MMP-2), offering an explanation for the sustained beneficial effect. Indeed, this study strongly points to multifactorial mechanisms of action involving: (1) a persistently low cell death rate; (2) a limited period of cell death and (3) ECM remodeling concomitant with stimulation of MMP-2 expression (Courtoy et al., 2015).
An in vitro study demonstrated another possible mechanism of action of UPA: inhibition of activin A expression and function in cultured leiomyoma cells (Ciarmela et al., 2014).
Novel approaches and algorithms, with a special emphasis on infertility
There is a clear need for alternatives to surgery, even the less invasive endoscopic techniques, especially when fertility preservation is the goal (Donnez et al., 2014a,b; Donnez et al. 2015b). There is no doubt that surgery remains indicated in some instances, but we must now establish whether SPRMs (UPA) allow less invasive surgery or even complete avoidance of surgery. On the other hand, it is clear that long-term intermittent use of UPA will change our approach to the management of uterine fibroids.
To address the question of which therapy to adopt, it is crucial to consider key factors determining the management of uterine fibroids: patient age, severity of symptoms (pain, bleeding and infertility), wish to preserve the uterus and/or fertility, localization of fibroids according to FIGO classification and myoma volume. The approaches described below are according to the FIGO classification (Munro et al., 2011).
Type 0 myomas
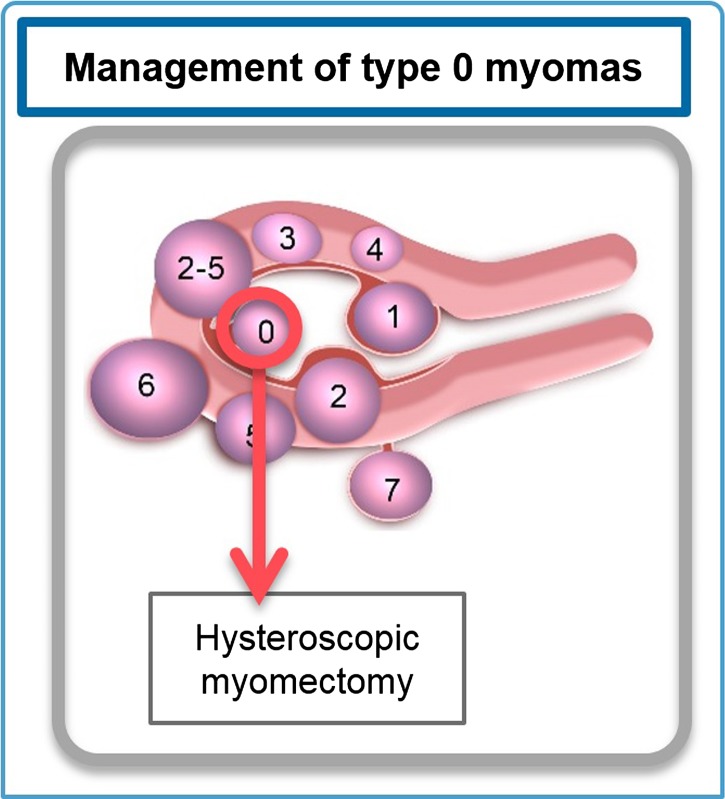
If type 0 myomas are present, cutting the pedicle by hysteroscopy is indicated (Fig. 7).
Figure 7.
Management of type 0 myomas. Hysteroscopic myomectomy is the most appropriate approach. Fibroid classification cartoon republished with permission from Munro et al. (2011).
Type 1 myomas
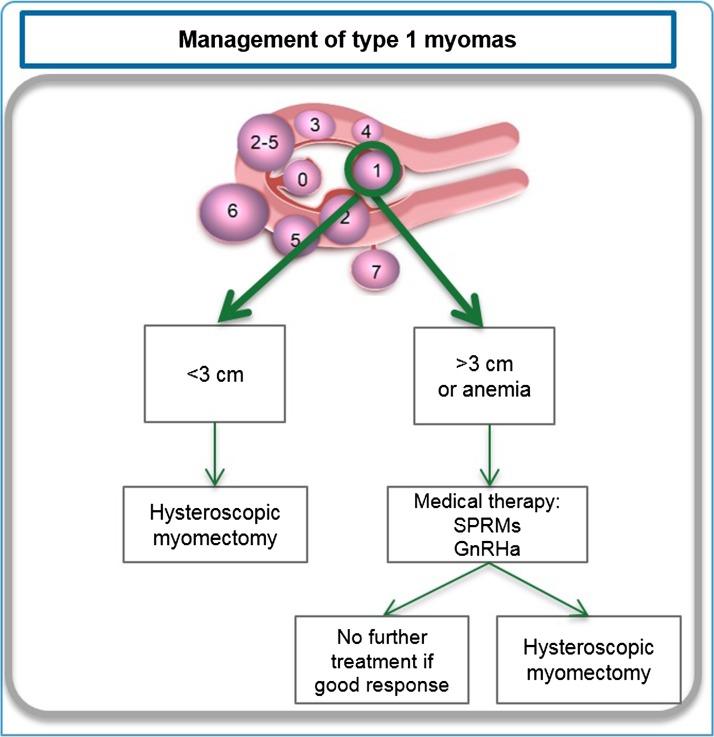
In the majority of cases, hysteroscopic myomectomy for type 1 myomas is relatively straightforward for experienced surgeons, especially in case of type 1 myomas less than 3 cm in size (Fig. 8). If a fibroid is of type 1 but larger than 3 cm, or if the patient presents with anemia, pre-hysteroscopic medical therapy (SPRMs or GnRH agonist) is indicated. Results in terms of subsequent fertility after hysteroscopic myomectomy were discussed earlier in this paper.
Figure 8.
Management of type 1 myomas. Depending on the myoma size, presence of anemia and the surgeon's skill, hysteroscopic myomectomy combined or not with ulipristal acetate(UPA) should be proposed. Fibroid classification cartoon republished with permission from Munro et al.(2011).
Medical therapy may be given in one or two courses of three months. In the vast majority of cases, type 1 myomas respond to this preoperative therapy and regress in size, enabling an easier hysteroscopic approach in better conditions (recovery of hemoglobin). It should be pointed out that in some cases, myomas regress so much that surgery may be avoided.
Type 2 or type 2–5 myomas (single or multiple) distorting the uterine cavity
Young infertile women of reproductive age and wishing to conceive
In case of type 2 myomas, medical therapy (SPRMs) can be proposed (Fig. 9). Myomas often respond to this preoperative therapy and regress in size. This reduction also allows a hysteroscopic approach that can be planned after the first menstrual bleed (Donnez et al., 2014a,b). In some cases (if myomas regress so much that they no longer distort the uterine cavity), surgery may not be required. If myomas are multiple (≥2) or of different types (type 2–5), as is frequently observed, medical therapy (SPRMs) can be given in two courses of three months, as described in clinical trials with UPA (Donnez et al., 2014a,b; Donnez et al., 2015,a,b). After these two courses of three months, there are three possible outcomes.
Figure 9.
Management in case of myomas or multiple myomas (type 2–5) in women of reproductive age, according to desire for pregnancy. In cases of infertility, two courses of three months are recommended (left panel). Subsequent therapy is determined depending on the response to treatment and restoration of the uterine cavity. If there is no desire to conceive (right panel), long–term (four courses) intermittent therapy may be proposed. In case of a good response in terms of fibroid volume reduction and bleeding, treatment is stopped and only restarted if symptoms recur. Fibroid classification cartoon republished with permission from Munro et al. (2011).
The most positive outcome would be that myoma regression is very significant (>50% decrease in volume). The uterine cavity is no longer distorted and the patient can try to conceive naturally or undergo assisted reproductive techniques, if indicated. A first series of pregnancies after UPA treatment was recently described, demonstrating that in some cases, surgical treatment is not required and patients can conceive and deliver healthy offspring (Luyckx et al., 2014) (Fig. 10). Other case reports have also been published (Monleon et al., 2014). In our series of pregnancies, patients were able to have unprotected sexual intercours or to start with ovarian stimulation after the second menstrual bleed (Luyckx et al., 2014). For those having to undergo IVF, a vaginal ultrasound was performed on day 3 of the second menstrual bleed to assess the absence of a thick endometrium. The second outcome would be that myoma regression is significant (≥25% but <50%). However, in some instances, if the uterine cavity remains distorted or if the myoma remains large due to great volume at baseline, the indication for surgery stands. In this case, medical treatment may allow surgery to be performed by a laparoscopic approach once the hemoglobin level is normalized, avoiding laparotomy.
Figure 10.
Considerable shrinkage of all myomas after four courses of intermittent ulipristal acetate (UPA) therapy. A patient aged 30 years presented with heavy menstrual bleedingand an unclear desire for pregnancy. (A) Before treatment, a midline sagittal T2-weighted magnetic resonance image (MRI) demonstrated the presence of multiple myomas: type 2, 3, 4 and 6. (B) Upon completion of treatment (intermittent UPA therapy (four courses of threemonths), the uterine cavity was no longer distorted. (C) One year after delivery of a healthy baby, no fibroid regrowth was observed after delivery.
The least outcome would be that the response to medical therapy is inadequate. In this case, surgery remains indicated.
Young women of reproductive age with symptomatic myomas and wishing to preserve their fertility but having no immediate desire for pregnancy
In these cases too, medical therapy can be proposed (Fig. 9), taking into account recent clinical trials with SPRMs demonstrating that four courses of three months induce a significant improvement, course upon course (decrease in myoma size and PBAC score) (Donnez et al., 2015,a,b, 2016). In the vast majority of cases, regression of myoma size (≥25% in 80% of patients) and control of bleeding (in >90% of patients) will allow avoidance of surgery and restoration of hemoglobin levels (Fig. 11).
Figure 11.
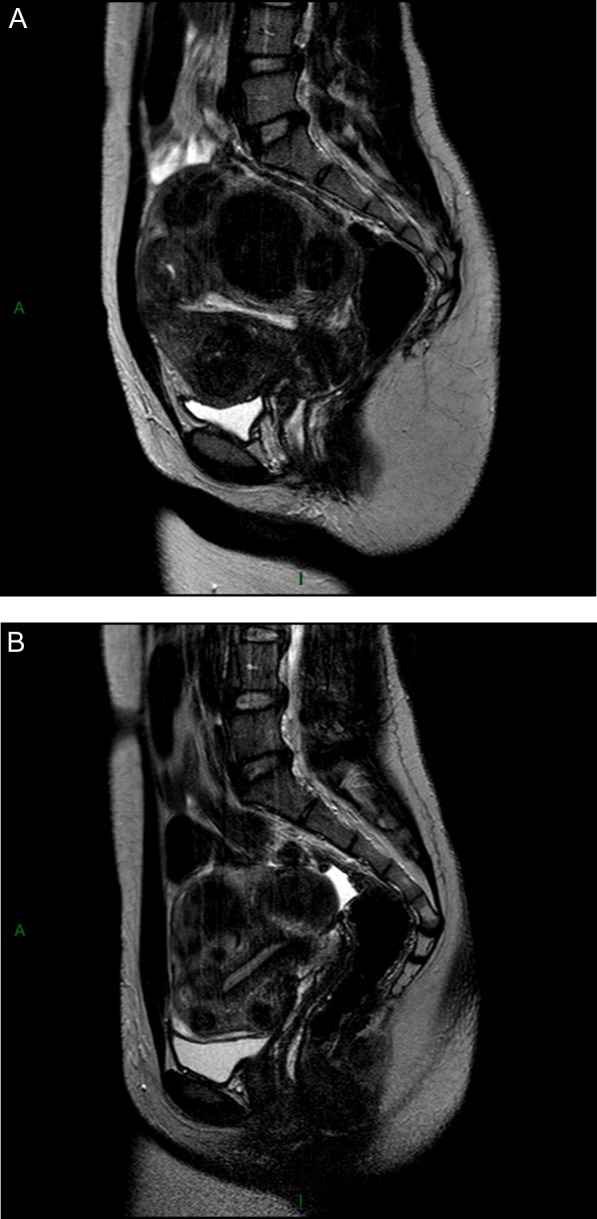
Important shrinkage of the submucosal myoma was obtained after two courses of three months of intermittent ulipristal acetate (UPA) therapy. (A) Coronal T2-weighted magnetic resonance image (MRI) image illustrated the presence of multiple myomas (type 2, type 2-5) distorting the uterine cavity in a 19-year-old nulligravid patient, who presented to the emergency department, with heavy menstrual bleeding and anaemia (haemoglobin level of 7.4 g/l).The patient received two courses of UPA (5 mg) and iron. (B) At the end of therapy, MRI demonstrated a significant reduction in myoma volume (<50%) and restoration of the uterine cavity. Amenorrhea was achieved, with a haemoglobin level of 11.9 g/l. The patient was free of symptoms and did not wish to conceive; therefore, surgery was avoided.
When there is no immediate wish to conceive, there is no pressing need for surgery (even if the uterine cavity remains distorted and/or large myomas are still present). In some cases, myomas will all but disappear. In case of symptom recurrence, medical therapy may be reinitiated. Myomectomy should only be considered when the patient wishes to become pregnant, and if really necessary according to the localization and volume of the fibroids still present. This is important to take into account, especially for women of African descent. Indeed, African and African-American women have a greater chance of developing symptomatic myomas at an earlier age than Caucasian women (Baird et al., 2003). It is widely known that the rate of recurrence of myomas after myomectomy can reach almost 60% after an interval of 4–5 years, and that the risk of pelvic adhesions is significantly increased after a repeated myomectomy (Malone, 1969; Donnez et al., 2014a). Medical treatment with SPRMs can thus be beneficial, since long-term intermittent therapy (repeated in case of symptom recurrence during the interval) may help to avoid or at least postpone the need for surgery until the patient wishes to conceive (Fig. 11).
Surgery therefore remains indicated only when the patient wishes to conceive, and if large myomas (>3–4 cm) distorting the uterine cavity are present, as these could be the cause of her infertility.
Asymptomatic women with myomas and undergoing IVF or oocyte donation
A meta-analysis by Pritts et al. (2009) evaluating 23 studies showed a significant drop in pregnancy and implantation rates in the presence of myomas, especially submucous and/or intramural myomas distorting the uterine cavity. In another meta-analysis, Sunkara et al. (2010) demonstrated their impact on fertility, even in case of intramural myomas not distorting the uterine cavity. A recent study by Yan et al. (2014) confirmed that intramural fibroids >2.85 cm in size significantly decreased the delivery rate of patients undergoing IVF/intracytoplasmic sperm injection (ICSI).
Moreover, some centers have large cohorts of patients of more than 40 years of age in oocyte donation programs (Cobo et al., 2015). In this group of women, the prevalence of myomas is higher than in women of 30 years of age.
It could be proposed that patients with myomas be treated with one or two courses of SPRMs before IVF or oocyte donation, in order to reduce the size of myomas and restore the uterine cavity and subsequently improve implantation rates. Clinical trials evaluating UPA before IVF or oocyte donation should be initiated to investigate this further.
Premenopausal women presenting with symptomatic myomas and with no desire for pregnancy but a wish to keep their uterus
Isolated type 2 fibroids are relatively rare in premenopausal women. In the majority of cases, patients with symptomatic myomas have an enlarged uterus with multiple myomas or large myomas of type 2–5 (Fig. 12).
Figure 12.
Management of type 2 to 5 myomas or multiple myomas (type 2–5) in premenopausal women wishing to preserve their uterus. In this case, long-term (four courses of three months) intermittent therapy with SPRMs is proposed. Fibroid classification cartoon republished with permission from Munro et al. (2011).
Our latest results (Donnez et al., 2015,a,b, 2016) led us to slightly modify previously published algorithms (Donnez et al., 2014a,b) for this group of women. Indeed, in subjects treated with 5 mg UPA for four courses of three months, the percentage of patients with a clinically significant volume reduction increased from 62.3% after 1 course to 78.1% after 4 courses, suggesting increased benefits with repeated courses. The percentage of women showing a clinically significant reduction of >50% also increased from course 1 (37.2%) to course 4 (63.8%). Moreover, the median PBAC score during the off-treatment period decreased with each subsequent course.
In case of a good response (characterized by a clinically significant volume reduction and/or control of bleeding), treatment can be stopped after four courses and the patient is re-evaluated (Donnez et al., 2015,a,b, 2016). Repeated therapy may be proposed when the symptoms recur, as no endometrial hyperplasia was diagnosed in subjects who took 5 mg UPA for eight courses of three months. In this context, the goal is to reach menopause without the need for surgery. Data indicating that SPRMs exert an anti-proliferative effect in breast tissue are also reassuring (Poole et al., 2006; Engman et al., 2008). Some studies reported anti-proliferative effects on the endometrium after SPRM courses of up to six months (Wilkens et al., 2009).
Uterine fibroid associated pathologies
Endometriosis and adenomyosis are frequently associated with uterine fibroids (Donnez et al., 2014a).
Endometriosis
In theory, induction of amenorrhea in women treated with SPRMs should also relieve endometriosis-associated pain. In mammalian models, SPRMs stop prostaglandin production by endometriotic lesions (Gemzeel-Danielson and Hamberg, 1994; Elger et al., 2004) and this direct effect may also serve to reduce pain.
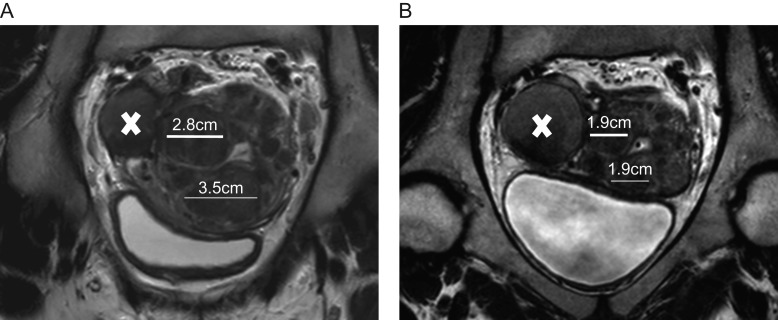
Nevertheless, it should be stressed that even if endometriosis and uterine fibroids are both estrogen-dependent diseases, they show a completely different response to progesterone: endometriosis is characterized by progesterone resistance (Donnez et al., 1996; Nisolle and Donnez, 1997; Bulun, 2009), while fibroids grow under the influence of progesterone (Kim and Sefton, 2012). Fig. 13 shows the excellent response (>50% volume reduction) to UPA obtained in fibroids, but the absence of response (or even a slight volume increase) in endometriomas.
Figure 13.
27 year-old women complaining of heavy menstrual bleeding and pelvic pain. A: Coronal T2-weighted MRI images illustrated the presence of type 2–5 and type 3 myomas distorting the uterine cavity and an endometrioma (indicated by X) of 4.3 cm in size. The white lines represent the diameter of the myomas. This patient received long-term intermittent therapy with 5 mg of UPA (2 courses of 3 months). B: At the end of therapy there was an important reduction in myoma volume, but not endometrioma volume.
The specific effects of SPRMs have yet to be determined in ectopic endometrium. Indeed, PAECs (Williams et al., 2007, 2012; Mutter et al., 2008) may be present in lesions, as observed in eutopic endometrium.
Adenomyosis
Adenomyomas and adenomyosis are two distinct clinical entities. Adenomyomas may respond very well to SPRMs, but severe full-thickness adenomyosis, characterized by the presence of numerous sites of ectopic endometrium in the myometrium of an enlarged uterus, is a specific entity that might have a completely different response.
SPRMs will probably be effective in reducing adenomyosis-associated pain by inducing amenorrhea, but are unlikely to be able to significantly reduce the size of the uterus. Clinical trials are ongoing to explore this particular context and the impact of endometrial modifications, as PAECs will also be present in ectopic intramyometrial endometrium.
Future prospectives for medical therapy
SPRMs have opened up new avenues to explore in fibroid medical therapy, to both treat symptoms and postpone or to eliminate the need for surgery. Future clinical trials should focus on prevention strategies, such as preventing occurrence in women genetically predisposed to this condition, and avoiding recurrence after surgery in women at high risk (i.e. those of a young age or with a family history) (Fig. 14).
Figure 14.
New avenues are emerging in medical fibroid therapy. The first goal of medical therapy is clearly to treat symptoms resulting from the presence of fibroids (heavy menstrual bleeding, pelvic pain, bulk symptoms, infertility, etc.), as well as to postpone or avoid surgery. Further avenues should be investigated by randomized trials, looking to avoid recurrence after surgery in women at high risk of recurrence, and to prevent occurrence of myomas in genetically predisposed women.
Conclusion
Symptomatic uterine fibroids require surgical and/or medical therapy according to the severity of symptoms, age, infertility, wish to preserve the uterus and FIGO classification (Fig. 14). Current strategies involve mainly surgical intervention, such as hysterectomy, myomectomy by hysteroscopy and myomectomy by laparoscopy or laparotomy. Hysterectomy provides the most effective treatment for fibroids, but is not appropriate in many cases. The choice between less invasive techniques (uterine-sparing options such as myomectomy) is guided by the size, number and location of fibroids as well as the personal experience of the gynecologist and available equipment. Other surgical techniques, such as laparoscopic cryomyolysis, thermocoagulation or uterine artery occlusion, are rarely used. Non-surgical interventions, such as UAE and MRgFUS, are also available but the desire for future pregnancy is a relative contraindication.
On the other hand, the need for medical therapy remains a reality. It is indeed essential that new treatments be developed to be able to offer as there is a pressing need for alternatives to surgical intervention, particularly when fertility preservation is the goal.
GnRH agonists have been used to shrink fibroids and restore hemoglobin levels in symptomatic women, but because of their side effects, they cannot be used for long periods of time. However, there is now growing evidence of the crucial role of progesterone in pathways in the pathophysiology of uterine fibroids by the use of SPRMs. UPA (one member of the SPRM compound family) has been studied in large clinical trials and its long-term intermittent administration has been evaluated, yielding promising results for new treatment perspectives. It was found that more than one three-month course of UPA maximizes its potential benefits in terms of bleeding control and fibroid volume reduction. Hence, depending on age and symptoms (infertility, bleeding, etc.), SPRMs should be considered an alternative to surgical therapy, or at least an adjunct to surgery, in some circumstances, as illustrated in the algorithms.
In conclusion, asymptomatic fibroids do not require treatment once the diagnosis is confirmed by ultrasonography or MRI. Women should be made aware of all available treatment options (medical, radiological and surgical) and why they may or may not be appropriate. Gynecologists now have new tools in their armamentarium (Fig. 15) opening up novel strategies for the management of uterine fibroids.
Figure 15.
Surgical, non-surgical and medical therapy for the management of fibroids: the current armamentarium.
Acknowledgements
The authors thank Dr. Latifa Fellah, MD, for the selection and preparation of MRI images, Mira Hryniuk, BA, for reviewing the English language of the manuscript and Deborah Godefroidt for her administrative assistance.
Authors’ roles
J.D. and M.M.D. contributed equally to the research and interpretation of data discussed in the manuscript and approved the final version.
Funding
No funding was received for this paper.
Conflicts of interest
J.D. has been a member of the Scientific Advisory Board (SAB) of PregLem S.A. since 2007. He held PregLem stocks, related to SAB activities, that he sold in October 2010 upon PregLem's full acquisition by the Gedeon Richter Group. There was no relationship between the stock payment value and future commercial performance of the studied drug.
M.M.D. has no conflict of interest to declare.
References
- Aarts JW, Nieboer TE, Johnson N, Tavender E, Garry R, Mol BW, Kluivers KB. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 2015; 8:CD003677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alborzi S, Ghannadan E, Alborzi S, Alborzi M.. A comparison of combined laparoscopic uterine artery ligation and myomectomy versus laparoscopic myomectomy in treatment of symptomatic myoma. Fertil Steril 2009;92:742–747. [DOI] [PubMed] [Google Scholar]
- Alessandri F, Lijoi D, Mistrangelo E, Ferrero S, Ragni N.. Randomized study of laparoscopic versus minilaparotomic myomectomy for uterine myomas. J Minim Invasive Gynecol 2006;13:92–97. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists ACOG practice bulletin: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol 2008;112:387–400. [DOI] [PubMed] [Google Scholar]
- Andreotti RF, Fleischer AC.. Practical applications of 3D sonography in gynecologic imaging. Radiol Clin North Am. 2014;52:1201–1213. [DOI] [PubMed] [Google Scholar]
- Bae JH, Chong GO, Seong WJ, Hong DG, Lee YS.. Benefit of uterine artery ligation in laparoscopic myomectomy. Fertil Steril 2011;95:775–778. [DOI] [PubMed] [Google Scholar]
- Bagaria M, Suneja A, Vaid NB, Guleria K, Mishra K.. Low–dose mifepristone in treatment of uterine leiomyoma: a randomised double–blind placebo–controlled clinical trial. Aust N Z J Obstet Gynaecol 2009;49:77–83. [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB.. Why is parity protective for uterine fibroids. Epidemiology. 2003;14:247–250. [DOI] [PubMed] [Google Scholar]
- Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM.. High cumulative invidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–107. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Lumsden MA, Fauser BC, Terrill P, Bestel E.. Individualized vaginal bleeding experience of women with uterine fibroids in the PEARL I randomized controlled trial comparing the effects of ulipristal acetate or placebo. Hum Reprod. 2014;29:480–489. [DOI] [PubMed] [Google Scholar]
- Berman JM, Bolnick JM, Pemueller RR, Garza Leal JG. Reproductive outcomes in women following radiofrequency volumetric thermal ablation of symptomatic fibroids. A retrospective case series analysis. J Reprod Med 2015;60:194e8. [PubMed] [Google Scholar]
- Bernardi TS,Radosa MP, Weisheit A, Diebolder H, Schneider U, Schleussner E, Runnebaum IB. Laparoscopic myomectomy: a 6-year follow-up single-center cohort analysis of fertility and obstetric outcome measures. Arch Gynecol Obstet 2014;290:87e91. [DOI] [PubMed] [Google Scholar]
- Bestel E, Donnez J.. The potential of selective progesterone receptor modulators for the treatment of uterine fibroids. Expert Rev Endocrinol Metab 2014;9:79–92. [DOI] [PubMed] [Google Scholar]
- Bettocchi S, Ceci O, Nappi L, Di Venere R, Masciopinto V, Pansini V, Pinto L, Santoro A, Cormio G.. Operative office hysteroscopy without anesthesia: analysis of 4863 cases performed with mechanical instruments. J Am Assoc Gynecol Laparosc 2004;11:59–61. [DOI] [PubMed] [Google Scholar]
- Bettocchi S, Nappi L, Ceci O, Selvaggi L.. What does ‘diagnostic hysteroscopy’ mean today? The role of the new techniques. Curr Opin Obstet Gynecol 2003;15:303–308. [DOI] [PubMed] [Google Scholar]
- Bhave Chittawar P, Franik S, Pouwer AW, Farquhar C.. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev 2014;10:CD004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz MJ, Rochelson B, Augustine S, Greenberg M, Sison CP, Vohra N.. Uterine fibroids at routine second-trimester ultrasound survey and risk of sonographic short cervix. J Matern Fetal Neonatal Med 2016;14:1–7. [DOI] [PubMed] [Google Scholar]
- Bojahr B, De Wilde RL, Tchartchian G.. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet 2015;292:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosteels J, Weyers S, Kasius J, Broekmans FJ, Mol BW, D'Hooghe TM.. Anti-adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane Database Syst Rev 2015;11:CD011110. [DOI] [PubMed] [Google Scholar]
- Bosteels J, Weyers S, Mathieu C, Mol BW, D'Hooghe T.. The effectiveness of reproductive surgery in the treatment of female infertility: facts, views and vision. Facts Views Vis Obgyn. 2010. a;2:232–252. Review [PMC free article] [PubMed] [Google Scholar]
- Bosteels J, Weyers S, Puttemans P, Panayotidis C, Van Herendael B, Gomel V, Mol BW, Mathieu C, D'Hooghe T.. The effectiveness of hysteroscopy in improving pregnancy rates in subfertile women without other gynaecological symptoms: a systematic review. Hum Reprod Update 2010. b;16:1–11. [DOI] [PubMed] [Google Scholar]
- Bouchard P. Selective progesterone receptor modulators: a class with multiple actions and applications in reproductive endocrinology, and gynecology. Gynecol Endocrinol. 2014;30:683–684. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Chabbert-Buffet N, Fauser BC. Selective progesterone receptor modulators in reproductive medicine; pharmacology, clinical efficacy and safety. Fertil Steril 2011;96:1175–1189. [DOI] [PubMed] [Google Scholar]
- Brohl AS, Li L, Andikyan V, Običan SG, Cioffi A, Hao K, Dudley JT, Ascher-Walsh C, Kasarskis A, Maki RG.. Age-stratified risk of unexpected uterine sarcoma following surgery for presumed benign leiomyoma. Oncologist 2015;20:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun S. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Bulun S. Uterine Fibroids. N Engl J Med 2013; 369:14. [DOI] [PubMed] [Google Scholar]
- Carbonnel M, Goetgheluck J, Frati A, Even M, Ayoubi JM.. Robot-assisted laparoscopy for infertility treatment: current views. Fertil Steril 2014;101:621–626. [DOI] [PubMed] [Google Scholar]
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH.. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, Roark M, Steinkampf MP.. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab 1993;76:1217–1223. [DOI] [PubMed] [Google Scholar]
- Casadio P, Youssef AM, Spagnolo E, Rizzo MA, Talamo MR, De Angelis D, Marra E, Ghi T, Savelli L, Farina A, et al. . Should the myometrial free margin still be considered a limiting factor for hysteroscopic resection of submucous fibroids? A possible answer to an old question. Fertil Steril 2011;95:1764–1768.e1. [DOI] [PubMed] [Google Scholar]
- Casini ML, Rossi F, Agostini R, Unfer V.. Effects of the position of fibroids on fertility. Gynecol Endocrinol 2006;22:106–109. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Ebser N, Bouchard P, Fibroid growth and medical options for treatment. Fertil Steril 2014;102:630–639. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM.. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update 2005;11:293–307. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Pintiaux A, Bouchard P. The immninent dawn of SPRMs in obstetrics and gynecology. Mol Cell Endocrinol 2012;02:21. [DOI] [PubMed] [Google Scholar]
- Cholkeri-Singh A, Miller CE.. Power morcellation in a specimen bag. J Minim Invasive Gynecol. 2015;22:160. [DOI] [PubMed] [Google Scholar]
- Ciarmela P, Bloise E, Gray PC, Carrarelli P, Islam MS, De Pascalis F, Severi FM, Vale W, Castellucci M, Petraglia F.. Activin-A and myostatin response and steroid regulation in human myometrium: disruption of their signalling in uterine fibroid. J Clin Endocrinol Metab. 2011. a;96:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarmela P, Carrarelli P, Islam MS, Janjusevic M, Zupi E, Tosti C, Castellucci M, Petraglia F.. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod Sci. 2014;21:1120–1125. [DOI] [PubMed] [Google Scholar]
- Ciarmela P, Islam MS, Reis FM, Gray PC, Bloise E, Petraglia F, Vale W, Castellucci M.. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Hum Reprod Update 2011. b;17:772–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NA, Mumford SL, Segars JH.. Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Curr Opin Obstet Gynecol 2014;26:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo A, Garrido N, Pellicer A, Remohí J.. Six years’ experience in ovum donation using vitrified oocytes: report of cumulative outcomes, impact of storage time, and development of a predictive model for oocyte survival rate. Fertil Steril 2015;104:1426–1434. [DOI] [PubMed] [Google Scholar]
- Courtoy G, Donnez J, Marbaix E, Dolmans MM.. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatmen. Fertil Steril 2015; 104:426–434. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Risinger JI, Chandramouli GV, Bushel PR, Baird DD, Peddada SD. Gene expression in uterine leiomyoma from tumors likely to be growing (from black women over 35) and tumors likely to be non-growing (from white women over 35). PLoS One 2013;8:e63909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Spiezio Sardo A, Bettocchi S, Spinelli M, Guida M, Nappi L, Angioni S, Sosa Fernandez LM, Nappi C.. Review of new office-based hysteroscopic procedures 2003–2009. J Minim Invasive Gynecol 2010;17:436–448. [DOI] [PubMed] [Google Scholar]
- Di Spiezio Sardo A, Calagna G, Di Carlo C, Guida M, Perino A,Nappi C.. Cold loops applied to bipolar resectoscope: A safe ‘one-step’ myomectomy for treatment of submucosal myomas with intramural development. J Obstet Gynaecol Res 2015;41:1935–1941. [DOI] [PubMed] [Google Scholar]
- Di Spiezio Sardo A, Mazzon I, Bramante S, Bettocchi S, Bifulco G, Guida M, Nappi C.. Hysteroscopic myomectomy: a comprehensive review of surgical techniques. Hum Reprod Update 2008;14:101–119. [DOI] [PubMed] [Google Scholar]
- Doherty L, Mutlu L, Sinclair D, Taylor H.. Uterine fibroids: clinical manifestations and contemporary management. Reprod Sci 2014;21:1067–1092. [DOI] [PubMed] [Google Scholar]
- Donnez J, Arriagada P, Donnez O, Dolmans MM.. Current management of myomas: the place of medical therapy with the advent of selective progesterone receptor modulators. Curr Opin Obstet Gynecol 2015. b;27:422–431. [DOI] [PubMed] [Google Scholar]
- Donnez O, Donnez J.. A series of 400 laparoscopic hysterectomies for benign disease: a single centre, single surgeon prospective study of complications confirming previous retrospective study. BJOG 2010;117:752–755. [DOI] [PubMed] [Google Scholar]
- Donnez J, Donnez O, Dolmans MM. With the advent of selective progesterone receptor modulators, what is the place of myoma surgery in current practice. Fertil Steril 2014. a;102:640–648. [DOI] [PubMed] [Google Scholar]
- Donnez O, Donnez J, Dolmans MM, Dethy A, Baeyens M, Mitchell J.. Low Pain Score After Total Laparoscopic Hysterectomy and Same-Day Discharge Within Less Than 5 Hours: Results of a Prospective Observational Study. J Minim Invasive Gynecol 2015. c;22:1293–1299. [DOI] [PubMed] [Google Scholar]
- Donnez J, Donnez O, Matule D, Ahrendt HJ, Hudecek R, Zatik J, Kasilovskiene Z,Dumitrascu MC, Fernandez H, Barlow DH, et al. . Long–term medical management of uterine fibroids with ulipristal acetate. Fertil Steril 2016;105:165–173. [DOI] [PubMed] [Google Scholar]
- Donnez J, Gillerot S, Bourgonjon D, Clerckx F, Nisolle M.. Neodymium: YAG laser hysteroscopy in large submucous fibroids. Fertil Steril 1990;54:999–1003. [DOI] [PubMed] [Google Scholar]
- Donnez J, Hudecek R, Donnez O, Matule D, Arhendt HJ, Zatik J, Kasilovskiene Z, Dumitrascu MC, Fernandez H, Barlow DH, et al. . Efficacy and Safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 2015. a;103:519–527 [DOI] [PubMed] [Google Scholar]
- Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate. Hum Reprod 2002;17:1424–1430. [DOI] [PubMed] [Google Scholar]
- Donnez O, Jadoul P, Squifflet J, Donnez J.. Iatrogenic peritoneal adenomyoma after laparoscopic subtotal hysterectomy and uterine morcellation. Fertil Steril 2006;86:1511–1512. [DOI] [PubMed] [Google Scholar]
- Donnez O, Jadoul P, Squifflet J, Donnez J.. A series of 3190 laparoscopic hysterectomies for benign disease from 1990 to 2006: evaluation of complications compared with vaginal and abdominal procedures. BJOG 2009;116:492–500. [DOI] [PubMed] [Google Scholar]
- Donnez J, Nisolle M, Smoes P, Gillet N, Beguin S, Casanas-Roux F.. Peritoneal endometriosis and ‘endometriotic’ nodules of the rectovaginal septum are two different entities. Fertil Steril 1996;66:362–368. [PubMed] [Google Scholar]
- Donnez J, Schrurs B, Gillerot S, Sandow J, Clerckx F.. Treatment of uterine fibroids with implants of gonadotropin-releasing hormone agonist: assessment by hysterography. Fertil Steril 1989;51:947–950. [DOI] [PubMed] [Google Scholar]
- Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J.. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol 2007;14:156–160. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Polet R, Nisolle M.. Laparoscopic myolysis. Hum Reprod Update 2000;6:609–613. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, et al. . Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl j Med 2012. a;366:409–420. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, Nouri K, Selvaggi L, Sodowski K, Bestel E, et al. . Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012. b;366:421–432. [DOI] [PubMed] [Google Scholar]
- Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, Barlow DH, Palacios S, Donnez O, Bestel E, et al. . Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014. b;101:1565–1573. [DOI] [PubMed] [Google Scholar]
- Drayer SM, Catherino WH.. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet 2015;131:117–122. [DOI] [PubMed] [Google Scholar]
- Dubuisson JB, Fauconnier A, Babaki-Fard K, Chapron C.. Laparoscopic myomectomy: a current view. Hum Reprod Update 2000;6:588–594. [DOI] [PubMed] [Google Scholar]
- Eggert SL, Huyck KL, Somasundaram P, Kavalla R, Stewart EA, Lu AT, Painter JN, Montgomery GW, Medland SE, Nyholt DR, et al. . Genome-wide linkage and association analyses implicate FASN in predisposition to uterine leiomyomata. Am J Hum Genet 2012;91:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger W, Ivell R, Nandy A, Rasch A, Triller A, Chwalisz K.. Modulation of uterine prostaglandin secretion by the selective progesterone receptor modulator (SPRM) asoprisnil, progestins, and antiprogestins in cycling and ovariectomized guinea pig. Fertil Steril 2004;82:S316. [Google Scholar]
- Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA.. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol 2014;210:194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund K, Blanck A, Gustavsson I, Lundkvist Y, Sjoblom P, Norgren A, Lindblom B.. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin endocrinol Metabol 1998;83:4092–4096. [DOI] [PubMed] [Google Scholar]
- Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell-Danielsson K.. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod 2009;24:1870–1879. [DOI] [PubMed] [Google Scholar]
- Engman M, Skoog L, Soderqvist G, Gemzell-Danielsson K.. The effect of mifepristone on breast cell proliferation in premenopausal women evaluated through fine needle aspiration cytology. Hum. Reprod 2008;23:2072–2079. [DOI] [PubMed] [Google Scholar]
- Exacoustos C, Zupi E, Marconi D, Romanini ME, Szabolcs B, Piredda A, Arduini D.. Ultrasound-assisted laparoscopic cryomyolysis: two- and three-dimensional findings before, during and after treatment. Ultrasound Obstet Gynecol 2005;25:393–400. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS.. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol. 2006;108:1381–1387. [DOI] [PubMed] [Google Scholar]
- Fischer K, McDannold NJ, Tempany CM, Jolesz FA, Fennessy FM.. Potential of minimally invasive procedures in the treatment of uterine fibroids: a focus on magnetic resonance-guided focused ultrasound therapy. Int J Womens Health. 2015;7:901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Tanaka T, Kamada M, et al. . Comparison of the perinatal outcomes after laparoscopic myomectomy versus abdominal myomectomy. Gynecol Obstet Invest 2013;76:203e8. [DOI] [PubMed] [Google Scholar]
- Flynn M, Jamison M, Datta S, Myers E.. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gyncol. 2006;195:955–964. [DOI] [PubMed] [Google Scholar]
- Galliano D, Bellver J, Días-Garcia C, Simón C, Pellicer A.. ART and uterine pathology: how revelant is the maternal side for implantation. Hum Reprod Update 2015;21:13–38. [DOI] [PubMed] [Google Scholar]
- Gargiulo AR, Srouji SS, Missmer SA, Correia KF, Vellinga TT, Einarsson JI.. Robot-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy. Obstet Gynecol. 2012;120:284–291. [DOI] [PubMed] [Google Scholar]
- Gemzell Danielsson K, Hamberg M.. The effect of antiprogestin (RU 486) and prostaglandin biosynthesis inhibitor (naproxen) on uterine fluid prostaglandin F2 alpha concentrations. Hum. Reprod 1994;9:1926–1630. [DOI] [PubMed] [Google Scholar]
- Gupta S, Jose J, Manyonda I.. Clinical presentation of fibroids. Best Pract Res Clin Obstet Gynaecol 2008;22:615–626. [DOI] [PubMed] [Google Scholar]
- Gupta JK, Sinha A, Lumsden MA, Hickey M.. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. [DOI] [PubMed] [Google Scholar]
- Gupta JK, Sinha A, Lumsden MA, Hickey M.. Uterine artery embolization for symptomatic uterine fibroids.Cochrane Database Syst Rev. 2014;12:CD005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann JN, Corson SL.. GnRH agonist therapy before myomectomy or hysterectomy. J Minim Invasive Gynecol 2005;12:529–537. [DOI] [PubMed] [Google Scholar]
- Hald K, Langebrekke A, Klow NE, Noreng HJ, Berge AB, Istre O.. Laparoscopic occlusion of uterine vessels for the treatment of symptomatic fibroids: Initial experience and comparison to uterine artery embolization. YMOB 2004;190:37–43. [DOI] [PubMed] [Google Scholar]
- Goodwin SC, Spies JB.. Uterine fibroid embolization. N Engl J Med 2009;13:690–697. [DOI] [PubMed] [Google Scholar]
- Higham JM, O'Brien PM, Shaw RW.. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol 1990;97:734–739. [DOI] [PubMed] [Google Scholar]
- Islam MS, Akhtar MM, Ciavattini A, Giannubilo SR, Protic O, Janjusevic M, Procopio AD, Segars JH, Castellucci M, Ciarmela P.. Use of dietary phytochemicals to target inflammation, fibrosis, proliferation, and angiogenesis in uterine tissues: promising options for prevention and treatment of uterine fibroids. Mol Nutr Food Res 2014;58:1667–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Protic O, Giannubilo SR, Toti P, Tranquilli AL, Petraglia F, Castellucci M, Ciarmela P. Uterine Leiomyoma: Available Medical Treatments and New Possible Therapeutic Options. J Clin Endocrinol Metab 2013;98;921–934. [DOI] [PubMed] [Google Scholar]
- Islam MS, Protic O, Stortoni P, Grechi G, Lamanna P, Petraglia F, Castellucci M, Ciarmela P.. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril 2013;100:178–193. [DOI] [PubMed] [Google Scholar]
- Jacoby VL, Kohi MP, Poder L, Jacoby A, Lager J, Schembri M, Rieke V, Grady D, Vittinghoff E, Coakley FV.. PROMISe trial: a pilot, randomized, placebo–controlled trial of magnetic resonance guided focused ultrasound for uterine fibroids. Fertil Steril. 2015;S0015–0282:02090–02097. [DOI] [PubMed] [Google Scholar]
- Johnson N, Barlow D, Lethaby A, Tavender E, Curr L, Garry R. Methods of hysterectomy: systematic review and meta-analysis of randomised controlled trials. BMJ 2005;330:1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanade TT, McKenna JB, Choi S, Tsai BP, Rosen DM, Cario GM, Chou D. Sydney contained in bag morcellation for laparoscopic myomectomy. J Minim Invasive Gynecol 2014;21:981. [DOI] [PubMed] [Google Scholar]
- Karmon AE, Cardozo ER, Rueda BR, Styer AK. MicroRNAs in the development and pathobiology of uterine leiomyomata: does evidence support future strategies for clinical intervention? Hum Reprod Update 2014;20:670–687. [DOI] [PubMed] [Google Scholar]
- Kaump GR, Spies JB.. The impact of uterine artery embolization on ovarian function.J Vasc Interv Radiol 2013;24:459–467. [DOI] [PubMed] [Google Scholar]
- Khan AT, Shehmar M, Gupta JK.. Uterine fibroids: current perspectives. Int J Womens Health 2014;6:95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Kim D, Lee MK, Lee CR, Kang SY, Chung YJ, Cho HH, Kim JH, Kim MR.. Three cases of complications after high-intensity focused ultrasound treatment in unmarried women. Obstet Gynecol Sci. 2015;58:542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Lim HK, Park MJ, Rhim H, Jung SH, Sohn I, Kim TJ, Keserci B.. Screening Magnetic Resonance Imaging-Based Prediction Model for Assessing Immediate Therapeutic Response to Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound Ablation of Uterine Fibroids. Invest Radiol. 2016;51:15–24. [DOI] [PubMed] [Google Scholar]
- Kim J, Sefton EC.. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol. 2012;358:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho KA, Brown DN.. Surgical Treatment of Uterine Fibroids Within a Containment System and Without Power Morcellation. Clin Obstet Gynecol. 2016;59:85–92. [DOI] [PubMed] [Google Scholar]
- Kroencke TJ, Scheurig C, Poellinger A, Gronewold M, Hamm B.. Uterine artery embolization for leiomyomas: percentage of infarction predicts clinical outcome. Radiology 2010;255:834–841. [DOI] [PubMed] [Google Scholar]
- Lam SJ, Best S, Kumar S.. The impact of fibroid characteristics on pregnancy outcome. Am J Obstet Gynecol 2014;211:395. [DOI] [PubMed] [Google Scholar]
- Lasmar RB, Barrozo PR, Dias R, Oliveira MA.. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—preliminary report. J Minim Invasive Gynecol 2005;12:308–311. [DOI] [PubMed] [Google Scholar]
- Laughlin SK, Schroeder JC, Baird DD.. New directions in the epidemiology of uterine fibroids. Semin Reprod Med 2010;28:204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Matsuzono T.. Hysteroscopic intrauterine morcellation of submucosal fibroids: preliminary results in Hong Kong and comparisons with conventional hysteroscopic monopolar loop resection. Hong Kong Med J 2016;10:12809. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Vollenhoven B, Sowter M.. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev 2001:4. [DOI] [PubMed] [Google Scholar]
- Lewis EI, Srouji SS, Gargiulo AR.. Robotic single-site myomectomy: initial report and technique. Fertil Steril 2015;103:1370–1377. [DOI] [PubMed] [Google Scholar]
- Lieng M, Berner E, Busund B.. Risk of morcellation of uterine leiomyosarcomas in laparoscopic supracervical hysterectomy and laparoscopic myomectomy, a retrospective trial including 4791 women. J Minim Invasive Gynecol 2015;22:410–414. [DOI] [PubMed] [Google Scholar]
- Lin G, Yang LY, Huang YT, Ng KK, Ng SH, Ueng SH, Chao A, Yen TC, Chang TC, Lai CH.. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma/smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J Magn Reson Imaging 2015;10:1002. [DOI] [PubMed] [Google Scholar]
- Liu JP, Yang H, Xia Y, Cardini F.. Herbal preparations for uterine fibroids.Cochrane Database Syst Rev 2013;4:CD005292. [DOI] [PubMed] [Google Scholar]
- Liu WM, Wang PH, Chou CS, Tang WL, Wang IT, Tzeng CR.. Efficacy of combined laparoscopic uterine artery occlusion and myomectomy via minilaparotomy in the treatment of recurrent uterine myomas. Fertil Steril 2007;87:356–361. [DOI] [PubMed] [Google Scholar]
- Lumsden MA, Hamoodi I, Gupta J, Hickey M.. Fibroids: diagnosis and management. BMJ. 2015;351:h4887. [DOI] [PubMed] [Google Scholar]
- Luyckx M, Squifflet JL, Jadoul P, Votino R, Dolmans MM, Donnez J.. First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids. Fertil Steril 2014;102:1404–1409. [DOI] [PubMed] [Google Scholar]
- Lykke R, Blaakær J, Ottesen B, Gimbel H.. Hysterectomy in Denmark 1977–2011: changes in rate, indications, and hospitalization. Eur J Obstet Gynecol Reprod Biol 2013;171:333–338. [DOI] [PubMed] [Google Scholar]
- Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, et al. . MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011;334:252–255. [DOI] [PubMed] [Google Scholar]
- Malone LJ. Myomectomy: reccurence after removal of solitary and multiple myomas. Obstet Gynecol 1969;34:200–209. [PubMed] [Google Scholar]
- Malzoni M, Tinelli R, Cosentino F, Iuzzolino D, Surico D, Reich H.. Laparoscopy versus minilaparotomy in women with symptomatic uterine myomas: short-term and fertility results. Fertil Steril 2010;93:2368–2373. [DOI] [PubMed] [Google Scholar]
- Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O.. Midterm clinical and first reproductive results of a randozimed controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol 2008;31;73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mara M, Kubinova K.. Embolization of uterine fibroids from the point of view of the gynecologist: pros and cons. Int J Womens Health. 2014;6:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marret H, Fritel X, Ouldamer L, Bendifallah S, Brun JL, De Jesus I, Derrien J, Giraudet G, Kahn V, Koskas M, et al. . Therapeutic management of uterine fibroid tumors: updated French guidelines. Eur J Obstet Gynecol Reprod Biol 2012;165:156–164. [DOI] [PubMed] [Google Scholar]
- Marsh EE, Chibber S, Wu J, Siegersma K, Kim J, Bulun S.. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 expression and regulation in uterine leiomyoma. Fertil Steril 2015;S0015–0282:02172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ.. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997;90:967–973. [DOI] [PubMed] [Google Scholar]
- Mazzon I, Favilli A, Grasso M, Horvath S, Di Renzo GC, Gerli S.. Is cold loop hysteroscopic myomectomy a safe and effective technique for the treatment of submucous myomas with intramural development? A series of 1434 surgical procedures. J Minim Invasive Gynecol. 2015;22:792–798. [DOI] [PubMed] [Google Scholar]
- McKenna JB, Kanade T, Choi S, Tsai BP, Rosen DM, Cario GM, Chou D.. The Sydney contained in bag morcellation technique. J Minim Invasive Gynecol 2014;21:984–985. [DOI] [PubMed] [Google Scholar]
- Mehine M, Kaasinen E, Aaltonen LA.. Chromothripsis in uterine leiomyomas. N Engl J Med 2013;369:2160–2161. [DOI] [PubMed] [Google Scholar]
- Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P.. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril 2014;102:621–629. [DOI] [PubMed] [Google Scholar]
- Metwally M, Cheong YC, Horne AW.. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev 2012;11:CD003857. [DOI] [PubMed] [Google Scholar]
- Metwally M, Farquhar CM, Li TC.. Is another meta-analysis on the effects of intramural fibroids on reproductive outcomes needed. Reprod Biomed Online 2011;23:2–14. [DOI] [PubMed] [Google Scholar]
- Mittal P, Shin YH, Yatsenko SA, Castro CA, Surti U, Rajkovic A.. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J Clin Invest 2015;125:3280–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monleón J, Martínez-Varea A, Galliano D, Pellicer A.. Successful pregnancy after treatment with ulipristal acetate for uterine fibroids. Case Rep Obstet Gynecol 2014;2014:314587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravek MB, Yin P, Ono M, Coon JS V, Dyson MT, Navarro A, Marsh EE, Chakravarti D, Kim JJ, Wei JL, et al. . Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum Reprod Update 2015;21:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni RM, Martins WP, Ferriani RA, Vieira CS, Nastri CO, Candido Dos Reis FJ, Brito LG.. Add-back therapy with GnRH analogues for uterine fibroids. CochraneDatabase Syst Rev 2015;3:CD010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Christie A.. Uterine artery embolization for heavy menstrual bleeding. Womens Health 2016;12:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro MG. Hysteroscopic Myomectomy of FIGO Type 2 Leiomyomas Under Local Anesthesia: Bipolar Radiofrequency Needle-Based Release Followed By Electromechanical Morcellation. J Minim Invasive Gynecol 2016;23:12–13. [DOI] [PubMed] [Google Scholar]
- Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO Working Group on Menstrual Disorders . FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011;113:1–2. [DOI] [PubMed] [Google Scholar]
- Mussalam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, Khreiss M, Dahdaleh FS, Khavandi K, Sfeir PM, et al. . Preoperative anaemia and postoperative outcomes in noncardiac surgery: a retrospective cohort study. Lancet 2011;378:1396–1407. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, Williams AR, Blithe DL.. The spectrum of endometrial pathology induced by progesterone receptormodulators. Mod Pathol 2008;21:591–598. [DOI] [PubMed] [Google Scholar]
- Nelson AL, Ritchie JJ.. Severe anemia from heavy menstrual bleeding requires heightened attention. Am J Obstet Gynecol 2015;213:97. [DOI] [PubMed] [Google Scholar]
- Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M.. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy-a retrospective matched control study. Fertil Steril 2009;91:556–559. [DOI] [PubMed] [Google Scholar]
- Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D, Wesley R, Armstrong A.. Symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril 2011;95:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisolle M, Donnez J.. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril 1997;68:585–596. [DOI] [PubMed] [Google Scholar]
- Nisolle M, Gillerot S, Casanas-Roux F, Squifflet J, Berliere M, Donnez J.. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum Reprod 1999;14:2844–2850. [DOI] [PubMed] [Google Scholar]
- Palomba S, Zupi E, Falbo A, Russo T, Marconi D, Tolino A, Manguso F, Mattei A, Zullo F.. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: reproductive outcomes. Fertil Steril 2007;88:933–941. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Tozzi L, Bianchi S.. Pregnancy outcome and uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2015;S1521–6934:00231–0023. [DOI] [PubMed] [Google Scholar]
- Park MJ, Kim YS, Rhim H, Lim HK.. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging-guided high-intensity focused US therapy. J Vasc Interv Radiol 2014;25:231–239. [DOI] [PubMed] [Google Scholar]
- Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. [DOI] [PubMed] [Google Scholar]
- Parker WH, Einarsson J, Istre O, Dubuisson JB.. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol 2010;17:551–554. [DOI] [PubMed] [Google Scholar]
- Parker WH, Feskanich D, Broder MS, et al. . Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol 2013;121:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WH, Kaunitz AM, Pritts EA, Olive DL, Chalas E, Clarke-Pearson DL, Berek JS. Leiomyoma Morcellation Review Group . U.S. Food and Drug Administration's Guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obstet Gynecol. 2016;127:18–22. [DOI] [PubMed] [Google Scholar]
- Parker WH, Pritts EA, Olive DL.. What is the future of open intraperitoneal power-morcellation of fibroids? Clin Obstet Gynecol 2016;59:73–84. [DOI] [PubMed] [Google Scholar]
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B, Baird DD. Growth of uterine leiomyomata among premenopausal blanck and white women. Proc Natl AcadSci USA 2008;105:19887–19892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira N, Bender JL, Hancock K, Lekovich JP, Elias RT, Kligman I, Rosenwaks Z.. Routine monitoring of liver, renal, and hematologic tests after single- or double-dose methotrexate treatment for ectopic pregnancies after in vitro fertilization. J Minim Invasive Gynecol 2015;22:1266–1270. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Serour GI, Chapron C.. The changing prevalence of infertility. Int J Gynaecol Obstet 2013;123:S4–S8. [DOI] [PubMed] [Google Scholar]
- Pitter MC, Gargiulo AR, Bonaventura LM, Lehman JS, Srouji SS.. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod 2013;28:99–108. [DOI] [PubMed] [Google Scholar]
- Pohl O, Osterloh I, Gotteland JP.. Ulipristal acetate - safety and pharmacokinetics following multiple doses of 10–50 mg per day. J Clin Pharm Ther 2013;38:314–320. [DOI] [PubMed] [Google Scholar]
- Pohl O, Zobrist RH, Gotteland JP.. The clinical pharmacology and pharmacokinetics of ulipristal acetate for the treatment of uterine fibroids. Reprod Sci 2015;22:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. Prevention of Brca-1 mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 2006;314:1467–1470. [DOI] [PubMed] [Google Scholar]
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons Myomas and reproductive function. Fertil Steril 2008;90:S125–S130. [DOI] [PubMed] [Google Scholar]
- Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv 2001;56:483–491. [DOI] [PubMed] [Google Scholar]
- Pritts EA, Parker WH, Olive DL.. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril 2009;91:1215–1223. [DOI] [PubMed] [Google Scholar]
- Pritts EA, Parker WH, Brown J, Olive DL.. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol 2015;22:26–33. [DOI] [PubMed] [Google Scholar]
- Protic O, Toti P, Islam MS, Occhini R, Giannubilo SR, Catherino WH, Cinti S, Petraglia F, Ciavattini A, Castellucci M, et al. . Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2015;26613601(PMID) [DOI] [PubMed] [Google Scholar]
- Rabinovici J, David M, Fukunishi H, Morita Y, Gostout BS, Stewart EA MRgFUS Study Group . Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril 2010;93:199e209. [DOI] [PubMed] [Google Scholar]
- Ravina JH, Herbreteau D, Ciraru-vigneron N, Bouret JM, Houdart E, Aymard A, Merland JJ.. Arterial embolization to treat uterine myomata. Lancet 1995;346:671–672. [DOI] [PubMed] [Google Scholar]
- Richards T, Musallam KM, Nassif J, Ghazeeri G, Seoud M, Gurusamy KS, Jamali FR.. Impact of preoperative anaemia and blood transfusion on postoperative outcomes in gynaecological surgery. PLoS One 2015;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran S, Manyonda IT.. Medical management of fibroids. Best Pract Res Clin obstet Gynaecol. 2008;22:655–676. [DOI] [PubMed] [Google Scholar]
- Saravelos SH, Yan J, Rehmani H, Li TC.. The prevalence and impact of fibroids and their treatment on the outcome of pregnancy in women with recurrent miscarriage. Hum Reprod 2011;26:3274e9. [DOI] [PubMed] [Google Scholar]
- Saridogan E. Surgical treatment of fibroids in heavy menstrual bleeding. Womens Health (Lond Engl) 2016;12:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segars JH, Parrott EC, Nagel JD, Guo XC, Gao X, Birnbaum LS, Pinn VW, Dixon D.. Proceedings from the third National Institutes of Health International Congress on advances in uterine leiomyoma research: comprehensive review, conference summary and future recommendations. Hum Reprod Update 2014;20:309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, El-Toukhy T, Douiri A, Jayaprakasan K, Khalaf Y.. Diagnostic accuracy of saline infusion sonography in the evaluation of uterine cavity abnormalities prior to assisted reproductive techniques: a systematic review and meta-analyses. Hum Reprod Update 2015;21:262–274. [DOI] [PubMed] [Google Scholar]
- Shavell VI, Thakur M, Sawant A, Kruger ML, Jones TB, Singh M, Puscheck EE, Diamond MP.. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids. Fertil Steril 2012;97:107–110. [DOI] [PubMed] [Google Scholar]
- Shen Q, Chen M, Wang Y, Zhou Q, Tao X, Zhang W, Zhu X.. Effects of laparoscopic versus minilaparotomic myomectomy on uterine leiomyoma: a meta-analysis. J Minim Invasive Gynecol 2015;22:177e84. [DOI] [PubMed] [Google Scholar]
- Shen Q, Hua Y, Jiang W, Zhang W, Chen M, Zhu X.. Effects of mifepristone on uterine leiomyoma in premenopausal women: a meta-analysis. Fertil Steril 2013;100:1722–1726. [DOI] [PubMed] [Google Scholar]
- Siedhoff MT, Wheeler SB, Rutstein SE, Geller EJ, Doll KM, Wu JM, Clarke-Pearson DL.. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol 2015;212:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DC, Mastroyannis A, Taylor HS.. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-β3. J Clin Endocrinol Metab 2011;96:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AM, Yang H, Du EX, Kelkar SS, Winkel C.. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141–160. [DOI] [PubMed] [Google Scholar]
- Somigliana S, Vercellini P, Daguati R, Pasin R, De Giorgi O, Crosignani PG.. Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update 2007;13:465–476. [DOI] [PubMed] [Google Scholar]
- Song H, Lu D, Navaratnam K, Shi G.. Aromatase inhibitors for uterine fibroids.Cochrane Database Syst Rev 2013;10:CD009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol 2016;59:93–102. [DOI] [PubMed] [Google Scholar]
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290–300. [DOI] [PubMed] [Google Scholar]
- Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol 2009;21:318–324. [DOI] [PubMed] [Google Scholar]
- Stamatellos I, Bontis J.. Hysteroscopic myomectomy. Eur Clin Obstet Gynecol 2007;3:17–23. [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet 2001;357:293–298 Review [DOI] [PubMed] [Google Scholar]
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646–1655. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Nicholson WK, Bradley L, Borah BJ.. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larch-rnt) 2013;22:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer AK, Rueda BR.. The Epidemiology and Genetics of Uterine Leiomyoma. Best Pract Res Clin Obstet Gynaecol 2015: S1521–6934:00232–1. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A.. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Human Reprod 2010;25:418–429. [DOI] [PubMed] [Google Scholar]
- Tan YH, Lethaby A.. Pre-operative endometrial thinning agents before endometrial destruction for heavy menstrual bleeding. Cochrane Database Syst Rev 2013;11. [DOI] [PubMed] [Google Scholar]
- Thomas RL, Winkler N, Carr BR, Doody KM, Doody KJ.. Abdominal myomectomy—a safe procedure in an ambulatory setting. Fertil Steril 2010;94:2277–2280. [DOI] [PubMed] [Google Scholar]
- Tian YC, Long TF, Dai YM. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res 2015;41:350e7. [DOI] [PubMed] [Google Scholar]
- Ton R, Kilic GS,Phelps JY.. A medical-legal review of power morcellation in the face of the recent FDA warning and litigation. J Minim Invasive Gynecol 2015;22:564–572. [DOI] [PubMed] [Google Scholar]
- Tristan M, Orozco LJ, Steed A, Ramírez-Morera A, Stone P.. Mifepristone foruterine fibroids. Cochrane Database Syst Rev 2012;8:CD007687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigkou A, Reis FM, Lee MH, Jiang B, Tosti C, Centini G, Shen FR, Chen YG, Petraglia F.. Increased progesterone receptor expression in uterine leiomyoma: correlation with age, number of leiomyomas, and clinical symptoms. Fertil Steril. 2015;104:170–175. [DOI] [PubMed] [Google Scholar]
- Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, Petraglia F.. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update 2016;22:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilos GA, Allaire C, Laberge PY, Leyland N; Special Contributors, Vilos AG, Murji A, Chen I.. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2015;37:157–181. [DOI] [PubMed] [Google Scholar]
- Vilos GA, Hollett-Caines J, Burbank F.. Uterine artery occlusion: what is the evidence? Clin Obstet Gynecol 2006;49:798–810. [DOI] [PubMed] [Google Scholar]
- Whitaker LH, Williams AR, Critchley HO.. Selective progesterone receptor modulators. Curr Opin Obstet Gynecol 2014;26:237–242. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Male V, Ghazal P, Forster T, Gibson DA, Williams AR, Brito-Mutunayagam SL, Craigon M, Lourenco P, Cameron IT, et al. . Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol 2013;191:2226–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens J, Williams AR, Chwalisz K, Han C, Cameron IT, Critchley HO.. Effect of asoprisnil on uterine proliferation markers and endometrial expression of the tumour suppressor gene, PTEN. Hum Reprod 2009;24:1036–1044. [DOI] [PubMed] [Google Scholar]
- Williams AR, Bergeron C, Barlox DH, Ferenczy A.. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol 2012;31:556–569. [DOI] [PubMed] [Google Scholar]
- Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, Chwalisz K.. The effects of the selective progesterone receptor modulator asoprisnil on themorphology of uterine tissues after 3 months treatment in patients withsymptomatic uterine leiomyomata. Hum Reprod 2007;22:1696–1704. [DOI] [PubMed] [Google Scholar]
- Wise LA, Laughlin-Tommaso SK.. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol 2016;59:2–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, Rosenberg L .. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women's Health Study. Hum Reprod 2004;19:1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Spiegelman D, Harlow BL, Stewart EA, Adams-Campbell LL, Rosenberg L.. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology 2005;16:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Radin RG, Palmer JR, Kumanyika SK, Boggs DA, Rosenberg L.. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am J Clin Nutr 2011;94:1620–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L, White N, Ramkrishna J, Araujo Júnior E, Meagher S, Costa Fda S. Three-dimensional imaging of the uterus: the value of the coronal plane. World J Radiol 2015;7:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JY, Gold EB, Johnson WO, Lee JS.. Circulating Sex Hormones and Risk of Uterine Fibroids: Study of Women's Health Across the Nation (SWAN). J Clin Endocrinol Metab 2016;101:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JD, Tergas AI, Burke WM, Cui RR, Ananth CV, Chen L, Hershman DL. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA 2014;312:1253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L MD, Ding L, Li C, Wang YD PH, Tang R, Chen ZJ.. Effect of fibroids not distorting the endometrial cavity on the outcome of in vitro fertilization treatment: a retrospective cohort study. Fertil Steril 2014;101:716–721. [DOI] [PubMed] [Google Scholar]
- Yin P, Ono M, Moravek MB, Coon JS V, Navarro A, Monsivais D, Dyson MT, Druschitz SA, Malpani SS, Serna VA, et al. . Human uterine leiomyoma stem/progenitor cells expressing CD34 and CD49b initiate tumors in vivo. J Clin Endocrinol Metab. 2015;100:E601–E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepiridis LI, Grimbizis GF, Tarlatzis BC.. Infertility and uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2015;S1521–6934:00235–00237. [DOI] [PubMed] [Google Scholar]
- Zupi E, Centini G, Sabbioni L, Lazzeri L, Argay IM, Petraglia F.. Nonsurgical alternatives for uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2015;S1521–6934:00227–00228. [DOI] [PubMed] [Google Scholar]
- Zupi E, Piredda A, Marconi D, Townsend D, Exacoustos C, Arduini D, Szabolcs B.. Directed laparoscopic cryomyolysis: a possible alternative to myomectomy and/or hysterectomy for symptomatic leiomyomas. YMOB 2004;190:639–643. [DOI] [PubMed] [Google Scholar]