Summary
This study examined associations between suicidality and genotypes that predict plasma efavirenz exposure among AIDS Clinical Trials Group study participants. Genotypes were associated with increased risk of suicidality. The association was strongest among white but nearly null among black participants.
Keywords: HIV, efavirenz, suicidality, pharmacogenetics, CYP2B6
Abstract
Background
We examined associations between suicidality and genotypes that predict plasma efavirenz exposure among AIDS Clinical Trials Group study participants in the United States.
Methods
Four clinical trials randomly assigned treatment-naive participants to efavirenz-containing regimens; suicidality was defined as reported suicidal ideation or attempted or completed suicide. Genotypes that predict plasma efavirenz exposure were defined by CYP2B6 and CYP2A6 polymorphisms. Associations were evaluated with weighted Cox proportional hazards models stratified by race/ethnicity. Additional analyses adjusted for genetic ancestry and selected covariates.
Results
Among 1833 participants, suicidality was documented in 41 in exposed analyses, and 34 in on-treatment analyses. In unadjusted analyses based on 12 genotype levels, suicidality increased per level in exposed (hazard ratio, 1.11; 95% confidence interval, .96–1.27) and on-treatment 1.16; 1.01–1.34) analyses. In the on-treatment analysis, the association was strongest among white but nearly null among black participants. Considering 3 metabolizer levels (extensive, intermediate and slow), slow metabolizers were at increased risk. Results were similar after baseline covariate-adjustment for genetic ancestry, sex, age, weight, injection drug use history, and psychiatric history or recent psychoactive medication.
Conclusions
Genotypes that predict higher plasma efavirenz exposure were associated with increased risk of suicidality. Strength of association varied by race/ethnicity.
Efavirenz is a frequently prescribed antiretroviral globally. Randomized clinical trials have demonstrated its efficacy [1–6]. However, some patients who start efavirenz experience central nervous system symptoms [7]. Among 5332 individuals who had been randomly assigned to start efavirenz-containing or efavirenz-free regimens in 4 AIDS Clinical Trials Group (ACTG) studies [8], efavirenz-containing regimens were associated with a 2-fold increased hazard of suicidality. In the START trial, patients who started efavirenz-containing regimens had a higher incidence of suicidality than those who deferred therapy, a difference not seen in those starting nonefavirenz regimens [9]. In contrast, no association with suicidality was apparent in the Food and Drug Administration Adverse Event Reporting System [10], or in United States administrative claims data for commercially and Medicaid-insured individuals [11]. In 2015, United States prescribing guidelines were updated to change efavirenz-containing regimens from recommended to alternative status as initial therapy for human immunodeficiency virus type 1 (HIV-1) infection [12].
Efavirenz is metabolized by cytochrome P450 (CYP) 2B6, with minor metabolism by CYP2A6 and CYP3A4/5 [13, 14], and direct N-glucuronidation by uridine diphosphate (UDP)-glucuronosyltransferase (UGT) 2B7 [15]. Several CYP2B6 polymorphisms, including 516G→T (rs3745274) [16–21], 983T→C (rs28399499) [21–24], and 15582C→T (rs4803419), predict increased plasma efavirenz exposure [21]. The per allele effect is greatest with 983T→C and least with 15582C→T [21], such that combinations of the 3 polymorphisms predict 10 plasma concentration strata that span an approximately 10-fold range [21]. Slow metabolizer CYP2B6 genotypes comprise the 3 highest strata, which are defined by either 516 T/T homozygosity, dual 516 G/T + 983 C/T heterozygosity, or 983 C/C homozygosity.
Polymorphisms beyond CYP2B6, particularly CYP2A6, also affect efavirenz pharmacokinetics [25, 26]. Plasma efavirenz concentrations are increased with CYP2A6 -48T→G (rs28399433) [25–28], but only with concomitant CYP2B6 slow metabolizer genotypes [25, 28]. A possible association has also been reported with UGT2B7 genotype [27] although the effect size was small [28]. We examined whether genotypes known to predict increased plasma efavirenz exposure are associated with increased suicidality among individuals who were randomized to receive efavirenz-containing regimens in 4 ACTG studies.
METHODS
Study Design and Participants
The study design was similar to that of previous nongenetic analyses characterizing relationships between efavirenz and suicidality in these ACTG studies [8]. Data were pooled from antiretroviral-naive individuals who started therapy in 4 studies that had randomly assigned participants to efavirenz-containing versus efavirenz-free regimens: A5095 (ClinicalTrials.gov: NCT00013520) [2, 29], A5142 (NCT00050895) [3], A5175 (NCT00084136) [30], and A5202 (NCT00118898) [6]. Other than nucleoside analogue choice in A5142, drug class components of the regimens were randomly assigned. The present analyses were also restricted to self-reported white, black, and Hispanic participants (classified according to National Institutes of Health categories) at ACTG sites in the United States (including Puerto Rico). Genetic testing was restricted to participants who consented under ACTG protocol A5128 [31]. Other race/ethnicity groups (eg, Asian) included too few individuals for analysis. Primary analyses were conducted among individuals assigned to efavirenz-containing regimens (hereafter called the efavirenz group), and a supplemental analysis included those assigned to efavirenz-free regimens for comparison (hereafter called the nonefavirenz group).
History of suicidal ideation or attempt was not exclusionary. Protocols required reporting of signs, symptoms, or diagnoses at each visit, which were recorded with open-text and data-entry codes. Each study required reporting of severe and life-threatening graded signs or symptoms per the Division of AIDS grading table [32], as well as signs or symptoms that led to change in study treatment. Diagnoses were not graded. Further, study A5142 required report of all moderate signs or symptoms, and A5095 and A5202 required report of moderate central nervous system symptoms. Site institutional review boards approved each study, and participants provided written informed consent.
Outcomes
The primary outcome was suicidality, defined as suicidal ideation or attempted or completed suicide and identified from signs, symptoms, diagnoses, adverse events, and death data as described in detail elsewhere [8]. Attempted or completed suicide was a secondary outcome, as was suicidality or fatal injury attributed to substance abuse, homicide, accident, or unknown cause. The at-risk period started when antiretroviral treatment was initiated. For efavirenz-exposed analyses, subsequent follow-up was included regardless of treatment status. For on-treatment analyses, follow-up was censored at discontinuation of the randomly assigned efavirenz strategy, plus 28 days for washout.
Covariates
Baseline covariates included for adjustment were self-reported race/ethnicity (or, alternatively, genetic ancestry), sex, age category, history of injection drug use, documented psychiatric history or use of psychoactive medication within 30 days before study entry, and body weight category. CD4 T-cell count, HIV-1 RNA, and history of AIDS-defining event were also measured at baseline. Genome-wide genotype data were used to generate principal components that were also used as covariates to adjust for genetic ancestry, to minimize confounding by unrecognized population stratification (see Statistical Analysis section for details).
Genetic Assays and Data
Genotypes for CYP2B6 516G→T, 983T→C, 15582C→T and CYP2A6 -48T→G were largely available from a MassARRAY iPLEX Gold (Sequenom) assay, generated by Vanderbilt Technologies for Advanced Genomics (VANTAGE) as described elsewhere [21]. For additional participants who consented to genetic testing but lacked such data, CYP2B6/CYP2A6 genotyping was attempted with the same assay. Genome-wide genotype data largely available from a previous immunogenomics project [33] were generated by Illumina HumanHap 650Y array for A5095 and by Illumina 1M duo array for A5142 and A5202. Quality control and imputation of genome-wide data were performed essentially as described elsewhere [34]. To assure that genotypes could be combined across assays, 65 samples previously genotyped with HumanHap 650Y array or Illumina 1M duo array were regenotyped with Illumina Expanded Multi-Ethnic Genotyping array (MEGAEX, with >2 million markers). There was high concordance across genotypes (r2 > 0.998) and the first 10 principal components (all r2 > 0.998), thus providing assurance. In addition, MEGAEX genotyping was attempted in participants with suicidality in whom genome-wide genotype data were not already available.
Efavirenz metabolizer categories were prespecified, and were defined by combinations of CYP2B6 and CYP2A6 polymorphisms [21, 28]. Two categorization approaches considered the full range metabolizer strata based on either CYP2B6 alone (10 strata) or both CYP2B6 and CYP2A6 (12 strata). A third approach collapsed the 12 strata into 3 groups, defined as extensive metabolizers (strata 1–2), intermediate metabolizers (strata 3–7), and slow metabolizers (strata 8 and higher). See Supplemental Material for details. Consent for genetic analysis was obtained under ACTG protocol A5128 [31], and the ACTG approved this use of DNA.
Statistical Analysis
Our aim was to estimate the association between efavirenz metabolizer genotype and suicidality among participants who started randomly assigned efavirenz-containing regimens for initial treatment of HIV-1 infection. The association between genotype level and time to suicidality outcomes was evaluated with a hazard ratio (HR) from a weighted Cox proportional hazards model, stratified by race/ethnicity group, with genotype level included as a linear covariate. To assess the linearity assumption, genotype level was also fit using a restricted quadratic spline with 4 equally spaced knots at the 20th, 40th, 60th and 80th percentiles [35]. Furthermore, metabolizer genotype was fit as 3 unordered groups versus linear with 3 levels (extensive, intermediate, slow); a likelihood ratio test did not provide evidence against the linear fit (P = .49 for efavirenz-exposed and P = .93 for efavirenz on-treatment analysis). Subsequent analyses include genotype as a linear covariate unless indicated otherwise.
Further analyses were conducted to adjust for genetic ancestry among the subset of participants with genome-wide genotype data. To quantify ancestry among samples, we estimated continuous axes of ancestry incorporating the intersection of common autosomal genotypes across all genotyped sets used (n = 74308 single-nucleotide polymorphisms) using the program EIGENSTRAT version 6.1.4 [36]. Samples from the International HapMap Project [37] phase 3 were also included to provide global reference populations. The principal components scree plots were visually examined to ensure that the components selected for analyses represented ancestral information. The first 4 genetic ancestry principal components were included as covariates in a weighted Cox model that was not stratified by self-reported race/ethnicity; additional principal components were not included to avoid model overfitting. Adjusted analyses included additional baseline covariates as described above. Race/ethnicity (and genetic ancestry) was the primary confounding factor and potential modifier of interest, and small event numbers limited the number of additional covariates in the adjustment set for multivariable analyses.
A supplemental analysis evaluated whether participants classified as efavirenz extensive metabolizers in the efavirenz group were at greater risk of suicidality than those who were randomly assigned to the nonefavirenz group. As with the efavirenz group, the nonefavirenz group was restricted to white, black, and Hispanic participants who enrolled in the United States or Puerto Rico and who consented to genetic testing. CYP2B6/CYP2A6 testing was not conducted in the nonefavirenz group. A 2-sided 0.05 statistical significance level was used, with no adjustment for multiple testing. Statistical analyses were conducted using Windows SAS (version 9.4) and R (version 3.3.1) software.
Weighting for Missing Data and Dropout
The 2239 participants who started randomly assigned efavirenz therapy were considered the full sample. Inverse probability missingness weights were used to adjust for 18% missing metabolizer genotype data and 38% missing genetic ancestry data [38]. Inverse probability censoring weights were used in Cox models to adjust for event-free dropout due to (1) premature study discontinuation for the exposed follow-up analyses and (2) premature discontinuation of the assigned efavirenz strategy or study follow-up for on-treatment analyses [39]. For the exposed follow-up analyses, using inverse probability weights, we aimed to estimate the association between genotype level and suicidality in a setting where all participants who started efavirenz therapy had genetic data available and also completed study follow-up. For the on-treatment analyses, we aimed to estimate this same association in a setting where participants who started efavirenz therapy had genetic data available and remained on the efavirenz regimen and in follow-up for their full possible duration of study. Participants who could not continue owing to death or research site defunding were considered study completers.
Logistic regression was used to estimate missingness weights, and pooled logistic regression to estimate censoring weights. The product of these 2 weights was applied in a weighted Cox model using the robust variance estimator [40]. Five fully observed baseline covariates were included to estimate weights: sex, self-reported race/ethnicity, age (restricted quadratic spline), history of injection drug use, and psychiatric history or psychoactive medication. The missingness weights model included these 5 covariates plus an indicator of suicidality outcome to account for differential data availability; the censoring weights model included these 5 covariates as well as CYP2B6/CYP2A6 metabolizer level. Main effects and 2-way interactions (linear interactions for age) were included in the models to estimate inverse probability of missingness and censoring weights.
RESULTS
Study Participants
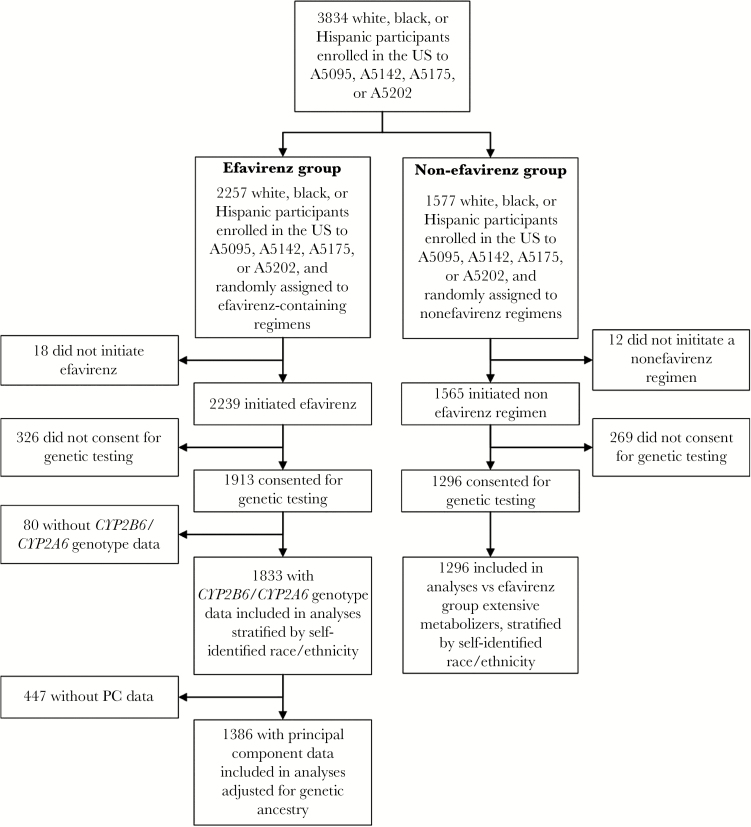
A total of 2257 white, black, or Hispanic participants from ACTG studies A5095, A5142, A5175, and A5202 were randomly assigned to receive efavirenz-containing regimens at research sites in the United States and Puerto Rico, of whom 2239 started efavirenz therapy. After exclusion of those who did not provide consent for genetic testing or were not successfully genotyped for CYP2B6 and CYP2A6, 1833 (82% of 2239) participants were evaluable for associations between CYP2B6/CYP2A6 genotype and suicidality. These included 41 (87% of 47) with suicidality and 1792 (82% of 2192) without suicidality. Of these 1833 participants, 1386 also had ancestry principal components data, including 40 (85% of 47) with suicidality and 1346 (61% of 2192) without suicidality, the latter lower percentage reflecting our decision to generate ancestry principal components in as many participants with suicidality as possible, while relying on previously available genotype data to generate principal components on those without suicidality. Participant disposition is presented in Figure 1.
Figure 1.
Derivation of the study sample. Selection of the study population is shown among white, black, and Hispanic participants who were randomly assigned to an efavirenz-containing or nonefavirenz regimen in A5095, A5142, A5175, or A5202 and enrolled in the United States (US) or Puerto Rico. PC, principal component.
Baseline characteristics and genotype frequencies are shown in Table 1. Among the 1833 participants with CYP2B6/CYP2A6 genotype data, the median age was 38 years, and 18% were female, generally consistent with the remaining 406 individuals who did not have CYP2B6/CYP2A6 genotype data, except that those with CYP2B6/CYP2A6 genotype data were more likely to be white. Genotype frequencies in each race/ethnicity group are presented in Table 2. Slow metabolizer genotypes were present in 50 (6%) of 781 white, 120 (18%) of 660 black, and 45 (11%) of 392 Hispanic participants.
Table 1.
Baseline Characteristics of Study Participants Assigned to an Efavirenz-Containing Regimen
| Characteristic | CYP2B6/CYP2A6 Genotype Data Available, No. (%)a | Overall, No. (%)a (n = 2239) | |
| Yes (n = 1833) | No (n = 406) | ||
| Parent study | |||
| A5095 | 635 (35) | 105 (26) | 740 (33) |
| A5142 | 403 (22) | 70 (17) | 473 (21) |
| A5175 | 104 (6) | 32 (8) | 136 (6) |
| A5202 | 691 (38) | 199 (49) | 890 (40) |
| Sex | |||
| Male | 1510 (82) | 316 (78) | 1826 (82) |
| Female | 323 (18) | 90 (22) | 413 (18) |
| Race/ethnicity | |||
| White non-Hispanic | 781 (43) | 121 (30) | 902 (40) |
| Black non-Hispanic | 660 (36) | 166 (41) | 826 (37) |
| Hispanic | 392 (21) | 119 (29) | 511 (23) |
| Age, y | |||
| Median (IQR) | 38 (31–44) | 38 (30–44) | 38 (31–44) |
| Range | 17–77 | 19–71 | 17–77 |
| CD4 T-cell count, cells/μLb | |||
| Median (IQR) | 218 (75–330) | 188 (64–295) | 212 (73–322) |
| Range | 0–1336 | 1–849 | 0–1336 |
| HIV-1 RNA, log10 copies/mL | |||
| Median (IQR) | 4.74 (4.39–5.24) | 4.74 (4.40–5.22) | 4.74 (4.39–5.24) |
| Range | 2.34–7.04 | 2.76–6.71 | 2.34–7.04 |
| History of AIDS | 326 (18) | 63 (16) | 389 (17) |
| History of injection drug use | 174 (9) | 49 (12) | 223 (10) |
| Psychiatric history or psychoactive medication | 711 (39) | 148 (36) | 859 (38) |
| Psychoactive medication | 310 (17) | 69 (17) | 379 (17) |
| Depression-related history or antidepressant medication | 459 (25) | 94 (23) | 553 (25) |
| Antidepressant medication | 239 (13) | 53 (13) | 292 (13) |
| Body mass index (kg/m2)c | |||
| Median (IQR) | 24.6 (22.0–27.8) | 24.9 (22.2–27.9) | 24.7 (22.1–27.8) |
| Range | 13.9–60.6 | 17.1–53.8 | 13.9–60.6 |
Abbreviations: HIV-1, human immunodeficiency virus type 1; IQR, interquartile range.
aData represent No. (%) of participants unless otherwise specified.
bThis analysis included 2237 participants (1831 with and 406 without CYP2B6/CYP2A6 genotype data available).
cThis analysis included 2205 participants (1810 with and 395 without CYP2B6/CYP2A6 genotype data available).
Table 2.
Genotype Frequencies According to Self-Identified Race/Ethnicity
| Metabolizer Level |
CYP2B6 | CYP2A6: -48T→G | White (n = 781) | Black (n = 660) | Hispanic (n = 392) | Overall (n = 1833) | ||
| 15582C→T | 516G→T | 983T→C | ||||||
| Extensive | 355 (45) | 218 (33) | 115 (29) | 688 (38) | ||||
| 1 | CC | GG | TT | - | 166 (21) | 137 (21) | 41 (10) | 344 (19) |
| 2 | CT | GG | TT | - | 189 (24) | 81 (12) | 74 (19) | 344 (19) |
| Intermediate | 376 (48) | 322 (49) | 232 (59) | 930 (51) | ||||
| 3 | TT | GG | TT | - | 94 (12) | 12 (2) | 68 (17) | 174 (9) |
| 4 | CC | GT | TT | - | 176 (23) | 198 (30) | 69 (18) | 443 (24) |
| 5 | CC | GG | CT | - | 0 (0) | 45 (7) | 1 (0) | 46 (3) |
| 6 | CT | GT | TT | - | 106 (14) | 57 (9) | 93 (24) | 256 (14) |
| 7 | CT | GG | CT | - | 0 (0) | 10 (2) | 1 (0) | 11 (1) |
| Slow | 50 (6) | 120 (18) | 45 (11) | 215 (12) | ||||
| 8 | CC | TT | TT | - | 47 (6) | 81 (12) | 40 (10) | 168 (9) |
| 9 | CC | GT | CT | - | 0 (0) | 15 (2) | 2 (1) | 17 (1) |
| 10 | CC | GG | CC | - | 0 (0) | 2 (0) | 0 (0) | 2 (0) |
| 11 | Level 8, 9, or 10 | GT | 2 (0) | 22 (3) | 3 (1) | 27 (1) | ||
| 12 | Level 8, 9, or 10 | GG | 1 (0) | 0 (0) | 0 (0) | 1 (0) | ||
| Total | ||||||||
Data are no. (%).
Associations With Suicidality
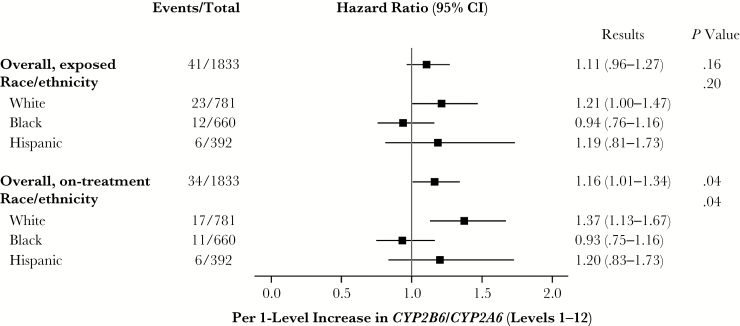
Among the 1833 participants with CYP2B6/CYP2A6 genotype data, suicidality was documented in 41 (2.2%) over 4743 person-years at risk in the efavirenz-exposed analysis (estimated incidence, 8.64/1000 person-years), and 34 (1.9%) over 3954 person-years at risk in the on-treatment analysis (8.60/1000 person-years). In unadjusted weighted analyses based on 12 genotype levels defined by CYP2B6/CYP2A6 genotypes (our most precise genetic estimate of plasma efavirenz exposure), the estimated hazard of suicidality was increased per level in both exposed (HR, 1.11; 95% confidence interval [CI], .96–1.27; P = .16) and on-treatment (1.16; 1.01–1.34; P = .04) analyses, although only the latter was statistically significant.
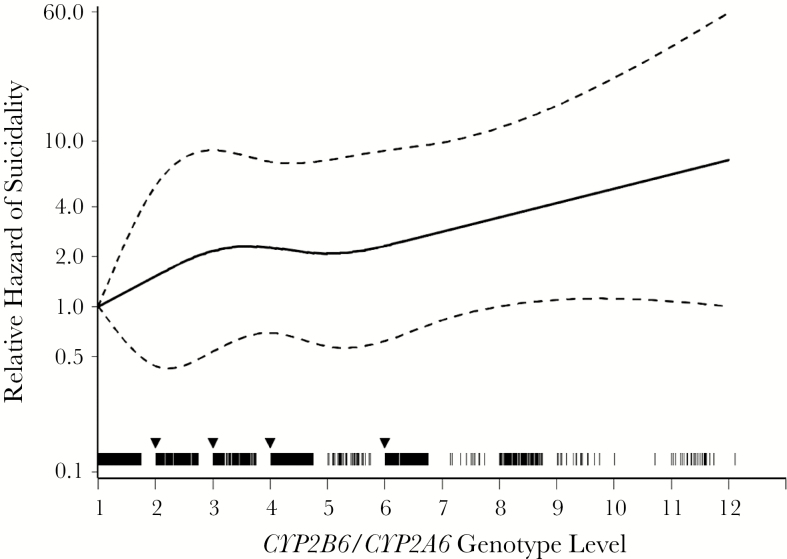
There was significant effect measure modification by race/ethnicity in the on-treatment analysis, with the positive association between genotype level and suicidality being strongest among white, intermediate among Hispanic, and not present among black participants (P = .04). In the exposed analysis the pattern was similar, although the association was partially attenuated among white participants, and significant effect measure modification was not detected (P = .20) (Figure 2). To assess the assumption of linearity of the 12 genotype levels (which predict progressively higher plasma efavirenz exposure) and suicidality, genotype level was also fit using a restricted quadratic spline. Visual inspection of the spline fit provided support for the assumption of a linear association with no apparent threshold effect (Figure 3).
Figure 2.
Relative hazard of suicidality by genotype level among participants randomly assigned to efavirenz-containing regimens. Each incremental CYP2B6/CYP2A6 genotype level (levels 1–12) is known to be associated with progressively greater plasma efavirenz exposure. The estimated relative hazard of suicidality is shown overall and within each race/ethnicity group, for both exposed and on-treatment risk periods. Overall P values test reflect the main effect of genotype level; race/ethnicity P values, a statistical interaction between genotype level and race/ethnicity group. Hazard ratios were estimated from a weighted Cox model stratified by race/ethnicity; robust Wald confidence intervals (CIs) and P values are shown.
Figure 3.
Genotype level fit with a quadratic spline. CYP2B6/CYP2A6 genotype level was fit with a quadratic spline with 4 equally spaced knots at levels 2, 3, 4, and 6, as indicated by downward arrows. Each hash mark represents the participant’s observed genotype level (n = 1833); solid line, the estimated relative hazard; and dashed lines, pointwise 95% Wald-type confidence intervals from a weighted Cox model stratified by race/ethnicity group. This provides support for the assumption of a linear association with no apparent threshold effect. The unweighted result was similar and slightly closer to linear (data not shown).
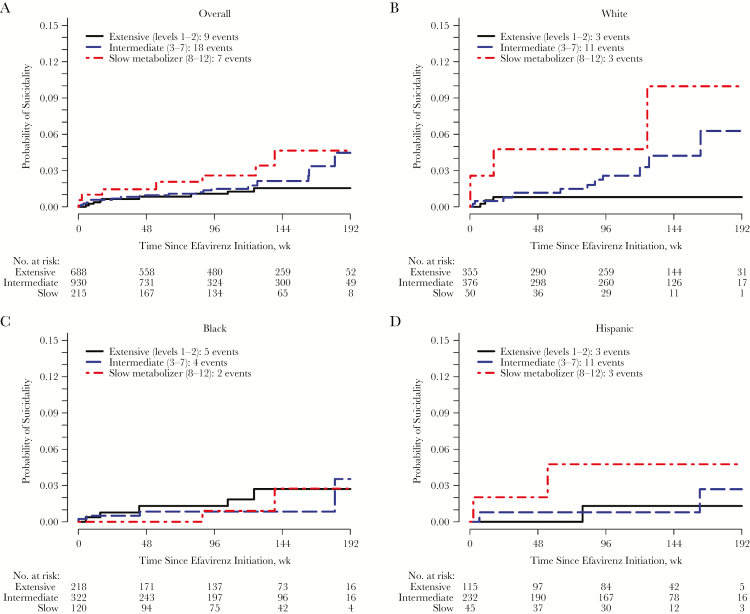
The estimated cumulative probability of suicidality based on 3 genotype levels is presented in Figure 4. Among all study participants, slow metabolizers were at numerically increased risk at each time point, with no apparent difference between intermediate and slow metabolizers during most of the study follow-up period (Figure 4A). This differed by race/ethnicity. Among black participants, neither slow nor intermediate metabolizers seemed to be at greater risk than extensive metabolizers, whereas among white and Hispanic participants, there seemed to be an association between metabolizer level and suicidality, although there were relatively few Hispanic participants (Figure 4B–4D). Table 3 presents results from weighted Cox models to estimate the association between genotype level and a suicidality outcome adjusted for self-identified race/ancestry, as well as for psychiatric history or psychoactive medication, injection drug use history, sex, age category, and body weight category.
Figure 4.
Cumulative probability of suicidality for each metabolizer group in on-treatment analysis, overall and by race/ethnicity. A, Estimated cumulative probability of suicidality for each metabolizer group is shown overall among all participants (A) and among white (B), black (C), and Hispanic (D) participants. Values for probability represent 1 minus the weighted Kaplan-Meier estimate; on-treatment analyses are shown. The unweighted number at risk is shown below each panel, and the number of suicidality events is provided in the key.
Table 3.
Associations Between Genotype level and Suicidality, Adjusted for Genetic Ancestry and Other Covariatesa
| Analysis Period, Adjustment, and Efavirenz Metabolizer Measure |
Estimated HR (95% CI) | Confidence Limit Ratio (Precision) | Wald P value |
| Efavirenz-exposed analysis | |||
| Self-identified race/ethnicity (n = 1831; 41 events) | |||
| 10 level | 1.12 (.97–1.29) | 1.3 | .13 |
| 12 level | 1.11 (.97–1.28) | 1.3 | .12 |
| 3 level | 1.48 (.85–2.56) | 3.0 | .17 |
| Principal components (n = 1384; 40 events) | |||
| 10 level | 1.10 (.96–1.27) | 1.3 | .17 |
| 12 level | 1.08 (.96– 1.23) | 1.3 | .21 |
| 3 level | 1.42 (.84– 2.41) | 2.9 | .19 |
| On-treatment analysis | |||
| Self-identified race/ethnicity (n = 1831; 34 events) | |||
| 10 level | 1.17 (1.01–1.36) | 1.3 | .04 |
| 12 level | 1.16 (1.01–1.33) | 1.3 | .04 |
| 3 level | 1.87 (1.05–3.31) | 3.2 | .03 |
| Principal components (n = 1384; 33 events) | |||
| 10 level | 1.15 (.99–1.32) | 1.3 | .06 |
| 12 level | 1.12 (.99–1.27) | 1.3 | .08 |
| 3 level | 1.74 (1.02–2.97) | 2.9 | .04 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
aEach weighted Cox model to estimate the association between genotype level and a suicidality outcome was adjusted either for self-identified race/ethnicity or for genetic ancestry using 4 principal components. Each analysis also adjusted for psychiatric history or psychoactive medication, injection drug use history, sex, age category, and body weight category. In the multivariable adjusted analyses the events to covariates ratio is low, with 33–41 events, and covariates using 12 degrees of freedom. Results adjusted only for race/ethnicity or principal components 1–4 were similar.
The above analyses were stratified by self-identified race/ethnicity, which can introduce unrecognized population stratification. Additional adjusted analyses controlled for genetic ancestry based on the first 4 principal components, as well as psychiatric history/psychoactive medication, history of injection drug use, sex, age category, and body weight category. Among 1384 participants (including white, black, and Hispanic participants) with both principal component and other covariate data, the HR estimate by genotype level for suicidality exceeded 1.0 in all analyses but was statistically significant only in on-treatment analysis based on 3 metabolizer levels (HR, 1.74; 95% CI, 1.02–2.97; P = .04). Results from weighted Cox models to estimate the association between genotype level and a suicidality outcome adjusted for the first 4 principal components, as well as for psychiatric history or psychoactive medication, injection drug use history, sex, age category, and body weight category, are presented in Table 3. Results of weighted Cox models were similar in analyses that considered not only suicidality but also death from substance abuse, accident, or unknown cause (data not shown).
Comparison to Efavirenz-Free Regimens
We explored risk of suicidality among CYP2B6/CYP2A6 extensive metabolizers versus individuals randomized to nonefavirenz regimens in these clinical trials, and who also consented to genetic testing. There were 12 (0.9%) suicidality events reported among 1296 individuals in the nonefavirenz group. In on-treatment analyses, the hazard of suicidality was numerically lower in the nonefavirenz group than in the extensive metabolizer group, although this difference was not statistically significant (HR, 0.70; 95% CI, .29–1.67; P = .42). The estimated probability of suicidality in the nonefavirenz group was numerically lower than in the extensive metabolizer group at every time point. Among white participants, the likelihood of suicidality was similar in the extensive metabolizer and nonefavirenz groups. Among black participants, the number with suicidality was small in both the extensive metabolizer and nonefavirenz groups. Cumulative probabilities of suicidality in relation to the nonefavirenz group are in the Supplemental Material.
DISCUSSION
In ACTG studies, randomization to initial treatment with efavirenz-containing regimens was previously reported to be associated with a 2-fold increased hazard of suicidality [8]. The present study showed that, in analyses limited to white, black, and Hispanic participants randomly assigned to receive an efavirenz-containing regimen in those same studies, genotypes that predict increased plasma efavirenz exposure were also associated with increased suicidality. This finding further supports the validity of the reported association between efavirenz and suicidality [8]. In addition, the genetic association with suicidality progressively increased by CYP2B6/CYP2A6 genotypes that predict progressively higher plasma efavirenz concentrations with no apparent threshold, suggesting that increased suicidality is not limited to CYP2B6 slow metabolizers. Our study also showed an apparent difference in the on-efavirenz treatment association between genetics and suicidality depending on race/ethnicity, with an association among white participants (HR. 1.37; 95% CI, 1.13–1.67) but not black participants (0.93; .75–1.16). This analysis included fewer Hispanic participants, but their risk seemed to be intermediate (HR, 1.20; 95% CI, .83–1.73).
This study replicates a difference by race first noted in a much smaller ACTG data set [24] in which there was an association between CYP2B6 slow metabolizer genotypes and increased central nervous system adverse events in 276 white participants (P = .04) but not in 217 black participants (P = .58). A subsequent observational study involving 563 patients who started efavirenz-containing regimens at a clinic in the southeastern United States found that slow metabolizer CYP2B6 genotypes were associated with efavirenz discontinuation for reported central nervous system symptoms in 335 white participants (P = .001) but not in 198 black participants (P = .27) [41]. These previous studies, together with the present study, suggest that among patients with intermediate or slow metabolizer genotypes who are prescribed efavirenz in the United States, central nervous system side effects, including suicidality, are more likely to be reported among white than among black participants. Among Hispanic participants, CYP2B6/CYP2A6 genotype level also seemed to be associated with increased suicidality, although this association was not statistically significant and the confidence interval was wide, reflecting the small number of events in these analyses.
We cannot explain the stronger association of CYP2B6/CYP2A6 slow metabolizer genotypes with suicidality among white compared with black participants. Black participants with suicidality may have been less likely to report such symptoms than white participants with similar symptoms, resulting in misclassification. Plasma efavirenz exposure is similar among black and white individuals with CYP2B6 slow metabolizer genotypes [21], so differences in such exposure are unlikely to explain the attenuated genetic association in blacks compared with whites. It is also possible that polymorphisms in genes beyond CYP2B6 affect susceptibility to suicidality with efavirenz.
Genetic testing has the potential to identify patients in whom drugs with unfavorable adverse event profiles may be safely prescribed. This was the rationale for exploring whether extensive metabolizers (ie, lower-risk subgroup) in the efavirenz group were still at greater risk of suicidality than participants in the nonefavirenz group. The numerically lower probability of suicidality in the nonefavirenz group at each study time point overall supports the validity of the association between CYP2B6 genotype level and suicidality in the efavirenz group. Although among white participants the likelihood of suicidality was similar in the extensive metabolizer and nonefavirenz groups, the small number of participants with suicidality in both groups prevents us from concluding that CYP2B6 genotyping can identify patients at no increased risk of suicidality with efavirenz versus nonefavirenz regimens.
Unrecognized population stratification can cause spurious associations in genetic epidemiology studies. We addressed this by not only considering self-identified race/ethnicity but by also separately adjusting for principal components generated from genome-wide genotype data. Associations persisted in the latter analyses. In addition, the genetic association persisted in multivariable models that also included sex, age category, history of injection drug use, psychiatric history or psychoactive medication, and body weight category (all measured at baseline).
Our study had limitations. Because data were from clinical trials that did not specifically focus on suicidality, we may have missed some suicidality cases. To help address the possibility that suicidality was underreported, we included suicidality plus death from substance abuse, accident, or unknown cause in sensitivity analyses. Because providers may not have referred participants considered to be at high risk for neurologic intolerance to studies with efavirenz-containing regimens, risk of suicidality may be underestimated. Analyses were limited to white, black, and Hispanic participants at sites in the United States, so findings may not translate to other countries or race/ethnicities, including Asians. The open-label design of 3 of the 4 studies might have biased investigators into reporting neuropsychiatric effects in patients randomized to efavirenz.
In summary, CYP2B6/CYP2A6 genotypes were associated with increased reported suicidality among participants who had been randomly assigned to receive efavirenz-containing regimens in clinical trials. This association was most apparent in white participants and attenuated in black participants.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We are grateful to the many persons with HIV infection who volunteered for A5095, A5142, A5175, A5202, and A5128. We also acknowledge the contributions of study teams and site staff for these protocols.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (grant U01AI068636); the National Institute of Mental Health; National Institute of Dental and Craniofacial Research; the ACTG, funded by the NIAID (grants AI-068636, AI-038858, AI-068634, and AI-038855); (NIAID grants AI-069439 and National Center for Translational Sciences (NCATS) TR-000445 to D. W. H.); the University of North Carolina at Chapel Hill Center for AIDS Research (grant P30 AI50410); and the Vanderbilt Molecular and Genetic Epidemiology of Cancer training program, funded by the US National Cancer Institute (grant R25 CA-160056; support to J. N. H.). Clinical research sites that participated in ACTG protocols A5095, A5142, A5175 and A5202 and collected DNA under protocol A5128 were supported by NIAID (grants AI069477, AI027675, AI073961, AI069474, AI069432, AI069513, AI069423, AI050410, AI069452, AI69450, AI054907, AI069428, AI069439, AI069467, AI045008, AI069495, AI069415, AI069556, AI069484, AI069424, AI069532, AI069419, AI069471, AI025859, AI069418, AI050409, AI069423, AI069501, AI069502, AI069511, AI069434, AI069465, AI069471, AI069494, AI069424, AI069472, AI069501, AI069470, AI046376, AI069511, AI072626, AI069472, AI038858, AI069472, AI027661, AI034853, AI069447, AI032782, AI027658, AI27666, AI058740, and AI046370) and by the National Center for Research Resources (grants RR00051, RR00046, RR025747, RR025777, RR024160, RR024996, and RR024156). Abbott Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline provided study medications.
Potential conflicts of interest. K. R. M. has received research support from grants awarded to the University of North Carolina at Chapel Hill from Merck, AbbVie, and Gilead. J. J. E. has received research support from grants awarded to the University of North Carolina at Chapel Hill from Bristol-Myers Squibb (BMS), AbbVie, Janssen, ViiV Healthcare, and Gilead and is a consultant to BMS, AbbVie, Janssen, ViiV Healthcare, Merck, and Gilead. R. H. is an employee of Gilead Sciences. P. E. S. has received research support from grants awarded to Brigham and Women’s Hospital from BMS, ViiV, and Gilead and is a consultant to BMS, AbbVie, Janssen, ViiV, Merck, and Gilead. T. B. C. has served as a consultant to Gilead Sciences. E. S. D. has received research support from grants awarded to Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center from Gilead, Merck, and ViiV and is a consultant for BMS, Janssen, Merck, Teva, and ViiV. Q. M. has received research support from grants awarded to the University at Buffalo from Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. van Leth F, Phanuphak P, Ruxrungtham K, et al. ; 2NN Study team Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet 2004; 363:1253–63. [DOI] [PubMed] [Google Scholar]
- 2. Gulick RM, Ribaudo HJ, Shikuma CM, et al. ; AIDS Clinical Trials Group (ACTG) A5095 Study Team Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA 2006; 296:769–81. [DOI] [PubMed] [Google Scholar]
- 3. Riddler SA, Haubrich R, DiRienzo AG, et al. ; AIDS Clinical Trials Group Study A5142 Team Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008; 358:2095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lennox JL, DeJesus E, Lazzarin A, et al. ; STARTMRK investigators Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 5. Cooper DA, Heera J, Goodrich J, et al. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis 2010; 201:803–13. [DOI] [PubMed] [Google Scholar]
- 6. Daar ES, Tierney C, Fischl MA, et al. ; AIDS Clinical Trials Group Study A5202 Team Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clifford DB, Evans S, Yang Y, et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med 2005; 143:714–21. [DOI] [PubMed] [Google Scholar]
- 8. Mollan KR, Smurzynski M, Eron JJ, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med 2014; 161:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arenas-Pinto A, Grund B, Sharma S et al. for the INSIGHT START Study Group. Increased risk of suicidal behaviour with use of efavirenz: results from the START trial [abstract 6199]. In: 21st International AIDS Society Conference, Durban, South Africa, 2016. [Google Scholar]
- 10. Napoli AA, Wood JJ, Coumbis JJ, Soitkar AM, Seekins DW, Tilson HH. No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database. J Int AIDS Soc 2014; 17:19214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nkhoma ET, Coumbis J, Farr AM, et al. No evidence of an association between efavirenz exposure and suicidality among HIV patients initiating antiretroviral therapy in a retrospective cohort study of real world data. Medicine (Baltimore) 2016; 95:e2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed Updated 14 July 2016.
- 13. Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 2003; 306:287–300. [DOI] [PubMed] [Google Scholar]
- 14. Desta Z, Saussele T, Ward B, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 2007; 8:547–58. [DOI] [PubMed] [Google Scholar]
- 15. Bélanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. Glucuronidation of the antiretroviral drug efavirenz by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine. Drug Metab Dispos 2009; 37:1793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004; 18:2391–400. [PubMed] [Google Scholar]
- 17. Tsuchiya K, Gatanaga H, Tachikawa N, et al. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun 2004; 319:1322–6. [DOI] [PubMed] [Google Scholar]
- 18. Rotger M, Colombo S, Furrer H, et al. ; Swiss HIV Cohort Study Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics 2005; 15:1–5. [DOI] [PubMed] [Google Scholar]
- 19. Haas DW, Smeaton LM, Shafer RW, et al. Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: an Adult Aids Clinical Trials Group Study. J Infect Dis 2005; 192:1931–42. [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez-Novoa S, Barreiro P, Rendón A, Jiménez-Nacher I, González-Lahoz J, Soriano V. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin Infect Dis 2005; 40:1358–61. [DOI] [PubMed] [Google Scholar]
- 21. Holzinger ER, Grady B, Ritchie MD, et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics 2012; 22:858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wyen C, Hendra H, Vogel M, et al. ; German Competence Network for HIV/AIDS Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother 2008; 61:914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J, Sönnerborg A, Rane A, et al. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet Genomics 2006; 16:191–8. [DOI] [PubMed] [Google Scholar]
- 24. Ribaudo HJ, Liu H, Schwab M, et al. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study. J Infect Dis 2010; 202:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. di Iulio J, Fayet A, Arab-Alameddine M, et al. ; Swiss HIV Cohort Study In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics 2009; 19:300–9. [DOI] [PubMed] [Google Scholar]
- 26. Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. CYP2B6 (c.516G–>T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol 2009; 67:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwara A, Lartey M, Sagoe KW, Kenu E, Court MH. CYP2B6, CYP2A6 and UGT2B7 genetic polymorphisms are predictors of efavirenz mid-dose concentration in HIV-infected patients. AIDS 2009; 23:2101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haas DW, Kwara A, Richardson DM, et al. Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes. J Antimicrob Chemother 2014; 69:2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gulick RM, Ribaudo HJ, Shikuma CM, et al. ; AIDS Clinical Trials Group Study A5095 Team Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med 2004; 350:1850–61. [DOI] [PubMed] [Google Scholar]
- 30. Campbell TB, Smeaton LM, Kumarasamy N, et al. ; PEARLS study team of the ACTG Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med 2012; 9:e1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haas DW, Wilkinson GR, Kuritzkes DR, et al. ; Adult AIDS Clinical Trials Group A multi-investigator/institutional DNA bank for AIDS-related human genetic studies: AACTG Protocol A5128. HIV Clin Trials 2003; 4:287–300. [DOI] [PubMed] [Google Scholar]
- 32. US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS table for grading the severity of adult and pediatric adverse events, Version 1.0. Updated August 2009. http://rsc.tech-res.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf. Accessed 13 February 2017.
- 33. Pereyra F, Jia X, McLaren PJ, et al. ; International HIV Controllers Study. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010; 330:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moore CB, Verma A, Pendergrass S, et al. Phenome-wide association study relating pretreatment laboratory parameters with human genetic variants in AIDS Clinical Trials Group Protocols. Open Forum Infect Dis 2015; 2:ofu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology 2011; 22:874–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38:904–9. [DOI] [PubMed] [Google Scholar]
- 37. International HapMap Consortium. The International HapMap Project. Nature 2003; 426:789–96. [DOI] [PubMed] [Google Scholar]
- 38. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013; 22:278–95. [DOI] [PubMed] [Google Scholar]
- 39. Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 2000; 56:779–88. [DOI] [PubMed] [Google Scholar]
- 40. Buchanan AL, Hudgens MG, Cole SR, Lau B, Adimora AA; Women’s Interagency HIV Study Worth the weight: using inverse probability weighted Cox models in AIDS research. AIDS Res Hum Retroviruses 2014; 30:1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leger P, Chirwa S, Turner M, et al. Pharmacogenetics of efavirenz discontinuation for reported central nervous system symptoms appears to differ by race. Pharmacogenet Genomics 2016; 26:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.