Abstract
The 2015–2016 epidemic of Zika virus (ZIKV) in the Americas and the Caribbean was associated with an unprecedented burden of neurological disease among adults. Clinically, Guillain-Barre syndrome (GBS) predominated among regions affected by the ZIKV epidemic, but the spectrum of neurological disease in the adults appears broader as cases of encephalopathy, encephalitis, meningitis, myelitis, and seizures have also been reported. A para-infectious temporal profile of ZIKV-associated GBS (ZIKV-GBS) has been described in clinical studies, which may suggest a direct viral neuropathic effect. However, ZIKV neuropathogenesis has not yet been fully understood. Mechanisms for ZIKV-GBS and other neurological syndromes have been hypothesized, such as adaptive viral genetic changes, immunological interactions with other circulating flaviviruses, and host and factors. This review summarizes the current evidence on ZIKV-associated neurological complications in the adults.
Keywords: Zika virus, Guillain-Barre syndrome, neurological disease, adult population
Zika virus (ZIKV) is a single-stranded, positive-sense enveloped RNA virus that belongs to the genus Flavivirus of the Flaviviridae family, which includes dengue virus (DENV) and the well-known neurotropic viruses, West Nile virus and Japanese encephalitis virus [1]. ZIKV is primarily transmitted to humans by certain Aedes species mosquitoes. Other routes are sexual transmission and vertical transmission from mother to fetus [2]. ZIKV was isolated for the first time in 1947 from blood of a febrile monkey in the Zika forest in Uganda [3]. The first ZIKV infection in humans was documented in Uganda in 1952, and subsequently sporadic cases were reported in Africa and Southeast Asia [4, 5]. Over the next few decades, ZIKV reached other areas of the world without any significant outbreak, and in the 21st century it continued its slow spread through islands in the Pacific Ocean. In 2007, the first ever known ZIKV epidemic occurred in Yap State (hereafter, “Yap”), a cluster of islands in the Federated States of Micronesia, where the infection was primarily self-limited and resembled other mosquito-borne flavivirus infections [6, 7]. It was not until 2013 when a ZIKV epidemic in French Polynesia was associated with an unusual increase in neurological disease in adults, mostly attributed to Guillain-Barre syndrome (GBS) [7, 8].
Then, in May 2015, the first cases of ZIKV infection were confirmed in the northeast region of Brazil. Cryptic transmission of ZIKV before detection of the epidemic is believed to have begun in late 2013 or early 2014 in the same region, after which ZIKV spread to Central and South America [9, 10]. Like the populations in Yap and French Polynesia, the population in the Americas was fully susceptible to ZIKV infection. Interestingly, an unprecedented burden of GBS in adults and microcephaly in newborns was geographically and temporarily associated with the ZIKV epidemic. What caused the marked difference in ZIKV infection outcomes in the Americas is still under investigation [11]. This review summarizes the current evidence on ZIKV-associated neurological complications in adults and the clinical spectrum of neurological disease observed in recent ZIKV epidemics.
ZIKV EPIDEMICS AND NEUROLOGICAL DISEASE
In 2007, investigators reported that 73% of the Yap population was exposed to ZIKV infection. The infection was mostly asymptomatic; symptomatic individuals presented with a self-limited, mild febrile illness associated with rash, joint pain, and conjunctivitis [6]. From early 2013 to late 2014, French Polynesia registered 8750 suspected cases of ZIKV disease among an estimated 32 000 people infected [12]. Seventy-four cases presented with neurological syndromes or other complications in the weeks/months following the acute febrile illness [12]. Of those, 42 corresponded to GBS, a number that overwhelmingly exceeded the expected GBS annual average of 3–8 cases [8]. Similarly, between 2015 and 2016, clusters of GBS emerged shortly after the ZIKV outbreak in the Americas, in a pattern that highlighted a temporal and geographical association between GBS cases and ZIKV transmission. In July 2015, the state of Bahia, Brazil, reported 42 cases of GBS, of which 42% had history consistent with ZIKV infection [12]. Later, in November 2015, researchers from Brazil confirmed ZIKV in 7 samples from patients with neurological disease who were initially believed to have dengue fever [12]. Between November 2015 and January 2016, Brazil registered 1708 GBS cases nationwide [13]. Similarly, in January 2016, El Salvador reported 46 GBS cases over 2 months, representing a 1.6-fold increase in the historical incidence for that region [14]; Venezuela also reported 252 GBS cases that were temporarily and geographically associated with ZIKV transmission [15]. Based on epidemiological studies, in 2016 the estimated GBS incidence increased between 2.0- and 9.8-fold in 7 countries in the Americas affected by the ZIKV epidemic [16].
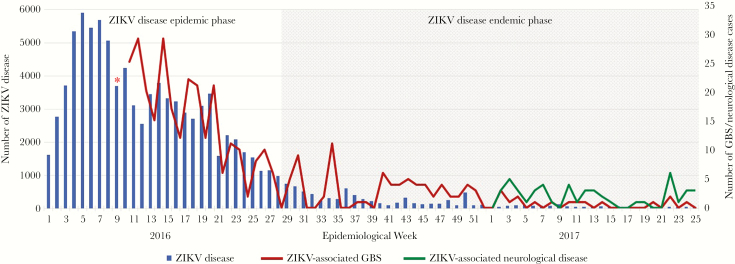
In Colombia, prior to the introduction of ZIKV, the average frequency of GBS was 242 cases per year, equivalent to an incidence of 0.49 cases/100 000 inhabitants per year. During November 2015–March 2016, Colombia reported 320 GBS cases, which represented a 211% increase in the GBS incidence as compared to the incidence before ZIKV introduction [16]. There was clear demonstration of a temporal association between the ZIKV epidemic and the marked increase in the number of GBS cases in this country (Figure 1) [15, 16]. By July 2016, Colombia transitioned to an endemic ZIKV transmission rate, and the magnitude of GBS cases decreased; however, ZIKV infection and neurological disease, preceded by a ZIKV-like febrile illness, continued to be reported (Figure 1). Moreover, during 2017, 67% of all neurological complications associated with a prior ZIKV infection corresponded to non-GBS diseases affecting the nervous system (Figure 1).
Figure 1.
Cases of Zika virus (ZIKV) disease, ZIKV-associated Guillain-Barre syndrome (GBS), and ZIKV-associated neurological disease in Colombia, by epidemiological week. The cumulative incidence of GBS from October 2015 to epidemiological week 9 of 2016 was 220 cases (red asterisk). Data are based on findings reported elsewhere [17].
From January 2013 to March 2017, the World Health Organization reported 23 countries with either increased incidence of GBS cases potentially associated with ZIKV infection or confirmed ZIKV infection among GBS cases [18].
NEUROLOGICAL SYNDROMES ASSOCIATED WITH ZIKV
ZIKV-Associated GBS (ZIKV-GBS)
Clinical Profile
GBS is an immune-mediated disorder of the peripheral nervous system that manifests as acute onset of ascending paralysis and sensory symptoms [19]. Accumulating epidemiological, serological, and virological evidence has supported ZIKV epidemics as temporally associated with the emergence of GBS clusters in the Pacific Islands and the Americas. Table 1 outlines the clinical profile of the patients described in the largest case series published on ZIKV-GBS since 2013 [20–25]. In all studies, GBS was slightly more common in men than in women, with patients between the third and fifth decades of life being more frequently affected. Limb weakness and areflexia/hyporeflexia were the most frequent clinical findings. Paresthesias were the most common sensory symptom reported, occurring in about two thirds of patients. Around half of patients in each series presented with cranial nerve deficits, predominantly facial palsy. Although the clinical profile of ZIKV-GBS follows the classical presentation of GBS, Parra et al reported 4 cases of Miller-Fisher syndrome, a GBS variant characterized by ataxia, ophthalmoplegia, and areflexia [21]. Few other cases of Miller-Fisher syndrome were also reported in Brazil [24]. Anaya et al also reported cases of the AMSAN (acute motor sensory axonal neuropathy), Bickerstaff’s encephalitis and pharyngeal-cervical-brachial variants of GBS [25]. One of the most interesting observations in ZIKV-GBS is rapid presentation of neurological symptoms after a viral prodrome. Almost all patients with ZIKV-GBS presented with a prior viral illness that occurred a median of 5–10 days before GBS onset (Table 1). This differs from the previously recognized classical form of GBS, in which almost two thirds of adult patients develop neurological symptoms 2–4 weeks after infection [26]. This observation suggests a parainfectious temporal profile, which contrasts to the postinfectious profile described in GBS associated with other infections [27]. While the rapid progression of neurological symptoms in ZIKV-GBS may suggest increased morbidity or mortality, the severity of the disease in the reported cases was similar to that described for classical forms of GBS, with one third of patients requiring mechanical ventilation and a mortality rate of 0%–4% (Table 1).
Table 1.
Clinical and Laboratory Findings Reported in 5 Studies of Patients With Guillain-Barre Syndrome Associated With Zika Virus (ZIKV) in 5 Countries
| Finding | Cao-Lormeau et al [20] | Parra et al [21] | Dirlikov et al [22] | Arias et al [23] | Da Silva et al [24] | Anaya et al. [25] |
|---|---|---|---|---|---|---|
| Site(s) | French Polynesia | Colombia | Puerto Rico | Colombia | Brazil | Colombia |
| Epidemic period | 2013–2014 | 2016 | 2016 | 2015–2016 | 2015–2016 | 2015–2016 |
| Cases, no. | 42 | 68 | 34 | 19 | 29 | 29 |
| Ratio of men to women | 3:1 | 1:1 | 1:1 | 2:1 | 2:1 | 1:1 |
| Age, y, median (IQR) | 42 (36–56) | 38 (35–57) | 55 (21–88) | 44 (27–59) | NAa | 42 (34–49) |
| Preceding viral prodrome | 37/42 (79) | 66/68 (97) | 30/32b (94) | 19/19 (100) | 24/29 (83) | NA |
| Time from viral prodrome to neurologic symptom onset, d, median (IQR) | 6 (4–10) | 7 (3–10) | 5 (0–17) | 10 (5–12) | 10 (4–22) | 7 (2–15) |
| Neurological findings | ||||||
| Any | 42/42 (100) | 68/68 (100) | 32/34 (94) | 19/19 (100) | 29/29 (100) | 29/29 (100) |
| Muscle weakness | 36 (86) | 66 (97) | NAc | 19 (100) | 29 (100) | NA*** |
| Hyporeflexia/areflexia | 20 (48) | 64 (94) | 31 (97) | 18d (95) | 26 (90) | NA^ |
| Facial palsy | 33 (79) | 34 (50) | 20 (63) | 8 (42) | 12 (41) | 20 (67) |
| Paresthesia | 35 (83) | 52 (77) | NAe | 14 (74) | NA | 26 (90) |
| Lumbar puncture | 42/42 (100) | 55/68 (81) | 25/34 (74) | 10/19 (53) | NA | 11 (38) |
| ACD | NAf | 45 (82) | 25 (100) | 8 (80) | NA | 10 (91) |
| ZIKV RT-PCR positive | 0/41 (0) | 17/42 (40) | 10/34 (29) | 1/1 (100) | 1/29 (3) | NA |
| Nerve conduction studies | ||||||
| Any | 37/42 (88) | 46/68 (68) | 5/34 (15) | 14/19 (74) | 27/29 (93) | 27/29 (93) |
| AIDP | 0 (0) | 36 (78) | 5 (100) | 0 (0) | 18 (67) | 16 (59) |
| AMAN | 37 (100) | 1 (2) | 0 (0) | 10 (71) | 2 (7) | 7 (26) |
| Treatment | ||||||
| Any | 42/42 (100) | 46/68 (68) | 34/34 (100) | 19/19 (100) | 28/29 (97) | 23/29 (79) |
| IVIG | 42 (100) | 42 (91) | 34 (100) | 16 (84) | 26 (93) | 21 (91) |
| Admitted to ICU | 16 (38) | 40 (59) | 21 (62) | 19 (100) | 6 (21) | 20 (69) |
| Mechanical ventilation | 12 (29) | 21 (31) | 12 (35) | 15 (79) | 3 (10) | 14 (48) |
| Died | 0/42 (0) | 3/68 (4) | 1/29 (3) | 0/19 (0) | 1/29 (3) | 0/29 (0) |
Data are no. (%) of patients or no. with the characteristic/no. evaluated (%), unless otherwise indicated. Studies from Parra et al, Arias et al, and Anaya et al reported patients from the city of Cucuta in Colombia during the 2015–2016 epidemic of ZIKV. Overlapping of patients from this city might have occurred between these studies.
Abbreviations: ACD, albuminocytological dissociation; AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; ICU, intensive care unit; IQR, interquartile range; IVIG, intravenous immunoglobulin; NA, not available; RT-PCR, reverse-transcription polymerase chain reaction.
aThe median age was 42 years (IQR, 22–67 years) for the ZIKV-positive group and 46 years (IQR, 45–47 years) for the ZIKV-negative group.
bIndicates acute illness not limited to viral prodrome.
cLeg weakness was reported in 31 cases (97%) and arm weakness in 24 (75%).
dOne patient had no lower limb areflexia.
eLeg paresthesia was reported in 24 cases (75%) and arm paresthesia in 19 (59%).
fIncreased protein concentration (>52 mg/dL) was reported in 39 cases (93%).
***Leg weakness was reported in 27 (93%) cases and arm weakness in 28 (97%) cases.
^Lower extremity hypo/areflexia was reported in 27 (93%) cases and upper extremity hypo/areflexia in 26 (90%) cases.
Laboratory and Neurophysiological Features
Cerebrospinal fluid (CSF) albuminocytological dissociation was found in >80% of reported ZIKV-GBS cases (Table 1). Negative results of serological tests for Campylobacter jejuni, human immunodeficiency virus, and various herpesviruses were reported by Cao-Lormeau et al in the French Polynesia case-control study [20]. Asialo-GM1 antibodies were found in 31% of patients, but the antiganglioside antibodies typically associated with GBS were rarely detected in this study [20]. Similarly, Anaya et al reported the presence of IgG against Mycoplasma pneumoniae as associated with GBS after ZIKV infection [25]. With respect to neurophysiological studies of ZIKV-GBS, publications have shown a variety of results. Data on GBS in Colombia, Puerto Rico, and French Polynesia prior to the introduction of ZIKV is scant. Although the classical acute inflammatory demyelinating polyneuropathy (AIDP) subtype of GBS has been described worldwide, the acute motor axonal neuropathy (AMAN) subtype, which occurs less frequently, has been found in Asia, Central and North America (particularly Mexico), and South America [28]. During the ZIKV epidemic in French Polynesia, 37 cases underwent nerve conduction studies (NCS) during the first week of GBS onset. All cases were documented to have neurophysiological changes consistent with the AMAN subtype, as the authors described prolonged distal motor latencies and reduction of the distal compound muscle action potential without significant decrease in the motor conduction velocity [20]. A similar pattern was reported by Arias et al in Colombia, although the mean time for the NCS was 35 days after the onset of GBS symptoms [23]. These 2 studies classified the patients as having the AMAN subtype of GBS, even though the distal motor latencies were as prolonged as in the demyelinating subtype. In contrast, Parra et al reported that almost 80% of the patients with NCS exhibited features of the AIDP subtype in Colombia; the median time of testing after the onset of GBS was 13 days [21]. Similarly, Da Silva et al reported that 67% of patients had AIDP, with a median time between the onset of GBS and NCS of 18 days [24]. Anaya et al also reported a predominant AIDP subtype which was exhibited by 59% of the cases [25]. A comprehensive analysis of published electrophysiology data suggests ZIKV-GBS is predominantly demyelinating, with significant involvement of the distal nerves [29]. Limitations of the reported studies include a heterogeneous set of criteria used for subtype classification and technical differences in the acquisition of neurophysiological data. The variations in time from the onset of GBS until testing is of importance because the demyelinating subtypes may depict axonal characteristics early in the disease [28]. A systematic review of the raw data would be required to better understand the electrophysiological characteristics of ZIKV-GBS and their clinical implications.
Evidence of Association Between GBS and ZIKV Infection
The confirmation of the association between GBS and ZIKV infection has posed a challenge to clinicians and epidemiologists owing to challenges in demonstrating ZIKV infection. Laboratory confirmation of ZIKV infection, similarly to that of other flavivirus infections, involves detection of viral RNA by reverse-transcription polymerase chain reaction (RT-PCR) analysis of blood specimens obtained during the first 3–5 days after disease onset [30]. After virus has faded from blood, the diagnosis of ZIKV infection is based on the detection of specific antibodies. Within the context of neurological disease, this approach to confirming ZIKV infection has several difficulties. First, by the time of neurological symptom presentation, ZIKV has cleared from blood in most cases [20, 21]. Second, since the CSF is not routinely sampled for flavivirus diagnosis, the duration and kinetics of ZIKV RNA detection in CSF is unknown. Third, serological diagnosis is limited by the extensive cross-reactivity with other flaviviruses, particularly when previous exposure to DENV has occurred [30]. Some of these limitations were evidenced in the French Polynesia retrospective case-control study during 2013–2014, in which the criteria for diagnosing ZIKV infection in GBS cases was different from that for ZIKV-infected controls. Viral RNA detection was limited in GBS cases because of constrains in the time of sample collection following the viral prodrome, and therefore a serological approach was conducted. However, the interpretation of serological anti-ZIKV immunoglobulin M (IgM) was also limited in 19% of GBS cases by cross-reactivity with anti-DENV IgM antibodies [20]. The first and largest study confirming the presence of the ZIKV RNA genome in subjects with presumed ZIKV-GBS was achieved in the study by Parra et al in Colombia, in which 17 of 42 patients (25%) had ZIKV detected by RT-PCR analysis, which most positive results (94%) involving urine specimens. Interestingly, only 1 patient had a positive blood specimens, and for 3 patients ZIKV RNA was detected in CSF specimens [21]. In summary, confirmation of ZIKV infection by RT-PCR analysis of blood or CSF specimens from patients with GBS or neurological disease may be limited by the time of sample collection and/or the low-level and transient viremia. These laboratory observations highlight the value of biological samples, such as urine, in the diagnosis of ZIKV infection, and they may support the hypothesis of the existence of viral reservoir organs, such as the kidney. Viral reservoirs may serve as potential amplifiers of viral replication and/or the antigenic source of an antiviral inflammatory response, with potential pathogenic significance in the development of neurological syndromes, even after viremia has faded away. Evidence of causation in ZIKV-GBS has also been limited by the lack of well-designed case-control studies, the lack of reliable epidemiological and laboratory data, poor historical data on the GBS baseline incidence where ZIKV epidemics occurred, and the absence of neuropathological studies on ZIKV-GBS.
Additional evidence on the relationship between ZIKV infection and GBS has been provided by several small studies and case reports. In a multicenter case series of 8 ZIKV-GBS cases in the intensive care unit setting, 1 patient had NCS findings consistent with AIDP, and 1 had findings consistent with AMAN [31]. A small case series from Martinique reported 2 young patients with positive ZIKV-specific PCR results who had NCS findings consistent with AIDP [32]. Eight case reports from different regions affected by the emergence of ZIKV, including French Polynesia [33], Brazil [34, 35], Haiti [36], the Netherlands [37], Spain [38], Honduras [39] and New Zealand [40], reported a link between GBS and ZIKV infection. ZIKV RNA was confirmed in 4 cases, and anti-ZIKV IgM was detected in the rest. NCS were done in 6 cases and demonstrated AIDP in 4 cases. Mild transient polyneuritis, which presented rapidly after febrile illness, has also been reported, but it may represent a milder form of GBS. This raises the possibility of underreporting of minor cases involving patients who do not require or seek medical care (Table 2) [41].
Table 2.
Studies on Guillain-Barre Syndrome (GBS) in Association With Zika Virus (ZIKV) Infection
| Study Type, Site(s) (Year[s]) | Findings | CSF Analysis and NCS | ZIKV Infection Testing |
|---|---|---|---|
| Case-control | |||
| French Polynesia (2013) | 42 cases with GBS (median age, 55 y; 88% had prior viral syndrome; median period from prodrome to GBS, 6 days; 29% required mechanical ventilation) and 98 controls (group 1) with nonfebrile illness | CSF with increased protein concentration in 93% of patients and a median of 4 cells/mm3 [1–7]; all 37 patients with NCS exhibited findings consistent with AMAN | Anti-ZIKV IgM/IgG found in 41 cases (98%) and 54 controls (56%); OR, 59.7 (P < .0001) |
| Colombia (2016) | Cases: 29 GBS patients; median age of 42 years; median period from prodrome to GBS 7 days; 48% required mechanical ventilation; control: 74 patients with ZIKV infection and no neurological involvement; M. pneumoniae IgG and low socioeconomic status were associated with GBS after ZIKV infection | CSF with ACD in 10 patients; NCS consistent with AIDP in 16/27 patients and AMAN in 7/27 patients | Anti-ZIKV/DENV IgG in all cases cases; anti-ZIKV IgM in 1 case (median of 108 days after the prodrome) |
| Case series | |||
| Colombia (2016) | 68 patients with GBS; median age, 47 y; 97% had prior febrile illness; median period from prodrome to GBS, 7 days; 31% required mechanical ventilation and 3 died | CSF with ACD in 45 patients; 36/46 had NCS findings consistent with AIDP | ZIKV RT-PCR done for 17 patients (urine samples for 16, CSF samples for 3, and a serum sample for 1); anti-flavivirus IgM/IgG found in 18 |
| Colombia (2016) | 19 patients with GBS; mean age, 44 y; all had preceding mild febrile illness; median period from prodrome to GBS, 10 days; 79% required mechanical ventilation | CSF with ACD in 8 patients and NCS findings consistent with AMAN in 10 | ZIKV RT-PCR done for 1 patient |
| Puerto Rico (2016) | 34 patients with GBS; median age, 55 y; 94% had prior acute febrile illness; median period from prodrome to GBS, 5 days; 35% required mechanical ventilation and 1 died | CSF with ACD in 25 patients; 5/5 had NCS findings consistent with AIDP | ZIKV RT-PCR done for 10 patients; either anti-ZIKV or anti-flavivirus IgM antibodies found in 24 |
| Brazil (2016) | 29 patients with GBS; 83% had prior viral symptoms; median period from prodrome to GBS, 10 days; 10% required mechanical ventilation and 1 died; outcomes at 3 mo included chronic pain in 55% and Hughes GBS disability scale of 1 in most patients | No CSF analysis reported; 18/27 patients had NCS findings consistent with AIDP, 2/27 had AMAN, and 6 had AMSAN | ZIKV RT-PCR done for 1 patient; anti-ZIKV IgM found in serum/ CSF for 1 and anti-DENV IgM negativity for 26 |
| Multicentera (2016) | 8 patients with GBS and 2 with encephalitis admitted to the ICU; 7 had exanthema on admission and 6 required mechanical ventilation | CSF with pleocytosis in 4 patients (2 with increased protein levels); NCS findings consistent with AIDP in 1 and with AMAN in 1 | ZIKV RT-PCR done for 10 patients |
| Martinique (2016) | 2 patients with GBS | CSF with ACD; NCS findings consistent with AIDP | ZIKV RT-PCR |
| Suriname (2016) | 3 patients with GBS; 2 aged 40 y and 1 aged 60 y | CSF with ACD in 1 patient; NCS findings consistent with AIDP in 2 | ZIKV RT-PCR done for 1 patient; anti-ZIKV IgG found in 2 patients |
| Case report | |||
| Brazil (2014, 2016); French Polynesia (2013); Haiti (2016); Honduras (2016); New Zealand (2016); Spain (2016); the Netherlands (2016) | 8 patients with GBS; age range, 24–62 y; female sex more common (n = 5); 1 was pregnant, 4 had preceding febrile illness, 1 developed weakness after a trip to Tonga, 5 had cranial nerve involvement, 1 had Miller-Fisher syndrome variant, and 1 had respiratory failure | CSF with CAD in 5 patients; NCS findings consistent with AIDP in 4 | ZIKV RT-PCR done for 4 patients; anti-ZIKV IgM found in 4 patients |
Abbreviations: ACD, albuminocytological dissociation; AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor sensory axonal neuropathy; CSF, cerebrospinal fluid; IgG, immunoglobulin G; IgM, immunoglobulin M; NCS, nerve conduction studies; OR, odds ratio; RT-PCR, reverse-transcription polymerase chain reaction.
aSites were in Colombia, Venezuela, El Salvador, Guatemala, Puerto Rico, Ecuador, Peru, and Chile.
ZIKV-Associated Central Nervous System (CNS) Involvement
In contrast to the developing CNS of the fetus, the mature adult brain appears to be less susceptible to the possible neuroinvasive effect of ZIKV. While congenital infection with ZIKV has been associated with encephalitis and microcephaly in newborns, very few cases of CNS involvement in adults have been reported. Encephalopathy, encephalitis, meningitis, myelitis, and/or seizures have been described in patients with ZIKV infection confirmed by RT-PCR analysis [42–47]. The frequency of cases is very low in comparison to the magnitude of ZIKV-GBS cases observed during the outbreak in the Americas. Ancillary workup is also notably inconsistent among cases. For example, brain magnetic resonance imaging has shown a variety of findings, which include meningeal inflammation, T2W hyperintensities in the deep white matter and subcortical deep gray matter nuclei, and lesions with restriction of diffusion suggesting ischemic events [31, 43, 46]. While electroencephalography has revealed abnormal slowing, no reports have linked ZIKV infection in adults to specific epileptogenic problems or epilepsy [44, 45]. Few cases of myelitis or myelopathy associated with ZIKV infection are available in the literature. In those cases, spinal cord magnetic resonance imaging has shown longitudinally extensive lesions throughout the spinal cord [42, 46]. In comparison to cases of ZIKV-GBS, CNS-associated ZIKV complications, such as encephalitis and myelitis, appear to have a predominant neuroinflammatory profile, because CSF analysis depicts lymphocytic pleocytosis and elevated protein levels [25, 42–46]. Although this evidence is scarce, it provides a valuable foundation of the spectrum of CNS involvement related to ZIKV infection.
ZIKV NEUROPATHOGENESIS IN ADULTS
Little is known about ZIKV neuropathogenesis in adult humans. The parainfectious profile in ZIKV-GBS may suggest a direct role of ZIKV in the development of neurological disease [20–22]. The detection of ZIKV RNA in CSF specimens from adult patients may also suggest a neuroinvasive feature of ZIKV, but the viral neurotropism has yet to be demonstrated in the adult nervous system. ZIKV strains isolated from humans or mosquitoes (during 1947–2014) have been classified by nucleotide sequence analysis into 2 genotypes, African and Asian [30, 48, 49]. ZIKV epidemic strains (isolated during 2007–2016) belong to the Asian genotype. The reemergent ZIKV Asian lineage that was introduced in French Polynesia in 2014 and later spread to Brazil and the Americas likely emerged from a common viral founder, which share s>98% nucleotide sequence homology and exhibits greater neurovirulence than the Asian lineage from Yap described in 2007 [10, 50]. However, genetic changes in the viral genome or amino acid substitutions on viral proteins cannot explain the switch in the neurovirulence of the Asian genotype, from the mild disease induced in the population of Yap to the dramatic surge in neurological complications associated with ZIKV in the Americas. Evaluation of a limited number of ZIKV genomes from microcephaly cases in Brazil showed nonshared amino acid mutations among them [9]. A genetic analysis of strains from patients with ZIKV-GBS has not been performed.
Although structural analysis of ZIKV by cryo–electron microscopy revealed its similarity to other flaviviruses, some insights on the structure of a ZIKV strain from French Polynesia revealed structural variations on the envelope (E) protein of ZIKV that are not coincident with the same structural region in DENV or other flaviviruses. Changes were observed at the E loop, adjacent to the fusion loop, a region that mediates virus entry and infectivity. An insertion of 5 amino acid residues in the loop surrounding the Asn154 glycosylation site changed the carbohydrate density and conformation in ZIKV E protein relative to DENV and other flaviviruses [51]. This suggest that differences in this region may be important for transmission and disease. Interestingly, ZIKV diverges from other flaviviruses in the precursor membrane protein (prM), E protein, and nonstructural protein 1 (NS1), all key players for viral infectivity or pathogenesis. Mutations in the genes encoding NS1, NS5, and NS4B occur between Asian and African ZIKV strains, which may be important in immune evasion and pathogenesis [52, 53]. However, it remains unknown whether there are viral structural determinants of neurological disease or neuroinvasiveness.
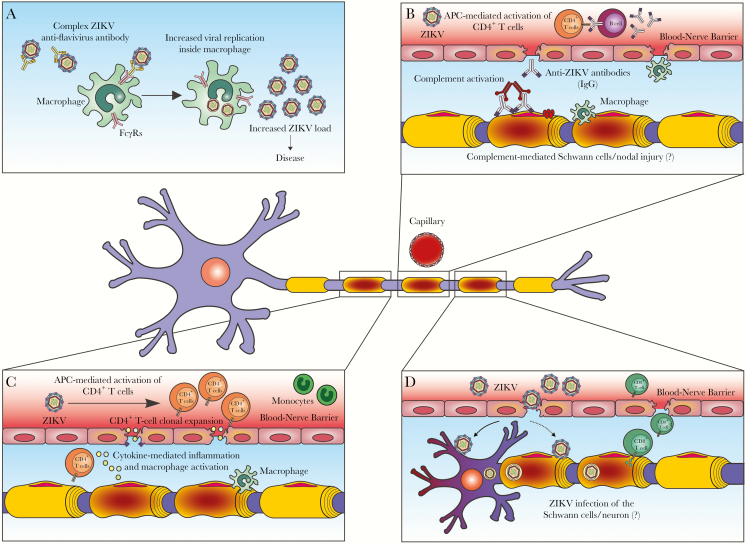
Important for neuropathogenesis is the possibility that, as for other flavivirus infections (eg, DENV-heterotypic infection), preexistent cross-reactive flavivirus antibodies may prompt disease-enhancing mechanisms during ZIKV infection via antibody-dependent enhancement (ADE) of infection. In ADE, subneutralizing levels of immunoglobulin G antibodies against E protein or the E protein–prM heterodimer on the virus surface elicited by prior flavivirus exposure fail to neutralize a new infection, and, instead, these virus-antibody complexes are endocytosed by cells expressing Fc receptors (FcγR), thereby boosting viral replication. This mechanism appears to be a factor leading to immune dysregulation associated with disease severity in DENV infection [54]. Stettler et al demonstrated in vitro enhancement of ZIKV and DENV infection by human cross-reactive heterologous antibodies against epitopes on the I/II domains of ZIKV E protein [55]. DENV-specific monoclonal antibody against a flavivirus–cross-reactive epitope in the E protein or low levels of DENV-specific antibodies in human plasma enable the in vitro infection of nonpermissive cells by ZIKV via ADE [56]. Therefore, it is possible that previous DENV immunity may pose a risk for neurological disease upon exposure to ZIKV through ADE (Figure 2A). However, ADE infection was not demonstrated to occur in 2 nonhuman in vivo models of ZIKV infection [57, 58].
Figure 2.
Speculated mechanisms for Zika virus (ZIKV)–associated neurological disease. A, Antibody-dependent enhancement (ADE) of infection. Anti-envelope (E) or anti-premembrane (prM) immunoglobulin G (IgG) antibodies elicited by previous flavivirus infection fail to neutralize ZIKV but instead enhance the capture of these complexes by cells expressing FcγR (eg, macrophages), which ultimately increases the ZIKV load, promoting disease. B, Anti-ZIKV IgG antibodies attach to antigens in the peripheral nerves (ie, cross-reactivity), which activates the complement system and triggers local inflammation driven by macrophages. C, Antigen-presenting cells (APCs) present ZIKV-derived peptides on class II major histocompatibility complex (MHC) molecules to naive CD4+ T lymphocytes, driving activation of these cells and inflammation. Cytokines and inflammatory cells are attracted to the peripheral nervous system, mediating peripheral nerve injury. D, ZIKV infects Schwann cells/neurons and causes cytopathic effects; viral infection activates CD8+ T lymphocytes, causing cytotoxicity and peripheral nerve injury.
In the case of ZIKV-GBS, the immune-mediated injury hypothesis seems the most plausible. On one side, it has been suggested that anti-ZIKV antibodies with cross-reactive activity against unknown peripheral nervous system antigens may induce damage through molecular mimicry (Figure 2B). This is a well-known mechanism for antiganglioside-mediated GBS, triggered by C. jejuni infection, which phenotypically manifests as AMAN [59]. However, for the demyelinating variant, which is the most frequent subtype in ZIKV-GBS, the main target antigens are still unknown. Clinically, the role of the humoral factors in ZIKV-GBS is also supported by the reported beneficial effect of the intravenous immunoglobulin and/or plasmapheresis. On the other hand, cellular immunity is likely involved, as previously demonstrated in human pathological studies of GBS and idiopathic polyneuritis [60, 61]. Based on what is known for GBS not associated with ZIKV, we speculate that, in ZIKV-GBS, particularly the AIDP variant, the affected nerves might present cellular-mediated inflammation and segmental demyelination induced by complement and macrophage activation (Figure 2C). Although still unclear, a direct viral pathogenic effect on Schwann cells or axonal structures in the PNS is possible according to the parainfectious profile of ZIKV-GBS (Figure 2D). ZIKV infection has been demonstrated in dorsal root ganglion cells in animal models, but no evidence of a direct viral role is available in humans [62].
Alternatively, the emergence of neurological complications associated with ZIKV infection may be explained by intrinsic host factors (eg, genetic susceptibility) or the magnitude of the outbreaks [63]. Low-risk neurological events such as GBS may be noticed in epidemics with large number of cases, such as those in French Polynesia, Brazil, and Colombia (>30.000–>1.000.000), but may go unrecognized when the number of cases is small, as might have occurred in Southeast Asia before 2007.
CONCLUSION
The recent emergence of ZIKV in the Pacific Islands and the Americas has revealed a new and unexpected spectrum of ZIKV-associated nervous system disease in the adult human population. Although a causal relationship has not been established, the demonstration of ZIKV infection in GBS cases is the most striking biological evidence of the association between these 2 conditions. Although the neuropathogenesis of ZIKV-GBS and other neurological syndromes is unclear, immune-mediated inflammation of the neural tissue is the leading hypothesis. Further investigations are crucial to understand the factors involved in the heavy burden of neurological disease in the Americas and to assess the global risk and impact of ZIKV. Health authorities should be aware of the potential neurological complications derived from ZIKV infection in regions with transmission of the virus.
Notes
Acknowledgments. We thank Eliza Gordon-Lipkin for the valuable comments, and members of the Neuroviruses Emerging in the Americas Study (NEAS) for their support.
Financial support. This work was supported by the Bart McLean Fund for Neuroimmunology Research and the Johns Hopkins Project Restore. The Neuroviruses Emerging in the Americas Study is supported in part by the European Union Zika Plan.
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Knipe DM. Fields virology. Vol 6 Alphen aan den Rijn, Netherlands: Wolters Kluwer Health, 2013. [Google Scholar]
- 2. Vorou R. Zika virus, vectors, reservoirs, amplifying hosts, and their potential to spread worldwide: what we know and what we should investigate urgently. Int J Infect Dis 2016; 48:85–90. [DOI] [PubMed] [Google Scholar]
- 3. Dick GW, Kitchen SF, Hadow AJ. Zika virus, I: isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:11. [DOI] [PubMed] [Google Scholar]
- 4. Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954; 48:139–45. [DOI] [PubMed] [Google Scholar]
- 5. Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956; 50:442–8. [PubMed] [Google Scholar]
- 6. Duffy MR, Chen TH, Hancock WT et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 7. Cao-Lormeau VM, Roche C, Teissier A et al. . Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watrin L, Ghawché F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barré Syndrome (42 Cases) occurring during a Zika virus outbreak in French Polynesia. Medicine (Baltimore) 2016; 95:e3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faria NR, Azevedo RDSDS, Kraemer MUG et al. . Zika virus in the Americas: early epidemiological and genetic findings. Science 2016; 352:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faria NR, Quick J, Claro IM et al. . Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017; 546:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lessler J, Chaisson LH, Kucirka LM et al. . Assessing the global threat from Zika virus. Science 2016; 353:aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. PAHO/WHO. Epidemiological update: neurological syndrome, congenital anomalies and Zika virus infection 17 January 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32879&lang=en. Accessed 3 July 2017.
- 13. WHO. Guillain-Barré syndrome—Brazil http://www.who.int/csr/don/8-february-2016-gbs-brazil/en/. Accessed 3 July 2017.
- 14. WHO. Guillain-Barré syndrome—El Salvador http://www.who.int/csr/don/21-january-2016-gbs-el-salvador/en/. Accessed 3 July 2017.
- 15. WHO. Guillain-Barré syndrome—Colombia and Venezuela http://www.who.int/csr/don/12-february-2016-gbs-colombia-venezuela/en/. Accessed 3 July 2017.
- 16. Dos Santos T, Rodriguez A, Almiron M et al. . Zika virus and the Guillain-Barré syndrome - case series from seven countries. N Engl J Med 2016; 375:1598–601. [DOI] [PubMed] [Google Scholar]
- 17. Colombian National Institute of Health. Boletin Epidemiologico Semanal Available at: http://www.ins.gov.co/boletin-epidemiologico/Paginas/default.aspx. Accessed 1 July 2017.
- 18. WHO. Situation report: Zika virus, microcephaly, Guillain-Barré syndrome http://apps.who.int/iris/bitstream/10665/254714/1/zikasitrep10Mar17-eng.pdf?ua=1. Accessed 14 June 2017.
- 19. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol 1990; 27:S21–4. [DOI] [PubMed] [Google Scholar]
- 20. Cao-Lormeau VM, Blake A, Mons S et al. . Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016; 387:1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parra B, Lizarazo J, Jiménez-Arango JA et al. . Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med 2016; 375:1513–23. [DOI] [PubMed] [Google Scholar]
- 22. Dirlikov E, Major CG, Mayshack M et al. . Guillain-Barré syndrome during ongoing Zika virus transmission - Puerto Rico, January 1-July 31, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:910–4. [DOI] [PubMed] [Google Scholar]
- 23. Arias A, Torres-Tobar L, Hernandez G et al. . Guillain-Barre syndrome in patients with a recent history of Zika in Cucuta, Colombia: a descriptive case series of 19 patients from December 2015 to March 2016. J Crit Care 2017; 37:19–23. [DOI] [PubMed] [Google Scholar]
- 24. da Silva IRF, Frontera JA, Bispo de Filippis AM, Nascimento O; Group R-G-ZR Neurologic complications associated with the Zika virus in Brazilian Adults. JAMA Neurol 2017; doi: 10.1001/jamaneurol.2017.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anaya JM, Rodriguez Y, Monsalve DM,. et al. A comprehensive analysis and immunobiology of autoimmune neurological syndromes during the Zika virus outbreak in Cucuta, Colombia. J Autoimmun 2017; 77:123–38. [DOI] [PubMed] [Google Scholar]
- 26. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet 2016; 388:717–27. [DOI] [PubMed] [Google Scholar]
- 27. van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 2014; 10:469–82. [DOI] [PubMed] [Google Scholar]
- 28. Hadden RD, Cornblath DR, Hughes RA et al. . Electrophysiological classification of Guillain-Barré syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Ann Neurol 1998; 44:780–8. [DOI] [PubMed] [Google Scholar]
- 29. Uncini A, Shahrizaila N, Kuwabara S. Zika virus infection and Guillain-Barré syndrome: a review focused on clinical and electrophysiological subtypes. J Neurol Neurosurg Psychiatry 2017; 88:266–71. [DOI] [PubMed] [Google Scholar]
- 30. Lanciotti RS, Kosoy OL, Laven JJ et al. . Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sebastián UU, Ricardo AVA, Alvarez BC et al. ; LACCTIN group Zika virus-induced neurological critical illness in Latin America: Severe Guillain-Barre Syndrome and encephalitis. J Crit Care 2017; 42:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rozé B, Najioullah F, Fergé JL et al. ; GBS Zika Working Group Zika virus detection in urine from patients with Guillain-Barré syndrome on Martinique, January 2016. Euro Surveill 2016; 21:30154. [DOI] [PubMed] [Google Scholar]
- 33. Oehler E, Watrin L, Larre P et al. . Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill 2014; 19:pii: 20720. [DOI] [PubMed] [Google Scholar]
- 34. Brasil P, Sequeira PC, Freitas AD et al. . Guillain-Barré syndrome associated with Zika virus infection. Lancet 2016; 387:1482. [DOI] [PubMed] [Google Scholar]
- 35. Fontes CA, Dos Santos AA, Marchiori E. Magnetic resonance imaging findings in Guillain-Barré syndrome caused by Zika virus infection. Neuroradiology 2016; 58:837–8. [DOI] [PubMed] [Google Scholar]
- 36. Kassavetis P, Joseph JM, Francois R, Perloff MD, Berkowitz AL. Zika virus-associated Guillain-Barré syndrome variant in Haiti. Neurology 2016; 87:336–7. [DOI] [PubMed] [Google Scholar]
- 37. van den Berg B, van den Beukel JC, Alsma J et al. . Guillain-Barré syndrome following infection with the Zika virus. Ned Tijdschr Geneeskd 2016; 160:D155. [PubMed] [Google Scholar]
- 38. Reyna-Villasmil E, López-Sánchez G, Santos-Bolívar J. Guillain-Barré syndrome due to Zika virus during pregnancy. Med Clin (Barc) 2016; 146:331–2. [DOI] [PubMed] [Google Scholar]
- 39. Medina MT, England JD, Lorenzana I et al. . Zika virus associated with sensory polyneuropathy. J Neurol Sci 2016; 369:271–2. [DOI] [PubMed] [Google Scholar]
- 40. Siu R, Bukhari W, Todd A, Gunn W, Huang QS, Timmings P. Acute Zika infection with concurrent onset of Guillain-Barré Syndrome. Neurology 2016; 87:1623–4. [DOI] [PubMed] [Google Scholar]
- 41. Nascimento OJM, Frontera JA, Amitrano DA, Bispo de Filippis AM, Da Silva IRF; RIO-GBS-ZIKV Research Group Zika virus infection-associated acute transient polyneuritis. Neurology 2017; 88:2330–2. [DOI] [PubMed] [Google Scholar]
- 42. Mécharles S, Herrmann C, Poullain P et al. . Acute myelitis due to Zika virus infection. Lancet 2016; 387:1481. [DOI] [PubMed] [Google Scholar]
- 43. Carteaux G, Maquart M, Bedet A et al. . Zika virus associated with Meningoencephalitis. N Engl J Med 2016; 374:1595–6. [DOI] [PubMed] [Google Scholar]
- 44. Soares CN, Brasil P, Carrera RM et al. . Fatal encephalitis associated with Zika virus infection in an adult. J Clin Virol 2016; 83:63–5. [DOI] [PubMed] [Google Scholar]
- 45. Roze B, Najioullah F, Signate A et al. . Zika virus detection in cerebrospinal fluid from two patients with encephalopathy, Martinique, February 2016. Euro Surveill 2016; 21:doi: 10.2807/1560-7917.ES.2016.21.16.30205. [DOI] [PubMed] [Google Scholar]
- 46. Galliez RM, Spitz M, Rafful PP et al. . Zika virus causing encephalomyelitis associated with immunoactivation. Open Forum Infect Dis 2016; 3:ofw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roth W, Tyshkov C, Thakur K, Vargas W. Encephalomyelitis following definitive Zika virus infection. Neurol Neuroimmunol Neuroinflamm 2017; 4:e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haddow AD, Schuh AJ, Yasuda CY et al. . Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6:e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faye O, Freire CC, Iamarino A et al. . Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014; 8:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Metsky HC, Matranga CB, Wohl S et al. . Zika virus evolution and spread in the Americas. Nature 2017; 546:411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dai L, Song J, Lu X et al. . Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 2016; 19:696–704. [DOI] [PubMed] [Google Scholar]
- 52. Cox BD, Stanton RA, Schinazi RF. Predicting Zika virus structural biology: challenges and opportunities for intervention. Antivir Chem Chemother 2015; 24:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sironi M, Forni D, Clerici M, Cagliani R. Nonstructural proteins are preferential positive selection targets in Zika virus and related flaviviruses. PLoS Negl Trop Dis 2016; 10:e0004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Halstead SB. Dengue antibody-dependent enhancement: knowns and unknowns. Microbiol Spectr 2014; 2:doi: 10.1128/microbiolspec.AID-0022-2014. [DOI] [PubMed] [Google Scholar]
- 55. Stettler K, Beltramello M, Espinosa DA et al. . Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016; 353:823–6. [DOI] [PubMed] [Google Scholar]
- 56. Dejnirattisai W, Supasa P, Wongwiwat W et al. . Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 2016; 17:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pantoja P, Pérez-Guzmán EX, Rodríguez IV et al. . Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 2017; 8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McCracken MK, Gromowski GD, Friberg HL et al. . Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog 2017; 13:e1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsunoda I, Omura S, Sato F et al. . Neuropathogenesis of Zika virus infection: potential roles of antibody-mediated pathology. Acta Med Kinki Univ 2016; 41:37–52. [PMC free article] [PubMed] [Google Scholar]
- 60. Asbury AK, Arnason BG, Adams RD. The inflammatory lesion in idiopathic polyneuritis. Its role in pathogenesis. Medicine (Baltimore) 1969; 48:173–215. [DOI] [PubMed] [Google Scholar]
- 61. Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet 2005; 366:1653–66. [DOI] [PubMed] [Google Scholar]
- 62. Oh Y, Zhang F, Wang Y et al. . Zika virus directly infects peripheral neurons and induces cell death. Nat Neurosci 2017; 20:1209–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev 2016; 29:487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]