Summary
Age-related differences in influenza B lineage infection were assessed by the community-based Canadian Sentinel Practitioner Surveillance Network between 2010–2011 and 2015–2016. Influenza B(Victoria) cases were on average 20 years younger than B(Yamagata) cases, with the latter showing a bimodal age distribution.
Keywords: influenza B virus, influenza B lineage, age, risk, birth cohort effects
Abstract
Age-related differences in influenza B lineage detection were explored in the community-based Canadian Sentinel Practitioner Surveillance Network (SPSN) from 2010–2011 to 2015–2016. Whereas >80% of B(Victoria) cases were <40 years old, B(Yamagata) cases showed a bimodal age distribution with 27% who were <20 years old and 61% who were 30–64 years old, but with a notable gap in cases between 20 and 29 years old (4%). Overall, the median age was 20 years lower for B(Victoria) vs B(Yamagata) cases (20 vs 40 years; P < .01). Additional phylodynamic and immuno-epidemiological research is needed to understand age-related variation in influenza B risk by lineage, with potential implications for prevention and control across the lifespan.
Influenza B viruses exist as 2 antigenically distinct lineages, represented by B/Victoria/2/1987 [“B(Victoria)”] and B/Yamagata/16/1988 [“B(Yamagata)”]. B(Yamagata) virus was first identified as a new antigenic variant in 1988 and the early winter of 1988–1989, associated with sporadic outbreaks in Asia [1]. The B(Yamagata) variant was not detected in North America during the 1988–1989 season, but became the predominant type B influenza virus circulating globally for most of the 1990s [1–3]. During that period, B(Victoria)–lineage viruses were detected almost exclusively in eastern Asia, but reemerged in North America in 2001 and have since co-circulated with B(Yamagata) viruses [4].
Trivalent influenza vaccines (TIVs) include 2 influenza A antigens but only a single influenza B antigen—representative since the 2001–2002 season of either the B(Victoria) or B(Yamagata) lineage—depending on which lineage/strain is predicted to contribute most to the annual influenza epidemic. Quadrivalent influenza vaccines (QIVs) containing both B(Victoria)– and B(Yamagata)–lineage antigens have been available in Canada since the 2014–2015 season but have represented <15% of the national influenza vaccine allotment up to and including the 2015–2016 season. Canada’s National Advisory Committee on Immunization recommends influenza vaccine for everyone ≥6 months of age but also that immunization programs focus on individuals at high risk for influenza-related complications [5]. Accordingly, about one-third of the Canadian population overall receives influenza vaccine annually, but rates are highest in older adults, particularly elderly people ≥65 years old, and those with chronic comorbidities [6].
B(Victoria) and B(Yamagata) lineages are considered genetically and antigenically distinct based on their surface hemagglutinin (HA) antigens [1, 4], although some cross-lineage interaction, including cross-protection, has been suggested [7–9]. Influenza B viruses evolve at a slower rate compared to influenza A [10, 11], but have adopted other evolutionary mechanisms to evade the human immune system, including nucleotide insertion/deletion mutations and frequent inter- and intralineage re-assortment events with the co-circulation of multiple lineages and antigenic variants [10–12].
These evolutionary mechanisms combined with variability in historic prime-boost (infection or immunization) exposures over time have likely created a complex immuno-epidemiological patchwork in the population, potentially corresponding with variable age-related risk for influenza B illness. Drawing on existing virological and epidemiological data sets of the community-based Canadian Sentinel Practitioner Surveillance Network (SPSN) [13–18], we explored age-related differences in influenza B infection by lineage during the 2010–2011 to 2015–2016 seasons.
METHODS
As detailed in previously published analyses [13–18], respiratory specimens were systematically collected from consenting outpatients presenting to the Canadian SPSN alongside epidemiological information, including patient age at time of specimen collection and self-reported vaccine status for current and prior season(s). All patients included in the analysis met a standardized case definition for influenza-like illness (ILI): fever and cough and at least 1 other symptom (arthralgia, myalgia, sore throat, or prostration). Fever was not a requirement for elderly patients after the 2010–2011 season. Vaccination status was defined as per usual by the receipt of current season’s influenza vaccine ≥2 weeks before ILI onset. Ethics approval was obtained from institutional review boards in each participating province.
Specimens were tested for influenza B viruses by reverse-transcription polymerase chain reaction (RT-PCR) assay according to standard protocols at public health reference laboratories in each participating province (British Columbia, Alberta, Ontario, and Quebec) [13–18]. During the first season of this analysis (2010–2011), lineage-level characterization was based on hemagglutination inhibition (HI) assay performed at Canada’s National Microbiology Laboratory (NML) alone. Thereafter, influenza B lineage determination was based on a combination of HI assay at the NML or hemagglutinin gene sequencing and/or an influenza B lineage–specific RT-PCR assay conducted at provincial public health reference laboratories.
Analyses were restricted to specimens collected from patients presenting within 7 days of ILI onset during the typical period for seasonal influenza circulation in the northern hemisphere (1 November to 30 April). Patients with unknown age or indeterminate laboratory results were excluded. Median age (in years) was derived overall and by influenza B lineage and compared across lineages and seasons using the nonparametric Wilcoxon rank-sum test. The proportion of patients by age group was compared across lineages and seasons using the χ2 test. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Influenza B viruses comprised about one-third of all influenza detections across included study seasons (range, 25%−51% by season). The influenza B detection (ie, test positivity) rate among ILI specimens ranged from 11% in 2010–2011 and 2012–2013 to 18% in 2015–2016 (Supplementary Table 1).
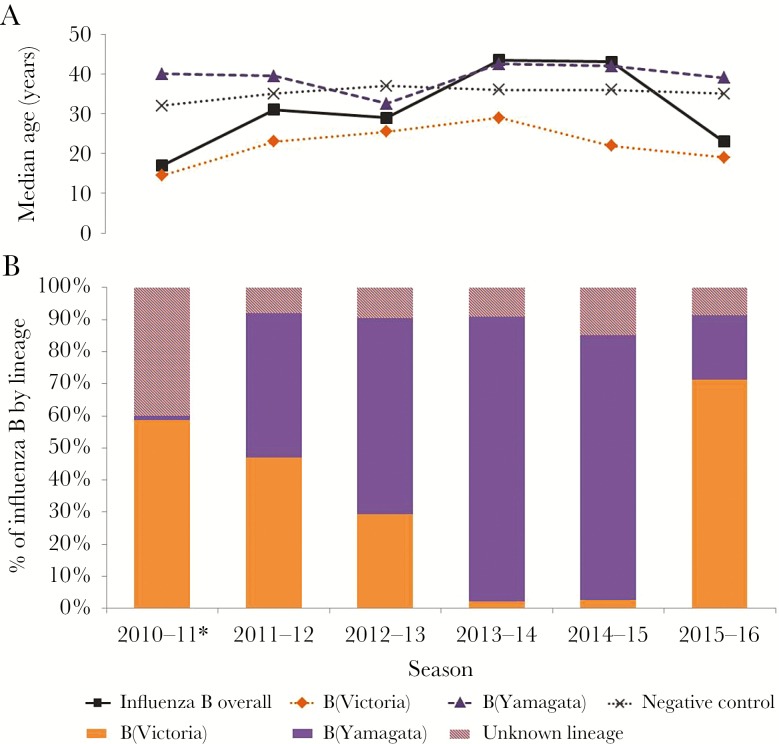
Influenza B(Victoria)–lineage viruses were dominant in 2010–2011 (98% of influenza B detections with known lineage) and 2015–2016 (78%), whereas B(Yamagata)–lineage viruses were dominant in 2013–2014 (98%) and 2014–2015 (97%) (Figure 1). Co-circulation of both lineages occurred in 2011–2012 (51% Victoria vs 49% Yamagata) and 2012–2013 with B(Yamagata) slightly more dominant in the latter mixed season (32% Victoria vs 68% Yamagata).
Figure 1.
Median age by influenza B lineage (A) and percentage distribution of influenza B cases by lineage and season (B), Canadian Sentinel Practitioner Surveillance Network, 2010–2011 to 2015–2016. *Sequencing and lineage-specific reverse-transcription polymerase chain reaction assay were not conducted on influenza B detections during the 2010–2011 season; lineage-level characterization was based only upon hemagglutination inhibition assay among isolates submitted to Canada’s National Microbiology Laboratory, accounting for a greater proportion of viruses with unknown lineage that season.
Overall, the median age of influenza B cases was 30 years (compared to 35 years among test-negative controls; P < .01). However, the median age of influenza B cases varied significantly by season and lineage—lowest during B(Victoria)–dominant seasons in 2010–2011 (17 years) and 2015–2016 (23 years) but highest during B(Yamagata)–dominant seasons in 2013–2014 (43.5 years) and 2014–2015 (43 years) (P < .01) (Figure 1). Across seasons combined (2010–2011 to 2015–2016), the median age of B(Victoria) cases was 20 years lower than the median age of B(Yamagata) cases (20 vs 40 years, respectively; P < .01).
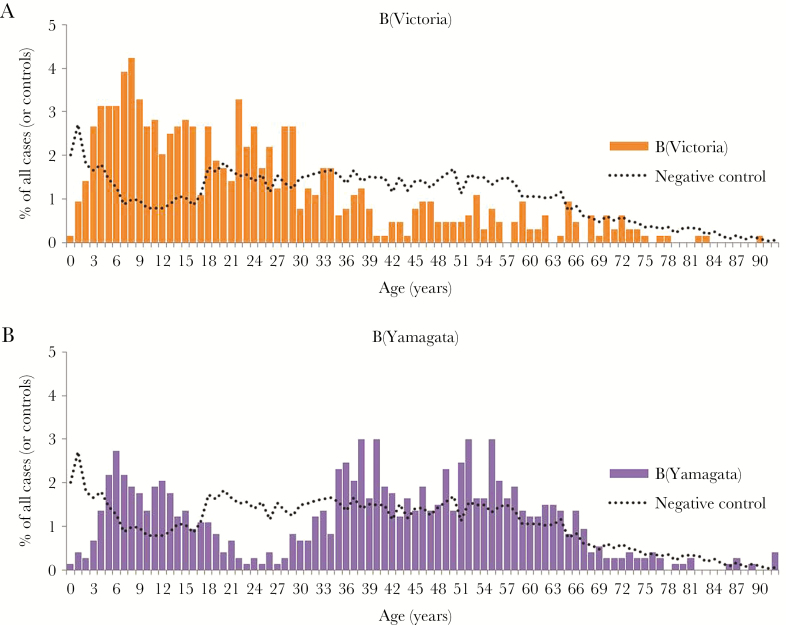
As shown by the proportionate distribution of cases by single year of age, B(Victoria)–lineage infections were skewed toward younger individuals with the majority (>80%) of cases <40 years old, including half (50%) who were children <20 years old (Figure 2). Conversely, B(Yamagata)–lineage infections displayed a bimodal age distribution with about one-quarter (27%) of cases <20 years old and a substantial proportion (61%) who were 30–64 years old, but with a notable gap in the distribution of cases between 20 and 29 years of age (4%) (Figure 2). The highest B(Victoria) detection rates were in children 5–19 years old followed by those 20–29 years old (Supplementary Table 2). Children 5–19 years old also had among the highest rates of detection of B(Yamagata), but these rates were more comparable to adults 30–64 years old (Supplementary Table 2).
Figure 2.
Percentage distribution of influenza B(Victoria) cases (A) and influenza B(Yamagata) cases (B) and test-negative controls by single-year age group, Canadian Sentinel Practitioner Surveillance Network (SPSN), 2010–2011 to 2015–2016. The percentage of all cases of the specified influenza B lineage belonging to a given age (in years) is displayed as a bar graph; the same information for test-negative controls is superimposed as a dotted line for comparison purposes to indicate the sampling distribution by age in the overall SPSN sample.
With stratification, a similar 20-year difference in median age for B(Victoria) vs B(Yamagata) cases was observed for unvaccinated (18 years vs 38 years, respectively; P < .01) or vaccinated (29 years vs 52 years, respectively; P < .01) participants. Patterns evident in the lineage-specific age distributions overall were driven by the preponderance of unvaccinated cases, but the notable paucity of B(Yamagata) cases among participants 20–29 years old was also evident among vaccinated cases (Supplementary Figure 1). The reduced sample size included in the distribution of vaccinated cases by age otherwise requires cautious interpretation (Supplementary Table 3). Among participants vaccinated in the current season (>80% of whom were also vaccinated the prior season), a greater proportion of both B(Victoria) and B(Yamagata) cases were older adults, notably elderly people ≥65 years old. This skew was also evident among vaccinated test-negative controls, consistent with vaccination program recommendations in Canada that preferentially target adults ≥65 years old and those with chronic comorbidities—resulting in higher influenza vaccine coverage rates in these older groups more generally [5, 6].
DISCUSSION
In this community-based study of patients presenting with ILI to an outpatient sentinel practitioner, we observed significant age-related differences in influenza B infection by lineage during the 2010–2011 to 2015–2016 seasons. Patients infected with B(Victoria)–lineage viruses were on average 20 years younger than those infected with B(Yamagata)–lineage viruses. While both lineages affected pediatric patients, with detection rates notably highest in school-aged children 5–19 years old, B(Yamagata) infections showed a bimodal age distribution skewed toward a greater number of cases in adults 30–64 years old.
Epidemiological studies of influenza B conducted elsewhere have also consistently found a younger age of infection associated with B(Victoria)–lineage compared with B(Yamagata)–lineage viruses, including studies in both the northern and southern hemispheres [10, 19–23]. Findings of higher detection rates associated with B(Victoria) in pediatric age groups, as reported in the current study, suggests that QIV products containing an additional B(Victoria) antigen might be preferentially targeted toward children during seasons where TIV would otherwise contain only the B(Yamagata) antigen. Conversely, both children and adults might benefit from TIV products containing B(Victoria) antigen or QIV with an additional B(Yamagata) antigen. Ultimately, however, given cross-lineage immunological interactions, including evidence of substantial cross-protection [7–9], the incremental value of QIV vs TIV may be limited and will depend upon other factors not assessed here.
Since the emergence of B(Yamagata) viruses in the mid-1980s, the varying contributions of both lineages (predominately Yamagata during the 1990s, followed by co-circulation of both lineages after 2001–2002 in North America) have likely created complex immuno-epidemiological landscapes across birth cohorts. In our data, the paucity of B(Yamagata) infections in young adults 20–29 years old—and correspondingly higher B(Victoria) detection rates—is consistent with the birth cohort that was likely primed during the 1990s with a related B(Yamagata)–like virus, which may have afforded some degree of preexisting cross-protection to contemporary B(Yamagata) strains [1–3]. The lower median age and smaller number of infections associated with B(Victoria)–lineage viruses in adults 30–64 years old may also be explained by birth (immunological) cohort effects reflecting protection conferred by more distant childhood exposure to a common B(Victoria)–like ancestral virus. Social mixing patterns alone are unlikely to explain differential age-related risk for B(Victoria) given the pattern of B(Yamagata) detection prominently involving the adult (30–64 years old) age group.
Similar immuno-epidemiological rationale has been hypothesized to explain the lower risk of infection among older adults during the 2009 influenza A(H1N1)pdm09 pandemic owing to childhood exposures to related ancestral A(H1N1) viruses decades earlier [24]. According to the underlying theory, individuals will preferentially recall cross-reactive, memory antibodies toward epitopes shared with ancestral viruses to which they were originally exposed in childhood, including potentially positive or negative effects on protection against contemporary viruses. Whether original childhood priming to a common B(Victoria)–like ancestral virus may explain lower risk of B(Victoria) illness in adults 30–64 years old—or conversely contribute to the bimodal wave of heightened B(Yamagata) risk in that age band—remains speculative but warrants further evaluation.
Sequence data for historical influenza B viruses are limited to inform priming epochs and potential birth (immunological) cohort effects over space or time. Between approximately 1973 and 1979, ancestral influenza B viruses began to diverge into 2 antigenically distinct lineages [1, 3]. However, ancestral influenza B viruses share properties of both contemporary B(Victoria)–like and B(Yamagata)–like lineages, whose relative influence is difficult to disentangle [1–3, 11]. Both B(Victoria) and B(Yamagata) lineages also undergo frequent reassortment events, including within and across lineages, as well as insertion–deletion mutations that further complicate our understanding of the effects of historical influenza B exposures on contemporary influenza B risk profiles by age [4, 10–12].
An alternative explanation for the observed age-related differences by influenza B lineage is that B(Victoria) viruses may induce a broader immune response and confer better protection in older age groups [10]. In their report, Vijaykrishna et al demonstrate that B(Victoria) viruses undergo stronger positive selection and have a higher effective reproductive number (Re) than B(Yamagata) viruses [10]. Like influenza A(H3N2) subtype viruses, B(Victoria) viruses may experience selective “bottlenecks” between seasons, followed by serial replacement with a new dominant antigenic variant [10]. In contrast, B(Yamagata) viruses, which have less seasonal fluctuation in their relative genetic diversity, tend to have slower and shorter transmission chains compared to B(Victoria), with multiple genetic clades co-circulating for longer periods of time [10]. This phenomenon can be seen in the Canadian sentinel surveillance data for the 2010–2011 to 2015–2016 seasons, with a single B(Victoria) strain, represented by B/Brisbane/60/2008 (clade 1A), dominant in certain seasons, but 2 genetic clades of B(Yamagata), represented by B/Massachusetts/2/2012 (clade 2) and B/Wisconsin/1/2010 (clade 3), co-circulating in varying proportions within the same season or across consecutive seasons (Supplementary Table 1).
In conclusion, our systematically collected community-based surveillance data show a younger profile for B(Victoria) compared to B(Yamagata) cases, with the latter instead showing a bimodal age distribution, with peaks in pediatric and adult age groups. Epidemiological differences between cases of B(Victoria) and B(Yamagata) likely reflect a combination of birth (immunological) cohort effects defined by variability in historic influenza B prime-boost epochs, as well as differences in influenza B phylodynamics, by lineage. Further research is needed to identify the immuno-epidemiological determinants of influenza B risk by lineage and age, with potential implications for prevention and control across the lifespan.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We gratefully acknowledge the contribution of those at the sentinel sites whose regular submission of specimens and data provided the basis of our analyses. We acknowledge the coordination and technical support provided by epidemiologic and laboratory staff in all participating provinces. We thank the following for network coordination and data entry activities in each province: Lisan Kwindt for national database management and sentinel network coordination activities in British Columbia; Elaine Douglas, Kinza Rizvi, Sandra Berzins, Kasim Qureshi, and Virginia Goetz for TARRANT in Alberta; Romy Olsha for Public Health Ontario; and Sophie Auger and Isabelle Petillot for the Institut national de santé publique du Québec. We thank those who provided laboratory support at the British Columbia Centre for Disease Control Public Health Laboratory, the Alberta Provincial Laboratory for Public Health (ProvLab), the Public Health Ontario Laboratory, the Laboratoire de santé publique du Québec, and the National Microbiology Laboratory in Manitoba.
Financial support. This work was supported by the Canadian Institutes of Health Research (grant number TPA-90193); the British Columbia Centre for Disease Control; Alberta Health and Wellness; Public Health Ontario; Ministère de la santé et des services sociaux du Québec; l’Institut national de santé publique du Québec; and the Public Health Agency of Canada. S. S. was partially funded by the Canadian Institutes of Health Research (grant number TPA-90193) and by the Public Health Agency of Canada.
Potential conflicts of interest. G. D. S. has received grants unrelated to influenza from GSK and Pfizer and travel reimbursement to attend an ad hoc advisory board meeting of GSK also unrelated to influenza; he has provided paid expert testimony in a grievance against a vaccinate-or-mask healthcare worker influenza vaccination policy for the Ontario Nurse Association. J. G. has received a research grant from Pfizer Inc to conduct microbiological surveillance of Streptococcus pneumoniae. M. K. has received research grants from Roche, Merck, Siemens, Hologic, and Boerhinger Ingelheim for unrelated studies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990; 175:59–68. [DOI] [PubMed] [Google Scholar]
- 2. Nerome R, Hiromoto Y, Sugita S, et al. Evolutionary characteristics of influenza B virus since its first isolation in 1940: dynamic circulation of deletion and insertion mechanism. Arch Virol 1998; 143:1569–83. [DOI] [PubMed] [Google Scholar]
- 3. Rota PA, Hemphill ML, Whistler T, Regnery HL, Kendal AP. Antigenic and genetic characterization of the haemagglutinins of recent cocirculating strains of influenza B virus. J Gen Virol 1992; 73(pt 10):2737–42. [DOI] [PubMed] [Google Scholar]
- 4. Shaw MW, Xu X, Li Y, et al. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000–2001 and 2001–2002 seasons. Virology 2002; 303:1–8. [DOI] [PubMed] [Google Scholar]
- 5. National Advisory Committee on Immunization (NACI). Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2015–2016. Ottawa: Public Health Agency of Canada, 2015. [Google Scholar]
- 6. Public Health Agency of Canada. Influenza vaccine uptake: results from the 2015/16 national influenza immunization coverage survey in Canada. Ottawa: Government of Canada, 2017. https://www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-uptake-results-2015-16-national-influenza-immunization-coverage-survey.html. Accessed 28 July 2017. [Google Scholar]
- 7. Skowronski DM, Hamelin ME, Janjua NZ, et al. Cross-lineage influenza B and heterologous influenza A antibody responses in vaccinated mice: immunologic interactions and B/Yamagata dominance. PLoS One 2012; 7:e38929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skowronski DM, Hottes TS, De Serres G, et al. Influenza Β/Victoria antigen induces strong recall of Β/Yamagata but lower Β/Victoria response in children primed with two doses of Β/Yamagata. Pediatr Infect Dis J 2011; 30:833–9. [DOI] [PubMed] [Google Scholar]
- 9. Tricco AC, Chit A, Soobiah C, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 2013; 11:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vijaykrishna D, Holmes EC, Joseph U, et al. The contrasting phylodynamics of human influenza B viruses. Elife 2015; 4:e05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamashita M, Krystal M, Fitch WM, Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology 1988; 163:112–22. [DOI] [PubMed] [Google Scholar]
- 12. McCullers JA, Wang GC, He S, Webster RG. Reassortment and insertion-deletion are strategies for the evolution of influenza B viruses in nature. J Virol 1999; 73:7343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skowronski DM, Janjua NZ, De Serres G, et al. A sentinel platform to evaluate influenza vaccinate effectiveness and new variant circulation, Canada, 2010–2011 season. Clin Infect Dis 2012; 55:332–42. [DOI] [PubMed] [Google Scholar]
- 14. Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. [DOI] [PubMed] [Google Scholar]
- 15. Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Skowronski DM, Chambers C, Sabaiduc S, et al. Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine-virus relatedness and effectiveness during the 2013-2014 influenza season. J Infect Dis 2015; 212:726–39. [DOI] [PubMed] [Google Scholar]
- 17. Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014-2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skowronski DM, Chambers C, Sabaiduc S, et al. Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015-2016 season in Canada. J Infect Dis 2017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barr IG, Vijaykrishna D, Sullivan SG. Differential age susceptibility to influenza B/Victoria lineage viruses in the 2015 Australian influenza season. Euro Surveill 2016; 21(4). doi:10.2807/1560-7917.ES.2016.21.4.30118. [DOI] [PubMed] [Google Scholar]
- 20. Seleka M, Treurnicht FK, Tempia S, et al. Epidemiology of influenza B/Yamagata and B/Victoria lineages in South Africa, 2005-2014. PLoS One 2017; 12:e0177655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharabi S, Drori Y, Micheli M, et al. Epidemiological and virological characterization of influenza B virus infections. PLoS One 2016; 11:e0161195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Socan M, Prosenc K, Ucakar V, Berginc N. A comparison of the demographic and clinical characteristics of laboratory-confirmed influenza B Yamagata and Victoria lineage infection. J Clin Virol 2014; 61:156–60. [DOI] [PubMed] [Google Scholar]
- 23. Cowling BJ, Wu P, Lo JY, et al. Population-based hospitalization burden of lineage specific influenza B children in Hong Kong, 2004-2014. Clin Infect Dis, 6 April 2017 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skowronski DM, Hottes TS, McElhaney JE, et al. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J Infect Dis 2011; 203:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.