SUMMARY
The underlying mechanisms how self-renewing cells are controlled in regenerating tissues and cancer remain ambiguous. PAF (PCNA-associated factor) modulates DNA repair via PCNA. Also, PAF hyperactivates Wnt/β-catenin signaling independently of PCNA interaction. We found that PAF is expressed in intestinal stem and progenitor cells (ISCs and IPCs) and markedly upregulated during intestinal regeneration and tumorigenesis. Whereas PAF is dispensable for intestinal homeostasis, upon radiation injury, genetic ablation of PAF impairs intestinal regeneration along with the severe loss of ISCs and Myc expression. Mechanistically, PAF conditionally occupies and transactivates c-Myc promoter, which induces the expansion of ISCs/IPCs during intestinal regeneration. In mouse models, PAF knockout inhibits Apc inactivation-driven intestinal tumorigenesis with reduced tumor cell stemness and suppressed Wnt/β-catenin signaling activity, supported by transcriptome profiling. Collectively, our results unveil that PAF-Myc signaling axis is indispensable for intestinal regeneration and tumorigenesis by positively regulating self-renewing cells.
Keywords: PAF, Myc, intestinal regeneration, stem/progenitor cells, intestinal stem cells, colorectal cancer, cancer stem cells, Wnt signaling, β-catenin
eTOC Blurb
Controlling the proliferation of self-renewing cells is crucial for tissue homeostasis and regeneration. Kim et al. show that PAF (PCNA-associated factor), dispensable for intestinal homeostasis, is required to positively regulate the proliferation of self-renewing cells via Myc transactivation during intestinal regeneration and tumorigenesis.
INTRODUCTION
Stem cells play key roles in tissue homeostasis and regeneration by self-renewing and repopulating progenitor cells (Fuchs et al., 2004; Morrison and Spradling, 2008). In the small intestine, two major ISCs co-exist. Crypt base columnar cells (CBC) ISCs marked by the high Lgr5 expression are highly proliferative and essential for the intestinal homeostasis (Barker et al., 2007). The other ISCs located at position 4 (+4) and labeled by Hopx, Lrig1, Bmi1, and Tert are quiescent during intestinal homeostasis, whereas conditionally activated upon tissue damage. (Montgomery et al., 2011; Powell et al., 2012; Sangiorgi and Capecchi, 2008; Takeda et al., 2011). Accumulating evidence suggests that +4 ISCs function as a reservoir of ISCs during regeneration (Buczacki et al., 2013; Tian et al., 2011). Additionally, the committed progenitor cells (Dll1+, Alpi+, and Krt19+) located above the position +4 cells also dedifferentiate into ISCs for intestinal regeneration (Asfaha et al., 2015; Tetteh et al., 2016; Van Es et al., 2012), implying the involvement of the cell plasticity in rebuilding intestinal epithelium. Although the extensive lineage tracing studies have been used to identify ISCs or reservoir ISCs/IPCs populations, still the underlying mechanisms how these ISCs/IPCs cells are activated and expanded during regeneration remain elusive.
It was proposed that cancer stem cells (CSCs) are a subpopulation of tumor cells, which drives tumor growth by self-renewing and giving rise to the daughter cells (Nguyen et al., 2012). The identities of CSCs are still controversial, however, it is plausible that CSCs might be related to therapeutic resistance and tumor recurrence (Dean et al., 2005; Kreso and Dick, 2014). Expression of CD44, CD133, and Lgr5 have been suggested as a maker for stemness of colorectal cancer (CRC) cells (O’Brien et al., 2007; Ricci-Vitiani et al., 2007; Schepers et al., 2012; Zeilstra et al., 2008; Zhu et al., 2009). Nonetheless, how CSCs are maintained and expanded were not fully understood.
PAF (also known as p15/KIAA0101/NS5ATP9/OEACT-1) was initially identified as a proliferating cell nuclear antigen (PCNA)-interacting protein (Yu et al., 2001). PAF is implicated in both DNA repair and cell proliferation (Emanuele et al., 2011; Povlsen et al., 2012). PAF binds to PCNA sliding clamp and regulates DNA replication and repair (De Biasio et al., 2015). Importantly, PAF expression is significantly upregulated in many human cancers (Cheng et al., 2013; Hosokawa et al., 2007; Jain et al., 2011; Jung et al., 2013; Kais et al., 2011; Kato et al., 2012; Mizutani et al., 2005; Wang et al., 2016; Yu et al., 2001; Yuan et al., 2007). In pancreatic cancer cells, PAF overexpression is necessary for pancreatic cancer cell proliferation (Hosokawa et al., 2007). Additionally, PAF is associated with MAPK hyperactivation via transcriptional activation of the late endosomal/lysosomal adaptor, MAPK and mTOR activator 3 (LAMTOR3), which is involved in the initiation of pancreatic intraepithelial neoplasia (Jun et al., 2013). Moreover, PAF hyperactivates Wnt/β-catenin signaling in CRC cells as a co-factor of β-catenin/EZH2 transcriptional complex, resulting in the development of intestinal adenoma (Jung et al., 2013).
In this study, we sought to interrogate how stem cells are activated by radiation injury. Our unbiased gene expression screening identified that, among DNA repair-related genes, PAF expression is associated with controlling ISCs/IPCs. Further comprehensive and genetic approaches revealed that PAF-Myc signaling axis is indispensable for intestinal regeneration and tumorigenesis by positively controlling the expansion of stem cells.
RESULTS
Upregulation of PAF Expression upon Radiation Injury
To identify essential genes associated with DNA repair during tissue regeneration, we conducted qRT-PCR array for DNA repair gene collections from irradiation (IR) treated mouse small intestine (1 day post-injury [1 dpi], 10 Gy) (Figures 1A and 1B). Among differentially expressed 79 genes, PAF expression was highly upregulated by IR (7th ranked). Additionally, IR upregulated PAF expression in the mouse small intestine in a dose- and time-dependent manner (Figures 1C and 1D), which led us to hypothesize that PAF plays crucial roles in intestinal regeneration.
Figure 1. Upregulation of PAF Expression upon Radiation Injury.
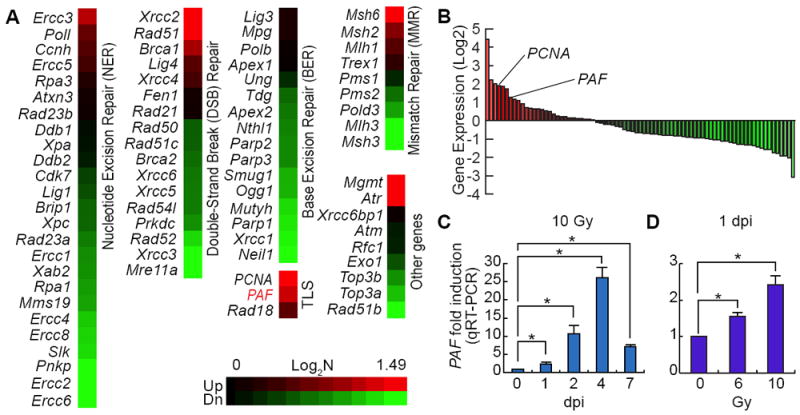
(A, B) Gene expression profiling of DNA repair genes upon radiation injury in mouse small intestine. After treatment of 10 Gy irradiation (1 day post-injury [1 dpi]), the whole small intestine samples were analyzed by qRT-PCR (N=3). PCNA is the fourth and PAF are the seventh upregulated genes among the 79 genes related to DNA repair.
(C) Time-dependent upregulation of PAF expression upon IR injury. At 0, 1, 2, 4, and 7 dpi, the small intestine samples were collected and analyzed by qRT-PCR (N=3).
(D) Dose-dependent upregulation of PAF expression upon injury. 6 and 10 Gy irradiation were used (1 dpi). Student’s t-test; error bars = S.E.M; asterisks: P< 0.05.
PAF Expression in Replenishing and Regenerating Intestinal Crypts
To elucidate the in vivo roles of PAF in intestinal regeneration, we generated PAF KO mouse model using clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 gene targeting system (Wang et al., 2013; Yang et al., 2013)(Figure S1). Of note, PAF KO mice are viable without any discernible phenotypes. To examine PAF expression in the crypt, we conducted immunohistochemistry (IHC) using two different PAF monoclonal antibodies (Figures 2A and S2B). We confirmed the specificity of PAF antibody using PAF KO mice as a negative control (Figures 2A and S2B). We located 5~10 cells of PAF positive (PAF+) cells existed in the one section of crypt (Figures 2A and 2B). PAF was expressed in 1~2 cells of CBC ISCs and 3~8 cells of transit-amplifying (TA) cells (Figures 2A and 2B), but not in the villi (Figure S2A). Most PAF+ cells belong to Ki67+ cells (17.71% of Ki67+ cells), a marker for cell proliferation (Figures 2C and 2E). Despite the additional role of PAF in DNA repair as a PCNA-interacting protein (Povlsen et al., 2012), only 23.01% of PAF+ cells were PCNA+ cells (Figures 2D and 2E). Furthermore, PAF KO mice did not display differences in the expression of PCNA in the crypts, compared to PAF wild-type (WT) mice (Figure 2F), implying the existence of PCNA-independent functions of PAF in the intestine.
Figure 2. PAF Expression in Normal and Regenerating Intestinal Crypts.
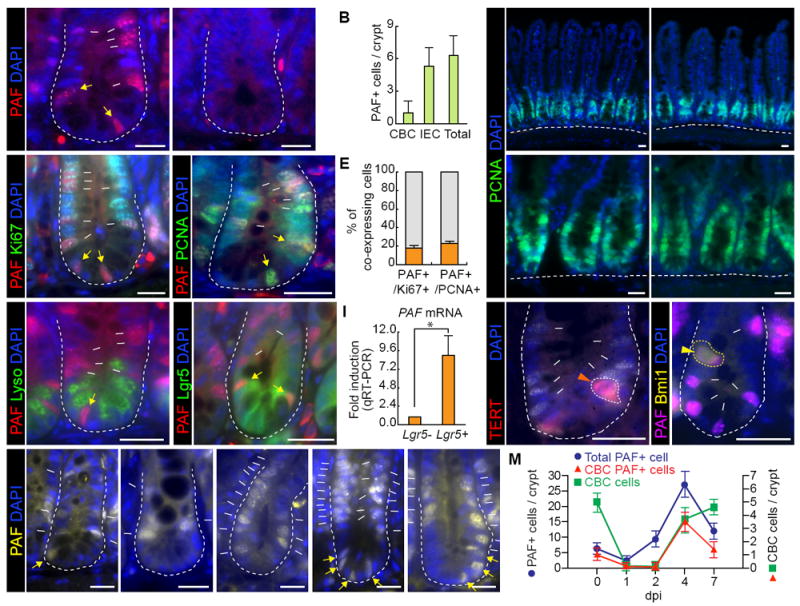
(A) PAF expression in the small intestine. PAF WT and KO mice were analyzed for immunofluorescent (IF) staining of PAF (arrows). Asterisks mark non-specific staining signals.
(B) Quantification of PAF+ cells in the small intestinal crypts.
(C) Co-immunostaining of mouse small intestine (PAF WT and KO) for PAF and Ki67. White arrows: PAF+:Ki67+ cells; yellow arrows: PAF+:Ki67+ CBC cells. Asterisks mark non-specific staining signals.
(D) Co-immunostaining of mouse small intestine (PAF WT and KO) for PAF and PCNA. White arrows: PAF+:PCNA+ cells; yellow arrows: PAF+:PCNA+ CBC cells; Asterisks mark non-specific staining signals.
(E) Quantification of PAF+ cells in K67+ or PCNA+ cell population. Of note, PAF+:Ki67- cells were rarely found (approximately 1/50 crypts).
(F) No effects of PAF KO on PCNA expression pattern. IF staining of the mouse small intestine for PCNA.
(G) No expression of PAF in the Paneth cells. Co-immunostaining of the small intestine for PAF and Lysozyme. White arrows: PAF+ cells; yellow arrows: PAF+ CBC cell.
(H) Co-expression of PAF and Lgr5 in the small intestine. Co-immunostaining of the small intestine of Lgr5-EGFP-CreERT2 mouse strain. White arrows: PAF+:Lgr5+ cells; yellow arrows: PAF+:Lgr5+ CBC cells. Asterisks indicate non-specific staining signals.
(I) PAF expression in Lgr5+ cells. FACS-isolated Lgr5+ cells were analyzed for qRT-PCR. Asterisk=P<0.05.
(J, K) PAF expression in TERT+ and Bmi1+ cells. Co-immunostaining of TERT-Tdtomato-CreERT2 or Bmi1-EGFP knock-in mouse intestine samples for PAF. Arrowheads: PAF+:TERT+ (J) and PAF+:Bmi1+ (K) cells; arrows: PAF+ cells. Asterisks mark non-specific staining signals.
(L, M) The increase of PAF+ cells upon radiation injury. Immunostaining of mouse intestine (0, 1, 2, 4, and 7 dpi; 10 Gy) for PAF (L). White arrows: PAF+ cells; yellow arrows: PAF+ CBC cells. Quantification of PAF+ cells in the regenerating crypts (M).
The representative images were shown from at least three independent experiments. Scale bars=20μm. See also Figures S1 and S2.
To determine whether PAF is expressed in ISCs, we used a Lgr5-EGFP-CreERT knock-in mouse. PAF+ cells were correlated with Lgr5+ CBC cells and Lgr5+ progenitor cells localized at transit amplifying zone (TA zone) (Figure 2H), confirmed by qRT-PCR of fluorescence-activated cell sorting (FACS)-isolated Lgr5+ cells (Figure 2I). The Paneth cells between CBC ISCs did not express PAF (Figure 2G). We further asked whether PAF is expressed in the quiescent ISCs at position 4, using TERT-tdTomato-CreERT2 (Jun et al., 2016) and Bmi1-EGFP (Hosen et al., 2007) knock-in mice. Some population of TERT+ (52.94%) and Bmi1+ cells (63.16%) expressed PAF (Figures 2J, 2K, S2D, and S2E). These results indicate that PAF+ cells include some population of CBC (Lgr5high) ISCs, position 4+ ISCs (TERT+ and Bmi1+), and TA progenitor (Lgr5low) cells.
Given the upregulation of PAF expression by IR (Figure 1), we monitored PAF+ cells in the regenerating intestinal crypts. Interestingly, upon IR exposure (10 Gy), the number of PAF+ cell was increased until 4 dpi and then decreased at 7 dpi (Figures 2L and 2M), which is consistent with PAF mRNA upregulation (Figures 1C and 1D). At 1~2 dpi, the remaining ISCs started to divide. At 4 dpi, the regenerating crypts were enlarged, and newly generating CBC cells and TA cells reappeared. At 7 dpi, the regeneration process was mostly completed (Jun et al., 2016; Suh et al., 2017). Intriguingly, PAF+ cells remained at position 4-6 and 8-9 at 1~2 dpi, whereas PAF+ CBC cells were lost by cell death at 1 dpi (Figures 2L and 2M). The newly generated CBC cells and TA cells highly expressed PAF at 4 dpi, and PAF+ cells were partially restored as CBC and TA cells (7 dpi) (Figures 2L and 2M). Given the high enrichment of PAF expression in the remaining and active ISCs/IPCs in the regenerating crypts, these results imply that PAF might be involved in controlling ISCs/IPCs during regeneration.
Impaired Intestinal Regeneration by PAF KO
To directly test whether PAF is engaged in intestinal regeneration, we utilized PAF KO mouse model. Prior to the experiments, we examined the intestinal morphology and several differentiated intestinal epithelial cell (IEC) markers in PAF KO mice. PAF KO mice displayed no differences in intestinal morphology and IEC differentiation (Figures S3A and S3B). To further address whether PAF KO affects intestinal homeostasis, we performed BrdU incorporation assays (Figures S3C and S3D). The number of BrdU incorporated cells in the crypts of PAF KO mice was the same as that in WT after 2h BrdU induction (Figure S3C). Furthermore, PAF KO mice showed a similar migration rate during 3 days of tracing of BrdU+ cells (Figure S3D). These data indicate that genetic ablation of PAF does not affect the proliferation and migration of IECs. Additionally, we analyzed the expression of various genes related to intestinal homeostasis by qRT-PCR (Figure S3E). Despite the slight decrease of c-Myc expression in PAF KO intestine (~36%), the expression of most genes was not altered in the crypts of WT and PAF KO mice. It is noteworthy that conditional deletion of c-Myc in the intestine has no impact on intestinal homeostasis (Bettess et al., 2005). These results suggest that PAF is dispensable for intestinal homeostasis.
Next, we asked whether PAF is required for tissue regeneration. We found that upon IR injury, PAF KO mice showed severe defects in the intestinal regeneration (Figures 3A-3D). At 4 dpi, the number of viable crypts was markedly reduced by 10 Gy (50.5% decrease) and 12 Gy (100% decrease) IR treatment (Figures 3B and 3E). The crypt structure, represented by lysozyme staining, was also severely disintegrated in PAF KO mice (Figures 3D and 3F) compared to that in WT mice. These results suggest that PAF is indispensable for intestinal regeneration.
Figure 3. Impaired Intestinal Regeneration by PAF KO.
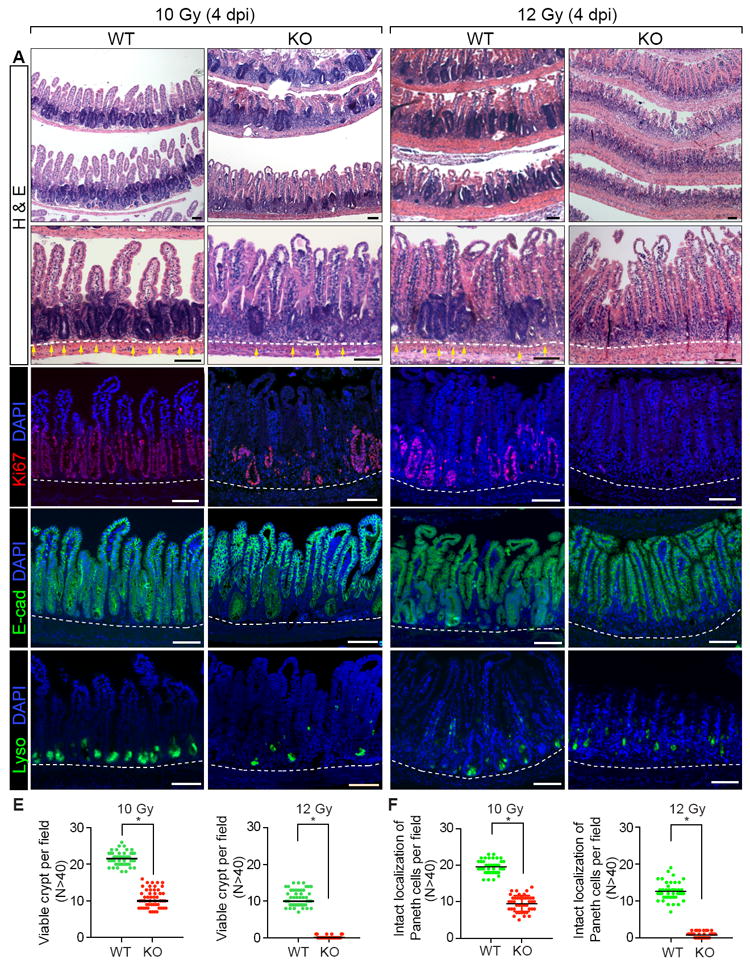
(A-D) Impaired intestinal regeneration after irradiation (4 dpi; 10 and 12 Gy). Hematoxylin and eosin staining of the mouse small intestine samples of PAF+/+ (WT) or PAF-/- (KO) mice (A); IHC of the small intestine samples for Ki67 (B); IHC of the small intestine samples for E-cadherin, a marker for epithelial cell (C); IHC of the small intestine samples for Lysozyme, a marker for Paneth cell (D); Yellow arrows: crypts.
(E) Quantification of the viable crypts. Crypts that showed five successive Ki67+ cells were counted as a viable crypt.
(D) Quantification of the intact localization of Lysozyme. Crypts possessing at least three Lyso+ cells localized at the crypt bottom were counted as intact localization.
The representative images were shown from at least three animals for each condition. Scale bars=100μm. See also Figures S3 and S4.
PAF Is Required for ISCs/IPCs Expansion in Regenerating Crypts
To understand the underlying mechanism of intestinal regeneration defects by PAF KO, we first examined DNA repair process in PAF KO mice. Interestingly, no significant increase of cell death (Apoptotic body and Active-Caspase 3 staining) was detected in PAF KO mice during 48h after IR (Figures S4A-S4D). Initial recognition of DNA damages, checked by ATM pS1981(1h) and phospho-γH2AX (6h) (Figures S4E, S4F, and S4G), and downstream targets of DNA damage responses were not changed in PAF KO mice (Figures S4H-S4K). DNA double-strand breaks repair (6h~48h after IR) represented by γ-H2AX was also similar between PAF WT and KO mouse intestine samples (Figures S4E and S4F). These data suggest that regeneration defects in PAF KO mice are not due to impaired DNA repair processes.
Next, given the specific expression of PAF in ISCs/IPCs (Figures 2H-2K), we asked whether PAF KO-induced defects in intestinal regeneration is due to the dysfunction of ISCs/IPCs. To test this, we performed crypt organoid culture assays. Interestingly, the crypt organoids derived from PAF KO mice showed the low efficiency of organoid formation (Figures 4A and 4B) as well as decreased growth and budding efficiency (Figures 4A, 4C, and 4D). These results imply that PAF might be required for intestinal regeneration possibly by controlling ISCs/IPCs in a cell-autonomous manner.
Figure 4. PAF Is Required for ISCs/IPCs Expansion in Regenerating Crypts.
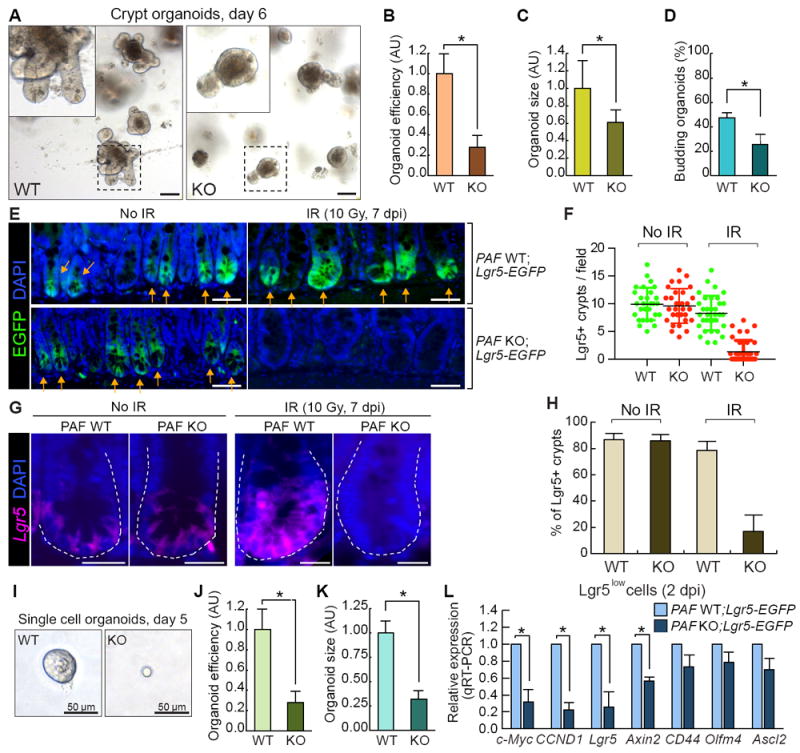
(A-D) Reduced organoid development by PAF KO. The crypt organoid growth assays from the small intestine samples of PAF WT and KO mice (A); quantification of organoid efficiency (N≥2000 from the three independent experiments) (B); size (5 days after seeding; N≥50) (C); budding efficiency (N≥50) (D). Asterisks=P< 0.01. Scale bars=100μm.
(E, F) Impaired ISCs/IPCs expansion by PAF KO. Visualization of Lgr5+ EGFP cells by GFP immunostaining of the small intestines from PAF WT;Lgr5-EGFP and PAF KO;Lgr5-EGFP mice at 0 and 7 dpi (E). Scale bars=50μm; Quantification Lgr5+ (EGFP+) existing crypts per field (F). At least 30 fields of view were counted; Arrows (EGFP+ crypts); asterisks (EGFP-regenerating crypts).
(G, H) Analysis of Lgr5 expression by FISH in PAF WT and PAF KO regeneration crypts. The representative images were shown. (G). Scale bars=20μm; % of Lgr5+ (EGFP+) crypts in the field (H). At least 10 fields of view were counted.
(I-K) Reduced single cell organoid development by PAF KO. Representative images of single cell (Lgr5+) organoids (day 5) derived from PAF WT;Lgr5-EGFP and PAF KO;Lgr5-EGFP mice (I). Scale bars=50μm; quantification of organoid development efficiency (J) (N≥5000 cells were analyzed for from three independent experiments); size (K) (N≥30 single cell organoids were analyzed). (L) Gene expression analysis of FACS-isolated Lgr5low (GFP+) cells in the IR-treated intestine from PAF WT;Lgr5-EGFP and PAF KO;Lgr5-EGFP mice (2 dpi, 10 Gy). qRT-PCR analysis. Asterisks=P< 0.05. See also Figure S5.
To further assess underlying mechanisms of dysregulated ISCs/IPCs in PAF KO mice during intestinal regeneration, we generated PAF KO;Lgr5-EGFP-CreERT mice and analyzed the Lgr5+ ISCs/IPCs population after radiation injury. After IR treatment, Lgr5high ISCs cells are depleted by DNA damage-induced apoptosis. However, a small population of Lgr5low cells (including quiescent ISCs and TA progenitor cells) survives and contributes to intestinal regeneration (Buczacki et al., 2013; Metcalfe et al., 2014; Muñoz et al., 2012). In the normal intestine of PAF KO mice, the number of Lgr5+ ISCs/IPCs was not changed (Figures S5A and S5B), which is consistent with the BrdU incorporation assay results (Figures S3C and S3D). Similarly, PAF KO did not affect the number of Lgr5low cells population upon IR (Figures S5C and S5D), suggesting PAF KO has no effects on Lgr5+ ISCs/IPCs on the overall cell viability in the early stage of regeneration (1 dpi, 10 Gy). However, PAF KO mice exhibited significantly delayed restoring the Lgr5+ cells in regenerating crypts at the late time point (7 dpi) (Figures 4E-4H). Of note, in the treatment of 10 Gy IR, PAF KO mice displayed about 50% of viable crypts (4 dpi) (see Figures 3A-3E). Although the regenerating crypts restored their morphology similar to WT in PAF KO mice (7 dpi), the number of Lgr5+ cells were not restored. To complement the in vivo result of PAF KO-decreased Lgr5+ ISCs/IPCs, we also employed single cell organoid cultures of FACS-isolated Lgr5+ cells from the mouse intestine. Strikingly, single cell organoids from PAF KO Lgr5+ cells showed three times lower efficiency in the organoid formation and displayed severe growth defects compared to PAF WT Lgr5+ organoids (Figures 4I-4K). PAF KO Lgr5+ cells grew markedly slowly during the 2 weeks in single cell organoid culture. These in vivo and in vitro results suggest that PAF is required for the expansion of ISCs/IPCs during intestinal regeneration.
PAF Transactivated c-Myc Is Required for ISCs/IPCs Expansion upon Radiation Injury
Next, we sought to determine the molecular mechanism of PAF-induced ISCs/IPCs expansion. We collected remaining Lgr5+ cells from IR-treated intestine samples of Lgr5-EGFP-CreERT and PAF KO mice and examined the expression of target genes of the Wnt, Notch, Hippo-YAP, Hedgehog, and TGF-β/BMP pathways (Figure 4L and S5E). In the regenerating crypts (1 and 2 dpi), remaining Lgr5low cells from PAF KO mice exhibited a marked decreased expression of Wnt target genes (c-Myc, Cyclin D1, and Lgr5) (Figure 4L and S5E). The well-established Wnt target gene, Axin2, was also decreased in the remaining Lgr5low cells at 2 dpi. Downregulation of Wnt target genes was also observed in whole crypts fraction at the late time point (4 dpi) (Figure S5F). IHC confirmed that c-Myc and Cyclin D1 expression was significantly downregulated in 4 dpi PAF KO mouse intestine (Figures 5A, 5B, and S5G-S5I). Additionally, we found notable PAF+:Myc+ cells in position 4-6 at 1 dpi, when ISCs are activated for repopulation (Figure 5C). Whereas c-Myc expression was upregulated during regeneration, PAF KO showed a decrease in c-Myc expression (Figures S5G-S5I). These results suggest that PAF might be required for c-Myc upregulation during intestinal regeneration.
Figure 5. Requirement of PAF-Myc Axis for ISCs/IPCs Expansion.
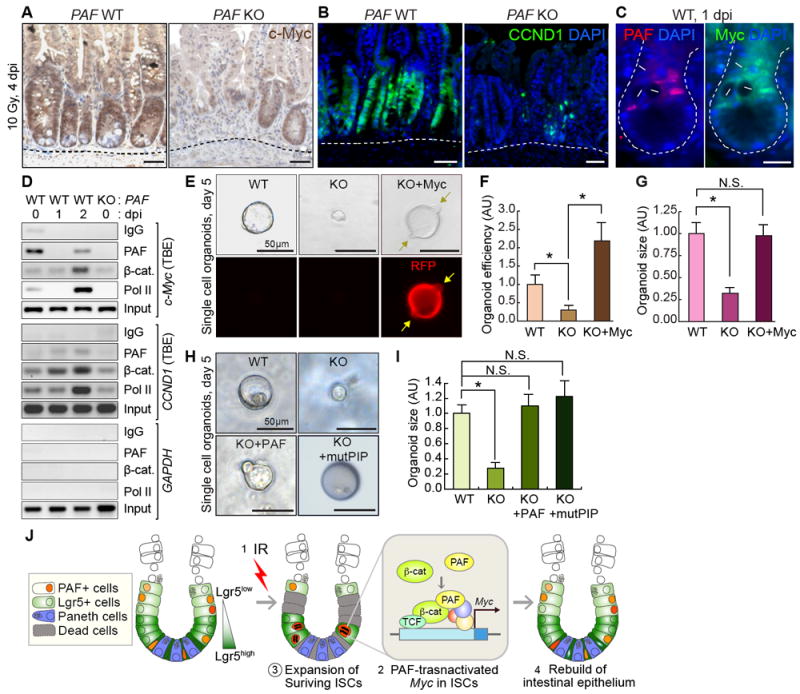
(A, B) Downregulation of c-Myc and Cyclin D1 in PAF KO crypts (4 dpi, 10 Gy). IHC for c-Myc or Cyclin D1. Hematoxylin or DAPI for nuclear counterstaining (blue).
(C) Co-expression of PAF and c-Myc in surviving cells in the regenerating crypts. Arrows: PAF+:Myc+ cells; asterisks: disappeared CBC cells.
(D) Conditional recruitment of PAF and β-catenin to TCF-binding elements (TBEs) in c-Myc and CCND1 (Cyclin D1) proximal promoter. Chromatin immunoprecipitation (ChIP) assays of the mouse small intestine (PAF WT and KO; 1 and 2 dpi; 10 Gy). IgG ChIP and PAF KO small intestine samples served as negative control. RNA Pol II ChIP (positive control for gene transactivation). GAPDH promoter served as negative control.
(E-G) Rescue of PAF KO-induced organoid growth failure by c-Myc expression. The Lgr5+ cells isolated from (PAF WT and KO) were transduced with Retroviruses encoding c-Myc and RFP (red fluorescent protein) and cultured for organoid development. Arrows indicate the budding. The representative images (E); quantification of organoid efficiency (F) and size (G). Asterisks: P<0.05 (N≥30). N.S. (not significant; P≥0.05).
(H, I) Rescue of PAF KO-induced organoid growth failure by ectopic expression of wild-type PAF and PIP mutant PAF (mutPIP-PAF). The Lgr5+ cells isolated from PAF KO;Lgr5-EGFP were transduced with retroviruses encoding wild-type PAF and mutPIP-PAF and cultured for organoid development. The representative images (H); Quantification of organoid size (I) Asterisks: P<0.05 (N≥20).
(J) Illustration of the working model. Upon irradiation injury, the highly proliferative cells (Lgr5high ISCs and some of Lgr5low TA cells) undergo apoptosis. PAF and β-catenin transactivate c-Myc in the surviving ISCs/IPCs (Lgr5low), which leads to the expansion of ISCs/IPCs and the subsequent rebuilding of the intestinal epithelium. Scale bars=50μm (A, B, E, H) and 20μm (C); the representative images were shown from at least three independent experiments. See also Figure S5
As a cofactor of β-catenin transcription complex, PAF upregulates c-Myc expression in CRC (Jung et al., 2013), which led us to determine whether PAF transactivates c-Myc in the regenerating intestine. To test this, we performed a chromatin immunoprecipitation (ChIP) assay of the mouse small intestine using PAF antibody. The small intestine samples from PAF KO mice served as a negative control (Figure 5D; Lane 4). We found that endogenous PAF occupied the TCF-binding element (TBE)-containing proximal promoter of c-Myc and CCND1 (Cyclin D1) in the normal intestine (Figure 5D; Lane 1, 0 dpi). It is noteworthy that despite the massive death of PAF+ cells upon IR, some PAF+ cells survived at 1 dpi (Figures 2L and 2M). Nonetheless, we found that IR conditionally induced the recruitment of PAF and β-catenin to the c-Myc promoter (Figure 5D; lane 3, 2 dpi). These results suggest that PAF is conditionally associated with and transactivates the c-Myc promoter during intestinal regeneration, similar to PAF-induced c-Myc transactivation in CRC cells (Jung et al., 2013).
Next, we asked whether c-Myc mediates PAF-controlled ISCs/IPCs expansion by rescue assay of the Lgr5+ single cell organoid culture. We found that ectopic expression of c-Myc using retrovirus rescued the reduction of organoid-forming efficiency and proliferation in PAF KO Lgr5+ organoids (Figures 5E-5G). These results suggest that PAF-transactivated c-Myc is required for ISCs/IPCs expansion during intestinal regeneration.
Additionally, we performed rescue experiments using wild-type PAF and PIP mutant PAF (mutPIP-PAF) (Jung et al., 2013). It has been suggested that the function of PAF in DNA repair is mediated by the interaction of PAF with PCNA via PIP, a PCNA-interacting protein motif (Emanuele et al., 2011; Povlsen et al., 2012). However, mutPIP-PAF expression was sufficient to recover PAF KO single cell organoids (Figures 5H and 5I), suggesting that PAF-mediated DNA repair through PCNA binding is not involved in growth defect of PAF KO organoids.
Attenuation of Intestinal Tumorigenesis by PAF KO
PAF is significantly upregulated in many human cancers (Cheng et al., 2013; Hosokawa et al., 2007; Jain et al., 2011; Jun et al., 2013; Jung et al., 2013; Kais et al., 2011; Kato et al., 2012; Mizutani et al., 2005; Wang et al., 2016; Yu et al., 2001; Yuan et al., 2007), indicating the potential roles of PAF in promoting tumorigenesis. Additionally, given the pivotal roles of PAF and c-Myc in controlling ISCs/IPCs activation (Figures 5 and S5G-S5I) and initiating intestinal tumorigenesis (Jung et al., 2013; Sansom et al., 2007), we next asked whether PAF contributes to intestinal tumorigenesis by positively modulating the cancer cell stemness. We assessed the expression of PAF in intestinal adenomas driven by Apc mutation using ApcMin/+ mouse model (Moser et al., 1990). IHC results showed that PAF was markedly upregulated in intestinal adenomas of ApcMin/+ mice (Figure 6A). sqRT-PCR of individually isolated intestinal adenomas and adjacent normal intestine samples of ApcMin/+ mice (age of 14 and 16 weeks) also showed the marked upregulation of PAF in Apc mutation-driven adenomas (Figure 6B), which confirms the upregulation of PAF in CRC (Jung et al., 2013). Of note, PAF+ cells were a subpopulation of Ki67+ or PCNA+ cells in adenomas (27.68% and 26.49%, respectively)(Figures S6A-S6D).
Figure 6. Attenuation of Intestinal Tumorigenesis by PAF KO.
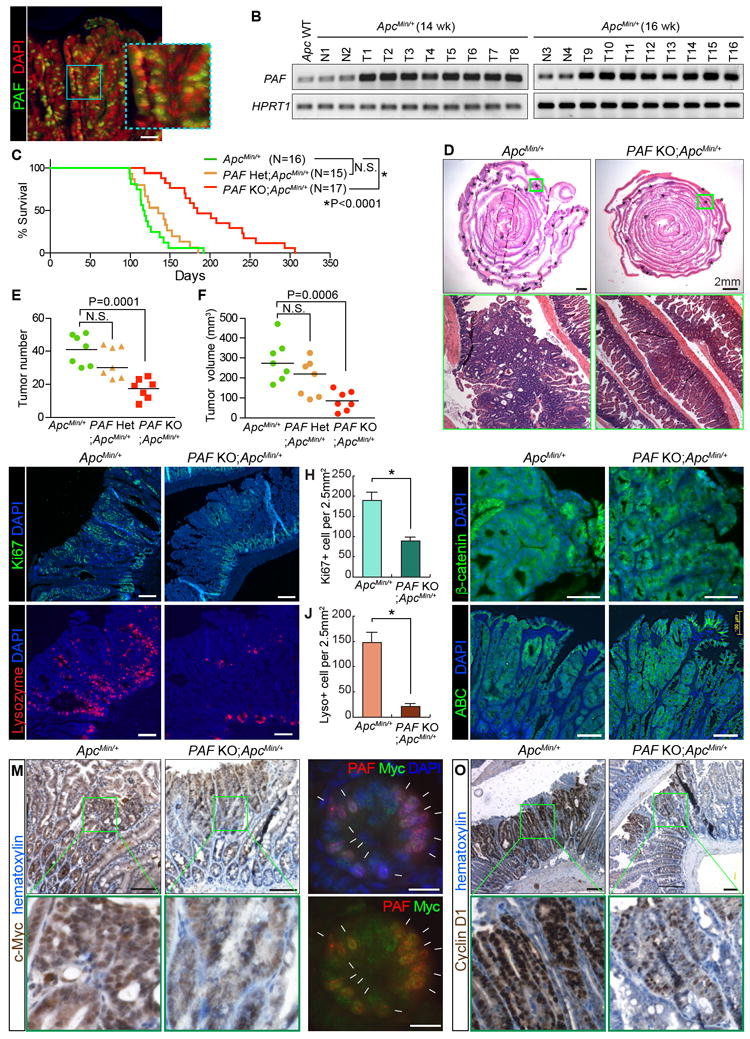
(A, B) Expression of PAF in ApcMin adenomas. Immunostaining of ApcMin/+ intestinal adenoma (16 wk old) (A), scale bar=50μm; semi-QT-RT-PCR (B). Apc WT: Wild-type (Apc+/+) intestine sample; N1-4: normal adjacent intestine samples; T1-16: intestinal adenomas.
(C) The extended life span of ApcMin/+ mice by PAF KO. Kaplan-Meier survival curve of ApcMin/+ (N=16), PAF Het;ApcMin/+ (N=15), and PAF KO;ApcMin/+ (N=17).
(D) H&E staining of the small intestines from ApcMin/+ and PAF KO;ApcMin/+ (age of 16 weeks). Asterisks indicate intestinal adenomas. Scale bars=2mm.
(E, F) Decreased tumor burden of ApcMin/+ mice by PAF KO. The number of tumors (≥1.5mm) (E) and tumor volumes (mm3) (F) were quantified; 16 wk old; N=7 for each group.
(G, H) Reduced cell proliferation of ApcMin tumors by PAF KO. Ki67 staining of small intestine adenomas from ApcMin/+ or PAF KO;ApcMin/+ (16 wk old) (G); quantification (H). Asterisk: P<0.001.
(I, J) Decreased differentiation of the Paneth cells of ApcMin tumors by PAF KO. Lysozyme staining of small intestine adenomas from ApcMin/+ or PAF KO;ApcMin/+ (16 wk old) (I); quantification (J). Asterisk: P<0.001.
(K, L) No change in β-catenin level and activity of ApcMin tumors by PAF KO. Immunostaining of small intestine adenomas from ApcMin/+ or PAF KO;ApcMin/+ (16wk old) for total β-catenin (K) and active (unphosphorylated) β-catenin (ABC) (L). Scale bars=100μm.
(M) Downregulation of c-Myc of ApcMin tumors by PAF KO. c-Myc IHC; 16 wk old. Scale bars=100μm.
(N) Co-expression of c-Myc and PAF in ApcMin tumors. Arrows: PAF+:Myc+ cells. Scale bars=20μm.
(O) Downregulation of Cyclin D1 of ApcMin tumors by PAF KO. Cyclin D1 IHC; 16 wk old. Scale bars=100μm.
The representative images (N≥3) were shown. See also Figure S6.
To test whether genetic ablation of PAF suppresses tumorigenesis in a mouse model, we established ApcMin/+ (control group) and PAF KO;ApcMin/+ compound strains (experimental group) and. The median survival of ApcMin/+ mice was 117 days (N=16). Surprisingly, PAF KO;ApcMin/+ mice displayed marked extended survival (median 184 days, N=17; P=0.0001) (Figure 6C). Moreover, PAF KO;ApcMin/+ mice showed a decreased number and size of adenomas, compared to those in ApcMin/+ mice (Figures 6D, 6F, and S6E). Of note, PAF heterozygous KO (PAF Het;ApcMin/+) did not affect mouse survival and the number and size of adenomas compared to PAF KO;ApcMin/+ (Figures 6C-6F). Ki67 IHC showed reduced cell proliferation in adenomas developed in PAF KO;ApcMin/+ mice compared to that in ApcMin/+ control mice (Figures 6G and 6H). The number of Paneth cells was also diminished in intestinal adenomas of PAF KO;ApcMin/+ mice (Figures 6I and 6J). Given that the Paneth cell differentiation is driven by Wnt/β-catenin signaling (van Es et al., 2005), it is possible that PAF KO might induce the overall downregulation of Wnt/β-catenin target genes in tumor cells. Based on our previous finding that PAF hyperactivates the Wnt signaling as a cofactor of the β-catenin transcriptional complex (Jung et al., 2013), we tested whether PAF KO suppresses Wnt/β-catenin signaling in intestinal adenomas. While PAF KO did not affect the level and activity of the β-catenin protein in adenomas (Figures 6K and 6L), PAF KO notably downregulated the expression of Wnt/β-catenin target genes, c-Myc and Cyclin D1, in intestinal adenomas (Figures 6M-6O and S6G). Furthermore, we found that PAF was co-expressed with c-Myc in intestinal adenomas of ApcMin/+ mice, similar to their co-expression in the regenerating crypts (Figure 6N and S6F). These results suggest that PAF KO attenuates intestinal tumorigenesis by downregulation of β-catenin target genes including c-Myc.
Reduced CRC cell stemness by PAF KO
Previously, we found that PAF-Wnt signaling axis is required for the maintenance of breast cancer cell stemness (Wang et al., 2016). Moreover, having determined that PAF is indispensable for ISCs/IPCs expansion in regeneration (Figures 4), we next examined the role of PAF in controlling the stemness of CRC cells. We analyzed the expression of several known CRC stemness makers (CD44, CD133, and Lgr5) (O’Brien et al., 2007; Ricci-Vitiani et al., 2007; Schepers et al., 2012; Zeilstra et al., 2008; Zhu et al., 2009). Adenomas from PAF KO;ApcMin/+ mice showed marked decreased expression of CRC stemness markers (CD44, CD133, and Lgr5)(Figures 7A-7D, S7A, and S7B). To better understand the role of PAF in CRC cell stemness, we cultured tumor organoids from ApcMin/+ and PAF KO;ApcMin/+ crypts. Owing to hyperactivation of Wnt signaling, ApcMin/+ organoids develop into the sphere shape (cystic) without budding (Sato et al., 2011a). Surprisingly, the organoids derived from PAF KO;ApcMin/+ mouse intestine showed severe defects in growth (Figures 7E and 7F). Although the initial organoid forming efficiency was similar (Figure 7G), PAF KO; ApcMin/+ organoids did not grow until 21 days, compared to the organoids from PAF WT;ApcMin/+ (Figures 7E and 7F). One copy deletion of PAF (PAF Het;ApcMin/+) did not suppress the cystic organoid growth (Figure S7C), consistent with in vivo results from PAF Het;ApcMin/+ mice (see Figures 6C-6F). We further assessed the effects of PAF knockdown on cancer cell stemness using human CRC cells. Colorectal CSCs exhibit the enrichment of CD44 and CD133 expression (Kemper et al., 2010; O’Brien et al., 2007; Ricci-Vitiani et al., 2007). We found the significantly decreased population of CD44+:CD133+ cells in PAF-depleted HT29 cells (Figure S7D). Furthermore, PAF knockdown inhibited colonosphere formation of HT29 (Figure S7E and S7F). These results suggest that PAF is required for the maintenance of CRC cell stemness.
Figure 7. Decreased CRC Cell Stemness by PAF KO.
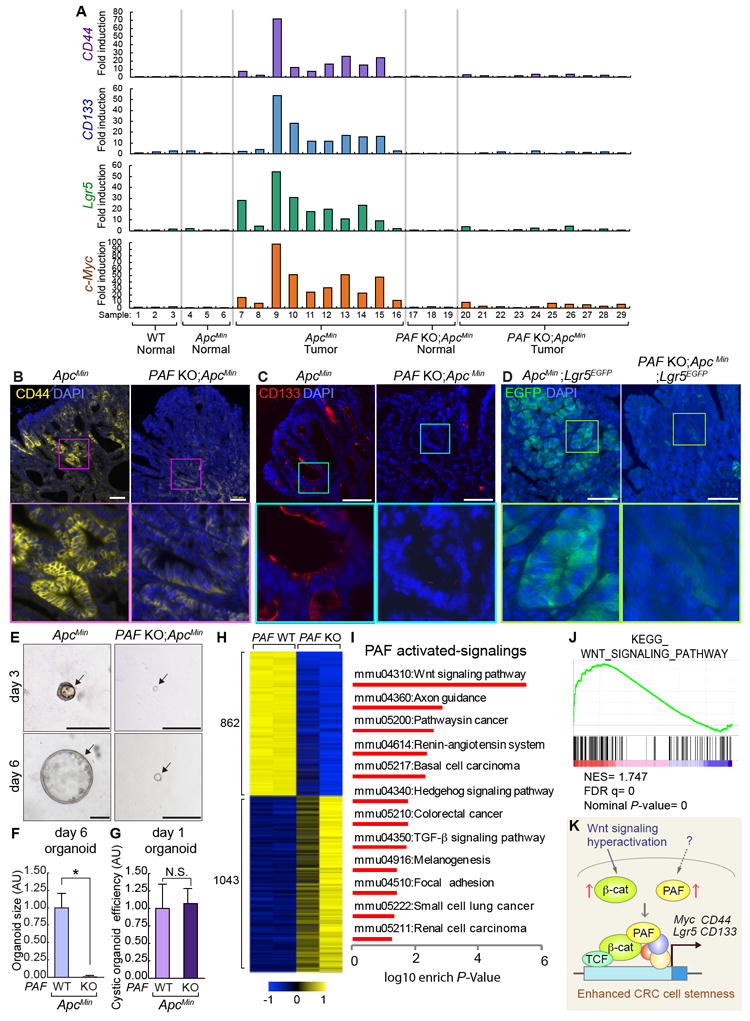
(A) Gene expression analysis of CD44, CD133, Lgr5, and c-Myc in the normal intestine or adenomas from ApcMin/+ and PAF KO;ApcMin/+ (16 wk old). At least three individual samples from WT and normal region of ApcMin/+ and PAF KO;ApcMin/+ were used as the control.
(B, C) Downregulation of CD44 and CD133 expression in ApcMin tumors by PAF KO. IF staining for CD44 and CD133; 16 wk old. Scale bars=100μm.
(D) Downregulation of Lgr5 in ApcMin tumors by PAF KO. IF for GFP (adenomas of ApcMin/+;Lgr5-EGFP and PAF KO;ApcMin/+;Lgr5-EGFP [16 wk old]). Scale bars=100 μm.
(E) Reduced cystic organoid development by PAF KO. Representative images of organoids (day 5) derived from ApcMin and PAF KO;ApcMin adenomas
(F, G) Quantification of organoid size (F) (N≥30 cystic organoids were analyzed); efficiency (G) (N≥5000 cells were analyzed for from three independent experiments). Scale bars=100μm. Asterisk: P<0.001.
(H) Heatmap gene expression profile generated by significant differential expression (P<0.05) of ApcMin/+ and PAF KO;ApcMin adenomas by RNA sequencing (N=2 per group).
(I) Significantly PAF upregulated signaling pathways identified by GSEA analysis (KEGG) using RNA-seq result. P<0.05.
(J) Gene set enrichment analysis (GSEA) for Wnt signaling pathway. NES: normalized enrichment score; FDR: False detection rate; P-value: Nominal P-value.
(K) Illustration of the working model. During tumorigenesis, Wnt/β-catenin signaling is hyperactivated and PAF is upregulated. PAF and β-catenin transactivate c-Myc and CRC stemness-related genes (CD44, CD133, and Lgr5), which leads to the increase of CRC stemness.
See also Figure S7, Tables S1 and S2.
Next, for an unbiased assessment of PAF-controlled transcriptome in intestinal tumors, we also performed RNA-seq of PAF WT and KO ApcMin mouse tumors (Figure 7H). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed that Wnt signaling is markedly downregulated in PAF KO tumor cells (Figures 7I, S7G, and Table S1), which was also confirmed by Gene Set Enrichment Analysis (GSEA) (Figure 7J and Table S2). These results strongly suggest that PAF positively modulates Wnt/β-catenin signaling in CRC, similar to that during intestinal regeneration.
DISCUSSION
Herein, our comprehensive approaches revealed that the PAF-Myc axis is required for ISCs/IPCs expansion during intestinal regeneration and tumorigenesis.
c-Myc is not required for normal intestine homeostasis but is indispensable for intestinal regeneration (Ashton et al., 2010; Bettess et al., 2005). However, it was unknown how c-Myc contributes to intestinal regeneration. In our experimental setting, ectopic expression of c-Myc rescued PAF KO-induced defects in Lgr5+ single cell organoid growth (see Figures 5E-5G), which strongly suggests that c-Myc mediates PAF-controlled ISCs/IPCs expansion during intestinal regeneration. Interestingly, PAF KO did not affect intestinal homeostasis, which requires constitutively active Wnt signaling in the crypts. Nonetheless, there were no changes in the expression of Wnt/β-catenin target genes (Cyclin D1, CD44, and Lgr5) in PAF KO intestine (without IR injury)(see Figure S3E). However, under specific physiologic or pathologic conditions such as regeneration or tumorigenesis when enhanced Wnt signaling is required (Cadigan and Waterman, 2012; Clevers and Nusse, 2012; Polakis, 2012), the highly upregulated PAF hyperactivates Wnt/β-catenin transcriptional complex and transactivates c-Myc, which subsequently triggers the activation of self-renewing cells (Figures 5J and 7K). This is also supported by the marked upregulation of PAF expression in the regenerating crypts (see Figures 1A-1C, and 2L) and CRC (Jung et al., 2013).
Accumulating evidence suggests the pivotal roles of c-Myc in regulating stem cells. c-Myc is necessary for efficient cellular reprogramming of induced pluripotent stem cells (iPSCs) (Araki et al., 2011; Takahashi et al., 2007). In ESCs, c-Myc modulates cell stemness and differentiation by amplifying protein biosynthesis (Nie et al., 2012; Varlakhanova et al., 2010). c-Myc also regulates the balance between self-renewing and differentiated/committed hematopoietic stem cells by controlling the interaction with their niche (Laurenti et al., 2008; Wilson et al., 2004). Given the specific and dynamic expression of PAF in ISCs/IPCs cells (see Figures 2) and PAF-transactivated c-Myc (see Figure 5), it is probable that PAF may play pivotal roles in governing various stem cells via c-Myc. Indeed, in the small intestine, PAF KO decreased the expression of stem cell marker, Lgr5, in the regenerating crypts accompanied with the downregulation of c-Myc. Furthermore, c-Myc rescued PAF KO-induced failure of organoid growth (see Figures 5E-5G), indicating that PAF functions as an upstream molecule of c-Myc in controlling the stem cells.
70% of human CRC displays a significant upregulation of c-Myc (Erisman et al., 1985; Sikora et al., 1987). In mouse models, genetic ablation of c-Myc suppresses intestinal tumorigenesis driven by Apc mutations (Sansom et al., 2007). Similarly, PAF KO inhibits intestinal adenoma development in ApcMin/+ mice with reduced c-Myc expression (see Figures 6M and S6G). Moreover, the depletion of PAF decreased the expression of CRC cell stemness markers, (CD44, CD133, and Lgr5) in ApcMin adenomas (see Figures 7A-7D) with downregulation of c-Myc (see Figures 6M and 7A). Although the effects of PAF KO on CRC cell stemness should be further elaborated with cell ablation, lineage-tracing, and serial transplantation assays, our results are somewhat similar to our previous study that PAF is required for the maintenance of breast cancer cell stemness (Wang et al., 2016). Although Myc depletion rescued phenotypes of tumorigenesis driven by APC mutation in the mouse model (Sansom et al., 2007), the ectopic expression of Myc failed to rescue the organoid growth in PAF KO;ApcMin/+ condition. These results imply that additional factors/pathways might be involved in PAF-controlled CRC cell stemness. Transcriptomic analysis of ApcMin/+ and PAF KO;ApcMin/+ adenomas revealed that in addition to Wnt signaling, other oncogenic pathways including Hedgehog and TGF-G signaling were modulated by PAF (Figures 7H-7J and S7H). Given the oncogenic function of Hedgehog and TGF-G signaling in CRC (Munoz et al., 2006; Takaku et al., 1998; Varnat et al., 2009), it is possible that PAF-controlled colorectal cancer stemness is mediated by several oncogenic pathways including Wnt-Myc, Hedgehog, and TGF-β signaling. Although we here limited our scope to PAF-activated Wnt-Myc axis, it is necessary to further examine how PAF is associated with various oncogenic signalings beyond Wnt/β-catenin pathway.
PAF directly binds to PCNA (De Biasio et al., 2015). Nonetheless, it is highly likely that PAF-controlled stem cells might be independent of PCNA interaction, which is supported by the following results: (a) Without IR treatment, PAF KO is sufficient to suppress organoid growth (Figures 4A-4D and 4I-4K), which rules out the potential involvement of PAF-mediated DNA repair in tissue regeneration; (b) PAF transactivates β-catenin target genes including c-Myc in a PCNA-independent manner (Jung et al., 2013); (c) Instead of DNA repair genes, c-Myc is sufficient to rescue the PAF KO phenotypes in the single cell organoid growth (see Figures 5E-5G); (d) Ectopic expression of PIP-mutated PAF fully rescues the defect of PAF KO organoid growth (see Figures 5H and 5I); (e) Only some of PCNA+ cells are PAF+ cells in the normal intestine (26.49%) and adenomas (23.01%) (see Figures 2D, 2E, S6C, and S6D), indicating the potential roles PAF in a PCNA-independent manner; (f) PAF KO mice showed no abnormality in PCNA expression and IECs growth in the crypts (see Figure 2F); (g) PAF KO did not affect the DNA double-strand breaks and DNA damage foci formation in the intestine (see Figures S4E and S4F), indicating that PAF is dispensable for genomic stability or DNA repair. Thus, these results strongly support that PAF-mediated intestinal regeneration is independent of PCNA and DNA repair pathway.
Although PAF+ cells partially mark ISCs and TA cells in the normal intestine, PAF KO mice did not display defects in tissue homeostasis (see Figures S3). Tissue injury upregulates PAF expression, which enhances c-Myc transcription for the subsequent expansion of ISCs/IPCs. This is also supported by co-expression of PAF and c-Myc in the surviving cells (not the Paneth cells) of regenerating crypts (see Figure 5C). Employing PAF reporter and lineage tracing mice will provide further insights into how PAF+ cells contribute to tissue regeneration and cancer.
Despite the crucial roles of PAF in regulating self-renewing cells in intestinal regeneration and tumorigenesis, it is still unclear how PAF is upregulated in the regenerating crypts and CRC. Due to the high expression of PAF in ApcMin/+ tumors (see Figures 6A and 6B), it is reasonable that Wnt/β-catenin signaling might directly transactivate PAF. Although the proximal promoter of PAF contains multiple TBEs (Wang et al., 2016), we found that manipulation of Wnt/β-catenin signaling did not affect the transcription of PAF in both IECs and CRC cells (Figures S7I and S7J). Given our previous finding that Oct4, Nanog, and Sox2 transactivate PAF in breast cancer cells and mammary epithelial cells (Wang et al., 2016), the involvement of such iPSC-inducing factors in PAF regulation should be addressed in future studies.
PAF is significantly upregulated in CRC and contributes to tumorigenesis, whereas dispensable for tissue homeostasis. Therefore, PAF might be a viable molecular target for cancer treatment with minimal damage to the normal cells. Conversely, tweaking PAF might provide new ways to manipulate tissue regeneration. Collectively, our findings unveil the essential role of the PAF-Myc signaling axis in controlling stem cell activation in regeneration and cancer.
STAR METHODS
CONTACT FOR REAGENT ANDRESOU RCESHARING
Additional information and requests for reagents should be directed to and will be fulfilled by the Lead Contact, Jae-Il Park at jaeil@mdanderson.org
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse Strains
We generated PAF KO strain using CRISPR/Cas9 gene targeting system. ApcMin/+ (JAX: 002020) and Lgr5-EGFP-IRES-CreERT2 (JAX: 008875) mice were obtained from the Jackson Laboratory. All mice were maintained on a C57BL/6 background except Bmi1EGFP mice (BKa.Cg-Ptprcb Bmi1tm1IlwThy1a/J). Both male and female of PAF KO, C57BL/6J (WT control), or Lgr5-EGFP compound strains were used for regeneration experiments in 8-10 weeks of age. For intestinal adenoma quantification and Kaplan-Meier survival curve, only male mice (ApcMin/+ and PAF KO;ApcMin/+ compound strains) were used. All mouse experiments were performed under MD Anderson guidelines and Association for Assessment and Accreditation of Laboratory Animal Care International standards.
METHOD DETAILS
Generation of PAF KO Mice
To establish PAF KO mice, two guide RNAs (gRNAs) targeting exon 1 of PAF gene and Cas9 mRNA (Sigma) were injected into the mouse zygotes (The Genetically Engineered Mouse Facility, MD Anderson). gRNA sequences were as follows: #1: 5’-GTTCCCGCCACCGTTTAAATGGG-3’, #2: 5’-ACCAAAGCAAACTACGTTCCAGG-3’. Four strains harboring PAF mutation (gene targeting efficiency: 4/7=57.14%) were identified. Two heterozygote strains (strain 4 and 7) carrying 118bp and 125bp deletion of PAF (PAF null) between the two gRNAs targeted region were selected as founders. Using the strain 7 as a founder, at least 5 times subsequent backcross with C57BL/6 was conducted to minimize the off-target effects. For PCR genotyping of PAF KO following primer pairs and cycling conditions were used: primers: #F: 5’ - AGAATCGAGGTTCTCAAGCG-3’; #R: 5’-CCTTCTAGCTGCTCAATGGG-3’, PCR conditions: 10 min at 95°C, followed by 40 cycles o f 95°C for 15 sec, 65°C for 15 sec, 72°C for 30 sec and post-elongation at 72°C for 5 m in. PAF WT makes 280 bp, and PAF KO makes 155bp (125bp deletion) of PCR product.
Radiation Injury
For intestinal damage, 8-10 weeks old mice were treated with γ-irradiation (10 or 12 Gy) using irradiator (Nasatron). The intestinal regeneration was assessed at 4 and 7 dpi.
Gene Expression Analysis
For small-scale screening of DNA repair gene expression in mice, mRNAs from the irradiated mouse small intestines (1 dpi, 10 Gy) were analyzed with RT2 Profiler™ PCR Array Mouse DNA Repair (Qiagen). Control and experimental mice were used for analysis (N=3, each). RNA was extracted from the mouse small intestine and organoids using the TRIzol (Invitrogen) as the manufacturer’s instructions. iScript cDNA synthesis kit (Biorad) with 1 μg of RNA was used for cDNA synthesis. For gene expression analysis of Lgr5+ cells, sorted 100-500 Lgr5+ cells were collected from Lgr5EGFP or PAF KO;Lgr5-EGFP mice in normal and IR-treated conditions. cDNA was synthesized using REPLI-g WTA Single Cell Kit (Qiagen #150063). The quantitative real-time PCR (qRT-PCR) was performed using a 7500 real-time PCR machine (Applied Biosystems) with specific primers listed in Table S3. Target gene expression was normalized by Hypoxanthine phosphoribosyltransferase 1 (HPRT1) or 18s rRNA. Comparative 2-ΔΔCt methods were used for quantification of qRT-PCR results.
Crypt Organoid Culture
Crypt organoid culture and Apc-mutated organoid culture were performed based on the previous studies (Sato et al., 2011b; Sato et al., 2009). Briefly, the small intestine samples were opened longitudinally and washed with PBS several times. For extracting the crypt, the small intestine was incubated with 5mM EDTA/PBS for 30 min and the crypt-rich supernatant was collected after vigorous shaking. After filtering through the 100 strainers, the same number (300~500) of crypts were seeded in 50 μl Matrigel (BD). 500 μl of ENR medium (Advanced DMEM/F12 media supplement with EGF (20 ng/ml, Peprotech), Noggin (100 ng/ml, Peprotech) and R-spondin1 (500 ng/ml, Peprotech) were added every two days. For single cell organoid culture, crypts fractions collected through the 70 μm (BD) cell strainer was incubated with Accumax (Stem cell technology 07921) and DNAse (0.8 mg/ml, Sigma) for 30 min. Passaging though the 40 μm strainer, ~5000 Lgr5high and Cytox Blue (Life technologies) negative cells were collected by cell sorting (MoFlo, Beckman Coulter) and seeded in Matrigel. ENR medium with Jagged-1 peptide (1 μM, AnaSpec) was supplied every 2 days. Organoid efficiency was calculated by counting viable crypts or Lgr5+ cells (at day 3) in total crypts (300~500 per well, crypt organoid) or total seeded cells (N=~2000 per well, single cell organoid). For ApcMin/+ organoids, adenomas were collected and suspended with Advanced DMEM/F12 media supplement with EGF (20 ng/ml) and Noggin (100 ng/ml). Fresh media were supplemented every 3 days until the next passage (10-14 days). Cystic organoid efficiency was calculated by counting viable cells (at day 3) in total suspended cells (Single cell organoid). The size of organoids was analyzed by measuring the area of the middle section of organoids under the microscope using AxioVision software (Zeiss) (at least 30 organoids per group for all experiments).
Organoid Retrovirus Infection
For organoid gene transduction, we utilized the modified method based on the previous reference (Onuma et al., 2013). FACS sorted Lgr5high single cells (~5000) from PAF WT and PAF KO mice were incubated with media containing retroviruses expressing c-Myc with RFP (MSCV-c-Myc-IRES-RFP, Addgene [#35395]), or wild-type PAF and mutPIP-PAF (Jung et al., 2013) for 6h at 37 °C with polybr ene (7 μg/ml) and Jagged-1 peptide (1 μM, AnaSpec). Then, infected cells were seeded on 50 μL Matrigel/well in a 12-well plate with conventional ENR media. RFP+ cells were able to observe 2 days after infection. Only RFP+ cells were considered as transfected cells. For selecting nt-PAF or mutPIP-PAF infected organoids, blasticidin (10 μg/ml) was treated.
Lgr5 mRNA Fluorescence In Situ Hybridization (FISH)
WT or PAF KO intestinal tissue sections (0 and 7 dpi) were processed for Lgr5 mRNA FISH according to the manufacturer’s protocol (Invitrogen, FISH TagTM RNA Green Kit, with Alexa Fluor® 488 dye). Probe was designed as 530 base pair length targeting the coding sequence of Lgr5. A probe for sense strand was used as a negative control.
Mammalian Cell Culture and Sphere Formation Assay
CRC Cell line (HT29) was maintained in DMEM media containing 10% fetal bovine serum. For gene depletion, lentiviruses encoding short hairpins against PAF mRNA (MISSION shRNA, Sigma) were stably transduced into target cells and selected using puromycin (2 μg/ml). For counting the CSC population, trypsinized each cell line was counted and incubated with antibodies: CD44v6-APC (1:100, BD-Pharmingen [G44-26]) and CD133-FITC (1: 200, Miltenyi Biotec). Dead cells were excluded by Cytox Blue staining. FACS analysis was performed using FacsJazz Cell Sorter (BD). For sphere formation assay, the limited number of HT-29 cells (5000 cells per ml) were plated in triplicate in the ultra-low attachment plates and grown for six days in serum-free stem cell medium (SCM) supplemented with B27 (Invitrogen), EGF (20 ng/ml, Invitrogen), and bFGF (10 ng/ml, Invitrogen). The number and size of spheres were quantified using AxioVision software (Zeiss).
Chromatin Immunoprecipitation Assay
The mouse small intestines (the duodenum) were minced and cross-linked with 1% formaldehyde for 15 min at room temperature. After quenching by glycine, samples were incubated with lysis buffer (0.5% NP40, HEPES 25 mM, KCl 150 mM, MgCl2 1.5 mM, 10% glycerol and KOH pH 7.5) containing proteinase inhibitors. Then the nuclear fractions were collected after centrifugation. Cell lysates were subjected to sonication (10 times, 30s on and 30s off, Bioruptor 300 [Diagenode]) with ChIP-lysis buffer (Tris 50 mM pH 8.0, NaCl 150 mM, 0.1% SDS, 0.5% deoxycholate, 1% NP40 and EDTA 1 mM). Supernatant from lysates was used for immunoprecipitation with the primary antibodies. The following antibodies were used for ChIP: RNA Polymerase II (1μg/ml, EMD Millipore [CTD4H8]), mouse-anti PAF (1 μg/ml, Abcam [ab56773]), and normal mouse IgG (1 μg/ml, Invitrogen). ChIP amplicons were detected by ChIP-PCR using the primers listed in STAR Methods.
RNA-sequencing
The total RNA from ApcMin/+ and PAF KO;ApcMin/+ adenomas (two biological replicas) were used for RNA-seq. The transcriptome sequencing was performed by BGI (www.bgi.com/global/) with BGISEQ-500. Reads are mapped by Tophat2 and Differential genes are defined by Cuffdiff with P<0.05. KEGG analysis was performed by DAVID functional annotation analysis. GSEA analysis is performed with normalized FPKM of all genes with default parameters (Number of permutations =1000, collapse dataset=true, permutation type=phenotype).
QUANTIFICATION AND STATISTICAL DETAILS
The Student t-test was applied for comparison of two samples. P-values below the 0.05 were considered significantly different. At least three biological and experimental replicas were used for statistical analyses, otherwise described in Figure legends. Error bars represent standard error (S.E.M).
DATA AND SOFTWARE AVAILABILITY
The accession number for RNA-seq data reported in this paper is GSE109209. All data is available upon request.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER | |
|---|---|---|---|
|
Antibodies
| |||
| Mouse anti-PAF (KIAA0101) | Santa Cruz Biotechnology | Cat# sc-390515 | |
|
| |||
| Mouse anti-PAF (KIAA0101) | Abcam | Cat# ab56773 | |
| RRID:AB_943922 | |||
|
| |||
| Rabbit anti-PCNA | Cell Signaling Technology | Cat# 13110 | |
|
| |||
| Rabbit anti-Bmi1 | Cell Signaling Technology | Cat# 6964S | |
| RRID:AB_10828713 | |||
|
| |||
| Rabbit anti-RFP | Rockland | Cat# 600-401-379S | |
| RRID:AB_11182807 | |||
|
| |||
| Rat anti-BrdU | Abcam | Cat# ab6326 | |
| RRID:AB_305426 | |||
|
| |||
| Rabbit anti-Ki67 | Abcam | Cat# ab16667 | |
| RRID:AB_302459 | |||
|
| |||
| Mouse anti-E-Cadherin | BD | Cat# 610182 | |
| Biosciences | RRID:AB_397581 | ||
|
| |||
| Rabbit anti-Lysozyme | Abcam | Cat# ab108508 | |
| RRID:AB_10861277 | |||
|
| |||
| Rabbit anti-Villin | Thermo Fisher Scientific | Cat# PA5-22072 | |
| RRID:AB_11155190 | |||
|
| |||
| Rabbit anti-Chromogranin A | Abcam | Cat# ab15160 | |
| RRID:AB_301704 | |||
|
| |||
| Rabbit anti-Phospho-Histone H2A.X (Ser139) (20E3) | Cell Signaling Technology | Cat# 9718S | |
| RRID:AB_2118009 | |||
|
| |||
| Rabbit anti-Cleaved Caspase-3 (Asp175) (5A1E) | Cell Signaling Technology | Cat# 9664 | |
| RRID:AB_2070042 | |||
|
| |||
| Rabbit anti-β-Catenin (D10A8) | Cell Signaling Technology | Cat# 9587S | |
| RRID:AB_10695312 | |||
|
| |||
| Rabbit anti-Non-phospho (Active) β-Catenin (D13A1) | Cell Signaling Technology | Cat# 8814s | |
| RRID:AB_11127203 | |||
|
| |||
| Rabbit anti-c-Myc (N262) | Santa Cruz Biotechnology | Cat# sc-764 | |
| RRID:AB_631276 | |||
|
| |||
| Rabbit anti-Cyclin D1 (92G2) | Cell Signaling Technology | Cat# 2978P | |
| RRID:AB_10839128 | |||
|
| |||
| Rat anti-CD44 | BD | Cat# 550538 | |
| Biosciences | RRID:AB_393732 | ||
|
| |||
| Rat anti-CD133 | eBioscience | Cat# 14-1331-82 | |
| RRID:AB_467471 | |||
|
| |||
| Mouse anti-P53 (Ab-1) | Thermo Fisher Scientific | Cat# MS-104-P0 | |
| RRID:AB_64407 | |||
|
| |||
| Rabbit anti-P21 (M-19) | Santa Cruz Biotechnology | Cat#sc-471 | |
| RRID:AB_632123 | |||
|
| |||
| Rabbit anti-Phospho-Chk1 (Ser345) | Cell Signaling Technology | Cat#2341 | |
| RRID:AB_330023 | |||
|
| |||
| Mouse anti-ATM pS1981 | Rockland | Cat# 200-301-500 | |
| RRID:AB_828098 | |||
|
| |||
|
Chemicals, Peptides, and Recombinant Proteins
| |||
| 5-Bromo-2’-deoxyuridine | Sigma | Cat# B5002 | |
|
| |||
| Y-27632 dihydrochloride | Sigma | Cat# Y0503 | |
|
| |||
| Recombinant Mouse R-Spondin 1 | R&D Systems | Cat# 3474-RS-050 | |
|
| |||
| Recombinant Murine Noggin | Peprotech | Cat# 250-38 | |
|
| |||
| Recombinant Murine EGF | Peprotech | Cat# 315-09 | |
|
| |||
| Jagged-1 peptide | AnaSpec | AS-61298 | |
|
| |||
|
Critical Commercial Assays
| |||
| REPLI-g WTA Single Cell Kit | Qiagen | Cat# 150063 | |
|
| |||
|
Experimental Models: Cell Lines
| |||
| FHC | ATCC | CRL-1831 | |
|
| |||
| RRID:CVCL_3688 | |||
|
| |||
| HCT116 | ATCC | CCL-247 | |
| RRID:CVCL_0291 | |||
|
| |||
| SW620 | ATCC | CCL-227 | |
| RRID:CVCL_0547 | |||
|
| |||
| HT29 | ATCC | HTB-38 | |
| RRID:CVCL_0320 | |||
|
| |||
|
Experimental Models: Organisms/Strains
| |||
| Mouse: Lgr5-EGFP-IRES-creERT2 (B6.129P2-Lgr5tm1(cre/ERT2)Cle/J) | The Jackson Laboratory | JAX: 008875 | |
| RRID:IMSR_JAX:008875 | |||
|
| |||
| Mouse: ApcMin/+ (C57BL/6J-ApcMin/J) | The Jackson Laboratory | JAX: 002020 | |
| RRID:IMSR_JAX:002020 | |||
|
| |||
| Mouse: TERTTCE/+ | Jun et al., 2016 | Jae-il Park, jaeil@mdanderson.org | |
|
| |||
| Mouse: Bmi1EGFP (BKa.Cg-Ptprcb Bmi1tm1Ilw Thy1a/J) | The Jackson Laboratory | JAX: 017351 | |
| RRID:IMSR_JAX:017351 | |||
|
| |||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664 | |
| RRID:IMSR_JAX:000664 | |||
|
| |||
|
Recombinant DNA
| |||
| MSCV-c-Myc-IRES-RFP | Kawauchi et al., 2012 | Addgene #35395 | |
|
| |||
|
Sequence-Based Reagents
| |||
| Primers for qPCR and ChIP, see Table S3 | This paper | N/A | |
|
| |||
|
Deposited Data
| |||
| RNA-seq data set | This paper | GEO: GSE109209 | |
HIGHLIGHTS.
PAF is expressed in intestinal stem cells and upregulated in regenerating crypts
PAF transactivates c-Myc in intestinal stem cells of the regenerating crypts
PAF-Myc axis is required for intestinal regeneration
PAF-Myc axis is required for stemness of colorectal cancer cells
Acknowledgments
We are grateful to Christopher Cervantes, Youn-Sang Jung, Seung-Hyo Lee, and Junjie Chen for helpful comments on the manuscript. This work was supported by the Cancer Prevention and Research Institute of Texas (RP140563), the National Institutes of Health (R01 CA193297-01), the Department of Defense (CA140572), the National Cancer Institute (P50 CA098258), the Duncan Family Institute Research Program, the University Cancer Foundation (IRG-08-061-01), the Center for Stem Cell and Developmental Biology (MD Anderson Cancer Center), an Institutional Research Grant (MD Anderson Cancer Center), a New Faculty Award (MD Anderson Cancer Center Support Grant), a Metastasis Research Center Grant (MD Anderson Cancer Center), and Uterine SPORE Career Enhancement Program (MD Anderson Cancer Center). The Genetically Engineered Mouse Facility was supported by the MD Anderson Cancer Center Support Grant (CA016672).
Footnotes
AUTHOR CONTRIBUTIONS
M.J.K. and J.-I.P. conceived the experiments; M.J.K., H.N.S., S.H.L., S.J., E.M.L., and J.Z. performed the experiments; B.X. and K.C. analyzed RNA-Seq results; M.J.K. and J.-I.P. analyzed the data; M.J.K. and J.-I.P. wrote the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
Supplemental Tables
Table S1. List of PAF up- and down-regulated signaling identified by KEGG analysis (Related to Figure 7 and Figure S7)
Table S2. Result of GSEA analysis of PAF KO cells (Related to Figure 7 and Figure S7)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki R, Hoki Y, Uda M, Nakamura M, Jincho Y, Tamura C, Sunayama M, Ando S, Sugiura M, Yoshida MA, et al. Crucial role of c-Myc in the generation of induced pluripotent stem cells. Stem Cells. 2011;29:1362–1370. doi: 10.1002/stem.685. [DOI] [PubMed] [Google Scholar]
- Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, Westphalen CB, von Burstin J, Mastracci TL, Worthley DL, et al. Krt19(+)/Lgr5(-) Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell. 2015;16:627–638. doi: 10.1016/j.stem.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Van Es JH, Kuipers J, Kujala P, Van Den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bettess MD, Dubois N, Murphy MJ, Dubey C, Roger C, Robine S, Trumpp A. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol Cell Biol. 2005;25:7868–7878. doi: 10.1128/MCB.25.17.7868-7878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczacki SJA, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Li K, Diao D, Zhu K, Shi L, Zhang H, Yuan D, Guo Q, Wu X, Liu D, et al. Expression of KIAA0101 protein is associated with poor survival of esophageal cancer patients and resistance to cisplatin treatment in vitro. Laboratory Investigation. 2013;93:1276–1287. doi: 10.1038/labinvest.2013.124. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- De Biasio A, de Opakua AI, Mortuza GB, Molina R, Cordeiro TN, Castillo F, Villate M, Merino N, Delgado S, Gil-Carton D, et al. Structure of p15(PAF)-PCNA complex and implications for clamp sliding during DNA replication and repair. Nat Commun. 2015;6:6439. doi: 10.1038/ncomms7439. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Emanuele MJ, Ciccia A, Elia AEH, Elledge SJ. Proliferating cell nuclear antigen (PCNA)-associated KIAA0101/PAF15 protein is a cell cycle-regulated anaphase-promoting complex/cyclosome substrate. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9845–9850. doi: 10.1073/pnas.1106136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman MD, Rothberg PG, Diehl RE, Morse CC, Spandorfer JM, Astrin SM. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol Cell Biol. 1985;5:1969–1976. doi: 10.1128/mcb.5.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Hosen N, Yamane T, Muijtjens M, Pham K, Clarke MF, Weissman IL. Bmi-1-green fluorescent protein-knock-in mice reveal the dynamic regulation of bmi-1 expression in normal and leukemic hematopoietic cells. Stem Cells. 2007;25:1635–1644. doi: 10.1634/stemcells.2006-0229. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Takehara A, Matsuda K, Eguchi H, Ohigashi H, Ishikawa O, Shinomura Y, Imai K, Nakamura Y, Nakagawa H. Oncogenic role of KIAA0101 interacting with proliferating cell nuclear antigen in pancreatic cancer. Cancer Research. 2007;67:2568–2576. doi: 10.1158/0008-5472.CAN-06-4356. [DOI] [PubMed] [Google Scholar]
- Jain M, Zhang L, Patterson EE, Kebebew E. KIAA0101 is overexpressed, and promotes growth and invasion in adrenal cancer. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0026866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S, Jung YS, Suh HN, Wang W, Kim MJ, Oh YS, Lien EM, Shen X, Matsumoto Y, McCrea PD, et al. LIG4 mediates Wnt signalling-induced radioresistance. Nat Commun. 2016;7:10994. doi: 10.1038/ncomms10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S, Lee S, Kim HC, Ng C, Schneider AM, Ji H, Ying H, Wang H, DePinho RA, Park JI. PAF-mediated MAPK signaling hyperactivation via LAMTOR3 induces pancreatic tumorigenesis. Cell Rep. 2013;5:314–322. doi: 10.1016/j.celrep.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HY, Jun S, Lee M, Kim HC, Wang X, Ji H, McCrea PD, Park JI. PAF and EZH2 induce wnt/β-catenin signaling hyperactivation. Molecular Cell. 2013;52:193–205. doi: 10.1016/j.molcel.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kais Z, Barsky SH, Mathsyaraja H, Zha A, Ransburgh DJR, He G, Pilarski RT, Shapiro CL, Huang K, Parvin JD. KIAA0101 interacts with BRCA1 and regulates centrosome number. Molecular Cancer Research. 2011;9:1091–1099. doi: 10.1158/1541-7786.MCR-10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Daigo Y, Aragaki M, Ishikawa K, Sato M, Kaji M. Overexpression of KIAA0101 predicts poor prognosis in primary lung cancer patients. Lung Cancer. 2012;75:110–118. doi: 10.1016/j.lungcan.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G, et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 2010;70:719–729. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Laurenti E, Varnum-Finney B, Wilson A, Ferrero I, Blanco-Bose WE, Ehninger A, Knoepfler PS, Cheng PF, MacDonald HR, Eisenman RN, et al. Hematopoietic Stem Cell Function and Survival Depend on c-Myc and N-Myc Activity. Cell Stem Cell. 2008;3:611–624. doi: 10.1016/j.stem.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe C, Kljavin NM, Ybarra R, De Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Onda M, Asaka S, Akaishi J, Miyamoto S, Yoshida A, Nagahama M, Ito K, Emi M. Overexpressed in anaplastic thyroid carcinoma-1 (OEATC-1) as a novel gene responsible for anaplastic thyroid carcinoma. Cancer. 2005;103:1785–1790. doi: 10.1002/cncr.20988. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk MEG, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Muñoz J, Stange DE, Schepers AG, Van De Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, et al. The Lgr5 intestinal stem cell signature: Robust expression of proposed quiescent ‘+4’ cell markers. EMBO Journal. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz NM, Upton M, Rojas A, Washington MK, Lin L, Chytil A, Sozmen EG, Madison BB, Pozzi A, Moon RT, et al. Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Res. 2006;66:9837–9844. doi: 10.1158/0008-5472.CAN-06-0890. [DOI] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Onuma K, Ochiai M, Orihashi K, Takahashi M, Imai T, Nakagama H, Hippo Y. Genetic reconstitution of tumorigenesis in primary intestinal cells. Proc Natl Acad Sci U S A. 2013;110:11127–11132. doi: 10.1073/pnas.1221926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31:2737–2746. doi: 10.1038/emboj.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlsen LK, Beli P, Wagner SA, Poulsen SL, Sylvestersen KB, Poulsen JW, Nielsen ML, Bekker-Jensen S, Mailand N, Choudhary C. Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass. Nature Cell Biology. 2012;14:1089–1098. doi: 10.1038/ncb2579. [DOI] [PubMed] [Google Scholar]
- Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, et al. The pan-ErbB negative regulator lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nature Genetics. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011a;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Sato T, Van Es JH, Snippert HJ, Stange DE, Vries RG, Van Den Born M, Barker N, Shroyer NF, Van De Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011b;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Sikora K, Chan S, Evan G, Gabra H, Markham N, Stewart J, Watson J. c-myc oncogene expression in colorectal cancer. Cancer. 1987;59:1289–1295. doi: 10.1002/1097-0142(19870401)59:7<1289::aid-cncr2820590710>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Suh HN, Kim MJ, Jung YS, Lien EM, Jun S, Park JI. Quiescence Exit of Tert(+) Stem Cells by Wnt/beta-Catenin Is Indispensable for Intestinal Regeneration. Cell Rep. 2017;21:2571–2584. doi: 10.1016/j.celrep.2017.10.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, Van Den Born M, Korving J, De Sauvage F, Van Es JH, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, De Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nature Cell Biology. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- Van Es JH, Sato T, Van De Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, Van Den Born M, Korving J, et al. Dll1 + secretory progenitor cells revert to stem cells upon crypt damage. Nature Cell Biology. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlakhanova NV, Cotterman RF, deVries WN, Morgan J, Donahue LR, Murray S, Knowles BB, Knoepfler PS. myc maintains embryonic stem cell pluripotency and self-renewal. Differentiation. 2010;80:9–19. doi: 10.1016/j.diff.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jung YS, Jun S, Lee S, Wang W, Schneider A, Sun Oh Y, Lin SH, Park BJ, Chen J, et al. PAF-Wnt signaling-induced cell plasticity is required for maintenance of breast cancer cell stemness. Nature Communications. 2016;7 doi: 10.1038/ncomms10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche AC, Knabenhans C, Macdonald HR, Trumpp A. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Huang B, Shen M, Lau C, Chan E, Michel J, Xiong Y, Payan DG, Luo Y. p15PAF, a novel PCNA associated factor with increased expression in tumor tissues. Oncogene. 2001;20:484–489. doi: 10.1038/sj.onc.1204113. [DOI] [PubMed] [Google Scholar]
- Yuan RH, Jeng YM, Pan HW, Hu FC, Lai PL, Lee PH, Hsu HC. Overexpression of KIAA0101 predicts high stage, early tumor recurrence, and poor prognosis of hepatocellular carcinoma. Clinical Cancer Research. 2007;13:5368–5376. doi: 10.1158/1078-0432.CCR-07-1113. [DOI] [PubMed] [Google Scholar]
- Zeilstra J, Joosten SPJ, Dokter M, Verwiel E, Spaargaren M, Pals ST. Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Research. 2008;68:3655–3661. doi: 10.1158/0008-5472.CAN-07-2940. [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


