Abstract
Mitochondrial dysfunction may be an important, if not essential, component of human glaucoma. Using transcriptomics followed by molecular and neurobiological techniques, we have recently demonstrated that mitochondrial dysfunction within retinal ganglion cells is an early feature in the DBA/2J mouse model of inherited glaucoma. Guided by these findings, we discovered that the retinal level of nicotinamide adenine dinucleotide (NAD, a key molecule for mitochondrial health) declines in an age-dependent manner. We hypothesized that this decline in NAD renders retinal ganglion cells susceptible to damage during periods of elevated intraocular pressure. To replete NAD levels in this glaucoma, we administered nicotinamide (the amide of vitamin B3). At the lowest dose tested, nicotinamide robustly protected from glaucoma (~70% of eyes had no detectable glaucomatous neurodegeneration). At this dose, nicotinamide had no influence on intraocular pressure and so its affect was neuroprotective. At the highest dose tested, 93% of eyes had no detectable glaucoma. This represents a ~10-fold decrease in the risk of developing glaucoma. At this dose, intraocular pressure still became elevated but there was a reduction in the degree of elevation showing an additional benefit. Thus, nicotinamide is unexpectedly potent at preventing this glaucoma and is an attractive option for glaucoma therapeutics. Our findings demonstrate the promise for both preventing and treating glaucoma via interventions that bolster metabolism during increasing age and during periods of elevated intraocular pressure. Nicotinamide prevents age-related declines in NAD (a decline that occurs in different genetic contexts and species). NAD precursors are reported to protect from a variety of neurodegenerative conditions. Thus, nicotinamide may provide a much needed neuroprotective treatment against human glaucoma. This manuscript summarizes human data implicating mitochondria in glaucoma, and argues for studies to further assess the safety and efficacy of nicotinamide in human glaucoma care.
Keywords: Glaucoma, NAD+, nicotinamide, axon degeneration, retinal ganglion cell, optic nerve head cupping
Glaucoma, mitochondrial dysfunction, and nicotinamide treatment
Affecting ~80 million people by the end of this decade, glaucoma is a leading cause of blindness worldwide 1. It represents a significant economic and health burden. Glaucoma is characterized by the progressive dysfunction and loss of retinal ganglion cells (RGCs) and their axons, which make up the neural tissue of the optic nerve. Major risk factors for glaucoma include increased intraocular pressure (IOP) and age. The DBA/2J (D2) mouse is an age-dependent, inherited model of high IOP and glaucoma 2–11. Ourselves and others have established that the key features of glaucomatous neurodegeneration in DBA/2J mice match those in human patients. This includes the progressive nature and specificity of RGC demise 12, 13, the location of a critical insult to RGC axons within the optic nerve head 14, the topographic pattern of cell loss 15 (fan-shaped from the optic disk 14), and the lessening of neurodegeneration by lowering IOP 7, 16–18. The similarities extend to molecular changes, with changes in specific pathways being demonstrated in both DBA/2J mice and human patients (including the endothelin pathway and its receptors and various complement pathway molecules 2, 12, 19–22).
In order to elucidate the earliest molecular changes that occur in glaucoma we have used RNA-sequencing (RNA-seq) to analyze D2 RGCs at different ages and stages of disease 23. We discovered that metabolic dysfunction and mitochondrial abnormalities occur prior to neurodegeneration, at a time-point that corresponds with early decreases in electrical activity as assessed by pattern electroretinogram (PERG). Guided by these results, we performed metabolic profiling, which identified an age-dependent decrease in the levels of retinal NAD. Targeting this metabolic decline, by repleting levels of NAD by administering its precursor nicotinamide, robustly protected eyes from glaucoma 23 (Fig. 1). This treatment protected all assessed RGC compartments including the axon, soma, and synapses 23, 24. Additionally, it protected from declines in the very early and sensitive measures of RGC dysfunction; PERG and axoplasmic transport. Some of the very earliest detectable changes in RGCs following periods of elevated IOP were also prevented (mitochondrial dysfunction and transcriptomic changes) (Fig. 2). To develop a ‘one-shot’ treatment for glaucoma, we tested a gene therapy (over expressing Nmnat1, coding a key NAD producing enzyme). Gene therapy has the advantage of overcoming compliance issues by being long-lasting (possibly life-long). This Nmnat1 gene therapy was sufficient to protect the majority of eyes from glaucoma. Combining this gene therapy with nicotinamide treatment was even more effective, protecting significantly more eyes 23.
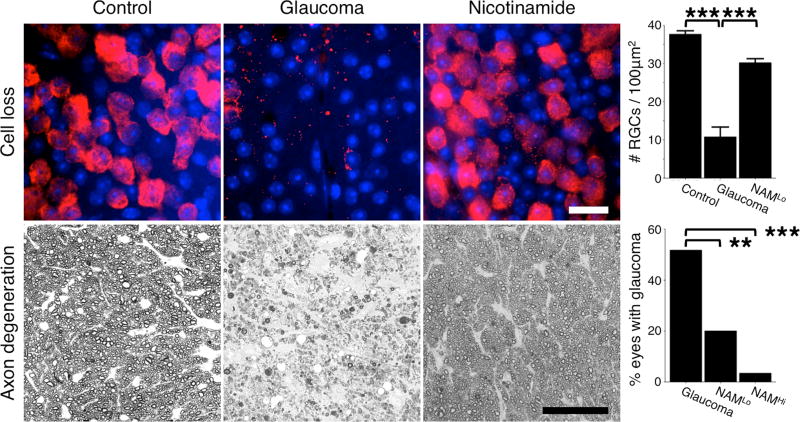
Figure 1. Retinal ganglion cell protection following nicotinamide treatment in the DBA/2J mouse model of inherited glaucoma.
Nicotinamide profoundly protects retinal ganglion cells and prevents optic nerve degeneration in a dose dependent manner. Top row shows flat-mounted retinas stained with an anti-RBPMS antibody that specifically labels retinal ganglion cells (red) and counterstained with DAPI that stains nuclei (blue). There is a significant loss of retinal ganglion cells following periods of elevated IOP (top row, middle panel), which is prevented by nicotinamide treatment (top row, right panel). Bottom row shows cross sections of the optic nerve stained with PPD. Following periods of elevated IOP retinal ganglion cell axons in the optic nerve degenerate and glial scars are formed (bottom row, middle panel). Nicotinamide treatment (NAMLo) robustly protected the axons, and the number of optic nerves with glaucoma was significantly decreased. At a higher dose (NAMHi) 93% of optic nerves did not develop glaucoma (bottom row, chart). Scale bars = 20μm (top row), 50μm (bottom row). ** = P < 0.01, *** = P < 0.001, Student’s t-test (top), Fisher’s exact test (bottom). All images are for mice that were 12 months of age. See references 23, 24 for more details.
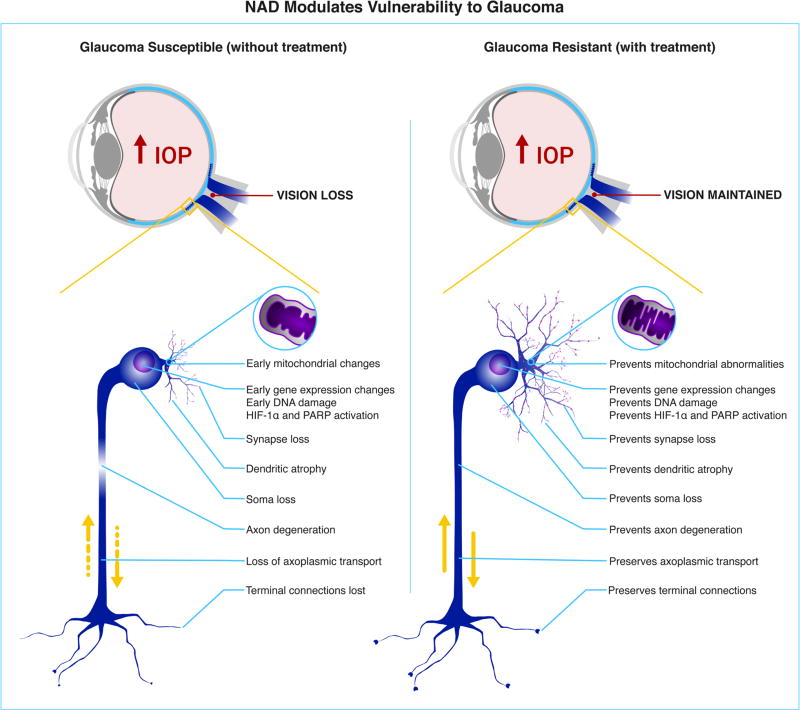
Figure 2. NAD protects from retinal ganglion cell degeneration and glaucoma.
NAD levels decrease with age rendering retinal ganglion cells vulnerable to IOP-induced damage. Preventing this age-dependent change by increasing NAD availability robustly protects from all assessed signs of glaucoma. Increasing NAD using nicotinamide 23, Nmnat1 gene therapy 23, the WldS allele 24, 29, or combinations of these robustly protect retinal ganglion cells from degeneration. This protection is neuroprotective, as neither the low dose that we used (NAMLo) or the other treatments changed the IOP. Higher doses of nicotinamide may have an additional benefit by also limiting the degree of IOP elevation 23. The inhibitory affect of nicotinamide on various enzymes may enhance its potency against glaucoma through different mechanisms (see text). Left shows a retinal ganglion cell undergoing glaucomatous changes due to high IOP. Right shows a retinal ganglion cell that is protected from the detrimental effects of IOP due to increased NAD levels presumably within the RGC. The affects of NAD on other cell types may also be important. See references 23, 24, 29 for more details.
Further evidence of the robust protection from glaucoma in mice with increased NAD levels is provided by our findings using D2 mice carrying the Wallerian degeneration slow allele (WldS, which encodes additional NMNAT1 activity) 14, 25–28. D2 mice with the WldS allele were protected from glaucoma to a similar degree as mice with nicotinamide treatment alone (~70% of eyes not developing glaucoma) 24. We also tested the effect of combining both enzyme and precursor (i.e. WLDS plus nicotinamide). This robustly prevented development of glaucoma more than either treatment alone (94% of eyes not developing glaucoma) 24. Importantly, these treatments protected from RGC synapse loss and prevented dendrite pruning out to the oldest ages tested 24, 29. Although further experiments with a variety of animal models are needed to assess how widespread and robust such NAD based protection may be in glaucoma, we also demonstrated protection against two experimentally induced models of RGC death and protection has been shown in other models by other groups 23. Expression of a cytoplasmically localized variant of NMNAT1 (in mice) or NMNAT3 (in rats) protected from experimentally induced models of RGC death and glaucoma 30, 31. Together, these data suggest that interventions that target NAD levels protect both RGC morphology and function, and may transform patient care for glaucoma.
Mitochondria and human glaucoma
When assessing the translational relevance of our findings, it is important to consider the potential importance of mitochondrial dysfunction in human glaucoma. Mitochondria have been considered relevant to glaucoma for some time 32–37. Our studies extend this by demonstrating that mitochondrial dysfunction occurs as one of the earliest detectable events within RGCs following IOP elevation in vivo in a chronic mouse model of glaucoma and that nicotinamide treatment is remarkably protective 23. As further discussed below, a growing literature suggests that mitochondrial functions are relevant to the human disease and that they may underlie both susceptibility and resistance to developing glaucoma. Together with our findings, this suggests that nicotinamide treatment may prove beneficial in human glaucoma and studies to directly evaluate it are of the utmost importance.
Although further studies are needed, emerging research suggests that a systemic vulnerability to mitochondrial abnormalities and metabolic demise exists in open-angle glaucoma patients compared to controls (in mitochondrial complexes I, III, IV, and V) 38. DNA analysis has demonstrated increased mitochondrial DNA content 38 as well as a spectrum of mtDNA mutations and mutations in nuclear-encoded mitochondrial protein-coding genes in both open-angle and normal tension glaucoma patients 39–45. Such mitochondrial abnormalities were present in peripheral blood leukocytes suggesting a systemic susceptibility to metabolic abnormalities (as opposed to mitochondrial changes in the eye as a consequence of high IOP) 46. Increased mitochondrial DNA content provides evidence of imbalance between mitochondrial and nuclear genomes that predispose to mitochondrial dysfunction. Decreased plasma citrate levels have been suggested as a biomarker for human glaucoma 47. Citrate is an important substrate in energy production within mitochondria. It is produced within mitochondria and so reduced mitochondrial activity may underlie the lower citrate levels. In another study, lymphoblasts from open-angle glaucoma patients had decreased mitochondrial complex I-mediated oxidative phosphorylation, again supporting systemic susceptibility of mitochondria 48. Such systemic susceptibility is expected to contribute to mitochondrial damage and increasing vulnerability to glaucoma with increasing age. Taken together with our data, this susceptibility would be predicted to increase the likelihood of an energetic crisis and RGC dysfunction when RGCs are subject to stresses induced by high IOP in human glaucoma. On the other hand, having more reliable or efficient mitochondria may protect from glaucoma when IOP is high. In fact, glaucoma resistant individuals who have not developed glaucomatous neuropathy despite years of high IOP are reported to have systemic mitochondrial efficiency 49, including increased rates of ADP phosphorylation by mitochondrial complexes I, II and IV as compared to both unaffected controls and glaucoma patients. Other lines of inquiry have demonstrated that OPA1 expression (which promotes mitochondrial stability) was decreased in open-angle glaucoma patients 50 and genome wide expression studies (GWAS) have linked a key mitochondrial gene (TXNRD2) to glaucoma susceptibility 51. A recent study has identified certain African (and African-American) mtDNA haplogroups as risk factors for primary open-angle glaucoma. These haplogroups contain ancestral variants for mitochondrial genes MT-RNR2 and MT-CO1 that have known roles in other degenerative diseases 52, 53. Another study found that groups of common variants related by shared membership in mitochondrial and metabolic pathways had associations with primary open-angle and normal tension glaucoma 54. Given all of these observations, our findings of mitochondrial damage as an early and key driver in D2 glaucoma, may well generalize to human patients. Taken as a whole, these studies support including glaucoma in the spectrum of mitochondrial optic neuropathies 55–57.
Choice of NAD precursor and safety
Therapies to increase NAD levels and improve metabolic reliability under stress may be effective against glaucoma 23, 24, 29, 58. Thus, the administration of NAD precursors offers promise for improving glaucoma prevention and care. As decreasing NAD levels appear to be a common feature of aging in different tissues and species 59–63, the use NAD precursors may be effective in a wide variety of glaucoma cases. The profound nicotinamide-mediated protection that we have demonstrated supports testing the use of nicotinamide in human glaucoma. Nicotinamide has a good safety profile especially when used at 3 g/d or below 64. Despite the extensive use of nicotinamide, human safety studies in aged glaucoma populations are necessary. In our studies, the lowest dose used (nicotinamide low dose; NAMLo) is equivalent to ~2.7 g/day for a 60 kg human (the human dose equivalent is based on a mouse dose of 550 mg/kg/d 65). At this dose structural and functional changes were prevented and the overall neural protection was robust, despite no impact on IOP elevation. The highest dose that we tested (nicotinamide high dose; NAMHi, 2000 mg/kg/d) is equivalent to ~9.8 g/day for a 60 kg human and was extremely protective against glaucoma. At this dosage, IOP became elevated but to a lesser magnitude than in untreated eyes, suggesting that NAM has effects on a variety of cell types and may have dual benefit in glaucoma.
Other NAD precursors must also be considered. The most appropriate or most effective NAD-precursor may depend on tissue and disease context. Compliance issues, including ease of dosing, are important when considering specific precursors, with more frequent dosing being more problematic, especially in the elderly. Of the commonly used precursors, nicotinic acid has the most unpleasant side effects and nicotinic acid (NA) is more noxious than nicotinamide (NAM) and nicotinamide riboside (NR) 64, 66. These side effects of NA include flushing and gut irritation that significantly impact compliance. Nevertheless, both NA and NAM have a long history of human use that demonstrates good safety with minimal adverse effects. Safety data are derived from studies evaluating high doses ~3–9 g/day (or more in some cases) for long periods of time (up to 5 years 64). In one study, there were only 3 cases of hepatotoxicity among 6000 patients on megadoses of NA and/or NAM. One of these cases normalized without withdrawing NA, while another normalized when concomitant phenothiazine was withdrawn but the patient was still on niacin. The other patient was on 9 g/day niacin and likely had individual susceptibility to this hepatotoxicity 64. It is important to note that the term niacin originally referred to NA, and this patient was likely on NA. The term niacin is confusing, however, as it is also used to refer to a mixture of NA and NAM or rarely NAM alone. Thus, caution must be taken when referring to niacin, nicotinic acid, or vitamin B3 in older literature. A case of hepatoxicity caused by 9g/day NAM has been reported, however, emphasizing the need to monitor for individual susceptibility when high doses are consumed 67.
Regarding ocular side effects, there is one case in which NA raised IOP 68. Despite the common long-term use of high doses of niacin (often 2–3 g/day) to lower cholesterol or treat other conditions, we have not found any other reports of this effect on IOP. In fact, niacin was reported to lower IOP in 12 AMD patients and our highest dose of NAM lessened the degree of IOP elevation in DBA/2J mice 23, 69. Macular edema without fluorescein leakage is a rare complication of NA (0.67% of patients treated for hyperlipidemia) that can be easily detected and reversed by stopping NA supplementation 70. It is not clear if NAM or NR ever induces this phenotype, however, and there are limited safety data for NR. Given its documented safety, tolerability and other potential advantages discussed below, we chose to work with NAM over NA or other NAD precursors.
In recent years, various studies have promoted the use of NR over NAM. NR can be converted to NAD independently of NAM (through NRKs) and is claimed to be more bioavailable and more effective at increasing NAD levels for a given dose. NR is also claimed to be superior to NAM because it does not inhibit the activity of sirtuins (SIRTs) 71. NAM is a physiological regulator that inhibits SIRTs. SIRTs are important NAD consuming enzymes that deacetylate lysines on proteins. They are key regulators of metabolism and mitochondrial reprogramming with aging. Their activities are known to be important for NAD mediated protection in various settings 62, 71–73. Although there is merit to these arguments, we do not necessarily agree that NR is superior for treating glaucoma (discussed in more detail below) and comparative tests are required to definitively test this.
A recent mouse and human study comparing NR and NAM claimed superior bioavailability of NR 66. NR was effective at raising NAD in liver, adipose tissue, and muscle shortly after administration, and NR was reported to be more bioavailable and more effective at raising NAD than NAM. From the reported data, it is clear that NR raised NAD levels more rapidly and to a greater degree than NAM in liver. However, when NAD totals are calculated as the area under the curve over a 12–14 hour period there is no clear difference (in liver 66). The area under the curve for both NR and NAM are almost identical with NAM providing a more sustained NAD increase (see Figure 5b of 66). Thus, NR may be better if frequent or continuous dosing is possible. However, due to the preference for a simpler less frequent administration protocol to enhance compliance, it could be argued that the more sustained NAD altering kinetics of NAM are preferable. Ultimately, the kinetics of NAD increase and duration in the retina and optic nerve (and possibly brain) are important for glaucoma. In mammalian cells, NRKs are necessary for the conversion of NR to NAD (and rate limiting), while NRKs are not required for conversion of NAM to NAD 74. Initial studies suggest that NRK protein levels are low in brain 74, but NR successfully increased NAD levels in whole brain by ~1.5-fold 66. In our previously published and publically available RNA-seq datasets, the transcript abundance of NRK genes (Nmrk1, Nmrk2) indicates that they are only lowly expressed in RGCs 23. Thus it is not clear that NR would be more effective against glaucoma than NAM, although whole retina effects and systemic effects cannot be discounted. Thus, although NR should not be discounted, NAM warrants serious consideration and may prove equally or even more effective against glaucoma. An overview of the major NAD precursors and the pathways involved are shown in Fig. 3.
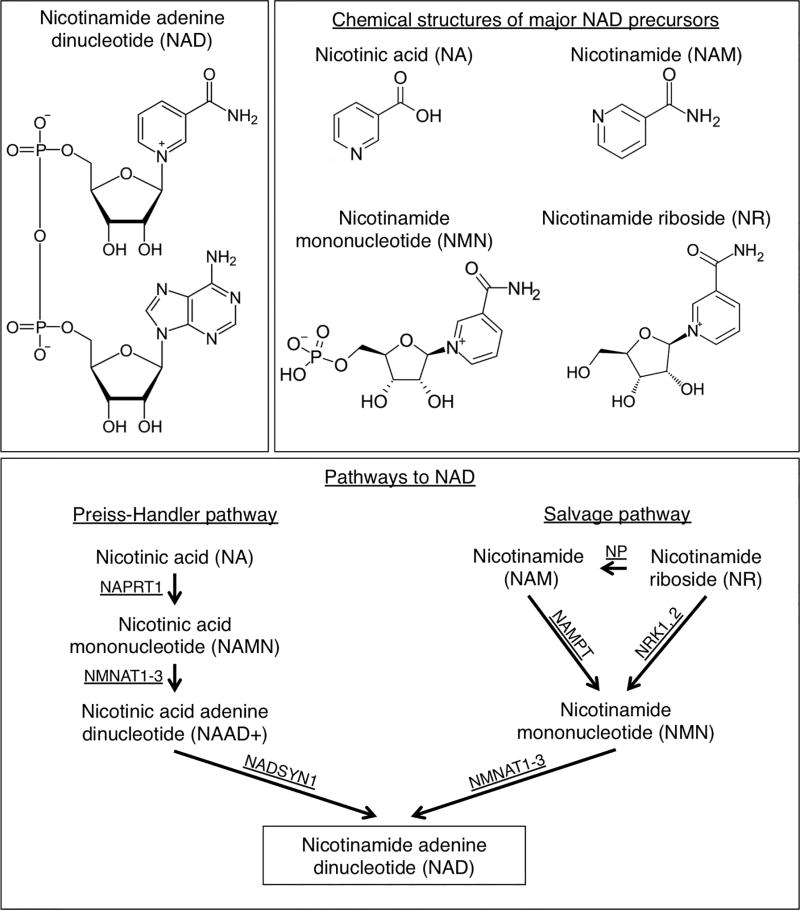
Figure 3. NAD synthesis.
The chemical structure of nicotinamide adenine dinucleotide (NAD) (top left) and four major NAD precursors; nicotinic acid (NA), nicotinamide (NAM), nicotinamide mononucleotide (NMN), and nicotinamide riboside (NR) (top right). NAD can be produced de novo from dietary tryptophan. Alternatively NAD can be produced through two other core pathways; the Preiss-Handler pathway from NA, or through the salvage pathway from NAM (bottom panel). NAM is available in diet and readily absorbed as a major NAD precursor through the salvage pathway in vivo. Enzymes: NAPRT, nicotinic acid phosphoribosyltransferase; NADSYN, NAD synthetase 1; NAMPT, nicotinamide phosphoribosyltransferase; NMNAT1, -2, and -3 nicotinamide nucleotide adenylytransferases 1, 2 and 3; NRK1, -2, nicotinamide riboside kinases (mouse gene names Nmrk1, -2); NP, purine nucleoside phosphorylase.
The current literature would suggest that NAM makes it into RGCs intact, thus there are several potential advantages of NAM treatment for glaucoma over other NAD precursors as discussed in the following paragraphs. Firstly, NAM administration is already shown to rapidly increase retinal NAD levels (an ~3-fold increase that is sustained using our NAMLo dose – 550 mg/kg/d) and to robustly prevent all assessed signs of glaucoma including RGC and optic nerve degeneration 23. If these changes hold true in glaucoma patients, then NAM will be an attractive treatment option.
Secondly, NAM is a unique in that it is a major natural precursor for NAD in mammals in vivo 75, 76. It is also unique among NAD precursors because it is a physiological inhibitor of the major NAD catabolic enzymes: (1) CD38 is a major NAD consumer, and CD38 is inhibited by NAM 77; (2) PARPs are upregulated in the retina during glaucoma and are major NAD consumers that deplete ATP under stress conditions, PARPs are inhibited by NAM 78; (3) SIRTs are major NAD consumers and SIRTs are inhibited by NAM 79, 80. The unique inhibitory effects of NAM may have multiple complex effects that are not easily predictable and some of these may be advantageous in a glaucoma setting. For example and by inhibiting PARP, NAM may protect from further ATP depletion until metabolism is normalized. Additionally, inhibition of NAD catabolism may be advantageous as it would be expected to allow more rapid or greater local increases in NAD. SIRTs are class III histone deacetylases (HDACs) and HDAC inhibition is at least partially protective in various models of neurodegenerative disease, including RGC death and DBA/2J glaucoma 81–83. On the other hand, NAMs inhibitory functions have been suggested to be detrimental as SIRT activity is required for NAD mediated protection in some settings 62, 71, 73, 84, 85. Further experiments are needed to determine whether or not inhibition of some HDACs or activation of specific SIRTs is required for the protection against glaucoma. It is also possible that the inhibition of potentially protective SIRTs by NAM is relieved at higher NAD concentrations. NAM based inhibition of SIRTs was initially thought to be a traditional noncompetitive base-exchange inhibition. However, more recent kinetic data demonstrate different inhibition characteristics between various SIRTs that include apparent competition between NAM and NAD+ 86. Differences in inhibition kinetics can be explained by differences in NAD+ binding affinity between specific enzymes.
Thirdly, NAM has documented effects on calcium channels and calcium signaling at least in part through inhibition of ADPRC and its target the ryanodine receptor 87. Calcium signaling/mobilization is important in axon degeneration and may impact glaucoma 88–90.
Fourthly, NAM has vasoactive and vasoprotective properties. Vasculature dysfunction is implicated in glaucoma 91, 92. NAM can improve endothelial function and stabilize blood flow by preventing transient flow interruptions 93. Endothelins are very potent vasoconstrictors that are implicated in glaucoma in humans and animal models, with endothelin receptor blockers protecting from glaucoma 19, 94–97. NAM can reverse endothelin-mediated vasoconstriction though its inhibitory actions on ADPRC 98–100.
Lastly, since NAMPT is the rate-limiting enzyme in the conversion of NAM to NAD 101. The NAD precursor NMN, which is an intermediate in this process, should not accumulate with NAM administration, as it should be rapidly converted to NAD (see Fig. 3). This is also true for NR, as NRKs are rate limiting in its conversion to NMN. Although administration of NMN protects from retinal photoreceptor degenerations induced by NAMPT deficiency or light exposure 102, potential accumulation of NMN may be detrimental for glaucoma as NMN can participate in axon degeneration 103 (axon injury and activation of axon intrinsic degeneration pathways are central in glaucoma). Thus NAM may have better efficacy than NMN in glaucoma. Based on the above considerations of NAM’s properties as well as existing data demonstrating a very robust protection in mice, we propose testing NAM as a neuroprotective agent to combat human glaucoma.
Conclusion
In conclusion, nicotinamide (NAM; the amide of vitamin B3) has promise to be a safe and potent neuroprotective agent in human glaucoma. Although further animal studies and human clinical trials are needed, the growing literature implicating mitochondrial dysfunction and systemic mitochondrial susceptibility as determinants of vulnerability to glaucoma support this. Nicotinamide may offer an attractive combinational therapeutic with agents that lower IOP, and may have additional benefit in normal tension glaucoma patients or glaucoma patients refractory to IOP lowering medications. The possibility that nicotinamide can prevent glaucomatous neurodegeneration is an exciting prospect, with potentially important implications for other age-related or ophthalmic diseases.
Acknowledgments
Funding
The Jackson Laboratory Fellowships (PAW, JMH), EY11721 (SWMJ), the Barbara and Joseph Cohen foundation, the Partridge Foundation, and the Lano Family Foundation (SWMJ). SWMJ is an Investigator of HHMI.
The Authors would like to thank members of the John Lab for their scientific contributions, Jane Cha for graphic design, and the late Dr. Oliver Smithies for his collaboration, guidance and enthusiasm for testing nicotinamide in glaucoma and other conditions.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby RT, Anderson MG, Pang IH, et al. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- 3.Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 2011;22:78–86. doi: 10.1097/ICU.0b013e32834372ec. [DOI] [PubMed] [Google Scholar]
- 4.Nickells RW. Ganglion cell death in glaucoma: from mice to men. Vet Ophthalmol. 2007;10(Suppl 1):88–94. doi: 10.1111/j.1463-5224.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes KA, Harder JM, Williams PA, et al. Using genetic mouse models to gain insight into glaucoma: Past results and future possibilities. Experimental eye research. 2015;141:42–56. doi: 10.1016/j.exer.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinnon SJ, Schlamp CL, Nickells RW. Mouse models of retinal ganglion cell death and glaucoma. Exp Eye Res. 2009;88:816–824. doi: 10.1016/j.exer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaraju M, Saleh M, Porciatti V. IOP-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Invest Ophthalmol Vis Sci. 2007;48:4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 9.Inman DM, Sappington RM, Horner PJ, Calkins DJ. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the DBA/2 mouse model of glaucoma. Investigative Ophthalmology & Visual Science. 2006;47:986–996. doi: 10.1167/iovs.05-0925. [DOI] [PubMed] [Google Scholar]
- 10.Scholz M, Buder T, Seeber S, Adamek E, Becker CM, Lütjen-Drecoll E. Dependency of intraocular pressure elevation and glaucomatous changes in DBA/2J and DBA/2J-Rj mice. Invest Ophthalmol Vis Sci. 2008;49:613–621. doi: 10.1167/iovs.07-0745. [DOI] [PubMed] [Google Scholar]
- 11.Anderson MG, Smith RS, Hawes NL, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nature Genetics. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- 12.Williams PA, Tribble JR, Pepper KW, et al. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol Neurodegener. 2016;11:26. doi: 10.1186/s13024-016-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams PA, Howell GR, Barbay JM, et al. Retinal ganglion cell dendritic atrophy in DBA/2J glaucoma. PLoS One. 2013;8:e72282. doi: 10.1371/journal.pone.0072282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howell GR, Libby RT, Jakobs TC, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. Journal of Cell Biology. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reichstein D, Ren L, Filippopoulos T, Mittag T, Danias J. Apoptotic retinal ganglion cell death in the DBA/2 mouse model of glaucoma. Exp Eye Res. 2007;84:13–21. doi: 10.1016/j.exer.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Schuettauf F, Quinto K, Naskar R, Zurakowski D. Effects of anti-glaucoma medications on ganglion cell survival: the DBA/2J mouse model. Vision Res. 2002;42:2333–2337. doi: 10.1016/s0042-6989(02)00188-8. [DOI] [PubMed] [Google Scholar]
- 17.Sawada K, Hiraoka M, Ohguro H. Effect of Antiglaucoma Medicine on Intraocular Pressure in DBA/2J Mice. Ophthalmic Res. 2016;55:205–211. doi: 10.1159/000444057. [DOI] [PubMed] [Google Scholar]
- 18.Wong AA, Brown RE. A neurobehavioral analysis of the prevention of visual impairment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2012;53:5956–5966. doi: 10.1167/iovs.12-10020. [DOI] [PubMed] [Google Scholar]
- 19.Howell GR, Macalinao DG, Sousa GL, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121:1429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–179. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- 21.Astafurov K, Elhawy E, Ren L, et al. Oral microbiome link to neurodegeneration in glaucoma. PLoS One. 2014;9:e104416. doi: 10.1371/journal.pone.0104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stasi K, Nagel D, Yang X, et al. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2006;47:1024–1029. doi: 10.1167/iovs.05-0830. [DOI] [PubMed] [Google Scholar]
- 23.Williams PA, Harder JM, Foxworth NE, et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355:756–760. doi: 10.1126/science.aal0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams P, Harder J, Foxworth N, Cardozo B, Cochran K, John S. Nicotinamide and WLDS act together to prevent neurodegeneration in glaucoma. Frontiers in Neuroscience. 2017;11 doi: 10.3389/fnins.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mack TG, Reiner M, Beirowski B, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 26.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conforti L, Gilley J, Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- 28.Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harder JM, Braine CE, Williams PA, et al. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1608769114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y, Zhang L, Sasaki Y, Milbrandt J, Gidday JM. Protection of mouse retinal ganglion cell axons and soma from glaucomatous and ischemic injury by cytoplasmic overexpression of Nmnat1. Invest Ophthalmol Vis Sci. 2013;54:25–36. doi: 10.1167/iovs.12-10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitaoka Y, Munemasa Y, Kojima K, Hirano A, Ueno S, Takagi H. Axonal protection by Nmnat3 overexpression with involvement of autophagy in optic nerve degeneration. Cell Death Dis. 2013;4:e860. doi: 10.1038/cddis.2013.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol. 2013;13:12–15. doi: 10.1016/j.coph.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Ju WK, Kim KY, Lindsey JD, et al. Elevated hydrostatic pressure triggers release of OPA1 and cytochrome C, induces apoptotic cell death in differentiated RGC-5 cells. Mol Vis. 2009;15:120–134. [PMC free article] [PubMed] [Google Scholar]
- 34.Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18:93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Van Bergen NJ, Kong GY, et al. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res. 2011;93:204–212. doi: 10.1016/j.exer.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Lascaratos G, Garway-Heath DF, Willoughby CE, Chau KY, Schapira AH. Mitochondrial dysfunction in glaucoma: understanding genetic influences. Mitochondrion. 2012;12:202–212. doi: 10.1016/j.mito.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Inman DM, Harun-Or-Rashid M. Metabolic Vulnerability in the Neurodegenerative Disease Glaucoma. Front Neurosci. 2017;11:146. doi: 10.3389/fnins.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533–2541. doi: 10.1167/iovs.05-1639. [DOI] [PubMed] [Google Scholar]
- 39.Opial D, Boehnke M, Tadesse S, et al. Leber's hereditary optic neuropathy mitochondrial DNA mutations in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 2001;239:437–440. doi: 10.1007/s004170100309. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee D, Banerjee A, Mookherjee S, et al. Mitochondrial genome analysis of primary open angle glaucoma patients. PLoS One. 2013;8:e70760. doi: 10.1371/journal.pone.0070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar M, Tanwar M, Faiq MA, et al. Mitochondrial DNA nucleotide changes in primary congenital glaucoma patients. Mol Vis. 2013;19:220–230. [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Amero KK, González AM, Osman EA, Larruga JM, Cabrera VM, Al-Obeidan SA. Susceptibility to primary angle closure glaucoma in Saudi Arabia: the possible role of mitochondrial DNA ancestry informative haplogroups. Mol Vis. 2011;17:2171–2176. [PMC free article] [PubMed] [Google Scholar]
- 43.Yu-Wai-Man P, Stewart JD, Hudson G, et al. OPA1 increases the risk of normal but not high tension glaucoma. J Med Genet. 2010;47:120–125. doi: 10.1136/jmg.2009.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeoung JW, Seong MW, Park SS, Kim DM, Kim SH, Park KH. Mitochondrial DNA variant discovery in normal-tension glaucoma patients by next-generation sequencing. Invest Ophthalmol Vis Sci. 2014;55:986–992. doi: 10.1167/iovs.13-12968. [DOI] [PubMed] [Google Scholar]
- 45.Collins DW, Gudiseva HV, Trachtman BT, et al. Mitochondrial sequence variation in African-American primary open-angle glaucoma patients. PLoS One. 2013;8:e76627. doi: 10.1371/journal.pone.0076627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundaresan P, Simpson DA, Sambare C, et al. Whole-mitochondrial genome sequencing in primary open-angle glaucoma using massively parallel sequencing identifies novel and known pathogenic variants. Genet Med. 2015;17:279–284. doi: 10.1038/gim.2014.121. [DOI] [PubMed] [Google Scholar]
- 47.Fraenkl SA, Muser J, Groell R, et al. Plasma citrate levels as a potential biomarker for glaucoma. J Ocul Pharmacol Ther. 2011;27:577–580. doi: 10.1089/jop.2011.0062. [DOI] [PubMed] [Google Scholar]
- 48.Van Bergen NJ, Crowston JG, Craig JE, et al. Measurement of Systemic Mitochondrial Function in Advanced Primary Open-Angle Glaucoma and Leber Hereditary Optic Neuropathy. PLoS One. 2015;10:e0140919. doi: 10.1371/journal.pone.0140919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lascaratos G, Chau KY, Zhu H, et al. Resistance to the most common optic neuropathy is associated with systemic mitochondrial efficiency. Neurobiol Dis. 2015;82:78–85. doi: 10.1016/j.nbd.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Bosley TM, Hellani A, Spaeth GL, et al. Down-regulation of OPA1 in patients with primary open angle glaucoma. Mol Vis. 2011;17:1074–1079. [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey JN, Loomis SJ, Kang JH, et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet. 2016;48:189–194. doi: 10.1038/ng.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins DW, Gudiseva HV, Trachtman B, et al. Association of primary open-angle glaucoma with mitochondrial variants and haplogroups common in African Americans. Mol Vis. 2016;22:454–471. [PMC free article] [PubMed] [Google Scholar]
- 53.Abu-Amero KK, Hauser MA, Mohamed G, et al. Mitochondrial genetic background in Ghanaian patients with primary open-angle glaucoma. Mol Vis. 2012;18:1955–1959. [PMC free article] [PubMed] [Google Scholar]
- 54.Khawaja AP, Cooke Bailey JN, Kang JH, et al. Assessing the Association of Mitochondrial Genetic Variation With Primary Open-Angle Glaucoma Using Gene-Set Analyses. Invest Ophthalmol Vis Sci. 2016;57:5046–5052. doi: 10.1167/iovs.16-20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carelli V, Ross-Cisneros FN, Sadun Aa. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochemistry international. 2002;40:573–584. doi: 10.1016/s0197-0186(01)00129-2. [DOI] [PubMed] [Google Scholar]
- 56.Carelli V, Chiara, Valentino ML, Barboni P, Ross-Cisneros FN, Sadun Aa. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochimica et biophysica acta. 2009;1787:518–528. doi: 10.1016/j.bbabio.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 57.Sadun AA. Metabolic optic neuropathies. Semin Ophthalmol. 2002;17:29–32. doi: 10.1076/soph.17.1.29.10290. [DOI] [PubMed] [Google Scholar]
- 58.Liebmann J, Cioffi G. Nicking Glaucoma with Nicotinamide? The New England Journal of Medicine. 2017;376:2079–2081. doi: 10.1056/NEJMcibr1702486. [DOI] [PubMed] [Google Scholar]
- 59.Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci U S A. 2016;113:4252–4259. doi: 10.1073/pnas.1603023113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H, Ryu D, Wu Y, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 61.Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 62.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knip M, Douek IF, Moore WP, et al. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 65.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trammell SA, Schmidt MS, Weidemann BJ, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winter SL, Boyer JL. Hepatic toxicity from large doses of vitamin B3 (nicotinamide) N Engl J Med. 1973;289:1180–1182. doi: 10.1056/NEJM197311292892208. [DOI] [PubMed] [Google Scholar]
- 68.Tittler EH, de Barros DS, Navarro JB, et al. Oral niacin can increase intraocular pressure. Ophthalmic Surg Lasers Imaging. 2008;39:341–342. doi: 10.3928/15428877-20080701-17. [DOI] [PubMed] [Google Scholar]
- 69.Barakat MR, Metelitsina TI, DuPont JC, Grunwald JE. Effect of niacin on retinal vascular diameter in patients with age-related macular degeneration. Curr Eye Res. 2006;31:629–634. doi: 10.1080/02713680600760501. [DOI] [PubMed] [Google Scholar]
- 70.Domanico D, Verboschi F, Altimari S, Zompatori L, Vingolo EM. Ocular Effects of Niacin: A Review of the Literature. Med Hypothesis Discov Innov Ophthalmol. 2015;4:64–71. [PMC free article] [PubMed] [Google Scholar]
- 71.Cantó C, Houtkooper RH, Pirinen E, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomes AP, Price NL, Ling AJ, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mouchiroud L, Houtkooper RH, Moullan N, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ratajczak J, Joffraud M, Trammell SA, et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun. 2016;7:13103. doi: 10.1038/ncomms13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Adv Enzymol Relat Areas Mol Biol. 1999;73:135–182. xi. doi: 10.1002/9780470123195.ch5. [DOI] [PubMed] [Google Scholar]
- 76.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 77.Chini EN. CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr Pharm Des. 2009;15:57–63. doi: 10.2174/138161209787185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 79.Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell. 2005;17:855–868. doi: 10.1016/j.molcel.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 80.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 81.Pelzel HR, Schlamp CL, Waclawski M, Shaw MK, Nickells RW. Silencing of Fem1cR3 gene expression in the DBA/2J mouse precedes retinal ganglion cell death and is associated with histone deacetylase activity. Invest Ophthalmol Vis Sci. 2012;53:1428–1435. doi: 10.1167/iovs.11-8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmitt HM, Schlamp CL, Nickells RW. Role of HDACs in optic nerve damage-induced nuclear atrophy of retinal ganglion cells. Neurosci Lett. 2016;625:11–15. doi: 10.1016/j.neulet.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Green KN, Steffan JS, Martinez-Coria H, et al. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cantó C, Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev. 2012;64:166–187. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guan X, Lin P, Knoll E, Chakrabarti R. Mechanism of inhibition of the human sirtuin enzyme SIRT3 by nicotinamide: computational and experimental studies. PLoS One. 2014;9:e107729. doi: 10.1371/journal.pone.0107729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sethi JK, Empson RM, Galione A. Nicotinamide inhibits cyclic ADP-ribose-mediated calcium signalling in sea urchin eggs. Biochem J. 1996;319(Pt 2):613–617. doi: 10.1042/bj3190613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways-a rôle for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–662. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Araie M, Mayama C. Use of calcium channel blockers for glaucoma. Prog Retin Eye Res. 2011;30:54–71. doi: 10.1016/j.preteyeres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 90.Kaushik S, Pandav SS, Ram J. Neuroprotection in glaucoma. J Postgrad Med. 2003;49:90–95. doi: 10.4103/0022-3859.917. [DOI] [PubMed] [Google Scholar]
- 91.Pasquale LR. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr Opin Ophthalmol. 2016;27:94–101. doi: 10.1097/ICU.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Resch H, Garhofer G, Fuchsjäger-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 93.Mokudai T, Ayoub IA, Sakakibara Y, Lee EJ, Ogilvy CS, Maynard KI. Delayed treatment with nicotinamide (Vitamin B(3)) improves neurological outcome and reduces infarct volume after transient focal cerebral ischemia in Wistar rats. Stroke. 2000;31:1679–1685. doi: 10.1161/01.str.31.7.1679. [DOI] [PubMed] [Google Scholar]
- 94.Howell GR, MacNicoll KH, Braine CE, et al. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol Dis. 2014;71:44–52. doi: 10.1016/j.nbd.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krishnamoorthy RR, Rao VR, Dauphin R, Prasanna G, Johnson C, Yorio T. Role of the ETB receptor in retinal ganglion cell death in glaucoma. Can J Physiol Pharmacol. 2008;86:380–393. doi: 10.1139/Y08-040. [DOI] [PubMed] [Google Scholar]
- 96.Prasanna G, Hulet C, Desai D, et al. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol Res. 2005;51:41–50. doi: 10.1016/j.phrs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Rosenthal R, Fromm M. Endothelin antagonism as an active principle for glaucoma therapy. Br J Pharmacol. 2011;162:806–816. doi: 10.1111/j.1476-5381.2010.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thai TL, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptors mediate endothelin ETA and ETB receptor-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol. 2008;295:F360–368. doi: 10.1152/ajprenal.00512.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li F, Fushima T, Oyanagi G, et al. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1614947113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geiger J, Zou AP, Campbell WB, Li PL. Inhibition of cADP-ribose formation produces vasodilation in bovine coronary arteries. Hypertension. 2000;35:397–402. doi: 10.1161/01.hyp.35.1.397. [DOI] [PubMed] [Google Scholar]
- 101.Preiss J, Handler P. Biosynthesis of diphosphopyridine nucleotide. I. Identification of intermediates. J Biol Chem. 1958;233:488–492. [PubMed] [Google Scholar]
- 102.Lin JB, Kubota S, Ban N, et al. NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell Rep. 2016;17:69–85. doi: 10.1016/j.celrep.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di Stefano M, Nascimento-Ferreira I, Orsomando G, et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 2015;22:731–742. doi: 10.1038/cdd.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]