Abstract
Epidemiological studies indicate an inverse correlation between the prevalence of the so-called western diseases, such as obesity and metabolic syndrome, and the exposure to helminths. Obesity, a key risk factor for many chronic health problems, is rising globally and is accompanied by low-grade inflammation in adipose tissues. The precise mechanism by which helminths modulate metabolic syndrome and obesity is not fully understood. We infected high fat diet (HFD)-induced obese mice with the intestinal nematode parasite Heligmosomoides polygyrus and observed that helminth infection resulted in significantly attenuated obesity. Attenuated obesity corresponded with marked upregulation of uncoupling protein 1 (UCP1), a key protein involved in energy expenditure, in adipose tissue, suppression of glucose and triglyceride levels, and alteration in the expression of key genes involved in lipid metabolism. Moreover, the attenuated obesity in infected mice was associated with enhanced helminth-induced Th2/Treg responses and M2 macrophage polarization. Adoptive transfer of helminth-stimulated M2 cells to mice that were not infected with H. polygyrus resulted in a significant amelioration of HFD-induced obesity and increased adipose tissue browning. Thus, our results provide evidence that the helminth-dependent protection against obesity involves the induction of M2 macrophages.
Introduction
The incidence of obesity is rising globally and remains one of the biggest threats to adults and children. More than one-third of U.S. adults suffer from obesity and 17% children were reported either overweight or obese in 20151. Rising obesity rates have significant health consequences, contributing to increased rates of metabolic diseases such as diabetes, steatosis, hypertension, and heart disease. Obesity is characterized by chronic low-grade inflammation in adipose tissues (AT) from accumulating inflammatory cells (Th1/Th17 cells, macrophages, etc)2 which represents the unique feature of this metabolic inflammatory state. AT inflammation was proposed as a central mechanism connecting obesity with its metabolic and vascular complications. Various mediators and cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1), released by the accumulating inflammatory cells and adipocytes, may contribute to the development of metabolic syndrome, thus exacerbating the consequences of obesity3.
In recent years, adipose tissue has emerged as a highly active body tissue integrating metabolic, endocrine, and immune functions into a single entity that exerts significant effects on whole body homeostasis. Under physiological conditions, the structural and functional integrity of AT are sustained by a meticulously orchestrated network of immune cells4. Resident immune cells constitute the second largest AT cellular component after adipocytes, and as such play important roles in the maintenance of AT homeostasis. Adipose tissue from lean individuals preferentially secretes anti-inflammatory adipokines such as adiponectin, transforming growth factor beta (TGFβ), IL-10, IL-4, IL-13, IL-1 receptor antagonist (IL-1Rα), and apelin. In contrast, obese adipose tissue mainly releases proinflammatory cytokines among other molecules such as TNF-α, IL-6, leptin, visfatin, resistin, angiotensin II, and plasminogen activator inhibitor 15. The metabolic role of adipose tissue is complex. White adipose tissue (WAT) stores energy in the form of triglycerides. Brown adipose tissue (BAT) dissipates stored energy as heat, due to the presence of uncoupling protein 1 (UCP1), an integral membrane protein unique to brown adipocyte mitochondria6, which plays an important role in energy balance. BAT, therefore, increases energy expenditure and resistance to obesity. It has been shown that UCP1 transgenic mice, which have an increased content of UCP1, are protected against diet-induced obesity7.
Helminth infections form a major group of tropical diseases that affect approximately 1.5 billion people worldwide (or 24% of the world’s population)8. Concomitant to the decrease in parasitism over the past half century in developed countries, the prevalence of the so-called western diseases, such as allergic and autoimmune diseases, and metabolic syndrome have spectacularly risen9. This inverse correlation of immune mediated diseases and metabolic syndrome with helminth infections suggests that helminths may play a protective role against these diseases. Existing data indicate that helminth infections induce the development of Th2 and/or Treg responses, which have been linked to the amelioration of Th1- or Th17-mediated diseases10,11. The immune modulatory capacity of helminths has sparked a great deal of interest within the research community in exploring their therapeutic potential in treating immune-mediated diseases. Helminth-based therapy has been tested for controlling allergic and intestinal inflammatory disorders2,12–16. Despite these advances, our understanding of the potential mechanisms by which helminths modulate the host metabolism and nutrition-associated disorders remains incomplete.
Obesity is associated with macrophage accumulation in adipose tissue. The number of macrophages within adipose tissue differs depending on the metabolic status. Macrophages can exhibit either pro- or anti-inflammatory phenotypes and are routinely classified into classically activated M1 phenotype and alternatively activated M2 phenotype, respectively. Pro-inflammatory M1 macrophages secrete high levels of proinflammatory cytokines (TNF-α, IL-6, IL-1β) and generate reactive oxygen and nitrogen species, such as nitric oxide via activation of inducible nitric oxide synthase (iNOS). The alternatively activated M2 macrophages, which express Arg 1, Ym1/2, Fizz1 and produce IL-10, are anti-inflammatory17–19. Prior observations, including our own, have shown that helminth infection induces the development of alternatively activated macrophages (AAM or M2) via Th2 cytokines17–19. In the current study, we examine the effect of infection with the helminth parasite Heligmosomoides polygyrus on high fat diet (HFD)-induced obesity in mice and show that helminth-induced M2 macrophages play an important role in modulation of energy balance.
Results
H. polygyrus infection attenuated body weight gain, blood glucose and triglyceride levels in the HFD-fed obese mice
To determine the effect of H. polygyrus infection on the HFD-induced obesity, C57BL/6 female mice were fed HFD or control diet followed by H. polygyrus or sham infection (Fig. 1). As expected, HFD feeding resulted in a significant increase in body weight gain in non-infected mice. In sharp contrast, as shown in Fig. 2a, H. polygyrus-infected, HFD fed mice gained significantly less weight compared to non-infected HFD fed mice. In fact, HFD-fed mice with helminth infection maintained their body weight at levels similar to mice fed with CD throughout the experimental period. H. polygyrus infection had no effect on body weight gain in CD fed mice.
Figure 1.
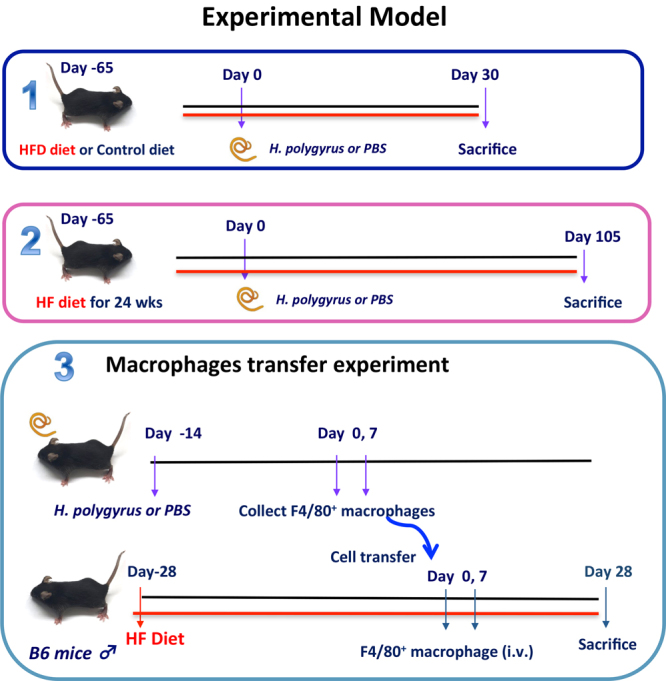
Experimental models. Schematic diagram illustrates H. polygyrus infection on HFD-fed mice. Schematic diagram illustrates H. polygyrus infection on long-term HFD experiment. The schematic diagram (3) illustrates the adoptive transfer of macrophage experiment in HFD-induced obesity; here the objective was to observe the potential regulatory role of helminth-induced M2 cells in HFD-obesity.
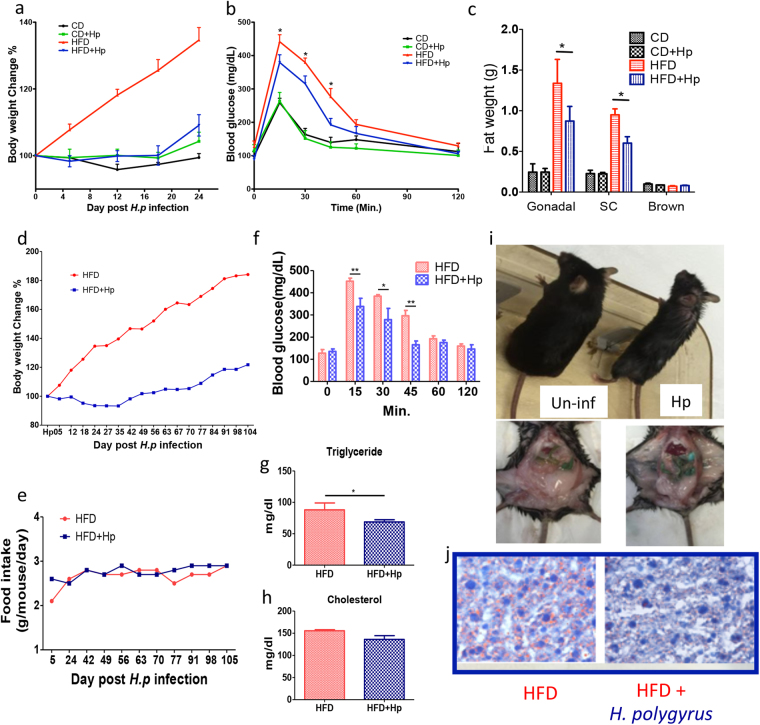
Figure 2.
H. polygyrus infection attenuates HFD-induced obesity in mice. C57BL/6 mice fed with the HFD showed steady gain in body weight than those HFD infected with H. polygyrus through the course of experiment (a). Blood glucose levels were attenuated by H. polygyrus infection (b). Mice on the HFD accumulated more gonadal and subcutaneous fat than HFD fed infected mice (c). In long term experiments, C57BL/6 mice on HFD gradually gained more body weight (red line) than those HFD but infected with H. polygyrus (blue line) along a 104-day course (d). No differences in food intakes of HFD obese mice with (blue line) or without (red line) H. polygyrus infection (e). Blood glucose levels showed significant differences at 15, 30 and 45 minutes, between the two groups of mice (f). Triglyceride and cholesterol levels significantly dropped in H. polygyrus infected mice (g and h). The physical appearance (body size) in HFD-fed mice with (Hp) and without infection (Un-inf) (i) and the histological analysis of liver section of HFD induced mice showing severe steatosis, with fat deposits shown in un-infected mice using ORO staining (j). (n = 5–6; *p < 0.05; **p < 0.01. Two-tailed Student’s t-test).
Obesity is shown to exert detrimental effects on glucose homeostasis and lipid metabolism. To test this in our model, we next examined whether helminth-mediated attenuation of obesity reversed the detrimental effects associated with obesity. The results from glucose tolerance tests showed that when challenged with glucose, non-infected mice had a higher blood glucose level (Fig. 2b) and H. polygyrus-infected mice showed an enhanced glucose tolerance apparent at various time points of the tolerance test. Analysis of the body fat distribution was proportional to and in agreement with reduced body weight gain. Hp-infected mice had marked reduction in gonadal and subcutaneous fat pads: approximately 30% reduction was observed compared to non-infected HFD induced obese mice (Fig. 2c). However, no differences were detected in brown fat weight (Fig. 2c) between infected and non-infected mice fed with HFD.
To determine whether helminth infection alleviated obesity in mice on long-term HFD-feeding regime, we fed both helminth-infected and non-infected mice with HFD for over 104 days and observed that the infected mice gained significantly less body weight than the HFD-fed non-infected mice (Fig. 2d). However, the body weight changes were not correlated with a decrease in food intake since we found food intake did not differ significantly between both helminth-infected and non-infected mice on HFD (Fig. 2e). In the long-term experiments, we also found that helminth infection resulted in reduced blood glucose levels (at 15, 30 and 45 minutes) (Fig. 2f), decreased serum triglyceride levels (Fig. 2g) and unchanged cholesterol level (Fig. 2h) in HFD fed mice. Moreover, marked differences in physical appearance of mice (body size) were detected between H. polygyrus-infected and non-infected mice (Fig. 2i). The body size of HFD-fed mice was almost double the size of HFD-fed mice with H. polygyrus infection. Histological analysis of liver section of HFD induced mice revealed severe steatosis (Fig. 2j left panel). In contrast, less fat deposits were detected in H. polygyrus-infected obese group (Fig. 2j right panel). Our collective data suggested that helminth infection attenuates HFD-induced obesity in mice.
H. polygyrus infection influences gene expression of key enzymes/mediators of lipid metabolism
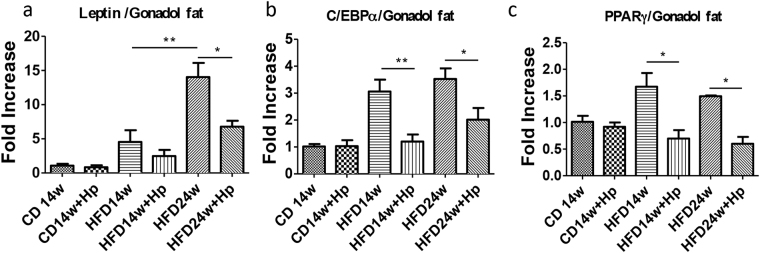
To determine the mechanism by which helminth infection may alter HFD-induced body weight gain, we analyzed gene expression of key regulators mediating lipid metabolism in gonadal fat, including leptin, C/EBPα, and PPARγ. Leptin is a hormone produced in AT that regulates the amount of stored fat in the body by balancing both the appetite and energy usage20, and this adipocytokine has also been associated with pro-inflammatory attributes and the ability to induce pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1220. PPARγ and C/EBPα are critical transcription factors in adipogenesis21. The results from our PCR analysis revealed that obese mice had higher leptin mRNA levels than the lean mice, and that HFD obese mice infected with H. polygyrus showed a marked decrease in leptin gene expression compared to that detected in non-infected HFD obese mice (Fig. 3a). Our results also showed that HFD-feeding resulted in significant increase in the expression of C/EBPα, which is prevented by helminth infection (Fig. 3b). The results suggest that helminth exposure in obese mice inhibited lipogenic gene expression. We further determined the impact of helminth infection on the expression of PPARγ and found that H. polygyrus infection significantly decreased the HFD-induced upregulation of PPARγ in obese mice (Fig. 3c). These results are in line with observations showing that PPARγ−/− mice fail to generate adipose tissue when fed HFD22. Our observations suggest that H. polygyrus infection may ameliorate diet-induced obesity by modulating gene expression of key mediators of lipid metabolism
Figure 3.
H. polygyrus infection influences gene expression of key enzymes/mediators of lipid metabolism. H. polygyrus infection results in decreased gene expression of leptin enzymes compared with normal mice in gonadal fat tissues: (a) leptin; (b) C/EBPα; and (c) PPARγ. (n = 5–6; *p < 0.05; **p < 0.01; two-tailed Student’s t-test).
H. polygyrus infection promotes the induction of UCP1 expression in both white adipose and brown adipose tissues
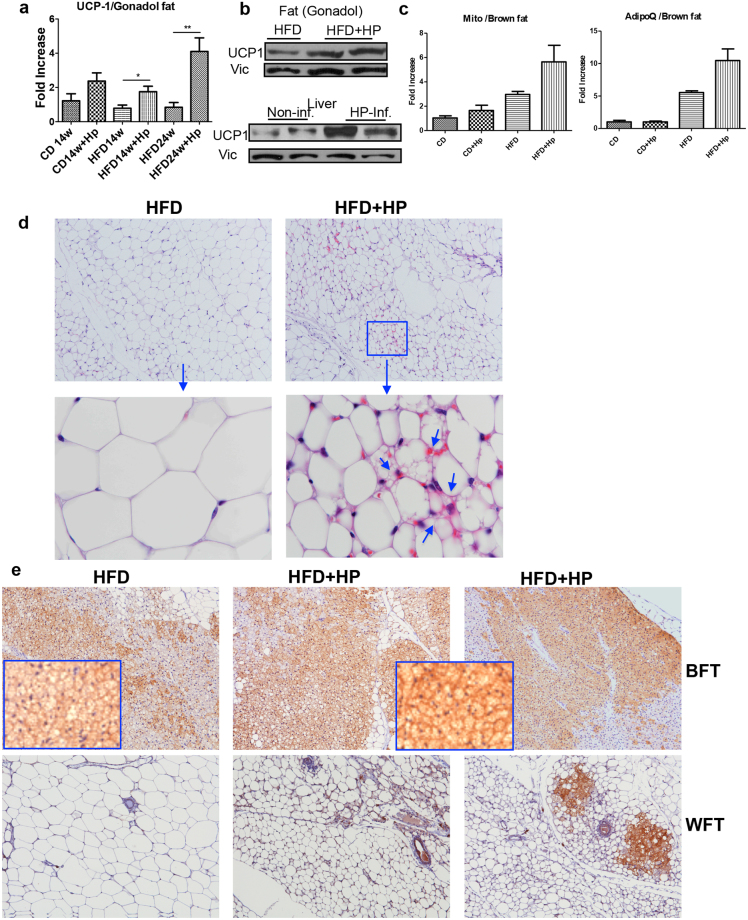
Uncoupling protein 1 (UCP1), which is exclusively expressed in adipose tissue, particularly in BAT23, is involved in uncoupling- mediated energy expenditure and is a marker for fat browning. In our subsequent experiments, we tested the hypothesis that H. polygyrus infection promoted adipose tissue browning and contributed to increased resistance to diet-induced obesity. To test this idea, we first quantified UCP1 expression in different fat depots by using real time quantitative PCR (qPCR), immunoblot and immunohistochemical approaches. As illustrated in Fig. 4a,b, qPCR and immunoblot results revealed significant upregulation of UCP-1 expression at both mRNA and protein levels in adipose tissues of helminth-infected mice compared to HFD-fed control animals. Our PCR data also showed helminth infection resulted in increased mitochondrial mass, characteristic feature of beige fat24. Immunoblot analysis further demonstrated that H. polygyrus infection resulted in increased UCP-1 expression in liver tissue (Fig. 4b).
Figure 4.
H. polygyrus infection results in upregulation of UCP1 expression and adipose tissue browning. qPCR analysis revealed that H. polygyrus infection results in a significant upregulation of UCP-1 expression in gonadal fat tissue at mRNA level (a). Immunoblot analysis showed increased UCP1 levels in adipose and liver tissue (b). qPCR results revealed significant upregulation of mitochondrial mass and UPC-1 expression (c). (n = 5–6; *p < 0.05; **p < 0.01; two-tailed Student’s t-test). (d and e) The adipose tissues showed marked reduction in the adipocyte size and increased expression of UCP1 in helminth-infected HFD fed mice (HFD-HP) compared to non-infected (HFD) mice. Adipose (including brown adipose, subcutaneous and gonadal adipose tissues) from HFD-fed mice with and without helminth infection collected. Ten-μm sections were cut on a Leica CM1850 Cryostat (Leica Biosystem) and were stained with hematoxylin and eosin (d) or anti-UCP1 antibody (e). (d) H&E staining: arrows indicate the presence of multilocular lipid droplets in adipocytes in the white adipose tissues of infected mice absent in uninfected obese mice. (e) Immunohistochemical staining of the brown (upper panel) and white (low panel) adipose tissues revealed a markedly intensified UCP1 expression in the adipose tissue of helminth-infected mice compared to non-infected HFD-fed mice.
Obesity induces “whitening” of brown adipose tissue,which is characterized by larger lipid droplets, inflammation, and decreased mitochondrial respiration25–27. We next determined if helminth infection induced browning of white adipose tissues in mice. Our hematoxylin and eosin (H&E) staining of the adipose tissues showed marked reduction in the adipocyte size in helminth-infected HFD fed mice compared to non-infected mice (Fig. 4d). Furthermore, we observed the presence of multilocular adipocytes in the white adipose tissues of infected mice and the absence in non-infected obese mice. Such tissue remodeling - presence of multilocular adipocytes - are characteristics of beige adipose cells (Fig. 4d).
Using immunohistochemical approach, we determined the UCP-1 expression in white adipose tissue. Our analysis of the brown adipose tissue revealed that UCP1 expression was substantially intensified in the adipose tissue of helminth-infected mice compared to non-infected HFD-fed mice (Fig. 4e). These observations therefore supported that helminth infection enhances the UCP-1 expression in brown adipose tissues, which in turn may contribute to increased energy expenditure and attenuated diet-induced obesity in helminth infected mice. As illustrated in Fig. 4e (lower panel), consistent with the observations of multilocular adipocytes, we observed more pronounced UCP1 positive cells in white adipose tissues in helminth-infected mice compared to non-infected control mice (Fig. 4e). These observations, therefore, demonstrated that H. polygyrus infection induces UCP1 expression, promoting the browning of white adipose tissue in mice.
H. polygyrus infection promotes a strong type 2 immune response resulting in cytokine-mediated modulation of lipid metabolism
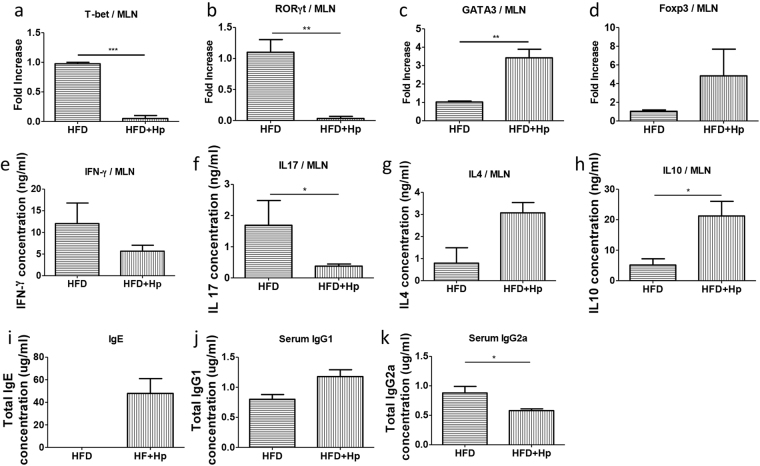
Cytokines TNFα, the IFNs, and IL-17A have been shown to regulate lipid metabolism28,29. In the liver, IFNs and other cytokines stimulate lipid synthesis and very-low-density lipoprotein (VLDL) production, which contribute to elevated serum triglyceride levels that characteristically occur during infections and inflammatory diseases. Evidence also showed that IL-17A is involved in lipid metabolism and pathogenesis of atherosclerosis, a chronic inflammatory arterial disease driven by both innate and adaptive immune responses to modified lipoproteins30. The helminth-induced immune responses mainly stem from the Th2 mediated cytokines IL-4, IL-10 and IL-13, the functions of which in nutrient metabolism and obesity are not fully understood and are an area of active research. In the current study, we collected MLN from different groups of mice and measured the expression levels of transcriptional factors responsible for the regulation and differentiation of Th1 (T-bet), Th2 (GATA3), Th17 (RORγt) and Treg (Foxp3) cells using real time qPCR. Our results show a clear suppression in the T-bet and RORγt mRNAs expression (Fig. 5a,b) and increase in the expression of GATA3 and Foxp3 mRNAs (Fig. 5c,d) in the MLN cells from HFD-fed H. polygyrus-infected mice. Furthermore, we re-stimulated the MLN cells in vitro and analyzed the cytokine profiles of the cells. Increased production of IL-4 and IL-10 were detected in the MLN cells from H. polygyrus-infected HFD-fed mice compared to non-infected obese host (Fig. 5g,h). The enhanced Th2 cytokine response in HFD-fed and helminth-infected mice corresponded with a marked reduction of IFN-γ and IL-17 production by MLN cells from these mice (Fig. 5e,f). In line with these observations, our further analysis of the impact of helminth infection on the immune system of HFD-fed mice revealed an enhanced systemic Th2 response and suppression of Th1 response, evidenced by increased serum IgE and IgG1 levels and decreased IgG2a levels (Fig. 5i,j,k). Taken together, our data showed a clear impact of on-going helminth infection on the effector T cell differentiation and cytokine production profile in mice fed HFD by promoting Th2/Treg and suppressing Th1/Th-17 cell responses.
Figure 5.
H. polygyrus infection promotes a strong type 2 immune response. Real time qPCR analysis shows a clear suppression in the T-bet and RORγt mRNAs expression (a and b) and increase in the expression of GATA3 and Foxp3 mRNAs (c and d) in the MLN cells from HFD-fed H. polygyrus-infected mice. H. polygyrus-infected obese mice showed decreases in IFN-γ and IL-17 secretions in the supernatant of cultured MLN cells (e and f). H. polygyrus-infected obese mice had higher production of cytokines IL-4 and IL-10 in MLN (g and h). H. polygyrus-infected obese mice had increased levels of serum IgE (i) and IgG1 (j) and a decreased IgG2a level (k). (n = 5–6; *p < 0.05; two-tailed Student’s t-test).
H. polygyrus infection enhances the ‘anti-inflammatory’ M2 levels by a balance shift in M1/M2 macrophage ratio
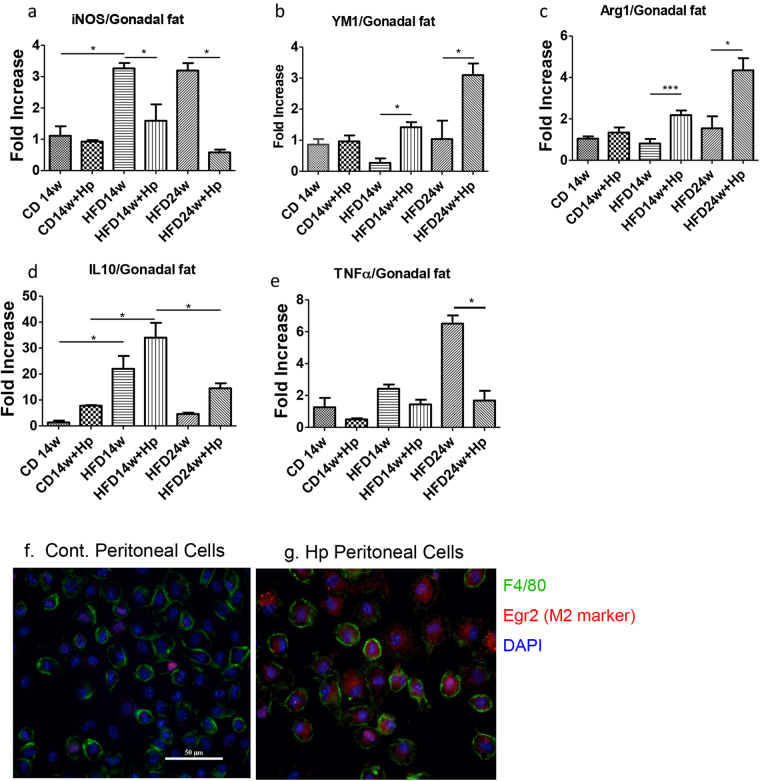
Obesity is associated with macrophage accumulation in adipose tissue. The number of macrophages within adipose tissue differs depending on the metabolic status. In the current study, we determined whether helminth infection induces an imbalance in the ratio of M1/M2 macrophages in gonadal fat tissue. The results from our qPCR analysis showed that HFD-feeding of non-infected obese mice leads to increased M1 cells which are evidenced by significant increase in iNOS expression and this increase is prevented by helminth infection (Fig. 6a). The results from our qPCR experiments further indicated that H. polygyrus infection resulted in increased M2 macrophages in gonadal fat tissue as evidenced by the detection of enhanced expression of Arg1 and Ym1 levels, genes that are expressed in M2 cells (Fig. 6b,c). We also observed that H. polygyrus-infection significantly up-regulated IL10 expression in gonadal adipose tissues (Fig. 6e). The increased M2 and IL-10 responses corresponded with the decreased expression levels of the pro-inflammatory cytokine, TNF-α (Fig. 6e) and iNOS (Fig. 6a), suggesting a suppression of M1 cells in obese adipose tissue in helminth-infected HFD-fed mice. H. polygyrus infection induced M2 cells were further shown in peritoneal cell population from helminth-infected host, which is evidenced by the detection of high frequency of F4/80 and Egr2 double positive cells from infected host relative to control mice (Fig. 6f,g). These results suggest that H. polygyrus may protect against obesity by modulating the polarization of macrophages (promoting anti-inflammatory M2 macrophages) in gonadal fat of obese mice.
Figure 6.
H. polygyrus-infection influences M1/M2 macrophage polarization in gonadal fat of obese mice towards anti-inflammatory M2 phenotype. (a) H. polygyrus infection results in reduced HFD-induced inducible oxide synthase (iNOS) expression, indicating inhibition M1 macrophages. (b and c) H. polygyrus infection results in increased expression of YM1 and Arg1, promoting the development of M2 cells in HFD-fed mice. (d and e) H. polygyrus infection results in increased IL-10 expression and decreased TNF-α expression. (n = 5–6; *p < 0.05; two-tailed Student’s t-test). (f and g) Peritoneal F4/80+ macrophages from normal control (g, mostly F4/80+EGR2− M1) and H. polygyrus-infected mice (h: most F4/80+EGR2+ M2 cells).
Adoptive transfer of helminth-induced M2 cells results in attenuated HFD-induced obesity
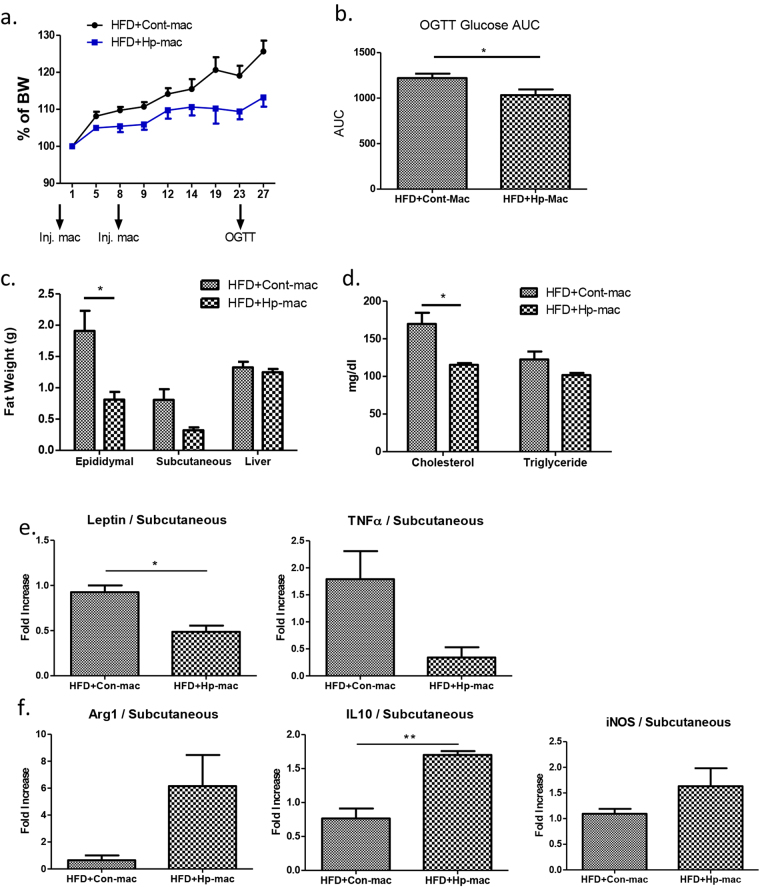
To demonstrate the functional role of M2 cells in the regulation of HFD-induced obesity in our model, we isolated macrophages from helminth infected and normal control animals and adoptively transferred into the HFD-pre-fed obese mice and examined the body weight gain for 4 weeks. As shown in Fig. 1, both male and female mice were used in the experiments and developed similarly enhanced weight gain on the high fat diet. Both sexes were also equivalently protected from high fat-induced weight gain by helminth infection. However, since male mice gained weight more rapidly than females, we used only males in the macrophage transfer experiments in order to shorten their duration. HFD-fed mice that received macrophages from normal control mice showed sustained body weight gain after cell transfer (Fig. 7a). In contrast, HFD-fed mice that received macrophages from helminth infected mice gained significantly less weight during the experimental period (Fig. 7a). The observations demonstrate the functional role of helminth-induced M2 cells in the attenuation of HFD-induced obesity. In line with the reduced weight gain, adoptive transfer of macrophages from helminth-infected donors resulted in reduced glucose, fat, cholesterol level, leptin, and TNF-α levels (Fig. 7b–e). M2 cell transfer from helminth infected donor mice increased IL-10 expression in HFD-fed recipient mice (Fig. 7f). The results confirm that macrophages stimulated by helminth infection play an important role in modulating host metabolism, contributing to the amelioration of diet-induced obesity.
Figure 7.
Adoptive transfer of M2 cells results in attenuated obesity in HFD-fed mice. (a) Body weigh change in mice received macrophages from normal and helminth-infected donor mice. (b) AUC of OGTT, Blood glucose level; (c) fat tissue and liver weight; (d) blood chemistry test; (e) M2 cell transfer alters gene expression of leptin and TNF-α; (f) M2 transfer displays M2 phenotype of increased IL-10 expression. (n = 5–6; *p < 0.05; **p < 0.01; two-tailed Student’s t-test).
Helminth-induced M2 cells promote UCP1 expression and browning of adipose tissues in mice
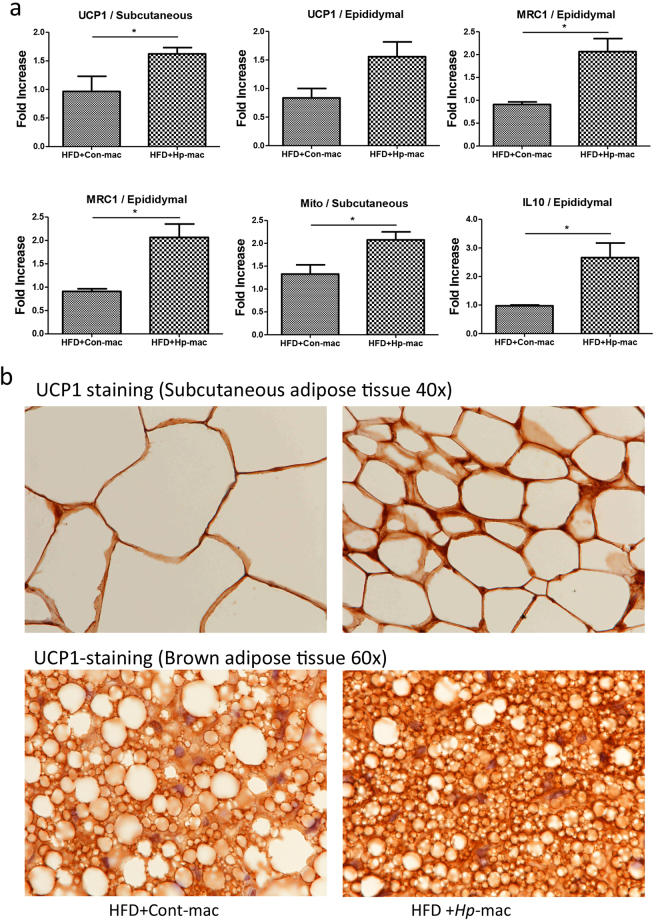
To test if H. polygyrus-induced M2 cells may promote adipose tissue browning, and contribute to increased resistance to dietary obesity, we examined the UCP1 expression and mitochondrial level in the recipient mice that were transferred with macrophages from normal or helminth infected donors. qPCR data confirmed a significant increase in UCP1 expression and mitochondrial levels in adipose tissues from mice that received helminth-stimulated M2 cells (Fig. 8a). qPCR analysis of the adipose tissue also confirmed that the recipient mice of macrophages from helminth infected donor showed M2 characteristics, as evidenced by increased MRC1 (CD206) and IL-10 expression (Fig. 8a), both of which are known to be expressed in M2 cells. Our immunohistochemical analysis of the adipose tissues showed marked reduction of the adipocyte size in mice that were subject to helminth-induced M2 cell transfer compared to mice that received cells from non-infected donor mice (Fig. 8b). Our results further showed that adoptive transfer of helminth-induced M2 cells increased UCP1 expression in the adipose tissue compared to mice harboring normal macrophages (Fig. 8b). The increased UCP-1 expression may contribute to enhanced thermogenesis. Overall the collective observations demonstrate a significant role for helminth-induced M2 cells in enhancing energy expenditure and attenuating dietary induced obesity in mice. These observations, therefore, suggest a new aspect of immune regulatory effects of helminth infection and provide mechanistic insights into our observation that H. polygyrus attenuate HFD-induced obesity.
Figure 8.
Adoptive transferring of H. polygyrus-induced M2 cells promotes adipose tissue browning. Purified F4/80+ macrophages from normal or H. polygyrus-infected donors were transferred into HFD-fed recipient mice. Adipose tissues were collected. (a) qPCR data showed significant increase in UCP1 expression and mitochondrial levels in adipose tissues from mice that received helminth-stimulated M2 cells. (n = 5–6; *p < 0.05; two-tailed Student’s t-test). (b) Representative of immunohistochemical stained sections showed marked reduction of the adipocyte size and increased intensity of UCP1 expression in the adipose tissue of the recipient mice transferred with helminth-induced M2 cells compared to mice harboring normal macrophages.
Discussion
Helminth infections form a major group of tropical diseases that affect approximately 1.5 billion people worldwide (or 24% of the world’s population)8. Success in eradicating helminth infection in the developed world significantly reduced helminth-associated morbidity and mortality, but is also correlated with increasing incidence of “western diseases”, including metabolic syndrome and type 2 diabetes31. This inverse correlation between the incidence of immune mediated diseases and metabolic syndrome and helminth infections suggests that helminths may play a protective role against these diseases. Our current findings show that infection with the small intestinal Th2-stimulating helminth parasite H. polygyrus ameliorates HFD-induced obesity. The amelioration of obesity in infected mice was accompanied by an intensified UCP1 expression, and increased browning of white adipose tissue, and altered gene expression of key regulators mediating lipid metabolism in gonadal fat. The results further showed that attenuated obesity in H. polygyrus-infected mice was associated with enhanced M2 macrophage polarization. By using adoptive cell transfer approach, we showed that transplantation of H. polygyrus-stimulated M2 macrophages to the HFD-fed recipient mice attenuated HFD-induced obesity, corresponding with enhanced UCP-1 expression in adipose tissues and an increase in number of beige cells (or browning) in adipose tissue. The results, therefore, demonstrate a functional role for helminth-induced M2 cells in orchestrating the host response to diet.
The mechanism of the relationship between helminth infection and nutrition is complex and remains to be fully elucidated. We show that a Th2-inducing intestinal helminth (H. polygyrus) infection has a beneficial effect on diet-induced obesity. It has been shown that injection of mice on a HFD with the Th2 cytokine IL-4 activates STAT 6 signaling pathway and attenuates adipose tissue inflammation, and improves insulin action32. IL-4 has also been suggested to play a pro-lipolytic role in lipid metabolism by enhancing the activity and translocation of hormone sensitive lipase (HSL) in mature adipocytes and by IL4-mediated inhibition of adipocyte differentiation and lipid accumulation, thereby promoting lipolysis in mature adipocytes (decreased lipid droplets)32. Eosinophils, which are associated with helminth infection, reportedly enhance glucose tolerance33. We have also observed helminth-infected HFD-fed mice have markedly elevated serum IgE levels, which have been associated with decreased fasting blood glucose and lipid levels34. Our results are consistent with prior reports that Th2-inducing nematode parasite Nippostrongylus brasiliensis35 and Schistosoma mansoni36 infection in HFD-fed mice resulted in decreased body weight gain and improved glucose metabolism. These results further support the inverse association between helminth infections and the incidence of metabolic syndrome37.
Diet-induced obesity can lead to a shift in the activation state of adipose tissue macrophages from an M2-polarized state in lean animals to an M1 proinflammatory state that contributes to insulin resistance38. This imbalance culminates in chronic inflammation and long-term metabolic dysfunction. The results from the current study showed that in HFD-fed mice H. polygyrus infection influences M1/M2 macrophage polarization in gonadal fat, shifting it towards a predominantly anti-inflammatory state or M2 phenotype. In line with our observation, M2 phenotype has also been detected in adipose tissue in mice with N. brasiliensis infection35. The detection of an M2-polorized state in helminth-infected HFD-fed lean mice may suggest that macrophages in helminth-infected mice migrating to adipose tissue upon high-fat feeding may contribute to altered lipid metabolism. To test this hypothesis, in the current study, we carried out the macrophage transfer experiments by collecting F4/80+ macrophages from helminth-infected or uninfected control donors and adoptively transferred the cells to HFD-fed recipient mice. We showed that adoptive transfer of helminth-stimulated M2 cells to mice that were not infected with H. polygyrus resulted in a significant amelioration of HFD-induced obesity and increased adipose tissue browning, demonstrating the functional contribution of helminth-induced M2 cells in the attenuation of HFD-induced obesity.
Our results showed that the food intake was not affected by helminth infection (Fig. 2e), suggesting the attenuated obesity in HFD-fed mice is independent of any pathology associated with helminth infection. Our results provide evidence that helminth infection results in marked upregulation of UCP-1 and mitochondrial expression, activated brown adipose tissue and increased browning of the white adipose tissues in HFD-fed mice, which may contribute to increased energy expenditure, consequently, limited adiposity (Fig. 4). These observations, therefore, provide new mechanistic insights into H. polygyrus induced attenuation of HFD-induced obesity. UCP1 allows brown adipose tissue to generate heat via uncoupling oxidative phosphorylation at the expense of ATP production. New evidence suggests that post transcriptionally stabilizing UCP1 mRNA protects mice from HFD-induced obesity38. Loss of UCP1 resulted in whitening of brown adipose tissue and exacerbates Western diet-induced glucose intolerance39. Brown adipose tissue has been associated with protection against inflammation and inversely associated with insulin resistance, Type 2 diabetes, and risk of cardiovascular disease40. In line with our observation that helminth infection stimulates the browning of white adipose tissues, this process has been shown to occur under certain environmental and pharmacological conditions. For example, cold exposure stimulates the browning of white adipose tissues41,42. Increased UCP1 activity and mitochondrial biogenesis in white AT have also been shown as results of exercise training43,44. During the exercise training, it was shown that β-adrenergic activation43,44 improved insulin sensitivity and increased energy expenditure45,46. Helminth infection may activate adipocyte precursors to adopt a beige fate through induction of the cytokines IL-4/IL-13 and ILC2s9, as these Th2 promoting molecules and cells have been implicated in the commitment and maturation of beige adipocytes47,48. In contrast, there is evidence from a recent study showing that loss of ILC2 and Treg did not affect uncoupled respiration in brown or beige adipocytes49.
Similar to our observations showing that helminth infection results in a marked upregulation of UCP-1 expression, the results from our macrophage transfer experiments provide strong evidence that transfer of helminth-induced M2 cells, in the absence of helminths in the recipient host, is sufficient to induce an attenuated obesity and enhanced UCP-1 expression in adipose tissues, leading to the activation of browning in adipose tissue and the potential for enhanced thermogenesis. These novel observations suggest that the observed helminth-induced protective metabolic effects involve browning of adipose tissue by helminth-stimulated M2 cells. Previous study has demonstrated that cold exposure promotes M2 cell development50. M2 cells have been implicated in activation of beige adipocytes to generate heat by producing catecholamines50. The question how precisely helminth-stimulated M2 regulates the UPC1 expression warranties a future investigation.
In our current study, an increased expression of the prototypical anti-inflammatory cytokine, IL-10, was observed in adipose tissues of helminth-infected obese mice. The same is true in M2 cell recipient mice, which may suggest that helminth-stimulated M2 cell derived IL-10 constitutes a major portion of the IL-10 in adipose tissue. Overexpression of IL-10 in macrophages suppresses atherosclerosis in hyperlipidemic mice51. IL10 has been shown to be able to ameliorate inflammation in obese adipose tissue and insulin resistance induced by proinflammatory cytokines, TNF-α and MCP-152,53. IL-10 can modulate lipid metabolism enhancing both uptake and efflux of cholesterol in macrophages, playing an antiatherogenic role54.
Our histological analysis of liver section of HFD induced mice revealed severe steatosis. However, much less fat deposits were detected in H. polygyrus-infected obese group (Fig. 2i). Although the precise mechanism by which helminth infection regulates liver disease is still unclear, we speculate that the marked attenuation of liver damage may be related to helminth-induced Th2/Treg cell homing to the lever, dampening Th1/Th17 inflammatory response. It has been suggested that mucosal DC-primed T cells can home to liver, and T cells activated by liver sinusoidal endothelial cells are capable to home to the intestine, termed as “two-way cross talk” in the gut-liver axis55. As over 80% the body’s macrophages reside in the liver56, it is highly likely that helminth-induced AAM may aid in the repair of damage liver tissues56.
The significant inhibitory effect of H. polygyrus infection on diet-induced obesity in our model supports the idea that helminth parasites, which infect millions of people worldwide, particularly in the developing world, may have beneficial metabolic effects. Our results also support the potential for helminths as a new class of biologics in treating inflammatory diseases and metabolic disorders. Further identification of the specific immunomodulatory components of helminths may catapult it to a more refined treatment strategy against obesity and other inflammatory diseases. Our recent published work showed that H. polygyrus infection induces significant alterations in intestinal microbiota composition and functionality57. However, the potential role for helminth-altered microbiota in the protection against HFD-induced obesity remains to be determined. Taken together, results from our study raise the intriguing possibility of using helminths as novel, safer and effective therapeutics in the treatment of obesity and other immune and metabolic disorders.
Methods and Materials
Mice and diet
Four-week-old female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME), fed a normal control diet (CD, 10% of kcal from fat) or given a high-fat diet (HFD, 60% of kcal from fat; Research Diets, New Brunswick, NJ) to induce obesity. These mice were maintained in a specific-pathogen-free (including helminths such as pinworm) facility at Massachusetts General Hospital. Animal care was provided in accordance with protocols approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital. At different time points after dietary treatment was started, some of the HFD- or CD-fed mice were infected with H. polygyrus. The dietary treatments were maintained throughout the experimental period (Fig. 1). The body weight changes were measured throughout the experimental period and represented as a percentage of weight gain by dividing each mouse’s initial weight.
H. polygyrus infection
H. polygyrus were propagated as previously described and stored at 4 °C until use58. Mice were inoculated orally with 200 third stage larvae.
Glucose and insulin tolerance
The mice were fasted overnight for 15 hrs and challenged with glucose solution (2 g/kg of body weight) by oral gavage. Prior to glucose load, blood glucose level was measured and then monitored at times 15, 30, 45, 60, and 120 min, using a Bayer contour (Bayer healthcare LLC, Mishawaka, IN).
Adiposity-specific gene expression
Total RNA was isolated from gonadal adipose tissue. Real time PCR (RT-PCR) was used to determine the expression levels of adipocyte specific genes (peroxisome proliferator-activated receptor gamma (PPARγ), leptin, cytokine genes (IL-6, IL-10 and TNF-α), M1 macrophage marker (iNOS2) and M2 macrophage markers (CD206, arginase1, Ym1). mRNA expression of markers of adipocyte differentiation (CCAAT/enhancer-binding protein alpha, C/EBPα) was measured in visceral fat depots (mesenteric fat) and brown fat. GAPDH was the housekeeping control.
Measurement of cytokine production
Th1 (IFN-γ), Th2 (IL-4), Treg (IL-10) and Th17 (IL-17) cytokines were assayed using ELISA as previously described59. ELISA capture (BVD4-1D11, IL-4; R4-6A2, IFN-γ; IL-10; IL-17) and biotinylated second Abs (BVD6-24G2, IL-4; XMG1.2, IFN-γ; IL-10; IL-17) was purchased from BD Pharmingen. Standard curves were obtained using recombinant murine IFN-γ; IL-4; IL-10; and IL-17 (Genzyme, Cambridge, MA, US).
Measurement of serum antibody
Each mouse was bled at sacrifice, and individual sera were assayed for total IgE, IgG1 and IgG2a by ELISA on plates coated with goat anti-mouse Ab to IgG1, IgG2a (BD PharMingen) or rat anti-mouse Ab to IgE (BD PharMingen). Blocked, washed plates were incubated with diluted serum samples in triplicate for 1.5 h at RT. After washing with PBS/Tween, IgG1 and IgG2a were detected with HRP-conjugated goat anti-mouse IgG1, IgG2a, respectively (PharMingen); IgE was detected with biotin-conjugated rat anti-mouse IgE (PharMingen) and peroxidase-conjugated streptavidin (Zymed Labs.). Both reactions were developed with OPD and read at 492 nm. OD values were converted to μg/ml of IgG1, IgG2a and IgE by comparison with standard curves of purified IgG1, IgG2a (PharMingen) or IgE (PharMingen) by linear regression analysis, and are expressed as the mean concentration for each group of mice ± SEM.
Histopathological examinations
At necropsy, liver and fat tissues from helminth-infected, non-infected were collected, frozen in Tissue Tek OCT compound (Miles, Inc., Elkhart, IN) and stored at −80 °C. Ten-μm sections were cut on a Leica CM1850 Cryostat (Leica Biosystem) and were stained with hematoxylin and eosin or anti-UCP1 antibody. For the fat tissues, the samples from brown adipose, subcutaneous and gonadal adipose tissues were prepared. Using the histopathological approach, we examined the sizes of adipocytes from the various groups of mice that were fed with HFD or CD with and without H. polygyrus infection. Both brown and white adipose tissue sections were processed and UCP1 expression of adipose tissues was determined using immunohistochemical staining with anti-UCP1 antibody (ab10983, Abcam, Cambridge, MA, US).
Macrophage isolation and adoptive transfer
Spleen and peritoneal cells from H. polygyrus-infected mice (2–3 weeks post infection) and non-infected mice were collected aseptically into complete DMEM as previously described59. The tissues were digested with collagenase (type IV; Worthington Biochemical) at 200 U/ml for 45 min at 37 °C. Macrophages were purified by positive selection over a magnetic cell-sorting column (MACS) using microbead-conjugated anti-F4/80 mAb (Miltenyi Biotec). Purified F4/80+ macrophages from normal or H. polygyrus infected donors were transferred into HFD-fed recipient mice (4 weeks on the dietary treatment) (2–3 × 106 cells per mouse) via tail vein injection. HFD treatment was sustained for 4 weeks after macrophage transplantation. Immunostaining with anti-F4/80 and anti- early growth response factor-2 (EGR2) was used to confirm the M2 phenotype of macrophages from helminth-infected mice.
Statistical analysis
All results were expressed as the mean ± standard error of the mean. N refers to the number of mice used. Statistical differences were determined using a two-tailed Student’s t-test (GraphPad Software, Inc., San Diego CA.). A p value of <0.05 was considered significant.
Data availability
All data generated or analysed during this study are included in this article.
Acknowledgements
This work was supported by grants from the National Institutes of Health–R01 DK082427 and R21 AI121997 (to HNS) and by the Nutrition Obesity Research Center at Harvard (P30 DK040561). CS was supported by The Pilot Feasibility Grant from Nutrition Obesity Research Center at Harvard (P30 DK040561). YL and SL were sponsored by the China Scholarship Council. WM was supported by the Massachusetts General Hospital for Children’s Digestive Disease Summer Research Program. The authors acknowledge Dr. Bobby J. Cherayil for critical review of the manuscript.
Author Contributions
C.S., C.C. performed most experimental work, acquired and analyzed results, and C.S. wrote the first draft of the manuscript. Y.L., S.L., W.M., D.K. performed experimental work and analyzed results. W.A.W. and H.S. designed, supervised, and interpreted the experimental data, critically revised the manuscript. H.S. wrote the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berbudi A, Ajendra J, Wardani AP, Hoerauf A, Hübner MP. Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes Metab Res Rev. 2016;32:238–250. doi: 10.1002/dmrr.2673. [DOI] [PubMed] [Google Scholar]
- 2.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 4.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 7.Kopecky J, Hodny Z, Rossmeisl M, Syrovy I, Kozak LP. Reduction of dietary obesity in aP2-Ucp transgenic mice: physiology and adipose tissue distribution. Am J Physiol. 1996;270:E768–775. doi: 10.1152/ajpendo.1996.270.5.E768. [DOI] [PubMed] [Google Scholar]
- 8.Jourdan, P. M., Lamberton, P. H. L., Fenwick, A. & Addiss, D. G. Soil-transmitted helminth infections. Lancet, 10.1016/s0140-6736(17)31930-x (2017). [DOI] [PubMed]
- 9.Guigas B, Molofsky AB. A worm of one’s own: how helminths modulate host adipose tissue function and metabolism. Trends Parasitol. 2015;31:435–441. doi: 10.1016/j.pt.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai S, Tezuka H, Fujita K. A factor of inducing IgE from a filarial parasite prevents insulin-dependent diabetes mellitus in nonobese diabetic mice. Biochem Biophys Res Commun. 2001;286:1051–1058. doi: 10.1006/bbrc.2001.5471. [DOI] [PubMed] [Google Scholar]
- 11.Zaccone P, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–1449. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 12.Summers RW, Elliott DE, Urban JF, Jr., Thompson RA, Weinstock J. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Sun S, et al. Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasites & vectors. 2011;4:186. doi: 10.1186/1756-3305-4-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whary, M. T. et al. Helminth co-infection in Helicobacter pylori infected INS-GAS mice attenuates gastric premalignant lesions of epithelial dysplasia and glandular atrophy and preserves colonization resistance of the stomach to lower bowel microbiota. Microbes Infect16, 345–55, 10.1016/j.micinf.2014.01.005 (2014). [DOI] [PMC free article] [PubMed]
- 15.Elliott D, Weinstock J. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Annals of the New York Academy of Sciences. 2012;1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstock J, Elliott D. Translatability of helminth therapy in inflammatory bowel diseases. International journal for parasitology. 2013;43:245–251. doi: 10.1016/j.ijpara.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng M, et al. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol. 2007;179:4721–4731. doi: 10.4049/jimmunol.179.7.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su CW, et al. Helminth infection impairs autophagy-mediated killing of bacterial enteropathogens by macrophages. J Immunol. 2012;189:1459–1466. doi: 10.4049/jimmunol.1200484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gainsford T, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci USA. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen ED, et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones JR, et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 25.Gao M, Ma Y, Liu D. High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One. 2015;10:e0119784. doi: 10.1371/journal.pone.0119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu I, et al. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest. 2014;124:2099–2112. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu I, Walsh K. The Whitening of Brown Fat and Its Implications for Weight Management inObesity. Curr Obes Rep. 2015;4:224–229. doi: 10.1007/s13679-015-0157-8. [DOI] [PubMed] [Google Scholar]
- 28.Hardardottir I, Grunfeld C, Feingold KR. Effects of endotoxin and cytokines on lipid metabolism. Curr Opin Lipidol. 1994;5:207–215. doi: 10.1097/00041433-199405030-00008. [DOI] [PubMed] [Google Scholar]
- 29.Khovidhunkit W, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Yu XH, et al. Interleukin-17A in lipid metabolism and atherosclerosis. Clin Chim Acta. 2014;431:33–39. doi: 10.1016/j.cca.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 31.de Ruiter, K. et al. Helminths, hygiene hypothesis and type 2 diabetes. Parasite Immunol39, 10.1111/pim.12404 (2017). [DOI] [PubMed]
- 32.Ricardo-Gonzalez RR, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiria AE, et al. Relationship between carotid intima media thickness and helminth infections on Flores Island, Indonesia. PLoS One. 2013;8:e54855. doi: 10.1371/journal.pone.0054855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, et al. Parasitic nematode-induced modulation of body weight and associated metabolic dysfunction in mouse models of obesity. Infect Immun. 2013;81:1905–1914. doi: 10.1128/IAI.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hussaarts L, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29(Jul):3027–3039. doi: 10.1096/fj.14-266239. [DOI] [PubMed] [Google Scholar]
- 37.Wiria AE, Djuardi Y, Supali T, Sartono E, Yazdanbakhsh M. Helminth infection in populations undergoing epidemiological transition: a friend or foe? Semin Immunopathol. 2012;34:889–901. doi: 10.1007/s00281-012-0358-0. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi A, et al. Post-transcriptional Stabilization of Ucp1 mRNA Protects Mice from Diet-Induced Obesity. Cell Rep. 2015;13:2756–2767. doi: 10.1016/j.celrep.2015.11.056. [DOI] [PubMed] [Google Scholar]
- 39.Winn NC, et al. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol. 2017;312:R74–R84. doi: 10.1152/ajpregu.00425.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 41.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 42.Vitali A, et al. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J Physiol. 2009;587:1607–1617. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trevellin E, et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes. 2014;63:2800–2811. doi: 10.2337/db13-1234. [DOI] [PubMed] [Google Scholar]
- 45.Stanford KI, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanford KI, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–2014. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MW, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu Y, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odegaard JI, et al. Perinatal Licensing of Thermogenesis by IL-33 and ST2. Cell. 2016;166:841–854. doi: 10.1016/j.cell.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han X, Kitamoto S, Wang H, Boisvert WA. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J. 2010;24:2869–2880. doi: 10.1096/fj.09-148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 53.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Han X, Kitamoto S, Lian Q, Boisvert WA. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J Biol Chem. 2009;284:32950–32958. doi: 10.1074/jbc.M109.040899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann K, et al. Connecting liver and gut: murine liver sinusoidal endothelium induces gut tropism of CD4+ T cells via retinoic acid. Hepatology. 2012;55:1976–1984. doi: 10.1002/hep.24816. [DOI] [PubMed] [Google Scholar]
- 56.Narayanan S, Surette FA, Hahn YS. The Immune Landscape in Nonalcoholic Steatohepatitis. Immune Netw. 2016;16:147–158. doi: 10.4110/in.2016.16.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su C, et al. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 2018;11(1):144–157. doi: 10.1038/mi.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen, C. C., Louie, S., McCormick, B. A., Walker, W. A. & Shi, H. N. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol176, 472–483 (2006). [DOI] [PMC free article] [PubMed]
- 59.Chen, C. C., Louie, S., McCormick, B., Walker, W. A. & Shi, H. N. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect Immun73, 5468–5481, 10.1128/iai.73.9.5468-5481.2005 (2005). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article.