Abstract
Early podocyte loss is characteristic of chronic kidney diseases (CKD) in obesity and diabetes. Since treatments for hyperglycemia and hypertension do not prevent podocyte loss, there must be additional factors causing podocyte depletion. The role of oxidative stress has been implicated in CKD but it is not known how exactly free radicals affect podocyte physiology. To assess this relationship, we investigated the effects of lipid radicals on podocytes, as lipid peroxidation is a major form of oxidative stress in diabetes. We found that lipid radicals govern changes in podocyte homeostasis through redox sensitive RhoA signaling: lipid radicals inhibit migration and cause loss of F-actin fibers. These effects were prevented by mutating the redox sensitive cysteines of RhoA. We therefore suggest that in diseases associated with increased lipid peroxidation, lipid radicals can determine podocyte function with potentially pathogenic consequences for kidney physiology.
Keywords: Lipid peroxidation, Reactive lipids, Podocyte, RhoA, Cysteine, Chronic kidney disease
Highlights
-
•
Lipid peroxyl radicals impact podocyte motility and cytoskeletal F-actin arrangement.
-
•
Lipid peroxyl radicals activate the small GTPase RhoA.
-
•
When the Cys residues of RhoA are mutated, lipid peroxyl radicals do not affect podocytes.
-
•
Lipid peroxidation likely contributes to podocyte injury.
1. Introduction
Podocytes are highly specialized cells in the glomeruli, and an integral part of a healthy glomerular filtration barrier. Any impairment in their adhesion to the glomerular basement membrane [1], [2] or in their foot processes [3], [4] can lead to cell deformity, foot process effacement and ultimately to the loss of the cell, thus compromising barrier function. Loss of more than ~30% of podocytes is detrimental to a glomerulus [5], [6], [7] leading to glomerulosclerosis and chronic kidney disease (CKD), which is a major complication in diabetes. Therefore, podocyte preservation is critical. However, treatments aimed at two major factors in diabetes, hyperglycemia or hypertension, do not prevent CKD. Podocyte loss is also typical in obesity-related glomerulopathy (ORG) in normoglycemic individuals [8]. Thus, besides high glucose and hypertension, there must be additional pathogenic factors that can cause podocyte depletion. Numerous studies suggested “oxidative stress” as a common denominator in the pathogenesis of kidney disease [9], [10], [11], [12], [13], [14], [15] and, at least in part, in podocyte loss [7], [16], [17], [18], [19]. NADPH oxidase or mitochondrial pathways have been proposed to be main sources of ROS generation in the kidney, resulting in podocyte injury [7], [20], [21]. ROS has also been shown to alter gene expression in podocytes and induce inflammation and injury through the granulocyte macrophage-colony-stimulating factor [22]. Mechanisms leading to podocyte dysfunction and specifications as to which “ROS” are able to elicit such a response remain elusive. To address this issue, we propose that a lipotoxic environment accompanying diabetes is conducive to the formation of lipid peroxyl radicals (LOO•) and their end-products, lipid hydroperoxides.
Lipid radicals are formed during lipid peroxidation [23], which is heightened in diabetes [24]. Their stable end-products, lipid hydroperoxides (LOOH), will cross biological membranes [25], [26]. Many of these end-products, termed as reactive lipid species (RLS) [27] are electrophilic (4-hydroxynonenal, F-isoprostanes) and readily react with nucleophile amino acid residues, particularly cysteine residues of proteins [27]. The key aspect of this notion is that even very low levels of oxidized lipids elicit biological response, through covalently modifying cysteine residues of proteins [27], [28]. Cysteine residues of protein thiols are particularly sensitive to oxidation, thus lipid radical metabolites modulate cellular processes through protein thiol signaling [29], [30]. Therefore, it is reasonable to suggest that lipid radicals/RLS initiating protein thiol signaling through cysteines may be a driving redox mechanism under conditions of oxidative stress leading to podocyte injury. Here, we address this hypothesis in vitro by exposing podocytes to lipid peroxyl radicals and propose a mechanism through investigating the role of RhoA, which is a redox sensitive [31] master regulator protein.
2. Methods
2.1. Materials
All chemicals were from Sigma (St. Louis, MO), purest grade available unless otherwise stated. Antibodies from various sources are described in each section specifically.
2.2. Cell culture
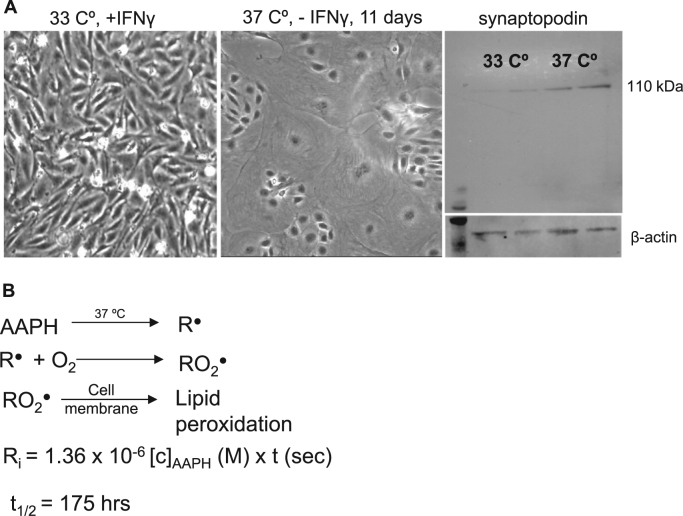
Conditionally immortalized SV-40T podocytes were a generous gift from Dr. Katalin Susztak's (University of Pennsylvania, Philadelphia, PA) and Dr. Farhad Danesh's (Baylor, Houston, TX) laboratories. They were cultured and differentiated as described by Shankland et al. [32]. Briefly, podocytes were maintained under growth permissive conditions at 33 °C with mouse IFNγ supplementation (20 U/ml) in RPMI 1640 media (Thermo Scientific, Waltham, MA). For differentiation, cells were switched to 37 °C without IFNγ for at least 11 days. Since these cells carry the temperature-sensitive variant of the SV40T antigen, it allows podocytes to proliferate at 33 °C. Inactivation of the large T antigen at 37 °C results in cell cycle exit and differentiation into mature podocytes with interdigitating processes. Differentiation was verified by synaptopodin expression. Experiments were performed with differentiated podocytes plated on 6 well plates or glass bottom dishes coated with collagen I.
2.3. RhoA mutation
Cys16, Cys20 or both cysteines (Cys16/20) on RhoA were mutated to alanine (C16A and C20A) using site-directed mutagenesis. These cDNAs were then subcloned into adenoviral shuttle vectors for the construction of recombinant adenoviral vectors, as described. pEGFP-RhoA was purchased from Addgene (Plasmid # 23224). RhoA cDNA was subcloned into the pAC.CMV shuttle vector using EcoRI and BamHI sites in both vectors. pAC.CMV RhoA was used as a template to generate the C16A and C20A mutations in RhoA. pAC.CMV RhoA C16A was used as a template to generate the RhoA C16A/C20A double mutant. All mutations were generated using the QuikChange Site-Directed Mutagenesis kit, according to the manufacturer's protocol, and verified by dideoxy sequencing (PBRC Genomics Core) prior to construction of recombinant adenoviral vectors. Primers used for site-directed mutagenesis are as follows:
C16A: (F) 5′ GTTGGTGATGGAGCCgcTGGAAAGACATGCTTG 3′ and (R) 5′ CAAGCATGTCTTTCCAgcGGCTCCATCACCAAC 3′
C20A: (F) 5′ GAGCCTGTGGAAAGACAgcCTTGCTCATAGTCTTCAG 3′ and (R) 5′ CTGAAGACTATGAGCAAGgcTGTCTTTCCACAGGCTC 3′
C16A/ C20A: (F) 5′' GAGCCgcTGGAAAGACAgcCTTGCTCATAGTCTTC 3′ and (R) 5′ GAAGACTATGAGCAAGgcTGTCTTTCCAgcGGCTC 3′
Recombinant adenoviruses were generated by cotransfection of the pAC.CMV RhoA shuttle vectors and pJM17 viral genome into HEK293 cells. All four adenoviruses were confirmed to be insert positive and E1A negative. To deliver the constructs into podocytes, cells were transduced with the adenoviral constructs at day 11, and incubated with the virus for 48 h. (Delivery and transduction efficiency were titrated and verified using an adenovirus-GFP construct and Western blots see Suppl. Fig. 2). Experiments were performed with cells containing mutated RhoA.
2.4. Podocyte migration and time lapse imaging of live cells
Podocytes were differentiated on collagen coated 6 well plates for 11 days, washed and switched to serum-free RPMI 1640 media before experiments. To study the effects of lipid radicals, cells were incubated with the alkyl radical donor 2,2′-azobis(2-amidino-propane) dihydrochloride (AAPH) at different concentrations (10 and 25 mM, for 4 h at 37 °C to produce 0.8 and 2 µmol/min radical generation, respectively). All incubations were in serum free RPMI 1640 media. After treatments, control or treated cells were washed twice with RPMI 1640 and placed into fresh media with 10% serum supplementation. A “wound” was scratched with a 200 µl sterile pipette tip onto the cell monolayer across each well. Pictures of the wounded area were taken on a Zeiss Axiovert 200 microscope at 72 h to count the number of cells migrating into the wound (4 different viewing areas per well, duplicate wells for each group, two independent experiments). In the live cell time lapse monitoring experiments, a 6-well plate of “wounded” podocyte cells was placed into a humidifying chamber with CO2 thermostat and temperature control (37 °C) of a Leica DM6000 microscope. Coordinates of four different view area positions per well were programmed into the microscope software and cell migration was followed real time for 72 h. A picture of each position was taken in every 30 min (total of 144 frames per position) and compiled into video files at the end of experiments (see online Supplement for videos). Time-lapse videos were then analyzed using an Image J Manual Tracking plug-in. At least two independent experiments were performed for each group.
2.5. F-actin fiber analysis
To assess podocyte cytoskeletal F-actin fiber changes, an F-actin specific green fluorescence conjugated (488 nm) antibody was used (Cytoskeleton Inc, Denver, CO). After exposing podocytes to AAPH for 4 h, cells were washed with sterile PBS, fixed in 10% buffered formaldehyde for 30 min and incubated with the anti-F-actin antibody for 1 h. Cells were washed with PBS three times and were observed in 6 well plates using a Leica DM6000 fluorescent microscope (10 pictures per well, random viewing areas, duplicate wells per group, three independent experiments). F-actin anisotropy (how parallel the fibers are) and orientation were evaluated using the Image J plug-in “FibrilTool”: confocal pictures of the actin fibers (green channel) were converted to greyscale images and at least 3–4 cells from each picture were analyzed with the plug-in.
2.6. RhoA activation
Podocytes were serum starved for 24 h before experiments to minimize endogenous RhoA amount and activity. Since activation of RhoA is a fast process, a time-course of RhoA activation was determined in preliminary experiments, and 5 min of activation was used with the radical donor AAPH. Cells were washed once with ice cold PBS and lysed rapidly on ice. The amount of active GTP-bound RhoA was measured from cell lysates equalized for protein content using a commercially available “G-LISA” assay (Cytoskeleton Inc., Denver, CO) that is specific for the active form of RhoA. A primary anti-RhoA (mouse) antibody provided in the assay kit was used (1:250). RhoA activity was expressed as fold-change, using untreated (normal or wild type) podocyte cell basal RhoA activity for normalization.
2.7. Statistical analysis
Experiments were done with podocytes that were closely similar in passage numbers (13−20) before differentiation. Data were expressed as mean ± SD. Statistical significance between groups was determined by one-way ANOVA followed by Bonferroni post-hoc test, or Student's t-test as appropriate. p < 0.05 was considered as statistically significant difference.
3. Results and discussion
To investigate the effects of lipid radicals on podocytes we have developed a unique, quantitative lipid radical generating system based on the work of Niki [33]. The use of the donor AAPH provides a precisely controlled environment for careful modeling of lipid peroxidation and lipid peroxyl radical formation. AAPH produces alkyl radicals (R•, Fig. 1B) by temperature sensitive decomposition at 37 °C. R• will react very fast with oxygen to generate alkyl peroxyl radicals (RO2•). Ultimately the decomposition of AAPH will generate, in aerobic media, a mix of alkyl, peroxyl and alcoxyl radicals, similarly to lipid peroxidation in vivo. In fact, lipid peroxidation happens exactly the same way: carbon-centered lipid radicals react with oxygen. The difference is that AAPH is quite inert to the attack by its own peroxyl/alcoxyl radicals, and therefore there is no self-amplification of the AAPH oxidation. RO2• only reacts with the cell membrane, creating lipid radicals. Other advantages when compared to various ROS generating systems (such as the xanthine/xanthine oxidase approach for superoxide production or adding H2O2 to cells) include: no off-targets or undesired activation of transcription factors, a very “naturally” occurring membrane lipid peroxidation with close resemblance to an in vivo situation (in terms of the process and also in terms of generating sub-lethal amounts of reactive lipids), minimal or no cell death, no significant pH change in the media (at least in the experimental conditions used herein). The system generates a mixture of lipid radicals, which is a likely scenario in vivo, while just increasing H2O2 concentration or generating only one specific ROS such as superoxide likely does not happen in vivo. The amount of radicals being generated is estimated with the equation in Fig. 1B [33]. The half-life of AAPH is ~ 175 h, therefore, the first few hours of the reaction in practical terms can be assumed to be constant. With this rationale, we chose a 4 h time for our experiments.
Fig. 1.
Alkyl (lipid peroxyl) radical generation in cultured podocytes. (A) Conditionally immortalized mouse podocytes were cultured as described in Methods. Representative pictures show podocytes maintained at 33 °C with IFNγ present and for differentiation cells were switched to non-permissive conditions (37 °C, no IFNγ). Podocytes were differentiated for at least 11 days or until increased synaptopodin levels were evident as verified by Western blot analysis. (B) The free radical donor AAPH was used to generate alkyl radicals and mimic lipid peroxidation. Equations show the temperature sensitive decomposition of AAPH, the resultant radical formation in the cell membrane and the method for calculating free radical production for the incubation periods.
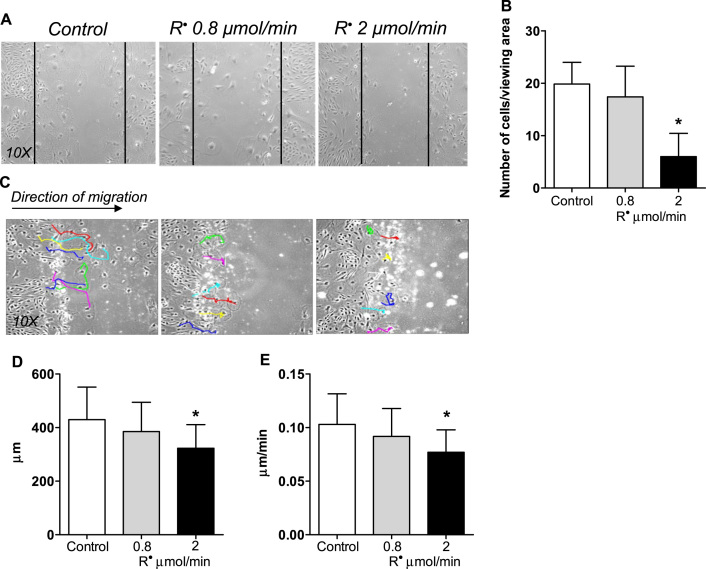
When podocytes were exposed to increasing amounts of LOO•, cells lost their normal motility (Fig. 2A-B). To further understand the migratory properties of podocytes under oxidative stress, we have developed an advanced migration assay using time-lapse imaging combined with computer-based tracking. Podocyte migration was followed for 72 h, with images collected every 30 min (Suppl. video). Cell trajectories, direction, distance and velocity were then analyzed in each group (Fig. 2C-E). These experiments revealed that increasing amounts of LOO• impair motility, and while normal cells maintain a mainly forward track, podocytes under lipid radical stress tend to be stationary or not maintain a forward track (Fig. 2C) and travel less total distance with less velocity (Fig. 2D-E).
Fig. 2.
Lipid peroxyl radicals influence podocyte migratory parameters. (A-B) “Wound” scratch assay shows impaired migration of podocytes exposed to lipid radicals generated at 2 µmol/min for 4 h. Representative pictures of duplicate experiments, number of cells migrating into the wound was counted after 72 h. (C) Track directions of untreated cells and podocytes exposed to lipid radicals (0.8 − 2 µmol/min) were followed in random individual cells (colored lines show representatives) for 72 h using an Image J manual cell tracking plug-in. (D-E) Total distance traveled and average velocity have been calculated from the tracks. Colored tracks show a more static phenotype in cells with 2 µmol/min R• treatment; with significantly less distance traveled, and lower velocity. Duplicate experiments, n = 12 viewing areas (4 per group), at least six cells tracked in each viewing area. *p < 0.05 vs. control (two-tailed Student's t-test).
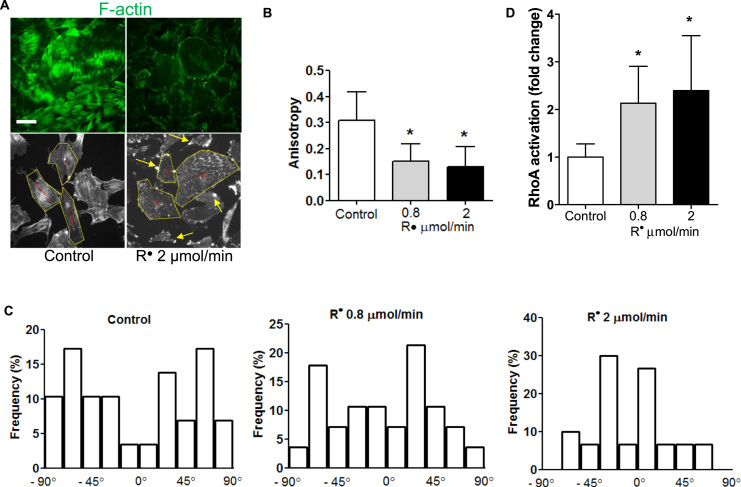
Cytoskeletal structure is essential for proper foot processes and maintaining the integrity of the filtration barrier [6]. When podocytes are exposed to lipid radicals, they lose their F-actin fiber cytoskeletal structure, cortically rearrange filaments or form aberrant filaments with actin-rich junctions (Fig. 3A-B). We have also analyzed fiber anisotropy and orientation. These measurements show that there is a significant loss of fiber anisotropy in podocytes treated with lipid radicals, together with a loss of random orientation (Fig. 3B-C). Well-developed F-actin fibers are seen in normal podocytes, while cortical rearrangement, loss of fibers, aberrant fiber development and actin-rich centers with radial fibers are typical in podocytes under stress [34], [35]. Our experiments with lipid radicals recapitulate this phenotype. Undifferentiated podocytes have also been described to show cortically arranged fibers [36]. These results suggest that under oxidative stress, lipid radicals may lead to aberrant podocyte formation or even dedifferentiation through affecting cytoskeletal properties and actin formation.
Fig. 3.
Lipid peroxyl radicals induce cytoskeletal rearrangements in podocytes and increase RhoA activation. (A) Upper panels: Representative images of untreated podocytes and podocytes incubated with AAPH to generate alkyl radicals (R•). After incubation (4 h), cells were stained for F-actin as described in Materials and methods. F-actin orientation was observed under a fluorescent microscope (10 pictures/group at random viewing areas). Podocytes exposed to lipid radicals display cortical rearrangement and loss of F-actin transversal fibers. Lower panels: representative greyscale images of control and R• treated podocytes for anisotropy and orientation analysis, showing the anisotropy vectors in red (Image J “FibrilTool” application). Yellow arrows show examples of actin-rich centers in podocytes exposed to lipid peroxidation. (B) Anisotropy analysis of control and lipid radical treated podocytes confirms significant loss of transversal fibers. (C) Orientation analysis of F-actin fibers, showing significant reorientation in podocytes exposed to lipid peroxidation. Orientation angles from − 90 to + 90 degrees were grouped into 20 degree intervals. Duplicate experiments in six well plates, n = at least 30 cells analyzed per group (3 cells each picture, 10 pictures each group), *p < 0.05 vs. control. (D) Lipid radicals generated from AAPH activate RhoA in a dose-dependent manner. RhoA activation was measured using an active (GTP-bound) RhoA specific “G-LISA” assay. Duplicate experiments, n = 4 per group, *p < 0.05 vs. control (one-way ANOVA for multiple comparisons).
Next, we aimed to elucidate the mechanism by which lipid radicals can cause such changes in podocytes. Because podocyte migratory and cytoskeletal properties were impacted by free radicals in our experiments, we examined known redox-sensitive regulatory proteins controlling cell migration. Specific examples include the small GTP-ase family: RhoA, Rac1 and Cdc42 [37]. Activation of RhoA in general promotes a stationary cell phenotype [38]. Previous investigations established that too much or too little RhoA activity is equally detrimental to the podocyte; through slightly different mechanisms, both increased and decreased RhoA activity causes proteinuric kidney disease [39], [40]. RhoA has a distinct motif in its phosphoryl binding loop, which contains two redox sensitive cysteine residues [31]. RhoA is activated not only by guanine nucleotide exchange factors (GEFs) but also by ROS in fibroblast cells [31]. We thus hypothesized that RhoA activity may be altered by lipid radicals, leading to the observed functional changes of podocytes. To test this hypothesis, we analyzed RhoA activation in podocytes treated with AAPH.
Lipid radicals activated RhoA in podocytes in a dose-dependent fashion (in a range of ~2–3.5-fold activation, Fig. 3D). The concentrations of radicals used in our experiments are in the pathophysiologically relevant range, which was determined by the kinetic equation shown on Fig. 1B and comparing the calculated concentrations to literature data on RLS levels [27]. We show that as lipid radical concentrations increase, RhoA activity increases hence the cells lose motility. As shown on the colored individual cell tracks on Fig. 2C, cells exposed to lipid radicals tend to remain stationary, with little or no forward movement. If we consider the cell migration assay as an in vitro surrogate measurement of foot processes [41], we can propose that podocyte injury from lipid radicals can translate into FP effacement and ultimately, proteinuria. In our experiments an overly active RhoA led to cortical rearrangement of F-actin fibers and to the loss of transversal fibers (Fig. 3A). Transversal fibers are part of a normal podocyte cytoskeletal structure, and a basal RhoA activity seems to be necessary to ensure the formation of such fibers. We can surmise that on the other hand, an overly active RhoA is disruptive and causes cytoskeletal rearrangement and loss of F-actin fibers. Our observations are in agreement with those where a stimuli that is known to be proteinuric in vivo (such as insulin) leads to the loss of F-actin fibers in vitro, and to RhoA activation in a similar fashion [42].
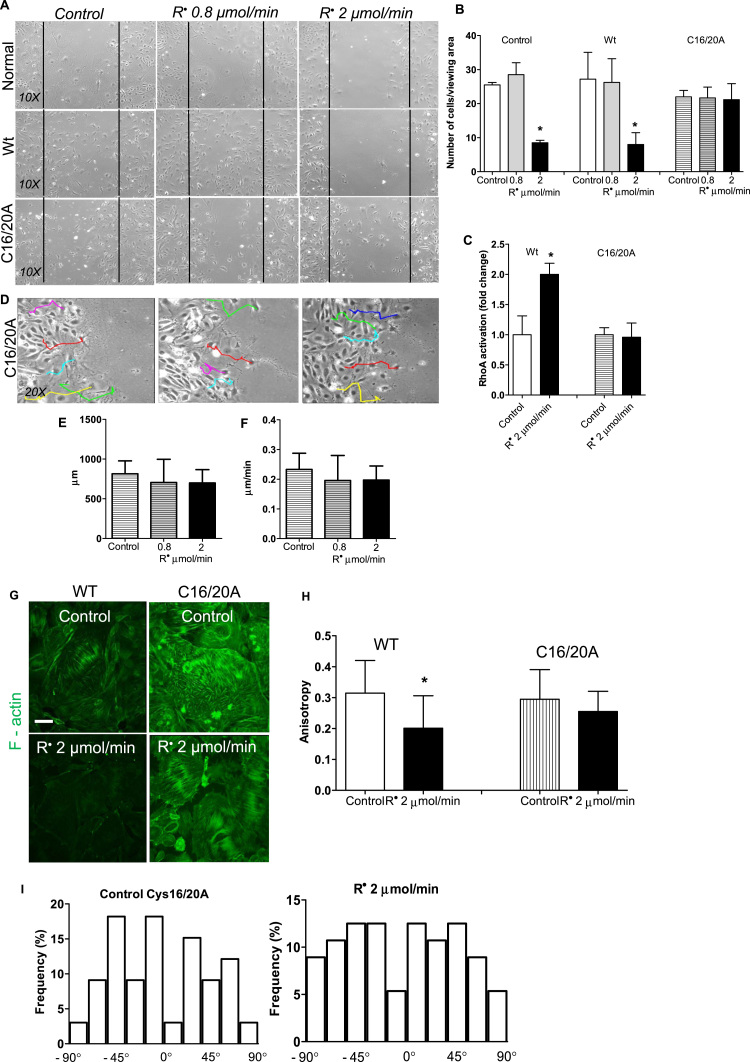
To unequivocally demonstrate that the mechanism of action of lipid radicals is through redox regulation of RhoA, we repeated the experiments with podocytes expressing RhoA proteins with cysteine residue(s) mutated to alanine. Podocytes were transduced with RhoA-expressing constructs (Supplement Fig. 2) to examine the impact of mutated RhoA proteins. A wild type RhoA-construct (Wt) was used as control. Podocytes with both cysteine 16 and cysteine 20 mutated on RhoA (C16/20 A) maintained normal migratory properties despite being exposed to lipid radicals (Fig. 4A-B). Neither number of cells migrating into the wound nor the track direction and velocity of cells were significantly different from normal (Wt-RhoA-transfected), untreated podocytes (Fig. 4D-F). Similarly, podocytes expressing RhoA with C16/20 A mutations maintained healthy F-actin fibers despite lipid peroxidation (Fig. 4G-I). While cells transfected with WT-RhoA, similarly to normal podocytes, had increased their RhoA activity about 2–2.5-fold upon exposure to lipid radicals, podocytes expressing RhoA with C16/20 A mutations maintained basal RhoA activity (Fig. 4C). When we conducted migration experiments by transducing podocytes with RhoA C16A or C20A single mutants, these podocytes responded to lipid radicals similarly to normal or Wt-RhoA-transfected cells (Suppl. Fig. 3). These experiments demonstrate that cysteine 16 and/or 20 are required for lipid radical-induced RhoA activation, and that cytoskeletal changes brought about by lipid radical exposure are downstream of RhoA and not a direct consequence of the exposure. We propose that through this mechanism, lipid peroxidation can impact podocytes by dysregulating RhoA activity. As our experiments with redox-insensitive RhoA proteins demonstrate, it appears that in podocytes the dominant effect of lipid radicals may be mediated by RhoA.
Fig. 4.
Podocytes sense lipid peroxyl radicals through the redox sensitive cysteine residues of RhoA. (A-B) Comparison of podocyte migration using the “wound” assay in normal cells, wild type RhoA transduced podocytes and in cells transduced with the mutant C16/20 A RhoA, after lipid peroxide radical exposure (0.8 − 2 µmol/min, 4 h). Number of cells migrating into the wound was counted in each group in duplicate experiments after 72 h. While normal or Wt podocytes have significantly impaired migration at 2 µmol/min radical exposure, C16/20 A RhoA mutant podocytes migrate normally. N= 4 viewing areas each group, * p < 0.05 vs. control. (C) Activation of RhoA by lipid radicals is blunted in podocytes bearing C16/20 A mutated RhoA as measure by a “G-LISA” assay (see also Fig. 2). (D) Track direction of C16/20 A RhoA transduced podocytes were followed in random individual cells (colored lines show representatives) for 72 h using an Image J manual cell tracking plug-in. (E-F) Total distance traveled and average velocity have been calculated from the tracks. Podocytes with mutant RhoA display normal migratory parameters. (G) F-actin fibers were visualized and (H-I) anisotropy and fiber orientation were measured similarly to as shown in Fig. 3, in wild type RhoA and C16/20 A RhoA transduced cells using the highest radical concentration. (One-way ANOVA analysis was used for multiple comparisons).
4. Conclusions
In summary, we found that lipid peroxidation impacts podocyte motility and cytoskeletal structure and that mechanistically this occurs through the redox sensitive cysteine residues of RhoA. Our results are consistent with our hypothesis and with literature data yet they also raise some important questions. For example, while RhoA tends to be at the tail end of the cell body, promoting the formation of actin-myosin containing fibers [37], Cdc42 and Rac1 are at the leading edge. In principle, when Cdc42 and Rac1 are activated, RhoA is less active and this concerted apparatus ensures physiological motility (healthy foot process in vivo). In general, if RhoA is overly active, cells have a more stationary phenotype. Our observations from the time lapse analyses of cell tracking and pathway direction experiments are consistent with this notion. RhoA activation by lipid radicals may be a part of the overall pathogenic mechanism. We are aware that other members of the RhoA family GTPases, such as Rac1 and Cdc42 also contain similar cysteine residues [43]. Hence, we suspect that lipid radicals may influence the activity of these GTPases as well. These effects likely depend on the localization and basal activity of these GTPases and could mean either activation or inactivation. Podocyte-specific loss of Cdc42 for example has been shown to lead to congenital nephropathy [44]. Furthermore, we recognize that in other cell types found in the kidney, disruption or cysteine-driven redox regulation of other redox sensitive proteins may be of higher importance. These effects may be cell type specific and similarly to small GTPases, likely depend on localization and compartmentalization.
Importantly, podocytes in our experiments were not exposed to hyperglycemic conditions, angiotensin II, free fatty acids or mechanical forces (all of which conditions have been shown to influence podocyte cell homeostasis) [7], [45], [46]. Thus, we suggest that lipid peroxidation is a new pathogenic factor that influences the migratory and cytoskeletal properties of podocytes, independently of other conditions. In metabolic diseases, lipid peroxidation therefore may significantly impact the integrity of the filtration barrier, contributing to the development of proteinuria. Since lipid peroxidation is significantly elevated in diabetes and end-products of lipid peroxidation are known to accumulate in human diabetes, including the glomeruli [47], [48], the implications of our findings may be of clinical relevance in diabetic CKDs.
Taken together, we conclude that lipid peroxyl radicals modulate structural and functional changes in podocytes through redox sensitive regulation of RhoA. Our experiments conclusively demonstrate a novel redox sensitive mechanism of podocyte RhoA dysregulation. Numerous studies suggested a role for oxidative stress in podocyte loss [7], [16], [19], but the mechanisms are less clear. Our findings provide a novel pathway that can be the first step to better understand how specific radicals affect podocyte function. Either selective targeting and removal of excess lipid peroxidation products or inhibiting reactions leading to their formation should be considered to minimize oxidative stress, to ensure normal RhoA activity levels and to prevent podocyte loss and consequent proteinuria in CKD.
Acknowledgments
Research for this project was supported partially by an NIH “DiaComp” grant 14GHSU1393 (K.S.), R01-HD085017 (J.M.S.) and the Pennington Foundation. The work utilized the facilities of the Cell Biology and Bioimaging Core that are supported in part by COBRE (NIH P20-RR021945) and NORC (NIH 1P30-DK072476) center grants from the NIH. The authors thank Drs. Katalin Susztak and Farhad Danesh for the podocyte cell line.
Acknowledgments
Declaration of interests
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.02.024.
Appendix A. Supplementary material
Supplementary material: Figure S2. Confirmation of RhoA construct delivery and transduction in podocytes. (A) Delivery of a GFP-labeled adenoviral construct was confirmed at increasing doses with fluorescent microscopy. (B) Western blot analyses of podocyte cell lysates 48 h after transduction with the RhoA constructs (showing RhoA at ~ 20 kDa). Figure S3. Cell migration experiments were conducted in podocytes with C16A or C20A (single cysteine RhoA) mutation. Similarly to podocytes with non-mutated RhoA, single cysteine mutant cells show impaired migration upon lipid radical exposure, demonstrating that single mutation is not sufficient to disrupt the redox sensitivity of RhoA. *p < 0.05 vs. control (one-way ANOVA for multiple comparisons).
Supplementary material: Figure S1. Representative time lapse video of untreated and lipid radical treated (2 µmol/min, 4 h) podocytes migrating over a 72 h time frame, recorded on a Leica DM6000 microscope. 144 frames total recorded at 30 min intervals were combined into an AVI file to monitor changes in podocyte cell motility. Track direction, distance and speed analyses shown on Fig. 2, Fig. 4 were conducted using these time lapse video file frames.
References
- 1.Sachs N., Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat. Rev. Nephrol. 2013;9:200–210. doi: 10.1038/nrneph.2012.291. [DOI] [PubMed] [Google Scholar]
- 2.Sugar T. Podocyte-specific deletion of NDST1, a key enzyme in the sulfation of heparan sulfate glycosaminoglycans, leads to abnormalities in podocyte organization in vivo. Kidney Int. 2014;85:307–318. doi: 10.1038/ki.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barisoni L., Mundel P. Podocyte biology and the emerging understanding of podocyte diseases. Am. J. Nephrol. 2003;23:353–360. doi: 10.1159/000072917. [DOI] [PubMed] [Google Scholar]
- 4.Endlich K., Kriz W., Witzgall R. Update in podocyte biology. Curr. Opin. Nephrol. Hypertens. 2001;10:331–340. doi: 10.1097/00041552-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Li J.J. Podocyte biology in diabetic nephropathy. Kidney Int. Suppl. 2007:S36–S42. doi: 10.1038/sj.ki.5002384. [DOI] [PubMed] [Google Scholar]
- 6.Shankland S.J. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 7.Susztak K., Raff A.C., Schiffer M., Bottinger E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 8.Camici M., Galetta F., Abraham N., Carpi A. Obesity-related glomerulopathy and podocyte injury: a mini review. Front. Biosci. 2012;4:1058–1070. doi: 10.2741/e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceriello A. Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes. 2000;49:2170–2177. doi: 10.2337/diabetes.49.12.2170. [DOI] [PubMed] [Google Scholar]
- 10.Decleves A.E., Mathew A.V., Cunard R., Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J. Am. Soc. Nephrol. 2011;22:1846–1855. doi: 10.1681/ASN.2011010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugan L.L. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Investig. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha H., Lee H.B. Oxidative stress in diabetic nephropathy: basic and clinical information. Curr. Diab Rep. 2001;1:282–287. doi: 10.1007/s11892-001-0047-1. [DOI] [PubMed] [Google Scholar]
- 13.Kakkar R., Mantha S.V., Radhi J., Prasad K., Kalra J. Antioxidant defense system in diabetic kidney: a time course study. Life Sci. 1997;60:667–679. doi: 10.1016/s0024-3205(96)00702-3. [DOI] [PubMed] [Google Scholar]
- 14.Ruggiero C., Ehrenshaft M., Cleland E., Stadler K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am. J. Physiol. Endocrinol. Metab. 2011;300:E1047–E1058. doi: 10.1152/ajpendo.00666.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh M. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2005;288:F1144–F1152. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 16.Breyer M.D. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 17.Brosius F.C., 3rd Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wicks S.E., Nguyen T.T., Breaux C., Kruger C., Stadler K. Diet-induced obesity and kidney disease – in search of a susceptible mouse model. Biochimie. 2016;124:65–73. doi: 10.1016/j.biochi.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng S. Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. J. Am. Soc. Nephrol. 2008;19:2077–2085. doi: 10.1681/ASN.2007080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daehn I. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J. Clin. Investig. 2014;124:1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J., Chen J.K., Harris R.C. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J. Am. Soc. Nephrol. 2015;26:1115–1125. doi: 10.1681/ASN.2014020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greiber S., Muller B., Daemisch P., Pavenstadt H. Reactive oxygen species alter gene expression in podocytes: induction of granulocyte macrophage-colony-stimulating factor. J. Am. Soc. Nephrol. 2002;13:86–95. doi: 10.1681/ASN.V13186. [DOI] [PubMed] [Google Scholar]
- 23.Niki E., Yamamoto Y., Komuro E., Sato K. Membrane damage due to lipid oxidation. Am. J. Clin. Nutr. 1991;53:201S–205S. doi: 10.1093/ajcn/53.1.201S. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B., Gutteridge J.M. Free radicals, lipid peroxidation, and cell damage. Lancet. 1984;2:1095. doi: 10.1016/s0140-6736(84)91530-7. [DOI] [PubMed] [Google Scholar]
- 25.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 26.Grimsrud P.A., Xie H., Griffin T.J., Bernlohr D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higdon A., Diers A.R., Oh J.Y., Landar A., Darley-Usmar V.M. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem. J. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levonen A.L. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renedo M. Modification and activation of Ras proteins by electrophilic prostanoids with different structure are site-selective. Biochemistry. 2007;46:6607–6616. doi: 10.1021/bi602389p. [DOI] [PubMed] [Google Scholar]
- 30.Wall S.B. Rac1 modification by an electrophilic 15-deoxy Delta(12,14)-prostaglandin J2 analog. Redox Biol. 2015;4:346–354. doi: 10.1016/j.redox.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aghajanian A., Wittchen E.S., Campbell S.L., Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009;4:e8045. doi: 10.1371/journal.pone.0008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankland S.J., Pippin J.W., Reiser J., Mundel P. Podocytes in culture: past, present, and future. Kidney Int. 2007;72:26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- 33.Niki E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990;186:100–108. doi: 10.1016/0076-6879(90)86095-d. [DOI] [PubMed] [Google Scholar]
- 34.Mundel P., Kriz W. Structure and function of podocytes: an update. Anat. Embryol. 1995;192:385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- 35.Endlich N. Podocytes respond to mechanical stress in vitro. J. Am. Soc. Nephrol. 2001;12:413–422. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- 36.Mundel P. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 37.Asanuma K. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat. Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 38.Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 39.Wang L. Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int. 2012;81:1075–1085. doi: 10.1038/ki.2011.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L., Jiang R., Aoudjit L., Jones N., Takano T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 2011;22:1621–1630. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiser J. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J. Biol. Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 42.Welsh G.I. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hobbs G.A., Zhou B., Cox A.D., Campbell S.L. Rho GTPases, oxidation, and cell redox control. Small GTPases. 2014;5:e28579. doi: 10.4161/sgtp.28579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott R.P. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J. Am. Soc. Nephrol. 2012;23:1149–1154. doi: 10.1681/ASN.2011121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kampe K., Sieber J., Orellana J.M., Mundel P., Jehle A.W. Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am. J. Physiol. Ren. Physiol. 2014;306:F401–F409. doi: 10.1152/ajprenal.00454.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu H.H. Downregulation of the antioxidant protein peroxiredoxin 2 contributes to angiotensin II-mediated podocyte apoptosis. Kidney Int. 2011;80:959–969. doi: 10.1038/ki.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horie K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Investig. 1997;100:2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki D. Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J. Am. Soc. Nephrol. 1999;10:822–832. doi: 10.1681/ASN.V104822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Figure S2. Confirmation of RhoA construct delivery and transduction in podocytes. (A) Delivery of a GFP-labeled adenoviral construct was confirmed at increasing doses with fluorescent microscopy. (B) Western blot analyses of podocyte cell lysates 48 h after transduction with the RhoA constructs (showing RhoA at ~ 20 kDa). Figure S3. Cell migration experiments were conducted in podocytes with C16A or C20A (single cysteine RhoA) mutation. Similarly to podocytes with non-mutated RhoA, single cysteine mutant cells show impaired migration upon lipid radical exposure, demonstrating that single mutation is not sufficient to disrupt the redox sensitivity of RhoA. *p < 0.05 vs. control (one-way ANOVA for multiple comparisons).
Supplementary material: Figure S1. Representative time lapse video of untreated and lipid radical treated (2 µmol/min, 4 h) podocytes migrating over a 72 h time frame, recorded on a Leica DM6000 microscope. 144 frames total recorded at 30 min intervals were combined into an AVI file to monitor changes in podocyte cell motility. Track direction, distance and speed analyses shown on Fig. 2, Fig. 4 were conducted using these time lapse video file frames.