Abstract
Ozone and obesity both increase IL-17A in the lungs. In mice, obesity augments the airway hyperresponsiveness and neutrophil recruitment induced by acute ozone exposure. Therefore, we examined the role of IL-17A in obesity-related increases in the response to ozone observed in obese mice. Lean wild-type and obese db/db mice were pretreated with IL-17A–blocking or isotype antibodies, exposed to air or ozone (2 ppm for 3 h), and evaluated 24 hours later. Microarray analysis of lung tissue gene expression was used to examine the mechanistic basis for effects of anti–IL-17A. Compared with lean mice, ozone-exposed obese mice had greater concentrations of BAL IL-17A and greater numbers of pulmonary IL-17A+ cells. Ozone-induced increases in BAL IL-23 and CCL20, cytokines important for IL-17A+ cell recruitment and activation, were also greater in obese mice. Anti–IL-17A treatment reduced ozone-induced airway hyperresponsiveness toward levels observed in lean mice. Anti–IL-17A treatment also reduced BAL neutrophils in both lean and obese mice, possibly because of reductions in CXCL1. Microarray analysis identified gastrin-releasing peptide (GRP) receptor (Grpr) among those genes that were both elevated in the lungs of obese mice after ozone exposure and reduced after anti–IL-17A treatment. Furthermore, ozone exposure increased BAL GRP to a greater extent in obese than in lean mice, and GRP-neutralizing antibody treatment reduced obesity-related increases in ozone-induced airway hyperresponsiveness and neutrophil recruitment. Our data indicate that IL-17A contributes to augmented responses to ozone in db/db mice. Furthermore, IL-17A appears to act at least in part by inducing expression of Grpr.
Keywords: gastrin-releasing peptide receptor, microarray, airway hyperresponsiveness, neutrophil, CXCL1
Clinical Relevance
Our data indicate that IL-17A contributes to the augmented responses to ozone (O3) observed in db/db mice and suggest that IL-17A may be mediating its effects on O3-induced airway hyperresponsiveness via changes in gastrin-releasing peptide receptor. The data suggest that IL-17A may contribute to the greater decrements in pulmonary function observed in obese versus lean human subjects after O3 exposure, though experiments in human subjects will be required to confirm that hypothesis.
Ozone (O3) is a common air pollutant that triggers asthma. O3 exposure initiates asthma symptoms, reduces lung function, and increases hospital admissions and emergency room visits for asthma (1, 2). O3 exposure causes lung epithelial injury leading to an inflammatory response that includes release of cytokines and chemokines, neutrophil recruitment, and airway hyperresponsiveness (AHR), a canonical feature of asthma (3, 4). The inflammation and AHR caused by O3 exposure likely contribute to the capacity of O3 to trigger asthma.
In the United States, approximately one-third of adults are obese, and another one-third are overweight. Importantly, obesity exacerbates O3-induced reductions in lung function, especially in subjects with AHR (5, 6). Obesity exacerbates the impact of O3 on asthma symptoms in children (7). Given that obesity is itself a risk factor for asthma (8), these data suggest that O3 can be a particular problem for the obese individuals with asthma.
Obesity exacerbates responses to acute O3 exposure in mice (9, 10). We have demonstrated that blocking IL-33 signaling reduces but does not abolish obesity-related increases in the response to O3 (9), indicating that although IL-33 plays a role in these events, other factors must also contribute. In mice, IL-17A augments the AHR and neutrophil recruitment induced by IL-33 (11), and O3 increases IL-17A expression (12, 13). IL-17A contributes to the pathogenesis of other comorbidities of obesity, such as glucose intolerance (14). Lung IL-17A is also elevated, both in obese versus lean mice (15–17) and in sputum of obese versus lean persons with asthma (18). Furthermore, IL-17A contributes to the innate AHR observed in mice with diet-induced obesity (15) and has also been shown to play a role in neutrophil recruitment induced by subacute (low-dose, long-duration) O3 exposure (19, 20). Therefore, we hypothesized that IL-17A could be contributing to obesity-related increases in the pulmonary responses to acute O3 exposure.
To test our hypothesis, we treated lean wild-type (WT) and obese db/db mice with anti–IL-17A or isotype antibody 24 hours before air or O3 exposure. Anti–IL-17A decreased O3-induced AHR in obese but not in lean mice. To examine the mechanism underlying the effect of IL-17A, we performed a microarray analysis to identify genes altered both by O3 and by anti–IL-17A in db/db mice. The gene for gastrin-releasing peptide receptor (GRPR), the receptor for gastrin-releasing peptide (GRP), a member of the bombesin family of peptides that is found in pulmonary neuroendocrine cells (NECs) (21), was among the genes identified. Importantly, obesity augmented O3-induced increases in BAL GRP, and treatment with anti-GRP neutralizing antibodies significantly reduced obesity-related increases in O3-induced AHR. The data indicate that in obesity, IL-17A contributes not only to innate AHR (15) but also to further increases in airway responsiveness induced by a common nonallergic asthma trigger. The data also show, for the first time, to our knowledge, that one mechanism by which IL-17A may be driving AHR is through increases in Grpr expression. The data suggest that GRP and GRPR might be good therapeutic targets for asthma phenotypes such as obese asthma, in which IL-17A appears to play a role.
Methods
Mice
Female db/db and WT (C57BL/6) mice at ages 6–8 weeks were purchased from The Jackson Laboratory and studied at 10 weeks of age. Breeding of Cpefat/TNFR2−/− mice is described elsewhere (16). Cpefat/TNFR2−/− mice at 12 weeks of age were studied. Mice on high-fat diets (HFDs) were rendered obese by feeding them a diet in which 60% of calories were derived from fat in the form of lard for 24 weeks, as described previously (9). Control mice were fed regular mouse chow for 24 weeks. All protocols were approved by the Harvard Medical Area Standing Committee on Animals.
Protocol
WT and db/db mice as well as Cpefat/TNFR2−/− mice were given intraperitoneal injections with either an IL-17A–neutralizing antibody (4 mg/kg, clone 50104; R&D Systems) or with an isotype antibody (4 mg/kg, clone 54447; R&D Systems) (22). Mice were exposed to room air or O3 (2 ppm for 3 h) 24 hours later. Airway responsiveness was measured; BAL was performed; and blood was collected for preparation of serum 24 hours after exposure. The lungs were then flushed with cold PBS via the vasculature to remove blood and harvested for preparation of RNA for microarray and qRT-PCR.
To examine the role of signaling through GRPR, WT and db/db mice were given injections of a monoclonal antibody, 2A11 (4 mg/kg i.p.) (23), which binds the C-terminal region of both human and mouse GRP with high affinity (24) and blocks GRP-mediated effects on lung cells (25), or with isotype antibody (clone MOPC-21; BioLegend) 1 hour before air or O3 exposure. Mice were studied 24 hours after the cessation of exposure, as described above.
Microarray
Lung RNA samples from 11 mice were used in this analysis. All were from db/db mice. Four were from air-exposed isotype antibody–treated mice, four were from O3-exposed isotype antibody–treated mice, and three were from O3-exposed anti–IL-17A–treated mice. Gene expression analyses of these 11 samples were performed using GeneChip Mouse Gene 1.0 ST arrays (Affymetrix), as described in the data supplement. Transcription factor binding enrichment for genes that were both affected by O3 and within the O3-affected genes, as well as affected by anti–IL-17A, were analyzed using MsigDB (Broad Institute) (see data supplement). Microarray results were confirmed using qRT-PCR. The microarray data have been deposited in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE81800). Methods of O3 exposure, measurements of airway responsiveness, RNA extraction, qRT-PCR, BAL, measurement of cytokines and chemokines, transcription factor binding enrichment, and flow cytometry are detailed in the data supplement.
Statistics
Statistica software (StatSoft) was used to analyze data by factorial ANOVA with mouse genotype, antibody treatment, and exposure, or with mouse genotype, diet, and exposure, as main effects. As a post hoc test, we used Fisher’s least significant difference test. BAL cells and flow cytometric data were log transformed before statistical analysis to conform to a normal distribution. A P value less than 0.05 was considered statistically significant.
Results
Obesity Augments O3-induced IL-17A Expression
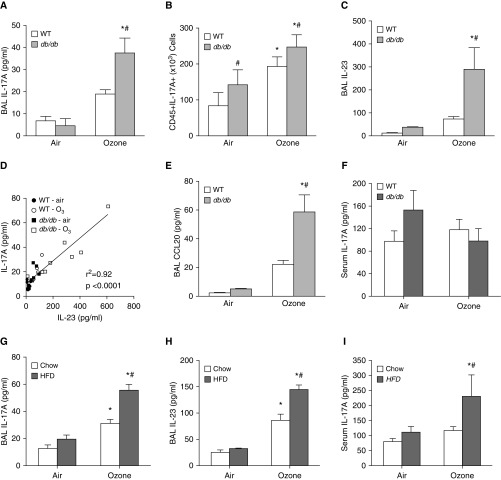
O3 caused significant increases in BAL IL-17A (Figure 1A) and in IL-17A+CD45+ lung cells (Figure 1B; and Figure E1 in the data supplement) that were greater in db/db than in WT mice. IL-17A+CD45+ lung cells were also greater in obese than in lean mice exposed to room air (Figure 1B). O3-induced increases in BAL IL-23 (Figure 1C), a cytokine that contributes to IL-17A expression (26), were also greater in db/db than in WT mice. Furthermore, there was a strong correlation between BAL IL-17A and BAL IL-23 (Figure 1D). Obesity also augmented O3-induced increases in BAL CCL20 (Figure 1E), a chemotactic factor for CCR6+ IL-17A–expressing cells (27). Obesity and O3 have systemic as well as pulmonary effects. Hence, we also examined serum IL-17A concentrations. Neither db/db genotype nor O3 exposure had any significant effect on serum IL-17A (Figure 1F).
Figure 1.
Ozone (O3) exposure increases BAL IL-17A. BAL concentrations of (A and G) IL-17A, (C and H) IL-23, and (E) CCL20, as well as (F and I) serum concentrations of IL-17A, were measured by ELISA. (B) Lung IL-17A+CD45+ cells were measured by flow cytometry. (D) Correlation between BAL IL-17A and BAL IL-23 is shown. Data are derived from (A–F) db/db and wild-type (WT) mice or from (G–I) C57BL/6 mice fed either a high-fat diet (HFD) or regular mouse chow for 24 weeks. Mice were exposed to air or ozone (2 ppm for 3 h) and examined 24 hours after exposure. Results are mean ± SE of five to nine mice per group. *P < 0.05 versus air; #P < 0.05 versus lean mice.
Essentially similar results were obtained with diet-induced obese mice: O3-induced increases in both BAL IL-17A and BAL IL-23 were greater in mice in which obesity was induced by HFD feeding than in lean control mice fed regular mouse chow (Figures 1G and 1H), and there was also a tight correlation between IL-17A and IL-23 in these mice (r2 = 0.89). Interestingly, O3 exposure also resulted in a significant increase in serum IL-17A in the HFD-fed mice but not in the chow-fed mice (Figure 1I). Taken together, the data indicate that after acute O3 exposure, obesity (regardless of how it was induced) created an environment conducive to the pulmonary recruitment and activation of IL-17A–expressing cells.
Effects of IL-17A Neutralization on Responses to O3 in Lean and in Obese Mice
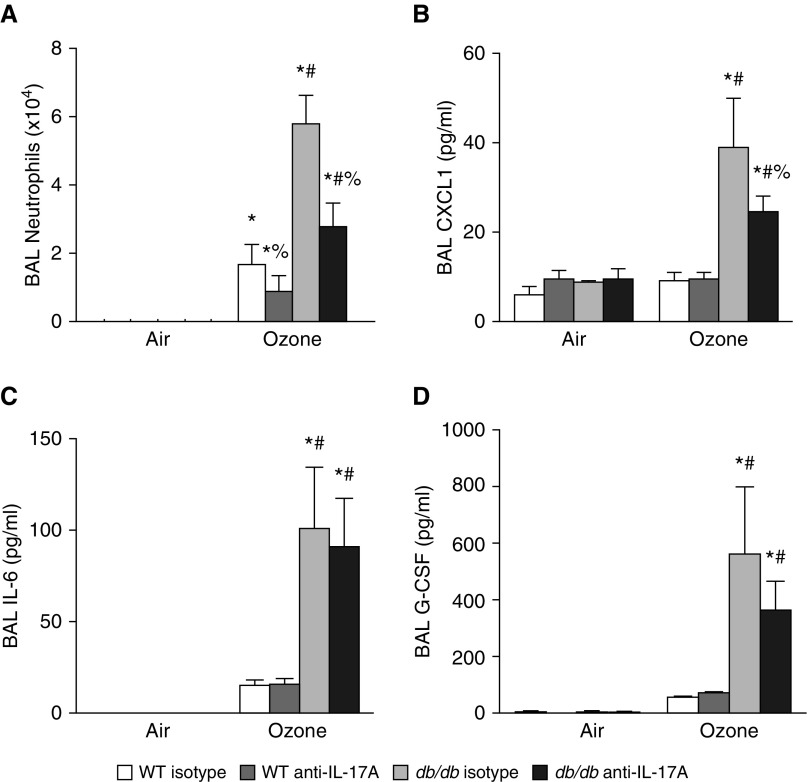
To examine the impact of O3-induced elevations in IL-17A, mice were treated with anti–IL-17A or isotype control antibodies before O3 or air exposure. In mice treated with isotype antibodies, O3-induced increases in BAL neutrophils were greater in db/db than in WT mice (Figure 2A), as previously described (9, 10). In O3-exposed db/db mice, anti–IL-17A significantly reduced BAL neutrophils toward amounts observed in WT mice (Figure 2A). IL-17A neutralization also reduced BAL neutrophils in WT mice. IL-17A recruits neutrophils via production of other neutrophil chemotactic and survival factors, including CXCL1, CXCL2, CXCL5, IL-6, and granulocyte-colony stimulating factor (G-CSF) (28–30). BAL concentrations of CXCL2 and CXCL5 were not significantly affected by O3 in either lean or obese mice (data not shown). However, in mice treated with isotype antibody, O3 exposure did increase BAL concentrations of CXCL1, IL-6, and G-CSF, and each was increased to a significantly greater extent in obese than in lean mice (Figures 2B–2D). BAL CXCL1 was significantly lower in O3-exposed db/db mice treated with anti–IL-17A than in those treated with isotype antibody, whereas BAL IL-6 and BAL G-CSF were not affected by anti–IL-17A treatment. No effect of anti–IL-17A on O3-induced increases in BAL CXCL1, IL-6, or G-CSF was observed in WT mice (Figures 2B–2D). Anti–IL-17A did not impact O3-induced changes in BAL macrophages or BAL protein, an index of O3-induced injury to the alveolar/capillary barrier (data not shown).
Figure 2.
Anti–IL-17A reduces BAL neutrophils and BAL CXCL1 in obese O3-exposed mice. (A) BAL neutrophils and BAL concentration of (B) CXCL1, (C) IL-6, and (D) granulocyte-colony stimulating factor (G-CSF) in db/db and WT mice treated intraperitoneally with IL-17A–neutralizing or isotype antibodies (4 μg/g) 24 hours before air or O3 exposure. Results are mean ± SE of five to eight mice per group. *P < 0.05 versus air-exposed mice with same genotype and treatment; #P < 0.05 versus WT mice with same exposure and treatment; %P < 0.05 versus isotype-treated mice with same genotype and exposure.
In air-exposed mice, airway responsiveness was significantly greater in db/db than in WT mice, consistent with previous reports (9), but there was no significant difference in airway responsiveness in db/db mice treated with isotype versus anti–IL-17A antibody (Figure 3). O3-induced increases in baseline pulmonary mechanics and in airway responsiveness were also greater in db/db than in WT mice (compare Figures 3A and 3B), consistent with previous reports (9). Shown in Figure 3 are changes in the coefficient of lung tissue damping, G. There were also effects of O3 on the coefficient of lung tissue elastance, H, in obese but not in lean mice (see Figure E2). G and H measure changes in the lung periphery, including airway closure, and dominate effects of O3 on airway responsiveness to aerosolized methacholine in mice (10). In contrast, there was no significant effect of obesity or O3 on Newtonian resistance, Rn, which reflects mainly changes in the central airways (see Figure E2). Compared with isotype antibody, anti–IL-17A had no significant effect on baseline pulmonary mechanics in either db/db or WT mice (PBS values in Figures 3A and 3B and Figure E2). However, anti–IL-17A reduced but did not abolish O3-induced AHR in db/db but not WT mice. Anti–IL-17A had no effect on airway responsiveness in either db/db or WT mice exposed to air (Figures 3A and 3B). Taken together, our data indicate that IL-17A contributes to part of the augmented response to O3 observed in obese mice.
Figure 3.
Anti–IL-17A reduces ozone-induced airway hyperresponsiveness in obese but not lean mice. To assess airway responsiveness (G), the coefficient of lung tissue damping, a measure of responses of the lung periphery, was measured by the forced oscillation technique in (A) WT mice or (B) db/db mice. Results are mean ± SE of five to eight mice per group. *P < 0.05 versus air-exposed mice with same genotype and treatment; #P < 0.05 versus WT mice with same exposure and treatment; %P < 0.05 versus isotype-treated mice with same genotype and exposure.
Because we have previously reported that IL-33 contributes to obesity-related increases in the response to O3 (9), we also examined the impact of anti–IL-17A on BAL IL-33 in O3-exposed db/db mice. Compared with isotype, anti–IL-17A did not significantly alter BAL IL-33 in db/db O3-exposed mice (85 ± 21 vs. 78 ± 15 pg/ml, respectively; nonsignificant result).
We have previously reported that another type of obese mouse, the Cpefat mouse, also exhibits greater O3-induced AHR than WT mice do (10). Cpefat mice are obese because they are genetically deficient in carboxypeptidase E (Cpe), an enzyme involved in processing neuropeptides involved in eating behavior. O3-induced AHR is even further augmented if the Cpefat mice also lack TNFR2 (Cpefat/TNFR2−/− mice) (10). Consequently, to determine whether the observed role for IL-17A in O3-induced AHR (Figure 3B) was unique to db/db mice or a general feature of obese mice, we also examined the impact of anti–IL-17A on O3-induced AHR in obese Cpefat/TNFR2−/− mice. Compared with isotype control mice, anti–IL-17 reduced O3-induced AHR in Cpefat/TNFR2−/− mice (Figure 4), similar to results obtained in db/db mice (Figure 3B).
Figure 4.
Effect of anti–IL-17A in obese Cpefat/TNFR2−/− mice exposed to ozone. Cpefat/TNFR2−/− mice were treated intraperitoneally with IL-17A–neutralizing or isotype antibodies 24 hours before ozone exposure. Airway responsiveness was evaluated using G, the coefficient of lung tissue damping, a measure of responses of the lung periphery. Results are mean ± SE of seven to nine mice per group. *P < 0.05 versus isotype-treated mice.
Effect of Anti–IL-17A on O3-induced Pulmonary Gene Expression in Obese Mice
To begin to understand the mechanistic basis for the impact of anti–IL-17A treatment on O3-induced AHR in db/db mice, we performed a microarray analysis on lung tissue from db/db mice. We first determined which genes were affected by O3 exposure. Compared with air, exposure of db/db mice to O3 resulted in significant changes in 869 Affymetrix IDs. Of these, 481 decreased with O3, and 388 increased. The list of increased genes contained several, including Il6, Cxcl5, Timp1, Mt2, and Tnc, that have also been reported to increase with acute O3 in lean mice, albeit with different exposure protocols (31, 32). To determine whether there were gene ontology categories that were enriched in these gene sets, we performed functional annotation clustering on those genes that were either significantly increased (108 genes) or decreased (190 genes) with O3 with a fold change of at least 2. (See Table E1 for a list of these genes.) For the genes that increased with O3, nine annotation clusters had significant enrichment scores (Table 1). These clusters included protease inhibitors, especially serpins; members of the epidermal growth factor family, including Areg, Ereg, and Hbegf; components of fibrinogen; genes involved in epithelial morphogenesis, including Igf1 and Pgf; genes related to ribosome biogenesis; and cell–cell adhesion molecules, including gap junction proteins Gjb4 and Gjb3. For genes that decreased with O3, 10 annotation clusters had significant enrichment scores (Table 2). With the sole exception of a category containing ribosome genes, all of these clusters were related to dynein and other components of cilia. Taken together, the results are consistent with substantial injury to and loss of the epithelium, especially the ciliated epithelium, and subsequent matrix remodeling and repair.
Table 1.
Functional Annotation Clusters of Genes Significantly Increased by Ozone with Fold Change of at Least 2
| Cluster | Enrichment Factor | Genes (n) | P Value |
|---|---|---|---|
| Extracellular region | 4.56 | 26 | 1.9E-7 |
| Areg, Ereg, Cpxm1, AI182371, Fgg, Fgl1, Hbegf, Igf1, Il24, Il6, Lcn2, Mmp19, Mmp3, Mia2, Pi15, Pgf, Plau, Sectm1b, Timp1, Serpine1, Serpina3n, Serpina3m, Serpina3k, Cxcl5, Tnc, Thbs1 | |||
| Peptidase inhibitor activity | 4.41 | 8 | 2.1E-5 |
| Timp1, Serpine1, Serpina3n, Serpina3m, Serpina3k, Serpina3f, pi15, AI182371 | |||
| Serpins | 3.51 | 5 | 4.9E-4 |
| Serpine1, Serpina3n, Serpina3m, Serpina3k, Serpina3f, | |||
| Fibrinogen, α/β/γ chain, C-terminal globular, subdomain 2 | 2.39 | 3 | 2.4E-4 |
| Tnc, Fgl1, Fgg | |||
| Response to peptide hormone stimulus | 2.35 | 5 | 1.5E-3 |
| Serpine1, Serpina3n, Serpina3m, Serpina3k, Serpina3f, Egr2 | |||
| EGF-like, type 3 | 2.25 | 6 | 2.9E-3 |
| Thbs1, Tnc, Plau, Hbegf, Ereg, Areg | |||
| Morphogenesis of an epithelium | 1.27 | 5 | 1.5E-2 |
| Tnc, Areg, Pgf, Igf1, Stil | |||
| Ribosome biogenesis | 1.21 | 3 | 2.1E-2 |
| Nhp2, Sdad1, Gm5633 | |||
| Cell–cell junction | 1.03 | 5 | 1.1E-2 |
| Tgm1, Gjb4, Gjb3, Cgn, 9030425E11Rik |
Definition of abbreviation: EGF = epidermal growth factor.
Functional annotation clustering performed using DAVID functional annotation clustering tool on high stringency.
Table 2.
Functional Annotation Clusters of Genes Significantly Decreased by Ozone with Fold Change of at Least 2
| Cluster | Enrichment Score | Genes (n) | P Value |
|---|---|---|---|
| Dynein complex | 8.63 | 10 | 1.2E-16 |
| Dnali1, Dnaic1, Dnahc9, Dnahc7a, Dnahc6, Dnahc5, Dnahc3, Dnahc11, Dnahc10, Dynlrb2 | |||
| Dynein heavy chain | 7.3 | 6 | 1.1E-9 |
| Dnahc9, Dnahc7a, Dnahc6, Dnahc5, Dnahc3, Dnahc11 | |||
| ATPases associated with various cellular activities (AAA-5) | 5.73 | 6 | 2.2E-7 |
| Dnahc9, Dnahc7a, Dnahc5, Dnahc3, Dnahc11 | |||
| Dynein heavy chain, N-terminal region 2 | 3.72 | 4 | 1.0E-5 |
| Dnahc9, Dnahc5, Dnahc3, Dnahc11 | |||
| Leucine-rich repeat | 2.99 | 8 | 8.9E-5 |
| Dnahc3, Fbxl13, 4930451C15Rik, Lrrc23, Lrrc36, Lrrc48, Spag17, Spef2 | |||
| WD40 | 1.77 | 5 | 4.1E-3 |
| Wdr78, Dnaic1, D19Ertd652e, Wdr65, Wdr63 | |||
| Ribosome | 1.63 | 4 | 1.2E-3 |
| Rpl31, Rps29, Rpl17, Rps2 | |||
| Ciliary or flagellar motility | 1.48 | 3 | 2.9E-4 |
| Dnahc11, Dnahc5, Tekt2 | |||
| Cilium | 1.4 | 4 | 2.4E-3 |
| Dnahc3, Sntn, Sapg17,Ttll6 | |||
| Leucine-rich repeat (LRR6) | 1.87 | 4 | 4.5E-2 |
| Dnahc3, Lrrc48, Fbxl13, 4930451C15Rik |
Functional annotation clustering performed using DAVID functional annotation clustering tool on high stringency.
To confirm these data, we used qRT-PCR on RNA extracted from lungs of isotype-treated WT and db/db mice exposed to air or O3 as described in Figure 3 to assay the relative expression of a subset of these genes. qRT-PCR confirmed the results of the microarray because compared with air, O3 increased Timp1 and Mt2 and decreased Dnahc6 and Scl4a5 mRNA abundances in db/db mice (Figure E3). In addition, O3-induced changes in each of these genes were greater in db/db than in WT mice.
To determine the effect of IL-17A on expression of these O3-responsive genes, we then examined the set of 869 O3-affected Affymetrix IDs in db/db mice that had been treated with isotype antibody versus anti–IL-17A. Among those genes significantly affected by O3 exposure, there were 108 genes that were also affected by anti–IL-17A treatment using a false discovery rate–adjusted P value less than 0.1 and a log2 fold change of 0.3 or greater as criteria (Table E2). We used the MsigDB Broad Institute software to determine which transcription factor binding sequences were enriched in that gene set. Among the transcription factors so identified were several that are known to induce IL-17A or to be activated by IL-17A signaling, including AP-1 (Table 3) (33).
Table 3.
Transcription Factor Analysis of Genes Affected by Ozone and by Anti–IL-17 in db/db Mice
| Transcription Factor | Sequence | FDR-adjusted P Value |
|---|---|---|
| MIF1 | NNGTTGCWWGGYAACNGS | 3.78E-02 |
| POUGF1 | GCATAAWTTAT | 3.78E-02 |
| SREBP | VNNVTCACCCYA | 3.78E-02 |
| TCF12* | RCCWGCTG | 3.78E-02 |
| USF* | NNRNCACGTGNYNN | 3.78E-02 |
| AP1† | TGANTCA | 3.78E-02 |
Definition of abbreviation: FDR = false discovery rate.
Activated by IL-17A.
Important for IL-17A expression.
Although there were effects of anti–IL-17A on other O3-responsive genes (Table E2), we were particularly interested in Grpr because our primary focus was O3-induced AHR, and GRP, the pulmonary NEC peptide for which GRPR serves as a receptor, causes contraction of small airways (34). Obesity and O3 both induce oxidative stress, and other conditions that induce oxidative stress, especially hyperoxia, have been associated with GRP release (35). In addition, AHR is observed in children with pulmonary NEC hyperplasia (36). Furthermore, others have shown interactions between GRP and IL-17A in the induction of experimental arthritis in mice (37). Consequently, we performed qRT-PCR to examine the pulmonary mRNA abundance of Grpr in the mice described in Figures 2 and 3. Consistent with the microarray analysis, the mRNA abundance of Grpr was significantly greater in lungs of O3- versus air-exposed db/db mice and significantly decreased in anti–IL-17A– versus isotype-treated db/db mice exposed to O3 (Figure 5A). Moreover, O3-induced increases in Grpr expression were greater in db/db mice than in WT mice, consistent with the greater IL-17A in the obese mice (Figures 1A, 1B, and 1G). Because our data indicated a significant impact of anti–IL-17A on AHR in Cpefat/TNFR2−/− mice exposed to O3 (Figure 4), we also examined pulmonary Grpr expression in those mice. Consistent with the impact of anti–IL-17A on Grpr expression in db/db mice exposed to O3 (Figure 5A), we also observed a reduction in Grpr mRNA abundance in lungs of anti–IL-17A– versus isotype-treated Cpefat/TNFR2−/− mice exposed to O3 (Figure 5B), confirming a role for IL-17A in the expression of this gene after O3 exposure in obese mice. We also observed a significant increase in the pulmonary mRNA expression of Grpr in db/db versus WT air-exposed mice (Figure 5A). However, in air-exposed mice, Grpr expression was not impacted by anti–IL-17A treatment (Figure 5A). qRT-PCR also confirmed that another gene on the list of O3- and anti–IL-17A–affected genes (Table E2), Nqo1, was also significantly increased by O3 in db/db versus WT mice and significantly reduced in anti–IL-17A– versus isotype-treated db/db mice (Figure E4), further validating the results of the microarray.
Figure 5.
Anti–IL-17A attenuates O3-induced increases in Grpr in obese mice. (A) Pulmonary mRNA abundance of Grpr in db/db and WT mice treated with IL-17A–neutralizing or isotype antibodies 24 hours before air or O3 exposure. Results are mean ± SE of five to nine mice per group. (B) Pulmonary mRNA abundance of Grpr in obese Cpefat/TNFR2−/− mice treated with IL-17A–neutralizing or isotype antibodies 24 hours before O3 exposure. Results are mean ± SE of seven to nine mice per group. *P < 0.05 versus air-exposed mice with same genotype; #P < 0.05 versus WT mice with same exposure and antibody treatment; %P < 0.05 versus isotype-treated mice with the same genotype and exposure. Grpr = gastrin-releasing peptide receptor.
Effect of Anti-GRP on Obesity-related Increases in Response to O3
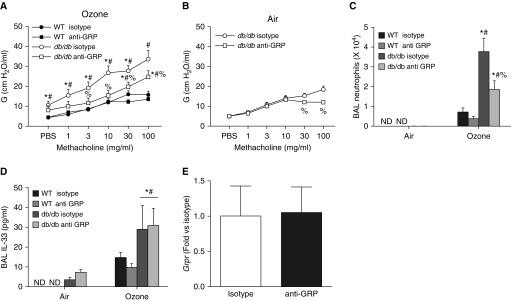
To assess a possible role for GRPR, we treated obese db/db mice with anti-GRP neutralizing antibodies or isotype antibodies. Compared with isotype treatment, anti-GRP treatment caused a significant decrease in AHR (Figure 6A) in obese O3-exposed mice, but it did not completely abolish the differences between lean and obese mice, similar to the effects of anti–IL-17A treatment (Figure 3B). There was also a small reduction in airway responsiveness in obese mice exposed to air (Figure 6B). BAL neutrophils were also reduced in anti-GRP– versus isotype-treated obese mice exposed to O3 (Figure 6C), whereas other BAL cells were unchanged (data not shown). Given the importance of IL-33 for O3-induced AHR in obese mice (9), we measured BAL IL-33 by ELISA in these mice. O3 caused a greater increase in BAL IL-33 in obese than in lean mice, as previously reported (9), but there was no effect of anti-GRP on BAL IL-33 in either obese or lean mice (Figure 6D), nor was Grpr expression itself affected by anti-GRP treatment (Figure 6E).
Figure 6.
Anti–gastrin-releasing peptide (anti-GRP) reduces responses to O3 in obese mice. (A) Airway responsiveness using G, the coefficient of lung tissue damping, in db/db and WT mice treated intraperitoneally with anti-GRP or isotype antibodies 1 hour before O3 exposure. (B) In db/db mice, airway responsiveness was also assessed after room air exposure. We also assessed (C) BAL neutrophils and (D) BAL IL-33 in the same mice. (E) Pulmonary Grpr mRNA abundance in db/db mice treated with anti-GRP or isotype antibody before O3 exposure. ND = experiments with WT mice exposed to air were not done; consequently, statistical analysis of the effect of O3 in the WT mice was not assessed. Results are mean ± SE of seven or eight mice per group. *P < 0.05 versus air-exposed mice with same genotype and treatment; #P < 0.05 versus WT mice with same exposure and treatment; %P < 0.05 versus isotype-treated mice with same genotype and exposure.
We also assessed BAL GRP in these mice (Figure 7A). Compared with air, exposure to O3 caused a significant increase in BAL GRP in db/db mice, and BAL GRP was significantly greater in O3-exposed db/db mice than in WT mice. O3-induced increases in BAL GRP were also greater in mice with diet-induced obesity than in chow-fed control mice (Figure 7B). To determine whether IL-17A could affect GRP release, we also measured BAL GRP in mice in the cohort of mice described above that were treated with anti–IL-17A versus isotype antibody (Figure 7C). O3-induced increases in BAL GRP were greater in db/db than in WT mice, consistent with the data in Figure 7A. However, compared with isotype antibody, anti–IL-17A had no effect on BAL GRP in either db/db or WT mice.
Figure 7.
Exposure to O3 causes greater increases in BAL GRP in obese than in lean mice. (A) BAL GRP in WT and db/db mice exposed to air or O3. N.D. = experiments with WT air-exposed mice were not done. (B) BAL GRP in HFD-fed versus chow-fed mice exposed to air or O3. (C) BAL GRP in db/db and WT mice exposed to air or O3 that had been pretreated with isotype versus anti–IL-17A antibodies. Results are mean ± SE of 4–14 mice per group. *P < 0.05 versus air-exposed mice with same genotype; #P < 0.05 versus WT mice with same exposure.
Reductions in the expression of inflammatory cytokines, including IL-17A, are observed after GRPR inhibition in experimental arthritis (37). However, there was no difference in BAL IL-17A in isotype- versus anti–GRP-treated db/db mice exposed to O3 (Figure E5). We also did not observe any effect of anti-GRP treatment on BAL concentrations of other cytokines and chemokines known to be elevated in obese versus lean mice exposed to O3 (Figure E5). The lack of impact on inflammatory cytokines of blocking GRP in the lungs of these obese O3-exposed mice versus the efficacy of blocking GRP signaling in the joints of mice with experimental arthritis (37) likely reflects differences in the nature of the inflammation induced in the two types of models.
Discussion
Our data indicate that IL-17A contributes to augmented responses to O3 in obese mice. BAL IL-17A and lung IL-17A+ cells were greater in O3-exposed obese mice than lean mice (Figures 1A, 1B, and 1G), and anti–IL-17A treatment attenuated obesity-related increases in the response to O3 (Figures 2–4). A multiplex assay identified CXCL1 as a chemokine downstream of IL-17A that may have contributed to the impact of IL-17A on BAL neutrophils (Figure 2B), whereas microarray data coupled with measurements of BAL GRP and studies with anti-GRP identified Grpr as a gene downstream of IL-17A that was involved in the impact of IL-17A on O3-induced AHR (Figures 5–7).
Anti–IL-17A reduced O3-induced AHR in obese mice (Figures 3B and 4). Consistent with these observations, others have reported that IL-17A can induce AHR, but usually only under circumstances in which IL-13 or IL-33 is also elevated (11, 38). We have shown that both IL-13 and IL-33 are induced to a greater extent in obese than in lean mice after acute O3 exposure (9, 10). Given that experiments using anti-ST2 and anti–IL-13 also indicated roles for IL-13 and IL-33 in response to O3 in obese mice (9, 10), the data suggest that the role of IL-17A in the augmented O3-induced AHR of obese mice (Figures 3B and 4) may be to amplify the effects of IL-33 and/or IL-13. However, the nature of this amplification is unlikely to include effects of IL-17A on IL-33 release, because BAL IL-33 was unchanged in isotype control– versus anti–IL-17–treated obese mice, nor did blocking the effects of IL-33 with anti-ST2 antibodies (9) affect BAL IL-17A (data not shown). Instead, interactions between IL-17A and IL-33 likely occur downstream from the release of these cytokines.
Whereas anti–IL-17A treatment reduced O3-induced AHR in obese mice (Figures 3B and 4), it had no effect in lean mice (Figure 3A), even though it did impact BAL neutrophils in lean mice (Figure 2A). The reason for the obesity-related difference in the impact of anti–IL-17 on O3-induced AHR is not clear, but it may involve type 2 cytokines. We have shown that IL-13 is induced by this acute O3 exposure in obese but not in lean mice (9, 10). As discussed above, the ability of IL-17A to promote O3-induced AHR in the obese mice may be related to interactions between IL-17A and IL-13 or IL-33 (which induces type 2 cytokines). In this context, it is interesting to note that IL-17A does contribute to O3-induced AHR in lean mice when the mice are exposed using a different O3 exposure paradigm involving several acute exposures, each separated by 2 days (13). With this latter type of O3 exposure, the lean mice also express type 2 cytokines in their lungs (13) (likely as a result of expression by recruited invariant natural killer T cells), and IL-4 and/or IL-13 is required for O3-induced AHR.
Though we cannot rule out the possibility that other IL-17A–dependent genes (Table E2) are also be involved, our data indicate that GRP/GRPR is likely part of the pathway by which IL-17A contributes to AHR in obese O3-exposed mice. Microarray analysis identified, and qRT-PCR confirmed, Grpr as a gene that was induced by O3 in an IL-17A–dependent fashion in obese mice (Table E2, Figure 5). Furthermore, exposure to O3 increased BAL GRP to a greater extent in obese than in lean mice (Figure 7), and anti-GRP attenuated obesity-related increases in O3-induced AHR (Figure 6A), just as anti–IL-17A did (Figures 3B and 4). However, because anti–IL-17A did not affect BAL GRP (Figure 7C), our data are consistent with the hypothesis that interactions between GRP and IL-17A involved IL-17A–mediated changes in Grpr expression rather than GRP release. To our knowledge, this is the first report of O3-induced GRP release in the lungs, though others have reported elevated levels in response to another pulmonary irritant, cigarette smoke (39). NECs are the primary source of GRP in the lungs (21), and it is possible that greater O3-induced release of GRP in obese than in lean mice (Figure 7) is the result of greater numbers of NECs in the lungs of the obese mice, because NEC hyperplasia occurs in a variety of lung diseases (40). To our knowledge, this is also the first report that IL-17A can affect Grpr expression. Because neutrophils express GRPR (41), and because anti–IL-17A reduced BAL neutrophils (Figure 2A), we considered the possibility that decrements in pulmonary Grpr expression induced by IL-17A were the result of fewer Grpr-expressing neutrophils in the lungs after anti–IL-17A treatment. However, treatment with anti-GRP had no effect on pulmonary Grpr expression (Figure 6E), even though it also caused a substantial reduction in BAL neutrophils (Figure 6C). The results suggest that direct effects of IL-17A on Grpr expression were instead involved, though we do not know which Grpr-expressing cells were the targets of IL-17A. In addition to neutrophils (41), many other cells in the lung express Grpr. For example, stimulation of GRPRs enhances phagocytosis and activation of alveolar macrophages (42). GRP causes contraction of small airways (34), suggesting that airway smooth muscle could be the target. Pulmonary fibroblasts undergo proliferation in response to GRP, as do endothelial cells and lung epithelial cells (40), and these cells also express IL-17A receptors (43). Further experiments will be required to determine which of these cells are involved in the IL-17A/GRPR-mediated responses observed in this study.
Though we cannot rule out the possibility that there were other unmeasured factors that also contributed, our data suggest that, of the neutrophil chemotactic factors measured, changes in CXCL1 may have contributed to the reduction in BAL neutrophils observed in anti–IL-17A–treated obese mice (Figure 2A). BAL CXCL1 was also reduced in anti–IL-17A– versus isotype-treated db/db mice exposed to O3 (Figure 2B), whereas other neutrophil chemotactic and survival factors were not (Figures 2C and 2D). IL-17A is known to stimulate CXCL1 production from macrophages (44), and others have also reported a role for IL-8 family chemokines in the IL-17A–dependent neutrophil recruitment induced by O3 in lean mice (45). IL-17A–dependent changes in Grpr expression (Figure 5A) could have also contributed to obesity-related changes in neutrophil recruitment, because anti-GRP also attenuated O3-induced increases in BAL neutrophils in obese mice (Figure 6C). We did not observe any changes in the BAL concentrations of CXCL1 or other neutrophil chemoattractants, including CXCL2, in anti-GRP– versus isotype-treated mice (Figure E5), suggesting that the effects of anti–IL-17A treatment on CXCL1 (Figure 2B) do not involve IL-17A–dependent changes in Grpr expression. Instead, the impact of anti-GRP on neutrophil recruitment (Figure 6C) may have resulted from blocking GRPR signaling within neutrophils, because GRP has direct chemotactic effects on neutrophils (41).
Whereas obesity increased pulmonary IL-17A+ cells even in the absence of O3 exposure (Figure 1B), obesity did not result in increases in BAL neutrophils unless mice were also exposed to O3 (Figures 2A and 6C). It is possible that the changes in IL-17A observed with obesity alone were of insufficient magnitude to evoke neutrophil recruitment. However, it is also possible that IL-17A is not released from IL-17A+ cells except under conditions of O3 exposure. Indeed, BAL IL-17A was significantly increased by O3 but not by obesity alone (Figures 1A and 1G).
Obesity not only augmented O3-induced AHR but also increased airway responsiveness, even in air-exposed mice. Anti–IL-17A did not attenuate this innate AHR (Figure 3), even though Kim and colleagues (15), using mice genetically deficient in IL-17A, did observe a role for IL-17A in the innate AHR of obesity caused by high-fat feeding. It is not clear exactly what accounts for this difference. It may be that IL-17A contributes to innate AHR in mice with dietary but not genetic obesity. However, it is also possible that the explanation lies in the sex of the mice: The db/db mice in the present study were female, and the mice in the study of Kim and colleagues were male.
We cannot rule out the possibility that the observed effects of anti–IL-17A (Figures 2–4) were the result of blocking systemic rather than pulmonary IL-17A or that systemic responses to IL-17A were involved. However, at least in db/db mice, the IL-17A that impacts responses to O3 likely has its origin in the lung rather than in the blood, because O3 increased BAL (Figure 1A) but not serum (Figure 1F) concentrations of IL-17A in db/db mice. However, there were increases in serum (Figure 1I) as well as BAL IL-17A (Figure 1G) in mice with diet-induced obesity after O3 exposure.
Some technical issues require consideration. First, the anti–IL-17A antibody used has some cross-reactivity with IL-17F. Hence, whereas we focused on IL-17A because of data in the literature suggesting it could play a role in obesity-related differences in the response to O3, we cannot rule out the possibility that IL-17F also contributed. Second, we used an acute O3 exposure (2 ppm for 3 h). We used this O3 exposure paradigm because our primary interest was the impact of O3 on AHR and because this exposure regimen is known to cause AHR in obese mice (9, 10). This concentration is higher than ambient concentrations of O3. However, as previously described (46), because of differences in dosimetry between mice and humans, the dose of O3 delivered by this exposure regimen is roughly equivalent to the dose employed in human chamber studies in which O3-induced AHR was assessed (47). Third, the mice in this study were female. We used female mice because increases in asthma prevalence with body mass index are stronger in women than in men (48). The gene that encodes GRPR is located on the X chromosome and escapes X inactivation, resulting in two transcribed alleles in women compared with only one in men (49). Moreover, lung epithelial GRPR is more readily induced by inhaled irritants such as cigarette smoke in women than in men (50). Thus, whether the GRP–GRPR axis also contributes to obesity-related increases in O3-induced AHR in males remains to be established.
One additional limitation of the study is that we did not evaluate the cellular source of the IL-17A that contributes to obesity-induced augmentation of O3-induced AHR. Many cells within the lungs, including CD4+ T cells, γδ T cells, type 3 innate lymphoid cells, and invariant natural killer T cells, have the capacity to produce IL-17A (13, 15, 17, 19, 26, 27). Better understanding of the cellular source of IL-17A could lead to additional insights into the mechanistic basis for the effects of obesity within the lungs.
In summary, our data indicate that IL-17A contributes to the augmented responses to O3 observed in db/db mice and suggest that IL-17A may mediate its effects on O3-induced AHR via changes in Grpr. The data suggest that IL-17A may contribute to the greater decrements in pulmonary function observed in obese than in lean human subjects after O3 exposure (5, 6), though experiments in human subjects will be required to confirm that hypothesis. IL-17A–neutralizing antibodies were found to have little effect in an asthma clinical trial in which subjects were recruited without regard to body mass index or allergic status (51). However, given that IL-17A is elevated in sputum of obese versus lean individuals with asthma (18), our data suggest that IL-17A might be a therapeutic target in a more restricted cluster of individuals with asthma, those with obesity-related asthma, especially for asthma triggers such as O3 that involve oxidative stress.
Footnotes
Supported by National Institutes of Health grants ES013307 (S.A.S.), F32ES02256 (J.A.M.), HL007118 (S.A.S.), ES024032 (B.D.L.), HL122531 (B.D.L.), and Harvard T. H. Chan School of Public Health ES000002.
Author Contributions: J.A.M., N.K., D.I.K., J.H., Y.C., J.D.B., A.S.W., A.P.W., L.R., F.C., M.E.S., B.D.L., and S.A.S.: conceived of and designed the experiments; J.A.M., N.K., D.I.K., J.H., Y.C., J.D.B., A.P.W., and L.R.: performed the experiments; J.A.M.: wrote the paper; and J.A.M., N.K., D.I.K., J.H., Y.C., J.D.B., A.S.W., A.P.W., L.R., F.C., M.E.S., B.D.L., and S.A.S.: reviewed, revised, and approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0071OC on September 28, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gleason JA, Fagliano JA. Associations of daily pediatric asthma emergency department visits with air pollution in Newark, NJ: utilizing time-series and case-crossover study designs. J Asthma. 2015;52:815–822. doi: 10.3109/02770903.2015.1033726. [DOI] [PubMed] [Google Scholar]
- 2.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- 3.Devlin RB, McDonnell WF, Mann R, Becker S, House DE, Schreinemachers D, et al. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol. 1991;4:72–81. doi: 10.1165/ajrcmb/4.1.72. [DOI] [PubMed] [Google Scholar]
- 4.Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18-20 h postexposure to ozone. J Appl Physiol (1985) 2000;89:1804–1810. doi: 10.1152/jappl.2000.89.5.1804. [DOI] [PubMed] [Google Scholar]
- 5.Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure and lung function: effect modified by obesity and airways hyperresponsiveness in the VA normative aging study. Chest. 2007;132:1890–1897. doi: 10.1378/chest.07-1126. [DOI] [PubMed] [Google Scholar]
- 6.Bennett WD, Hazucha MJ, Folinsbee LJ, Bromberg PA, Kissling GE, London SJ. Acute pulmonary function response to ozone in young adults as a function of body mass index. Inhal Toxicol. 2007;19:1147–1154. doi: 10.1080/08958370701665475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong GH, Qian Z, Liu MM, Wang D, Ren WH, Fu Q, et al. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the Seven Northeastern Cities study. Int J Obes. 2013;37:94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]
- 8.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909, quiz 910. doi: 10.1016/j.jaci.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 9.Mathews JA, Krishnamoorthy N, Kasahara DI, Cho Y, Wurmbrand AP, Ribeiro L, et al. IL-33 drives augmented responses to ozone in obese mice. Environ Health Perspect. 2017;125:246–253. doi: 10.1289/EHP272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams AS, Mathews JA, Kasahara DI, Chen L, Wurmbrand AP, Si H, et al. Augmented pulmonary responses to acute ozone exposure in obese mice: roles of TNFR2 and IL-13. Environ Health Perspect. 2013;121:551–557. doi: 10.1289/ehp.1205880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizutani N, Nabe T, Yoshino S. IL-17A promotes the exacerbation of IL-33-induced airway hyperresponsiveness by enhancing neutrophilic inflammation via CXCR2 signaling in mice. J Immunol. 2014;192:1372–1384. doi: 10.4049/jimmunol.1301538. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara DI, Mathews JA, Park CY, Cho Y, Hunt G, Wurmbrand AP, et al. ROCK insufficiency attenuates ozone-induced airway hyperresponsiveness in mice. Am J Physiol Lung Cell Mol Physiol. 2015;309:L736–L746. doi: 10.1152/ajplung.00372.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams AS, Chen L, Kasahara DI, Si H, Wurmbrand AP, Shore SA. Obesity and airway responsiveness: role of TNFR2. Pulm Pharmacol Ther. 2013;26:444–454. doi: 10.1016/j.pupt.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathews JA, Wurmbrand AP, Ribeiro L, Neto FL, Shore SA. Induction of IL-17a precedes development of airway hyperresponsiveness during diet-induced obesity and correlates with complement factor D. Front Immunol. 2014;5:440. doi: 10.3389/fimmu.2014.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marijsse GS, Seys SF, Schelpe AS, Dilissen E, Goeminne P, Dupont LJ, et al. Obese individuals with asthma preferentially have a high IL-5/IL-17A/IL-25 sputum inflammatory pattern. Am J Respir Crit Care Med. 2014;189:1284–1285. doi: 10.1164/rccm.201311-2011LE. [DOI] [PubMed] [Google Scholar]
- 19.Mathews JA, Williams AS, Brand JD, Wurmbrand AP, Chen L, Ninin FM, et al. γδ T cells are required for pulmonary IL-17A expression after ozone exposure in mice: role of TNFα. PLoS One. 2014;9:e97707. doi: 10.1371/journal.pone.0097707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Che L, Jin Y, Zhang C, Lai T, Zhou H, Xia L, et al. Ozone-induced IL-17A and neutrophilic airway inflammation is orchestrated by the caspase-1-IL-1 cascade. Sci Rep. 2016;6:18680. doi: 10.1038/srep18680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson DE, Georgieff MK. Pulmonary neuroendocrine cells: their secretory products and their potential roles in health and chronic lung disease in infancy. Am Rev Respir Dis. 1989;140:1807–1812. doi: 10.1164/ajrccm/140.6.1807. [DOI] [PubMed] [Google Scholar]
- 22.Shen N, Wang J, Zhao M, Pei F, He B. Anti-interleukin-17 antibodies attenuate airway inflammation in tobacco-smoke-exposed mice. Inhal Toxicol. 2011;23:212–218. doi: 10.3109/08958378.2011.559603. [DOI] [PubMed] [Google Scholar]
- 23.Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, et al. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 24.Avis IL, Kovacs TO, Kasprzyk PG, Treston AM, Bartholomew R, Walsh JH, et al. Preclinical evaluation of an anti-autocrine growth factor monoclonal antibody for treatment of patients with small-cell lung cancer. J Natl Cancer Inst. 1991;83:1470–1476. doi: 10.1093/jnci/83.20.1470. [DOI] [PubMed] [Google Scholar]
- 25.Siegfried JM, Krishnamachary N, Gaither Davis A, Gubish C, Hunt JD, Shriver SP. Evidence for autocrine actions of neuromedin B and gastrin-releasing peptide in non-small cell lung cancer. Pulm Pharmacol Ther. 1999;12:291–302. doi: 10.1006/pupt.1999.0210. [DOI] [PubMed] [Google Scholar]
- 26.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton CE, Mielke LA, Mills KH. IL-17-producing γδ T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 28.Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 29.Kasahara DI, Kim HY, Mathews JA, Verbout NG, Williams AS, Wurmbrand AP, et al. Pivotal role of IL-6 in the hyperinflammatory responses to subacute ozone in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2014;306:L508–L520. doi: 10.1152/ajplung.00235.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasahara DI, Kim HY, Williams AS, Verbout NG, Tran J, Si H, et al. Pulmonary inflammation induced by subacute ozone is augmented in adiponectin-deficient mice: role of IL-17A. J Immunol. 2012;188:4558–4567. doi: 10.4049/jimmunol.1102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasu VT, Oommen S, Lim Y, Valacchi G, Hobson B, Eirserich JP, et al. Modulation of ozone-sensitive genes in α-tocopherol transfer protein null mice. Inhal Toxicol. 2010;22:1–16. doi: 10.3109/08958370902838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams AS, Issa R, Leung SY, Nath P, Ferguson GD, Bennett BL, et al. Attenuation of ozone-induced airway inflammation and hyper-responsiveness by c-Jun NH2 terminal kinase inhibitor SP600125. J Pharmacol Exp Ther. 2007;322:351–359. doi: 10.1124/jpet.107.121624. [DOI] [PubMed] [Google Scholar]
- 33.Benderdour M, Tardif G, Pelletier JP, Di Battista JA, Reboul P, Ranger P, et al. Interleukin 17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: differential activation of AP-1 members by IL-17 and IL-1β. J Rheumatol. 2002;29:1262–1272. [PubMed] [Google Scholar]
- 34.Lach E, Haddad EB, Gies JP. Contractile effect of bombesin on guinea pig lung in vitro: involvement of gastrin-releasing peptide-preferring receptors. Am J Physiol. 1993;264:L80–L86. doi: 10.1152/ajplung.1993.264.1.L80. [DOI] [PubMed] [Google Scholar]
- 35.Chang LY, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, et al. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2003;167:57–64. doi: 10.1164/rccm.200203-232OC. [DOI] [PubMed] [Google Scholar]
- 36.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40:157–165. doi: 10.1002/ppul.20243. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira PG, Grespan R, Pinto LG, Meurer L, Brenol JC, Roesler R, et al. Protective effect of RC-3095, an antagonist of the gastrin-releasing peptide receptor, in experimental arthritis. Arthritis Rheum. 2011;63:2956–2965. doi: 10.1002/art.30486. [DOI] [PubMed] [Google Scholar]
- 38.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguayo SM, Kane MA, King TE, Jr, Schwarz MI, Grauer L, Miller YE. Increased levels of bombesin-like peptides in the lower respiratory tract of asymptomatic cigarette smokers. J Clin Invest. 1989;84:1105–1113. doi: 10.1172/JCI114273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degan S, Lopez GY, Kevill K, Sunday ME. Gastrin-releasing peptide, immune responses, and lung disease. Ann NY Acad Sci. 2008;1144:136–147. doi: 10.1196/annals.1418.022. [DOI] [PubMed] [Google Scholar]
- 41.Czepielewski RS, Porto BN, Rizzo LB, Roesler R, Abujamra AL, Pinto LG, et al. Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils. Proc Natl Acad Sci USA. 2012;109:547–552. doi: 10.1073/pnas.1110996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meloni F, Ballabio P, Bianchi L, Mangiarotti P, Grassi G, Bignamini A, et al. Bombesin enhances monocyte and macrophage activities: possible role in the modulation of local pulmonary defenses in chronic bronchitis. Respiration. 1996;63:28–34. doi: 10.1159/000196512. [DOI] [PubMed] [Google Scholar]
- 43.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barin JG, Baldeviano GC, Talor MV, Wu L, Ong S, Quader F, et al. Macrophages participate in IL-17-mediated inflammation. Eur J Immunol. 2012;42:726–736. doi: 10.1002/eji.201141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M, Fei X, Zhang GQ, Zhang PY, Li F, Bao WP, et al. Role of neutralizing anti-murine interleukin-17a monoclonal antibody on chronic ozone-induced airway inflammation in mice. Biomed Pharmacother. 2016;83:247–256. doi: 10.1016/j.biopha.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 46.Shore SA, Williams ES, Chen L, Benedito LA, Kasahara DI, Zhu M. Impact of aging on pulmonary responses to acute ozone exposure in mice: role of TNFR1. Inhal Toxicol. 2011;23:878–888. doi: 10.3109/08958378.2011.622316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, et al. Ozone dose and effect in humans and rats: a comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. 1994;150:676–683. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- 48.Akinbami LJ, Fryar CD. Current asthma prevalence by weight status among adults: United States, 2001–2014. NCHS Data Brief. 2016;(239) [PubMed] [Google Scholar]
- 49.Ishikawa-Brush Y, Powell JF, Bolton P, Miller AP, Francis F, Willard HF, et al. Autism and multiple exostoses associated with an X;8 translocation occurring within the GRPR gene and 3′ to the SDC2 gene. Hum Mol Genet. 1997;6:1241–1250. doi: 10.1093/hmg/6.8.1241. [DOI] [PubMed] [Google Scholar]
- 50.Shriver SP, Bourdeau HA, Gubish CT, Tirpak DL, Davis AL, Luketich JD, et al. Sex-specific expression of gastrin-releasing peptide receptor: relationship to smoking history and risk of lung cancer. J Natl Cancer Inst. 2000;92:24–33. doi: 10.1093/jnci/92.1.24. [DOI] [PubMed] [Google Scholar]
- 51.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]