Abstract
Rationale: Short- and long-term fine particulate matter (particulate matter ≤2.5 μm in aerodynamic diameter [PM2.5]) pollution is associated with asthma development and morbidity, but there are few data on the effects of long-term exposure to coarse PM (PM10–2.5) on respiratory health.
Objectives: To understand the relationship between long-term fine and coarse PM exposure and asthma prevalence and morbidity among children.
Methods: A semiparametric regression model that incorporated PM2.5 and PM10 monitor data and geographic characteristics was developed to predict 2-year average PM2.5 and PM10–2.5 exposure during the period 2009 to 2010 at the zip-code tabulation area level. Data from 7,810,025 children aged 5 to 20 years enrolled in Medicaid from 2009 to 2010 were used in a log-linear regression model with predicted PM levels to estimate the association between PM exposure and asthma prevalence and morbidity, adjusting for race/ethnicity, sex, age, area-level urbanicity, poverty, education, and unmeasured spatial confounding.
Measurements and Main Results: Exposure to coarse PM was associated with increased asthma diagnosis prevalence (rate ratio [RR] for 1-μg/m3 increase in coarse PM level, 1.006; 95% confidence interval [CI], 1.001–1.011), hospitalizations (RR, 1.023; 95% CI, 1.003–1.042), and emergency department visits (RR, 1.017; 95% CI, 1.001–1.033) when adjusting for fine PM. Fine PM exposure was more strongly associated with increased asthma prevalence and morbidity than coarse PM. The estimates remained elevated across different levels of spatial confounding adjustment.
Conclusions: Among children enrolled in Medicaid, exposure to higher average coarse PM levels is associated with increased asthma prevalence and morbidity. These results suggest the need for direct monitoring of coarse PM and reconsideration of limits on long-term average coarse PM pollution levels.
Keywords: particulate matter, asthma, air pollution
At a Glance Commentary
Scientific Knowledge on the Subject
Long- and short-term exposure to fine particulate matter (particulate matter ≤2.5 μm in aerodynamic diameter [PM2.5]) is associated with asthma morbidity, but little is known about the long-term effects of coarse PM (PM10–2.5) on asthma prevalence or morbidity.
What This Study Adds to the Field
This study found that coarse PM exposure was associated with higher asthma prevalence and morbidity among U.S. children enrolled in Medicaid and that this association was independent of fine PM exposure. This finding suggests that long-term limits on coarse PM exposure be reconsidered.
Asthma affects more than 7 million U.S. children and is responsible for more than 3,000 deaths, 400,000 hospitalizations, and 1.8 million emergency department (ED) visits per year in the United States (1). Particulate matter (PM) air pollution has been shown repeatedly to have significant short- and long-term effects on both the development of asthma and asthma morbidity (2). To date, most of the research has focused on fine particles (particulate matter ≤2.5 μm in aerodynamic diameter [PM2.5]), for which epidemiologic studies have now provided enough evidence for the Environmental Protection Agency (EPA) to determine that both long- and short-term exposure are likely to be causally related to negative respiratory health outcomes (3).
In contrast, the coarse fraction of PM (PM10–2.5) is generally believed to be less harmful than fine PM, both because of the particle size, which limits penetration deep into the lungs, and because the sources of coarse PM are believed to be less harmful (4). However, coarse PM can deposit in the upper airways involved in obstructive lung diseases such as asthma and chronic obstructive pulmonary disease, and there is emerging evidence that short-term coarse PM exposure may be associated with cardiovascular and respiratory morbidity (5–7). Little is known about long-term effects of coarse PM on respiratory health (2).
One of the reasons for the relative lack of data about the health effects of coarse PM is the paucity of monitor locations that measure PM10 and PM2.5 simultaneously. Concentrations of coarse PM are not directly measured but instead are calculated by subtracting the concentrations of directly measured PM2.5 from PM10 at collocated monitors. Because fewer than half of the monitoring locations measure both PM2.5 and PM10, studies that rely on observed coarse PM data from collocated monitors are limited in geographic scope.
Here, we estimate long-term average fine and coarse PM concentrations using an exposure prediction model based on monitor observations and geographic data. We apply these predictions to healthcare use data from children enrolled in Medicaid across the United States during 2009 to 2010 to assess the relationship between long-term exposure to PM and asthma morbidity and prevalence.
Methods
Participants
Subjects were children aged 5 to 20 years old enrolled in Medicaid in the United States between 2009 and 2010. As previously described (8), the data were obtained from the Research Data Assistance Center (University of Minnesota, Minneapolis, MN). Medicaid data were collected and aggregated on the state level and then processed by the Centers for Medicare and Medicaid into the Medicaid Analytic Extract (MAX). Use of the data was approved by the Johns Hopkins School of Medicine Institutional Review Board.
Children were only included if they were enrolled for the full 24-month period. Six states were excluded from the analysis because of concerns about utilization data quality: Maine, which had incomplete utilization data, and Pennsylvania, Ohio, Idaho, Arkansas, and Kansas, which all had rates of asthma care use that were either abnormally low and inconsistent with other sources of data (9–12) (Pennsylvania, Arkansas, and Ohio) or had large inconsistencies in asthma care use between 2009 and 2010 (Idaho and Kansas). Further examination showed that most of the abnormally low rate of asthma care use reported in Pennsylvania was due to very low rates of asthma care use in the Pittsburgh and Philadelphia areas (0.6% and 0.3% prevalence from utilization data, respectively), which is not consistent with external data on asthma prevalence in this area (15% and 18% self-reported prevalence, from one source [13]). Alaska and Hawaii were excluded because of the difficulty in predicting PM in the noncontiguous states. Because prior investigations, including our own investigation of this Medicaid data, have shown that race/ethnicity is strongly associated with asthma prevalence and morbidity (8, 14), eight states were excluded because more than 10% of subjects had missing data for race/ethnicity. These states were: Colorado, Iowa, Massachusetts, New Jersey, Rhode Island, Vermont, Washington, and Wisconsin.
PM Data
Twenty-four-hour average measurements of PM2.5 and PM10 for the period January 1, 2009, through December 31, 2010, were obtained from the EPA Air Quality System (AQS) database (15). We restricted to monitors using Federal Reference Methods. For both PM2.5 and PM10, the annual average concentration was computed for locations with at least 28 observations and gaps of no more than 30 days between measurements. A long-term concentration at each PM2.5 and PM10 site for the period 2009 to 2010 was created by averaging together the 2009 and 2010 annual averages, using the value for a single year when one year was missing.
Exposure Prediction
We developed a semiparametric regression model to predict long-term average PM concentrations across the entire contiguous United States. We built separate models for PM2.5 and PM10 and used the difference of predictions to compute PM10–2.5. By building separate models for each fraction, we can incorporate monitoring locations that only measure one type of PM. The mean component of the prediction models comprised penalized spatial splines and principal component analysis (PCA) scores derived from geographic variables. Generalized additive models of this form, and related approaches such as land-use regression and universal kriging, have been used throughout the literature to predict long-term average air pollution concentrations for epidemiological analyses (16–20).
Four types of publicly available geographic data were incorporated in the models. Population density at the county and zip-code tabulation area (ZCTA) level was obtained from Census 2010 data (21). Primary and secondary road network data were also obtained from the 2010 Census (22). Satellite measurements of impervious surface, which can indicate anthropogenic development, were obtained from the National Land Cover Database (23). Data on point source emissions were obtained from the 2008 National Emission Inventory (24). Within circular buffers of varying radii, we computed the sum of road lengths, the sum of PM2.5 emissions, the sum of PM10 emissions, and the percentage of impervious surface. PCA was then performed on these buffer measures and log-transformed county- and ZCTA-level population density to obtain a set of five PCA scores. This procedure allows information from multiple buffers for each covariate to be included, without requiring a manual variable selection procedure.
The PCA scores were combined with thin-plate regression splines (TPRS) (25) as the mean component of the generalized additive model. The model was fit via the mgcv package in R, and the coefficients for the TPRS were penalized using a generalized cross-validation criterion (26). The number of PCA scores (from between 1 and 5) and degrees of freedom (df) for the splines before penalization (from 25 to 400) were selected via tenfold cross-validation (CV). The performance of CV models was assessed via mean-squared error-based R2 (CV R2), which incorporates precision and bias (27, 28).
Predictions from the fitted models were made at 10 randomly selected locations within each ZCTA. The average of the PM2.5 predictions was taken as the ZCTA-level predicted exposure value. The average of the difference between the PM10 and PM2.5 predictions was taken as the ZCTA-level PM10–2.5 predicted exposure.
Outcome
Asthma hospitalizations were defined as hospitalizations with a primary diagnosis of an asthma-related condition (International Classification of Diseases, Ninth Revision diagnosis code of an asthma-related condition [493.x]; see Table E1 in the online supplement). ED visits were defined as outpatient visits occurring in hospital-based EDs with a primary or secondary diagnosis code of an asthma-related condition. Prevalent diagnosed asthma was defined as having at least one asthma-related outpatient visit (defined as an outpatient visit with a primary or secondary diagnosis code of an asthma-related condition), ED visit, or hospitalization during the 24-month period.
Analysis
To account for area-level confounding due to socioeconomic status and other factors, we obtained data on county-level urbanicity and ZCTA-level poverty and education. Urbanicity was quantified using the six-level scale developed by the National Center for Health Statistics, which ranges from “large central metropolitan” counties to rural, “non-core” counties (29). The percentage of families below the poverty level and the percentage of adults with highest education level of high school or below were obtained from the U.S. Census Bureau (30, 31). To allow for possible nonlinear relationships, we represented poverty and education in the models using natural splines with 4 df.
We estimated associations between long-term PM exposure and asthma prevalence and morbidity using generalized estimating equations with a logarithmic link function and clustering within ZCTA. For the prevalence analysis, the number of subjects with asthma was the outcome variable, and an offset was included for the total number of children enrolled in Medicaid. Separate morbidity models were fit for hospital admissions and ED visits, with the number of events included as the outcome variable and number of person-months at risk, equivalent to 24 months times the number of enrollees, included via offset. We fit models that included additive terms for both fractions of PM, as well as models that included each fraction separately. The models were adjusted for the individual variables age category (5–8, 9–11, 12–14, 15–17, or 18–20 yr), sex (male or female), and race/ethnicity (Asian, black, Hispanic, white, or other) and the area-level variables of urbanicity, poverty and its interaction with urbanicity, education, and state. The models further included an unpenalized TPRS with 15 df to account for unmeasured, large-scale spatial differences across the country.
Sensitivity analyses
We considered the sensitivity to the spatial confounding adjustment by fitting models without spatial splines and with TPRS with 100 df, which approximately accounts for medium-scale spatial differences within states. In addition, we explored restricting the cohort to persons 11 years of age or younger and examined including adjustment for estimated county-level adult smoking prevalence (32).
Results
Exposure Assessment
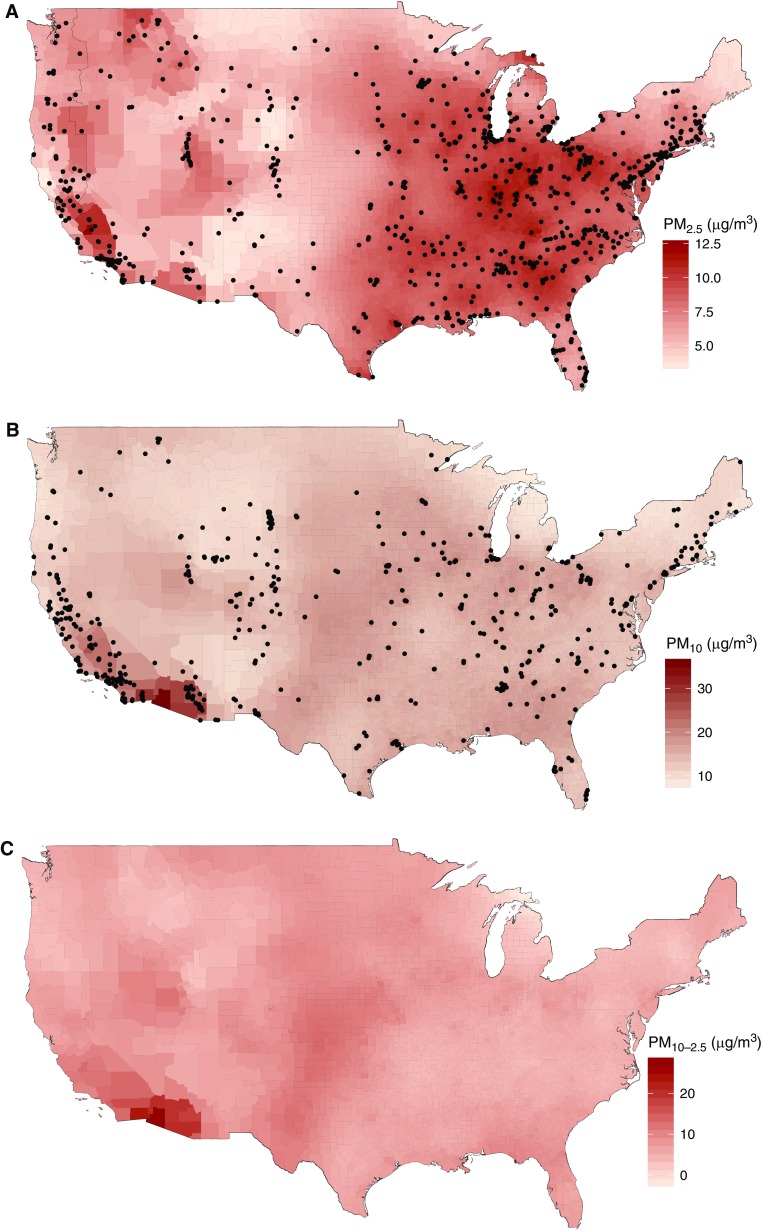
There were 860 PM2.5 monitors and 581 PM10 monitors with data for the 2009 to 2010 period that met inclusion criteria, corresponding to 834 and 518 distinct ZCTAs, respectively. The mean long-term average concentration at monitor locations was 9.4 and 18.7 μg/m3 for PM2.5 and PM10, respectively. The models with the best CV performance included four PCA scores and 350 df TPRS for PM2.5 and four PCA scores and 250 df TPRS for PM10. The corresponding CV R2 values were 0.75 (root-mean-squared error, 1.13 μg/m3) and 0.51 (root-mean-squared error, 4.85 μg/m3), respectively. Scatterplots of CV predictions and monitor observations are provided in Figure E1. Prediction model accuracy was generally better in the eastern United States (Figure E2). Maps of the predicted values of PM2.5 and PM10, and the derived PM10–2.5, aggregated by county for presentation, are shown in Figure 1. The correlation between PM2.5 and PM10 predictions was similar to correlation between observations at collocated monitors (Table E2). The mean (SD) predicted ZCTA-average concentration across all 48 contiguous states was 8.44 μg/m3 (2.01) for PM2.5 and 6.87 μg/m3 (2.89) for PM10–2.5.
Figure 1.
Predicted average (A) particulate matter less than or equal to 2.5 μm in aerodynamic diameter (PM2.5), (B) PM10, and (C) PM10–2.5 for the period 2009 to 2010 across the contiguous United States. Dots represent monitor locations.
Characteristics of the Cohort
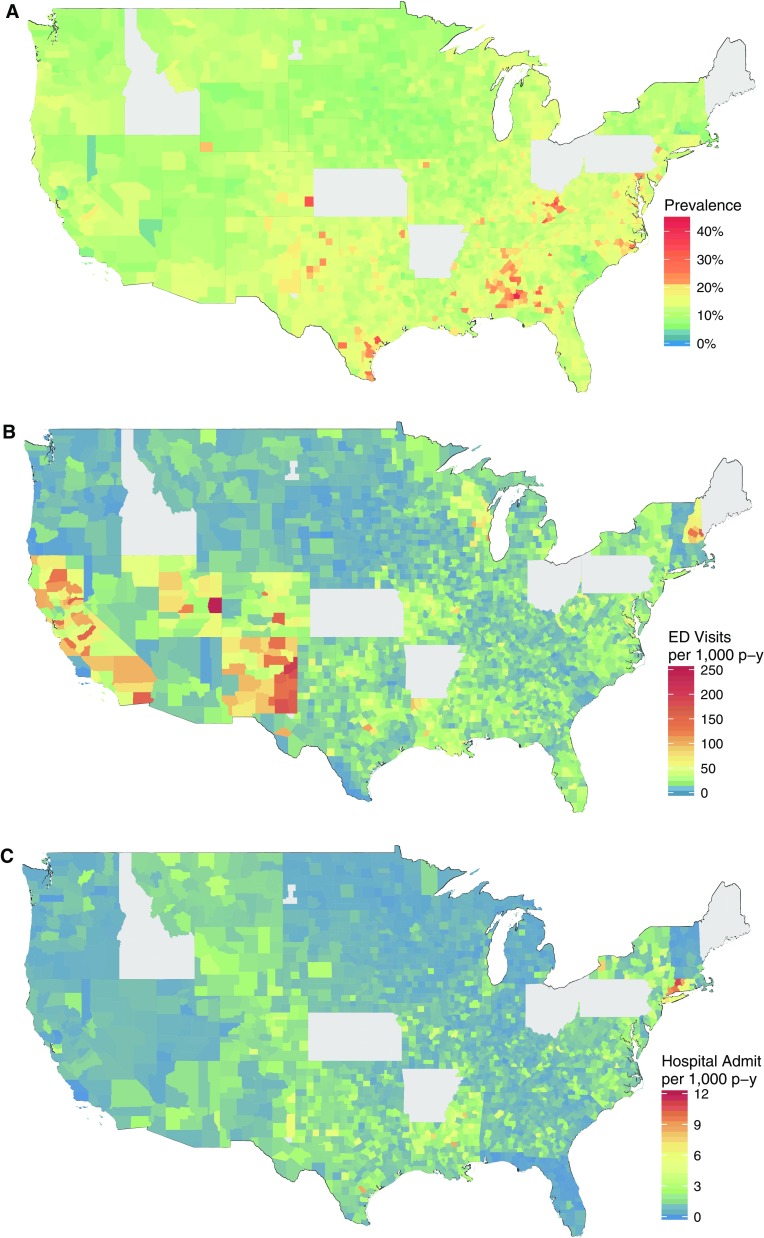
A total of 7,810,025 subjects were included in the analysis. Demographics of included subjects are in Table 1. The overall prevalence of asthma was estimated to be 12.8%. On average, there were two hospitalizations and 32 emergency department visits per 1,000 person-years (Table 2), and these rates were higher among children aged 5 to 11 years (Table E2). As can be seen from Figure 2, there is substantial variation in asthma prevalence and morbidity throughout the United States.
Table 1.
Demographics of Study Cohort
| Characteristic | Full Cohort (N = 7,810,025) |
|
|---|---|---|
| n | % | |
| Age, yr | ||
| 5–8 | 2,154,581 | 28 |
| 9–11 | 1,944,164 | 25 |
| 12–14 | 1,724,497 | 22 |
| 15–17 | 1,567,168 | 20 |
| 18–20 | 419,615 | 5 |
| Sex | ||
| Female | 3,821,081 | 49 |
| Male | 3,988,944 | 51 |
| Race/ethnicity | ||
| Asian | 198,149 | 3 |
| Black | 2,292,236 | 29 |
| Hispanic | 1,771,789 | 23 |
| White | 2,589,294 | 33 |
| Other | 958,557 | 12 |
| Urbanicity | ||
| Large central metro | 2,973,197 | 38 |
| Large fringe metro | 1,280,101 | 16 |
| Medium metro | 1,583,297 | 20 |
| Small metro | 679,014 | 9 |
| Micropolitan | 721,913 | 9 |
| Noncore | 572,503 | 7 |
| Prevalent asthma | 996,843 | 12.8 |
Table 2.
Summary of Asthma Events
| Event Type | Full Cohort |
|
|---|---|---|
| Count | Rate per 1,000 Person-Years | |
| Hospital admissions | 31,122 | 2.0 |
| Emergency department visits | 492,730 | 31.5 |
Figure 2.
(A) Asthma prevalence, (B) emergency department (ED) visits, and (C) hospitalizations by county among children enrolled in Medicaid in the contiguous United States. Data are smoothed to account for variation in the number of Medicaid enrollees. p-y = person-years.
Associations between Predicted PM and Asthma Diagnosis Prevalence
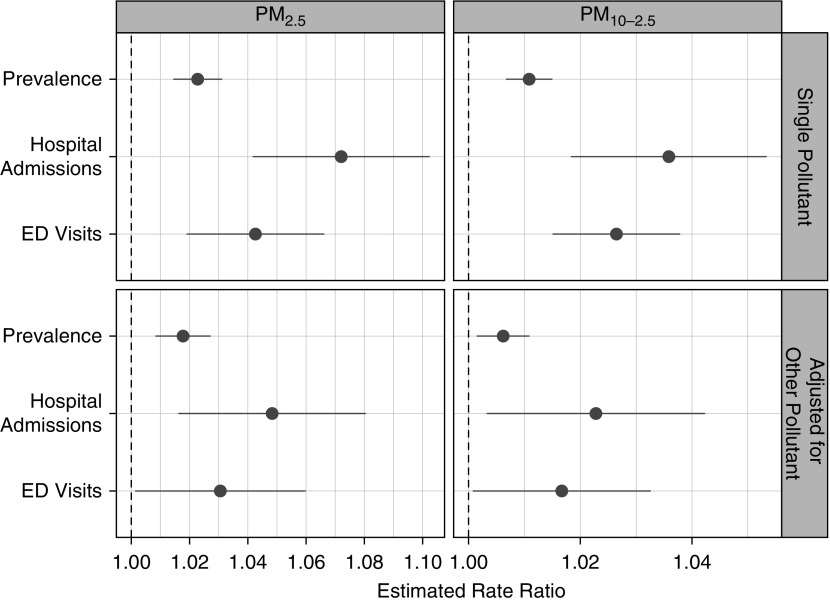
An average increase of 1 μg/m3 predicted PM2.5 was associated with a 2.3% increase in the prevalence of diagnosed asthma (rate ratio [RR], 1.023; 95% confidence interval [CI], 1.014–1.031; P < 0.001), whereas an increase of 1 ug/m3 predicted PM10–2.5 was associated with a 1.1% increase in asthma prevalence (RR, 1.011; 95% CI, 1.007–1.015; P < 0.001). These relationships were robust to inclusion of the other pollutant in the model; the adjusted relative rates were 1.018 for PM2.5 (95% CI, 1.008–1.027; P < 0.001) and 1.006 for PM10–2.5 (95% CI, 1.001–1.011; P = 0.01) (Figure 3).
Figure 3.
Estimated ratios of asthma prevalence and rates of asthma morbidity associated with a 1-μg/m3 difference in particulate matter less than or equal to 2.5 μm in aerodynamic diameter (PM2.5) or PM10–2.5. Single pollutant: not adjusted for PM2.5 or PM10–2.5, respectively. Adjusted for other pollutant: model including both PM2.5 and PM10–2.5. ED = emergency department.
Associations between Predicted PM2.5 and PM10–2.5 and Asthma Morbidity
An average increase of 1 μg/m3 predicted PM2.5 was associated with a 7.2% increase in asthma hospitalizations (RR, 1.072; 95% CI, 1.042–1.102; P < 0.001) and a 4.2% increase in asthma ED visits (RR, 1.042; 95% CI, 1.019–1.066; P < 0.001) (Figure 3). An average increase of 1 μg/m3 PM10–2.5 was associated with a 3.6% increase in asthma hospitalizations (RR, 1.036; 95% CI, 1.018–1.053; P < 0.001) and 2.6% increase in ED visits (RR, 1.026; 95% CI, 1.015–1.038; P < 0.001).
In a model that included both PM2.5 and PM10–2.5, associations between asthma morbidity and both fractions of PM were somewhat attenuated but remained statistically significant. After adjustment, PM2.5 was associated with an RR of 1.048 (95% CI, 1.016–1.081; P = 0.003) for hospitalizations and 1.030 (95% CI, 1.001–1.060; P = 0.040) for ED visits, and PM10–2.5 was associated with an RR of 1.023 (95% CI, 1.003–1.042; P = 0.02) for hospitalizations and 1.017 (95% CI, 1.001–1.033; P = 0.040) for ED visits. (Figure 3).
Sensitivity Analyses
Associations between both pollutants and asthma morbidity and prevalence were sensitive to the scale of spatial adjustment (Table E4) but remained positive for all measures and ranges of spatial adjustment. When we restricted to those aged 11 years and younger, all observed associations between both pollutants and asthma morbidity and prevalence were stronger (Table E5). When county-level smoking prevalence was included, the estimated associations were slightly stronger (Table E6).
Discussion
In this analysis of children across the United States enrolled in Medicaid, we found that it is not only fine PM (PM2.5) but also coarse PM (PM10–2.5) that is associated with long-term effects on asthma diagnosis prevalence and morbidity. For each 1-μg/m3 increase in average coarse PM, there was a 0.6% increase in asthma prevalence, 2.3% more asthma hospitalizations, and 1.7% more ED visits. These associations are adjusted for exposure to fine PM and suggest that there is an effect of coarse PM on asthma-related outcomes that is independent of fine PM.
Our findings fill a key gap in the evidence that long-term coarse PM pollution negatively affects respiratory heath in children. The most recent provisional Integrated Science Assessment by the EPA found that there was insufficient evidence to conclude that coarse PM exposure causes negative health effects, and the 2012 rule-making did not include specific limits on coarse PM but only provided daily PM10 limits (3, 33). However, recent data, including our findings here, suggest that coarse PM may have both short- and long-term effects on human health, with potentially stronger effects on respiratory health (33). Short-term effects have been demonstrated in several time series studies in a variety of communities that have linked daily changes in coarse PM to mortality (34–36), hospitalizations (2, 5, 6, 37), cardiac events (38), and asthma admissions (39). Evidence for long-term effects is much sparser. The few studies published on chronic coarse PM exposure and cardiovascular disease or mortality have failed to find an association (40–43). In contrast, long-term coarse PM exposure was associated with decreased lung function and increased bronchitic symptoms in Southern Californian children (44, 45) and with bronchitis in children in four Chinese cities (46). This study expands those findings in a national-level analysis of long-term coarse PM, finding associations with both prevalent asthma and asthma morbidity.
The composition of coarse PM and fine PM are distinct, reflecting different pollution sources. Fine PM is typically generated by combustion or through reactions in the atmosphere, whereas coarse PM is commonly formed by grinding and resuspension of solid materials. Thus includes crustal elements and organic debris from soil in rural areas as well as heavy metals and roadway-derived particles (e.g., from brake wear) in urban areas (33, 47, 48). Roadway and crustal sources impact coarse PM composition in most areas, although the precise elemental profile can vary between cities (48, 49). Differences in composition could cause regional heterogeneity in the association between PM and asthma morbidity.
The biologic rationale for negative pulmonary effects of coarse PM is strong. Notwithstanding compositional differences, controlled exposure of healthy adult volunteers to coarse PM leads to systemic and pulmonary inflammation similar in magnitude to that of fine PM (50–52) and may lead to skewing of the immune system that predisposes to allergy (53).
That our findings were stronger for children 11 years of age and younger might be expected, as it is at younger ages that asthma develops. In addition, younger children may be more susceptible to outdoor air pollution, both for biologic reasons and because they spend more time outdoors (2). Finally, because younger children have had less time to change residences than older children, exposure over the 2 years studied may correlate more closely with lifetime exposure in younger than in older children.
Our finding that long-term higher average fine PM exposure was associated with asthma prevalence and morbidity is consistent with a large body of literature showing long-term respiratory effects of fine PM exposure in children (3). Data from regional and national studies have shown higher rates of asthma diagnosis, asthma symptoms, respiratory infections, and hay fever with higher fine PM exposure (45–63). Even more importantly, when fine PM concentrations have dropped, respiratory symptoms and infections in children have also decreased (64, 65). Although less novel than the coarse PM analysis, our findings emphasize that despite overall decreases in fine PM over the past decades with the Clean Air Act (66), we still see respiratory morbidity attributed to fine PM exposure among children.
In general, the asthma diagnosis prevalence found here is somewhat higher than reported in national surveys (for example, the estimate of asthma prevalence in 2010 by the CDC on the basis of self-report was 9.4% [67] compared with our prevalence of 12.8%). This is not surprising, both because asthma prevalence is higher in low-income children (14), such as those enrolled in Medicaid, and because not all families who have received a visit diagnosis of asthma may consider their child to have asthma. Demographic risk factors for asthma, including black race/ethnicity and male sex, are consistent between the Medicaid population and national surveys, as detailed in previous analyses of similar data (8).
One challenge of estimating health effects of long-term exposure to coarse PM is the limited amount of monitoring data available. Because federal monitors do not directly assess PM10–2.5, measurement of this fraction requires collocated PM2.5 and PM10 monitors. Such monitors are rare, as the majority of PM monitoring locations only measure one of PM2.5 and PM10. Exposure prediction models based on monitoring data are widely used to estimate long-term PM exposures for air pollution epidemiology cohorts (18, 20, 68, 69). Such models allow estimation of health effects of pollutants in areas where there is not direct monitoring of both pollutants simultaneously. The accuracy of our prediction models (CV R2 of 0.75 and 0.51 for PM2.5 and PM10, respectively) is similar to spatial accuracy reported for exposure prediction models in other studies across the United States, which ranged from 0.62 to 0.88 for PM2.5 (20, 68, 70) and 0.55 to 0.69 for PM10 (19, 71). PM10, because of its greater mass, has shorter residence times in the air than PM2.5. This makes it more spatially heterogeneous than PM2.5 and more difficult to predict. Predictive accuracy for PM10 is also impacted by the decline in the number of operational PM10 monitors since the widespread PM2.5 monitoring began in 1999, which is likely why the prediction accuracy of our PM10 model is somewhat less than reported in earlier studies.
Limitations to our analysis include the inherent limitations of using claims data to measure disease activity. Access to health care, health behaviors such as compliance with medications, and individual health choices can all influence whether an asthma exacerbation leads to an ED visit or hospitalization, although one of the benefits of using Medicaid data is that all subjects should be able to access all types of care. Miscoding or missing data could add bias; we excluded a number of states where important data were missing (such as for race) or where utilization data showed very improbable rates of asthma care use, but we cannot exclude the possibility that other data were flawed. We were unable to adjust for individual-level economic status, household tobacco exposure, or other individual-level factors that could confound the relationship between pollution exposure and asthma, although we did adjust for race and zip-code–level education and poverty and did sensitivity analyses adjusting for county-level tobacco exposure. Our results are limited by the assumption of a linear exposure–response relationship, although in sensitivity analyses we did not find evidence that the linearity assumption was violated. Furthermore, although nonlinearity of exposure response is of great interest across large exposure ranges, the limited range of exposures in the current data make it difficult to detect. The prediction of exposures introduces correlated measurement error in point estimates (72, 73), and calculation of coarse PM as the difference of PM10 and PM2.5 predictions can introduce additional measurement error (74). However, for linear health models with a single pollutant, analytic measurement error corrections have identified relatively small amounts of bias (16, 75). Differences in the spatial distribution of monitors and cohort subjects has been shown to introduce bias in some settings (73, 76), but its overall impact is unclear (77, 78). Finally, our analysis may not be generalizable to non–low-income children, but our focus on this population may be a strength, as there is evidence that low-income children are particularly vulnerable to the effects of pollution (2).
Our findings were sensitive to the extent of spatial adjustment. The long-range spatial adjustment chosen for our primary analysis can account for unmeasured differences that occur on a spatial scale similar to the differences between states, but in a smooth manner not restricted by state boundaries. Our choice of adjustment scale also preserves smaller-scale variation in the PM exposure, which provides informative contrasts between ZCTAs that allow us to estimate the association of interest. The finer-scale adjustment in the sensitivity analysis effectively removes a large proportion of the spatial variability in the exposure surfaces. For predicted coarse PM, which was more spatially smooth than predicted PM2.5 (Figure 1), this reduces power and results in attenuated point estimates. In contrast, predicted PM2.5 had smaller-scale contrasts that were not removed by the greater adjustment, and the estimates were larger in the sensitivity analysis. Nonetheless, the estimates for exposure to both fractions of PM were in the same direction for all levels of adjustment. Further research should consider the application of automated procedures for selecting the extent of spatial confounding adjustment. Algorithms such as minimizing quasi-likelihood information criteria (79), which target the modeled outcome, may introduce additional bias and are not necessarily appropriate (80, 81).
The sensitivity of results to adjustment for unmeasured spatial confounding and additional pollutants merits further investigation in future research. This includes considering other pollutants, such as ozone, that have been associated with asthma morbidity (82). Ozone in particular can present challenges for the exposure modeling framework, because there are limited year-round measurements. Additional avenues of further inquiry are possible nonlinearity of the exposure–response relationship and spatial heterogeneity of the effect because of compositional differences in coarse PM.
Conclusions
Among children enrolled in Medicaid in the United States between 2009 and 2010, we found that long-term exposure to coarse PM was independently associated with higher rates of prevalent asthma, asthma hospitalizations, and asthma ED visits. This first-ever analysis of the long-term effects of coarse PM on asthma in a nationwide sample of U.S. children provides evidence supporting the harmful effects of coarse PM on respiratory health. Our results suggest that direct monitoring of coarse PM may need to be implemented and that long-term coarse PM standards should be reconsidered.
Footnotes
Supported by National Institute of Allergy and Infectious Diseases grants K23AI103187 and R21AI107085 and U.S. Environmental Protection Agency (EPA) grant RD835871. This work has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. The EPA does not endorse any products or commercial services mentioned in this publication.
Author Contributions: C.A.K. planned the project, acquired the data, helped interpret the data, and wrote the manuscript. J.P.K. contributed to the conception of the work, performed statistical analyses, interpreted the results, and helped write the manuscript. R.D.P. conceived and planned the project, acquired the data, analyzed the data, interpreted the results, and critically reviewed the manuscript. All authors gave final approval of the work to be published and agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201706-1267OC on December 15, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention. Asthma facts–CDC’s National Asthma Control Program grantees. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 2.United States Environmental Protection Agency. Integrated science assessment (ISA) for particulate matter (final report, Dec 2009) Washington, DC: U.S. Environmental Protection Agency; 2009. [Google Scholar]
- 3.National Center for Environmental Assessment RTP Division. Provisional assessment of recent studies on health effects of particulate matter exposure. Research Triangle Park: NC: U.S. Environmental Protection Agency; 2012. [Google Scholar]
- 4.United States Environmental Protection Agency. How does PM affect human health? 2017 [accessed 2017 Sept 7]. Available from: https://www3.epa.gov/region1/airquality/pm-human-health.html.
- 5.Powell H, Krall JR, Wang Y, Bell ML, Peng RD. Ambient coarse particulate matter and hospital admissions in the Medicare Cohort Air Pollution Study, 1999-2010. Environ Health Perspect. 2015;123:1152–1158. doi: 10.1289/ehp.1408720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng RD, Chang HH, Bell ML, McDermott A, Zeger SL, Samet JM, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA. 2008;299:2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Wang S, Lang L, Huang C, Ma W, Lin H. Ambient fine and coarse particulate matter pollution and respiratory morbidity in Dongguan, China. Environ Pollut. 2017;222:126–131. doi: 10.1016/j.envpol.2016.12.070. [DOI] [PubMed] [Google Scholar]
- 8.Keet CA, Matsui EC, McCormack MC, Peng RD. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J Allergy Clin Immunol. 2017;140:822–827. doi: 10.1016/j.jaci.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck AF, Moncrief T, Huang B, Simmons JM, Sauers H, Chen C, et al. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J Pediatr. 2013;163:574–580. doi: 10.1016/j.jpeds.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott JP, Harrison C, Konopka C, et al. Pharmacist-led screening program for an inner-city pediatric population. J Am Pharm Assoc. 2003;2015:413–418. doi: 10.1331/JAPhA.2015.14273. [DOI] [PubMed] [Google Scholar]
- 11.Elliott JP, Marcotullio N, Skoner DP, Lunney P, Gentile DA. An asthma sports camp series to identify children with possible asthma and cardiovascular risk factors. J Asthma. 2014;51:267–274. doi: 10.3109/02770903.2013.867349. [DOI] [PubMed] [Google Scholar]
- 12.Pesek RD, Vargas PA, Halterman JS, Jones SM, McCracken A, Perry TT. A comparison of asthma prevalence and morbidity between rural and urban schoolchildren in Arkansas. Ann Allergy Asthma Immunol. 2010;104:125–131. doi: 10.1016/j.anai.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennsylvania Department of Health. Pennsylvania asthma prevalence report. 2015 [accessed 2017 May 15]. Available from: http://www.health.pa.gov/My%20Health/Diseases%20and%20Conditions/A-D/Asthma/Documents/2015%20PENNSYLVANIA%20ASTHMA%20PREVALENCE%20REPORT%20UPDATED%20FEB%2023%202016.pdf.
- 14.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: rethinking the inner-city asthma epidemic. J Allergy Clin Immunol. 2015;135:655–662. doi: 10.1016/j.jaci.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Environmental Protection AgencyAir Quality System Database: Query AirData. 2016 [accessed 2016 Nov 8]. Available from: https://aqs.epa.gov/api
- 16.Bergen S, Szpiro AA. Mitigating the impact of measurement error when using penalized regression to model exposure in two-stage air pollution epidemiology studies. Environ Ecol Stat. 2015;22:601–631. [Google Scholar]
- 17.Bergen S, Sheppard L, Sampson PD, Kim SY, Richards M, Vedal S, et al. A national prediction model for PM2.5 component exposures and measurement error-corrected health effect inference. Environ Health Perspect. 2013;121:1017–1025. doi: 10.1289/ehp.1206010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoek G, Beelen R, de Hoogh K, Briggs DJ. A review of land-use regression models to assess spatial variation of outdoor air pollution. Atmos Environ. 2008;42:7561–7578. [Google Scholar]
- 19.Hart JE, Yanosky JD, Puett RC, Ryan L, Dockery DW, Smith TJ, et al. Spatial modeling of PM10 and NO2 in the continental United States, 1985-2000. Environ Health Perspect. 2009;117:1690–1696. doi: 10.1289/ehp.0900840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampson PD, Richards M, Szpiro AA, Bergen S, Sheppard L, Larson TV, et al. A regionalized national universal kriging model using partial least squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ (1994) 2013;75:383–392. doi: 10.1016/j.atmosenv.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Census Bureau. DP-1: Profile of general population and housing characteristics: 2010. 2010 [accessed 2016 Oct 26]. Available from: https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?
- 22.U.S. Census Bureau. 2010 TIGER/Line Shapefiles: primary and secondary roads. 2010 [accessed 2016 Nov 21]. Available from: ftp://ftp2.census.gov/geo/tiger/TIGER2010/PRISECROADS/
- 23.Xian G, Homer C, Dewitz J, Fry J, Hossain N, Wickham J. The change of impervious surface area between 2001 and 2006 in the conterminous United States. Photogramm Eng Remote Sensing. 2011;77:758–762. [Google Scholar]
- 24.United States Environmental Protection Agency. 2008 National Emissions Inventory [digital data set]. 2008 [accessed 2017 Feb 17]. Available from: ftp://ftp.epa.gov/EmisInventory/2008v3/2008neiv3_facility.zip.
- 25.Wood SN. Thin plate regression splines. J R Stat Soc B. 2003;65:95–114. [Google Scholar]
- 26.Wood SN. Generalized additive models: an introduction with R. Boca Raton: Chapman & Hall/CRC; 2006. [Google Scholar]
- 27.Keller JP, Olives C, Kim S-Y, Sheppard L, Sampson PD, Szpiro AA, et al. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the multi-ethnic study of atherosclerosis and air pollution. Environ Health Perspect. 2015;123:301–309. doi: 10.1289/ehp.1408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindström J, Szpiro AA, Sampson PD, Oron AP, Richards M, Larson TV, et al. A flexible spatio-temporal model for air pollution with spatio-temporal covariates. Environ Ecol Stat. 2014;21:411–433. doi: 10.1007/s10651-013-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingram DD, Franco SJ. 2013 NCHS urban-rural classification scheme for counties. Vital Health Stat 2. 2014;166:1–73. [PubMed] [Google Scholar]
- 30.U.S. Census Bureau. S1702: Poverty status in the past 12 months of families. 2011 [accessed 2016 Oct 26]. Available from: https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?
- 31.U.S. Census Bureau. S1501: Educational attainment. 2011 [accessed 2016 Oct 26]. Available from: https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?
- 32.Dwyer-Lindgren L, Mokdad AH, Srebotnjak T, Flaxman AD, Hansen GM, Murray CJL. Cigarette smoking prevalence in US counties: 1996-2012. Popul Health Metr. 2014;12:5. doi: 10.1186/1478-7954-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adar SD, Filigrana PA, Clements N, Peel JL. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Curr Environ Health Rep. 2014;1:258–274. doi: 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117:898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yorifuji T, Kashima S, Doi H. Associations of acute exposure to fine and coarse particulate matter and mortality among older people in Tokyo, Japan. Sci Total Environ. 2016;542:354–359. doi: 10.1016/j.scitotenv.2015.10.113. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Honda Y, Hashizume M, Guo YL, Wu CF, Kan H, et al. Short-term exposure to fine and coarse particles and mortality: a multicity time-series study in East Asia. Environ Pollut. 2015;207:43–51. doi: 10.1016/j.envpol.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Cheng MH, Chiu HF, Yang CY. The effects of coarse particles on daily mortality: a case-crossover study in a subtropical city, Taipei, Taiwan. Int J Environ Res Public Health. 2016;13:13. doi: 10.3390/ijerph13030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia R, Zhou G, Zhu T, Li X, Wang G. Ambient air pollution and out-of-hospital cardiac arrest in Beijing, China. Int J Environ Res Public Health. 2017;14:423. doi: 10.3390/ijerph14040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng MH, Chiu HF, Yang CY. Coarse particulate air pollution associated with increased risk of hospital admissions for respiratory diseases in a tropical city, Kaohsiung, Taiwan. Int J Environ Res Public Health. 2015;12:13053–13068. doi: 10.3390/ijerph121013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonnell WF, Nishino-Ishikawa N, Petersen FF, Chen LH, Abbey DE. Relationships of mortality with the fine and coarse fractions of long-term ambient PM10 concentrations in nonsmokers. J Expo Anal Environ Epidemiol. 2000;10:427–436. doi: 10.1038/sj.jea.7500095. [DOI] [PubMed] [Google Scholar]
- 41.Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puett RC, Hart JE, Suh H, Mittleman M, Laden F. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect. 2011;119:1130–1135. doi: 10.1289/ehp.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117:1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gauderman WJ, McConnell R, Gilliland F, London S, Thomas D, Avol E, et al. Association between air pollution and lung function growth in southern California children. Am J Respir Crit Care Med. 2000;162:1383–1390. doi: 10.1164/ajrccm.162.4.9909096. [DOI] [PubMed] [Google Scholar]
- 45.McConnell R, Berhane K, Molitor J, Gilliland F, Künzli N, Thorne PS, et al. Dog ownership enhances symptomatic responses to air pollution in children with asthma. Environ Health Perspect. 2006;114:1910–1915. doi: 10.1289/ehp.8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang JJ, Hu W, Wei F, Wu G, Korn LR, Chapman RS. Children’s respiratory morbidity prevalence in relation to air pollution in four Chinese cities. Environ Health Perspect. 2002;110:961–967. doi: 10.1289/ehp.02110961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomson EM, Breznan D, Karthikeyan S, MacKinnon-Roy C, Vuong NQ, Dabek-Zlotorzynska E, et al. Contrasting biological potency of particulate matter collected at sites impacted by distinct industrial sources. Part Fibre Toxicol. 2016;13:65. doi: 10.1186/s12989-016-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sturtz TM, Adar SD, Gould T, Larson TV. Constrained source apportionment of coarse particulate matter and selected trace elements in three cities from the multi-ethnic study of atherosclerosis. Atmos Environ (1994) 2014;84:65–77. doi: 10.1016/j.atmosenv.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung K, Daher N, Kam W, et al. Spatial and temporal variation of chemical composition and mass closure of ambient coarse particulate matter (PM10-2.5) in the Los Angeles area. Atmos Environ. 2011;45:2651–2662. [Google Scholar]
- 50.Graff DW, Cascio WE, Rappold A, Zhou H, Huang YC, Devlin RB. Exposure to concentrated coarse air pollution particles causes mild cardiopulmonary effects in healthy young adults. Environ Health Perspect. 2009;117:1089–1094. doi: 10.1289/ehp0900558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellavia A, Urch B, Speck M, Brook RD, Scott JA, Albetti B, et al. DNA hypomethylation, ambient particulate matter, and increased blood pressure: findings from controlled human exposure experiments. J Am Heart Assoc. 2013;2:e000212. doi: 10.1161/JAHA.113.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Behbod B, Urch B, Speck M, Scott JA, Liu L, Poon R, et al. Endotoxin in concentrated coarse and fine ambient particles induces acute systemic inflammation in controlled human exposures. Occup Environ Med. 2013;70:761–767. doi: 10.1136/oemed-2013-101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, et al. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol. 2006;117:1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 54.Parker JD, Akinbami LJ, Woodruff TJ. Air pollution and childhood respiratory allergies in the United States. Environ Health Perspect. 2009;117:140–147. doi: 10.1289/ehp.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nachman KE, Parker JD. Exposures to fine particulate air pollution and respiratory outcomes in adults using two national datasets: a cross-sectional study. Environ Health. 2012;11:25. doi: 10.1186/1476-069X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhattacharyya N. Air quality influences the prevalence of hay fever and sinusitis. Laryngoscope. 2009;119:429–433. doi: 10.1002/lary.20097. [DOI] [PubMed] [Google Scholar]
- 57.Kloog I, Coull BA, Zanobetti A, Koutrakis P, Schwartz JD. Acute and chronic effects of particles on hospital admissions in New-England. PLoS One. 2012;7:e34664. doi: 10.1371/journal.pone.0034664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neupane B, Jerrett M, Burnett RT, Marrie T, Arain A, Loeb M. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am J Respir Crit Care Med. 2010;181:47–53. doi: 10.1164/rccm.200901-0160OC. [DOI] [PubMed] [Google Scholar]
- 59.Meng YY, Rull RP, Wilhelm M, Lombardi C, Balmes J, Ritz B. Outdoor air pollution and uncontrolled asthma in the San Joaquin Valley, California. J Epidemiol Community Health. 2010;64:142–147. doi: 10.1136/jech.2009.083576. [DOI] [PubMed] [Google Scholar]
- 60.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118:1021–1026. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akinbami LJ, Lynch CD, Parker JD, Woodruff TJ. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001-2004. Environ Res. 2010;110:294–301. doi: 10.1016/j.envres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Carlsten C, Dybuncio A, Becker A, Chan-Yeung M, Brauer M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med. 2011;68:291–295. doi: 10.1136/oem.2010.055152. [DOI] [PubMed] [Google Scholar]
- 63.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noonan CW, Navidi W, Sheppard L, Palmer CP, Bergauff M, Hooper K, et al. Residential indoor PM2.5 in wood stove homes: follow-up of the Libby changeout program. Indoor Air. 2012;22:492–500. doi: 10.1111/j.1600-0668.2012.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhattacharyya N, Shapiro NL. Air quality improvement and the prevalence of frequent ear infections in children. Otolaryngol Head Neck Surg. 2010;142:242–246. doi: 10.1016/j.otohns.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 66.Ross K, Chmiel JF, Ferkol T. The impact of the Clean Air Act. J Pediatr. 2012;161:781–786. doi: 10.1016/j.jpeds.2012.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Center for Health Statistics, CDC. 2010 National Health Interview Survey (NHIS) data. Compiled 2012 Jan 3 [accessed 2017 May 15]. Available from: https://www.cdc.gov/asthma/nhis/2010/table2-1.htm.
- 68.Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang Y, Schwartz J. Assessing PM2.5 exposures with high spatiotemporal resolution across the continental United States. Environ Sci Technol. 2016;50:4712–4721. doi: 10.1021/acs.est.5b06121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanosky JD, Paciorek CJ, Suh HH. Predicting chronic fine and coarse particulate exposures using spatiotemporal models for the Northeastern and Midwestern United States. Environ Health Perspect. 2009;117:522–529. doi: 10.1289/ehp.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu X, Waller LA, Lyapustin A, Wang Y, Liu Y. 10-year spatial and temporal trends of PM2.5 concentrations in the southeastern US estimated using high-resolution satellite data. Atmos Chem Phys. 2014;14:6301–6314. doi: 10.5194/acp-14-6301-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health. 2014;13:1–15. doi: 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopiano KK, Young LJ, Gotway CA. Estimated generalized least squares in spatially misaligned regression models with Berkson error. Biostatistics. 2013;14:737–751. doi: 10.1093/biostatistics/kxt011. [DOI] [PubMed] [Google Scholar]
- 73.Szpiro AA, Paciorek CJ. Measurement error in two-stage analyses, with application to air pollution epidemiology. Environmetrics. 2013;24:501–517. doi: 10.1002/env.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang HH, Peng RD, Dominici F. Estimating the acute health effects of coarse particulate matter accounting for exposure measurement error. Biostatistics. 2011;12:637–652. doi: 10.1093/biostatistics/kxr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergen S, Sheppard L, Kaufman JD, Szpiro AA. Multipollutant measurement error in air pollution epidemiology studies arising from predicting exposures with penalized regression splines. J R Stat Soc Ser C Appl Stat. 2016;65:731–753. doi: 10.1111/rssc.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keller JP, Chang HH, Strickland MJ, Szpiro AA. Measurement error correction for predicted spatiotemporal air pollution exposures. Epidemiology. 2017;28:338–345. doi: 10.1097/EDE.0000000000000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Antonelli J, Cefalu M, Bornn L. The positive effects of population-based preferential sampling in environmental epidemiology. Biostatistics. 2016;17:764–768. doi: 10.1093/biostatistics/kxw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee A, Szpiro A, Kim SY, Sheppard L. Impact of preferential sampling on exposure prediction and health effect inference in the context of air pollution epidemiology. Environmetrics. 2015;26:255–267. doi: 10.1002/env.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan W. Model selection in estimating equations. Biometrics. 2001;57:529–534. doi: 10.1111/j.0006-341x.2001.00529.x. [DOI] [PubMed] [Google Scholar]
- 80.Peng RD, Dominici F, Zeger SL. Reproducible epidemiologic research. Am J Epidemiol. 2006;163:783–789. doi: 10.1093/aje/kwj093. [DOI] [PubMed] [Google Scholar]
- 81.Speckman P. Kernal smoothing in partial linear models. J R Stat Soc B. 1988;50:413–436. [Google Scholar]
- 82.United States Environmental Protection Agency. Integrated science assessment (ISA) of ozone and related photochemical oxidants (final report, Feb 2013) Washington, DC: U.S. Environmental Protection Agency; 2013. [Google Scholar]