Abstract
Historically, immune-based therapies have played a leading role in the treatment of hematologic malignancies, with the efficacy of stem cell transplantation largely attributable to donor immunity against malignant cells. As new and more targeted immunotherapies have developed, their role in the treatment of hematologic malignancies is evolving and expanding. Herein, we discuss approaches for antigen discovery and review known and novel tumor antigens in hematological malignancies. We further explore the role of established and investigational immunotherapies in hematologic malignancies, with a focus on personalization of treatment modalities such as cancer vaccines and adoptive cell therapy. Finally, we identify areas of active investigation and development. Immunotherapy is at an exciting crossroads for the treatment of hematologic malignancies, with further investigation aimed at producing effective, targeted immune therapies that maximize anti-tumor effects while minimizing toxicity.
Keywords: Neo-antigens, antigens, cancer immunotherapy, mutation, genome sequencing, immunopeptidome, hematologic malignancies
Introduction
The recent successes of targeting immune checkpoint blockade for the treatment of solid tumors have led to the current broad adoption of immune-based therapy across diverse malignancies, with immunotherapy now anticipated to remain a steady part of the therapeutic armamentarium against cancer. Long before this current age, the effectiveness of immune-based therapy for the treatment of hematologic malignancies was widely demonstrated through decades of research and clinical experience. In particular, the curative experience of using allogeneic hematopoietic stem cell transplantation (HSCT) to treat leukemia demonstrated both the potent impact of the immune system to target malignant cells, but also indicated the possibility of developing significant immune-based toxicities. Indeed, over 25 years ago, Horowitz and colleagues elegantly showed that the presence of donor T cells during bone marrow transplantation for leukemia decreased the probability of relapse, providing early support for the use of competent immune elements to combat hematologic malignancies1. An immune basis for the anti-leukemic activity of donor allografts was further supported by the successful use of post-allograft infusions of lymphocytes from the original donor (DLI), which continues to provide an established means for effectively treating leukemic relapse after HSCT, often in the absence of further cytotoxic agents2,3.
Identifying and characterizing the targets of the anti-leukemia responses induced by HSCT/DLI were amongst the earliest efforts to gain understanding of the mechanisms underlying these therapeutic responses, and continues to provide a rational path towards developing newer therapies. For DLI, although the precise mechanism by which anti-leukemia control is mediated has remained elusive, collective evidence has supported the idea that effective graft-versus-leukemia (GvL) responses relate to coordinated leukemia antigen-specific cellular and humoral immunity and to local reversal of CD8+ T cell exhaustion within the bone marrow4–11. Thus, the process of antigen discovery has provided the means to dissect out the paths to therapeutic benefit. The demonstration of immune activity against leukemia from the HSCT/DLI experience has paved the way for the new area of cellular adoptive therapy with chimeric antigen receptor (CAR) T cells. At the same time, the concepts encompassed by HSCT of combinatorially incorporating immunologic help and focusing immune responses on cancer-specific antigens have foreshadowed the current active efforts in immune checkpoint blockade (CPB) and cancer vaccines in oncology. Characterization of antigen specificities has also provided insight into the mechanistic basis of the major immunologic complication of GvL, namely the donor immune responses against normal recipient cells, termed graft-versus-host disease (GvHD)12,13. The presentation and spectrum of targets of GvHD and its clinical management have likewise foreshadowed the severest complications of the new immune checkpoint inhibitors, namely autoimmune toxicities. In the current age, maximizing anti-tumor benefit (GvL) effect while minimizing toxicity (GvHD) remains an important goal, with these efforts extending to new CPB-based efforts14,15.
Herein, we discuss prior and current methods for antigen discovery, including known tumor antigens in hematologic malignancies, with commentary on current immune-based therapies. In particular, we discuss known and proposed mechanisms for generating neo-antigens in hematologic malignancies, with a focus on identifying immunogenic neo-antigens for therapeutic targeting.
Identifying Tumor Antigens in Hematologic Malignancies
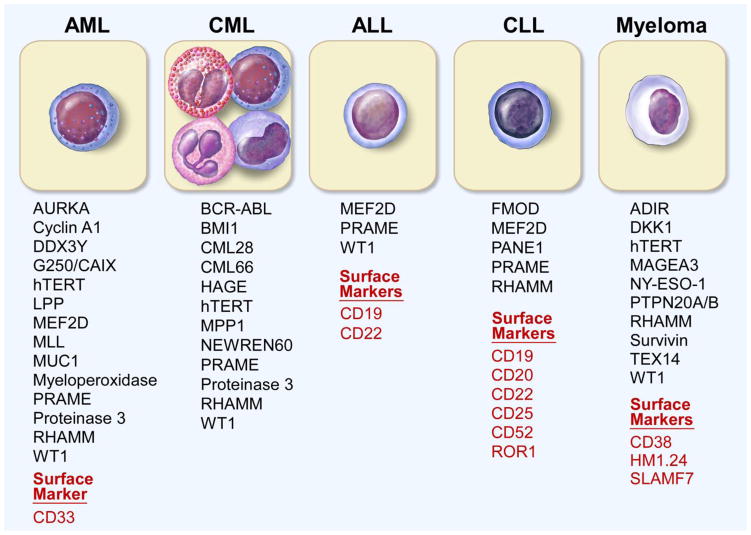
A major priority in cancer research has been the identification of tumor antigens, with the aim of targeting them through immune-based therapies. The methods used to identify such antigens have evolved over decades (Figure 1). Overall, resource intensive cell-based and biochemical-based approaches have now led to higher throughput approaches based on systematic evaluation of DNA, RNA and protein to search for suitable antigens. Examples of some of the tumor antigens identified in acute myeloid leukemia (AML)16–30, chronic myeloid leukemia (CML)19,24,31–41, acute lymphoblastic leukemia (ALL)19,30,42,43, chronic lymphocytic leukemia (CLL)19,29,30,44–47, and multiple myeloma21,22,27,30,48–52 are shown in Figure 2.
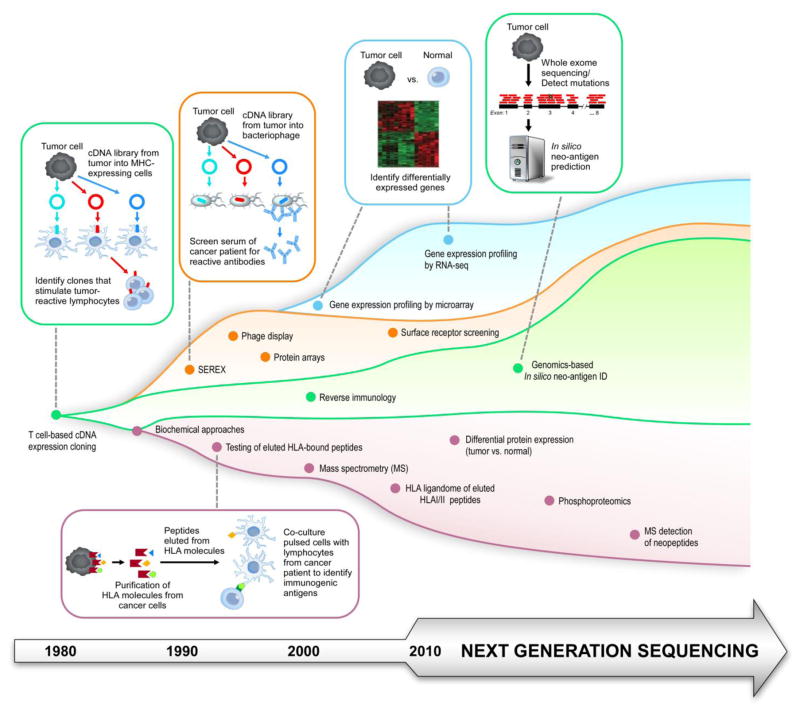
Figure 1. Evolution in the Methods of Tumor of Antigen Discovery.
Historical and contemporary methods for tumor antigen identification, identifying T-cell based (green), serology-based (orange), gene expression based (blue) and biochemical/proteomic-based (purple) approaches.
Figure 2. Examples of Tumor Antigens in Hematologic Malignancies.
A selection (not exhaustive) of tumor antigens and cell surface markers in a variety of hematologic malignancies.
Experimental Approaches to Tumor Antigen Identification
The discovery of lineage-defining cell surface markers on human immune cells following the development of hybridoma technology54, has been the starting point of much studies of human immunity. Although these findings were not specifically geared at discovering tumor antigens per se, these defining markers have become highly suitable antigen targets of current B and T cell-based immunotherapeutic approaches (described in the next section, and noted in Figure 2). For example, malignant B cells were found to express a unique variable region on cell surface immunoglobulin (“idiotype”)53, and monoclonal antibodies could be subsequently generated to therapeutically target these tumor idiotypes55,56. This type of technology was similarly used to identify lineage-defining cell surface markers on human immune cells, including CD19 and CD20, expressed on normal and malignant B cells57. Now, monoclonal antibodies directed against cell surface markers29,58,59 expressed by lymphoma cells have become a standard approach in the treatment of a wide variety of lymphomas60,61,29,62 (i.e. rituximab, the monoclonal antibody targeting CD2063). More recently CD19 surface expression on certain ALL cells has been targeted effectively with CAR T cell therapy64,65. Ongoing investigative work to identify novel surface markers will likely continue to prove fruitful, as exemplified by the recent identification of restricted ROR1 surface expression on B-lineage ALL cells66, and subsequent efforts to therapeutically target this surface antigen with CAR T cells67,68.
Many investigative efforts have been focused on the identification of tumor antigens that could generate a classical T cell response. The earliest approach to bona fide tumor antigen discovery was through T cell-based screening of tumor cDNA expression libraries, pioneered by Boon and colleagues in the early 1980s, which led to the identification of the MAGE family of melanoma-associated antigens69,70. This time- and labor-intensive technique involves the generation of cDNA libraries from tumor cells, transfection into cells also expressing the appropriate MHC molecule, and then screening with tumor-reactive T cells, with cells transfected with an immunogenic antigen leading to T cell stimulation and cytokine release69–76. While this technique was initially successful in identifying melanoma antigens, subsequent application of this method has led to the identification of a number of leukemia-associated antigens, including minor histocompatibility antigens (mHAs) with leukemia-restricted expression77–80. Another early approach involved separating cell fractions (using reversed-phase liquid chromatography and gel electrophoresis), identification of fractions containing an immunogenic antigen and finally protein sequencing of the identified analyte81. Biochemical approaches like this were used to identify the first minor histocompatibility antigen HA-1 in hematopoietic cells82. Similarly, testing of HLA-bound peptides on tumor cells (isolated on the basis of immunoaffinity purification and subsequent elution of HLA molecules83–85), pulsed on antigen-presenting cells against autologous lymphocytes, has led to the identification of antigens such as ADIR in multiple myeloma86.
Humoral immunity may cooperative with T cell responses as part of a complex immune response, as demonstrated by the known protective effects of antibody responses in infectious immunity, and also through coordinated B cell and T cell responses identified in blood malignancies in settings of effective clinical response. For example, in patients with CML who received DLI, generation of high titer antibodies against CML antigens correlated with disease remission, suggesting that effective humoral immunity may serve as a positive biomarker for response to therapy87. To take advantage of higher serologic responses to tumors, Pfreundschuh and colleagues pioneered an alternative system for antigen identification, termed serological analysis of recombinant cDNA expression libraries or SEREX88–90 in the 1990’s. In this technique, a cDNA library is constructed from tumor cells, and transfected into prokaryotic cells. The recombinant proteins are then screened using the patient’s serum, allowing for the detection of tumor antigens which generate a high-titer IgG antibody response in the patient. The clones that are identified can then have their DNA sequenced for identification of the tumor antigen. This technique has detected the antigens PRAME in AML91, cTAGE-1 in cutaneous T cell lymphoma92, and the cancer testis antigen NY-ESO-193 found in multiple myeloma94, among others. More recently, this approach has been extended further with the use of high-density protein microarrays to identify antigens in CLL, CML, and multiple myeloma95–97. These serologic-based approaches have the advantage of providing a rapid method for identifying a broad array of potential tumor antigens, but also have the limitations that these antigens may be byproducts of tumor cytolysis but not tumor rejection antigens per se. In addition, this approach may fail to detect important tumor antigens that depend on post-translational modifications or conformational changes that do not occur in a prokaryote system93,98,99.
More recently, antigens have been identified on the basis of differential gene expression profiling. Gene expression analysis seeks to identify differentially or aberrantly expressed genes in tumor cells compared to normal tissue99,100. These early studies began with cDNA microarray and SAGE (serial analysis of gene expression) platforms. These techniques have been used to successfully identify candidate tumor antigens in CLL (including ROR1)101 and multiple myeloma (such as TEX14, PTPN20A/B, among others)102. While DNA microarray analysis typically requires a priori knowledge of transcript sequence, RNA-seq (and genome sequencing with computational prediction tools) allows for the identification of novel transcripts103–106. However, as gene expression may not correlate with protein expression, and does not take into account post-translational modifications that may contribute to immunogenicity.
Some of these limitations can be overcome with modern proteomic approaches. These approaches typically use mass spectrometry to identify and quantify peptide fragments100,107. Modifications to this technique include an initial immunoaffinity purification step to isolate HLA molecules and bound peptides, prior to mass spectrometry analysis. This technique allows for the identification of HLA-bound peptides, including mutated or tumor-specific HLA-bound peptides (such as a BCR-ABL peptide in CML108), which constitute the “immunopeptidome” or “HLA ligandome” for a tumor20,109,110. A related approach can enrich for phosphorylated peptides, and analysis of the phosphoproteome of a tumor may similarly reveal novel antigens111, and has led to the identification of MLL, LPP, and MEF2D, among others112, in leukemias and lymphomas.
Overall, these and other approaches have identified unique tumor antigens, overexpressed or tumor-associated antigens, and cancer-testis antigens (which are, under normal circumstances, only expressed in an immune privileged environment)113–115 across cancers19. Still another approach to tumor antigen discovery has been through “reverse immunology” techniques, where peptides are selected and synthesized based on a computationally predicted or experimentally determined ability to bind HLA molecules, and then are tested for their ability to elicit a T-cell response. In the hematologic malignancies113, this strategy has led to the identification of leukemia-specific antigens linked to the GVL response, including proteinase 3 (PRTN3)116,117, Wilms’ tumor protein 1 (WT1)118, and the BCR-ABL fusion peptides35. As a further extension of this approach, exome-wide DNA sequencing data has been combined with computational prediction tools (such as NetMHC) to effectively predict a class of antigens called neo-antigens arising from tumor-specific genomic alterations. These somatic alterations include missense mutations (single nucleotide variants, or SNVs), or insertions or deletions leading to frameshift mutations (indels) and potential new open reading frames (neo-ORFs)105,106,119,120.
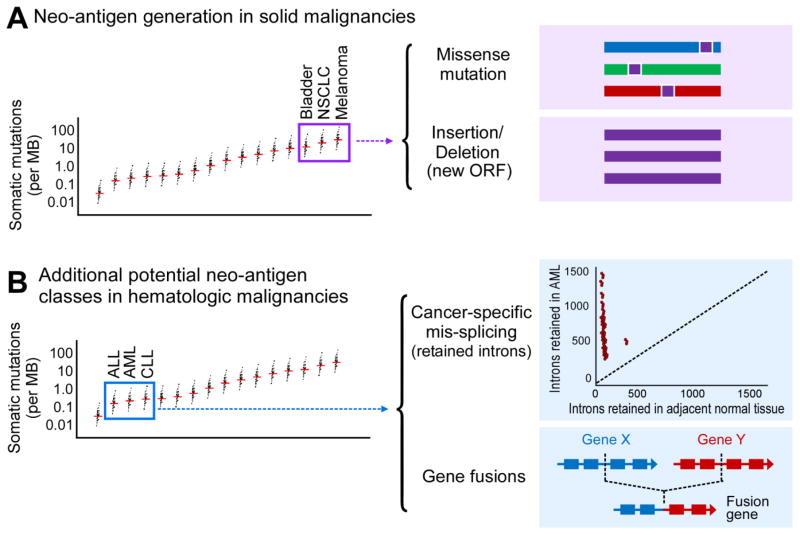
For carcinogen-driven solid malignancies, such as melanoma126–128, bladder cancer129,130, and non-small cell lung cancer131,132, where the somatic mutation loads (primarily from SNVs and indels) are high, more neoantigens have been predicted, and both spontaneous immunity and response to checkpoint blockade inhibition have been associated with increased neoantigen load, effective immune response (Figure 3A)133.
Figure 3. Potential sources of Neo-antigens in the Hematologic Malignancies.
(A) Solid malignancies responsive to immunotherapies tend to have a higher mutational load, with more missense mutations and insertions/deletions, leading to a high number of neo-antigens. (B) Hematologic malignancies tend to have a lower number of somatic mutations, yet are often still able to generate immune responses. Other possible mechanisms for generating neo-antigens in the setting of low somatic mutation burden are gene fusions and alterations in RNA splicing leading to retain introns. Graph of somatic mutation number adapted from ref. 134.
In contrast, the somatic mutation burden for most hematologic malignancies is relatively low134 and corresponding by lower numbers of neo-antigens have been predicted. Do alternative mechanisms for generating neo-antigens exist in hematologic malignancies (Figure 3B)? One possible source could be through the generation of neo-antigens through novel gene fusions. A canonical example is BCR-ABL, occurring in CML and some cases of ALL, which is known to be presented on certain HLA molecules and generate a T cell response135–137, previously tested as a target of therapeutic peptide vaccines138–140. Another possibility involves abnormal splicing, with retention of introns leading to the generation of neo-antigens141,142. Spliceosome mutations are relatively common in AML (and myelodysplastic syndrome). Dvinge and colleagues demonstrated that AML cells have a higher number of retained introns (and, presumably, neo-antigens) than adjacent normal tissue (Figure 3B, adapted from ref. 142). Therefore, even with a relatively low somatic mutation load, hematologic malignancies may employ alternate mechanisms for neo-antigen generation, and offer immunogenic tumor-specific targets for immune-based therapies.
Immunotherapies for Hematologic Malignancies
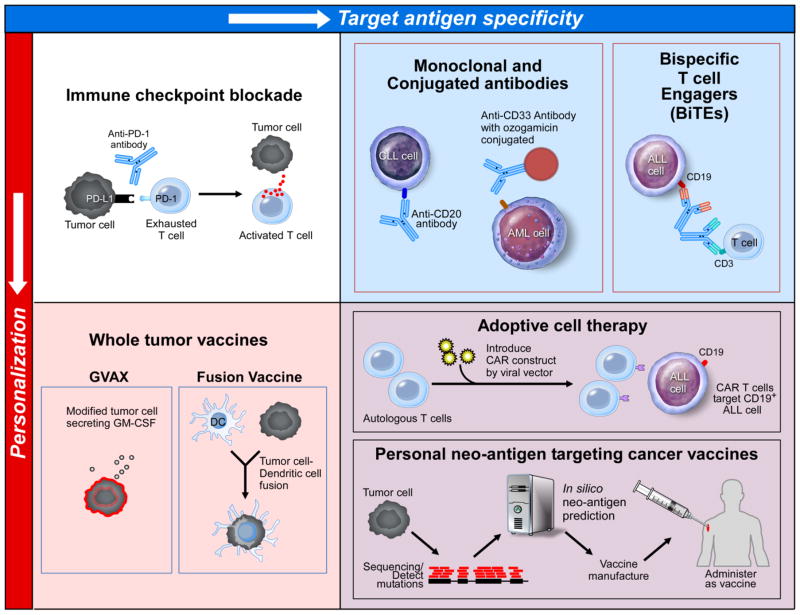
For hematologic malignancies, a number of therapeutic approaches are currently under investigation, that vary in the degree to which they specifically target an antigen (or antigens), and to which they are “personalized” for each patient’s individual tumor (Figure 4).
Figure 4. Immune based therapies in the Hematologic Malignancies vary in degree of antigen-targeting personalization.
Immunotherapeutic strategies for hematologic malignancies can be categorized based on whether they are personalized for an individual patient/tumor (bottoms row), and whether they target antigen(s) are known/specified (right column). In the top left panel, immune checkpoint blockade with an anti-PD-1 antibody is depicted. Typically, tumor cells may express a ligand, PD-L1, which binds PD-1 on T cells and ultimately inhibits T cell effector function. Anti-PD-1 antibodies inhibit this effect, leading to T cell activation and effective tumor cell killing. This strategy is not personalized for an individual patient, and the specific target tumor antigen is not known. In the bottom left panel, whole tumor vaccines are depicted, including lethally irradiated tumor cells engineered to secrete GM-CSF to attract APCs (GVAX, left image), and DC/tumor cell fusions, which also leads to antigen presentation and activation of the native immune system. These strategies require personalized products, but the target tumor antigen is not known. In the top right panel, a variety of monoclonal antibody therapies are depicted, including conventional monoclonal antibody therapy against CD20, antibody-drug conjugate targeted against CD33-expressing cells, and the bispecific T cell engages blinatumomab which transiently cross-links T cells with CD19 expressing ALL cells. These strategies target specific antigens, but are not personalized for the individual patient. In the top right panel, CD19-targeting CAR-T cell therapy (bottom) and neo-antigen therapeutic peptide vaccine (top) strategies are depicted. The therapeutic approaches require personalization for the individual patient/tumor, and are targeted against known tumor antigen(s).
Immune checkpoint blockade (CPB) represents a therapeutic strategy that potently enhance immunity in a non-antigen directed fashion nor personalized inhibits naturally occurring negative regulators of T cell activation and function, effectively “cutting the brakes” on T cells, leading to an antitumor response143–147. There have been several notable examples of success with this approach. In Hodgkin’s lymphoma, the PDL1 and PDL2 genes, located on chromosome 9p24.1, are frequently amplified, suggesting a possible susceptibility to PD-1 blockade. Impressively, in a cohort of patients with relapsed or refractory Hodgkin’s lymphoma (following autologous stem cell transplant and treatment with the antibody-drug conjugated brentuximab vedotin), treatment with the anti-PD-1 antibody nivolumab led to at least a partial response in the majority of patients148,149, leading to FDA approval for this indication in 2016. For other relapsed or refractory hematologic malignancies (non-Hodgkin’s lymphoma, acute leukemias, and myelodysplastic syndrome), early trial data for nivolumab and for the anti-CTLA4 antibody ipilimumab have shown responses in at least some patients150,151. On the other hand, there are notable failures of this approach, including in multiple myeloma152. Further studies are needed to understand why certain malignancies fail to respond to these therapies. Although CPB is not an antigen-directed approach per se, response to checkpoint blockade has been shown to amplify the T cell response to personal neoantigens153,154.
In a very different approach, exquisite antigen-specific targeting therapies directed at B and T cell responses have been devised for hematologic malignancies. Indeed, frequently used “off-the-shelf” antibody-based therapies targeting a specific tumor antigen were pioneered in the hematologic malignancies, with Levy and colleagues reporting in 1982 the case of a patient with a B cell lymphoma who had complete remission following administration of an anti-idiotype antibody56. Later, the anti-CD20 antibody rituximab became the first monoclonal antibody approved for cancer therapy, and remains a mainstay of treatment for many B cell lymphomas60,63,155,156. Rituximab functions primarily through activation of the complement cascade (i.e. complement-dependent cytotoxicity, or CDC) and by antibody-dependant cell-mediated cytotoxicity (ADCC). More recently approved anti-CD20 monoclonal antibodies, obinutuzumab and ofatumumab, have modified structures that increase programmed cell death (PCD) and ADCC, or increase CDC, respectively157. Other monoclonal antibodies targeting different surface antigens have been used a variety of hematologic malignancies, such as daratumumab (targeting CD38) in multiple myeloma158,159, among others.
The modification of traditional monoclonal antibodies has led to further therapeutic opportunities. One such alteration involves the conjugation of a cytotoxic agent, with the goal of targeted delivery of the cytotoxic molecule to the target cells. The first antibody-drug conjugate (ADC), gemtuzumab ozogamicin, which combined an anti-CD33 (targeting a surface antigen on AML cells) with the cytotoxic agent calicheamicin, appeared effective in inducing complete remission in AML160, but was later withdrawn from market over concerns about toxicity (specifically veno-occlusive disease)161. Currently, the anti-CD30 ADC brentuximab vedotin is available for relapsed CD30-positive lymphomas162, and more recently the anti-CD22 ADC inotuzumab ozogamicin has shown promising early results in relapsed ALL163. An alternative modification uses antibody engineering to combine the short peptide binding domains of two antibodies with different specificities, with the goal of bringing tumor cells into close proximity with T cells (termed bispecific T cell engagers, or BiTEs)164. Blinatumomab, which has specificity for both CD3 (found on T cells) and CD19 (found on ALL cells and some lymphomas), is the first FDA-approved BiTE, and has demonstrated efficacy in relapsed ALL165,166.
On the T cell side, the field of adoptive antigen-specific T cell therapies has evolved greatly. Whereas initial adoptive cell therapies involved the ex vivo expansion of tumor-infiltrating lymphocytes using interleukin-2 (and thus, did not necessarily have a known target antigen), more recent efforts have focused on genetically modifying autologous T cells to express a chimeric antigen receptor (CAR) with specificity for a tumor antigen (such as CD19 for ALL)167. Current generation CAR constructs link an antigen-specific, extracellular single-chain variable fragment (ectodomain), with the intracellular signaling component of the T cell receptor CD3ζ (endodomain) and at least one stimulation domain (such as CD28 or 4-1BB) 58,59. These constructs have shown great promise in the treatment of relapsed and refractory CLL168, ALL64,65, and multiple myeloma169. While this new therapy has generated considerable excitement, it has also had notable associated toxicity, and future generations of CAR constructs are being devised that attempt to balance efficacy and adverse effects170–172.
Cancer vaccines provide an opportunity to focus the immune response in an antigen-specific fashion. Whole tumor cell vaccines represent a therapy that is personalized (by using the patient’s individual tumor as a source of antigen), though the precise target antigen is typically not known. The aim of these therapies is to stimulate active immunity against tumor cells through presentation of tumor antigens by antigen presenting cells (APCs) and activation of the native immunity. In one therapeutic approach, autologous tumor cells are lethally irradiated and then either genetically engineered to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) or mixed with other GM-CSF secreting cells, and then re-administered to the patient, with the goal of recruiting APCs. This approach has been tested in AML and CLL, with some evidence of immunologic (and potentially clinical) responses173,174. An alternative whole tumor vaccine approaches involves the fusion of tumor cells with autologous dendritic cells (DCs). This DC/tumor cell fusion approach is under investigation for multiple myeloma175,176, and has already shown some promising results in AML177–179.
Neo-antigen directed therapeutic vaccines represent a truly personalized, antigen-specific therapeutic strategy. In this approach, targets of vaccination are identified on the basis of individual tumor-specific DNA sequence analysis of somatic mutations predicted to generate peptides that can bond to personal HLA molecules. As vaccines, this approach is anticipated to expand the breadth and repertoire of tumor-specific T cells that can participate in the anti-tumor immune response. This approach, while promising, is still in the early phases of investigation, with multiple clinical trials ongoing (see NCT00683670103,180, NCT01970358181, and NCT02035956182 in melanoma). Combination of this approach with CPB is expected to synergize together, and active testing of this strategy is in progress. Additional open questions include: How many neo-peptides must be administered to ensure an immune response? What immune adjuvant should be used? What is the optimal dosing and scheduling of these therapies? What toxicities will we observe, and will they be limiting? With multiple clinical trials ongoing, we will hopefully begin to answer some of these important questions, and be able to effectively direct a patient’s immune response to maximize therapeutic benefit and minimize adverse outcomes.
Acknowledgments
This work is support by the Blavatnik Family Foundation, the National Institutes of Health, National Cancer Institute grant 1RO1CA155010-02, and National Heart, Lung, and Blood Institute grant 5R01HL103532-03. C.J.W. is a Scholar of the Leukemia and Lymphoma Society.
Footnotes
CONFLICTS OF INTEREST: D.A.B. reports no relevant conflict of interest. C.J.W. is a founder of Neon Therapeutics and a member of its scientific advisory board.
References
- 1.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. [PubMed] [Google Scholar]
- 2.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5. [PubMed] [Google Scholar]
- 3.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–44. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 4.Bachireddy P, Hainz U, Rooney M, et al. Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123:1412–21. doi: 10.1182/blood-2013-08-523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Choi J, Zeng W, et al. Graft-versus-leukemia antigen CML66 elicits coordinated B-cell and T-cell immunity after donor lymphocyte infusion. Clin Cancer Res. 2010;16:2729–39. doi: 10.1158/1078-0432.CCR-10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 7.Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol. 2006;90:133–73. doi: 10.1016/S0065-2776(06)90004-2. [DOI] [PubMed] [Google Scholar]
- 8.Bachireddy P, Wu CJ. Understanding anti-leukemia responses to donor lymphocyte infusion. Oncoimmunology. 2014;3:e28187. doi: 10.4161/onci.28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkenburg JH, Wafelman AR, Joosten P, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999;94:1201–8. [PubMed] [Google Scholar]
- 10.Smit WM, Rijnbeek M, van Bergen CA, Fibbe WE, Willemze R, Falkenburg JH. T cells recognizing leukemic CD34(+) progenitor cells mediate the antileukemic effect of donor lymphocyte infusions for relapsed chronic myeloid leukemia after allogeneic stem cell transplantation. Proc Natl Acad Sci U S A. 1998;95:10152–7. doi: 10.1073/pnas.95.17.10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenq RR, van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10:213–21. doi: 10.1038/nrc2804. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–33. [PubMed] [Google Scholar]
- 14.Rezvani K, Barrett AJ. Characterizing and optimizing immune responses to leukaemia antigens after allogeneic stem cell transplantation. Best Pract Res Clin Haematol. 2008;21:437–53. doi: 10.1016/j.beha.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ofran Y, Ritz J. Targets of tumor immunity after allogeneic hematopoietic stem cell transplantation. Clin Cancer Res. 2008;14:4997–9. doi: 10.1158/1078-0432.CCR-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochi T, Fujiwara H, Suemori K, et al. Aurora-A kinase: a novel target of cellular immunotherapy for leukemia. Blood. 2009;113:66–74. doi: 10.1182/blood-2008-06-164889. [DOI] [PubMed] [Google Scholar]
- 17.Ochsenreither S, Majeti R, Schmitt T, et al. Cyclin-A1 represents a new immunogenic targetable antigen expressed in acute myeloid leukemia stem cells with characteristics of a cancer-testis antigen. Blood. 2012;119:5492–501. doi: 10.1182/blood-2011-07-365890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greiner J, Schmitt M, Li L, et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood. 2006;108:4109–17. doi: 10.1182/blood-2006-01-023127. [DOI] [PubMed] [Google Scholar]
- 19.Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. 2012;26:2186–96. doi: 10.1038/leu.2012.145. [DOI] [PubMed] [Google Scholar]
- 20.Berlin C, Kowalewski DJ, Schuster H, et al. Mapping the HLA ligandome landscape of acute myeloid leukemia: a targeted approach toward peptide-based immunotherapy. Leukemia. 2015;29:647–59. doi: 10.1038/leu.2014.233. [DOI] [PubMed] [Google Scholar]
- 21.Greiner J, Schmitt A, Giannopoulos K, et al. High-dose RHAMM-R3 peptide vaccination for patients with acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma. Haematologica. 2010;95:1191–7. doi: 10.3324/haematol.2009.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt M, Schmitt A, Rojewski MT, et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood. 2008;111:1357–65. doi: 10.1182/blood-2007-07-099366. [DOI] [PubMed] [Google Scholar]
- 23.Greiner J, Li L, Ringhoffer M, et al. Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood. 2005;106:938–45. doi: 10.1182/blood-2004-12-4787. [DOI] [PubMed] [Google Scholar]
- 24.Greiner J, Ringhoffer M, Taniguchi M, et al. Receptor for hyaluronan acid-mediated motility (RHAMM) is a new immunogenic leukemia-associated antigen in acute and chronic myeloid leukemia. Exp Hematol. 2002;30:1029–35. doi: 10.1016/s0301-472x(02)00874-3. [DOI] [PubMed] [Google Scholar]
- 25.Casalegno-Garduno R, Meier C, Schmitt A, et al. Immune responses to RHAMM in patients with acute myeloid leukemia after chemotherapy and allogeneic stem cell transplantation. Clin Dev Immunol. 2012;2012:146463. doi: 10.1155/2012/146463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greiner J, Ringhoffer M, Taniguchi M, et al. mRNA expression of leukemia-associated antigens in patients with acute myeloid leukemia for the development of specific immunotherapies. Int J Cancer. 2004;108:704–11. doi: 10.1002/ijc.11623. [DOI] [PubMed] [Google Scholar]
- 27.Oka Y, Tsuboi A, Fujiki F, et al. WT1 peptide vaccine as a paradigm for “cancer antigen-derived peptide”-based immunotherapy for malignancies: successful induction of anti-cancer effect by vaccination with a single kind of WT1 peptide. Anticancer Agents Med Chem. 2009;9:787–97. doi: 10.2174/187152009789056958. [DOI] [PubMed] [Google Scholar]
- 28.Brossart P, Schneider A, Dill P, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;61:6846–50. [PubMed] [Google Scholar]
- 29.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 30.Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15:201–15. doi: 10.1038/nrc3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber JM, Qin L, Kowalski J, et al. Characterization of chronic myeloid leukemia stem cells. Am J Hematol. 2011;86:31–7. doi: 10.1002/ajh.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang XF, Wu CJ, McLaughlin S, et al. CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 2001;98:7492–7. doi: 10.1073/pnas.131590998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang XF, Wu CJ, Chen L, et al. CML28 is a broadly immunogenic antigen, which is overexpressed in tumor cells. Cancer Res. 2002;62:5517–22. [PMC free article] [PubMed] [Google Scholar]
- 34.Bocchia M, Wentworth PA, Southwood S, et al. Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood. 1995;85:2680–4. [PubMed] [Google Scholar]
- 35.Bocchia M, Korontsvit T, Xu Q, et al. Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood. 1996;87:3587–92. [PubMed] [Google Scholar]
- 36.Yong AS, Stephens N, Weber G, et al. Improved outcome following allogeneic stem cell transplantation in chronic myeloid leukemia is associated with higher expression of BMI-1 and immune responses to BMI-1 protein. Leukemia. 2011;25:629–37. doi: 10.1038/leu.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams SP, Sahota SS, Mijovic A, et al. Frequent expression of HAGE in presentation chronic myeloid leukaemias. Leukemia. 2002;16:2238–42. doi: 10.1038/sj.leu.2402732. [DOI] [PubMed] [Google Scholar]
- 38.Greiner J, Ringhoffer M, Taniguchi M, et al. Characterization of several leukemia-associated antigens inducing humoral immune responses in acute and chronic myeloid leukemia. Int J Cancer. 2003;106:224–31. doi: 10.1002/ijc.11200. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt M, Li L, Giannopoulos K, et al. Chronic myeloid leukemia cells express tumor-associated antigens eliciting specific CD8+ T-cell responses and are lacking costimulatory molecules. Exp Hematol. 2006;34:1709–19. doi: 10.1016/j.exphem.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld C, Cheever MA, Gaiger A. WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia. 2003;17:1301–12. doi: 10.1038/sj.leu.2402988. [DOI] [PubMed] [Google Scholar]
- 41.Riley CL, Mathieu MG, Clark RE, McArdle SE, Rees RC. Tumour antigen-targeted immunotherapy for chronic myeloid leukaemia: is it still viable? Cancer Immunol Immunother. 2009;58:1489–99. doi: 10.1007/s00262-009-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding K, Wang XM, Fu R, Ruan EB, Liu H, Shao ZH. PRAME Gene Expression in Acute Leukemia and Its Clinical Significance. Cancer Biol Med. 2012;9:73–6. doi: 10.3969/j.issn.2095-3941.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber G, Caruana I, Rouce RH, et al. Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia--implications for immunotherapy. Clin Cancer Res. 2013;19:5079–91. doi: 10.1158/1078-0432.CCR-13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giannopoulos K, Li L, Bojarska-Junak A, et al. Expression of RHAMM/CD168 and other tumor-associated antigens in patients with B-cell chronic lymphocytic leukemia. Int J Oncol. 2006;29:95–103. [PubMed] [Google Scholar]
- 45.Mayr C, Bund D, Schlee M, et al. Fibromodulin as a novel tumor-associated antigen (TAA) in chronic lymphocytic leukemia (CLL), which allows expansion of specific CD8+ autologous T lymphocytes. Blood. 2005;105:1566–73. doi: 10.1182/blood-2004-04-1233. [DOI] [PubMed] [Google Scholar]
- 46.Proto-Siqueira R, Figueiredo-Pontes LL, Panepucci RA, et al. PRAME is a membrane and cytoplasmic protein aberrantly expressed in chronic lymphocytic leukemia and mantle cell lymphoma. Leuk Res. 2006;30:1333–9. doi: 10.1016/j.leukres.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 47.Tembhare PR, Marti G, Wiestner A, et al. Quantification of expression of antigens targeted by antibody-based therapy in chronic lymphocytic leukemia. Am J Clin Pathol. 2013;140:813–8. doi: 10.1309/AJCPYFQ4XMGJD6TI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walz S, Stickel JS, Kowalewski DJ, et al. The antigenic landscape of multiple myeloma: mass spectrometry (re)defines targets for T-cell-based immunotherapy. Blood. 2015;126:1203–13. doi: 10.1182/blood-2015-04-640532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rapoport AP, Aqui NA, Stadtmauer EA, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117:788–97. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hobo W, Strobbe L, Maas F, et al. Immunogenicity of dendritic cells pulsed with MAGE3, Survivin and B-cell maturation antigen mRNA for vaccination of multiple myeloma patients. Cancer Immunol Immunother. 2013;62:1381–92. doi: 10.1007/s00262-013-1438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian J, Xie J, Hong S, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110:1587–94. doi: 10.1182/blood-2007-03-082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–21. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevenson GT, Stevenson FK. Antibody to a molecularly-defined antigen confined to a tumour cell surface. Nature. 1975;254:714–6. doi: 10.1038/254714a0. [DOI] [PubMed] [Google Scholar]
- 54.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 55.Hatzubai A, Maloney DG, Levy R. The use of a monoclonal anti-idiotype antibody to study the biology of a human B cell lymphoma. J Immunol. 1981;126:2397–402. [PubMed] [Google Scholar]
- 56.Miller RA, Maloney DG, Warnke R, Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;306:517–22. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 57.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678–85. [PubMed] [Google Scholar]
- 58.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–20. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359:613–26. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 61.Zappasodi R, de Braud F, Di Nicola M. Lymphoma Immunotherapy: Current Status. Front Immunol. 2015;6:448. doi: 10.3389/fimmu.2015.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadler LM, Ritz J, Hardy R, Pesando JM, Schlossman SF, Stashenko P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest. 1981;67:134–40. doi: 10.1172/JCI110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–95. [PubMed] [Google Scholar]
- 64.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dave H, Anver MR, Butcher DO, et al. Restricted cell surface expression of receptor tyrosine kinase ROR1 in pediatric B-lineage acute lymphoblastic leukemia suggests targetability with therapeutic monoclonal antibodies. PLoS One. 2012;7:e52655. doi: 10.1371/journal.pone.0052655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger C, Sommermeyer D, Hudecek M, et al. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol Res. 2015;3:206–16. doi: 10.1158/2326-6066.CIR-14-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19:3153–64. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 70.Traversari C, van der Bruggen P, Luescher IF, et al. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992;176:1453–7. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brichard V, Van Pel A, Wolfel T, et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–95. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–9. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boel P, Wildmann C, Sensi ML, et al. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–75. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 74.Coulie PG, Lehmann F, Lethe B, et al. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci U S A. 1995;92:7976–80. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robbins PF, el-Gamil M, Kawakami Y, Stevens E, Yannelli JR, Rosenberg SA. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994;54:3124–6. [PubMed] [Google Scholar]
- 76.Wang RF, Robbins PF, Kawakami Y, Kang XQ, Rosenberg SA. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J Exp Med. 1995;181:799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosinski KV, Fujii N, Mito JK, et al. DDX3Y encodes a class I MHC-restricted H-Y antigen that is expressed in leukemic stem cells. Blood. 2008;111:4817–26. doi: 10.1182/blood-2007-06-096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Warren EH, Fujii N, Akatsuka Y, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–78. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brickner AG, Evans AM, Mito JK, et al. The PANE1 gene encodes a novel human minor histocompatibility antigen that is selectively expressed in B-lymphoid cells and B-CLL. Blood. 2006;107:3779–86. doi: 10.1182/blood-2005-08-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bleakley M, Otterud BE, Richardt JL, et al. Leukemia-associated minor histocompatibility antigen discovery using T-cell clones isolated by in vitro stimulation of naive CD8+ T cells. Blood. 2010;115:4923–33. doi: 10.1182/blood-2009-12-260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monach PA, Meredith SC, Siegel CT, Schreiber H. A unique tumor antigen produced by a single amino acid substitution. Immunity. 1995;2:45–59. doi: 10.1016/1074-7613(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 82.den Haan JM, Meadows LM, Wang W, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279:1054–7. doi: 10.1126/science.279.5353.1054. [DOI] [PubMed] [Google Scholar]
- 83.Cox AL, Skipper J, Chen Y, et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–9. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 84.Wolpert E, Franksson L, Karre K. Dominant and cryptic antigens in the MHC class I restricted T cell response across a complex minor histocompatibility barrier: analysis and mapping by elution of cellular peptides. Int Immunol. 1995;7:919–28. doi: 10.1093/intimm/7.6.919. [DOI] [PubMed] [Google Scholar]
- 85.Wolpert EZ, Grufman P, Sandberg JK, Tegnesjo A, Karre K. Immunodominance in the CTL response against minor histocompatibility antigens: interference between responding T cells, rather than with presentation of epitopes. J Immunol. 1998;161:4499–505. [PubMed] [Google Scholar]
- 86.van Bergen CA, Kester MG, Jedema I, et al. Multiple myeloma-reactive T cells recognize an activation-induced minor histocompatibility antigen encoded by the ATP-dependent interferon-responsive (ADIR) gene. Blood. 2007;109:4089–96. doi: 10.1182/blood-2006-08-043935. [DOI] [PubMed] [Google Scholar]
- 87.Wu CJ, Yang XF, McLaughlin S, et al. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J Clin Invest. 2000;106:705–14. doi: 10.1172/JCI10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sahin U, Tureci O, Schmitt H, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A. 1995;92:11810–3. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr Opin Immunol. 1997;9:709–16. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 90.Preuss KD, Zwick C, Bormann C, Neumann F, Pfreundschuh M. Analysis of the B-cell repertoire against antigens expressed by human neoplasms. Immunol Rev. 2002;188:43–50. doi: 10.1034/j.1600-065x.2002.18805.x. [DOI] [PubMed] [Google Scholar]
- 91.Greiner J, Ringhoffer M, Simikopinko O, et al. Simultaneous expression of different immunogenic antigens in acute myeloid leukemia. Exp Hematol. 2000;28:1413–22. doi: 10.1016/s0301-472x(00)00550-6. [DOI] [PubMed] [Google Scholar]
- 92.Eichmuller S, Usener D, Dummer R, Stein A, Thiel D, Schadendorf D. Serological detection of cutaneous T-cell lymphoma-associated antigens. Proc Natl Acad Sci U S A. 2001;98:629–34. doi: 10.1073/pnas.021386498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–8. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Rhee F, Szmania SM, Zhan F, et al. NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood. 2005;105:3939–44. doi: 10.1182/blood-2004-09-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biernacki MA, Tai YT, Zhang GL, et al. Novel myeloma-associated antigens revealed in the context of syngeneic hematopoietic stem cell transplantation. Blood. 2012;119:3142–50. doi: 10.1182/blood-2011-11-388926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Biernacki MA, Marina O, Zhang W, et al. Efficacious immune therapy in chronic myelogenous leukemia (CML) recognizes antigens that are expressed on CML progenitor cells. Cancer Res. 2010;70:906–15. doi: 10.1158/0008-5472.CAN-09-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marina O, Hainz U, Biernacki MA, et al. Serologic markers of effective tumor immunity against chronic lymphocytic leukemia include nonmutated B-cell antigens. Cancer Res. 2010;70:1344–55. doi: 10.1158/0008-5472.CAN-09-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawakami Y, Fujita T, Matsuzaki Y, et al. Identification of human tumor antigens and its implications for diagnosis and treatment of cancer. Cancer Sci. 2004;95:784–91. doi: 10.1111/j.1349-7006.2004.tb02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graziano DF, Finn OJ. Tumor antigens and tumor antigen discovery. Cancer Treat Res. 2005;123:89–111. doi: 10.1007/0-387-27545-2_4. [DOI] [PubMed] [Google Scholar]
- 100.Even-Desrumeaux K, Baty D, Chames P. State of the art in tumor antigen and biomarker discovery. Cancers (Basel) 2011;3:2554–96. doi: 10.3390/cancers3022554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daneshmanesh AH, Mikaelsson E, Jeddi-Tehrani M, et al. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int J Cancer. 2008;123:1190–5. doi: 10.1002/ijc.23587. [DOI] [PubMed] [Google Scholar]
- 102.Condomines M, Hose D, Reme T, et al. Gene expression profiling and real-time PCR analyses identify novel potential cancer-testis antigens in multiple myeloma. J Immunol. 2009;183:832–40. doi: 10.4049/jimmunol.0803298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hundal J, Carreno BM, Petti AA, et al. pVAC-Seq: A genome-guided in silico approach to identifying tumor neoantigens. Genome Med. 2016;8:11. doi: 10.1186/s13073-016-0264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barrett CL, DeBoever C, Jepsen K, Saenz CC, Carson DA, Frazer KA. Systematic transcriptome analysis reveals tumor-specific isoforms for ovarian cancer diagnosis and therapy. Proc Natl Acad Sci U S A. 2015;112:E3050–7. doi: 10.1073/pnas.1508057112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res. 2013;1:11–5. doi: 10.1158/2326-6066.CIR-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rajasagi M, Shukla SA, Fritsch EF, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453–62. doi: 10.1182/blood-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bassani-Sternberg M, Coukos G. Mass spectrometry-based antigen discovery for cancer immunotherapy. Curr Opin Immunol. 2016;41:9–17. doi: 10.1016/j.coi.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Clark RE, Dodi IA, Hill SC, et al. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 2001;98:2887–93. doi: 10.1182/blood.v98.10.2887. [DOI] [PubMed] [Google Scholar]
- 109.Bassani-Sternberg M, Braunlein E, Klar R, et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun. 2016;7:13404. doi: 10.1038/ncomms13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kowalewski DJ, Schuster H, Backert L, et al. HLA ligandome analysis identifies the underlying specificities of spontaneous antileukemia immune responses in chronic lymphocytic leukemia (CLL) Proc Natl Acad Sci U S A. 2015;112:E166–75. doi: 10.1073/pnas.1416389112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abelin JG, Trantham PD, Penny SA, et al. Complementary IMAC enrichment methods for HLA-associated phosphopeptide identification by mass spectrometry. Nat Protoc. 2015;10:1308–18. doi: 10.1038/nprot.2015.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cobbold M, De La Pena H, Norris A, et al. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci Transl Med. 2013;5:203ra125. doi: 10.1126/scitranslmed.3006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zilberberg J, Feinman R, Korngold R. Strategies for the identification of T cell-recognized tumor antigens in hematological malignancies for improved graft-versus-tumor responses after allogeneic blood and marrow transplantation. Biol Blood Marrow Transplant. 2015;21:1000–7. doi: 10.1016/j.bbmt.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vigneron N, Stroobant V, Van den Eynde BJ, van der Bruggen P. Database of T cell-defined human tumor antigens: the 2013 update. Cancer Immun. 2013;13:15. [PMC free article] [PubMed] [Google Scholar]
- 115.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–93. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 116.Molldrem JJ, Clave E, Jiang YZ, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90:2529–34. [PubMed] [Google Scholar]
- 117.Clave E, Molldrem J, Hensel N, Raptis A, Barrett AJ. Donor-recipient polymorphism of the proteinase 3 gene: a potential target for T-cell alloresponses to myeloid leukemia. J Immunother. 1999;22:1–6. doi: 10.1097/00002371-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 118.Gao L, Bellantuono I, Elsasser A, et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–203. [PubMed] [Google Scholar]
- 119.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125:3413–21. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–9. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shukla SA, Rooney MS, Rajasagi M, et al. Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nat Biotechnol. 2015;33:1152–8. doi: 10.1038/nbt.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fritsch EF, Hacohen N, Wu CJ. Personal neoantigen cancer vaccines: The momentum builds. Oncoimmunology. 2014;3:e29311. doi: 10.4161/onci.29311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 124.Zhang GL, Ansari HR, Bradley P, et al. Machine learning competition in immunology - Prediction of HLA class I binding peptides. J Immunol Methods. 2011;374:1–4. doi: 10.1016/j.jim.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 125.Lundegaard C, Lund O, Nielsen M. Prediction of epitopes using neural network based methods. J Immunol Methods. 2011;374:26–34. doi: 10.1016/j.jim.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 131.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Braun DA, Burke KP, Van Allen EM. Genomic Approaches to Understanding Response and Resistance to Immunotherapy. Clin Cancer Res. 2016;22:5642–50. doi: 10.1158/1078-0432.CCR-16-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yasukawa M, Ohminami H, Kojima K, et al. HLA class II-restricted antigen presentation of endogenous bcr-abl fusion protein by chronic myelogenous leukemia-derived dendritic cells to CD4(+) T lymphocytes. Blood. 2001;98:1498–505. doi: 10.1182/blood.v98.5.1498. [DOI] [PubMed] [Google Scholar]
- 136.Yasukawa M, Ohminami H, Kaneko S, et al. CD4(+) cytotoxic T-cell clones specific for bcr-abl b3a2 fusion peptide augment colony formation by chronic myelogenous leukemia cells in a b3a2-specific and HLA-DR-restricted manner. Blood. 1998;92:3355–61. [PubMed] [Google Scholar]
- 137.Bosch GJ, Joosten AM, Kessler JH, Melief CJ, Leeksma OC. Recognition of BCR-ABL positive leukemic blasts by human CD4+ T cells elicited by primary in vitro immunization with a BCR-ABL breakpoint peptide. Blood. 1996;88:3522–7. [PubMed] [Google Scholar]
- 138.Cathcart K, Pinilla-Ibarz J, Korontsvit T, et al. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood. 2004;103:1037–42. doi: 10.1182/blood-2003-03-0954. [DOI] [PubMed] [Google Scholar]
- 139.Pinilla-Ibarz J, Cathcart K, Korontsvit T, et al. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood. 2000;95:1781–7. [PubMed] [Google Scholar]
- 140.Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, Roberts W, Scheinberg DA. Synthetic peptide analogs derived from bcr/abl fusion proteins and the induction of heteroclitic human T-cell responses. Haematologica. 2005;90:1324–32. [PubMed] [Google Scholar]
- 141.Dvinge H, Kim E, Abdel-Wahab O, Bradley RK. RNA splicing factors as oncoproteins and tumour suppressors. Nat Rev Cancer. 2016;16:413–30. doi: 10.1038/nrc.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dvinge H, Bradley RK. Widespread intron retention diversifies most cancer transcriptomes. Genome Med. 2015;7:45. doi: 10.1186/s13073-015-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 144.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517–9. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 145.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pardoll D. Releasing the brakes on antitumor immune response. Science. 1996;271:1691. doi: 10.1126/science.271.5256.1691. [DOI] [PubMed] [Google Scholar]
- 147.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 148.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–94. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol. 2016;34:2698–704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med. 2016;375:143–53. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suen H, Brown R, Yang S, Ho PJ, Gibson J, Joshua D. The failure of immune checkpoint blockade in multiple myeloma with PD-1 inhibitors in a phase 1 study. Leukemia. 2015;29:1621–2. doi: 10.1038/leu.2015.104. [DOI] [PubMed] [Google Scholar]
- 153.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–42. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–32. [PubMed] [Google Scholar]
- 156.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 157.Suresh T, Lee LX, Joshi J, Barta SK. New antibody approaches to lymphoma therapy. J Hematol Oncol. 2014;7:58. doi: 10.1186/s13045-014-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015;373:1207–19. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 159.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375:754–66. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 160.Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–54. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 161.McKoy JM, Angelotta C, Bennett CL, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res. 2007;31:599–604. doi: 10.1016/j.leukres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 162.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 163.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375:740–53. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nelson MH, Paulos CM. Novel immunotherapies for hematologic malignancies. Immunol Rev. 2015;263:90–105. doi: 10.1111/imr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 166.Topp MS, Gokbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–7. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- 167.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Garfall AL, Maus MV, Hwang WT, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373:1040–7. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13:370–83. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johnson LA, June CH. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2017;27:38–58. doi: 10.1038/cr.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ho VT, Vanneman M, Kim H, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci U S A. 2009;106:15825–30. doi: 10.1073/pnas.0908358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Burkhardt UE, Hainz U, Stevenson K, et al. Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J Clin Invest. 2013;123:3756–65. doi: 10.1172/JCI69098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rosenblatt J, Vasir B, Uhl L, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood. 2011;117:393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rosenblatt J, Avivi I, Vasir B, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19:3640–8. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stone RM, Uhl L, Neuberg DS, et al. Clinical Trial Evaluating DC/AML Fusion Cell Vaccination In AML Patients. Blood. 2013;122:3928. [Google Scholar]
- 178.Rosenblatt J, Stone RM, Uhl L, et al. DC/Aml Fusion Cell Vaccination Administered to AML Patients Who Achieve a Complete Remission Potently Expands Leukemia Reactive T Cells and Is Associated with Durable Remissions. Blood. 2015;126:2549. [Google Scholar]
- 179.Rosenblatt J, Stone RM, Uhl L, et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci Transl Med. 2016;8:368ra171. doi: 10.1126/scitranslmed.aag1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Carreno BM, Magrini V, Becker-Hapak M, et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fritsch EF, Rajasagi M, Ott PA, Brusic V, Hacohen N, Wu CJ. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol Res. 2014;2:522–9. doi: 10.1158/2326-6066.CIR-13-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sahin U. Abstract IA06: Targeting the mutanome for individualized cancer immunotherapy. Cancer Immunology Research. 2016;4:IA06-IA. [Google Scholar]