Abstract
Research into therapeutic transcranial magnetic stimulation (TMS) for major depression has dramatically increased in the last decade. Understanding the mechanism of action of TMS is crucial to improve efficacy and develop the next generation of therapeutic stimulation. Early imaging research provided initial data supportive of widely held assumptions about hypothesized inhibitory or excitatory consequences of stimulation. Early work also indicated that while TMS modulated brain activity under the stimulation site, effects at deeper regions, and in particular the subgenual anterior cingulate cortex, were associated with clinical improvement. Concordant with earlier findings, functional connectivity studies also demonstrated that clinical improvements were related to changes distal, rather than proximal, to the site of stimulation. Moreover, recent work suggests that TMS modulates and potentially normalizes functional relationships between neural networks. An important observation that emerged from this review is that similar patterns of connectivity changes are observed across studies regardless of TMS parameters. Though promising, we stress that these imaging findings must be evaluated cautiously given the widespread reliance on modest sample sizes and little implementation of statistical validation. Additional limitations include use of imaging before and after a course of TMS, which provides little insight into changes that might occur during the weeks of stimulation. Furthermore, as studies to date have focused on depression, it is unclear whether observations are related to mechanisms of action of TMS for depression, or represent broader patterns of functional brain changes associated with clinical improvement.
Keywords: repetitive transcranial magnetic stimulation, major depressive disorder, functional magnetic resonance imaging, resting state functional connectivity, theta burst stimulation, mechanisms of action, default mode network
Introduction
Research into non-invasive brain stimulation is one of the fastest growing areas of psychiatric inquiry. Of these, repetitive transcranial magnetic stimulation (rTMS, hereafter simply TMS) is an important and relatively new treatment. TMS has been clinically available since 2008 when it was cleared by the U.S. Food and Drug Administration for pharmacoresistant major depressive disorder (MDD). TMS uses a pulsed magnetic field to induce neuronal depolarization in a targeted brain region. Since the initial multisite studies (1, 2), a number of groups have published on the efficacy of TMS in naturalistic samples (3), durability of effect (4), and efficacy across the lifespan (5, 6, 7). Furthermore, there is emerging literature supporting the use of TMS for other psychiatric conditions including schizophrenia (8, 9) and posttraumatic stress disorder (PTSD)(10, 11), and outside of psychiatry in areas such as tinnitus, migraine and pain syndromes (e.g., (12, 13, 14)).
While the putative therapeutic mechanism of action of TMS remains unknown, recent neuroimaging studies have set out to discover what is changing in the brain when a depressed patient receives multiple daily sessions of TMS delivered to the prefrontal cortex. This area of research is necessarily complex, requiring an interdisciplinary approach inclusive of expertise from neuroimaging, clinical research, engineering, etc. Given the myriad of approaches to data collection, processing, and analysis involved in human neuroimaging studies, and potential effects of the analytical decision-making process on study observations, a strong grounding in the fundamentals of neuroimaging methods and statistics is needed to appreciate the strength (or lack thereof) of evidence within the TMS/neuroimaging mechanisms literature.
In this review we synthesize findings from the key functional and resting state connectivity studies to identify potential mechanisms of action of TMS for MDD (see supplemental information for search details). To maintain a focus on clinically relevant mechanisms, all studies described below used therapeutic TMS. We considered performing a meta-analysis of these studies, but after reviewing the available literature we concluded that the vast heterogeneity of variables, including treatment parameters (stimulation site, number of sessions, etc.), imaging modalities (metabolic, resting state) and imaging analytic approaches (region of interest versus whole brain analyses), precluded the use of meta-analytic methods. To constrain the breadth of the review, we do not describe studies designed to test or manipulate neurotransmitter levels related to TMS. We acknowledge several studies reported TMS might be associated with increased dopamine release (reported in (15, 16), although not observed in (17, 18) and changes in gamma-aminobutryic acid (GABA) levels (e.g., (19, 20)). Our review also does not include diffusion or morphometry research, although there is a nascent literature suggesting TMS can impact these domains (e.g., (21, 22)).
The review begins with an overview of TMS to provide the reader context for the applications used in imaging studies. We then describe neuroimaging observations using metabolic approaches, followed by the more recent resting state functional connectivity studies. The review ends with an integrative summary of the current data, highlights important design limitations and conceptual assumptions, and suggests directions for future research.
TMS Overview
TMS for MDD starts with motor threshold (MT) determination, which calibrates the stimulator to an individual’s cortical excitability. During MT determination, a clinician delivers single pulse TMS to the motor cortex, and records the amount of stimulator output necessary to induce movement in the contralateral hand in 50% of delivered pulses. Following calibration, a course of TMS is delivered to the prefrontal cortex at 120% of MT on a daily basis for up to 30 (or more) sessions, often followed by a taper phase (for a review of clinical TMS see (23, 24)).
TMS parameters may vary, including stimulation intensity, location, frequency, and duration. These parameters have shifted over time, generally favoring higher stimulation intensity (i.e., increase from 100% (25) to 120% of MT (1), informed by research demonstrating that increased stimulation intensity was required to overcome coil-to-cortex variability associated with age and other factors (26)). Protocols have also evolved to incorporate more TMS sessions (i.e., increase from 10 (25) to >20 (1)), or multiple sessions given in a single day (termed accelerated TMS (e.g., (27))). Regarding location, in earlier studies TMS was delivered to the dorsolateral prefrontal cortex (DLPFC), usually the left DLPFC. This target was initially determined using a so-called “5cm rule,” where the TMS coil was moved 5cm anteriorly along the parasaggital line from the motor cortex. Follow up studies showed the 5cm rule could miss the DLPFC (28). Alternative targeting approaches utilize skull-based landmarks (29) or MRI-based neuronavigation (i.e., using MRI images co-localized with the TMS coil to enable placement over the DLPFC; e.g., (30)), and some evidence indicates that landmark-based techniques and neuronavigation approximate the same location (31). In recent years, various groups demonstrated the efficacy of different stimulation targets, including the dorsomedial prefrontal cortex (DMPFC) (32) and broader prefrontal cortex (33). When considering frequency, TMS pulses are typically considered to be either “high” (≥5Hz) or “low” (≤1Hz), where these frequencies are considered excitatory and inhibitory, respectively. These designations arose from corticospinal excitability studies measuring the size of motor evoked potentials following TMS to motor cortex (reviewed in (34)). This relationship was corroborated by metabolic neuroimaging (positron emission tomography (PET) or single-photon computed emission tomography (SPECT)) that suggested low frequency TMS reduced motor cortex activity, and higher frequency stimulation increased activity (35, 36, 37). Comparable results were observed in the DLPFC using near infrared spectroscopy (38). This apparently bimodal relationship between frequency and activity likely represents an oversimplification of stimulation-related brain changes, as connectivity studies in healthy controls suggest a more complicated relationship (39).
Resting State SPECT/PET Studies of TMS
SPECT/PET imaging studies are listed in Tables 1 & 2. TMS to the DLPFC was initially conceptualized as a way to reverse hypofrontality observed in depression (40, 41) and post-stroke depression (42). In the first TMS imaging study, Teneback et al. (43)(N=22) measured changes in regional cerebral blood flow (rCBF) in MDD patients scanned before and after two weeks of 20Hz or 5Hz TMS to the left DLPFC. They reported that increased inferior frontal lobe activity predicted subsequent TMS clinical response, and that active stimulation was associated with increased blood flow in the prefrontal cortex and limbic/paralimbic regions. Observed changes occurred under the TMS coil and distal to the stimulation site. Speer et al. (44)(N=10) then measured TMS-related rCBF changes before and after 10 sessions of 20Hz or 1Hz TMS to the left DLPFC. 20Hz TMS was associated with increased rCBF under the coil and in the amygdala, insula, hippocampus, parahippocampus, thalamus and cerebellum. 1Hz TMS was associated with distal reductions in rCBF in the right prefrontal cortex, left medial temporal cortex and amygdala. Changes in rCBF correlated with mood changes, and individuals whose mood improved with one frequency worsened with the other. Nahas et al. (45)(N=23; participants shared with (43)) delivered 5 sessions of 20Hz or 5Hz TMS to the DLPFC. They found higher frequency TMS caused greater prefrontal rCBF, relative to lower frequency stimulation, and that significant rCBF increases were observed both under the TMS coil and in distal regions. They also reported greater coil-to-cortex distance was associated with reduced brain activation, confirming observations by Kozel et al. (26). Loo et al. (46)(N=18) found similar results scanning during 15Hz and 1Hz TMS to the left DLPFC, with effects generally observed distal from the site of stimulation.
Table 1.
SPECT/PET TMS Studies: Experimental Design
| Study | N per Group | Neuroimaging Methods (Imaging Interval) | Participants | Study Design | TMS Parameters | ||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Frequency | Intensity (%MT) | Total Sessions a | Total Pulses a | |||||
| Teneback et al., 1999 (43) | Active 13 Sham 9 Total 22 |
SPECT (Pre/Post) | MDD (n=14), Bipolar (n=8) | Sham-controlled (parallel) | L DLPFC | 5Hz or 20Hz | 100 | 10 | 16000 |
| Speer et al., 2000 (44) | Total 10 | PET (Pre/Post) b | TRD (n=8), Bipolar (n=2) | Sham-controlled (crossover) | L DLPFC | 1Hz and 20Hz | 100 | 20 | 16000 per frequency |
| Nahas et al., 2001 (45) | 5Hz 5 20Hz 9 Sham 9 Total 23 |
SPECT (Pre/During 5th session) | MDD (n=16), Bipolar (n=7) | Sham-controlled (crossover) | L DLPFC | 5Hz and 20Hzc | 100 | 10 | 16000 |
| Loo et al., 2003 (46) | Sham/1Hz 9 Sham/15Hz 9 Total 18 |
SPECT (During Sham/Active) d | MDD (n=12), melancholic subtype (n=4); Bipolar (n=2) | Sham-controlled (mixed) | L DLPFC | 1Hz or 15Hz | 90 | 1 | 1Hz: 360 15Hz: 675 |
| Kito et al., 2008 (47) | Total 12 | SPECT (Pre/Post) | TRD | Unblinded | R DLPFC | 1Hz | 100 | 12 | 3600 |
| Baeken et al., 2009 (50) | Total 21 | PET (Pre/Post) | TRD, melancholic subtype | Unblinded | L DLPFC | 10Hz | 110 | 10 | 15600 |
| Kito et al., 2011 (48) | Total 26 | SPECT (Pre/Post) | TRD | Unblinded | R DLPFC | 1Hz | 100 | 12 | 3600 |
| Kito et al., 2012 (49) | Total 26 | SPECT (Pre) | TRD | Unblinded | R DLPFC | 1Hz | 100 | 12 | 3600 |
| Baeken et al., 2015 (51) | Active/sham 9 Sham/active 6 Total 15 |
PET (Pre/Post) b | TRD, melancholic subtype | Sham-controlled (crossover) | L DLPFC | 20Hz | 110 | 20 d | 31200 |
Key: SPECT, single-photon emission computed tomography; PET, positron emission tomography; TMS, transcranial magnetic stimulation; MDD, major depressive disorder; MT, motor threshold; TRD, treatment-resistant depression; L, left; R, right; DLPFC, dorsolateral prefrontal cortex.
Upper limit of sessions/pulses
Patients completed 2 post-TMS scans, one at the completion of each treatment arm
Participants switched to 10Hz at minute 18
First SPECT scan took place on day one during sham TMS, 2nd scan took place on day two during active TMS. Scans occurred on consecutive days, 24 hours apart.
Five TMS sessions per day (separated by 15–20 minutes)
Table 2.
SPECT/PET TMS Studies: Regions of Interest and Results
| Study | Region(s) of Interest | Pretreatment Predictor(s) of Improvement | Post-treatment TMS Effect(s) |
|---|---|---|---|
| Teneback et al., 1999 (43) | DLPFC, cingulate, caudate, anterior temporal poles, inferior frontal cortex, OFC, medial temporal cortex | Higher frontal lobe activity | Increased frontal lobe activity in responders vs. non-responders Reduced correlation between depression severity and limbic/prefrontal activity |
| Speer et al., 2000 (44) | Whole brain | - | Only 20Hz TMS increased global blood flow 20Hz: Increased prefrontal cortex (L>R), cingulate gyrus (L>R), L amygdala, bilateral insula, basal ganglia, uncus, hippocampus, parahippocampus, thalamus, cerebellum 1Hz: Reduced rCBF in R prefrontal cortex, L basal ganglia, L amygdala No correlation between clinical improvement and rCBF change |
| Nahas et al., 2001 (45) | L prefrontal cortex (stimulation site), paralimbic cortex | - | Increased rCBF at stimulation site and R medial frontal lobe Decreased ACC and anterior temporal activity 20 Hz with increased activity directly beneath TMS coil Blood flow under the TMS coil declined with increasing distance |
| Loo et al., 2003 (46) | Frontal cortex, then DLPFC, ACC, basal ganglia, thalamus. | - |
15Hz: increased rCBF in inferior frontal cortex (L>R), R DMPFC, R posterior cingulate/parahippocampus; reduced rCBF to R OFC, uncus, R subcallosal gyrus 1Hz: increased rCBF in dACC, L insula, L somatosensory, precuneus, L cerebellum, R insula |
| Kito et al., 2008 (47) | Whole brain | Higher rCBF in L prefrontal, OFC, sgACC, anterior insula, parahippocampus and amygdala, inferior parietal regions | Bilateral prefrontal (including DLPFC), OFC, anterior insula, R sgACC, L parietal cortex |
| Baeken et al., 2009 (50) | DLPFC, dorsal and ventral ACC | Higher activity in DLPFC and ACC | Clinical response correlated with increased ACC activity |
| Kito et al., 2011 (48) | Whole brain | - | Decreased rCBF in prefrontal cortex, OFC, sgACC, basal ganglia, insula. Efficacy correlated with reduced rCBF in R prefrontal cortex, R putamen, R anterior insula. |
| Kito et al., 2012 (49) | DLPFC, VMPFC | Higher VMPFC rCBF | - |
| Baeken et al., 2015 (51) | sgACC (as a predictor), whole-brain (follow-up analysis) | Higher sgACC activity | Clinical efficacy associated with reduction in sgACC activity |
Key: SPECT, single-photon emission computed tomography; PET, positron emission tomography; TMS, transcranial magnetic stimulation; L, left; R, right; DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; rCBF, regional cerebral blood flow; ACC, anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; DMPFC, dorsomedial prefrontal cortex; VMPFC, ventromedial prefrontal cortex
Several SPECT/PET studies described predictors of response to TMS alongside effects of stimulation, potentially identifying requisite neural circuits for clinical improvement. Kito et al. (47, 48, 49)(total N=26) found that treatment response to 1Hz TMS was predicted by increased rCBF to the ventromedial prefrontal cortex (VMPFC). Efficacy was associated with reduced rCBF in the prefrontal cortex (including under the TMS coil), orbitofrontal cortex, subcallosal cingulate, putamen and anterior insula. Baeken et al. (50)(N=21) delivered 10 sessions of 10Hz TMS to the left DLPFC, and found that higher baseline activity in the DLPFC and anterior cingulate predicted superior clinical outcomes. Efficacy was associated with increased post-treatment activity in the anterior cingulate, bordering on the subgenual anterior cingulate cortex (sgACC). Recently, Baeken et al. (51)(N=15) delivered 20 sessions of sham-controlled accelerated TMS. They found that higher baseline sgACC activity predicted superior clinical outcomes, and clinical response was associated with reduced sgACC activity.
In summary, metabolic imaging studies found generally consistent effects of TMS. Higher frequency stimulation was associated with increased brain activity, and therapeutic efficacy was associated with changes in brain regions associated with emotion processing or mood regulation. Observed changes often occurred under the coil (DLPFC), in regions with direct anatomical connections (e.g., orbitofrontal cortex, ventromedial prefrontal cortex, basal ganglia), and regions with polysynaptic relationships to the DLPFC (e.g., sgACC and posterior cingulate cortex (52); reviewed in (53)). The sgACC has been implicated in a number of these studies, and most observed reduced sgACC activity following stimulation. The principle limitations of these studies are those associated with early TMS use, including lower stimulation intensity, fewer sessions, and modest sample sizes.
Resting State Functional Connectivity and Neural Networks in TMS
Resting state functional connectivity has been favored by recent studies examining TMS from a neural network perspective. The brain is organized into functional networks (54, 55), and capacity for dynamic network change in response to changing demands (environmental or cognitive) is a hallmark of healthy brain function (e.g., (55)). Network relationships are disrupted in MDD; patients consistently exhibit some degree of default mode network (DMN) dysfunction, typically hyperconnectivity (56), alongside disruptions in the frontoparietal executive control network (ECN) and the attention/limbic or salience network (SN). Taking the DMN as an example, pathological connectivity observed at the group level is typically ascribed to rumination since DMN regions are implicated in introspection in healthy controls (57). It is hypothesized that pathological sgACC activity in depression induces broader DMN dysfunction (57). While hypotheses at the population level may not extend to all patients with MDD, this model-driven approach provides a conceptual framework to examine the interplay between clinical phenotypes and imaging observations.
Two key studies by Fox et al. (58, 59) implicated sgACC-to-DLPFC connectivity in the mechanism of action of TMS, and laid the foundation for future network-related investigations. These studies built upon reports linking MDD treatment response (see Table 1 in (58)) to reduced sgACC hyperactivity (60, 61). In the first study, connectivity relationships between different DLPFC TMS targets (extracted from TMS efficacy studies) and sgACC were evaluated in data from 98 healthy controls and 13 MDD patients (58). Superior clinical outcomes were associated with targets exhibiting the greatest DLPFC-to-sgACC negative connectivity (described as “anticorrelation”). The importance of this result was underscored by their next report (59)(n=98 healthy controls used in (58); n=42 new healthy controls scanned 68±54 days apart; and n=2 MDD patients scanned before and after a course of TMS), where individual differences in DLPFC-to-sgACC connectivity were large and reproducible across imaging sessions. These papers suggested that remote suppression of the sgACC via DLPFC stimulation may be an antidepressant mechanism of TMS, and that connectivity could be utilized to optimize TMS therapy at the individual level.
Prospective Resting State Connectivity TMS Studies
TMS to the DLPFC
Resting state functional connectivity studies are described in Tables 3 & 4. Of these, most delivered TMS to the left DLPFC. Baeken et al. (62)(N=20) acquired resting state images before and after accelerated TMS to evaluate sgACC functional connectivity, though only a subset of imaging data was available (n=12; five responders and seven non-responders). At baseline, future TMS responders displayed greater negative connectivity between the sgACC and superior medial prefrontal cortex, including portions of the DMPFC. After TMS, responders demonstrated reduced negative connectivity between the sgACC and medial prefrontal cortex (MPFC). No sgACC changes were observed in non-responders.
Table 3.
Resting State TMS Studies: Experimental Design
| Study | N per Group | Neuroimaging Methods (Imaging Interval) | Participants | Study Design | TMS Parameters | ||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Frequency | Intensity (%MT) | Total Sessions a | Total Pulses a | |||||
| Baeken et al., 2014 (62) | Active/sham 10 Sham/active 10 Total 20 |
RSFC (Pre/Post)b | TRD | Sham-controlled (crossover) | L DLPFC | 20Hz | 110 | 20c | 31200 |
| Liston et al., 2014 (63) | Total 17 | RSFC (Pre/Post) | TRD (n=14), Bipolar II (n=3) | Unblinded | L DLPFC | 10Hz | 120 d | 25 | 75000 |
| Downar et al., 2014 (72) | Total 47 | RSFC (Pre) | TRD (n=38), Bipolar I (n=2), Bipolar II (n=7) | Unblinded | DMPFC | 10Hz | 120 | 30 | 90000 |
| Salomons et al., 2014 (74) | Total 25 h | RSFC (Pre/Post) | TRD (n=22), Bipolar I (n=1), Bipolar II (n=3) | Unblinded | DMPFC | 10Hz | 120 | 30 | 90000 |
| Kang et al., 2016 (68) | Active 13 Sham 11 Total 24 |
RSFC (Pre/Post) | TRD | Sham-controlled (parallel) | L DLPFC | 10Hz | 110 | 10 | 10000 |
| Baeken et al., 2017 (65) | Active/sham 21 Sham/active 23 Total 44 |
RSFC (Pre/Post)b | TRD | Sham-controlled (crossover) | L DLPFC | aiTBS | 110 | 20c | 32400 |
| Ge et al., 2017 (70) | 10Hz 9 iTBS 9 Total 18 |
RSFC (Pre) | TRD | Active-controlled (parallel) | L DLPFC | 10Hz or iTBS | 120 | 30 | 10Hz: 90000 iTBS: 18000 |
| Avissar et al., 2017 (71) | Total 27 | RSFC (Pre/Post) | TRD (n=24), Bipolar II (n=3) | Unblinded | L DLPFC | 10Hz | 120 e | 25 | 75000 |
| Philip et al., 2017 (67) | Total 33 h | RSFC (Pre/Post) | Comorbid PTSD/TRD | Unblinded | L DLPFC | 5Hz | 120 | 40 | 165000 |
| Taylor et al i | Active 16 Sham 16 Total 32 |
RSFC (Pre/Post) | TRD | Sham-controlled (parallel) | L DLPFC | 10Hz | 120 | 25 | 75000 |
Key: TMS, transcranial magnetic stimulation; RSFC, resting state functional connectivity; MT, motor threshold; TRD, treatment-resistant depression; L, left; DLPFC, dorsolateral prefrontal cortex; MDE, major depressive episode; MDD, major depressive disorder; iTBS, intermittent theta-burst stimulation; aiTBS, accelerated iTBS; PTSD, posttraumatic stress disorder; DMPFC, dorsomedial prefrontal cortex
Upper limit of sessions/pulses
Patients completed 2 post-TMS scans, one at the completion of each treatment arm
Five TMS sessions per day (separated by 15–20 minutes)
Protocol for 120% of MT, average 86.5%, range 50–109
Protocol for 120% of MT, range 80–120 with 2 patients outside range (unreported MT)
n=25 with pre/post imaging
personal communication
Table 4.
Resting State TMS Studies: Regions of Interest and Results
| Study | Seeds/Region(s) of Interest | Pretreatment Predictor(s) of Improvement | Posttreatment TMS Effect(s) |
|---|---|---|---|
| Baeken et al., 2014 (62) | sgACC | Greater negative connectivity between sgACC and superior medial prefrontal cortex/DMPFC | Reduced negative connectivity between sgACC-to-MPFC No sgACC changes observed in non-responders |
| Liston et al., 2014 (63) | sgACC DLPFC |
Greater sgACC to-DMN and -ECN connectivity | Reduced sgACC-to-DMN connectivity Reduced DLPFC-to-DMN (MPFC) connectivity |
| Downar et al., 2014 (72) | Graph analysis; 516 atlas-based nodes | Greater reward circuit (ventral tegmental area, striatum, VMPFC), connectivity | - |
| Salomons et al., 2014 (74) | DMPFC (midcingulate) sgACC |
Midcingulate: positive connectivity with sgACC, negative connectivity to thalamus, hippocampus, amygdala sgACC: Higher sgACC-to-DLPFC connectivity; lower sgACC-to-insula, putamen and parahippocampus/amygdala connectivity |
Midcingulate: Reduced connectivity to insula and parahippocampus/amygdala sgACC: reduced midcingulate to ventral striatum connectivity; best responders had increased negative connectivity between midcingulate and sgACC |
| Kang et al., 2016 (68) | Bilateral DLPFC | Reduced DLFPC-to-caudate connectivity | Reduced DLFPC-to-caudate connectivity |
| Baeken et al., 2017 (65) | sgACC | Greater sgACC-to-OFC connectivity | Reduced connectivity with middle frontal gyrus and motor cortex, and increased with VMPFC |
| Ge et al., 2017 (70) | ICA-derived resting state networks | Stronger connectivity between DMN and SN regions | - |
| Avissar et al., 2017 (71) | Frontostriatal connectivity | Higher DLPFC to striatum connectivity | - |
| Philip et al., 2017 (67) a | sgACC, DLPFC, hippocampus, and amygdala; MVPA | Higher amygdala-to-MPFC connectivity MVPA: dorsal ACC, insula, inferior parietal lobule |
Reduced sgACC to DMN connectivity; reduced hippocampus to insula connectivity MVPA: insula, dorsal ACC |
| Taylor et al b | Affective network (sgACC, amygdala) DMN (PCC) SN (dACC) ECN (DLPFC) |
Higher connectivity between the posterior cingulate and insula predicted non-response | Reduced sgACC connectivity in all responders (sham and active), not observed in non-responders |
Key: TMS, transcranial magnetic stimulation; sgACC, subgenual anterior cingulate cortex; DMPFC, dorsomedial prefrontal cortex; MPFC, medial prefrontal cortex; DLPFC, dorsolateral prefrontal cortex (left DLPFC unless specified otherwise); DMN, default mode network; ECN, executive control network; VMPFC, ventromedial prefrontal cortex; OFC, orbitofrontal cortex; SN, salience network; MVPA, multivoxel pattern activation; dACC, dorsal anterior cingulate cortex.
results shown as related to MDD
personal communication
Liston et al. (63)(N=17 patients; 35 controls) delivered 10Hz TMS to the left DLPFC and imaged patients before and after TMS. Seeds were based on (58) and networks were small volume-corrected using the Shirer atlas (64). Compared to controls at baseline, MDD patients exhibited greater within-DMN connectivity (sgACC-to-DMN), reduced ECN connectivity (DLPFC-to-ECN), and disrupted between-network connectivity (reduced DLPFC-to-DMN connectivity and increased sgACC-to-ECN connectivity). These results were broadly consistent with other MDD imaging research (e.g., (56)). After MDD patients received TMS, sgACC-to-DMN connectivity was attenuated; sgACC-to-ECN connectivity changes were not observed. TMS also reduced connectivity between the DLPFC and the MPFC/VMPFC. From these results, the authors posited that TMS acts by reducing sgACC-to-DMN connectivity and inducing negative connectivity between the DLPFC and DMN. They also reported greater baseline sgACC connectivity predicted superior clinical outcomes, consistent with literature reviewed above.
Several important points arose from the study by Liston et al. (36). First, TMS selectively reduced pathological DMN connectivity and reduced ECN-to-DMN connectivity, without significant changes within the ECN. Another important observation was that “excitatory” 10Hz TMS was associated with reduced connectivity, emphasizing that simple assumptions regarding frequency and directionality of downstream effects may not be appropriate for connectivity studies. Importantly, no connectivity changes were associated with clinical improvement, which complicates the interpretation of their findings.
Baeken et al. (65)(N=44 patients, 44 controls) imaged before and after accelerated intermittent theta burst stimulation (aiTBS) as part of a larger inconclusive efficacy study (66). Compared to controls at baseline, patients had greater sgACC connectivity with the DLPFC and precuneus (consistent with (56)). Greater baseline connectivity between the sgACC and orbitofrontal cortex predicted clinical response. After TMS, sgACC connectivity with the middle frontal gyrus and motor cortex was reduced, and increased with the VMPFC. They did not observe TMS-induction of negative connectivity (i.e., increased anticorrelations) between the sgACC and prefrontal regions (e.g., (58, 63)).
We recently imaged participants with comorbid MDD+PTSD before and after 5Hz TMS (67)(N=33). Using a combination of seed-based and data-driven analyses followed by leave-one-out cross validation, we found baseline SN connectivity predicted subsequent clinical response, and MDD symptom reduction was associated with reduced sgACC-to-DMN connectivity. Interestingly, PTSD symptom improvement was associated with TMS-induced negative connectivity between the sgACC and DLPFC, similar to mechanisms proposed for TMS in MDD (58).
Several pilot or unpublished studies are relevant to potential mechanisms of TMS. Kang et al. (68)(N=24) delivered low-dose TMS and reported reduced DLFPC-to-caudate connectivity after treatment, using jackknife procedures (69) to validate their findings. Post-hoc analysis indicated DLPFC-to-caudate connectivity predicted improvement. Ge et al. (70)(N=20 patients, 21 controls) examined biomarkers of response, where patients received iTBS or 10Hz TMS. Responders (regardless of stimulation type) demonstrated stronger baseline DMN-to-SN connectivity. Avissar et al. (71)(N=27 patients, 27 controls; including a subset from (63)) reported higher left DLPFC to striatum connectivity predicted TMS response. Taylor et al. (personal communication)(N=32) delivered 10Hz TMS to the left DLPFC; while they observed no significant differences between active and sham TMS, post-treatment sgACC connectivity was reduced in all responders (i.e., sham and active) but not non-responders. Higher baseline connectivity between the posterior cingulate and insula predicted non-response.
TMS to the DMPFC
Downar et al. (72)(N=47) delivered 10Hz stimulation to the DMPFC, with treatment target based on prior work (32, 73). Non-responders demonstrated lower connectivity in reward pathways (ventral tegmental area, striatum, VMPFC), suggesting intact reward circuit function was necessary for response. Salomons et al. (74) (N=25, a subset of participants from (72)) delivered TMS to the DMPFC and compared connectivity before and after treatment. They seeded the DMPFC (composed of surface and midcingulate seeds from (75)) and a post-hoc defined sgACC; only midcingulate connectivity led to subsequent significant results. Several connectivity patterns predicted clinical response, including positive midcingulate-to-sgACC/MPFC connectivity, and negative midcingulate-to-thalamus, -hippocampus and -amygdala connectivity. Higher sgACC-to-DLPFC connectivity, and lower sgACC-to-insula, -putamen and -parahippocampus/amygdala connectivity also predicted clinical response. When evaluating TMS effects, they observed reduced DMPFC connectivity with the insula and parahippocampus/amygdala. When testing the sgACC seed, TMS reduced connectivity between the DMPFC and ventral striatum, and participants with the best clinical response developed more negative connectivity between the DMPFC and sgACC. These DMPFC studies use a less common treatment target but nevertheless reiterate the central role of the sgACC in mechanisms of action of TMS, and similar to DLPFC studies, they highlight how changes in regions and networks distal from the stimulation site are associated with clinical improvement.
New Approaches to Understanding Mechanisms of Action: Computational Psychiatry
Recently, novel computational approaches brought new insights into TMS mechanisms of action. In a recent study Drysdale et al. (76) used hierarchical clustering and machine learning to describe “biotypes” of depression using previously published neuroimaging data from multiple sites (N=1,188). While this was not formally a TMS treatment study, several findings are relevant to TMS mechanisms. Each of the 4 depression biotypes demonstrated distinct functional connectivity and clinical symptomatology profiles (see Figure 2d & 3c in (76)). Within this study, a subsample (n=124) had imaging prior to TMS to the DMPFC (other relevant findings described in (72) and (74)). A biotype characterized clinically by anhedonia, relatively severe anxiety, early insomnia, and middle insomnia (“biotype 4”) characterized patients who had the most robust clinical response to stimulation. Patients stimulated at other TMS targets were not included, so it is unknown whether this biotype is specifically responsive to DMPFC stimulation. Importantly, the biotypes defined by this analysis were stable over time (n=50, MRI scans 4–6 weeks apart), indicating that imaging changes observed after a course of TMS could be attributable to treatment and not biotype fluctuation. The authors also tested whether biotypes transcended categorical diagnostic boundaries by analyzing a cohort of patients with generalized anxiety disorder (GAD) (n=39) without comorbid MDD. Over two thirds of GAD patients were classified as belonging to a biotype, with nearly 60% assigned to the TMS-responsive biotype. This suggests functional circuits in mechanisms of TMS response might be independent of categorical diagnosis, and has implications for the nascent field of TMS for anxiety disorders (e.g., (77)). Whether conceptually similar results could be obtained when looking at more diagnostically heterogeneous groups (e.g., depressed patients with schizophrenia, substance use disorders, etc.) remains an important question.
Discussion and Future Directions
In the last ten years, research into mechanisms of TMS has rapidly expanded. The most commonly observation is that TMS applied near the cortical surface induces changes distal from the stimulation site and involves multiple neural networks. Interestingly, a similar thread emerges from studies using different stimulation sites, intensities, and frequencies: TMS is consistently associated with changes to the DMN (46, 62, 63, 67, 74) and induces some degree of change in multinetwork relationships. This unexpected consistency is poorly understood, though the common use of resting state scanning – designed to elicit the DMN – is a likely contributor. Distinguishing specific effects of TMS from non-specific changes related to symptom improvement will require more sham-controlled imaging studies. Despite direct stimulation of the ECN, the absence of significant ECN change across studies is also noteworthy. While statistical power considerations can lead to negative imaging results, it is also possible that local changes in metabolic demand induced by TMS complicate BOLD-based assessment of ECN connectivity. Regardless, the most parsimonious explanation for these convergent results is that changes near the cortical surface are less important for therapeutic response than those at distal locations and networks, inclusive of the subgenual cingulate.
Many important questions remain, and to this end, it is important to consider and challenge prior assumptions. Much emphasis has been placed on TMS stimulation site (DLPFC, DMPFC) and approach (high- versus low-frequency stimulation; repetitive, accelerated, and theta burst TMS)(Figure 1), yet there are strikingly few direct comparisons of these methods. Based on the general convergence of findings, it is possible that TMS parameters such as target site, intensity, and frequency either do not matter or are not relevant at the group level; without prospective data it will be impossible examine this issue. Assumptions about the nature and direction of connectivity effects from TMS, particularly as a function of pulse frequency, merit further testing. Summary findings from the metabolic imaging literature comparing higher and lower-frequency stimulation suggest opposing effects, whereas in connectivity studies higher frequency TMS is generally associated with reduced connectivity, an observation supported by a follow up analysis of participants (51) exhibiting increased GABA signal after 20Hz TMS (20).
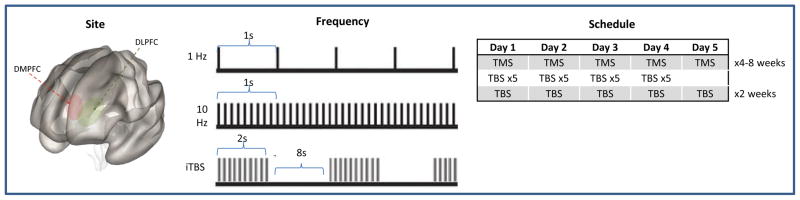
Figure 1. Clinical Variables in TMS Neuroimaging Research.
Several important variables in TMS neuroimaging research include site of stimulation, stimulation frequency and treatment schedule. Each of these may impact imaging findings, the use of differing approaches complicates interpretation of the current literature.
Abbreviations: DMPFC, dorsomedial prefrontal cortex; DLFPC, dorsolateral prefrontal cortex; TMS, transcranial magnetic stimulation; TBS, theta burst stimulation
Like many emerging fields, sample sizes are modest (mean n=~24), which is likely related to the financial resources required for imaging and TMS. It is important to note that effects of limited power are more substantial when contrasting subgroups (i.e., responders versus non-responders). Beyond increased type II error, using small sample sizes in conjunction with stringent alpha correction negatively impacts predictive value and reproducibility (78, 79). Synthesis of this collective work is also limited by the heterogeneity of connectivity methods (Supplemental Table S1). For example, global signal regression, a preprocessing approach that complicates the interpretation of connectivity results (80, 81), has occasionally been utilized. Stringent motion correction methods (82, 83, 84) have often not been implemented, and many studies used multiple comparisons correction procedures recently associated with false positive inflation (85, 86). Only two reports utilized statistical cross validation (67, 68), a procedure that should be adopted to enhance the rigor of future TMS imaging studies.
Inconsistencies in reporting direction(s) of connectivity effects also complicate interpretation of this area. The use of “anticorrelation” can be confusing and it is unclear whether different groups utilize the term consistently. Furthermore, “positive connectivity” observed after treatment could reflect either an increase in Z scores, a reduction in positive Z scores that remains greater than zero, or a change from negative to positive Z scores. Greater negative connectivity could also be described as an increased correlation. Additionally, when papers describe directionality of effects it is not always clear whether they refer to raw BOLD timeseries relationships or whether covariates were incorporated.
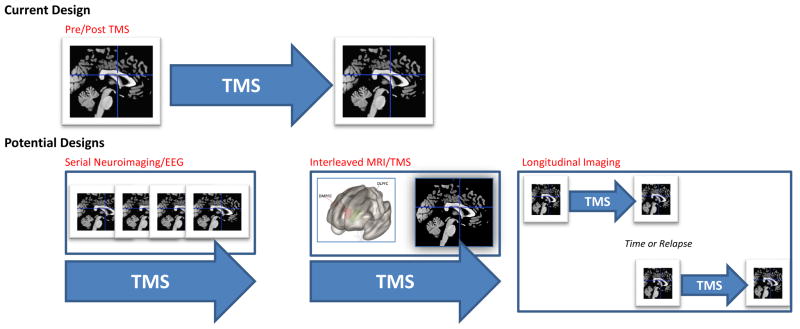
Review of this literature highlights a number of recommendations for future work. One important consideration is selection of study designs that will generate data to fill important gaps in the current knowledge base. The majority of studies reviewed above imaged participants before and after TMS. This design provides information regarding overall changes, yet sheds little light on changes occurring over the course of stimulation (Figure 2). We have little information about how treatment-related changes in connectivity, neuronal activity, or regional metabolism might shift over time, or about the durability of such changes over the longitudinal course of MDD in remission, upon relapse, or in the context of persistent chronic depression. Serial imaging, interleaved MRI/TMS (e.g., (87)) or ambulatory (e.g., EEG) assessment methods capturing data at different treatment intervals in conjunction with behavioral data will be essential for elucidating answers to these questions. Also, because the field has relied heavily on resting state designs that target the DMN, future investigations should also include task-based imaging. For example, one research study suggested brain activation during a planning task might predict TMS response (88). Integration of multimodal neuroimaging measures to assess TMS-related structural changes is also an important and relatively understudied area. Preliminary evidence of increased fractional anisotropy after TMS has been observed (21), and exploratory morphometry studies observed small increases in DMN and salience regional volume after TMS (22), and pointed to baseline hippocampal volume as a possible predictor of TMS response (89).
Figure 2. Current and Future Approaches to TMS Neuroimaging.
The most common approach in TMS neuroimaging research is to scan participants prior to and following TMS procedures. While this approach captures change over time, it does not provide information on what happens during the stimulation itself. Future designs may include a) serial neuroimaging, where multiple scans or other imaging modalities are performed at multiple timepoints during a course of TMS, b) causal assessments of neural network function using interleaved MRI/TMS, and c) testing the durability of neuroimaging findings across the course of depressive illness (e.g., over time or in the context of clinical relapse, etc.).
Abbreviations: TMS, transcranial magnetic stimulation; EEG, electroencephalography; MRI, magnetic resonance imaging
As noninvasive neuromodulation expands to new disease indications and additional imaging data becomes available, it is critical to advance our understanding of the specificity of effects. It is possible the findings summarized above are not unique to MDD and instead represent broader epiphenomena of neural networks moving from a state of disease to a state of wellness. It is also possible we have incorrectly interpreted imaging findings as putative mechanisms of TMS antidepressant action simply because most TMS research to date has examined effects in depressed samples. Testing this hypothesis requires prospective comparisons in different disease groups and across various treatment conditions, e.g., TMS versus, medications, psychotherapy, or other neuromodulatory interventions (e.g., (90)).
In summary, this review reflects a body of work in its infancy, but one that has already revealed important findings on which to rationally build the next steps of research. Ongoing and future methodological innovations hold tremendous potential for shedding important new insights into the putative mechanisms of TMS and advancing our understanding of neural mechanisms of disease more broadly.
Supplementary Material
Table S1. Data Analyses in Prospective Resting State Imaging Studies of TMS
Acknowledgments
This work was supported in part by a Career Development Award (IK2 CX000724) from the U.S. Department of Veterans Affairs (Clinical Sciences Research and Development), and the Center for Neurorestoration and Neurotechnology at the Providence VA Medical Center (Rehabilitation Research and Development). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
FINANCIAL DISCLOSURES
Drs. Philip and Carpenter have received grant support from Neuronetics, Neosync and Janssen through clinical trial contracts, and Dr. Carpenter is a consultant for Magstim. Dr. Philip has been an unpaid scientific advisory board member for Neuronetics. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Reardon J, Solvason H, Janicak P, Sampson S, Isenberg K, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 2.George M, Lisanby S, Avery D, McDonald W, Durkalski V, Pavlvicova M, et al. Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch Gen Psychiatry. 2010;67(5):507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter LL, Janicak P, Aaronson S, Boyadjis T, Brock D, Cook I, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587–596. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- 4.Philip NS, Dunner DL, Dowd SM, Aaronson ST, Brock DG, Carpenter LL, et al. Can Medication Free, Treatment-Resistant, Depressed Patients Who Initially Respond to TMS Be Maintained Off Medications? A Prospective, 12-Month Multisite Randomized Pilot Study. Brain Stimul. 2016;9(2):251–257. doi: 10.1016/j.brs.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Sabesan P, Lankappa S, Khalifa N, Krishnan V, Gandhi R, Palaniyappan L. Transcranial magnetic stimulation for geriatric depression: Promises and pitfalls. World J Psychiatry. 2015;5(2):170–181. doi: 10.5498/wjp.v5.i2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wall CA, Croarkin PE, Maroney-Smith MJ, Haugen LM, Baruth JM, Frye MA, et al. Magnetic Resonance Imaging-Guided, Open-Label, High-Frequency Repetitive Transcranial Magnetic Stimulation for Adolescents with Major Depressive Disorder. J Child Adolesc Psychopharmacol. 2016;26(7):582–589. doi: 10.1089/cap.2015.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conelea CA, Philip NA, Yip AG, Barnes JL, Niedzwiecki MJ, Greenberg BD, et al. Transcranial magnetic stimulation for treatment-resistant depression: Naturalistic treatment outcomes for younger versus older patients. J Affect Disord. 2017;217:42–47. doi: 10.1016/j.jad.2017.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasan A, Guse B, Coredes J, Wölwer W, Winterer G, Gaebel W, et al. Cognitive Effects of High-Frequency rTMS in Schizophrenia Patients With Predominant Negative Symptoms: Results From a Multicenter Randomized Sham-Controlled Trial. Schizophr Bull. 2016;42(3):608–618. doi: 10.1093/schbul/sbv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paillère-Martinot ML, Galinowski A, Plaze M, Andoh J, Bartrés-Faz D, Bellivier F, et al. Active and placebo transcranial magnetic stimulation effects on external and internal auditory hallucinations of schizophrenia. Acta Psychiatr Scand. 2017;135(3):228–238. doi: 10.1111/acps.12680. [DOI] [PubMed] [Google Scholar]
- 10.Watts BV, Landon B, Grott A, Young-Xu Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012;5(1):38–43. doi: 10.1016/j.brs.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Philip NS, Ridout SJ, Albright SE, Sanchez G, Carpenter LL. 5-Hz Transcranial Magnetic Stimulation for Comorbid Posttraumatic Stress Disorder and Major Depression. J Trauma Stress. 2016;29(1):93–96. doi: 10.1002/jts.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noh TS, Kyong JS, Chang MY, Park MK, Lee JH, Oh SH, et al. Comparison of Treatment Outcomes Following Either Prefrontal Cortical-only or Dual-site Repetitive Transcranial Magnetic Stimulation in Chronic Tinnitus Patients: A Double-blind Randomized Study. Otol Neurotol. 2017;38(2):296–303. doi: 10.1097/MAO.0000000000001266. [DOI] [PubMed] [Google Scholar]
- 13.Kalita J, Laskar S, Bhoi SK, Misra UK. Efficacy of single versus three sessions of high rate repetitive transcranial magnetic stimulation in chronic migraine and tension-type headache. J Neurol. 2016;263(11):2238–2246. doi: 10.1007/s00415-016-8257-2. [DOI] [PubMed] [Google Scholar]
- 14.Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST, George MS. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain. 2011;152(11):2477–2484. doi: 10.1016/j.pain.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pogarell O, Koch W, Pöpperl G, Tatsch K, Jokob F, Zwanzger P, et al. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: Preliminary results of a dynamic [123I] IBZM SPECT study. J Psychiatr Res. 2006;40(4):307–314. doi: 10.1016/j.jpsychires.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Pogarell O, Koch W, Pöpperl G, Tatsch K, Jokob F, Mulert C, et al. Acute prefrontal rTMS increases striatal dopamine to a similar degree as D-amphetamine. Psychiatry Res. 2007;156(3):251–255. doi: 10.1016/j.pscychresns.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda Y, Motohashi N, Ito H, Ito S, Takano A, Nishikawa T, Suhara T. Effects of repetitive transcranial magnetic stimulation on [11C]raclopride binding and cognitive function in patients with depression. J Affect Disord. 2006;95(1–3):35–42. doi: 10.1016/j.jad.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda Y, Motohashi N, Ito H, Ito S, Takano A, Takahashi H, et al. Chronic repetitive transcranial magnetic stimulation failed to change dopamine synthesis rate: Preliminary L-[β-11C]DOPA positron emission tomography study in patients with depression. Psychiatry Clin Neurosci. 2010;64(6):659–662. doi: 10.1111/j.1440-1819.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 19.Dubin MJ, Mao X, Banerjee S, Goodman Z, Lapidus KA, Kang G, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci. 2016;41(3):E37–E45. doi: 10.1503/jpn.150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baeken C, Lefaucheur JP, Van Schuerbeek P. The impact of accelerated high frequency rTMS on brain neurochemicals in treatment-resistant depression: Insights from 1H MR spectroscopy. Clin Neurophysiol. 2017;128(9):1664–1672. doi: 10.1016/j.clinph.2017.06.243. [DOI] [PubMed] [Google Scholar]
- 21.Kozel FA, Johnson KA, Nahas Z, Nakonezny PA, Morgan PS, Anderson BS, et al. Fractional anisotropy changes after several weeks of daily left high-frequency repetitive transcranial magnetic stimulation of the prefrontal cortex to treat major depression. J ECT. 2011;27(1):5–10. doi: 10.1097/YCT.0b013e3181e6317d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M. Transcranial Magnetic Stimulation of Left Dorsolateral Prefrontal Cortex Induces Brain Morphological Changes in Regions Associated with a Treatment Resistant Major Depressive Episode: An Exploratory Analysis. Brain Stimul. 2016;9(4):577–583. doi: 10.1016/j.brs.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016;9(3):336–346. doi: 10.1016/j.brs.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J Clin Psychiatry. 2017 doi: 10.4088/JCP.16cs10905. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li XB, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48(10):962–970. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- 26.Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12(3):376–384. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- 27.Holtzheimer PE, 3rd, McDonald WM, Mufti M, Kelley ME, Quinn S, Corso G, Epstein CM. Accelerated repetitive transcranial magnetic stimulation for treatment-resistant depression. Depress Anxiety. 2010;27(10):960–963. doi: 10.1002/da.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009;66(5):509–515. doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 29.Beam W, Borckardt J, Reeves S, George M. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2(1):50–54. doi: 10.1016/j.brs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahdab R, Avache SS, Brugières P, Goujon C, Lefaucheur JP. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin. 2010;40(1):27–36. doi: 10.1016/j.neucli.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, et al. Concordance Between BeamF3 and MRI-neuronavigated Target Sites for Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex. Brain Stimul. 2015;8(5):965–973. doi: 10.1016/j.brs.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Downar J, Daskalakis ZJ. New targets for rTMS in depression: A review of convergent evidence. Brain Stimul. 2013;6(3):231–240. doi: 10.1016/j.brs.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Levkovitz Y, Harel EV, Roth Y, Braw Y, Most D, Katz LN, et al. Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul. 2009;2(4):188–200. doi: 10.1016/j.brs.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148(1):1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 35.Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, et al. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54(4):956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- 36.Siebner H, Peller M, Bartenstein P, Willoch F, Rossmeier C, Schwaiger M, Conrad B. Activation of frontal premotor areas during suprathreshold transcranial magnetic stimulation of the left primary sensorimotor cortex: a glucose metabolic PET study. Hum Brain Mapp. 2001;12(3):157–167. doi: 10.1002/1097-0193(200103)12:3<157::AID-HBM1012>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siebner HR, Takano B, Peinemann A, Schwaiger M, Conrad B, Drzezga A. Continuous transcranial magnetic stimulation during positron emission tomography: a suitable tool for imaging regional excitability of the human cortex. Neuroimage. 2001;14(4):883–890. doi: 10.1006/nimg.2001.0889. [DOI] [PubMed] [Google Scholar]
- 38.Cao TT, Thomson RH, Bailey NW, Rogasch NC, Segrave RA, Maller JJ, et al. A near infra-red study of blood oxygenation changes resulting from high and low frequency repetitive transcranial magnetic stimulation. Brain Stimul. 2013;6(6):922–924. doi: 10.1016/j.brs.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA. 2011;108(52):21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchsbaum MS, Wu J, DeLisi LE, Holcomb H, Kessler R, Johnson J, et al. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. J Affect Disord. 1986;10(2):137–152. doi: 10.1016/0165-0327(86)90036-4. [DOI] [PubMed] [Google Scholar]
- 41.Martinot J, Hardy P, Feline A, Huret J, Mazoyer B, Attar-Levy D, et al. Left Prefrontal Glucose Hypometabolism in the Depressed State: A Confirmation. Am J Psychiatry. 1990;147(10):1313–1317. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- 42.Starkstein SE, Robinson RG. Affective disorders and cerebral vascular disease. Br J Psychiatry. 1989;154:170–182. doi: 10.1192/bjp.154.2.170. [DOI] [PubMed] [Google Scholar]
- 43.Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings LE, Spicer KM, et al. Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci. 1999;11(4):426–435. doi: 10.1176/jnp.11.4.426. [DOI] [PubMed] [Google Scholar]
- 44.Speer AM, Kimbrell TA, Wassermann EM, Repella DJ, Willis MW, Herscovitch P, Post RM. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000;48(12):1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- 45.Nahas Z, Teneback CC, Kozel A, Speer AM, DeBrux C, Molloy M, et al. Brain effects of TMS delivered over prefrontal cortex in depressed adults: role of stimulation frequency and coil-cortex distance. J Neuropsychiatry Clin Neurosci. 2001;13(4):459–470. doi: 10.1176/jnp.13.4.459. [DOI] [PubMed] [Google Scholar]
- 46.Loo CK, Sachdev PS, Haindl W, Wen W, Mitchell PB, Croker VB, Malhi GS. High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psychol Med. 2003;33(6):997–1006. doi: 10.1017/s0033291703007955. [DOI] [PubMed] [Google Scholar]
- 47.Kito S, Fujita K, Koga Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology. 2008;58(1):29–36. doi: 10.1159/000154477. [DOI] [PubMed] [Google Scholar]
- 48.Kito S, Hasegawa T, Koga Y. Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Clin Neurosci. 2011;65(2):175–182. doi: 10.1111/j.1440-1819.2010.02183.x. [DOI] [PubMed] [Google Scholar]
- 49.Kito S, Hasegawa T, Koga Y. Cerebral blood flow in the ventromedial prefrontal cortex correlates with treatment response to low-frequency right prefrontal repetitive transcranial magnetic stimulation in the treatment of depression. Psychiatry Clin Neurosci. 2012;66(2):136–145. doi: 10.1111/j.1440-1819.2011.02312.x. [DOI] [PubMed] [Google Scholar]
- 50.Baeken C, De Raedt R, Van Hove C, Clerinx P, De Mey J, Bossuyt A. HF-rTMS treatment in medication-resistant melancholic depression: results from 18FDG-PET brain imaging. CNS Spectr. 2009;14(8):439–448. doi: 10.1017/s1092852900020411. [DOI] [PubMed] [Google Scholar]
- 51.Baeken C, Marinazzo D, Everaert H, Wu G, Van Hove C, Audenaert K, et al. The Impact of Accelerated HF-rTMS on the Subgenual Anterior Cingulate Cortex in Refractory Unipolar Major Depression: Insights From 18FDG PET Brain Imaging. Brain Stimul. 2015;8(4):808–815. doi: 10.1016/j.brs.2015.01.415. [DOI] [PubMed] [Google Scholar]
- 52.Klein JC, Rushworth MF, Behrens TE, Mackay CE, de Crespigny AJ, D’Arceuil H, Johansen-Berg H. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage. 2010;51(2):555–564. doi: 10.1016/j.neuroimage.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219(1):2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 54.Fox MD, Snyder AZ, Vincnet JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Academy Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–36. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 56.Cooney RE, Joormann J, Eugéne F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cog Affect Behave Neurosci. 2010;10(4):470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72(7):595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:150–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baeken C, Marinazzo D, Wu GR, Van Schuerbeek P, De Mey J, Marchetti I, et al. Accelerated HF-rTMS in treatment-resistant unipolar depression: Insights from subgenual anterior cingulate functional connectivity. World J Biol Psychiatry. 2014;15(4):286–297. doi: 10.3109/15622975.2013.872295. [DOI] [PubMed] [Google Scholar]
- 63.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leutcher B, et al. Default mode network mechanisms of transcranial magnetic stimulation. Biol Psychiatry. 2014;76(7):517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. Cereb Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baeken C, Duprat R, Wu G, De Raedt R, van Heeringen K. Subgenual Anterior Cingulate–Medial Orbitofrontal Functional Connectivity in Medication-Resistant Major Depression: A Neurobiological Marker for Accelerated Intermittent Theta Burst Stimulation Treatment? Biol Psychiatry: CNNI. 2017 doi: 10.1016/j.bpsc.2017.01.001. In Press. [DOI] [PubMed] [Google Scholar]
- 66.Duprat R, Desmyter S, Rudi D, van Heeringen K, Van den Abbeele D, Tandt H, et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: A fast road to remission? J Affect Disord. 2016;200:6–14. doi: 10.1016/j.jad.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 67.Philip NS, Barredo J, v’ant Wout M, Almeida J, Tyrka A, Price L, Carpenter LL. Network Mechanisms of Clinical Response to Transcranial Magnetic Stimulation in Posttraumatic Stress and Major Depressive Disorders. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.07.021. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang JI, Lee H, Jhung K, Kim KR, An SK, Yoon KJ, et al. Frontostriatal Connectivity Changes in Major Depressive Disorder After Repetitive Transcranial Magnetic Stimulation: A Randomized Sham-Controlled Study. J Clin Psychiatry. 2016;77(9):e1137–e1143. doi: 10.4088/JCP.15m10110. [DOI] [PubMed] [Google Scholar]
- 69.Tukey JW. Bias and confidence in not quite large enough samples. Ann Math Statist. 1958;29(2):614–623. [Google Scholar]
- 70.Ge R, Blumberger DM, Downar J, Daskalakis ZJ, Dipinto AA, Tham JCW, et al. Abnormal functional connectivity within resting-state networks is related to rTMS-based therapy effects of treatment resistant depression: a pilot study. J Affect Discord. 2017;218:75–81. doi: 10.1016/j.jad.2017.04.060. [DOI] [PubMed] [Google Scholar]
- 71.Avissar M, Powell F, Ilieva I, Respino M, Gunning FM, Liston C, Dubin MJ. Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimul. 2017;10(5):919–925. doi: 10.1016/j.brs.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, et al. Anhedonia and Reward-Circuit Connectivity Distinguish Nonresponders from Responders to Dorsomedial Prefrontal Repetitive Transcranial Magnetic Stimulation in Major Depression. Biol Psychiatry. 2014;76(3):176–185. doi: 10.1016/j.biopsych.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 73.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRL in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, Downar J. Resting-State Portico-Thalamic-Striatal Connectivity Predicts Response to Dorsomedial Prefrontal rTMS in Major Depressive Disorder. Neuropsychopharmacology. 2014;39(2):488–498. doi: 10.1038/npp.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012;33(8):1914–1928. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri FM, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diefenbach GJ, Bragdon LB, Zertuche L, Hyatt CJ, Hallion LS, Tolin D, et al. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: a pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. 2016;209(3):222–228. doi: 10.1192/bjp.bp.115.168203. [DOI] [PubMed] [Google Scholar]
- 78.Yarkoni T. Big Correlations in Little Studies: Inflated fMRI Correlations Reflect Low Statistical Power-Commentary on Vul et al. (2009) Perspect Psychol ScI. 2009;4(3):294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 79.Yarkoni T, Braver TS. aCognitive Neuroscience Approaches to Individual Differences in Working Memory and Executive Control: Conceptual and Methodological Issues. In: Gruszka A, Matthews G, Szymura B, editors. Handbook of Individual Differences in Cognition. New York, NY: Springer; 2010. Spring Series on Human Exceptionality. [Google Scholar]
- 80.Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Power JD, Barnes KA, Snyder AZ, Schlagger BL, Petersen SE. Spurious by systematic correlations in functional connectivity in MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA. 2013;110(49):19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzgeald PB, Sritharan A, Daskalakis ZJ, de Castella AR, Kulkarni J, Egan G. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol. 2007;25(5):488–492. doi: 10.1097/jcp.0b013e318151521c. [DOI] [PubMed] [Google Scholar]
- 89.Furtado CP, Hoy KE, Maller JJ, Savage G, Daskalakis ZJ, Fitzgerald PB. Cognitive and volumetric predictors of response to repetitive transcranial magnetic stimulation (rTMS) – a prospective follow-up study. Psychiatry Res. 2012;202(1):12–19. doi: 10.1016/j.pscychresns.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 90.Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL. Low-Intensity Transcranial Current Stimulation in Psychiatry. Am J Psychiatry. 2017;174(7):628–639. doi: 10.1176/appi.ajp.2017.16090996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Data Analyses in Prospective Resting State Imaging Studies of TMS