Abstract
Voltage gated sodium channels (VGSCs) were first identified in terms of their role in the upstroke of the action potential. The underlying proteins were later identified as saxitoxin and scorpion toxin receptors consisting of α and β subunits. We now know that VGSCs are heterotrimeric complexes consisting of a single pore forming α subunit joined by two β subunits; a noncovalently linked β1 or β3 and a covalently linked β2 or β4 subunit. VGSC α subunits contain all the machinery necessary for channel cell surface expression, ion conduction, voltage sensing, gating, and inactivation, in one central, polytopic, transmembrane protein. VGSC β subunits are more than simple accessories to α subunits. In the more than two decades since the original cloning of β1, our knowledge of their roles in physiology and pathophysiology has expanded immensely. VGSC β subunits are multifunctional. They confer unique gating mechanisms, regulate cellular excitability, affect brain development, confer distinct channel pharmacology, and have functions that are independent of the α subunits. The vast array of functions of these proteins stems from their special station in the channelome: being the only known constituents that are cell adhesion and intra/extracellular signaling molecules in addition to being part of channel complexes. This functional trifecta and how it goes awry demonstrates the power outside the pore in ion channel signaling complexes, broadening the term channelopathy beyond defects in ion conduction.
Introduction
Voltage gated sodium channels (VGSCs) were first identified functionally in terms of their role in the upstroke of the action potential (AP) (Hille 2001). The underlying proteins were later identified as saxitoxin and scorpion toxin receptors consisting of α and β subunits (Hartshorne, Messner et al. 1982, Messner and Catterall 1985). We now know that VGSCs are heterotrimeric complexes consisting of a single pore forming α subunit joined by two β subunits; a noncovalently linked β1 or β3 and a covalently linked β2 or β4 subunit (Isom and De Jongh 1992, Isom, Ragsdale et al. 1995, Morgan, Stevens et al. 2000, Yu, Westenbroek et al. 2003). Mammalian genomes contain 9 VGSC α subunits, encoded by the SCN(1-10)A genes, and 5 β subunits, encoded by the SCN(1-4)B genes (Catterall WA and Waxman 2005). VGSC α subunits contain all the machinery necessary for channel cell surface expression, ion conduction, voltage sensing, gating, and inactivation, in one central, polytopic, transmembrane protein (Auld, Goldin et al. 1988). Early electrophysiological results led to the misconception that β subunits are simple accessories to α subunits (Goldin, Snutch et al. 1986). However, in the 25 years since the original cloning of the first β subunit, their role has expanded immensely (Isom and De Jongh 1992). VGSC β subunits confer unique gating mechanisms, regulate cellular excitability, affect brain development, confer distinct channel pharmacology, and have functions that are independent of the α subunits. The vast array of functions of these proteins stems from their special station in the channelome: being the only known constituents that are cell adhesion and intra/extracellular signaling molecules in addition to being part of channel complexes (Isom, De Jongh et al. 1994, Brackenbury and Isom 2011). This functional trifecta and how it goes awry demonstrates the power outside the pore in ion channel signaling complexes, broadening the term channelopathy beyond defects in ion conduction. This review will focus on VGSC β subunits in the nervous system. To read about β subunit function in heart, cancer, and beyond see: (Brackenbury and Isom 2011, Bao and Isom 2014, Martin, Ufodiama et al. 2015, O'Malley and Isom 2015).
Section 1: Structure and Expression of β Subunits
Excluding β1b, the VGSC β subunits have type 1 membrane protein topology consisting of an extracellular N-terminal domain, a single transmembrane segment, and a C-terminal tail (Fig. 1) (Isom and De Jongh 1992, Isom, Ragsdale et al. 1995, Kazen-Gillespie, Ragsdale et al. 2000, Morgan, Stevens et al. 2000, Yu, Westenbroek et al. 2003). The array of functions and interactions imparted by each of these domains make β subunits immensely powerful for such small proteins. β subunit functionality in brain is expanded even further if one takes into account their expression profiles in terms of developmental time course, cell type specificity, and subcellular localization. This section will examine the structure and expression of β subunits in the nervous system.
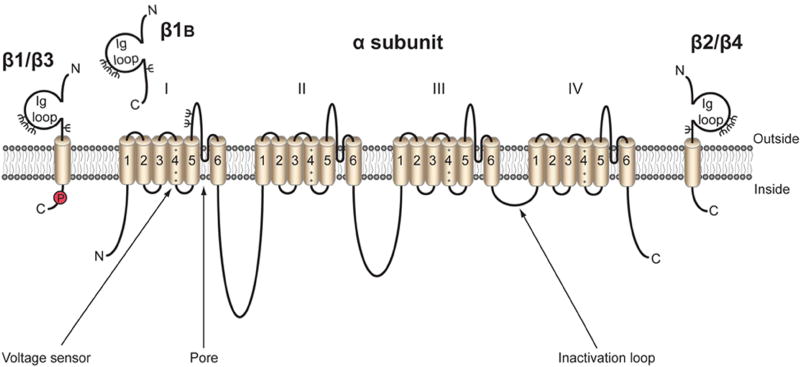
Figure 1. Topology of the voltage-gated Na+ channel α and β subunits.
VGSCs contain a pore-forming α subunit consisting of four homologous domains of six transmembrane segments (Catterall 2012). Segment 4 contains the voltage sensor. VGSCs also contain one or more β subunits. β1, β2, β3, and β4 contain an extracellular immunoglobulin (Ig) loop, transmembrane domain, and an intracellular C-terminal domain (O'Malley and Isom 2015). β1B also contains an Ig loop, but has a different C-terminus lacking a transmembrane domain, and is thus a soluble, secreted protein (Patino, Brackenbury et al. 2011). β1 contains a tyrosine phosphorylation site in its C-terminus (Malhotra, Thyagarajan et al. 2004) ψ, glycosylation sites. β1 and β3 are non-covalently linked to α, whereas β2 and β4 are covalently linked through disulfide bonds (O'Malley and Isom 2015). Figure was reproduced from (Brackenbury and Isom 2011).
Section 1 Part 1: The β Subunit Immunoglobulin Domain
Each VGSC β subunit features an extracellular N-terminal domain that contains homology to V-type immunoglobulin (Ig) loops common to the Ig superfamily of cell adhesion molecules (CAMs) (Isom and De Jongh 1992, Isom, Ragsdale et al. 1995, Kazen-Gillespie, Ragsdale et al. 2000, Morgan, Stevens et al. 2000, Yu, Westenbroek et al. 2003). In the β subunits, these domains are capable of both interaction with VGSC α subunits and cell adhesion (Brackenbury and Isom 2011). Each Ig domain consists of antiparallel β sheets stabilized by an intrasubunit disulfide bond (McCormick, Isom et al. 1998, Gilchrist, Das et al. 2013, Namadurai, Balasuriya et al. 2014, Das, Gilchrist et al. 2016, Shimizu, Miyazaki et al. 2016). Early on, β subunit structure was modeled based on the crystal structure of the extracellular domain of myelin P0, a protein containing a single V type Ig loop and single transmembrane domain with modest homology to β1 and β3 (Isom, Ragsdale et al. 1995, McCormick, Isom et al. 1998, Morgan, Stevens et al. 2000). Crystal structures of β2, β3, and β4 were recently solved (Gilchrist, Das et al. 2013, Namadurai, Balasuriya et al. 2014, Das, Gilchrist et al. 2016). These structures inform and confirm many insights into β subunit Ig domains garnered from previous biochemical work and show that each subunit contains unique structural features. Approximately one-third of the molecular mass of each β subunit is due to N-linked glycosylation moieties in the extracellular domain (Isom and De Jongh 1992, Isom, Ragsdale et al. 1995, McCormick, Isom et al. 1998, Morgan, Stevens et al. 2000, Yu, Westenbroek et al. 2003, Johnson, Montpetit et al. 2004). Sialic acid terminated glycosylation is necessary for modulation of α subunit gating by β1, suggesting that electrostatic interactions with voltage sensing domains may occur through modification of these residues (Johnson, Montpetit et al. 2004).
VGSC β1 and β3 subunits share the highest sequence homology to each other, while β2 and β4 share more homology to each other than to either β1 or β3 (Isom and De Jongh 1992, Isom, Ragsdale et al. 1995, Morgan, Stevens et al. 2000, Yu, Westenbroek et al. 2003). Studies using chimeric subunits revealed that β1 modulates the gating of Nav1.2 via association with the extracellular domain IV S5-S6 region of that α subunit (McCormick, Srinivasan et al. 1999, Qu, Rogers et al. 1999). This may extend to β3, given its high sequence homology to β1, although see (Namadurai et al. 2014). The site of association between β1 and Nav1.4 was localized to the S5-S6 segments connecting domains I and IV of that α subunit, implicating channel specific regions of interaction (Makita, Bennett et al. 1996). β2 and β4 bind to VGSC α subunits differently than β1 and β3. A key difference between the Ig loops of the β subunits is the presence of an exposed free cysteine residue in β2 and β4 that forms a disulfide bond with α subunits (Isom, Ragsdale et al. 1995, Yu, Westenbroek et al. 2003). Mutation of this residue in β2 or β4 abolishes α subunit interactions (Chen, Calhoun et al. 2012, Gilchrist, Das et al. 2013). The cysteine residues in β2 (Cys-26) or β4 (Cys-58) responsible for covalent association with α were determined to bind to a corresponding cysteine residue in Nav1.2 located in the domain II S5-S6 loop (Das, Gilchrist et al. 2016). This residue is absent from the tetrodotoxin-resistant (TTX-R) α subunits Nav1.5, Nav1.8 and Nav1.9, although Nav1.5 and β2 have been shown to associate through disulfide bonds in cardiac tissue and β2 modifies Nav1.5 currents in heterologous systems and in vivo (Malhotra, Chen et al. 2001, Johnson and Bennett 2006, Bao, Willis et al. 2016). β2 and β4 differentially affect channel modulation of α subunits by extracellular μ-, μO- and μO§-conotoxins relative to β1 and β3 (Wilson, Zhang et al. 2011, Zhang, Wilson et al. 2013, Gajewiak, Azam et al. 2014, Wilson, Zhang et al. 2015). In the case of μO§-conotoxins, association with α subunits occurs through the same cysteine through which these subunits covalently bind to VGSC α subunits. β1 and β3 have no influence on μO§-conotoxin effects (Gajewiak, Azam et al. 2014). Thus, as expected from early data showing that VGSCs in brain are heterotrimers, these results suggest that the binding sites for β1 and β3 vs β2 and β4 on α subunits are mutually exclusive.
In addition to channel modulation, β subunits, in particular β1, participate in homophilic and heterophilic cell adhesion through their extracellular Ig domains. This property allows β subunits to interact with a wide variety of proteins in both cis and trans configurations, including with identical β subunits in the same membrane or on adjacent cells (Brackenbury and Isom 2011). Importantly, β subunit-mediated cell adhesive interactions can occur both in the presence and absence of VGSC α subunits (Malhotra, Kazen-Gillespie et al. 2000). β subunit-mediated cell adhesion is most well characterized for β1, which associates homophilically in vitro in trans, and heterophilically with the Ig CAMs VGSC β2, contactin-1, Nf-186, and NrCam, with the adhesion molecule N-cadherin, and with the extracellular matrix molecule tenascin-R (Isom and Catterall 1996, Malhotra, Kazen-Gillespie et al. 2000, Kazarinova-Noyes, Malhotra et al. 2001, Ratcliffe, Westenbroek et al. 2001, McEwen and Isom 2004). β2 associates homophilically as well as heterophilically with β1 and tenascin-C, but not with contactin (Srinivasan, Schachner et al. 1998, Xiao, Ragsdale et al. 1999, McEwen and Isom 2004). β4 engages in homophilic cell-cell adhesion but its ability to function as a heterophilic CAM has not been tested (Shimizu, Miyazaki et al. 2016). Crystal structure guided photocrosslinking experiments with β1 and β4 revealed the specific residues responsible for homophilic cell adhesion, further elucidating the structure of VGSC complexes (Shimizu, Miyazaki et al. 2016). Interestingly, β3 is predicted to form trimeric complexes that would obscure the analogous residues necessary for trans homophilic adhesion (Namadurai, Balasuriya et al. 2014). These results are consistent with earlier data showing that, while β1 or β2 subunit expression in Drosphila S2 cells resulted in the formation of high affinity aggregates, β3 expression did not (McEwen, Chen et al. 2009). Overall, however, data on the cell adhesive properties of β3 are conflicting, possibly as an effect of differential trimerization based on expression system (Yereddi, Cusdin et al. 2013).
Section 1 Part 2: The Transmembrane and C-terminal Domains of β subunits
β subunits contain a single, α helical transmembrane domain that can confer unique functional properties. For example, the structure of the β3 transmembrane domain is proposed to mediate cis homophilic interactions allowing for trimerization (Namadurai, Balasuriya et al. 2014). Trimeric β3 complexes are capable of forming oligomers with Nav1.5, a departure from the canonical heterotrimeric VGSC structure (Namadurai, Balasuriya et al. 2014). For Scn1b, retention of intron 3 through alternative splicing results in the polypeptide β1b, a secreted, soluble CAM. In place of a transmembrane domain, β1b contains an alternate C-terminus with an amino acid sequence that is species specific (Kazen-Gillespie, Ragsdale et al. 2000). β1b acts as an extracellular CAM ligand. It is also capable of modulation of Nav1.5 but not Nav1.1 or Nav1.3 in heterologous systems (Watanabe, Koopmann et al. 2008, Patino, Brackenbury et al. 2011). β subunits can also shed their Ig domain following sequential cleavage by β-Site APP-cleaving enzyme 1 (BACE1) and γ-secretase (Wong, Sakurai et al. 2005, Gersbacher, Kim et al. 2010). The resulting soluble extracellular fragment is likely to behave similarly to β1b.
β subunit intracellular C-terminal domains are short sequences predicted to have disordered structure (Cusdin, Nietlispach et al. 2010, Lewis and Raman 2011, Namadurai, Balasuriya et al. 2014). Along with the transmembrane segment, the intracellular tails confer additional interactions with VGSC α subunits. Co-expression of β1 or β3 with Nav1.3 in heterologous cells attenuates lidocaine binding to the channel, suggesting that the β subunit C-terminal domains may occlude the α subunit local anesthetic binding site, located on the S6 helix of domain IV, and accessible only from the intracellular side (Lenkowski, Shah et al. 2003, Catterall 2012). Mutations in Scn1a identified an additional α-β1 subunit interaction site in the β1 C-terminal tail (Spampanato, Kearney et al. 2004). The intracellular domain of β2 modulates Nav1.5 gating and the C-terminal tail of β4 contains residues capable of intracellular blockade of α subunits to generate resurgent sodium current (INa) (described in section 2) (Grieco, Malhotra et al. 2005, Zimmer and Benndorf 2007). β subunit intracellular domains may also impart intracellular regulatory control of α and β subunit activity. For example, phosphorylation of β3 residue S161 is necessary for modulation of Nav1.2 INa (Merrick, Kalmar et al. 2010). The β1 intracellular tyrosine residue Y181 is required for β1 association with the scaffolding proteins ankyrin-B and ankyrin-G at points of cell-cell contact following trans homophilic cell adhesion (Malhotra, Kazen-Gillespie et al. 2000, Merrick, Kalmar et al. 2010). Phosphorylation of this residue prevents ankryin binding (Malhotra, Koopmann et al. 2002).
Section 1 Part 3: Expression Profile of the β Subunits
The β subunits differ in their expression and posttranslational modifications within different stages of development, in different tissues, between cell types, and subcellular locations. This imparts a specificity that is crucial for normal development and excitability. β subunits are expressed in both excitable and non-excitable cells in brain (O'Malley and Isom 2015). In whole brain, β1b and β3 mRNA and protein expression are high during the prenatal and early postnatal period in mammals (Kazen-Gillespie, Ragsdale et al. 2000, Shah, Stevens et al. 2001, Patino, Brackenbury et al. 2011). These subunits then give way to increased expression of β1, β2, and β4 mRNA protein in the first postnatal week and this expression persists through adulthood (Sutkowski and Catterall 1990, Isom and De Jongh 1992, Isom, Ragsdale et al. 1995, Sashihara, Greer et al. 1996, Lein, Hawrylycz et al. 2007). Different cell types in the brain express different β subunits at various levels. In situ hybridization and immunohistochemistry show that Scn1b and β1 expression, respectively, are ubiquitous throughout the brain but with modest heterogeneity (Sashihara, Greer et al. 1996, Blackburn-Munro and Fleetwood-Walker 1999, Lein, Hawrylycz et al. 2007, Brackenbury and Isom 2011, Wimmer, Harty et al. 2015). β1 immunohistochemical studies show subcellular localization in similar patterns to that found for α subunits, with high density at axon initial segments (AISs) and nodes of Ranvier in myelinated axons (Chen, Westenbroek et al. 2004, Wimmer, Reid et al. 2010, Kruger, O'Malley et al. 2016). This subcellular localization pattern allows for specialized functions. Exemplary of the importance of subcellular localization is the action of β1 at nodes of Ranvier. β1 is highly enriched in the nodal gap where it is presumed to modulate VGSC α subunit cell surface expression and gating. However, in the paranodal region β1 is engaged in a trimeric axonal-glial cell adhesion complex consisting of axonal β1, contactin, and Caspr binding in trans to glial neurfascin-155 in the absence of VGSC α subunits (Kazarinova-Noyes, Malhotra et al. 2001, McEwen and Isom 2004). β1 expression at the growth cones of developing neurons mediates several important functions where it is proposed to act both as a CAM and as a modulator of INa (Brackenbury, Calhoun et al. 2010). Scn1b is also the mostly highly expressed β subunit gene in the peripheral nervous system (PNS), where it is particularly enriched in medium and large diameter A fibers with lower expression in C fibers (Blackburn-Munro and Fleetwood-Walker 1999, Lopez-Santiago, Pertin et al. 2006, Zhao, O'Leary et al. 2011).
Scn2b expression is similarly ubiquitous in adult brain as assessed through in situ hybridization (Blackburn-Munro and Fleetwood-Walker 1999, Lein, Hawrylycz et al. 2007). Similar to β1, β2 protein is highly expressed at the AIS and nodes of Ranvier. Scn2b is also expressed in the PNS, where its deletion results in loss of responsiveness to inflammatory and neuropathic pain but not mechanosensation, again suggesting cell type specific functions (Lopez-Santiago, Pertin et al. 2006, Zhao, O'Leary et al. 2011).
While Scn3b mRNA and protein are expressed ubiquitously throughout the developing CNS, in adult mice expression is greatly attenuated except in specific structures, most highly in hippocampus and others (Morgan, Stevens et al. 2000, Lein, Hawrylycz et al. 2007). This differs in human brain, where SCN3B expression remains highly expressed through adulthood similarly to SCN3A (Chen, Dale et al. 2000, Lein, Hawrylycz et al. 2007). PNS expression of Scn3b is especially high in unmyelinated C fibers and is low in the other fiber types (Lopez-Santiago, Pertin et al. 2006, Zhao, O'Leary et al. 2011).
Scn4b is the most restricted of the β subunits in its expression profile. Scn4b mRNA and protein are highly expressed in several neuronal populations, many of which are known for spontaneous or burst firing APs (Lein, Hawrylycz et al. 2007, Lewis and Raman 2014). The highest expression of β4 protein and Scn4b mRNA are found in the striatum and cerebellar purkinje neurons but are also highly expressed in other restricted brain regions (Lein, Hawrylycz et al. 2007, Miyazaki, Oyama et al. 2014). β4 subcellular localization is similar to that of the other β subunits, being enriched at the AIS, nodes of Ranvier, and along the length unmyelinated axons (Buffington and Rasband 2013, Miyazaki, Oyama et al. 2014). In the PNS β4 is most highly expressed in medium and large A fibers (Lopez-Santiago, Pertin et al. 2006, Zhao, O'Leary et al. 2011).
While not much is known about the trafficking of β subunits down the axon, this process likely involves interactions with VGSC α subunits. Disruption of the disulfide bond between α subunits and β2 or β4 impairs the ability of β subunits to traffic to axons and nodes of Ranvier (Chen, Calhoun et al. 2012, Buffington and Rasband 2013). Similarly, an Scn1b mutation that interferes with α-β1 association results in failure of the mutant β1 to traffic from the soma to the AIS and nodes of Ranvier (Kruger, O'Malley et al. 2016). This suggests β1 subunit enrichment in axonal domains is dependent on trafficking of α–β complexes rather than to β subunit independent trafficking. This would imply dynamic association of β1 with α subunits, as β1 in the paranode of nodes of Ranvier is not associated with α subunits (Kazarinova-Noyes, Malhotra et al. 2001, McEwen and Isom 2004). β subunits are also expressed in various glia where they may function as cell adhesion guides and cues for neurodevelopment, including coordinating neurite outgrowth, axonal fasciculation, and neuronal migration (Brackenbury and Isom 2011).
Section 2: β Subunits in Physiology and Development
The cell type specificity of β subunit expression, β subunit specific interactions with other proteins, and unique β subunit structural features outlined in section 1 are important for cell type and brain region specific control of excitability and development. In the following section we will examine the physiological functions of the β subunits at the level of control of VGSC and voltage gated potassium (VGKC) channel currents, structural development in the nervous system, and impacts on excitability.
Section 2 Part 1: β Subunits Alter VGSC Properties
VGSCs undergo a series of conformational changes resulting in several channel states necessary for neuronal AP firing. The β subunits have specific effects on these states and contribute unique gating mechanisms. The “closed” (deactivated) state dominates at negative membrane potentials. In this state, channels do not pass ions. Upon membrane depolarization, the channels undergo conformational shifts to the “open” state, allowing passage of ions. Channels may then revert back to the closed state or undergo the process of “inactivation,” whereby the intracellular face of the channel is blocked by an inactivating “particle” and ion passage is blocked. Relief from inactivation occurs upon hyperpolarization for extended periods of time and is much slower than deactivation, leading to the refractory period between APs (Hille 2001). Early work expressing α subunits in heterologous systems showed that α subunits alone contain all the machinery necessary for these processes (Noda, Ikeda et al. 1986, Tomiko, Rosenberg et al. 1986, Recio-Pinto, Duch et al. 1987). However, the activation and inactivation kinetics of TTX-sensitive α subunits expressed in Xenopus oocytes without β subunits are slower than properties observed for native VGSC expression. The voltage dependence of INa is also altered (Goldin, Snutch et al. 1986, Auld, Goldin et al. 1988, Trimmer, Cooperman et al. 1989, Joho, Moorman et al. 1990). The first indication that β subunits were necessary to reconstitute native channel properties was found by coinjection of low molecular weight brain mRNA (containing β subunit transcripts) with high molecular weight mRNA (containing α subunit transcripts). The low molecular weight mRNA fraction conferred native kinetics and voltage dependence on INa expressed by the high molecular weight mRNA fraction (Krafte, Snutch et al. 1988). Cloning of Scn1b and Scn2b cDNAs and their coexpression with α subunits in Xenopus oocytes revealed that β subunits are indeed responsible for these differences as well as promotion of the surface expression of α subunits (Isom and De Jongh 1992, Isom, Ragsdale et al. 1995).
The effects of β subunits on channel cell surface expression, activation, and inactivation are dependent on the particular β subunit expressed, the chosen experimental expression system, and which α subunit is coexpressed. An overview of the effects of β subunits on channel gating and trafficking in heterologous cells is provided in (Calhoun and Isom 2014). Some possible reasons for these differences include background expression of other β subunits in some heterologous cell lines and cell line dependent differential posttranscriptional processing of β subunits. Sialic acid terminated glycosylation in particular seems to be important for modulation of α subunit gating by β1. Sialic acid terminated glycosylation on residues within the β1 Ig loop may confer electrostatic interactions with residues in α subunit voltage sensing domains. In addition, the presence of a high degree of sialic acid terminated glycosylation in Nav1.4 is thought to confer resistance to modulation by or association with β1. This is restored by mutating Scn4a cDNA to contain non-glycosylatable residues. This modification is also necessary for the effects of β subunits in promoting Nav1.4 cell surface expression, and thus may facilitate intersubunit association rather than mediate gating differences in skeletal muscle (Johnson, Montpetit et al. 2004). Differential sialic acid modification was also found to affect β2 modulation of Nav1.5 while, in the same study, Nav1.2 was modulated regardless of α subunit sialylation status (Johnson and Bennett 2006). The epilepsy-linked β1-C121W mutation in Scn1b results in decreased β1 subunit glycosylation and loss of its capacity to modulate VGSC properties and to traffic out of the soma to the axon in mouse neurons (Meadows, Malhotra et al. 2002, Tammaro, Conti et al. 2002, Kruger, O'Malley et al. 2016). This raises the possibility that control of glycosylation of β subunits, especially sialyation, may be important in subcellular targeting and α-β subunit association. Interestingly β1 and β3 oppositely affect glycosylation of Nav1.7 in heterologous systems, showing β subunits can also control gating of α subunits via regulation of their posttranscriptional processing (Laedermann, Syam et al. 2013). Modulation of INa by β subunits is also dependent on phosphorylation status. Phosphorylation of S161 in the C-terminal tail of β3 is necessary for modulation of Nav1.2 INa in heterologous cells (Merrick, Kalmar et al. 2010). Phosphomimetic mutation of residue Y181, located in the β1 C-terminal tail, results in the loss of β1’s capacity to modulate Nav1.2 mediated INa and surface expression, without interfering with α-β subunit association (McEwen, Meadows et al. 2004). Tyrosine phosphorylation of β1 is likely functionally relevant in neurons, as the β1 intracellular domain is known to interact with receptor protein tyrosine phosphatase β (RPTP-β) and likely Fyn kinase in mouse brain, as Fyn null neurons lack β1-mediated neurite outgrowth (Ratcliffe, Qu et al. 2000, Brackenbury, Davis et al. 2008). In contrast to β1, β2 does not interact with RPTP-β, suggesting β subunit specificity of association with certain phosphorylation enzymes (Ratcliffe, Qu et al. 2000). Thus, differential posttranscriptional modifications to β subunits, including glycosylation and phosphorylation, may lend modularity to channel activity in a cell type and subcellular region specific manner.
Section 2 Part 2: In Vivo Effects of β subunits on VGSC Function
Surprisingly, despite high expression throughout the hippocampus and large effects on INa in heterologous systems, acutely dissociated Scn1b null whole hippocampal pyramidal neurons or CA3 bipolar neurons show no changes in INa density or voltage dependent properties (Chen, Westenbroek et al. 2004, Patino, Claes et al. 2009). This may be due to the process of dissociation, compensation by other β subunit gene expression, or only subsets of neurons being affected by Scn1b deletion. The latter is likely, since the mix of several pyramidal neuron populations present in whole hippocampal neuron dissociations could obscure differences in specific hippocampal fields. Notably, in Scn1b null CA3 but not CA1 pyramidal neurons or dentate granule neurons, there is a reduction in the expression of Nav1.1 and an increase in the expression of Nav1.3 measured by immunohistochemistry (Chen, Westenbroek et al. 2004). Fittingly, acutely dissociated dentate granule neurons and CA1 pyramidal neurons show no changes in INa density, kinetics, or voltage dependent properties (Uebachs, Opitz et al. 2010, Uebachs, Albus et al. 2012). In contrast, acutely dissociated Scn1b null dorsal root ganglion (DRG) neurons show a depolarizing shift in the voltage dependence of TTX-S INa inactivation, reduced cell surface expression of Nav1.9, and a prolonged recovery from inactivation of TTX-R INa compared to wildtype (WT) (Lopez-Santiago, Brackenbury et al. 2011). While cultured cerebellar granule neurons show no change in the amount or voltage dependence of transient INa, they do have differences in α subunit expression and decreased resurgent INa, as discussed in more detail in section 2 parts 3 and 4 (Brackenbury, Calhoun et al. 2010).
Scn2b deletion also shows cell type dependent effects in native neurons. Cultured hippocampal neurons from Scn2b null mice have reduced surface expression of TTX-S VGSCs. Acutely dissociated Scn2b null hippocampal pyramidal neurons, but not dentate granule neurons, have reduced INa density (Chen, Bharucha et al. 2002, Uebachs, Opitz et al. 2010). Scn2b deletion also affects the gating properties of VGSCs, slowing the kinetics of activation and inactivation in dissociated hippocampal neurons. Scn2b null small fast DRG neurons show a 50% reduction in transient INa, reduced Nav1.7 expression, and slowed kinetics of activation and inactivation. These effects of Scn2b appear to be specific to TTX-S channels, as TTX-R INa in the PNS are unchanged, perhaps due to the lack of β2-binding cysteine residues in the TTX-R PNS channels Nav1.8 and Nav1.9, as discussed in section 1 (Lopez-Santiago, Pertin et al. 2006). Differential effects of Scn2b deletion on VGSC α subunit subtypes within the same neuron demonstrate that β subunits modulate channels in precise ways in vivo.
The effects of β3 in the nervous system in vivo are not well studied, however Scn3b deletion results in reduced transient INa and hyperpolarized voltage dependence of inactivation in cardiomyocytes, suggesting the effects of β3 observed in heterologous system may hold in vivo (Hakim, Gurung et al. 2008).
Scn4b knockdown in cultured cerebellar granule neurons results in reduced transient INa and hyperpolarization of the voltage dependence of transient INa availability (Bant and Raman 2010). Scn4b null mice show no changes in transient INa in striatum, but see below for effects on special gating schemes (Miyazaki, Oyama et al. 2014). Overexpression of Scn4b cDNA in cultured hippocampal CA3 neurons, which do not natively express β4, hyperpolarizes and decreases the slope factor of the voltage dependence of availability, suggesting effects of β4 in heterologous systems may translate to neurons (Aman, Grieco-Calub et al. 2009).
Section 2 Part 3: Role of β subunits in Resurgent and Persistent Sodium Current
β subunits play roles in special gating schemes that differ from the traditional transient INa described by Hodgkin and Huxley (Hille 2001). Resurgent INa is a specialized current in which traditional inactivation is impaired by blockade of the intracellular face of the pore, which is relieved more quickly by hyperpolarization than traditional inactivation. Upon membrane potential repolarization, the channel is able to quickly convert to the open state without the prolonged recovery interval imparted by traditional inactivation. This current activates quickly upon repolarization during an AP and promotes cell type dependent spontaneous, repetitive, or burst firing of APs (Lewis and Raman 2014). The C-terminal tail of β4 is the only known endogenous molecule to generate resurgent INa (Grieco, Malhotra et al. 2005, Lewis and Raman 2014). The residues KKLITFILKKTREK in the C-terminal tail of the mouse β4 subunit are required for resurgent INa in cerebellar purkinje and granule neurons (Grieco, Malhotra et al. 2005). Consistent with this result, Scn4b null mice lose resurgent INa in striatal projection neurons (Miyazaki, Oyama et al. 2014). While β4 is the only identified mediator of resurgent INa to date, there are neuronal populations that express this current but do not express β4, suggesting the involvement of other proteins (Lewis and Raman 2014). The ability of β4 to generate resurgent INa is α subunit dependent, with Nav1.6 carrying proportionally more than the other α subunits (Lewis and Raman 2014). Scn1b null cultured granule neurons have reduced resurgent INa, likely due to decreased expression of this α subunit (Brackenbury, Calhoun et al. 2010).
In addition to reduced resurgent INa, Scn4b null striatal projection neurons also have reduced persistent INa, defined as the fraction of transient INa that does not inactivate (Miyazaki, Oyama et al. 2014). Persistent INa promotes repetitive firing, facilitates the summation of excitatory postsynaptic currents, and contributes to determination of firing threshold (Crill 1996). Knockdown of β4 in cultured cerebellar granule neurons reduced both resurgent and persistent INa (Aman, Grieco-Calub et al. 2009). Inclusion of the C-terminal β4 peptide restored resurgent INa in these neurons and induced it in neurons that do not typically have resurgent INa (Grieco, Malhotra et al. 2005). Interestingly, in contrast to the β4 C-terminal peptide, expressing the full-length β4 protein does not generate resurgent INa in heterologous systems. It does, however, destabilize inactivation; tripling persistent INa amplitude (Aman, Grieco-Calub et al. 2009). β4 is not unique in its ability to impact persistent INa. β3, but not β1 or β2, when coexpressed in heterologous systems with Nav1.2, increases persistent INa (Qu, Curtis et al. 2001). Scn1b has effects on both persistent and resurgent INa in vitro and in vivo. Scn1b null CGNs have reduced resurgent INa and Scn1b null DRGs have reduced persistent TTX-R INa (Brackenbury, Calhoun et al. 2010, Lopez-Santiago, Brackenbury et al. 2011). This effect in CGNs may be due more to the loss of effects of Scn1b on Nav1.6 subunit expression, which is known to disproportionality carry resurgent and persistent INa relative to other α subunits (Lewis and Raman 2014).
In summary, many neurons undergo dynamic changes in their firing profile, which may be controlled by the levels of persistent and resurgent INa expressed (Krahe and Gabbiani 2004, Paul, DeWoskin et al. 2016). β subunits may play critical roles in neuronal firing based on their impacts to these currents, and thus impart dynamic control over neuronal firing properties.
Section 2 Part 4. β Subunits Affect Trafficking and Expression of VGSC α subunits In Vivo
The early heterologous expression studies of β1 and β2 revealed that, in addition to modulation of gating, these subunits also impact channel surface expression, largely through effects on trafficking and cell surface half-life of channels rather than through their transcriptional control (but see below) (Schmidt, Rossie et al. 1985, Schmidt and Catterall 1986, Isom and De Jongh 1992, Isom, Ragsdale et al. 1995). Association with β2 is the final step in VGSC biosynthesis, promoting α subunit insertion into the plasma membrane (Schmidt, Rossie et al. 1985, Schmidt and Catterall 1986). In contrast, “free” α subunits not associated with a disulfide-linked β subunit are located inside the cell and are inactive (Schmidt, Rossie et al. 1985). In addition, α subunits covalently associated with β subunits are removed from the cell surface more slowly than free α subunits (Schmidt and Catterall 1986). Consistent with these data, hippocampal neuron cultures from Scn2b null mice show a reduction in α subunits expressed at the cell surface (Chen, Bharucha et al. 2002). Interestingly, the function of β2 on VGSC surface expression in mammalian cells appears to be dependent on β1, and this point is discussed in more detail in the next section. In contrast to β2, β4 does not appear to contribute to surface expression of α subunits; Scn4b null neurons show no apparent reductions in transient sodium current (Miyazaki, Oyama et al. 2014).
Scn1b impacts α subunit expression and trafficking in an isoform specific manner in vivo. In CGNs, Scn1b deletion in mice causes a reduction in Nav1.6 and an increase in Nav1.1 expression at the CGN AIS, leaving total current density unchanged (Brackenbury, Calhoun et al. 2010). In rat brain, while “free” α subunits not disulfide linked to β subunits account for 21% of VGSCs in the soma, they are wholly undetectable in synaptosomes. This suggests that β2 or β4 subunits may play roles in directing α subunits to the synapse (Schmidt, Rossie et al. 1985, Schmidt and Catterall 1986). More recent work shows that β3 mediates clustering of Nav1.5 channels in heterologous systems, possibly through cis homophilic interactions resulting in β3 trimer formation (Namadurai, Balasuriya et al. 2014). VGSC α subunit single molecule tracking microscopy in fetal rat hippocampal neuron cultures showed that VGSCs exist in clustered microdomains (Akin, Solé et al. 2016). While the role of β subunits in this clustering is unknown, data showing the effects of β3 on VGSC clustering in culture may lend important insights into this process, since β3 is highly expressed in fetal and postnatal rodent brain. β3 also may affect α subunit expression through masking of an endoplasmic reticulum retention/retrieval motif (Zhang, Li et al. 2008). Scn4b deletion does not affect axonal expression of Nav1.2 in striatal projection neurons, suggesting that it may not play roles in channel trafficking but instead its function may be limited to specialized impacts on persistent and resurgent sodium currents (Miyazaki, Oyama et al. 2014).
β subunits can affect VGSC α subunit transcription or translation. In Scn1b null hippocampus, CA3, but not CA1 or dentate gyrus, pyramidal neurons have reduced Nav1.1 and increased Nav1.3 expression (Chen, Westenbroek et al. 2004). In addition, Nav1.9 protein is reduced in Scn1b null DRGs (Lopez-Santiago, Brackenbury et al. 2011). β2 is also able to affect expression of specific α subunits. In DRGS, mRNA for Nav1.7, Nav1.6 and Nav1.3 is reduced, while Nav1.1 and Nav1.7 protein are reduced, with no change in Nav1.6 (Lopez-Santiago, Pertin et al. 2006). Many of these effects on specific VGSC α subunit expression may be due to transcriptional regulatory effects of β subunits, as discussed in the next section.
Section 2 Part 5. Proteolytic Processing of β subunits
VGSC β subunits are targets for proteolytic cleavage, with the resulting products demonstrating specific autonomous functions. The β subunits contain cleavage sties for sequential cleavage by BACE1 and γ-secretase (Wong, Sakurai et al. 2005, Gersbacher, Kim et al. 2010). β2, and likely the other β subunits, contains an additional cleavage site for α-secretase enzyme ADAM10 in the extracellular portion (Kim, Ingano et al. 2005). BACE1 cleavage results in shedding of the extracellular β subunit domain. For β1, this cleaved polypeptide fragment is thought to be capable of functioning as a ligand for cell adhesion, similar to β1b (Kim, Ingano et al. 2005, Wong, Sakurai et al. 2005). Interestingly, while expression of full-length β4 subunits with α subunits in heterologous systems does not recapitulate resurgent INa, cleavage of β4 by BACE1 slows the decay of resurgent INa, suggesting that proteolytic processing of β4 may be involved in generation of resurgent INa (Huth, Rittger et al. 2011). Because the β4 residues responsible for generating resurgent INa are located within the C-terminal tail, sequential cleavage by γ-secretase, following the action of BACE1, may also be important in this mechanism. Formation and nuclear translocation of the γ-secretase cleaved β2 C-terminal fragment has been shown to lead to increased Nav1.1 subunit mRNA and protein expression in neurons, suggesting a role for β subunits in the regulation of α subunit transcription (Kim, Carey et al. 2007). While similar roles for the other β subunits have not been investigated, their processing by this cleavage pathway and effects on specific α subunit expression may suggest similar mechanisms. Thus, β subunits may also affect neuronal firing through the modulation of channel transcription.
Section 2 Part 6. β Subunit Crosstalk
VGSC β subunits can also impact the actions of each other. Coexpression of β1 and β2 in oocytes with Nav1.2 increases INa density greater than either β subunit alone, suggesting synergistic action (Isom, Ragsdale et al. 1995). In Chinese hamster lung fibroblasts, β2 expression does not increase the surface expression of Nav1.2 unless β1 is also expressed (Kazarinova-Noyes, Malhotra et al. 2001, McEwen, Meadows et al. 2004). This seem to be the case for endogenous expression as well; mice that are homozygous for both the Scn1b and Scn2b null alleles have reduced INa density in cerebellar purkinje neurons but INa density is not different in mice null for Scn1b or Scn2b alone (Grieco, Malhotra et al. 2005). This result suggests there is a capacity for β1 and β2 to compensate for each other in vivo, in spite of evidence that these subunits bind to different locations on α subunits. This may be the case for other subunits as well. Scn3b null mice have apparently normal nervous system development, suggesting compensatory Scn1b expression (Hakim, Gurung et al. 2008, Hakim, Brice et al. 2010). Coexpression of β1 with β4 overcomes β4 induced persistent INa in heterologous cells and neurons. Additionally, the β4 mediated hyperpolarized activation and inactivation of Nav1.1 INa are antagonized by β1. In contrast, β1 functions appear to be relatively unaffected by β4 co-expression, as cells expressing both subunits match the electrophysiological profile of β1 expression alone. These data suggest opposing roles of β1 and β4 in the same trimeric channel complex, with β4 acting as an excitation accelerator and β1 acting as an overriding brake (Aman, Grieco-Calub et al. 2009). While the binding sites on α subunits for β1 and β3 are predicted to be competitive, there is evidence that β1 and β3 coexpression can generate properties not present with either alone when coexpressed with Nav1.5 (Ko, Lenkowski et al. 2005). This may be specific to the α subunit, as similar experiments with Nav1.8 show the opposing shifts in channel availability with β1 and β3 seem to cancel when coexpressed in the same oocyte. In the same study, β1 and β2 coexpression revealed a depolarizing voltage shift in availability, contrary to the hyperpolarizing shift of β1 alone or the absence of effect of β2 alone (Vijayaragavan, Powell et al. 2004). β subunits can also affect expression of other β subunits in vivo. Quantification of β subunit proteins in Scn4b null mice showed increased expression of β1 and reduced β2 in spinal cord and cerebellum, with no effect on their expression levels in striatum or cortex (Miyazaki, Oyama et al. 2014). In DRG neurons, Scn2b null mice have decreased expression of Scn3b and Scn4b but not Scn1b mRNA (Lopez-Santiago, Pertin et al. 2006). This cell type specific regulation of β subunit mRNA expression by other β subunits further highlights the precise control of β subunit function in vivo.
Section 2 Part 7: β Subunits Modulate Potassium Channels
β subunits also affect some potassium currents (IK), creating the potential for cross-talk between VGKCs and VGSCs. β1 subunits interact with Kv4.2 and Kv4.3, major contributors to A-type IK in brain. This interaction was first uncovered in heterologous expression studies (Deschênes and Tomaselli 2002). Later, an unbiased proteomic approach designed to discover novel Kv4.2 interacting partners in rodent brain identified VGSC β1. In the same study, investigators found that heterologous co-expression of β1 with Kv4.2 increases IK density. In addition, shRNA knockdown of β1 in cultured cortical neurons decreased A-type IK (Marionneau, Carrasquillo et al. 2012). β1 also modulates several other VGKCs when co-expressed in Xenopus oocytes, including Kv4.3, Kv1.1, Kv1.3, Kv1.6, and Kv7.2. β1 did not, however, modulate Kv3.1 in this study, suggesting that interactions are moderately specific to channel type (Nguyen, Miyazaki et al. 2012). It will be important to test these interactions in native neurons and cardiomyocytes because β1 interactions are known to be dependent on cell type and these interactions may be limited to heterologous overexpression systems. VGKC modulation by β1 may be cell type dependent as well. CA1 pyramidal neurons express a high level of Kv4.2 mediated A-type IK but their excitability is largely unaffected in Scn1b null or homozygous mutant neurons (Chen, Yuan et al. 2006, Patino, Claes et al. 2009, Uebachs, Opitz et al. 2010, Reid, Leaw et al. 2014). Scn1b null mice exhibit reduced transient outward IK in DRG neurons, demonstrating that the effects of β1 on VGKCs is not limited to the brain (Lopez-Santiago, Brackenbury et al. 2011). Potential effects of other VGSC β subunits on VGKCs in the nervous system have not been investigated, however, large effects on IK in Scn2b null mouse cardiomyocytes suggest that other β subunits may also modulate VGKC function (Bao, Willis et al. 2016).
Section 2 part 8: β Subunit Function in Brain Development
VGSC β subunits function in nervous system development on several levels, including neurite outgrowth, axonal pathfinding, dendritic arborization, neuronal patterning, and cell migration. Neurite extension of WT CGNs plated on β1 expressing fibroblast monolayers is increased compared to control monolayers and requires β1- β1 trans homophilic cell adhesion. In contrast, β2 expressing fibroblast monolayers reduce neurite outgrowth of WT CGNs and β4 has no detectable effects. β1 function in neurite outgrowth requires both cell-cell adhesion and sodium channel activity, particularly current carried by Nav1.6. Deletion of Cntn1, Fyn, or Scn8a or the addition of TTX abrogated β1-mediated neurite outgrowth in CGNs. Unlike other CAMs, β1 dependent neurite outgrowth is independent of the FGF mediated neurite outgrowth pathway. Scn1b null mice have several nervous system structural defects that may be directly related to altered neurite outgrowth. These include cerebellar parallel fiber defasiculation, CGN axonal mistargeting, dentate granule neuron axonal mistargeting, corticospinal tract defasiculation, and decreased dendritic arborization in pyramidal neurons of the subiculum (Brackenbury, Davis et al. 2008, Brackenbury, Calhoun et al. 2010, Brackenbury, Yuan et al. 2013, Reid, Leaw et al. 2014). Structural defects in Scn1b null brain go beyond the regulation of neuronal process extension, also impacting cell migration, proliferation, and neuronal patterning defects. In the cerebellum there is an expansion of the external germinal layer and in the hippocampus there is increased proliferation of dentate granule neurons, ectopic Prox1 positive neurons, and increased thickness of the dentate gyrus granule cell layer with a reduction in dentate granule neuron number (Brackenbury, Davis et al. 2008, Brackenbury, Yuan et al. 2013).
Section 2 part 9: Differential Effects of β Subunits on Excitability
The wide array of β1-mediated effects on neuronal excitability is consistent with the multi-functionality of β1. Several of these effects can be linked directly to modulation of VGSC α subunit expression and function, while others are attributable to VGKC modulation or altered neuronal morphology. Scn1b deletion in mice results in differential effects on excitability at the single neuron level with some populations showing increased excitability, some decreased, and others unaffected. Cultured Scn1b null CGNs show a decrease in the number of APs fired in response to depolarizing current injections compared to WT (Brackenbury, Calhoun et al. 2010). There are no measureable changes in the level of transient INa in these cells, but instead a decrease in the level of resurgent INa, which is known to be important in repetitive firing of these neurons (Brackenbury, Calhoun et al. 2010, Lewis and Raman 2014). Many Scn1b null neuron populations, including dentate granule neurons, CA1 pyramidal neurons, and hippocampal interneurons, have unaffected excitability profiles compared to WT (Patino, Claes et al. 2009, Uebachs, Opitz et al. 2010, Uebachs, Albus et al. 2012). Scn1b null optic nerve has reduced speed of the fastest component of the compound AP, suggesting decreased excitability (Chen, Westenbroek et al. 2004).
The reported differential effects on excitability appear to be dependent on different functions of Scn1b. Scn1b null cortical layer 5 pyramidal neurons fire more APs compared to WT with an increased action potential half width, indicating loss of β1 modulation of Kv4.2 may be a factor (Marionneau, Carrasquillo et al. 2012). However, in homozygous knockin mice expressing the epilepsy causing Scn1b-C121W mutation, this population is unaffected (Reid, Leaw et al. 2014). While these results are seemingly at odds, they may provide clues to even more nuanced differences in the effects of Scn1b on neuronal excitability. The β1-C121W mutation interferes with both α subunit interaction and cell adhesion but with unknown impact on modulation of VGKCs (Meadows, Malhotra et al. 2002, Kruger, O'Malley et al. 2016). This may indicate subpopulations of the same neuron type in different regions of cortex may be affected in different ways or that C121W mutants may modulate excitability differently than total loss of Scn1b. The latter may be the case because C121W heterozygous mice have increased seizure susceptibility relative to Scn1b+/− mice (Kruger, O'Malley et al. 2016). Hyperexcitability has been found in layer 2/3 pyramidal neurons and pyramidal neurons of the subiculum in mice homozygous for the β1-C121W mutation. These increases in excitability can be attributed to differences in structural development of these neurons, as they have increased input resistance and reduced cell surface area, measured by whole cell capacitance and immunohistochemistry, respectively (Reid, Leaw et al. 2014). Interestingly, this may be a way that reductions in VGSC function can drive hyperexcitability via cross-talk between INa and neuronal development.
The effects of Scn2b on excitability are not as well studied as those of Scn1b. We do know, however, that Scn2b null mice have increased threshold and reduction in amplitude of extracellularly recorded compound APs in the optic nerve, suggesting that reduced levels of VGSCs at the cell surface result in reduced excitability. Scn2b deletion also increases susceptibility to pilocarpine-induced seizures, suggesting aberrant neuronal excitability elsewhere in the CNS (Chen, Bharucha et al. 2002). While the effects of Scn2b deletion on DRG AP firing have not been measured, altered VGSC function and reduced pain sensitivity in Scn2b null mice suggest reduced DRG neuron excitability (Lopez-Santiago, Pertin et al. 2006).
The effects of Scn3b on nervous system excitability have not been reported, but there is extensive work showing impacts on cardiomyocyte excitability (Hakim, Gurung et al. 2008, Hakim, Brice et al. 2010).
Scn4b’s effects on neuronal excitability appear to be largely related to its role in regulating persistent and resurgent INa. Scn4b null mice have reduced repetitive firing of APs in striatal projection neurons and Scn4b knockdown in cultured CGNs causes reduced excitability, suggesting that reductions in resurgent INa impact excitability (Bant and Raman 2010, Miyazaki, Oyama et al. 2014). In a similar manner, reduced persistent and resurgent current in Scn1b null CGNs decrease excitability (Brackenbury, Calhoun et al. 2010).
Section 3: β Subunits in Diseases of the Nervous System
β subunits play important roles in both the mechanism and treatment of several disorders of the nervous system. The underlying links between β subunit gene mutations and these disparate diseases result from the numerous biochemical and physiological functions outlined in sections 1 and 2. Some can be linked to effects on neuronal and network excitability, while others may be associated with the effects of β subunits in brain development. The most direct link to disease is between mutations in SCN1B and epilepsy. SCN1B mutations in human patients are linked to two pediatric epilepsies: generalized epilepsy with febrile seizures plus (GEFS+) and the pediatric epileptic encephalopathy Dravet syndrome (DS). Links also exist between the β subunits and several other disorders including neuropathic pain, neurodegenerative diseases, neurodevelopmental disorders, and sudden infant death syndrome (SIDS). Thus, β subunits are important and novel therapeutic targets due to their unique expression profiles, functions, and pharmacology.
Section 3 Part 1: β Subunits and Epilepsy
Epilepsy is a condition characterized by unprovoked recurrent seizures (Fisher, Acevedo et al. 2014). Up to 40% of epilepsy cases have unknown origin (Beck papers for citation). Exceptions to this are the genetic epilepsies, which can often be linked directly to specific ion channel gene mutations. Mutations in several VGSC genes, including SCN1B, are known to cause epilepsy (Meisler, O’brien et al. 2010). One of the first identified mutations linked to human epilepsy was SCN1B-C121W that results in GEFS+ (Wallace, Wang et al. 1998). GEFS+ is characterized by early pediatric seizures associated with fever followed by febrile and afebrile seizures. GEFS+ is one of a multitude of genetic pediatric epilepsies associated with VGSC mutations. The most extreme of these VGSC related epilepsies is Dravet syndrome, a debilitating epileptic encephalopathy resulting in severe epilepsy, ataxia, cognitive impairment, autism, sleep abnormalities, and a high risk of sudden unexpected death in epilepsy (SUDEP) (Scheffer, Zhang et al. 2010, Skluzacek, Watts et al. 2011). More than 70% of DS cases are caused by de novo loss-of-function mutations in SCN1A, however a small subset has been identified to have inherited homozygous mutations in SCN1B (Patino, Claes et al. 2009, Scheffer, Zhang et al. 2010, Ogiwara, Nakayama et al. 2012, Ramadan, Patel et al. 2017). The first of the SCN1B associated DS cases identified was a loss of function mutation, SCN1B-R125C that results in failure of β1 subunits to traffic to the cell surface (Patino, Claes et al. 2009). Simple loss-of-function is a viable explanation for the mechanism of SCN1B-linked DS, as Scn1b null mice recapitulate the full DS phenotype, including frequent spontaneous seizures, ataxia, developmental delay, and early lethality (Chen, Westenbroek et al. 2004). This phenotype is more severe than is found in Scn1a DS mice. 100% of Scn1b null mice die by postnatal day (P)21, which may explain the limited detection of patients with homozygous SCN1B mutations in the human population (Chen, Westenbroek et al. 2004). Even though β1-C121W polypeptides are expressed at the neuronal cell surface, mice homozygous for the GEFS+ mutation, Scn1b-C121W, recapitulate the phenotype of Scn1b null mice, suggesting loss-of-function of a cell surface expressed subunit (Reid, Leaw et al. 2014, Kruger, O'Malley et al. 2016). Interestingly, mice heterozygous for the Scn1b-C121W allele have increased susceptibility to febrile seizures compared to mice heterozygous for the Scn1b null allele (Kruger, O'Malley et al. 2016). Differences in excitability at the single neuron level are not always consistent between the Scn1b null and Scn1b-C121W homozygous mouse models (Marionneau, Carrasquillo et al. 2012, Reid, Leaw et al. 2014). Several defects in AP waveforms in Scn1b-C121W heterozygous mice are absent in Scn1b-C121W homozygous mice (Wimmer, Reid et al. 2010, Reid, Leaw et al. 2014). Thus, SCN1B associated GEFS+ and DS likely have distinct mechanisms.
A SCN1B mutation specific to β1b, G25R, was identified in multiple pedigrees with idiopathic epilepsy (Patino, Brackenbury et al. 2011). Excluding this mutation, all the SCN1B epilepsy-causing mutations are within or near the Ig domain, which is important in both α subunit modulation and cell adhesion as described in section 1. Importantly, in the Scn1b null mouse, developmental defects appear prior to the onset of seizures or evidence of altered neuronal excitability, suggesting a causative effect of aberrant brain development on altered excitability (Brackenbury, Yuan et al. 2013). As described in section two, the hyperexcitability of homozygous Scn1b-C121W subicular pyramidal neurons and cortical layer 2/3 pyramidal neurons appears to be a result of decreased cell size (Reid, Leaw et al. 2014). Importantly, specific deletion of Scn1a in forebrain GABAergic interneurons is sufficient to phenocopy DS in mice (Cheah, Frank et al. 2012, Ogiwara, Iwasato et al. 2013). Little is known about altered interneuron function in Scn1b models of DS, but initial experiments in hippocampal interneurons suggest that they are not affected (Patino, Claes et al. 2009, Reid, Leaw et al. 2014). Given the brain region and cell type specificity of β1 subunit expression discussed in section 2, it is important to expand our understanding of β1 function in the CNS to different neuron types. This may be a useful guide for understanding the exact microcircuit components that are altered in DS and GEFS+ and help us to understand how similar epileptic phenotypes may arise from different mechanisms in specific cell populations.
β subunits are also important in the treatment and pathophysiology of other epilepsies. Approximately 30% of epilepsy patients lack adequate seizure control with existing antiepileptic drugs (AEDs) for unknown reasons (Kwan and Brodie 2000). The actions of β subunits affect the efficacy of two known AEDs, carbamazepine and phenytoin (Lucas, Meadows et al. 2005, Uebachs, Opitz et al. 2010, Uebachs, Albus et al. 2012). A GEFS+-linked SCN1B mutation causes a reduction in the efficacy of phenytoin, attenuating the drug’s effects on frequency dependent block of sodium current (Lucas, Meadows et al. 2005). The effects of carbamazepine are also affected by β subunits. Scn1b null neurons show a paradoxical increase in persistent current when treated with carbamazepine, abolishing the usual spiking attenuation caused by this drug (Uebachs, Opitz et al. 2010, Uebachs, Albus et al. 2012).
VGSC β subunit expression is differentially impacted in several forms of epilepsy. In pilocarpine induced seizures, there is a downregulation of Scn1b and Scn2b mRNA following status epilepticus with impacts on sodium current consistent with known functions of these two subunits (Gastaldi, Robaglia-Schlupp et al. 1998, Ellerkmann, Remy et al. 2003). This result may be model or species dependent, as there is no evidence for similar increases in temporal lobe epilepsy patients (Gassen, Koen et al. 2008). There is however, a reduction in SCN3B and β3 expression in the hippocampus of temporal lobe epilepsy patients and increased SCN1B and β1 in reactive astrocytes of both epileptic and non-epileptic brain trauma patients. (Gorter, Van Vliet et al. 2002, Aronica, Troost et al. 2003, Gassen, Koen et al. 2008, Das, Wallace et al. 2012).
Section 3 Part 2: β Subunits in Sudden Unexpected Death in Epilepsy and Sudden Infant Death Syndrome
Sudden Unexpected Death in Epilepsy (SUDEP) is defined as death in patients with epilepsy that is not due to status epilepticus, trauma or drowning (Nashef, So et al. 2012). SUDEP occurs at a high rate among DS patients, with a 6-fold increase in risk above other epilepsies and 24-fold higher risk of sudden death than in the general population (Skluzacek, Watts et al. 2011). As mentioned above, 100% of Scn1b-DS mice die by P21. This phenotype is more severe than for Scn1a DS mouse models and other models of SUDEP, such as the Kcna1 null mouse, which exhibit death rates of ~50% and ~25%, respectively (Chen, Westenbroek et al. 2004, Frank, Mantegazza et al. 2006, Glasscock, Yoo et al. 2010). This positions the Scn1b null mouse as a reliable model in the study of SUDEP mechanisms. While the mechanism of SUDEP is not understood, it likely involves a “perfect storm” of catastrophic events. Proposed mechanisms of SUDEP include the effects of seizures on the brain and other organ systems resulting in spreading depression to the brain stem, autonomic dysfunction, cardiac arrhythmias, apnea, or pulmonary edema. SUDEP in DS patients typically occurs in the night and thus may involve defects in the autonomic nervous system, resulting in shutdown of breathing or arousal centers, as well as cardiac arrhythmias (Goldman, Glasscock et al. 2009). In addition to severe seizures, Scn1b null mice have cardiac arrhythmias, the combination of which may result in sudden death (Lin, O'malley et al. 2015). SIDS may also be related to β subunit dysfunction. The cause of SIDS remains unclear and likely varies. Sleep position is a known SIDS risk factor but genetic factors including β subunit gene mutations are now known to contribute (Krous, Beckwith et al. 2004, Tan, Pundi et al. 2010) . Several instances of SIDS have been linked to SCN3B and SCN4B mutations (Tan, Pundi et al. 2010). Scn4b null mice have spontaneous unexplained death without an apparent seizure phenotype, but this occurs post development and may be unrelated to SIDS (Miyazaki, Oyama et al. 2014). Mutations in β subunits may be related to SIDS through malfunctions of their roles in the heart, but there are also several overlaps between CNS developmental malformations and reactive astrogliosis in SIDS and the deficits seen in β subunit null models (Kinney, Brody et al. 1991, Cruz-Sánchez, Lucena et al. 1997, Gorter, Van Vliet et al. 2002, Aronica, Troost et al. 2003, Paine, Jacques et al. 2014).
Section 3 Part 3: β subunits and Pain
β subunits are involved in both nociceptive and neuropathic pain, and are expressed in fiber types responsible for pain sensation, as outlined in section 2. Thus, the normal pain sensing pathways that encode information through firing rates may be dependent on the differential expression of β subunits. Deletion of Scn1b results in DRG neuron hyperexcitability, predicting that these mice have allodynia (Lopez-Santiago, Brackenbury et al. 2011). In contrast, Scn2b deletion results in insensitivity to inflammatory and neuropathic pain paradigms (Lopez-Santiago, Pertin et al. 2006). Several pain models show perturbations of β subunit protein or mRNA expression. Scn1b mRNA is increased in dorsal horn neurons in a constrictive nerve injury model (Blackburn-Munro and Fleetwood-Walker 1999). In several injury models the expression levels of β2 mRNA and protein are perturbed in a cell type and injury specific manner, suggesting unique pathways for β2 regulation in different cell types and injury paradigms (Coward, Jowett et al. 2001, Pertin, Ji et al. 2005, Lopez-Santiago, Pertin et al. 2006). Scn3b is upregulated in a fiber type dependent manner in several different nerve injury models, further suggesting that the cell type specificity of β subunit expression is important in the generation of acute and chronic pain (Shah, Stevens et al. 2000, Takahashi, Kikuchi et al. 2003). Scn4b siRNA delivered to the DRG interferes with mechanical hypersensitivity in a radicular pain model. In this model, Scn4b siRNA interferes with increases in transient and resurgent current present in cultured DRG neurons following inflammation induction (Xie, Tan et al. 2016). β4 may be mechanistically important in SCN9A-linked paroxysmal extreme pain disorder. In this model, there is an increase in DRG excitability and resurgent current sodium current carried by Nav1.7, which may be regulated in vivo by β4 (Theile, Jarecki et al. 2011). The unique expression profile of each β subunit may make them valuable targets for pain therapeutics. For example, because β1 subunits are already known to impact the pharmacology of pain related drugs such as lidocaine and several neurotoxins, they may be readily targetable.
Section 3 Part 4: β Subunits in Demyelinating and Neurodegenerative Disorders
There are several lines of evidence to show that β subunits are important in demyelinating and neurodegenerative disorders. Scn1b null mice have defects in myelination in the CNS and PNS, including a reduction in the number of nodes of Ranvier in the optic nerve, improper paranodal development in CNS and PNS, brain and spinal cord dysmyelination, and increased axonal degeneration (Chen, Westenbroek et al. 2004). While the axo-glial cell adhesive complexes at paranodes involving β1, contactin-1, Caspr, and NF-155 are obvious candidates for this defect, the impact of sodium current defects may also be important, as channel activity is involved in the establishment of myelination (Gibson, Purger et al. 2014). While Scn2b null mice have normal myelination, Scn2b deletion ameliorates disease severity in a mouse model of experimental allergic encephalomyelitis (EAE), showing decreased axonal degeneration, fewer demyelinated and dysmyelinated fibers, reduced death, and reduced symptom severity compared to WT mice (Chen, Bharucha et al. 2002, O'Malley, Shreiner et al. 2009). Interestingly, MS patient cerebrospinal fluid has decreased BACE1 activity, suggesting that cleavage of the β subunits may be impacted and thus clinically relevant, as disease severity is correlated with the degree of BACE1 reduction (Mattsson, Axelsson et al. 2009).
Sequential BACE1 and γ-secretase cleavage of β subunits may also be important in Alzheimer’s disease. In Alzheimer’s disease, cleavage of amyloid precursor protein releases Amyloid β peptide, which accumulates in the brain and forms aggregates (Evin, Barakat et al. 2010). Cleavage of the VGSC β subunits involves this same pathway and may underlie the hyperexcitability and increased seizure risk observed in Alzheimer’s (Amatniek, Hauser et al. 2006). β subunits are also indicated in the pathophysiology of several other neurodegenerative diseases. In an amyotrophic lateral sclerosis model, there is downregulation of Scn1b and upregulation of Scn3b in the ventral dorsal horn, potentially accounting for observed neuronal hyperexcitability there (Nutini, Spalloni et al. 2011). Scn4b may be important in Parkinson’s disease and Huntington’s disease, potentially related to its high expression in neurons in the substantia nigra and striatum (Lein, Hawrylycz et al. 2007, Miyazaki, Oyama et al. 2014). In a model of Parkinson’ disease, Scn4b mRNA expression, β4 protein expression, and the extent of β4 glycosylation are increased (Zhou, Zhang et al. 2012). In a model of Huntington’s disease, β4 expression is reduced in neurons expressing mutant huntingtin (Oyama, Miyazaki et al. 2006). Scn4b null mice have decreased resurgent sodium current and hypoexcitability in the striatal projection neurons, representing a possible role of altered excitability in striatal neurodegeneration in Huntington’s disease (Miyazaki, Oyama et al. 2014).
Section 3 Part 5: β Subunits in Neurodevelopmental and Neuropsychiatric Disorders
Many epilepsy patients have comorbidities including autism, intellectual disability, impaired sleep, and mood disorders (Steffenburg, Gillberg et al. 1996, Levisohn 2007, Louis 2011, Lau, Ettinger et al. 2012). DS in particular is characterized as having high incidences of autism and intellectual disability and Scn1a+/− mice model autism spectrum disease (Han, Tai et al. 2012, Villeneuve, Laguitton et al. 2014). It is unclear if intellectual disability in DS is the result of epilepsy, single neuron excitability, or altered neuronal development. Altered CNS development in Scn1b null mice may predict intellectual disability and precede the onset of epilepsy and neuronal hyperexcitability (Brackenbury, Yuan et al. 2013). Mood disorders are a common comorbidity in epilepsy patients. Mutations in Ank3, encoding ankyrin G, have been found in bipolar disorder (Ferreira, O'Donovan et al. 2008). Recent work has shown hypoexcitability of parvalbumin positive interneurons in an Ank3 mutant mouse model of bipolar disorder that may link epilepsy and mood disorders (Lopez, Wang et al. 2016). Because ankyrin G is a VGSC β1 interacting protein and reductions in VGSC function in axons are proposed to underlie hypoexcitability of parvalbumin positive interneurons in this model, it is feasible that β subunits play a role in this connection.
Conclusion
The VGSC β subunits are key players in the development of excitability in the nervous system (Fig. 2). β subunits are more than simple accessories of the pore-forming α subunits and instead confer several unique properties to VGSCs that allow precise control of excitability in a cell type specific manner. β subunit multifunctionality allows for crosstalk between VGSC and VGKC function in the CNS and PNS. The cell adhesive properties of β subunits link neuronal development to excitability. Sodium current impacts brain structural development through β subunit dependent pathways and defects in structure resulting from β subunit gene deletion can drive hyperexcitability. These important properties implicate the VGSC β subunits in the mechanisms and potential treatments of several diseases of the nervous system. The cell type dependent properties and unique pharmacology of β subunits are also emerging as informative research tools to study disease mechanisms and to develop novel therapeutics. While the term channelopathy implies defects in the pore-forming subunit of the VGSC signaling complex, the non-pore-forming components are also critical in physiology and disease.
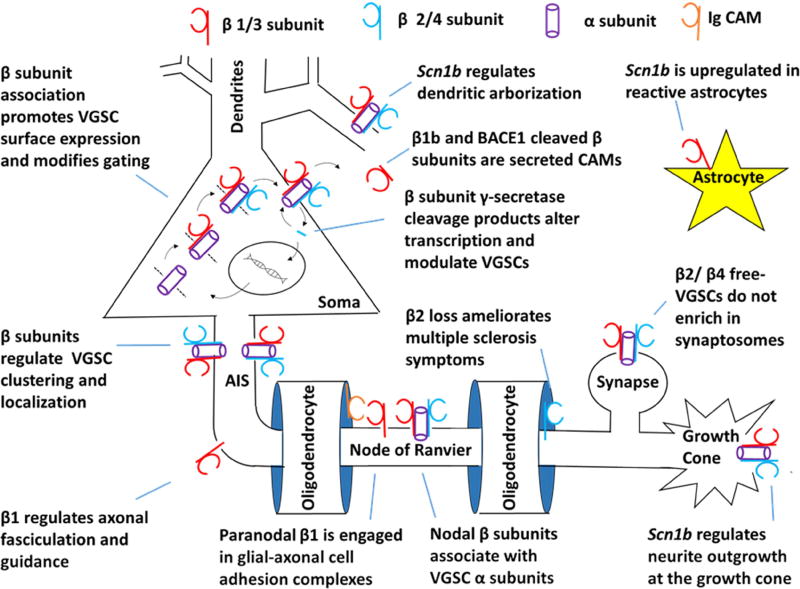
Figure 2. A multitude of functions of VGSC β subunits in the nervous system.
β subunits modulate VGSC function and trafficking to the cell surface (Brackenbury and Isom 2011). Sequential cleavage of β subunits by BACE1 and γ secretase alters transcription, including that of VGSC α subunits, and releases the Ig loop (similar to β1B) as an extracellular signaling molecule (Wong, Sakurai et al. 2005, Gersbacher, Kim et al. 2010, Patino, Brackenbury et al. 2011). β subunits alter the AIS localization of specific α subunits and can mediate clustering of α subunits (Brackenbury, Calhoun et al. 2010, Namadurai, Balasuriya et al. 2014). Loss of β1 expression alters axonal fasiculation and pathfinding (Brackenbury and Isom 2011). β1 subunits at nodes of Ranvier associate with α subunits, likely modifying their function. β1 in paranodes is engaged in heterophilic cell adhesive complexes independnetly of VGSC α subunits (Kazarinova-Noyes, Malhotra et al. 2001, McEwen and Isom 2004). Loss of β2 ameliorates symptoms of multiple sclerosis, likely through axonal-oligodendrocyte interactions (O'Malley, Shreiner et al. 2009). α subunits in the absence of β2 or β4 fail to enrich in synaptosomes, suggesting roles in VGSC expression at the synapse (Schmidt, Rossie et al. 1985, Schmidt and Catterall 1986). β1 facilitates neurite outgrowth at growth cones, requiring INa (Brackenbury, Calhoun et al. 2010). Mutation of Scn1b decreases dendritic arborization, influencing neuronal input resistance and excitability (Reid, Leaw et al. 2014). Finally, β1 is upregulated in reactive astrocytes (Aronica, Troost et al. 2003).
Highlights.
Sodium channel β subunits are not merely accessories to α subunits.
β subunits are channel modulators, cell adhesion molecules, and transcriptional regulators.
Mutations in β subunit genes result in neurological disease.
β subunit-linked diseases broaden the term ‘channelopathy’ beyond defects in ion conduction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akin EJ, Solé L, Johnson B, el Beheiry M, Masson J-B, Krapf D, Tamkun MM. Single-Molecule Imaging of Na v 1.6 on the Surface of Hippocampal Neurons Reveals Somatic Nanoclusters. Biophysical Journal. 2016;111(6):1235–1247. doi: 10.1016/j.bpj.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM. Regulation of persistent Na current by interactions between β subunits of voltage-gated Na channels. Journal of Neuroscience. 2009;29(7):2027–2042. doi: 10.1523/JNEUROSCI.4531-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47(5):867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, Troost D, Rozemuller AJ, Yankaya B, Jansen GH, Isom LL, Gorter JA. Expression and regulation of voltage-gated sodium channel β1 subunit protein in human gliosis-associated pathologies. Acta neuropathologica. 2003;105(5):515–523. doi: 10.1007/s00401-003-0677-2. [DOI] [PubMed] [Google Scholar]
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel α subunit with novel gating properties. Neuron. 1988;1(6):449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Bant JS, Raman IM. Control of transient, resurgent, and persistent current by open-channel block by Na channel β4 in cultured cerebellar granule neurons. Proceedings of the national academy of sciences. 2010;107(27):12357–12362. doi: 10.1073/pnas.1005633107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Isom LL. Na v 1.5 and regulatory β subunits in cardiac sodium channelopathies. Cardiac Electrophysiology Clinics. 2014;6(4):679–694. [Google Scholar]
- Bao Y, Willis BC, Frasier CR, Lopez-Santiago LF, Lin X, Ramos-Mondragón R, Auerbach DS, Chen C, Wang Z, Anumonwo J. Scn2b Deletion in Mice Results in Ventricular and Atrial Arrhythmias. Circulation: Arrhythmia and Electrophysiology. 2016;9(12):e003923. doi: 10.1161/CIRCEP.116.003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn-Munro G, Fleetwood-Walker S. The sodium channel auxiliary subunits β1 and β2 are differentially expressed in the spinal cord of neuropathic rats. Neuroscience. 1999;90(1):153–164. doi: 10.1016/s0306-4522(98)00415-1. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Calhoun JD, Chen C, Miyazaki H, Nukina N, Oyama F, Ranscht B, Isom LL. Functional reciprocity between Na+ channel Nav1. 6 and β1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proceedings of the National Academy of Sciences. 2010;107(5):2283–2288. doi: 10.1073/pnas.0909434107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, Dickendesher TL, Ranscht B, Isom LL. Voltage-gated Na+ channel β1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. Journal of Neuroscience. 2008;28(12):3246–3256. doi: 10.1523/JNEUROSCI.5446-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Isom LL. Na+ channel β subunits: overachievers of the ion channel family. Frontiers in pharmacology. 2011;2:53. doi: 10.3389/fphar.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Yuan Y, O’Malley HA, Parent JM, Isom LL. Abnormal neuronal patterning occurs during early postnatal brain development of Scn1b-null mice and precedes hyperexcitability. Proceedings of the National Academy of Sciences. 2013;110(3):1089–1094. doi: 10.1073/pnas.1208767110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Rasband MN. Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier. Journal of Neuroscience. 2013;33(14):6191–6202. doi: 10.1523/JNEUROSCI.4051-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun JD, Isom LL. Voltage Gated Sodium Channels. Springer; 2014. The role of non-pore-forming β subunits in physiology and pathophysiology of voltage-gated sodium channels; pp. 51–89. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. The Journal of physiology. 2012;590(11):2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, A G, Waxman S. International union of pharmacology. xlvii. Nomenclature and structure–function relationships of voltage-gated sodium channels. Pharmacological Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Cheah CS, Frank HY, Westenbroek RE, Kalume FK, Oakley JC, Potter GB, Rubenstein JL, Catterall WA. Specific deletion of NaV1. 1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proceedings of the National Academy of Sciences. 2012;109(36):14646–14651. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, Kazen-Gillespie KA. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel β2-subunits. Proceedings of the National Academy of Sciences. 2002;99(26):17072–17077. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Calhoun JD, Zhang Y, Lopez-Santiago L, Zhou N, Davis TH, Salzer JL, Isom LL. Identification of the cysteine residue responsible for disulfide linkage of Na+ channel α and β2 subunits. Journal of Biological Chemistry. 2012;287(46):39061–39069. doi: 10.1074/jbc.M112.397646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O'Malley HA, Bharucha V, Meadows LS. Mice lacking sodium channel β1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. Journal of Neuroscience. 2004;24(16):4030–4042. doi: 10.1523/JNEUROSCI.4139-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan L-L, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4. 2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 2006;26(47):12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Dale TJ, Romanos MA, Whitaker WR, Xie XM, Clare JJ. Cloning, distribution and functional analysis of the type III sodium channel from human brain. European journal of neuroscience. 2000;12(12):4281–4289. [PubMed] [Google Scholar]
- Coward K, Jowett A, Plumpton C, Powell A, Birch R, Tate S, Bountra C, Anand P. Sodium channel β1 and β2 subunits parallel SNS/PN3 α-subunit changes in injured human sensory neurons. Neuroreport. 2001;12(3):483–488. doi: 10.1097/00001756-200103050-00012. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annual review of physiology. 1996;58(1):349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Cruz-Sánchez FF, Lucena J, Ascaso C, Tolosa E, Quintò L, Rossi ML. Cerebellar cortex delayed maturation in sudden infant death syndrome. Journal of Neuropathology & Experimental Neurology. 1997;56(4):340–346. doi: 10.1097/00005072-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Cusdin FS, Nietlispach D, Maman J, Dale TJ, Powell AJ, Clare JJ, Jackson AP. The sodium channel β3-subunit induces multiphasic gating in NaV1. 3 and affects fast inactivation via distinct intracellular regions. Journal of Biological Chemistry. 2010;285(43):33404–33412. doi: 10.1074/jbc.M110.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Wallace G, Holmes C, McDowell ML, Smith JA, Marshall JD, Bonilha L, Edwards JC, Glazier SS, Ray SK. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012;220:237–246. doi: 10.1016/j.neuroscience.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Gilchrist J, Bosmans F, Van Petegem F. Binary architecture of the Nav1. 2-β2 signaling complex. Elife. 2016;5:e10960. doi: 10.7554/eLife.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes I, Tomaselli GF. Modulation of Kv4. 3 current by accessory subunits. FEBS letters. 2002;528(1–3):183–188. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- Ellerkmann R, Remy S, Chen J, Sochivko D, Elger C, Urban B, Becker A, Beck H. Molecular and functional changes in voltage-dependent Na+ channels following pilocarpine-induced status epilepticus in rat dentate granule cells. Neuroscience. 2003;119(2):323–333. doi: 10.1016/s0306-4522(03)00168-4. [DOI] [PubMed] [Google Scholar]
- Evin G, Barakat A, Masters CL. BACE: Therapeutic target and potential biomarker for Alzheimer's disease. The international journal of biochemistry & cell biology. 2010;42(12):1923–1926. doi: 10.1016/j.biocel.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nature genetics. 2008;40(9):1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J, Forsgren L, French JA, Glynn M. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Frank HY, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nature neuroscience. 2006;9(9):1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Gajewiak J, Azam L, Imperial J, Walewska A, Green BR, Bandyopadhyay PK, Raghuraman S, Ueberheide B, Bern M, Zhou HM. A disulfide tether stabilizes the block of sodium channels by the conotoxin μO § -GVIIJ. Proceedings of the National Academy of Sciences. 2014;111(7):2758–2763. doi: 10.1073/pnas.1324189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen V, Koen L, De Wit M, Koerkamp MJ, Rensen MG, Van Rijen PC, Holstege FC, Lindhout D, De Graan PN. Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia. 2008;49(6):1055–1065. doi: 10.1111/j.1528-1167.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- Gastaldi M, Robaglia-Schlupp A, Massacrier A, Planells R, Cau P. mRNA coding for voltage-gated sodium channel β2 subunit in rat central nervous system: cellular distribution and changes following kainate-induced seizures. Neuroscience letters. 1998;249(1):53–56. doi: 10.1016/s0304-3940(98)00394-2. [DOI] [PubMed] [Google Scholar]
- Gersbacher MT, Kim DY, Bhattacharyya R, Kovacs DM. Identification of BACE1 cleavage sites in human voltage-gated sodium channel beta 2 subunit. Molecular neurodegeneration. 2010;5(1):61. doi: 10.1186/1750-1326-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J, Das S, Van Petegem F, Bosmans F. Crystallographic insights into sodium-channel modulation by the β4 subunit. Proceedings of the National Academy of Sciences. 2013;110(51):E5016–E5024. doi: 10.1073/pnas.1314557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1. 1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. Journal of Neuroscience. 2010;30(15):5167–5175. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL, Snutch T, Lübbert H, Dowsett A, Marshall J, Auld V, Downey W, Fritz LC, Lester HA, Dunn R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proceedings of the National Academy of Sciences. 1986;83(19):7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A, Glasscock E, Yoo J, Chen T, Klassen T, Noebels J. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Science translational medicine. 2009;1(2):2ra6–2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, Van Vliet EA, Da Silva FHL, Isom LL, Aronica E. SHORT COMMUNICATION Sodium channel β1-subunit expression is increased in reactive astrocytes in a rat model for mesial temporal lobe epilepsy. European Journal of Neuroscience. 2002;16(2):360–364. doi: 10.1046/j.1460-9568.2002.02078.x. [DOI] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel β4 as a mechanism for resurgent sodium current. Neuron. 2005;45(2):233–244. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Hakim P, Brice N, Thresher R, Lawrence J, Zhang Y, Jackson A, Grace A, Huang CH. Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta physiologica. 2010;198(1):47–59. doi: 10.1111/j.1748-1716.2009.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim P, Gurung IS, Pedersen TH, Thresher R, Brice N, Lawrence J, Grace AA, Huang CL-H. Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Progress in biophysics and molecular biology. 2008;98(2):251–266. doi: 10.1016/j.pbiomolbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Frank HY, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, Horacio O, Catterall WA. Autistic-like behaviour in Scn1a+/−mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489(7416):385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R, Messner D, Coppersmith J, Catterall WA. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical beta subunits. Journal of Biological Chemistry. 1982;257(23):13888–13891. [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sinauer Sunderland, MA: 2001. [Google Scholar]
- Huth T, Rittger A, Saftig P, Alzheimer C. β-Site APP-cleaving enzyme 1 (BACE1) cleaves cerebellar Na+ channel β4-subunit and promotes Purkinje cell firing by slowing the decay of resurgent Na+ current. Pflügers Archiv-European Journal of Physiology. 2011;461(3):355–371. doi: 10.1007/s00424-010-0913-2. [DOI] [PubMed] [Google Scholar]
- Isom L, Ragsdale D, De Jongh K, Westenbroek RE, Reber B, Scheuer T, Catterall WA. Structure and function of the β2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83(3):433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- Isom LL, Catterall WA. Na+ channel subunits and Ig domains. Nature. 1996;383(6598):307. doi: 10.1038/383307b0. [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh K. Primary Structure and Functional Expression of the (Beta1) Subunit of the Rat Brain Sodium Channel. Science. 1992;256(5058):839. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12(6):1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Johnson D, Bennett ES. Isoform-specific effects of the β2 subunit on voltage-gated sodium channel gating. Journal of Biological Chemistry. 2006;281(36):25875–25881. doi: 10.1074/jbc.M605060200. [DOI] [PubMed] [Google Scholar]
- Johnson D, Montpetit ML, Stocker PJ, Bennett ES. The sialic acid component of the β1 subunit modulates voltage-gated sodium channel function. Journal of Biological Chemistry. 2004;279(43):44303–44310. doi: 10.1074/jbc.M408900200. [DOI] [PubMed] [Google Scholar]
- Joho RH, Moorman JR, Vandongen AM, Kirsch GE, Silberberg H, Schuster G, Brown AM. Toxin and kinetic profile of rat brain type III sodium channels expressed in Xenopus oocytes. Molecular Brain Research. 1990;7(2):105–113. doi: 10.1016/0169-328x(90)90087-t. [DOI] [PubMed] [Google Scholar]
- Kazarinova-Noyes K, Malhotra JD, McEwen DP, Mattei LN, Berglund EO, Ranscht B, Levinson SR, Schachner M, Shrager P, Isom LL. Contactin associates with Na+ channels and increases their functional expression. Journal of Neuroscience. 2001;21(19):7517–7525. doi: 10.1523/JNEUROSCI.21-19-07517.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazen-Gillespie KA, Ragsdale DS, D'Andrea MR, Mattei LN, Rogers KE, Isom LL. Cloning, localization, and functional expression of sodium channel β1A subunits. Journal of Biological Chemistry. 2000;275(2):1079–1088. doi: 10.1074/jbc.275.2.1079. [DOI] [PubMed] [Google Scholar]
- Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM-Y, Woolf CJ. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nature cell biology. 2007;9(7):755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Ingano LAM, Carey BW, Pettingell WH, Kovacs DM. Presenilin/γ-secretase-mediated cleavage of the voltage-gated sodium channel β2-subunit regulates cell adhesion and migration. Journal of Biological Chemistry. 2005;280(24):23251–23261. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Finkelstein DM, Vawter GF, Mandell F, Gillies FH. Delayed central nervous system myelination in the sudden infant death syndrome. Journal of Neuropathology & Experimental Neurology. 1991;50(1):29–48. doi: 10.1097/00005072-199101000-00003. [DOI] [PubMed] [Google Scholar]
- Ko S-H, Lenkowski PW, Lee HC, Mounsey JP, Patel MK. Modulation of Nav1. 5 by β1-and β3-subunit co-expression in mammalian cells. Pflügers Archiv. 2005;449(4):403–412. doi: 10.1007/s00424-004-1348-4. [DOI] [PubMed] [Google Scholar]
- Krafte DS, Snutch TP, Leonard JP, Davidson N, Lester HA. Evidence for the involvement of more than one mRNA species in controlling the inactivation process of rat and rabbit brain Na channels expressed in Xenopus oocytes. Journal of Neuroscience. 1988;8(8):2859–2868. doi: 10.1523/JNEUROSCI.08-08-02859.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe R, Gabbiani F. Burst firing in sensory systems. Nature Reviews Neuroscience. 2004;5(1):13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, Corey T, Cutz E, Hanzlick R, Keens TG, Mitchell EA. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114(1):234–238. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- Kruger LC, O'Malley HA, Hull JM, Kleeman A, Patino GA, Isom LL. β1-C121W Is Down But Not Out: Epilepsy-Associated Scn1b-C121W Results in a Deleterious Gain-of-Function. Journal of Neuroscience. 2016;36(23):6213–6224. doi: 10.1523/JNEUROSCI.0405-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Laedermann CJ, Syam N, Decosterd I, Abriel H. β1-and β3-voltage-gated sodium channel subunits modulate cell surface expression and glycosylation of Nav1. 7 in HEK293 cells. Frontiers in cellular neuroscience. 2013;7:137. doi: 10.3389/fncel.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Ettinger AB, Hamberger S, Fanning K, Reed ML. Do mood instability symptoms in epilepsy represent formal bipolar disorder? Epilepsia. 2012;53(2):e37–e40. doi: 10.1111/j.1528-1167.2011.03372.x. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lenkowski PW, Shah BS, Dinn AE, Lee K, Patel MK. Lidocaine block of neonatal Na v 1.3 is differentially modulated by co-expression of β1 and β3 subunits. European journal of pharmacology. 2003;467(1):23–30. doi: 10.1016/s0014-2999(03)01595-4. [DOI] [PubMed] [Google Scholar]
- Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(s9):33–35. doi: 10.1111/j.1528-1167.2007.01399.x. [DOI] [PubMed] [Google Scholar]
- Lewis AH, Raman IM. Cross-species conservation of open-channel block by Na channel β4 peptides reveals structural features required for resurgent Na current. Journal of Neuroscience. 2011;31(32):11527–11536. doi: 10.1523/JNEUROSCI.1428-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AH, Raman IM. Resurgent current of voltage-gated Na+ channels. The Journal of physiology. 2014;592(22):4825–4838. doi: 10.1113/jphysiol.2014.277582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, O'malley H, Chen C, Auerbach D, Foster M, Shekhar A, Zhang M, Coetzee W, Jalife J, Fishman GI. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. The Journal of physiology. 2015;593(6):1389–1407. doi: 10.1113/jphysiol.2014.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A, Wang X, Xu M, Maheshwari A, Curry D, Lam S, Adesina A, Noebels J, Sun Q, Cooper E. Ankyrin-G isoform imbalance and interneuronopathy link epilepsy and bipolar disorder. Molecular Psychiatry. 2016 doi: 10.1038/mp.2016.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Brackenbury WJ, Chen C, Isom LL. Na+ channel Scn1b gene regulates dorsal root ganglion nociceptor excitability in vivo. Journal of Biological Chemistry. 2011;286(26):22913–22923. doi: 10.1074/jbc.M111.242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Pertin M, Morisod X, Chen C, Hong S, Wiley J, Decosterd I, Isom LL. Sodium channel β2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. Journal of Neuroscience. 2006;26(30):7984–7994. doi: 10.1523/JNEUROSCI.2211-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis EKS. Sleep and epilepsy: strange bedfellows no more. Minerva pneumologica. 2011;50(3):159. [PMC free article] [PubMed] [Google Scholar]
- Lucas PT, Meadows LS, Nicholls J, Ragsdale DS. An epilepsy mutation in the β1 subunit of the voltage-gated sodium channel results in reduced channel sensitivity to phenytoin. Epilepsy research. 2005;64(3):77–84. doi: 10.1016/j.eplepsyres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Makita N, Bennett PB, George AL., Jr Molecular determinants of β1 subunit-induced gating modulation in voltage-dependent Na+ channels. Journal of Neuroscience. 1996;16(22):7117–7127. doi: 10.1523/JNEUROSCI.16-22-07117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Chen C, Rivolta I, Abriel H, Malhotra R, Mattei LN, Brosius FC, Kass RS, Isom LL. Characterization of sodium channel α-and β-subunits in rat and mouse cardiac myocytes. Circulation. 2001;103(9):1303–1310. doi: 10.1161/01.cir.103.9.1303. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel β subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. Journal of Biological Chemistry. 2000;275(15):11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Koopmann MC, Kazen-Gillespie KA, Fettman N, Hortsch M, Isom LL. Structural requirements for interaction of sodium channel β1 subunits with ankyrin. Journal of Biological Chemistry. 2002;277(29):26681–26688. doi: 10.1074/jbc.M202354200. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel β1 subunits are differentially localized in cardiac myocytes. Journal of Biological Chemistry. 2004;279(39):40748–40754. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- Marionneau C, Carrasquillo Y, Norris AJ, Townsend RR, Isom LL, Link AJ, Nerbonne JM. The sodium channel accessory subunit Navβ1 regulates neuronal excitability through modulation of repolarizing voltage-gated K+ channels. Journal of Neuroscience. 2012;32(17):5716–5727. doi: 10.1523/JNEUROSCI.6450-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Ufodiama C, Watt I, Bland M, Brackenbury WJ. Therapeutic value of voltage-gated sodium channel inhibitors in breast, colorectal, and prostate cancer: A systematic review. Frontiers in pharmacology. 2015;6 doi: 10.3389/fphar.2015.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Axelsson M, Haghighi S, Malmeström C, Wu G, Anckarsäter R, Sankaranarayanan S, Andreasson U, Fredrikson S, Gundersen A. Reduced cerebrospinal fluid BACE1 activity in multiple sclerosis. Multiple Sclerosis Journal. 2009;15(4):448–454. doi: 10.1177/1352458508100031. [DOI] [PubMed] [Google Scholar]
- McCormick KA, Isom LL, Ragsdale D, Smith D, Scheuer T, Catterall WA. Molecular determinants of Na+ channel function in the extracellular domain of the β1 subunit. Journal of Biological Chemistry. 1998;273(7):3954–3962. doi: 10.1074/jbc.273.7.3954. [DOI] [PubMed] [Google Scholar]
- McCormick KA, Srinivasan J, White K, Scheuer T, Catterall WA. The extracellular domain of the β1 subunit is both necessary and sufficient for β1-like modulation of sodium channel gating. Journal of Biological Chemistry. 1999;274(46):32638–32646. doi: 10.1074/jbc.274.46.32638. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Chen C, Meadows LS, Lopez-Santiago L, Isom LL. The voltage-gated Na+ channel β3 subunit does not mediate trans homophilic cell adhesion or associate with the cell adhesion molecule contactin. Neuroscience letters. 2009;462(3):272–275. doi: 10.1016/j.neulet.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DP, Isom LL. Heterophilic interactions of sodium channel β1 subunits with axonal and glial cell adhesion molecules. Journal of Biological Chemistry. 2004;279(50):52744–52752. doi: 10.1074/jbc.M405990200. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Meadows LS, Chen C, Thyagarajan V, Isom LL. Sodium channel β1 subunit-mediated modulation of Nav1. 2 currents and cell surface density is dependent on interactions with contactin and ankyrin. Journal of Biological Chemistry. 2004;279(16):16044–16049. doi: 10.1074/jbc.M400856200. [DOI] [PubMed] [Google Scholar]
- Meadows LS, Malhotra J, Loukas A, Thyagarajan V, Kazen-Gillespie KA, Koopman MC, Kriegler S, Isom LL, Ragsdale DS. Functional and biochemical analysis of a sodium channel β1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. Journal of Neuroscience. 2002;22(24):10699–10709. doi: 10.1523/JNEUROSCI.22-24-10699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, O’brien JE, Sharkey LM. Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. The Journal of physiology. 2010;588(11):1841–1848. doi: 10.1113/jphysiol.2010.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick EC, Kalmar CL, Snyder SL, Cusdin FS, Ester JY, Sando JJ, Isakson BE, Jackson AP, Patel MK. The importance of serine 161 in the sodium channel β3 subunit for modulation of NaV1. 2 gating. Pflügers Archiv-European Journal of Physiology. 2010;460(4):743–753. doi: 10.1007/s00424-009-0739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner DJ, Catterall WA. The sodium channel from rat brain. Separation and characterization of subunits. Journal of Biological Chemistry. 1985;260(19):10597–10604. [PubMed] [Google Scholar]
- Miyazaki H, Oyama F, Inoue R, Aosaki T, Abe T, Kiyonari H, Kino Y, Kurosawa M, Shimizu J, Ogiwara I. Singular localization of sodium channel β4 subunit in unmyelinated fibres and its role in the striatum. Nature communications. 2014;5:5525. doi: 10.1038/ncomms6525. [DOI] [PubMed] [Google Scholar]
- Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K. β3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proceedings of the National Academy of Sciences. 2000;97(5):2308–2313. doi: 10.1073/pnas.030362197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namadurai S, Balasuriya D, Rajappa R, Wiemhöfer M, Stott K, Klingauf J, Edwardson JM, Chirgadze DY, Jackson AP. Crystal structure and molecular imaging of the Nav channel β3 subunit indicates a trimeric assembly. Journal of Biological Chemistry. 2014;289(15):10797–10811. doi: 10.1074/jbc.M113.527994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012;53(2):227–233. doi: 10.1111/j.1528-1167.2011.03358.x. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Miyazaki H, Hoshi N, Smith BJ, Nukina N, Goldin AL, Chandy KG. Modulation of voltage-gated K+ channels by the sodium channel β1 subunit. Proceedings of the National Academy of Sciences. 2012;109(45):18577–18582. doi: 10.1073/pnas.1209142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Ikeda T, Suzuki H, Takeshima H, Takahashi T, Kuno M, Numa S. Expression of functional sodium channels from cloned cDNA. Nature. 1986;322(6082):826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Nutini M, Spalloni A, Florenzano F, Westenbroek RE, Marini C, Catterall WA, Bernardi G, Longone P. Increased expression of the beta3 subunit of voltage-gated Na+ channels in the spinal cord of the SOD1G93A mouse. Molecular and Cellular Neuroscience. 2011;47(2):108–118. doi: 10.1016/j.mcn.2011.03.005. [DOI] [PubMed] [Google Scholar]
- O'Malley HA, Isom LL. Sodium channel β subunits: emerging targets in channelopathies. Annual review of physiology. 2015;77:481–504. doi: 10.1146/annurev-physiol-021014-071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley HA, Shreiner AB, Chen G-H, Huffnagle GB, Isom LL. Loss of Na+ channel β2 subunits is neuroprotective in a mouse model of multiple sclerosis. Molecular and Cellular Neuroscience. 2009;40(2):143–155. doi: 10.1016/j.mcn.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Iwasato T, Miyamoto H, Iwata R, Yamagata T, Mazaki E, Yanagawa Y, Tamamaki N, Hensch TK, Itohara S. Nav1. 1 haploinsufficiency in excitatory neurons ameliorates seizure-associated sudden death in a mouse model of Dravet syndrome. Human molecular genetics. 2013;22(23):4784–4804. doi: 10.1093/hmg/ddt331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Nakayama T, Yamagata T, Ohtani H, Mazaki E, Tsuchiya S, Inoue Y, Yamakawa K. A homozygous mutation of voltage-gated sodium channel βI gene SCN1B in a patient with Dravet syndrome. Epilepsia. 2012;53(12):e200–e203. doi: 10.1111/epi.12040. [DOI] [PubMed] [Google Scholar]
- Oyama F, Miyazaki H, Sakamoto N, Becquet C, Machida Y, Kaneko K, Uchikawa C, Suzuki T, Kurosawa M, Ikeda T. Sodium channel β4 subunit: down-regulation and possible involvement in neuritic degeneration in Huntington's disease transgenic mice. Journal of neurochemistry. 2006;98(2):518–529. doi: 10.1111/j.1471-4159.2006.03893.x. [DOI] [PubMed] [Google Scholar]
- Paine SM, Jacques TS, Sebire NJ. Review: neuropathological features of unexplained sudden unexpected death in infancy: current evidence and controversies. Neuropathology and applied neurobiology. 2014;40(4):364–384. doi: 10.1111/nan.12095. [DOI] [PubMed] [Google Scholar]
- Patino GA, Brackenbury WJ, Bao Y, Lopez-Santiago LF, O'Malley HA, Chen C, Calhoun JD, Lafrenière RG, Cossette P, Rouleau GA. Voltage-gated Na+ channel β1B: a secreted cell adhesion molecule involved in human epilepsy. Journal of Neuroscience. 2011;31(41):14577–14591. doi: 10.1523/JNEUROSCI.0361-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, O'Malley HA, Gray CB, Miyazaki H, Nukina N. A functional null mutation of SCN1B in a patient with Dravet syndrome. Journal of Neuroscience. 2009;29(34):10764–10778. doi: 10.1523/JNEUROSCI.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul JR, DeWoskin D, McMeekin LJ, Cowell RM, Forger DB, Gamble KL. Regulation of persistent sodium currents by glycogen synthase kinase 3 encodes daily rhythms of neuronal excitability. Nature Communications. 2016;7 doi: 10.1038/ncomms13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertin M, Ji R-R, Berta T, Powell AJ, Karchewski L, Tate SN, Isom LL, Woolf CJ, Gilliard N, Spahn DR. Upregulation of the voltage-gated sodium channel β2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons. Journal of Neuroscience. 2005;25(47):10970–10980. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Curtis R, Lawson D, Gilbride K, Ge P, DiStefano P, Silos-Santiago I, Catterall WA, Scheuer T. Differential modulation of sodium channel gating and persistent sodium currents by the β1, β2, and β3 subunits. Molecular and Cellular Neuroscience. 2001;18(5):570–580. doi: 10.1006/mcne.2001.1039. [DOI] [PubMed] [Google Scholar]
- Qu Y, Rogers JC, Chen S-F, McCormick KA, Scheuer T, Catterall WA. Functional roles of the extracellular segments of the sodium channel α subunit in voltage-dependent gating and modulation by β1 subunits. Journal of Biological Chemistry. 1999;274(46):32647–32654. doi: 10.1074/jbc.274.46.32647. [DOI] [PubMed] [Google Scholar]
- Ramadan W, Patel N, Anazi S, Kentab AY, Bashiri FA, Hamad MH, Jad L, Salih MA, Alsaif H, Hashem M. Confirming the recessive inheritance of SCN1B mutations in developmental epileptic encephalopathy. Clinical Genetics. 2017 doi: 10.1111/cge.12999. [DOI] [PubMed] [Google Scholar]
- Ratcliffe CF, Qu Y, McCormick KA, Tibbs VC, Dixon JE, Scheuer T, Catterall WA. A sodium channel signaling complex: modulation by associated receptor protein tyrosine phosphatase β. Nature neuroscience. 2000;3(5):437–444. doi: 10.1038/74805. [DOI] [PubMed] [Google Scholar]
- Ratcliffe CF, Westenbroek RE, Curtis R, Catterall WA. Sodium channel β1 and β3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J Cell Biol. 2001;154(2):427–434. doi: 10.1083/jcb.200102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Pinto E, Duch DS, Levinson SR, Urban BW. Purified and unpurified sodium channels from eel electroplax in planar lipid bilayers. The Journal of general physiology. 1987;90(3):375–395. doi: 10.1085/jgp.90.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Leaw B, Richards KL, Richardson R, Wimmer V, Yu C, Hill-Yardin EL, Lerche H, Scheffer IE, Berkovic SF. Reduced dendritic arborization and hyperexcitability of pyramidal neurons in a Scn1b-based model of Dravet syndrome. Brain. 2014;137(6):1701–1715. doi: 10.1093/brain/awu077. [DOI] [PubMed] [Google Scholar]
- Sashihara S, Greer CA, Oh Y, Waxman SG. Cell-Specific Differential Expression of Na+-Channel b1-Subunit mRNA in the Olfactory System during Postnatal Development and after Denervation. Journal of Neuroscience. 1996;16(2):702–713. doi: 10.1523/JNEUROSCI.16-02-00702.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Zhang YH, Gecz J, Dibbens L. Genetics of the epilepsies: Genetic twists in the channels and other tales. Epilepsia. 2010;51(s1):33–36. doi: 10.1111/j.1528-1167.2009.02440.x. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Rossie S, Catterall WA. A large intracellular pool of inactive Na channel alpha subunits in developing rat brain. Proceedings of the National Academy of Sciences. 1985;82(14):4847–4851. doi: 10.1073/pnas.82.14.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JW, Catterall WA. Biosynthesis and processing of the α subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell. 1986;46(3):437–445. doi: 10.1016/0092-8674(86)90664-1. [DOI] [PubMed] [Google Scholar]
- Shah B, Stevens E, Gonzalez M, Bramwell S, Pinnock R, Lee K, Dixon A. β3, a novel auxiliary subunit for the voltage-gated sodium channel, is expressed preferentially in sensory neurons and is upregulated in the chronic constriction injury model of neuropathic pain. European Journal of Neuroscience. 2000;12(11):3985–3990. doi: 10.1046/j.1460-9568.2000.00294.x. [DOI] [PubMed] [Google Scholar]
- Shah B, Stevens E, Pinnock R, Dixon A, Lee K. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit β3, in rat CNS. The Journal of physiology. 2001;534(3):763–776. doi: 10.1111/j.1469-7793.2001.t01-1-00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Miyazaki H, Ohsawa N, Shoji S, Ishizuka-Katsura Y, Tosaki A, Oyama F, Terada T, Sakamoto K, Shirouzu M. Structure-based site-directed photo-crosslinking analyses of multimeric cell-adhesive interactions of voltage-gated sodium channel β subunits. Scientific reports. 2016;6 doi: 10.1038/srep26618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skluzacek JV, Watts KP, Parsy O, Wical B, Camfield P. Dravet syndrome and parent associations: the IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52(s2):95–101. doi: 10.1111/j.1528-1167.2011.03012.x. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Kearney J, De Haan G, McEwen D, Escayg A, Aradi I, MacDonald B, Levin S, Soltesz I, Benna P. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for β subunit interaction. Journal of Neuroscience. 2004;24(44):10022–10034. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J, Schachner M, Catterall WA. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proceedings of the National Academy of Sciences. 1998;95(26):15753–15757. doi: 10.1073/pnas.95.26.15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Steffenburg U. Psychiatric disorders in children and adolescents with mental retardation and active epilepsy. Archives of Neurology. 1996;53(9):904–912. doi: 10.1001/archneur.1996.00550090114017. [DOI] [PubMed] [Google Scholar]
- Sutkowski E, Catterall WA. Beta 1 subunits of sodium channels. Studies with subunit-specific antibodies. Journal of Biological Chemistry. 1990;265(21):12393–12399. [PubMed] [Google Scholar]
- Takahashi N, Kikuchi S, Dai Y, Kobayashi K, Fukuoka T, Noguchi K. Expression of auxiliary β subunits of sodium channels in primary afferent neurons and the effect of nerve injury. Neuroscience. 2003;121(2):441–450. doi: 10.1016/s0306-4522(03)00432-9. [DOI] [PubMed] [Google Scholar]
- Tammaro P, Conti F, Moran O. Modulation of sodium current in mammalian cells by an epilepsy-correlated β1-subunit mutation. Biochemical and biophysical research communications. 2002;291(4):1095–1101. doi: 10.1006/bbrc.2002.6570. [DOI] [PubMed] [Google Scholar]
- Tan B-H, Pundi KN, Van Norstrand DW, Valdivia CR, Tester DJ, Medeiros-Domingo A, Makielski JC, Ackerman MJ. Sudden infant death syndrome–associated mutations in the sodium channel beta subunits. Heart Rhythm. 2010;7(6):771–778. doi: 10.1016/j.hrthm.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Jarecki BW, Piekarz AD, Cummins TR. Nav1. 7 mutations associated with paroxysmal extreme pain disorder, but not erythromelalgia, enhance Navβ4 peptide-mediated resurgent sodium currents. The Journal of physiology. 2011;589(3):597–608. doi: 10.1113/jphysiol.2010.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiko SA, Rosenberg RL, Emerick MC, Agnew WS. Fluorescence assay for neurotoxin-modulated ion transport by the reconstituted voltage-activated sodium channel isolated from eel electric organ. Biochemistry. 1986;25(8):2162–2174. doi: 10.1021/bi00356a047. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Cooperman SS, Tomiko SA, Zhou J, Crean SM, Boyle MB, Kalen RG, Sheng Z, Barchi RL, Sigworth FJ. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989;3(1):33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Uebachs M, Albus C, Opitz T, Isom L, Niespodziany I, Wolff C, Beck H. Loss of β1 accessory Na+ channel subunits causes failure of carbamazepine, but not of lacosamide, in blocking high-frequency firing via differential effects on persistent Na+ currents. Epilepsia. 2012;53(11):1959–1967. doi: 10.1111/j.1528-1167.2012.03675.x. [DOI] [PubMed] [Google Scholar]
- Uebachs M, Opitz T, Royeck M, Dickhof G, Horstmann M-T, Isom LL, Beck H. Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel β subunits via paradoxical effects on persistent sodium currents. Journal of Neuroscience. 2010;30(25):8489–8501. doi: 10.1523/JNEUROSCI.1534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaragavan K, Powell AJ, Kinghorn IJ, Chahine M. Role of auxiliary β 1-, β 2-, and β 3-subunits and their interaction with Na v 1.8 voltage-gated sodium channel. Biochemical and biophysical research communications. 2004;319(2):531–540. doi: 10.1016/j.bbrc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Villeneuve N, Laguitton V, Viellard M, Lépine A, Chabrol B, Dravet C, Milh M. Cognitive and adaptive evaluation of 21 consecutive patients with Dravet syndrome. Epilepsy & behavior. 2014;31:143–148. doi: 10.1016/j.yebeh.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel β1 subunit gene SCN1B. Nature genetics. 1998;19(4):366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott J-J, Demolombe S, Probst V, Anselme F, Escande D. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. The Journal of clinical investigation. 2008;118(6):2260–2268. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MJ, Zhang M-M, Azam L, Olivera BM, Bulaj G, Yoshikami D. Navβ subunits modulate the inhibition of Nav1. 8 by the analgesic gating modifier μO-conotoxin MrVIB. Journal of Pharmacology and Experimental Therapeutics. 2011;338(2):687–693. doi: 10.1124/jpet.110.178343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MJ, Zhang M-M, Gajewiak J, Azam L, Rivier JE, Olivera BM, Yoshikami D. α-And β-subunit composition of voltage-gated sodium channels investigated with μ-conotoxins and the recently discovered μO § -conotoxin GVIIJ. Journal of neurophysiology. 2015;113(7):2289–2301. doi: 10.1152/jn.01004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Harty RC, Richards KL, Phillips AM, Miyazaki H, Nukina N, Petrou S. Sodium channel β1 subunit localizes to axon initial segments of excitatory and inhibitory neurons and shows regional heterogeneity in mouse brain. Journal of Comparative Neurology. 2015;523(5):814–830. doi: 10.1002/cne.23715. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Reid CA, Mitchell S, Richards KL, Scaf BB, Leaw BT, Hill EL, Royeck M, Horstmann M-T, Cromer BA. Axon initial segment dysfunction in a mouse model of genetic epilepsy with febrile seizures plus. The Journal of clinical investigation. 2010;120(8):2661–2671. doi: 10.1172/JCI42219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H-K, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. β Subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and γ-secretase. Journal of Biological Chemistry. 2005;280(24):23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- Xiao Z-C, Ragsdale DS, Malhotra JD, Mattei LN, Braun PE, Schachner M, Isom LL. Tenascin-R is a functional modulator of sodium channel β subunits. Journal of Biological Chemistry. 1999;274(37):26511–26517. doi: 10.1074/jbc.274.37.26511. [DOI] [PubMed] [Google Scholar]
- Xie W, Tan Z-Y, Barbosa C, Strong JA, Cummins TR, Zhang J-M. Upregulation of the sodium channel NaVβ4 subunit and its contributions to mechanical hypersensitivity and neuronal hyperexcitability in a rat model of radicular pain induced by local dorsal root ganglion inflammation. Pain. 2016;157(4):879–891. doi: 10.1097/j.pain.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yereddi NR, Cusdin FS, Namadurai S, Packman LC, Monie TP, Slavny P, Clare JJ, Powell AJ, Jackson AP. The immunoglobulin domain of the sodium channel β3 subunit contains a surface-localized disulfide bond that is required for homophilic binding. The FASEB Journal. 2013;27(2):568–580. doi: 10.1096/fj.12-209445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23(20):7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Wilson MJ, Azam L, Gajewiak J, Rivier JE, Bulaj G, Olivera BM, Yoshikami D. Co-expression of NaVβ subunits alters the kinetics of inhibition of voltage-gated sodium channels by pore-blocking μ-conotoxins. British journal of pharmacology. 2013;168(7):1597–1610. doi: 10.1111/bph.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-N, Li Q, Liu C, Wang H-B, Wang Q, Bao L. The voltage-gated Na+ channel Nav1. 8 contains an ER-retention/retrieval signal antagonized by the β3 subunit. Journal of cell science. 2008;121(19):3243–3252. doi: 10.1242/jcs.026856. [DOI] [PubMed] [Google Scholar]
- Zhao J, O'Leary ME, Chahine M. Regulation of Nav1. 6 and Nav1. 8 peripheral nerve Na+ channels by auxiliary β-subunits. Journal of neurophysiology. 2011;106(2):608–619. doi: 10.1152/jn.00107.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T-t, Zhang Z-w, Liu J, Zhang J-p, Jiao B-h. Glycosylation of the sodium channel β4 subunit is developmentally regulated and involves in neuritic degeneration. International journal of biological sciences. 2012;8(5):630. doi: 10.7150/ijbs.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer T, Benndorf K. The Intracellular Domain of the β2 Subunit Modulates the Gating of Cardiac Na v 1.5 Channels. Biophysical journal. 2007;92(11):3885–3892. doi: 10.1529/biophysj.106.098889. [DOI] [PMC free article] [PubMed] [Google Scholar]