Abstract
The heat shock factor 90 (hsp90) complex has long been associated with neuropathological phenotypes linked to Parkinson’s disease (PD) and its inhibition is neuroprotective in disease models. Hsp90 is conventionally believed to act by suppressing induction of hsp70. Here, we report a novel hsp70-independent mechanism by which Hsp90 may also contribute to PD-associated neuropathology. We previously reported that inhibition of the enzyme prolyl hydroxylase domain 2 (PHD2) in conjunction with increases in hypoxia-inducible factor 1 alpha (HIF1α) results in protection of vulnerable dopaminergic substantia nigra pars compacta (DAergic SNpc) neurons in in vitro and in vivo models of PD. We discovered an increased interaction between PHD2 and the p23:Hsp90 chaperone complex in response to mitochondrial stress elicited by the mitochondrial neurotoxin 1-methyl-4-phenylpyridine (MPP+) within cultured DAergic cells. Genetic p23 knockdown was found to result in decreases in steady-state PHD2 protein and activity and reduced susceptibility to MPP+ neurotoxicity. Administration of the p23 inhibitor gedunin was also neuroprotective in these cells as well as in human induced pluripotent stem cell (iPSC)-derived neurons. Our data suggests that mitochondrial stress-mediated elevations in PHD2 interaction with the p23-hsp90 complex have detrimental effects on the survival of DAergic neurons, while p23 inhibition is neuroprotective. We propose that neurotoxic effects are tied to enhanced PHD2 stabilization by the hsp90-p23 chaperone complex that is abrogated by p23 inhibition. This demonstrates a novel connection between two independent pathways previously linked to PD, hsp90 and PHD2-HIF1α, which could have important implications for here-to-fore unexplored mechanisms underlying PD neuropathology.
Keywords: Heat shock protein 90, prolyl hydroxylase domain protein 2, co-chaperone p23, hypoxia-inducible factor 1 alpha, Parkinson disease, mitochondria, dopamine
1. Introduction
Prolyl hydroxylase domain protein 2 (PHD2) is the primary regulator of steady-state levels of the transcription factor hypoxia-inducible factor 1α (HIF1α) {Bruick et al., 2001; Harten et al., 2010; Miyata et al., 2010; Fong et al., 2008; Nagel et al., 2010}. HIF-1α is itself responsible for up-regulating the expression of genes involved in response to hypoxia and other forms of cellular stress {Siddiq, et al., 2005; Greer et al., 2012; Nakayama et al., 2009}. HIF1α is marked for the proteasome degradation pathway through hydroxylation of proline-564 and proline-402 by PHD2. Prolyl hydroxylation is critical for promoting binding of HIF1α to the Von Hippel Lindau (VHL) E3 ligase, resulting in its polyubiquitination and subsequent proteasomal degradation. We previously reported that PHD inhibition in the context of either acute or chronic in vivo mouse models of PD resulted in protection of vulnerable DAergic SNpc neurons via increases in HIF1α levels {Lee et al., 2009; Rajagopalan et al., 2014; Rajagopalan et al., 2016}. Levels of PHD2 have been reported to be elevated within affected human PD SNpc tissues in conjunction with reduced levels of HIF1α, suggesting that chronically elevated levels of PHD2 may contribute to neurodegenerative events associated with the disorder {Mandel et al., 2008; Grunblatt et al., 2004; Elstner et al., 2011; Rajagopalan et al., 2016}.
Hsp90 inhibition has been widely studied as a potential therapeutic target for PD, largely in the context of its ability to enhance hsp70 induction in response to alpha-synuclein neurotoxicity or mitochondrial stress. Hsp70 overexpression has been shown to suppress alpha-synuclein aggregation and neurotoxicity in various synucleinopathy models as well as neurodegeneration associated with the mitochondrial neurotoxins rotenone and 1-methyl-4-phenyl-2,3,6-tetrahydropyridine (MPTP) {Zhou et al., 2003; Klucken et al., 2004; McLean et al., 2004; Cantuti-Castelvetri et al., 2005; Shin et al., 2005; Flower et al., 2005; Falsone et al., 2009; Chaari et al., 2013}. There are conflicting reports, however, which demonstrate that induction of hsp70 alone is not sufficient to prevent alpha-synuclein or MPTP-mediated neurotoxicity in vivo {Shimshek et al., 2010; Li et al., 2012}. This suggests that the hsp90 chaperone complex may play alternative roles in these neurodegenerative PD-associated phenotypes.
PHD2 has recently been reported to be capable of interacting with the hsp90 co-chaperone p23, resulting in its recruitment and stabilization by the hsp90 chaperone complex {Song et al., 2013; Song et al., 2014}. Here we report that under conditions of mitochondrial stress elicited by the MPTP metabolite MPP+, PHD2 becomes associated with the hsp90-p23 chaperone complex within cultured DAergic cells. In these same cells, p23 knockdown results in select reductions in steady-state levels of the PHD2 isoform corresponding with its increased activation and protection against MPP+-mediated neurotoxicity. Administration of the p23 inhibitor gedunin also elicits neuroprotection against MPP+ in these cells as well as in human iPSC-derived neurons. We propose that p23 via its ability to initiate chaperone-mediated PHD2 stabilization may contribute to mitochondrial stress-related events associated with PD. This suggests a novel connection between two pathways previously independently associated with PD neuropathology via the hsp90 co-factor p23.
2. Materials and methods
2.1. Experimental procedures
2.1.1. Cells and treatments
N27 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin and incubated at 37° C with 5% CO2. Cells were treated with 500 μM MPP+ for 24 hours prior to processing of cell lysates for immunoprecipitation (IP), western blotting or cell viability analyses. Human iPSC-derived DAergic neurons were purchased from XCell Science Inc. where they were subject strict quality control analyses including dopaminergic differentiation per the manufacturer’s specifications. Cells were treated with different concentrations of MPP+ (0.5 – 1 mM) for 24 hrs prior to analysis of cell viability. For gedunin experiments, N27 and iPSC-derived neurons were pre-treated with 1 μM and 5 μM concentrations of the drug respectively, 1hour prior to addition of 500 μM MPP+.
2.1.2. PHD2 IP
N27 cells were grown to confluency on 10 cm plates followed by growth in either 500 μM MPP+ or vehicle for 24 hrs. Cell pellets were collected following centrifugation at 500 × g for 2 min followed by lysis in RIPA buffer (20 mM Tris/HCl, pH 7.8, 50 mM KCl, 2 mM DTT, 0.1% Nonidet P-40, 10 mM NaF, 1mM NaVO4, 2 mM β-glycerophosphate, 2 mM sodium pyrophosphate) supplemented with a protease inhibitor mixture (Roche Applied Science). Immunoprecipitation was carried out by treating 500 μg of cell lysate with protein A immobilized on cross-linked 4% beaded agarose (Sigma, P2545) to remove endogenous immunoglobulins, followed by incubation with PHD2 primary antibody (Cell Signaling), and precipitation with protein A macrobeads (Sigma, P6486) in 20 mM phosphate buffered saline (pH 7.0; PBS) overnight at 4°C. The protein-antibody complex was washed three times with cold PBS and then boiled with SDS-PAGE sample buffer to extract the antigenic proteins for SDS-PAGE separation. Protein complexes were resolved on SDS-PAGE (10% gel) and analyzed by Western blotting (see below).
2.1.3. Knockdown of p23 in DAergic cells
N27 cells were transfected with various concentrations (0–80 nM) of p23 siRNA (Eurofins) versus scrambled control via Lipofectamine 3000 reagent (Invitrogen). Transfection efficiency and reductions in p23 levels were verified by western blot analysis using p23 antibodies.
2.1.4 Nuclear sub-fractionation
Nuclear fractions were obtained from different treatment groups using NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Scientific) as per manufacturer’s instructions. Briefly, the cell pellet was obtained by centrifugation at 500 × g for 5 min. The pellet was resuspended in reagents CER I followed by CER II, vortexed and centrifuged to get cytoplasmic fraction in the supernatant. The pellet was vortexed with NER reagent for 15 seconds over a period of 40 min. Nuclear fraction was obtained in the supernatant after centrifuging at 16,000 × g for 10 min.
2.1.5 Western blotting
Cellular lysates were homogenized in buffer containing 50 mM Tris/HCl, pH 8.0, 150 mM NaCl, 0.25% sodium deoxycholate, and 1 mM EDTA followed by centrifugation at 10,000 × g for 10 min. Supernatant was taken and protein concentration estimated via the Bradford assay. Aliquots of 25 ug protein were boiled at 95°C in Lamelli buffer for 5 min and loaded onto a 10% SDS-PAGE gel. Following electrophoresis, bands were transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were next incubated in primary antibodies (PHD1, 1:1000 dilution, Novus; PHD2, 1:1000 dilution, Cell Signaling; PHD3, 1:1000 dilution, Novus; p23, 1:1000, BD Biosciences; hsp90, 1:1000, Cell Signaling Technologies; HIF1α, 1:500, Novus; Hsp70, 1:1000, Cell Signaling; βactin, 1:5000 dilution, Millipore) for 24 hrs, 4°C. This was followed by incubation in appropriate HRP-conjugated secondary antibodies for 1 hr at RT. Immunoblots were developed using Enhanced Chemiluminescence (ECL) reagent and analyzed using a ChemDoc system from BioRad.
2.1.6. HIF1α immunocytochemistry
Cells were fixed with 4% paraformaldehyde and processed for immunocytochemistry to determine nuclear translocation of HIF-1α. Briefly, fixed cells were incubated in primary HIF1α antibody (1:200, Novus) overnight at 4 °C followed by Alexa-conjugated secondary antibody. Images were captured on a Zeiss LSM 510 confocal microscope for quantitation.
2.1.7 Quantitative real-time RT-PCR
A pure RNA tissue kit (Roche) was used to isolate RNA and HIF1α expression determined via qPCR using SYBR Green master mix (Roche). The primer sets used were as follows: HIF1α forward: 5′-AAG CAC TAG ACA AAG CTC ACC - 3′; HIF1α reverse: 5′-TTG ACC ATA TCG CTG TCC AC - 3′. Data were normalized to βactin.
2.1.8. Cell viability
Cell viability was assessed via the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay and the Abcam cell proliferation assay.
2.1.9. HPLC to assess cellular MPP+ uptake
Cell pellet was sonicated in 300 μL of 0.1M perchloric acid (PCA), and a 20 μL of sonicated lysate was collected in a chilled 1.5 ml vials for protein content determination. The remaining sonicated samples were centrifuged at 14000 rpm for 20 min at 4°C and the supernatant collected was again centrifuged with same parameters. The supernatant were diluted and an aliquot of the diluted supernatant was injected onto a terra MS C18 5uM (4.6 × 250 mm) column (W22071K019, Waters). Samples were eluted isocratically with the mobile phase that composed of 20 mM boric acid–sodium borate buffer, pH 7.75, containing 3 mM tetrabutylammonium hydrogen sulfate, 0.25mM 1-heptanesulfonic acid, and 10% isopropanol. The fluorescence detector set for excitation at 295 nm and emission at 375 nm was used to detect MPP+ levels {Yang et al., 2009; Kaidery et al., 2013}. Concentrations of MPP+ were expressed as nanograms per milligrams tissue weight.
2.2 Statistics
Results are presented as means +/− SEM. Two-way Anova followed by Tukey’s multiple comparison test were performed to analyze statistical significance of in vivo data and one-way Anova followed by Tukey’s multiple comparison for in vitro N27 studies and in human iPSC-derived neurons, all run using PRISM software. A value of p<0.05 was considered significant.
3. Results
3.1. Mitochondrial stress elicited by MPP+ in cultured DAergic N27 cells results in increased association of PHD2 with p23-hsp90
PHD2 inhibition results in reductions in DAergic neurodegeneration in both acute and age-related chronic in vivo models of PD-associated mitochondrial stress {Lee et al., 2009; Rajagopalan et al., 2014; Rajagopalan et al., 2016}. Alleles of PHD2 present in the human genome residing within its coding region have recently been linked to reduced binding of the protein to the p23 co-chaperone, preventing PHD2’s subsequent recruitment and stabilization by hsp90 and reduced ability to down-regulate HIF1α signaling {Song et al., 2014}. This suggested to us that p23 could serve as a potential link between the hsp90 and PHD2-HIF1α pathways under conditions of mitochondrial DAergic stress associated with PD.
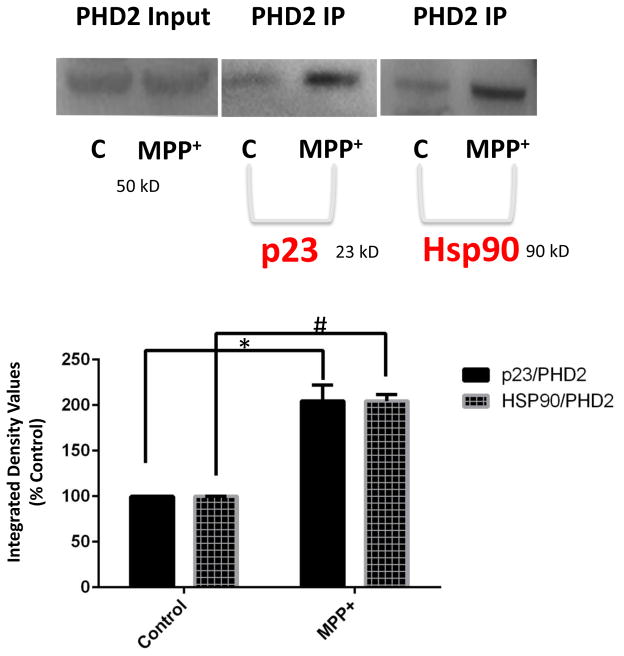
We first tested whether interactions between PHD2 and the hsp90-p23 complex were increased in the context of a published DAergic cell model of mitochondrial stress in which neurotoxicity is prevented by PHD2 inhibition involving the treatment of cultured rat DAergic N27 cells with the mitochondrial neurotoxin MPP+ {Lee et al., 2009}. Administration of MPP+ was found to result in increased levels of both p23 and hsp90 which co-immunoprecipitated with the PHD2 protein (Fig. 1). This suggests that under conditions of PD-related mitochondrial stress, there is an increased association between PHD2 and the p23-hsp90 chaperone complex that could impact on PHD stability and subsequent function.
FIGURE 1.
Mitochondrial-mediated stress elicited by the neurotoxin MPP+ in DAergic N27 cells results in increased association of PHD2 with both p23 and hsp90. Immunoprecipitation (IP) of PHD2 was performed using a monoclonal antibody specific for the PHD2 isoform followed by western blot analyses in cells treated with 500 μM MPP+ for 24 hrs versus untreated controls (C). Top left panel, PHD2 input; top middle panel, p23 western of PHD2 IP; top right panel; Hsp90 western of PHD2 IP. Below, densitometric quantitation of p23 and Hsp90 levels in Con versus MPP+ cells; #p<0.05 versus Con.
3.2. Genetic knockdown of p23 levels in DAergic cells results in selective reductions in PHD2 protein and activity levels
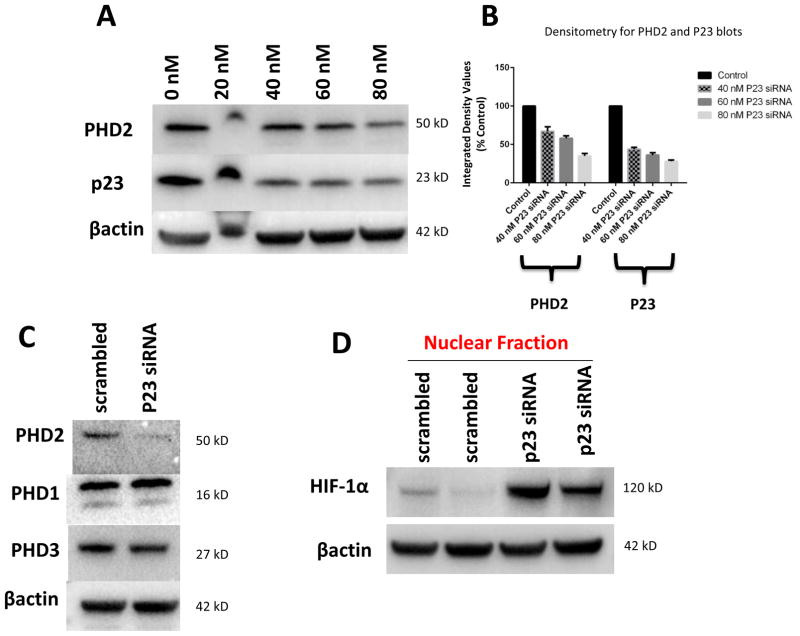
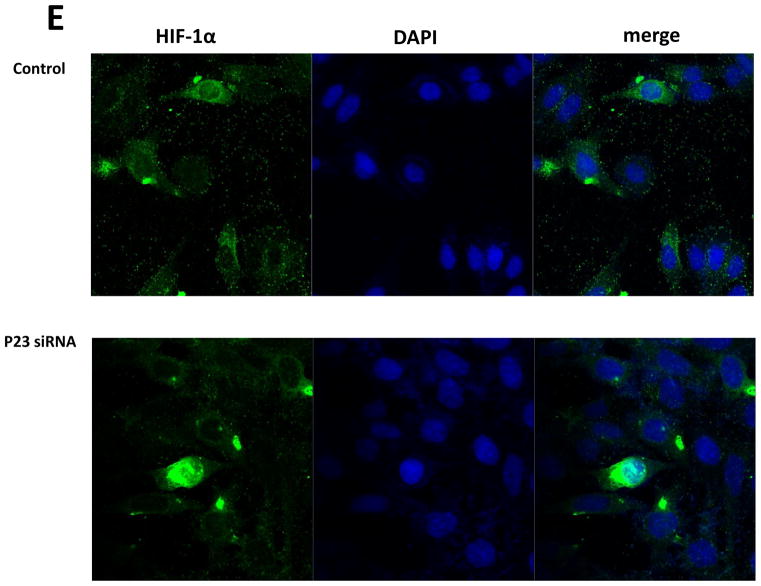
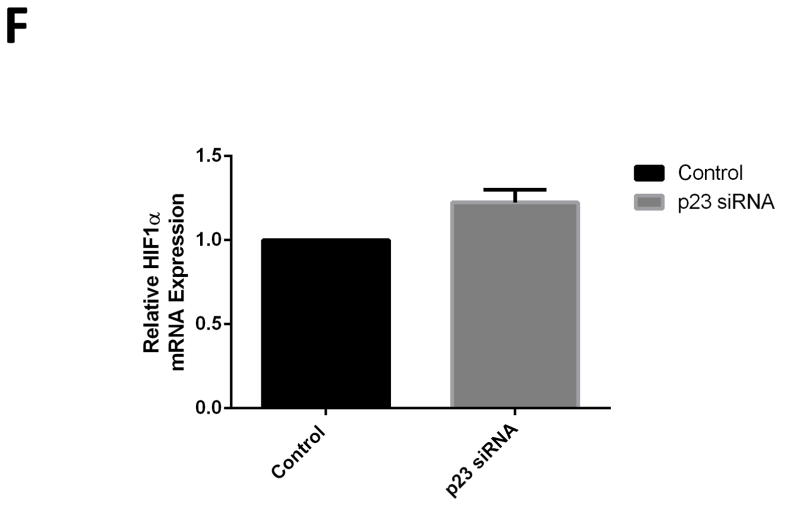
PHD2 contains a highly conserved zinc finger domain that has recently been reported to interact with a binding motif within p23, allowing its recruitment to and stabilization by hsp90 {Song et al., 2014}. We noted that this domain was absent in other known PHD isoforms. We hypothesized that reductions in p23 levels in our DAergic cell model should therefore selectively interfere with the stability of PHD2 protein levels. Selective knockdown of p23 was found to result in a dose-dependent reduction in steady-state PHD2 protein levels (Fig. 2 A–B). This did not however impact on protein levels of other known PHD isoforms (PHD1 and PHD3) at dosages that elicited effects on PHD2 levels (Fig. 2 C). Knockdown in p23 eliciting select reductions in PHD2 protein levels in turn corresponded with reduced PHD2 activity as indicated by increases in nuclear HIF1α translocation (Fig. 2 D, E). We verified that this was independent of induction of HIF1α gene expression via qPCR in p23 knockdown cells versus scrambled controls (Fig. 2 F)
FIGURE 2.
Knockdown of p23 via siRNA results in reductions in p23 protein levels in DAergic N27 cells coinciding with select decreased in steady-state levels of PHD2 protein and activity. A, Representative western blot analyses of cells transfected with increasing dosages (0–80 nM) p23 siRNA, 24 hrs. Top panel, PHD2; middle panel, p23; lower panel, actin as loading control. B, Densitometric quantitation of PHD2 and p23 steady-state protein levels normalized to actin. C, Representative westerns of effects of 24 hr p23 siRNA transfection at 80 nM on PHD2 (top panel) versus PHD1 and PHD3 levels (middle panels); actin was used as a loading control (lower panel). D, PHD2 activity as measured by HIF1α westerns of nuclear fractions from p23 siRNA versus scrambled transfected cells. E. Representative laser confocal microcopy images show translocation of HIF-1α (green) within nuclei stained with DAPI (blue stain) in cells treated with p23 siRNA (80nM) for 24 hrs.
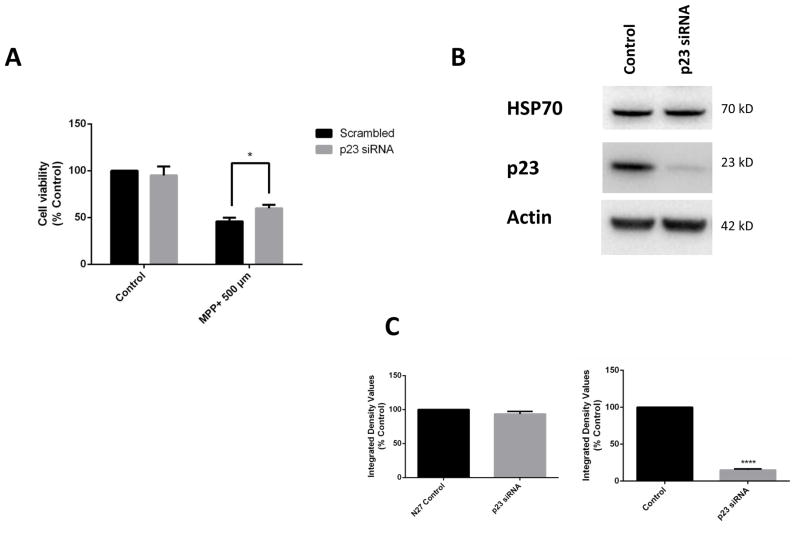
3.3. Genetic p23 knockdown in DAergic N27 cells prevents MPP+-mediated neurotoxicity
Next we asked whether genetic knockdown of p23 in DAergic cells could prevent neurotoxicity mediated by MPP+ treatment. In comparison to scrambled controls, expression of p23-specific siRNA which resulted in significant reductions in its levels as well as those of PHD2 was found to prevent DAergic cell loss associated with MPP+ administration (Fig. 3 A). To verify that this was not due to inductions in Hsp70 levels, we performed western blot analyses in p23 knockdown versus control cells (Fig. 3 B,C). Knockdown of p23 in DAergic N27 cells did not alter HSP70 levels, indicating that the neuroprotective effects are independent of HSP70 induction.
FIGURE 3.
Genetic p23 knockdown in DAergic N27 cells protects against MPP+-mediated neurotoxicity. Cell viability in p23 versus scrambled siRNA or untransfected controls in MPP+-treated (dark grey bars; 500 μM, 24 hrs) versus untreated cells (light grey bars);* p<0.05 versus untreated. B. Representative western blot analyses of HSP70 in N27 cells transfected with p23 siRNA (80 nM), 24 hrs. Top panel, HSP70; middle panel, p23; lower panel, actin as a loading control. C, Densitometric quantitation of HSP70 and p23 steady-state protein levels normalized to actin.
3.4. Inhibition of p23 activity via gedunin prevents MPP+-mediated neurotoxicity in both DAergic N27 cells and in human iPSC-derived neurons
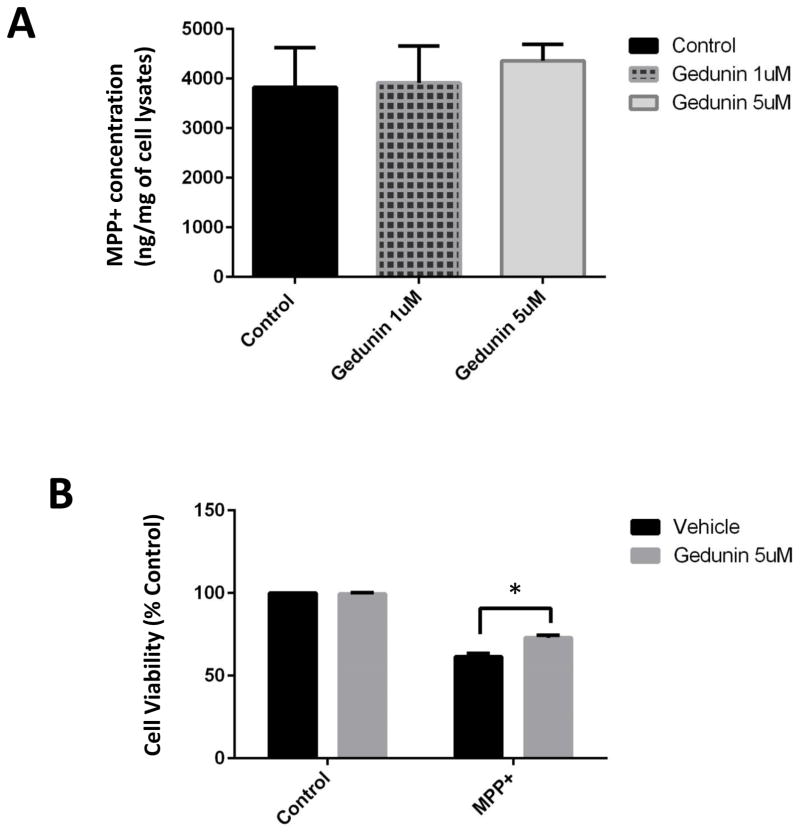
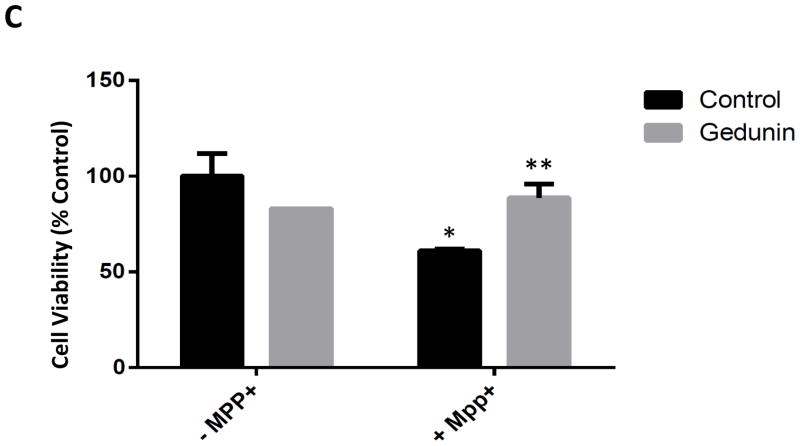
The miscible flavone compound gedunin has been demonstrated to act via direct interaction with p23, preventing it from binding to hsp90. In this manner, gedunin acts as a select inhibitor of hsp90 chaperone activity without interfering with the activities of other Hsp90 co-chaperones {Patwardhan, 2013}. Gedunin treatment of DAergic N27 cells did not impact on MPP+ uptake by the cells and was found to significantly attenuate MPP+-mediated cell loss (Fig. 4 A, B). It was also attenuated cell loss associated with MPP+ treatment of human iPSC-derived DAergic neurons (Fig. 4 C).
FIGURE 4.
Administration of the pharmacological p23 inhibitor gedunin protects against MPP+ neurotoxicity in both N27 cells and in human iPSC-derived neurons. A, MPP+ levels were measured in whole cell lysates from N27 cell treated with 500 μM MPP+ alone and cells co-treated with MPP+ and Gedunin (1 and 5 μM) for 24 h via HPLC with electrochemical detection. MPP+ was extracted from cells homogenized in the presence of 0.4N perchloric acid and measured against a known standard. Values are presented as ng MPP+/mg protein, n = 3 per treatment condition. B, Cell viability in N27 cells treated with gedunin (5 μM, 24 hrs) versus untreated +/− MPP+. *p<0.01 versus untreated control; **p<0.05 versus MPP+ alone. C, iPSC-derived neurons exposed to gedunin (5 μM, 24 hrs) versus untreated +/− MPP+. *p<0.01 versus untreated control; **p<0.05 versus MPP+ alone. Cell viability was calculated via MTT assay in a minimum of three different wells and 4 separate fields in each well; *p<0.01 versus control; **p<0.05 versus MPP+. Values are presented as mean +/− SEM of % Con.
4. Discussion
The hsp90 chaperone complex has been widely reported to be associated with PD and its inhibition to protect against neurodegenerative phenotypes in a variety of disease models {Uryu et al., 2006; Falsone et al., 2009}. Hsp90 inhibitors are largely believed to elicit their effects via induction of hsp70, although hsp70 induction alone is not neuroprotective in all models of PD including following systemic administration of MPTP {Zhou et al., 2004; McLean et al., 2004; Klucken et al., 2004; Cantuti-Castelvetri et al., 2005}. This suggests that the hsp90 chaperone complex may play additional roles in neurodegenerative phenotypes associated with PD.
PHD2 levels have been demonstrated to be aberrantly elevated within affected DAergic neurons in post-mortem SNpc tissues isolated from PD patients versus age-matched controls, in conjunction with reduced HIF1α levels {Mandel et al., 2005; Grunblatt et al., 2004; Elstner et al., 2011}. We previously reported that PHD inhibition acting via inductions in HIF1α signaling results in significant neuroprotection of vulnerable DAergic SNpc neurons in both acute and chronic in vivo mouse models of the disease {Lee et al., 2009; Rajagopalan, 2014; Rajagopolan et al., 2016}. This was found to be associated with reductions in levels of mitochondrial dysfunction in these models (Lee et al., 2009; Rajagopalan et al., 2014). PHD inhibitors have also been implicated as therapeutics for neuroprotection within the central nervous system in association with other conditions including stroke and ischemic injury and in chronic models of both Alzheimer’s and Huntington’s disease (Uryu et al., 2006; Natarajan et al., 2009; Siddiq et al., 2007; Siddiq et al., 2008; Siddiq et al., 2009; Niatsetskaya et al., 2010; Kant et al., 2013; de Lemos et al., 2013). PHD inhibitors are however broad-acting and may have effects on other PHD isoforms.
The p23 protein has recently been reported to specifically recruit PHD2 protein to the Hsp90 chaperone complex via select binding to a zinc finger domain that is found only within the PHD2 isoform {Song et al., 2013}. Human PHD2 alleles within the zinc finger binding site are associated with reduced p23 binding and decreased PHD2 protein stabilization by the hsp90 chaperone complex {Song et al., 2014}. Our current data demonstrates that mitochondrial stress elicited by the mitochondrial neurotoxin MPP+ result in elevated levels of PHD2 protein in cultured DAergic N27 cells in conjunction with increased interaction with the hsp90-p23 chaperone complex. Genetic p23 knockdown in these cells was found to selectively reduce PHD2 protein levels and subsequent activity resulting in increased HIF1α activity and prevention of MPP+-mediated neurotoxicity in a manner independent of Hsp70 induction. This is in keeping with previous data from our laboratory where we demonstrated that over-expression of wildtype p23 in N27 cells resulted in increased sensitivity to the neurotoxic effects of paraquat which was reduced in cells expressing mutant p23 (Chinta et al., 2008). Neuronal expression of wildtype p23 in vivo has been reported to result in neurological defects that track with p23 expression levels (Gong et al., 2011).
The p23 inhibitor gedunin was also found to protect against neurotoxicity associated with MPP+ in DAergic N27 cells. The ability to reiterate these results in iPSC-derived neurons suggests that our findings are relevant in a non-transformed human cell model of the disease. Based on these data, we propose that hsp90 may elicit toxic effects on DAergic neurons in the face of mitochondrial stress via interaction with p23 that acts to recruit PHD2 to the hsp90 complex resulting in stabilization of the PHD2 protein. This in turn results in reductions in neuroprotective HIF1α levels. HIF1α knockdown both in murine midbrain-derived neural precursor cells (NPCs) and in DAergic SN neurons in vivo has been reported to result in neuronal cell loss, suggesting that maintenance of HIF1α levels are important for survival of these cells {Milosevic et al., 2007; Lin et al., 2014}.
Small molecule inhibitors of hsp90 such as geldanamycin that act by binding the ATP pocket of the protein have been demonstrated to prevent neurotoxicity associated with various PD mouse models, including following in vivo MPTP administration {Shimshek et al., 2010; Li et al., 2012}. Though results in animal models have been promising, clinical utility of these inhibitors have to date been limited by poor solubility, blood-brain-barrier penetration, toxicity issues, or failure to protect against frank loss of nigrostriatal DAergic neurons {McFarland et al., 2014}. The miscible flavone compound gedunin directly binds to p23 and prevents its interaction with hsp90, thereby inhibiting hsp90 chaperone activity without interfering with the activities of any of the other ~20 known Hsp90 co-chaperones {Patwardhan et al., 2013}. Gedunin is a natural product isolated from the Meliacae family of medicinal plants and has been extensively used for treatment of malaria and other infectious diseases in the context of traditional Indian medicine. Gedunin or its derivatives including 7-oxo-gedunin could provide a novel alternative for use in the treatment of PD that may have less broad effects than other hsp90 inhibitors that have been explored to date {Patwardhan et al., 2013}. The gedunin derivative deosygedunin has indeed previously been reported to be neuroprotective against in vivo MPTP neurotoxicity (although not confirmed using stereological cell counts) {Nie et al., 2015}. Neuroprotection was found to correlate with modest increases in the activation of TrkB receptors within the SNpc, although definitive proof of trkB activation as the direct cause of drug-mediated neuroprotection (e.g. by its genetic knockdown) was not pursued in these studies {Nie et al., 2015}. This could however still constitutes an alternative or additional explanation for the neuroprotective effects elicited by gedunin in our in vitro studies. An important caveat to the use of gedunin and its derivatives as a treatment for PD is the reported modulatory activity of p23 on certain steroid receptors which could result in unwanted ‘off-target’ effects in non-neuronal tissues which suggest that direct brain targeting may be required {Ros-Bernal et al., 2011, Reebye et al., 2012}.
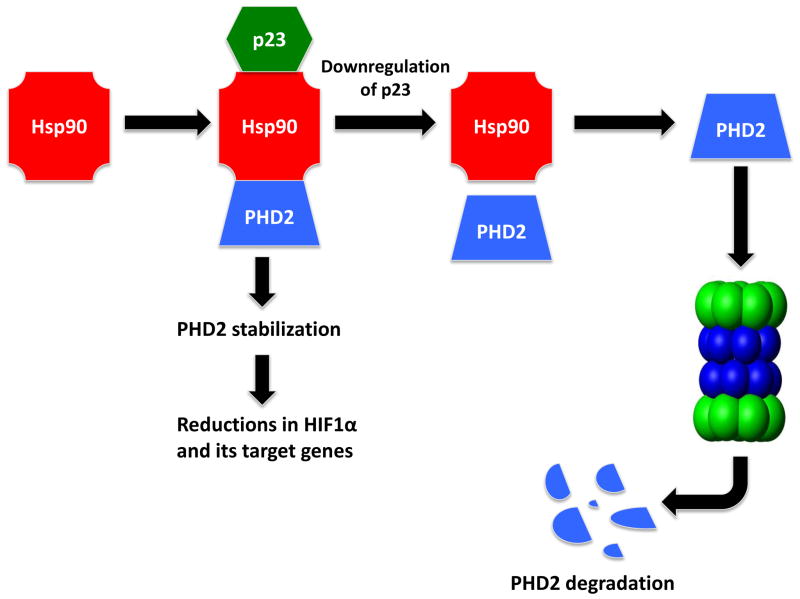
In summary, these data potentially provide a novel link between two entities whose dysfunction have been independently associated with PD-associated neurodegeneration, hsp90 and PHD2-HIF1α, via interaction with the hsp90 co-factor p23 (Fig. 5). This could have important implications for mechanisms underlying the involvement of these pathways in PD neuropathology and suggests that dopaminergic p23 inhibition be considered as a novel therapeutic target for the disorder.
FIGURE 5.
Schematic representation of hsp90 and its interaction with PHD2-HIF1α and regulation by co-factor p23.
Highlights.
Mitochondrial stress (MPP+) increases interaction between PHD2 and p23:Hsp90 chaperone complex.
p23 inhibitor Gedunin prevents MPP+ mediated dopaminergic cell death destabilization of PHD2-P23-HSP90 complex.
Acknowledgments
This work was supported by National Institutes of Health GrantsR01 NS101967 and Parkinson Support Group (Thomas B).
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions: A.R., S.J.C., and J.K.A. contributed to the study concept and design. S.R., A.R., and S.J.C. were involved in acquisition of data. All of the authors contributed to the interpretation of the data. A.R. S.R., and S.J.C. performed the statistical analysis. S.J.C. and J.K.A. drafted the manuscript. All of the authors revised the article critically for important intellectual content and approved the final version of the manuscript submitted for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bruick RK. A Conserved Family of Prolyl-4-Hydroxylases That Modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I, Klucken J, Ingelsson M, Ramasamy K, McLean PJ, Frosch MP, Hyman BT, Standaert DG. Alpha-Synuclein and Chaperones in Dementia With Lewy Bodies. Journal of Neuropathology and Experimental Neurology. 2005;64:1058–1066. doi: 10.1097/01.jnen.0000190063.90440.69. [DOI] [PubMed] [Google Scholar]
- Chaari A, Hoarau-Véchot J, Ladjimi M. Applying chaperones to protein-misfolding disorders: Molecular chaperones against α-synuclein in Parkinson’s disease. International Journal of Biological Macromolecules. 2013;60:196–205. doi: 10.1016/j.ijbiomac.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Rane A, Poksay KS, Bredesen DE, Andersen JK, Rao RV. Coupling endoplasmic reticulum stress to the cell death program in dopaminergic cells: effect of paraquat. Neuromolecular medicine. 2008;10:333–342. doi: 10.1007/s12017-008-8047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos ML, de la Torre AV, Petrov D, Brox S, Folch J, Pallàs M, Lazarowski A, Beas-Zarate C, Auladell C, Camins A. Evaluation of hypoxia inducible factor expression in inflammatory and neurodegenerative brain models. The International Journal of Biochemistry & Cell Biology. 2013;45:1377–1388. doi: 10.1016/j.biocel.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Elstner M, Morris CM, Heim K, Bender A, Mehta D, Jaros E, Klopstock T, Meitinger T, Turnbull DM, Prokisch H. Expression analysis of dopaminergic neurons in Parkinson’s disease and aging links transcriptional dysregulation of energy metabolism to cell death. Acta Neuropathologica. 2011;122:75–86. doi: 10.1007/s00401-011-0828-9. [DOI] [PubMed] [Google Scholar]
- Falsone SF, Kungl AJ, Rek A, Cappai R, Zangger K. The Molecular Chaperone Hsp90 Modulates Intermediate Steps of Amyloid Assembly of the Parkinson-related Protein α-Synuclein. Journal of Biological Chemistry. 2009;284:31190–31199. doi: 10.1074/jbc.M109.057240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower TR, Chesnokova LS, Froelich CA, Dixon C, Witt SN. Heat Shock Prevents Alpha-synuclein-induced Apoptosis in a Yeast Model of Parkinson’s Disease. Journal of Molecular Biology. 2005;351:1081–1100. doi: 10.1016/j.jmb.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Fong GH, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death and Differentiation. 2008;15:635–641. doi: 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- Gong P, Roseman J, Fernandez CG, Vetrivel KS, Bindokas VP, Zitzow LA, Kar S, Parent AT, Thinakaran G. Transgenic neuronal overexpression reveals that stringently regulated p23 expression is critical for coordinated movement in mice. Molecular neurodegeneration. 2011;6:87. doi: 10.1186/1750-1326-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. The EMBO Journal. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenblatt E, Mandel S, Jacob-Hirsch J, Zeligson S, Amariglo N, Rechavi G, Li J, Ravid R, Roggendorf W, Riederer P, Youdim MBH. Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. Journal of Neural Transmission. 2004;111:1543–1573. doi: 10.1007/s00702-004-0212-1. [DOI] [PubMed] [Google Scholar]
- Harten SK, Ashcroft M, Maxwell PH. Prolyl Hydroxylase Domain Inhibitors: A Route to HIF Activation and Neuroprotection. Antioxidants & redox signaling. 2010;12:459–480. doi: 10.1089/ars.2009.2870. [DOI] [PubMed] [Google Scholar]
- Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, Liby KT, Williams CR, Yamamoto M, Kensler TW, Ratan RR, Sporn MB, Beal MF, Gazaryan IG, Thomas B. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxidants & redox signaling. 2013;18:139–157. doi: 10.1089/ars.2011.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant R, Bali A, Singh N, Jaggi AS. Prolyl 4 Hydroxylase: A Critical Target in the Pathophysiology of Diseases. The Korean Journal of Physiology & Pharmacology. 2013;17:111. doi: 10.4196/kjpp.2013.17.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 Reduces -Synuclein Aggregation and Toxicity. Journal of Biological Chemistry. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- Lee DW, Rajagopalan S, Siddiq A, Gwiazda R, Yang L, Beal MF, Ratan RR, Andersen JK. Inhibition of Prolyl Hydroxylase Protects against 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Neurotoxicity: MODEL FOR THE POTENTIAL INVOLVEMENT OF THE HYPOXIA-INDUCIBLE FACTOR PATHWAY IN PARKINSON DISEASE. Journal of Biological Chemistry. 2009;284:29065–29076. doi: 10.1074/jbc.M109.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lee J, Parsons D, Janaurajs K, Standaert DG. Evaluation of TorsinA as a Target for Parkinson Disease Therapy in Mouse Models. PloS one. 2012;7:e50063. doi: 10.1371/journal.pone.0050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wadlington NL, Chen L, Zhuang X, Brorson JR, Kang UJ. Loss of PINK1 Attenuates HIF-1 Induction by Preventing 4E-BP1-Dependent Switch in Protein Translation under Hypoxia. Journal of Neuroscience. 2014;34:3079–3089. doi: 10.1523/JNEUROSCI.2286-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Grunblatt E, Riederer P, Amariglio N, Hirsch JJ, Rechavi G, Youdim MBH. Gene Expression Profiling of Sporadic Parkinson’s Disease Substantia Nigra Pars Compacta Reveals Impairment of Ubiquitin-Proteasome Subunits, SKP1A, Aldehyde Dehydrogenase, and Chaperone HSC-70. Annals of the New York Academy of Sciences. 2008;1053:356–375. doi: 10.1196/annals.1344.031. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Dimant H, Kibuuka L, Ebrahimi-Fakhari D, Desjardins CA, Danzer KM, Danzer M, Fan Z, Schwarzschild MA, Hirst W, McLean PJ. Chronic Treatment with Novel Small Molecule Hsp90 Inhibitors Rescues Striatal Dopamine Levels but Not α-Synuclein-Induced Neuronal Cell Loss. PloS one. 2014;9:e86048. doi: 10.1371/journal.pone.0086048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean PJ, Klucken J, Shin Y, Hyman BT. Geldanamycin induces Hsp70 and prevents α-synuclein aggregation and toxicity in vitro. Biochemical and Biophysical Research Communications. 2004;321:665–669. doi: 10.1016/j.bbrc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Milosevic J, Maisel M, Wegner F, Leuchtenberger J, Wenger RH, Gerlach M, Storch A, Schwarz J. Lack of Hypoxia-Inducible Factor-1 Impairs Midbrain Neural Precursor Cells Involving Vascular Endothelial Growth Factor Signaling. Journal of Neuroscience. 2007;27:412–421. doi: 10.1523/JNEUROSCI.2482-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Takizawa S, van Ypersele de Strihou C. Hypoxia. 1. Intracellular sensors for oxygen and oxidative stress: novel therapeutic targets. AJP: Cell Physiology. 2010;300:C226–C231. doi: 10.1152/ajpcell.00430.2010. [DOI] [PubMed] [Google Scholar]
- Nagel S, Talbot NP, Mecinović J, Smith TG, Buchan AM, Schofield CJ. Therapeutic Manipulation of the HIF Hydroxylases. Antioxidants & redox signaling. 2010;12:481–501. doi: 10.1089/ars.2009.2711. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Cellular Signal Transduction of the Hypoxia Response. Journal of Biochemistry. 2009;146:757–765. doi: 10.1093/jb/mvp167. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Salloum FN, Fisher BJ, Smithson L, Almenara J, Fowler AA. Prolyl hydroxylase inhibition attenuates post-ischemic cardiac injury via induction of endoplasmic reticulum stress genes. Vascular Pharmacology. 2009;51:110–118. doi: 10.1016/j.vph.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Niatsetskaya Z, Basso M, Speer RE, McConoughey SJ, Coppola G, Ma TC, Ratan RR. HIF Prolyl Hydroxylase Inhibitors Prevent Neuronal Death Induced by Mitochondrial Toxins: Therapeutic Implications for Huntington’s Disease and Alzheimer’s Disease. Antioxidants & redox signaling. 2010;12:435–443. doi: 10.1089/ars.2009.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie S, Xu Y, Chen G, Ma K, Han C, Guo Z, Zhang Z, Ye K, Cao X. Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology. 2015;99:448–458. doi: 10.1016/j.neuropharm.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BSJ, Chadli A. Gedunin Inactivates the Co-chaperone p23 Protein Causing Cancer Cell Death by Apoptosis. Journal of Biological Chemistry. 2013;288:7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Chinta S, Andersen J. Pharmacological Prolyl Hydroxylase Domain Inhibition as a Therapeutic Target for Parkinson’s Disease. CNS & Neurological Disorders - Drug Targets. 2014;13:120–125. doi: 10.2174/18715273113126660131. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Rane A, Chinta SJ, Andersen JK. Regulation of ATP13A2 via PHD2-HIF1alpha Signaling Is Critical for Cellular Iron Homeostasis: Implications for Parkinson’s Disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:1086–1095. doi: 10.1523/JNEUROSCI.3117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reebye V, Querol Cano L, Lavery DN, Brooke GN, Powell SM, Chotai D, Walker MM, Whitaker HC, Wait R, Hurst HC, Bevan CL. Role of the HSP90-associated cochaperone p23 in enhancing activity of the androgen receptor and significance for prostate cancer. Molecular endocrinology. 2012;26:1694–1706. doi: 10.1210/me.2012-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros-Bernal F, Hunot S, Herrero MT, Parnadeau S, Corvol JC, Lu L, Alvarez-Fischer D, Carrillo-de Sauvage MA, Saurini F, Coussieu C, Kinugawa K, Prigent A, Hoglinger G, Hamon M, Tronche F, Hirsch EC, Vyas S. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6632–6637. doi: 10.1073/pnas.1017820108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshek DR, Mueller M, Wiessner C, Schweizer T, van der Putten PH. The HSP70 Molecular Chaperone Is Not Beneficial in a Mouse Model of α-synucleinopathy. PloS one. 2010;5:e10014. doi: 10.1371/journal.pone.0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y, Klucken J, Patterson C, Hyman BT, McLean PJ. The Co-chaperone Carboxyl Terminus of Hsp70-interacting Protein (CHIP) Mediates α-Synuclein Degradation Decisions between Proteasomal and Lysosomal Pathways. Journal of Biological Chemistry. 2005;280:23727–23734. doi: 10.1074/jbc.M503326200. [DOI] [PubMed] [Google Scholar]
- Siddiq A. Prolyl 4-hydroxylase activity-responsive transcription factors: From hydroxylation to gene expression and neuroprotection. Frontiers in Bioscience. 2008;13:2875. doi: 10.2741/2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Aminova LR, Ratan RR. Hypoxia Inducible Factor Prolyl 4-Hydroxylase Enzymes: Center Stage in the Battle Against Hypoxia, Metabolic Compromise and Oxidative Stress. Neurochemical Research. 2007;32:931–946. doi: 10.1007/s11064-006-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Aminova LR, Troy CM, Suh K, Messer Z, Semenza GL, Ratan RR. Selective Inhibition of Hypoxia-Inducible Factor (HIF) Prolyl-Hydroxylase 1 Mediates Neuroprotection against Normoxic Oxidative Death via HIF- and CREB-Independent Pathways. Journal of Neuroscience. 2009;29:8828–8838. doi: 10.1523/JNEUROSCI.1779-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Ayoub IA, Chavez JC, Aminova L, Shah S, LaManna JC, Patton SM, Connor JR, Cherny RA, Volitakis I, Bush AI, Langsetmo I, Seeley T, Gunzler V, Ratan RR. Hypoxia-inducible Factor Prolyl 4-Hydroxylase Inhibition: A TARGET FOR NEUROPROTECTION IN THE CENTRAL NERVOUS SYSTEM. Journal of Biological Chemistry. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Li Ls, Arsenault PR, Tan Q, Bigham AW, Heaton-Johnson KJ, Master SR, Lee FS. Defective Tibetan PHD2 Binding to p23 Links High Altitude Adaption to Altered Oxygen Sensing. Journal of Biological Chemistry. 2014;289:14656–14665. doi: 10.1074/jbc.M113.541227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Li LS, Heaton-Johnson KJ, Arsenault PR, Master SR, Lee FS. Prolyl Hydroxylase Domain Protein 2 (PHD2) Binds a Pro-Xaa-Leu-Glu Motif, Linking It to the Heat Shock Protein 90 Pathway. Journal of Biological Chemistry. 2013;288:9662–9674. doi: 10.1074/jbc.M112.440552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Richter-Landsberg C, Welch W, Sun E, Goldbaum O, Norris EH, Pham C-T, Yazawa I, Hilburger K, Micsenyi M, Giasson BI, Bonini NM, Lee VMY, Trojanowski JQ. Convergence of Heat Shock Protein 90 with Ubiquitin in Filamentous α-Synuclein Inclusions of α-Synucleinopathies. The American Journal of Pathology. 2006;168:947–961. doi: 10.2353/ajpath.2006.050770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, Musiek ES, Morrow JD, Sporn M, Beal MF. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PloS one. 2009;4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. Tyrosine-to-Cysteine Modification of Human -Synuclein Enhances Protein Aggregation and Cellular Toxicity. Journal of Biological Chemistry. 2003;279:10128–10135. doi: 10.1074/jbc.M307563200. [DOI] [PubMed] [Google Scholar]