Abstract
Animals learn to reduce their intake of a tastant when its ingestion is followed by the administration of an anesthesia-inducing drug. To determine the nature of this intake suppression, the current study examined whether ketamine/xylazine (Experiment 1) and pentobarbital (Experiment 2) also conditionally reduce taste palatability. Using lick pattern analysis, we found that pairing saccharin with either drug reduced total licks, lick cluster size, and initial lick rate. Given that both lick cluster size and initial lick rate are indices of palatability, this pattern of results indicates that anesthesia-inducing drugs also induce conditioned taste aversions.
Keywords: Ketamine/xylazine, Pentobarbital, Taste palatability, Aversion-avoidance, Rat
1. Introduction
Palatability, or hedonic value, is one of the most important determinants of food intake (e.g., Cabanac, 1989; Le Magnen, 1977). However, palatability is not a fixed property of a food. Rather, taste palatability can be modulated by postingestive consequences. In particular, when consumption of a novel food is followed by an aversive systemic experience (e.g., gastrointestinal malaise) the food can become unpalatable or disgusting. This learned reduction in palatability is termed a conditioned taste aversion (CTA) and is considered to result from the acquisition of a Pavlovian association between the taste of the food (conditioned stimulus or CS) and the aversive post-ingestive consequence (unconditioned stimulus or US; e.g., Garcia, 1989; Garcia, Hankins & Rusiniak, 1974; for reviews see Barker, Best & Domjan, 1977; Braveman & Bronstein, 1985; Reilly & Schachtman, 2009). By conditionally lowering taste palatability, CTA learning protects us from the repeated ingestion of poisonous foods (for reviews see Lin, Arthurs & Reilly, 2014, 2017).
Garcia, Kovner and Green (1970) proposed that another form of aversive taste learning, based on the operation of a qualitatively different mechanism, could also lead to food rejection. These investigators evaluated the effect of pairing a taste CS with either an internal malaise-inducing US (i.e., lithium chloride, LiCl) or an external pain-inducing US (i.e., footshock). Post-conditioning tests revealed that the LiCl-induced CTA was not context dependent. On the other hand, the footshock-paired taste was avoided in the conditioning context but not elsewhere (e.g., home cage). This pattern of results encouraged Garcia and colleagues to advocate a distinction between CTA (which involves a reduction in both CS palatability and intake) and taste avoidance learning (TAL; which involves a reduction in CS intake only). According to their analysis of TAL, the taste CS is avoided because it has become a signal for impending danger. Supportive of this interpretation, Pelchat, Grill, Rozin and Jacobs (1983) reported evidence that footshocks, unlike lithium toxicosis, do not cause a downshift in the palatability of the associated taste CS1.
The present article is concerned with the nature of the taste learning supported by anesthesia-inducing drugs. Although having a pharmacological action that is different from those of poisons and toxins, these drugs can function as a US to reduce intake of a taste CS. The aversion-avoidance dichotomy suggests that the nature of aversive taste learning depends upon the type of US – poisons (e.g., LiCl) support CTA whereas external pain-inducing stimuli (e.g., footshocks) support TAL. Anesthesia-inducing drugs may support taste suppression learning via either mechanism. To investigate this issue, we employed lick pattern analysis methodology because it allows simultaneously measurement of intake and palatability during the voluntary drinking trials of the standard taste learning protocol (Lin et al., 2012, 2014).
When voluntarily drinking, rats make runs of licks that are interrupted by pauses. These runs of licks are termed clusters and the number of licks in a cluster defines the size of a cluster. Among the various measures that can be extracted from lick pattern analysis, lick cluster size accurately reflects taste palatability while other measures (e.g., number of licks, number of clusters, inter-cluster interval) are correlated with amount consumed or post-ingestive consequences (for reviews see Dwyer, 2012; Lin et al., 2014). For example, lick cluster size displays a positive monotonic relationship with the concentration of palatable solutions like sucrose and polycose (e.g., Davis, 1996; Davis & Smith, 1992) but a negative monotonic relationship with increasing concentrations of unpalatable solutions like quinine (Hsiao & Fan, 1993; Spector & St. John, 1998). Importantly, lick cluster size is not directly related to amount consumed, which bears an inverted U-shaped function to concentration (i.e., sucrose intake peaks at intermediate concentrations and diminishes as concentration increases; Davis & Smith, 1992). Like cluster size, initial lick rate also reflects taste palatability (Davis & Levine, 1977). Furthermore, initial lick rate is considered to represent palatability that is purely conveyed by the orosensory aspect of the taste stimulus (e.g., Davis & Perez, 1993) because the brief sampling period (first 1 to 3 min of licking) limits the influence of post-ingestive feedback. Accordingly, we used lick cluster size and initial lick rate as dependent measures for taste palatability.
In this study, ketamine/xylazine and pentobarbital were the anesthetics that served as the USs because they are known to function as USs to reduce the intake of a taste CS (ketamine/xylazine: Aguado, del Valle, & Perez, 1997; Misanin, Christianson, Anderson, Giovanni, & Hinderliter, 2004; pentobarbital: Taukulis, 1983). Furthermore, the two USs differentially induce emesis: whereas ketamine/xylazine (xylazine in particular) can cause vomiting (Fox, Corcoran, & Brizzee, 1990; Kolahian & Jarolmasjed, 2012), pentobarbital appears to attenuate nausea and vomiting (e.g., Wheelock, 1989). The aversion-avoidance dichotomy states that only nausea/vomiting-inducing USs can induce CTAs whereas other USs support TAL (Pelchat et al., 1983; for reviews see Parker 2003, 2014; Parker, Limebeer & Rana, 2009). Accordingly, evaluating the effects of ketamine/xylazine and pentobarbital on taste learning is theoretically important as it refines our understanding of the distinction between CTA and TAL.
In Experiment 1, two doses of ketamine/xylazine (10/0.5 and 20/1.0 mg/kg) were tested, both of which are lower than that used to induce general anesthesia (100/10 mg/kg). These lower doses were chosen because in a preliminary study we found nearly complete intake suppression after a single conditioning trial with a 100/10 mg/kg ketamine/xylazine, which prevented the collection of sufficient data for meaningful lick pattern analysis. Experiment 2 evaluated the conditioning effect of pentobarbital, a barbiturate aesthetic, on taste-evoked consummatory behavior. As in Experiment 1, we used sub-anesthetic doses of the US (15 and 30 mg/kg) to prevent rapid intake suppression. In both experiments water-deprived rats were allowed to consume 0.1% saccharin for 15 min and, 5 min later, were injected with saline or the drug US. Based on the aversion-avoidance account, we expect that ketamine/xylazine, but not pentobarbital, will support CTAs. That is, both taste intake and palatability are predicted to decline over repeated pairings when ketamine/xylazine serves as the US. On the other hand, we expect to find a significant reduction only in intake but no change in taste palatability (TAL learning) following contingent pentobarbital injections.
2. Materials and methods
2.1. Subjects
Experimentally naïve male Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA) were used as subjects. On arrival, the rats were individually housed in plastic cages (Ancare, Inc. Bellmore, NY) in a vivarium that was maintained at ~70°F with a 12:12 light:dark cycle (light on at 7:00 am). Food and water were available ad libitum until the experiment started. At that time, the rats were placed on a water deprivation schedule (see Procedure section); lab chow (Teklad global 18% protein; Envigo, IN) was always available in the home cages. The University of Illinois at Chicago Animal Care and Users Committee approved the procedures employed in the current study. At all times, rats were treated in accord with the guidelines recommended by the American Psychological Association (1996) and the National Institutes of Health (1996).
2.2. Apparatus
Eight identical drinking chambers (Med Associates, St. Albans, VT; 30.5 cm × 24.0 cm × 29.0 cm) were employed, each housed in a sound resistant cubicle fitted with a ventilation fan. The sidewalls of each chamber were made of aluminum, the front wall, back wall and ceiling were clear Plexiglas and the floor consisted of 19 stainless rods. A house light and a white noise generator (providing a masking noise level of 80 dB) were mounted at the top of the left wall in the chamber. Fluid was presented in a retractable sipper tube accessible through an oval hole (1.3 cm × 2.6 cm) in the middle of the right wall. In its extended position, the tip of the sipper tube was ~3 mm outside the wall. Each chamber was equipped with a lickometer circuit that allowed collection of lick times with a resolution of 10 milliseconds. Stimulus presentation and data collection from the drinking chambers were carried out on-line with a computer running Med-PC software (Med Associates) located in an adjacent room.
2.3. Procedure
2.3.1. Experiment 1
Following 7 days of environmental acclimation, the rats were deprived of water by giving 15-min water access in the morning in the drinking chambers and 15-min access each afternoon in the home cages. Once morning water intake stabilized, the experiment began. Counterbalanced by baseline water intake, the rats were assigned to three groups based on the dose of the US, which was a mixture of ketamine (ketamine HCl; Hospira Inc. Lake Forest, IL) and xylazine (Lloyd Inc. Shenandoah, IA). Training occurred in 3-day cycles. On Day 1 of each cycle, rats were allowed to consume saccharin (0.1%, w/v) for 15 min in the morning followed, 5 min later, with an IP injection of physiological saline (Group KX0; n = 9), 10/0.5 mg/kg (Group KX-d1; n = 10) or 20/1.0 mg/kg (Group KX-d2; n = 9) of ketamine/xylazine; all injections were 1 ml/kg. On Day 2, following the 15-min morning water access, Groups KX-d1 and KX-d2 received saline injections while Group KX0 was given ketamine/xylazine injections (n = 4 and 5 for high and low doses, respectively). On Day 3, rats were treated as Day 2, except there were no injections. This 3-day cycle was repeated once and followed, 24 hr later, by a single taste-only test trial (see Table 1).
Table 1.
Experimental Design.
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|---|
| Control | Sac-Sal | Water-US | Water | Sac-Sal | Water-US | Water | Sac |
| Experimental | Sac-US | Water-Sal | Water | Sac-US | Water-Sal | Water | Sac |
Note. Sac = saccharin CS; Sal = saline; US = unconditioned stimulus
2.3.2. Experiment 2
A new set of naïve male rats was obtained from Charles River Laboratory. The conditioning procedure was identical to that of Experiment 1, except that the US was pentobarbital (Lundbeck Inc. Deerfield, IL). The US doses were 15 and 30 mg/kg for, respectively, Group P-d1 (n = 7) and Group P-d2 (n = 9). As in Experiment 1, Group P0 (n = 9) received contingent injections of saline.
2.4. Dependent measures and data analysis
To characterize the nature of taste-guided learning, each experiment included three dependent variables: total licks, lick cluster size, and initial lick rate (total licks in the 3 min that followed the first lick). To afford comparability with prior studies that examined contingent palatability changes following US administrations (e.g., Dwyer, Boakes, & Hayward, 2008; Lin et al., 2013), a cluster was defined as a run of licks separated by inter-lick intervals of less than 0.5 s. Data were analyzed with 2-way mixed design analyses of variance (ANOVAs) with Group as the between-subject variable and Trial as the within-subject variable. Significant main effects or interaction were followed by post hoc comparisons with Fisher LSD tests. The alpha level for significance was set at .05. All analyses were conducted with statistical software (Statistica 6.0; StatSoft Inc. Tulsa, OK).
3. Results
3.1. Experiment 1
Baseline morning water data from the two days prior to the first conditioning trial were analyzed for each dependent measure. These analyses found no significant main effects of Group or Trial and no significant Group X Trial interaction for total licks, lick cluster size, or initial lick rate (ps > .1). Thus, water intake was stable prior to the saccharin trials. Averaged across trials for presentational purposes, the baseline water performance of each group in Experiment 1 is summarized in Table 2.
Table 2.
Baseline water results from Experiments 1 and 2 in terms of three dependent measures: total licks, lick cluster size, and initial lick rate. For each measure, the data (mean ±SE) were averaged across the two water days that preceded the first conditioning trial.
| Experiment | Group | Total Licks | Lick Cluster Size | Initial Lick Rate |
|---|---|---|---|---|
| 1 | KX0 | 2951.2 ±78.28 | 106.1 ±15.89 | 1145.0 ±12.11 |
| KX-d1 | 2773.3 ±167.34 | 136.4 ±22.19 | 1088.8 ±33.47 | |
| KX-d2 | 2947.7 ±118.33 | 107.4 ±10.49 | 1128.4 ±29.81 | |
| 2 | P0 | 2677.5 ±154.69 | 110.9 ±10.28 | 1092.3 ±22.24 |
| P-d1 | 2311.6 ±215.85 | 105.6 ±17.61 | 1034.5 ±38.97 | |
| P-d2 | 2818.4 ±148.99 | 124.4 ±13.37 | 1118.8 ±19.93 |
Note. Groups were defined by the dose of US given during the conditioning trials in each experiment. In Experiment 1, Groups KX0, KX-d1, and KX-d2 received saline, 10/0.5 mg/kg and 20/1.0 mg/kg ketamine/xylazine as the US. In Experiment 2, Groups P0, P-d1, and P-d2 received saline, 15 mg/kg and 30 mg/kg pentobarbital as the US.
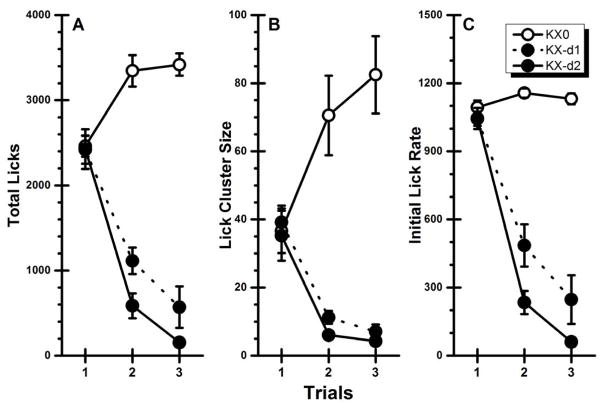
CS-directed behaviors over the two conditioning trials and the single taste-only test trial are presented in Figure 1. Inspection of the figure shows that contingent injections of the ketamine/xylazine US suppressed both the intake (total licks) and the palatability (lick cluster size and initial lick rate) of the taste CS across trials. These observations were supported by analysis, which, for total licks (Figure 1A) found a significant main effects of Group, F(2, 25) = 73.80, p < .05, and Trial, F(2, 50) = 43.65, p < .05, and a significant Group x Trial interaction, F(4, 50) = 42.34, p < .05. Post hoc analyses revealed that the control group, Group KX0, increased intake from Trial 1 to Trial 2 (p < .05) and that consumption was stable on Trial 2 and Trial 3 (p > .05). On the other hand, both experimental groups showed significant decreases in total licks from Trial 1 to Trial 2 to Trial 3 (ps < .05).
Fig. 1.
Experiment 1: Mean (±SE) CS-directed performance across two conditioning trials and one taste-only test trial for rats receiving contingent administrations of physiological saline (KX0), 10/0.5 mg/kg (KX-d1), or 20/1.0 mg/kg (KX-d2) of ketamine/xylazine. A: Total licks; B: Lick cluster size; C: Initial lick rate.
An overall similar pattern of results was found when the palatability data were subject to statistical analyses. The initial ANOVA conducted on the cluster size data (Figure 1B) found a significant main effect of Group, F(2, 25) = 25.42, p < .05, and a significant Group x Trial interaction, F(4, 50) = 25.94, p < .05; the main effect of Trial was not significant (p > .05). Post hoc comparisons indicated that the significant interaction was due to the performance of the control group and the two experimental groups changing in opposite directions – upwards for Group KX0 and downwards for Groups KX-d1 and KX-d2. Specifically, cluster size in the control group increased numerically across all three trials with the difference being significant from Trial 1 to Trial 2 (p < .05) but not from Trial 2 to Trial 3 (p > .05). The performances of the two experimental groups were virtually identical with each group showing a significant decrease in cluster size from Trial 1 to Trial 2 (p < .05) and no significant decrease between Trial 2 to Trial 3 (p > .05). In terms of the initial lick rate data (Figure 1C), the ANOVA found significant main effects of Group, F(2, 25) = 85.53, p < .05, Trial, F(2, 50) = 92.52, p < .05, and a significant Group x Trial interaction, F(4, 50) = 26.94, p < .05. Post hoc analysis revealed that Group KX0 showed no significant changes in initial lick rate across all three trials (ps > .05) while both drug-paired groups showed significant decreases in initial lick rate from Trial 1 to Trial 2 to Trial 3 (ps < .05). This conditional reduction in cluster size and initial lick rate (i.e., palatability downshift) indicates that ketamine/xylazine functions like a LiCl US to induce a CTA.
3.2. Experiment 2
Summarized in Table 2, the water baseline data from Experiment 2 were analyzed in the same way as described in Experiment 1. As in the previous experiment there were no significant main effects of Group or Trial and no significant Group X Trial interaction (ps > .10) for each performance measure. Thus, all three dependent measures were stable prior to the first conditioning trial.
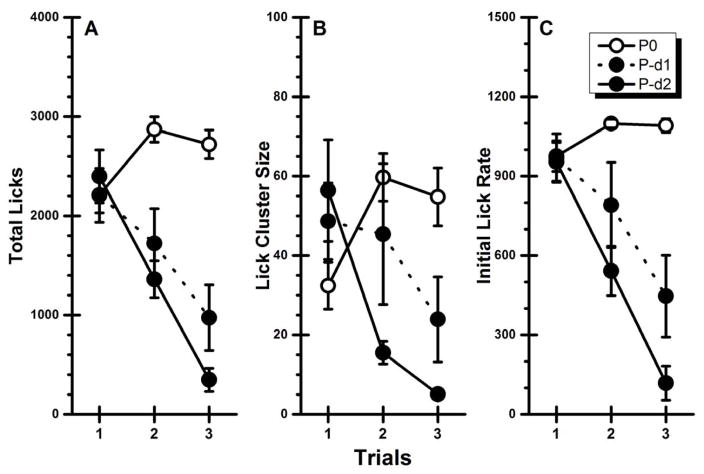
Inspection of Figure 2A suggests that contingent injections of the pentobarbital US suppressed both the intake (total licks) and the palatability (lick cluster size and initial lick rate) of the taste CS across trials. These observations were confirmed by statistical analysis that found significant main effects of Group, F(2, 22) = 12.44, p < .05, and Trial, F(2, 44) = 35.78, p < .05, and a significant Group X Trial interaction, F(4, 44) = 25.61, p < .05. Post hoc analyses further revealed that the control group (Group P0) consumed significantly more of the taste CS on Trials 2 and 3 than on Trial 1 (ps < .05), and that intake did not differ on the latter two trials (p > .05). On the other hand, both experimental groups showed the same pattern of decreasing CS intake across trials. That is, the rats in Group P-d1 and Group P-d2 significantly decreased total licks for Trial 1 to Trial 2 to Trial 3 (ps < .05).
Fig. 2.
Experiment 2: Mean (±SE) CS-directed performance across two conditioning trials and one taste-only test trial for rats receiving contingent administrations of physiological saline (P0), 15 mg/kg (P-d1) or 30 mg/kg (P-d2) of sodium pentobarbital. A: Total licks; B: Lick cluster size; C: Initial lick rate.
For lick cluster size (Figure 2B), analysis revealed a significant main effect of Trial, F(2, 44) = 6.26, p < .05 and a significant Group X Trial interaction, F(4, 44) = 11.85, p < .05. However, the main effect of Group was not significant (p = .08). Post hoc analyses of the interaction found that Group P0 increased cluster size from Trial 1 to Trial 2 (p < .05) but not from Trial 2 to Trial 3 (p > .05). The experimental groups showed a different pattern of performance to that of the control group. Specifically, cluster size for Group P-d1 was decreased across trials and achieved significance on Trial 3 (p < .05); there was no difference between Trials 1 and 2 (p > .05). For Group P-d2, the reduction in cluster size was significant between Trials 1 and 2 (p < .05) but not between Trials 2 and 3 (p > .05). For initial lick rate (Figure 2C), the ANOVA found significant main effects of Group, F(2, 22) = 13.25, p < .05, Trial, F(2, 44) = 46.79, p < .05, and a significant Group X Trial interaction, F(4, 44) = 23.00, p < .05. While post hoc analyses revealed no significant differences over trials in the control group (presumably due to a ceiling effect in licking over the first 3 min), the initial lick rate in each experimental group significantly decreased over successive trials (ps < .05). Similar to the results of Experiment 1 with the ketamine/xylazine US, the results of Experiment 2 indicate that pentobarbital contingently suppressed both intake and palatability of the associated saccharin CS, and thus supported the acquisition of a CTA.
In both Experiments 1 and 2, we also replicated our previous results (e.g., Lin et al., 2012) that taste neophobia involves a reduction in taste palatability. That is, we found that the rats that received unpaired US injections showed significantly shorter lick clusters (less palatable) when the taste was novel and potentially dangerous (i.e., Trial 1) than when the taste was familiar and safe after repeated benign exposures (i.e., Trials 2 and 3). One may wonder why, if initial lick rate also reflects palatability, this dependent measure did not significantly increase across trials. This lack of increase in initial lick rate, we believe, was likely due to a ceiling effect because of the limitation of how many licks can be made within a brief time period (e.g., Spector, Klumpp, & Kaplan, 1998). Therefore, in certain circumstances, cluster size is a more useful index than initial lick rate for palatability assessment.
4. Discussion
Using lick pattern analysis to evaluate conditioned shifts in taste palatability, the present study is the first to empirically investigate the nature of the taste learning caused by anesthesia-inducing drugs. Experiment 1 found that a ketamine/xylazine US suppressed both the intake (total licks) and the palatability (lick cluster size and initial lick rate) of the saccharin CS. This result was expected since the dissociative anesthetic, like classic poisons, can cause vomiting. This conditioned devaluation effect was also found in Experiment 2 with the pentobarbital US, a drug that has little vomiting-inducing action, supported a similar suppressive effect on CS intake and palatability. This later finding is inconsistent with the aversion-avoidance account (Parker et al., 2009; Pelchat et al., 1983). Rather, it indicates that anesthesia-inducing drugs, regardless of whether they support emesis or not, when administered following consumption of a taste CS, can function as USs to produce CTAs.
Over the past 30 plus years, Parker and colleagues have published a large body of work in which they propose that some drugs induce CTAs whereas other drugs cause TAL. Of particular relevance to the present research, it has been claimed that pentobarbital supports TAL (Parker, 2003; Parker, Limebeer & Rana, 2009). To our knowledge, however, there are no published data relevant to this claim. In the absence of experimental details in their review articles, it is not possible to speculate how their data were obtained. However, since the doses of pentobarbital used in Experiment 2 covered the dose range (1–20 mg/kg) that was reported in Parker (2003) to support TAL, the CTA-inducing effect of pentobarbital demonstrated here should not be attributed to the dosage of the US.
Pentobarbital has different pharmacological profiles from poisons and toxins (e.g., LiCl), USs that are considered to produce CTAs via their adverse effects involving gastrointestinal malaise (e.g., nausea; Balleine, Garner, & Dickinson, 1995). At the doses used in the current study, the pentobarbital US does not induce general anesthesia, but, at best, only sedated the rats. That is, following US administration, the rats gradually lay down with their eyes closed. This sedative state lasted about 10–20 min, during which the rats were capable of reacting to environmental stimulation by opening their eyes and/or raising their head. In addition to the sedative effect, there is evidence suggesting that the barbiturate anesthetic may also have rewarding properties in some behavioral paradigms (i.e., support place preference learning; e.g., Bossert & Franklin, 2001). How could pentobarbital functions like poisons and toxins to produce robust CTA acquisition within one or two conditioning trials?
In approaching the answer to this question it should be noted that, from the traditional perspective of taste aversion learning, anesthesia-inducing drugs are not the only atypical agents that are capable of supporting CTA acquisition. Using lick pattern analysis, we also found that CTAs are acquired when drugs of abuse (e.g., morphine and amphetamine; Arthurs et al., 2012; Lin et al., 2012; but see Dwyer, Boakes, & Hayward, 20082 ) and internal pain-inducing stimuli (e.g., gallamine and hypertonic saline; Lin et al., 2013) serve as the US. Although each of these agents has its own pharmacological effects, they nevertheless all support CTA learning as shown by the conditioned devaluation of the taste CS. These findings with drugs of abuse and internal pain-inducing agents are at odds with the aversion-avoidance account that claims CTAs only can be caused by nausea-inducing agents (e.g., LiCl; Parker et al., 2009; Pelchat et al., 1983). Contrary to this latter interpretation, we have proposed that the CTA mechanism must be very broadly tuned so that it can defend the internal milieu against a diverse array of poisons and toxins that have very different internal effects. As presented below, this new analysis emphasizes that to account for the CTAs induced by USs that are not considered as traditional poisons, we must acknowledge the important function that taste neophobia has in priming expectations about potential poisoning, a role that has not previously been appreciated (for detailed discussion see Lin et al., 2014, 2017).
Taste neophobia refers to the reluctance to consume a novel food because of fear that it may be poisonous (Barnett, 1956, 1958; Corey, 1978; Domjan, 1977). Intake reduction, however, does not fully characterize taste neophobia. We (Lin, Amodeo, Arthurs & Reilly, 2012) discovered that a novel food is not only avoided but its palatability is precipitously downshifted relative to when the food eventually becomes familiar and safe following benign exposures; the pleasure of eating or drinking increases as taste neophobia habituates. This novel finding indicates that taste neophobia is, in fact, an innate aversive response that ensures that the new food will not be consumed in large quantities. In addition, we proposed that the fear evoked by detection of an unknown food renders the individual highly vigilant about ensuing changes in their internal milieu (e.g., Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004; Paulus & Stein, 2006) and lowers the threshold for the detection of signs that the system has been compromised, which, in turn, ensures that the CTA mechanism will be triggered sooner than otherwise would occur.
The sole function of CTA learning is to prevent the ingestion of food-borne poisons. This mechanism functions over a range that spans from the highly toxic to doses that are so low that they produce no overt signs of poisoning (e.g., Nachman & Ashe, 1973; Smith, 1971; see also Physician’s Desk Reference, 1972). That CTAs can be acquired at such low doses is particularly important because it provides evidence that the mechanism can be engaged before dangerous amounts of poisons enter the body. This, we suggest, is a critical feature of the CTA mechanism. Although this degree of sensitivity will undoubtedly render the CTA mechanism prone to false positives, such CTAs are the price of survival where, on the other hand, a single failure to detect a poison may have fatal consequences. Given the myriad types of poisons it seems unlikely that the CTA mechanism could be sensitive to each poison. Rather, we propose that, primed by taste neophobia, the CTA mechanism is sensitive to any unexpected post-prandial deviation from the normal state of the internal milieu. According to this analysis, the novel body state (i.e., sedation) that occurs consequent to the administration of anesthesia-inducing drugs in the present experiments is taken as indication of the early onset sign of poisoning and, as such, triggers the CTA mechanism which devalues the associated taste CS.
Acknowledgments
This work was supported by grants DC06456 from the National Institute of Deafness and Other Communication Disorders. Jian-You Lin is now at the Department of Psychology, Brandeis University, Waltham, MA 02453, USA; Joe Arthurs is now at the Department of Biochemistry, University of Washington, Seattle, WA 98195, USA.
Footnotes
There were some notable procedural differences between the LiCl and footshock experiments in the Pelchat et al. study. For example, the LiCl experiment involved two conditioning trials, each spaced 3 days apart, in which 10-min access to sucrose preceded LiCl administration by intragastric gavage. The footshock experiment, on the other hand, involved one 10-min conditioning trial per day, for a total of 20–25 days. On each of these trials rats had access to two stimulus bottles (sucrose and water), and a footshock was delivered within a few seconds of the start of each bout of sucrose drinking; no shocks were delivered for drinking water. Although procedural differences may play some role, the distinctive nature of the USs has been considered the dominant cause contributing to the development of CTA or TAL (e.g., Grigson, 1997; Parker, Limebeer & Rana, 2009; Pelchat et al., 1983).
Dwyer et al. (2008) reported that contingent administration of amphetamine failed to suppress palatability of the associated taste. As discussed elsewhere (e.g., Lin, Arthurs et al., 2012; Lin et al., 2014), there were some between-study procedural differences that may account for the null effect reported by Dwyer et al. That said, it should be noted that the null finding of Dwyer et al. is also at odds with Parker’s results (e.g., Parker, 1991) showing that amphetamine at the dose used by Dwyer et al. significantly suppressed ingestive taste reactivity responses, which can be taken as evidence of a conditioned reduction in palatability.
References
- Aguado L, del Valle R, Perez L. The NMDA-receptor antagonist ketamine as an unconditioned stimulus in taste aversion learning. Neurobiology of Learning and Memory. 1997;68:189–196. doi: 10.1006/nlme.1997.3773. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Guidelines for ethical conduct in the care and use of animals. Washington DC: American Psychological Association; 1996. [Google Scholar]
- Arthurs J, Lin JY, Amodeo LR, Reilly S. Reduced palatability in drug-induced taste aversion: II. Aversive and rewarding unconditioned stimuli. Behavioral Neuroscience. 2012;126:433–444. doi: 10.1037/a0027676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Self-administration of barbiturates and benzodiazepines: a review. Pharmacology, Biochemistry and Behavior. 1987;27:391–398. doi: 10.1016/0091-3057(87)90588-0. [DOI] [PubMed] [Google Scholar]
- Balleine B, Garner C, Dickinson A. Instrumental outcome devaluation is attenuated by the anti-emetic ondansetron. The Quarterly Journal of Experimental Psychology. 1995;48:235–251. [PubMed] [Google Scholar]
- Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. Waco, Texas: Baylor University Press; 1977. [Google Scholar]
- Barnett SA. Behaviour components in the feeding of wild and laboratory rats. Behaviour. 1956;9:24–43. [Google Scholar]
- Barnett SA. Experiments on ‘neophobia’ in wild and laboratory rats. British Journal of Psychology. 1958;49:195–201. doi: 10.1111/j.2044-8295.1958.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Biskin RS, Franklin KB. Systemic and intracerebroventricular administration of sodium barbital induced a place preference in rats. Behavioural Pharmacology. 2003;14:517–523. doi: 10.1097/00008877-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Franklin KB. Pentobarbital-induced place preference in rats is blocked by GABA, dopamine and opioid antagonists. Psychopharmacology. 2001;157:115–122. doi: 10.1007/s002130100772. [DOI] [PubMed] [Google Scholar]
- Braveman NS, Bronstein P, editors. Experimental assessments and clinical applications of conditioned food aversions. Vol. 443. New York: New York Academy of Sciences; 1985. [PubMed] [Google Scholar]
- Cabanac M. Palatability of food and the ponderostat. Annals of the New York Academy of Sciences. 1989;575:340–352. doi: 10.1111/j.1749-6632.1989.tb53255.x. [DOI] [PubMed] [Google Scholar]
- Corey DT. The determinants of exploration and neophobia. Neuroscience and Biobehavioral Reviews. 1978;2:235–253. [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davis JD. Microstructural analysis of the ingestive behavior of the rat ingesting polycose. Physiology and Behavior. 1996;60:1557–1563. doi: 10.1016/s0031-9384(96)00321-6. [DOI] [PubMed] [Google Scholar]
- Davis JD, Levine MW. A model for the control of ingestion. Psychological Review. 1977;84:379–412. [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation-and palatability-induced microstructural changes in ingestive behavior. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1993;264:R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience. 1992;106:217–228. [PubMed] [Google Scholar]
- Domjan M. Attenuation and enhancement of neophobia for edible substances. In: Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. Waco, Texas: Baylor University Press; 1977. pp. 151–179. [Google Scholar]
- Dwyer DM. Licking and liking: The assessment of hedonic responses in rodents. The Quarterly Journal of Experimental Psychology. 2012;65:371–394. doi: 10.1080/17470218.2011.652969. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Boakes RA, Hayward AJ. Reduced palatability in lithium-and activity-based, but not in amphetamine-based, taste aversion learning. Behavioral Neuroscience. 2008;122:1051–1060. doi: 10.1037/a0012703. [DOI] [PubMed] [Google Scholar]
- Fox RA, Corcoran M, Brizzee KR. Conditioned taste aversion and motion sickness in cats and squirrel monkeys. Canadian Journal of Physiology and Pharmacology. 1990;68:269–278. doi: 10.1139/y90-041. [DOI] [PubMed] [Google Scholar]
- Garcia J. Food for Tolman: cognition and cathexis in concert. In: Archer T, Nilsson L-G, editors. Aversion, avoidance and anxiety. Hillsdale, NJ: Lawrence Erlbaum Associates; 1989. pp. 45–85. [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kovner R, Green KF. Cue properties vs palatability of flavors in avoidance learning. Psychonomic Science. 1970;20:313–314. [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: A reinterpretation. Behavioral Neuroscience. 1997;111:129–136. [PubMed] [Google Scholar]
- Hsiao S, Fan RJ. Additivity of taste-specific effects of sucrose and quinine: microstructural analysis of ingestive behavior in rats. Behavioral Neuroscience. 1993;107:317–326. doi: 10.1037//0735-7044.107.2.317. [DOI] [PubMed] [Google Scholar]
- Kolahian S, Jarolmasjed SH. Antiemetic efficacy of promethazine on xylazine-induced emesis in cats. Canadian Veterinary Journal. 2012;53:193–195. [PMC free article] [PubMed] [Google Scholar]
- Le Magnen J. Hunger and food palatability in the control of feeding behavior. In: Katsuki U, Sato M, Takagi S, Oomura Y, editors. Food intake and chemical senses. Tokyo: University of Tokyo Press; 1977. pp. 263–280. [Google Scholar]
- Li F, Fang Q, Liu Y, Zhao M, Li D, Wang J, Lu L. Cannabinoid CB(1) receptor antagonist rimonabant attenuates reinstatement of ketamine conditioned place preference in rats. European Journal of Pharmacology. 2008;589:122–126. doi: 10.1016/j.ejphar.2008.04.051. [DOI] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Amodeo LR, Reilly S. Reduced palatability in drug-induced taste aversion: I. Variations in the initial value of the conditioned stimulus. Behavioral Neuroscience. 2012;126:423–432. doi: 10.1037/a0027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S. Reduced palatability in pain-induced conditioned taste aversions. Physiology and Behavior. 2013;119:79–85. doi: 10.1016/j.physbeh.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S. Conditioned taste aversion, drugs of abuse and palatability. Neuroscience and Biobehavioral Reviews. 2014;45:28–45. doi: 10.1016/j.neubiorev.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S. Conditioned taste aversions: From poisons to pain to drugs of abuse. Psychonomic Bulletin & Review. 2017;24:335–351. doi: 10.3758/s13423-016-1092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Christianson JP, Anderson MJ, Giovanni LM, Hinderliter CF. Ketaset-Rompun extends the effective interstimulus interval in long-trace taste-aversion conditioning in rats. Behavioural Processes. 2004;65:111–121. doi: 10.1016/j.beproc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiology and Behavior. 1973;10:73–78. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Parker LA. Taste reactivity responses elicited by reinforcing drugs: a dose response analysis. Behavioral Neuroscience. 1991;105:955–964. doi: 10.1037//0735-7044.105.6.955. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learning & Behavior. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rana SA. Conditioned disgust, but not conditioned taste avoidance, may reflect conditioned nausea in rats. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 92–113. [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. Journal of Comparative Psychology. 1983;97:140–153. [PubMed] [Google Scholar]
- Physician’s Desk Reference. 261. Medical Economics Inc; Oradell, New Jersey: 1972. [Google Scholar]
- Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. [Google Scholar]
- Reyes JC, Negron JL, Colon HM, Padilla AM, Millan MY, Matos TD, Robles RR. The emerging of xylazine as a new drug of abuse and its health consequences among drug users in Puerto Rico. Journal of Urban Health. 2012;89:519–526. doi: 10.1007/s11524-011-9662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC. Radiation: Its detection and its effects on taste preferences. In: Stellar E, Sprague JM, editors. Progress in Physiological Psychology. Vol. 4. New York: Academic Press; 1971. pp. 53–118. [Google Scholar]
- Spector AC, St John SJ. Role of taste in the microstructure of quinine ingestion by rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 1998;274:R1687–R1703. doi: 10.1152/ajpregu.1998.274.6.R1687. [DOI] [PubMed] [Google Scholar]
- Taukulis HK. Thyrotropin-releasing hormone (TRH) potentiates pentobarbital-based flavor aversion learning. Behavioral and Neural Biology. 1983;39:135–139. doi: 10.1016/s0163-1047(83)90739-2. [DOI] [PubMed] [Google Scholar]
- Wheelock JB. Pentobarbital’s effect in a combination antiemetic regimen for cisplatin induced nausea and vomiting. Journal of the Mississippi State Medical Association. 1989;30:5–8. [PubMed] [Google Scholar]