Abstract
Head and neck cancer patients treated with irradiation often present irreversible salivary gland hypofunction for which no conventional treatment exists. We recently showed that recombinant neurturin, a neurotrophic factor, improves epithelial regeneration of mouse salivary glands in ex vivo culture after irradiation by reducing apoptosis of parasympathetic neurons. Parasympathetic innervation is essential to maintain progenitor cells during gland development and for regeneration of adult glands. Here, we investigated whether a neurturin-expressing adenovirus could be used for gene therapy in vivo to protect parasympathetic neurons and prevent gland hypofunction after irradiation. First, ex vivo fetal salivary gland culture was used to compare the neurturin adenovirus with recombinant neurturin, showing they both improve growth after irradiation by reducing neuronal apoptosis and increasing innervation. Then, the neurturin adenovirus was delivered to mouse salivary glands in vivo, 24 hr before irradiation, and compared with a control adenovirus. The control-treated glands have ∼50% reduction in salivary flow 60 days post-irradiation, whereas neurturin-treated glands have similar flow to nonirradiated glands. Further, markers of parasympathetic function, including vesicular acetylcholine transporter, decreased with irradiation, but not with neurturin treatment. Our findings suggest that in vivo neurturin gene therapy prior to irradiation protects parasympathetic function and prevents irradiation-induced hypofunction.
Keywords: gene therapy, adenovirus, irradiation, xerostomia, salivary gland, neurturin
Introduction
Every year approximately 630,000 new head and neck cancer patients are diagnosed globally.1 Therapeutic irradiation (IR) is one of the primary treatments, and ∼64% of survivors treated with IR suffer from permanent xerostomia, the subjective symptom of dry mouth.2 There is also an acute reduction in salivary gland flow rates of ∼50% within a week of IR, which can diminish to ∼20% by 7 weeks.3 This salivary hypofunction is often accompanied by xerostomia, although xerostomia may not always correlate with salivary flow rates.4 The primary pathophysiological mechanism that causes salivary hypofunction is not clear, although the loss of acinar cells and disruption of muscarinic receptor-stimulated water secretion have been proposed.4 Currently, there are no therapies to prevent or permanently repair this IR damage.2, 4
We previously showed that during murine submandibular gland (SMG) development the parasympathetic ganglion (PSG) is critical to maintain a population of epithelial keratin 5-positive (K5+) progenitors, which are required for gland development.5 Acetylcholine signaling from the PSG, via muscarinic M1 receptors and EGFR in the epithelium, increased K5+ progenitor proliferation. We then showed that IR of the developing SMG in ex vivo culture reduced innervation by causing apoptosis of ∼30% of distal endbud epithelial cells that produced neurturin (NRTN), a neurotrophic factor. NRTN binds its receptors GFRα2/RET and is important for parasympathetic nervous system development and function.6, 7 Nrtn−/− mice have reduced PSG and defects in SMG innervation.8 In our previous experiments, the loss of the NRTN-expressing endbud cells disrupted epithelial-neuronal interactions, resulting in a loss of innervation, and subsequently, 3 days after IR, apoptosis of PSG neurons occurred. However, when IR SMGs were treated with recombinant NRTN, which binds GFRα2 on the nerves, reduced neuronal apoptosis was observed. Consequently, NRTN restored parasympathetic function, which improved innervation and epithelial regeneration after IR.9 It has also been shown, using reversible duct ligation as a model to study gland regeneration after damage, that epithelial regeneration after removal of the ligature only occurred if the parasympathetic innervation was intact. The remaining epithelium regenerated in a similar manner to gland development, i.e., by branching morphogenesis.10 Taken together, these data suggest that NRTN could be a candidate for treatment of IR-induced salivary hypofunction by targeting the parasympathetic innervation to stimulate epithelial regeneration and saliva secretion.
Salivary glands are directly accessible through ducts in the oral cavity, and gene transfer to the gland epithelia is accomplished by delivery of a vector by retro-ductal cannulation. Several studies demonstrated the success of non-integrating adenovirus serotype 5 vectors in transducing adult mouse SMGs following retrograde ductal cannulation.11, 12, 13, 14 Adenoviral vectors show sustained transgene expression in the SMGs for up to 6 months after exposure to fractionated IR.14 As a result, a phase 1 clinical trial was completed, using adenoviral-mediated transfer of aquaporin-1 cDNA to the salivary epithelium in patients previously treated by IR for head and neck cancer. The study confirmed that gene therapy is feasible in humans to potentially increase fluid secretion and relieve xerostomia experienced by patients after IR.15, 16
A gene therapy vector expressing NRTN was used in human clinical trials in the central nervous system as a potential treatment for Parkinson’s disease.17 Here, we delivered a serotype 5 adenovirus expressing NRTN (AdNRTN) prior to fractionated IR of SMGs. The AdNRTN-treated SMGs produced similar amounts of saliva as control non-IR mice 60 days after IR. We propose that NRTN protects the PSG from IR damage by promoting PSG neuronal survival, subsequently allowing regeneration of the remaining epithelium. Thus, NRTN gene therapy may be a potential strategy to prevent permanent IR-induced hypofunction in humans being treated for head and neck cancer.
Results
AdNRTN and Recombinant NRTN Protein Have Similar Effects in Fetal SMG after IR
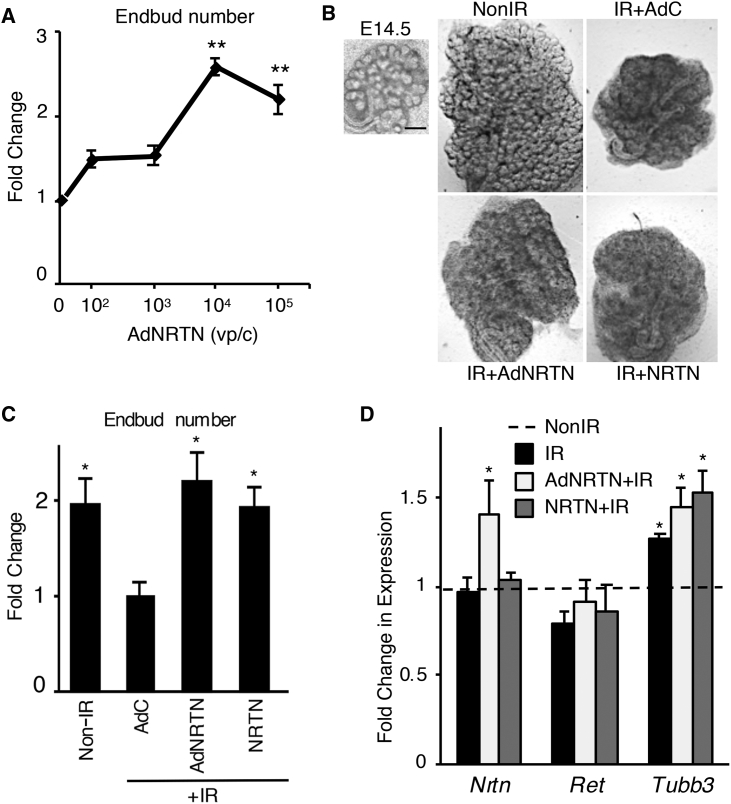
Our initial experiments were to determine whether AdNRTN treatment was similar to recombinant NRTN treatment in ex vivo fetal SMG culture. We hypothesized the virus may infect both mesenchyme and epithelium, which would then secrete NRTN to improve innervation after IR. First, we tested a range of viral doses by treating ex vivo embryonic day 13.5 (E13.5) SMGs for 24 hr with concentrations of AdNRTN ranging from 102 to 105 viral particles/cell (vp/c) and then a 5 Gy dose of IR. We calculated the average cell number in an E13.5 SMG (20,000 cells/gland) to determine the vp/c. After a further 72 hr of ex vivo culture, there was a significant increase in endbud number with an AdNRTN concentration of 104 vp/c compared with IR SMGs without AdNRTN treatment (Figure 1A). The endbud number is a measure of branching morphogenesis and SMG growth, and is expressed as a fold change over the number of endbuds at the beginning of the experiment. Further increases in the dose of AdNRTN virus did not improve SMG growth post-IR.
Figure 1.
An Adenovirus Expressing Neurturin Improves Ex Vivo SMG Growth after Irradiation Similar to Recombinant NRTN
(A) E13.5 SMGs were treated for 24 hr with increasing doses of AdNRTN, then IR (5 Gy) and cultured for a further 72 hr. The endbuds were counted and normalized to baseline. AdNRTN 104 vp/c was used for subsequent experiments. N = 12 SMG/group. Error bars represent SEM. ANOVA with post hoc Dunnett’s test, **p < 0.001 when compared with IR control without AdNRTN treatment. (B) Bright-field images of E14 SMGs at T0 and again at 72 hr showing the increase in size after treatment with AdNRTN at 104 vp/c and NRTN before IR. SMGs were treated for 24 hr with a control vector (AdC), AdNRTN (104 vp/c), NRTN (1 ng/mL), then IR (5 Gy) and cultured for a further 72 hr. Scale bars: 200 μm. (C) The number of endbuds from (B) were counted and normalized to the number of endbuds at time 0. N = 4. Error bars represent the SEM. ANOVA with post hoc Dunnett’s test, *p < 0.01 when compared with IR control. (D) There is an increase in Nrtn expression with AdNRTN treatment, no change in Ret, and increased Tubb3 expression with IR. Gene expression relative to non-IR control and normalized to ribosomal protein S29 (Rps29) shows N = 3. Error bars represent the SEM. ANOVA compared with non-IR control, *p < 0.05. vp/c, viral particles per cell.
For the following experiments, we used E14.5 SMGs in ex vivo culture (Figure 1B) to directly compare the AdNRTN (104 vp/c) with treatment with recombinant NRTN protein. This assay was similar to our previous studies, where we showed that a 5 Gy IR dose reduced endbud number due to apoptosis of the proliferating epithelium, but allowed survival of the PSG and axonal bundles.9 Both AdNRTN and recombinant NRTN protein treatment 24 hr before IR improved gland growth 72 hr post-IR (a total of 96 hr ex vivo culture), and the morphology of the epithelial endbuds appeared more similar to non-IR glands (Figure 1B). The irradiated SMGs treated with either AdNRTN or recombinant NRTN also had a 2-fold increase in endbud number compared with those treated with a control GFP-expressing virus (AdC) (Figure 1C). Moreover, viral infection alone did not stimulate endbud growth because AdC treatment was similar to IR alone (data not shown).
We measured gene expression with qPCR 72 hr post-IR and compared non-IR SMGs and IR SMGs treated with either AdNRTN or recombinant NRTN with the AdC treatment (Figure 1D). We measured expression of Nrtn its coreceptor, Ret, and the neuronal marker Tubb3. Overall, Nrtn expression was not reduced by 5 Gy IR, and the surviving cells expressed similar levels of Nrtn as recombinant NRTN-treated SMGs. Although we confirmed that AdNRTN treatment increased Nrtn expression (∼1.5-fold) after IR, Ret expression was similar in all groups. The expression of Tubb3 is relatively increased after 5 Gy IR (Figure 1D), because IR has direct effects on the epithelium, increasing apoptosis. Thus, the surviving neurons, which are more resistant to IR, result in increased Tubb3 expression by qPCR.
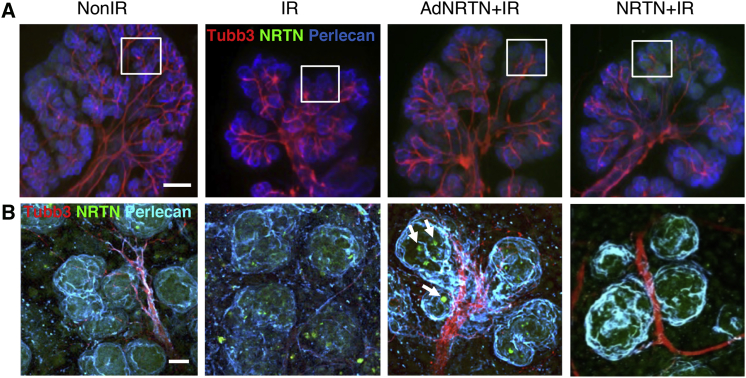
We next used whole-mount immunostaining to assess PSG axonal outgrowth and NRTN expression 72 hr post-IR after AdNRTN treatment. After 5 Gy IR, the PSG and axons are clearly present along the ducts, although the epithelium is reduced in size (Figure 2A). At higher magnification (Figure 2B) it is clear that the defasciculation of axons around the distal endbuds are reduced with IR. Both AdNRTN and recombinant NRTN increased epithelial budding and increased axonal projections surrounding them (Figure 2A). The PSG axon bundles (red, TUBB3+) appeared thicker surrounding the buds in SMGs treated with either AdNRTN or NRTN. NRTN protein (green, NRTN) was also present in the epithelial buds of AdNRTN- and NRTN-treated SMGs (higher magnification, Figure 2B). There appeared to be groups of cells with high NRTN expression after AdNRTN treatment (white arrows, Figure 2B), suggesting that the virus may be preferentially infecting a subpopulation of cells. These findings suggest that AdNRTN may protect the PSG after IR during ex vivo SMG culture to improve innervation and distal endbud regeneration.
Figure 2.
AdNRTN Treatment Increases Parasympathetic Innervation of Ex Vivo SMG Culture after Radiation
(A) Whole-mount image of a SMGs cultured for 72 hr after IR. The SMGs were immunostaining for nerves (Tubb3), NRTN, and perlecan, which stain the basement membrane. The area in the white box is shown in (B). (B) Higher magnification of terminal endbuds and nerves. Individual endbud cells with increased NRTN expression are apparent with AdNRTN treatment (white arrows). Scale bars: 250 μm (A); 50 μm (B).
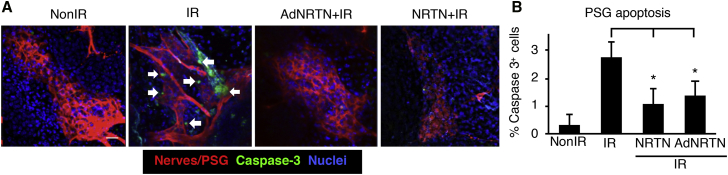
AdNRTN Reduces Apoptosis and Promotes PSG Survival in Fetal SMGs after IR
We previously showed that recombinant NRTN treatment reduces neuronal apoptosis and restores parasympathetic function after irradiation,9 and we needed to confirm that AdNRTN treatment would have a similar effect. Therefore, we measured immunostaining of cleaved caspase-3, an apoptotic marker, in the PSG after IR and treatments. Both AdNRTN and NRTN reduced the number of caspase-3-positive neuronal cell bodies (TUBB3+) in the PSG of IR SMGs in ex vivo culture (Figure 3A). Quantitation of fluorescence showed there was a significant ∼2.5-fold reduction in apoptotic cells in the PSG when IR SMGs were treated with either AdNRTN or NRTN (Figure 3B). Thus, AdNRTN and NRTN have similar effects in reducing neuronal apoptosis after IR.
Figure 3.
AdNRTN Treatment Reduces Apoptosis of Parasympathetic Ganglia in Irradiated E14 SMG
(A) SMG immunostained to visualize the PSG cell bodies (Tubb3, red), apoptotic cells (cleaved caspase-3, green), and nuclei (Hoechst, blue). White arrows show cells in the IR group that express both caspase-3 and Tubb3. Scale bar: 20 μm. (B) There is a reduction in apoptosis 48 hr after IR with AdNRTN and recombinant NRTN. Scale bar: 20 μm. N = 3. Error bars represent SEM. ANOVA compared with IR, *p < 0.05.
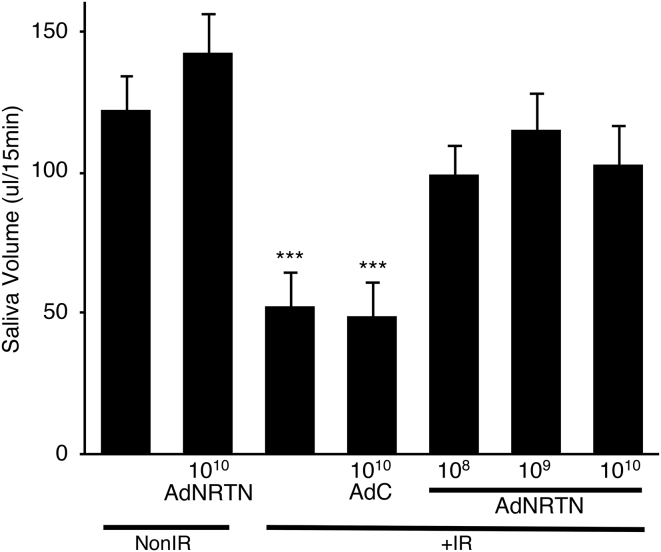
In Vivo AdNRTN Treatment followed by Fractionated IR Rescues Saliva Secretion 60 Days Post-IR
We then tested whether AdNRTN treatment before IR has a similar effect in adult murine SMGs in vivo. Adult mice were treated with a retro-ductal infusion of AdNRTN (108, 109, or 1010 vp/gland [vp/g]) or an empty vector adenoviral control (AdC) by ductal cannulation into both SMGs. The following day, ∼24 hr later, the SMGs were treated for 5 consecutive days with fractionated IR (5 × 6 Gy). After 60 days, the whole stimulated salivary volume was measured for 15 min by using capillary tubing to drain saliva from the floor of the mouth after intraperitoneal pilocarpine administration (Figure 4). It has been previously shown that by 60 days post-IR there is a reproducible chronic loss of saliva.18, 19, 20 As expected, IR significantly reduced saliva secretion more than 50% compared with non-IR controls (Figure 4), and treatment with the control vector (AdC+IR) did not improve saliva secretion. However, treatment with all three doses of AdNRTN before IR improved salivary secretion so that it was similar to non-IR control levels (Figure 4). Saliva secretion did not change in non-IR mice treated with AdNRTN 1010 vp/g (AdNRTN1010 vp/g non-IR). These data suggest that treatment with AdNRTN before IR may be a useful strategy to protect secretory function.
Figure 4.
AdNRTN Treatment of SMGs before IR Increases Salivary Flow Rates Similar to the Non-IR Group
Pilocarpine-stimulated whole saliva (in μL/15 min) was measured 60 days after IR. There was ∼60% reduction in saliva flow with IR compared with the non-IR group, and the control vector (AdC) did not increase saliva after IR. Non-IR SMGs were also treated with AdNRTN (1010 vp/g), and the AdNRTN (108, 109, and 1010 vp/g) treatment of IR SMGs improved saliva volume similar to the non-IR group. N = 8 mice, except non-IR AdNRTN10 vp/g, which had N = 4. Error bars represent the SEM. ANOVA with post hoc Dunnett’s test, ***p < 0.001 compared with non-IR.
In Vivo AdNRTN Treatment Does Not Alter SMG Histology but Increases Markers of Parasympathetic Vesicular Transport
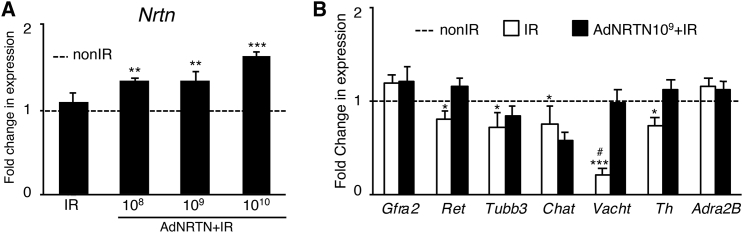
After in vivo saliva collection, the SMGs were harvested and analyzed by qPCR, histology, and immunostaining. We first confirmed that we could detect increased expression of Nrtn 60 days post-IR, which correlated with increasing doses of AdNRTN treatment (Figure 5A). We also confirmed we could detect adenovirus DNA in the SMGs 60 days after treatment (Figure S1). We used qPCR to show there was also a significant increase in expression of Ret, the kinase receptor that is a coreceptor with GFRα2 for NRTN. Ret expression decreased ∼0.7-fold after IR and increased to ∼1.2-fold of non-IR levels with AdNRTN treatment (Figure 5B). Furthermore, expression levels of parasympathetic and sympathetic genes, Vacht and Th, respectively, were restored with AdNRTN treatment 60 days after IR damage (Figure 5B). There were no significant differences in the expression of Gfra2 and Tubb3 or other parasympathetic and sympathetic markers, Chat and Adra2b, respectively, between the IR and IR with AdNRTN groups (Figure 5B).
Figure 5.
Treatment of SMGs In Vivo with Increasing Doses of AdNRTN Increases Nrtn Transcripts within the Gland and Improves Some Neuronal Markers after IR
(A) qPCR analysis of Nrtn transcripts in SMGs 60 days after IR and treatment with increasing doses of AdNRTN. (B) Additional qPCR of genes related to NRTN signaling in IR SMGs treated with AdNRTN 109 compared with IR treatment alone. The expression of the NRTN receptors Gfra2 and Ret kinase, neuronal markers Tubb3, and the parasympathetic markers Chat and Vacht and the sympathetic markers Th and Adra2b. Gene expression was normalized to non-IR SMGs and the ribosomal housekeeping gene Rps29 (data not shown). qPCRs were done in triplicate, and N = 11–12 SMGs analyzed. #Vacht expression was not detected in 5 of 11 SMGs. Error bars represent the SEM. ANOVA with post hoc Dunnett’s test compared with non-IR, *p < 0.05; **p < 0.01; ***p < 0.001.
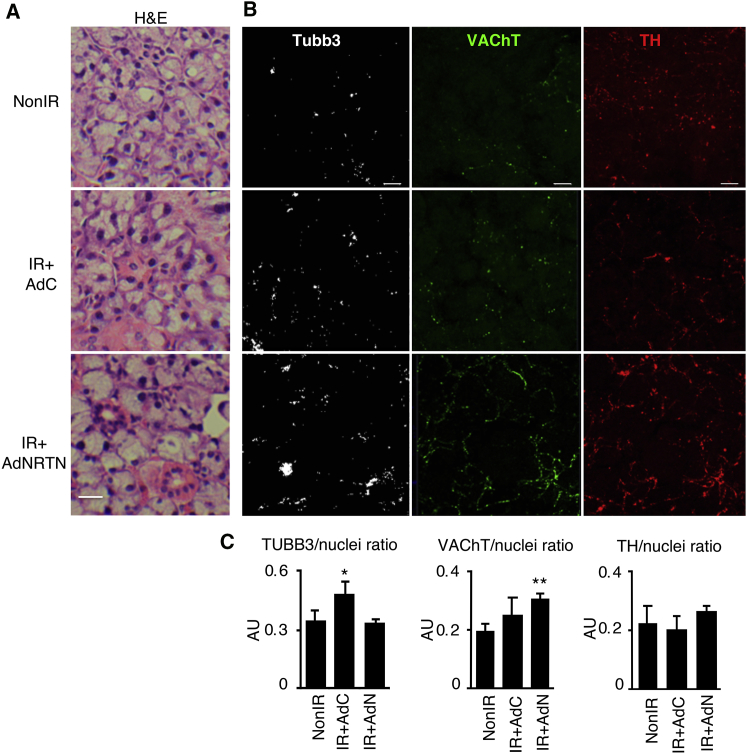
We then analyzed adult SMG tissue histology and markers of innervation after AdNRTN treatment. The cellular morphology of H&E-stained SMG tissues appeared comparable among all in vivo treatments (Figure 6A). These observations are consistent with previous reports, where major atrophy and tissue fibrosis are absent 2 months after IR damage in this strain of mice and appear a few months later.21 In addition, after staining SMGs for TUBB3, a pan-neuronal marker, increased innervation was observed with IR (Figures 6B and 6C). However, the protein expression of the parasympathetic marker, vesicular acetylcholine transporter (VAChT), but not the sympathetic marker tyrosine hydroxylase (TH), significantly increased with in vivo AdNRTN treatment compared with non-IR (Figures 6B and 6C). VAChT and TH are essential for acetylcholine neurotransmitter release and for catecholamine synthesis, respectively.22, 23 Therefore, changes in VAChT suggest that AdNRTN is acting directly upon the parasympathetic nerves.
Figure 6.
AdNRTN Treatment of Adult SMG In Vivo Also Increases Staining of the Neuronal Markers VAChT and TH
(A) H&E staining of adult SMG appears similar among all treatment groups after IR of mouse SMGs. (B) Sections were also immunostained for nerves (Tubb3), VAChT, a marker of parasympathetic function, TH, a sympathetic marker, and nuclei (Hoechst). (C) Staining was quantified (five sections/gland) and normalized to nuclear staining. N = 3–4 SMGs/group. Error bars represent SEM. ANOVA compared with non-IR, *p < 0.05; **p < 0.01. Scale bars: 20 μm.
NRTN Does Not Increase Proliferation in Head and Neck Cancer Cell Lines
A possible concern using gene therapy with a neurotrophic factor is the potential off-target proliferative effects on any remaining head and neck squamous carcinoma cells. To begin to address this issue, we treated 3 different head and neck cancer cell lines (WSU-HN12, WSU-HN6, WSU-HN824) with recombinant NRTN and measured cell proliferation (Figure S2). These cell lines were previously analyzed by RNA-sequencing (RNA-seq) analysis24 and did not express GFRα2, the receptor for NRTN. Here, we found that the direct treatment of the tumor cells with recombinant NRTN did not induce off-target proliferation.
Discussion
Here, we provide proof-of-principle evidence that gene therapy in vivo with an AdNRTN protects the parasympathetic innervation of the murine SMG 60 days after IR, resulting in stimulated saliva production similar to non-IR controls. There was no discernable difference in the histology of the SMGs at 60 days post-IR with any of the treatments, although there was also an overall increase in neuronal staining and an increase in staining of markers of both sympathetic and parasympathetic nerves in the AdNRTN-treated SMGs. We speculate that the mechanism by which AdNRTN protects saliva production may be similar to that reported in our previous ex vivo experiments. We previously proposed that recombinant NRTN was a survival factor and reduced apoptosis of the PSG neurons 3 days after IR-induced apoptosis of the Nrtn-expressing epithelial cells.9 Whether PSG apoptosis occurs 3 days after IR in vivo remains to be determined. The increase in neuronal staining with markers of both sympathetic and parasympathetic nerves suggests that the balance of innervation by these two autonomic pathways may be important for regeneration. Moreover, our previous studies with fetal SMGs showed that IR reduced parasympathetic innervation, which was restored with exogenous recombinant NRTN. However, the ex vivo fetal SMG at E13.5 is not innervated by sympathetic nerves at this stage, so the balance of sympathetic and parasympathetic innervation could not be studied. Despite this limitation, we did show that biopsies of IR human salivary glands had an overall increase in innervation.5 Understanding how both parasympathetic and sympathetic innervation function together to restore gland homeostasis requires further investigation.
The use of cholinergic parasympathomimetics agents, such as pilocarpine, to treat IR-induced salivary hypofunction is one of the current clinical treatment options.25, 26 Pilocarpine improves saliva production and reduces the symptoms of xerostomia in ∼50% of cancer patients,27 but patients must take this drug on a daily basis (3–4 times a day) for life. Unfortunately, common side effects are sweating, headaches, and gastric ulcers, and they have limited use for patients with cardiovascular disease because of their vasodilation effects.27, 28, 29 If given concomitantly with IR therapy, pilocarpine did not improve xerostomia symptoms more than placebo.30 Alternatively, sympathetic agonists acting on α1- or α2-adrenoreceptors have also been tested in rodents during IR therapy with conflicting results. Protection from IR damage occurred only when adrenoreceptor agonists were combined with muscarinic agonists and when the SMG was targeted 30 or 60 minutes before IR.31, 32, 33 However, the combination of adrenergic and muscarinic agonists only protected the SMG against IR damage on a short term, suggesting life-long use may be required. This raises another concern, because long-term use of sympathomimetics may prompt life-threatening cardiac side effects such as systemic vasoconstriction and vasodilation when using α1- or α2-adrenoreceptors, respectively.32 Therefore, finding a more permanent and effective treatment for IR-induced salivary hypofunction remains an important research goal.
A gene therapy trial to treat IR-induced xerostomia was aimed at repairing fluid secretion in the surviving ductal epithelial cells long after the IR damage. The phase 1 clinical trial reported some success using an Aquaporin-1-expressing adenovirus.15, 16 Aquaporin-1 is a water channel not normally expressed in salivary ductal epithelium and was hypothesized to increase salivary fluid flow from the surviving undamaged ductal cells post-IR.34 Our proof-of-principle in vivo study using AdNRTN before IR is based on the hypothesis that providing neurotropic support for the parasympathetic neurons will allow improved salivary function and regeneration of surviving epithelial progenitors after IR. We speculate that the improved salivary function may be a result of reduced neuronal apoptosis and an increase in PSG survival via neuronal NRTN/GFRα2/RET signaling. This is supported by the increased expression of VAChT in neurons after AdNRTN treatment. Studies have shown a strong relationship between the levels of VAChT expression and acetylcholine release into the synaptic cleft in the peripheral nervous system, suggesting that NRTN is increasing parasympathetic activity.35 Moreover, the increase in TH by AdNRTN treatment also denotes a potential increase in the synthesis of catecholamines, markers of sympathetic neurotransmission. Sympathetic signaling was reported to have inhibitory effects on epithelial regeneration by blocking muscarinic-induced EGFR activation.36 Nevertheless, these changes in VAChT and TH suggest that AdNRTN delivery directly affects GFRα2-expressing parasympathetic nerves and indirectly affects sympathetic nerves, although the mechanisms of this crosstalk remains to be investigated.
The murine SMG is used as a model to study how to prevent, repair, or regenerate function after IR damage. However, a caveat with the murine SMG model at 60 days post-IR is that it does not present the atrophy, fibrosis, and inflammatory infiltrates, which are a histopathological hallmark of human gland post-IR. The pathophysiological mechanisms causing hypofunction in the absence of histological damage are not clear. A likely mechanism relates to disruption of muscarinic signaling that drives salivary fluid secretion.4 We speculate that NRTN stimulates parasympathetic innervation and restores secretion of neurotransmitters, increasing cholinergic signaling via muscarinic receptors. While our studies are promising, future studies will be required using animal models such as the minipig, which respond to IR in a similar manner to human glands. Whether stimulation of parasympathetic innervation and subsequent epithelial regeneration can prevent the fibrotic and inflammatory response, or whether AdNRTN could be used to treat IR-induced hypofunction long after the initial IR damage remain to be determined.
Materials and Methods
Ex Vivo SMG Culture Treatment and Irradiation
Fetal SMG explants from ICR embryos were cultured as previously described.37 E13.5 or E14.5 SMGs were placed in culture dishes and incubated with fresh medium containing BSA, treated with either a single dose of recombinant mouse NRTN or adenovirus expressing NRTN cDNA. The optimal dose of recombinant NRTN was previously determined to be 1 ng/mL.9 For adenovirus treatment, 102–105 vp/c or an empty control vector were added to the media. Explants were irradiated 24 hr later in a Gammacell 1000 irradiator with a single dose of irradiation (5 Gy). SMGs were cultured for a further 24–48 hr. Explants were either lysed for RNA or fixed for immunostaining. The endbud number was counted at the beginning of the experiment (time 0) and at the end of the assay (72 hr) and expressed as a ratio (endpoint/T0) and normalized to the control irradiated treatment (% IR control). All experiments showing endbud numbers were obtained using 4 SMGs/group, and each experiment repeated at least three times.
Adenovirus Vector Construction
The AdLTR2EF1α-hNRTN vector used for these experiments, here abbreviated to AdNRTN for simplicity, is a hybrid E1-deleted serotype 5 recombinant adenoviral vector with a human NRTN cDNA (hNRTN; Origene Technologies, Rockville, MD, USA), which can persist long-term transgene expression in the salivary gland.14 In brief, to construct plasmid pACLTR2EF1α-hNRTN, the plasmid pACLTR2EF1α were digested with BamHI filled in with T4 DNA polymerase and then digested with EcoRI to ligate ∼0.6 kb of hNRTN; pCMV6-XL5-hNRTN (Origene) was digested with Xba I and filled in with T4 DNA polymerase and digested with EcoRI to obtain the hNRTN cDNA. The recombinant vector AdLTR2EF1α-hNRTN was produced by homologous recombination of pACLTR2EF1a-hNRTN with pJM17 in C7 cells. The titers (particles/mL) of purified vectors were determined by qPCR using primers from the E2 region.13
Gene Expression Analysis
Real-time PCR was performed as previously described.38 Primers were designed using Beacon Designer software. Melt-curves and primer efficiency were determined as previously described. Gene expression was normalized to Rps29 and to the corresponding experimental control, and reactions were run in triplicate and repeated three times.
In Vivo Experiments
All animal experiments were approved by the National Cancer Institute Animal Care and Use Committee. Eight-week-old female C3H mice (National Cancer Institute Animal Production Area) were used for this study. Mice were anesthetized with intraperitoneal ketamine (60 mg/kg) and xylazine (8 mg/kg). The AdLTR2EF1α-hNRTN vector (AdNRTN) was delivered to both SMGs at 108, 109, or 1010 vp/g by retrograde ductal infusion.21, 39 During the cannulation, 0.5 mg/kg atropine was intramuscularly used to inhibit saliva secretion in order to increase transduction efficiency. The three control groups were non-IR, IR only, and IR plus adenovirus control empty vector (AdC). This latter vector has the same backbone of AdLTR2EF1α-hNRTN, but without any transgene. Each group had three or four mice, and the experiment was repeated.
For the irradiated mice, only head and neck area was irradiated by placing each animal in a specially built Lucite jig so the animal could be immobilized without the use of anesthetics.40 Additionally, the jig was fitted with a Lucite cone surrounding the head and preventing head movement during radiation. Fractionated irradiation at 5 × 6 Gy (6 Gy/day for 5 days) was delivered 24 hr after vector delivery by a Therapax DXT300 X-ray irradiator (Pantak). After irradiation, animals were removed from the jig, housed (four animals per cage) in a climate- and light-controlled environment, and allowed free access to food and water.
Blood, saliva, and tissue were collected on day 60. For saliva collections, anesthetized mice were stimulated using 1 μL/g body weight of a pilocarpine solution subcutaneously (0.5 mg/mL). Whole saliva was collected with a 75-mm hematocrit tube (Drummond) into 1.5 mL pre-weight Eppendorf tubes for 15 min and frozen immediately. Blood samples were collected from the retro-orbital sinus after the saliva collection. To separate the serum phase from blood, we centrifuged samples at 12,000 rpm for 3 min, and the serum supernatant was collected and stored at −80°C. Mice were sacrificed in a carbon monoxide chamber and the SMGs were removed.
Histopathology
Intact SMGs were surgically removed from each mouse following euthanasia. SMGs were either lysed for RNA or protein, cryopreserved, or fixed in paraformaldehyde (PFA) 4%. After PFA fixation, 4-μm paraffin sections were stained with H&E and visualized in an Axiovert 25 light microscope (Zeiss, NY, USA) at 40× magnification. H&E images were taken with a Nikon 5000 camera (Nikon, Japan). Cryopreserved in vivo SMG tissues were used for immunofluorescence analysis.
Immunofluorescence Analysis
Whole-mount fetal SMG immunofluorescence analysis has been described previously.5 Ex vivo SMGs and PSGs were fixed with 4% PFA for 20 min at room temperature and/or ice-cold acetone/methanol for 5 min. In vivo SMGs were cryosectioned at 200 μm and fixed similar to the ex vivo SMGs. Tissues were blocked overnight with 10% donkey serum (Jackson Laboratories, ME, USA), 1% BSA, and mouse on mouse (MOM) immunoglobulin G (IgG) blocking reagent (Vector Laboratories, CA) in 0.01% PBS-Tween 20. SMG tissues were incubated with primary antibodies overnight at 4°C: goat anti-GFRα2 (1:100; R&D Systems), goat anti-NRTN (AB477, 1:100; R&D Systems), mouse anti-TUBB (clone TUJ1 at 1:200; R&D Systems), goat anti-vesicular acetylcholine transporter (VAChT; 1:100; Millipore), chicken anti-TH (1:400; Neuromics), rabbit anti-caspase-3 (1:200; Cell Signaling), rat anti-perlecan (1:400; Chemicon), and rabbit anti-E-cadherin (1:200; Cell Signaling). Nuclei were stained with Hoechst C10337 (Invitrogen). Antibodies were detected using Cy2-, Cy3-, or Cy5-conjugated secondary Fab fragment antibodies (Jackson Laboratories, ME, USA). Fluorescence was analyzed using a Zeiss 710 LSM microscope (Zeiss, NY, USA). Quantitation of the caspase-3-positive cells in the PSG of fetal SMGs was performed according to our previous publication.9 Quantitation of Tubb3, VAChT, TH, GFRα2, and Hoechst fluorescence in the in vivo adult SMG sections was calculated from z stacks consisting of eight confocal images separated 3 μm apart and scanned through the section at four different random quadrants (N = 4). The Tubb3, GFRα2, VAChT, and TH fluorescent staining were normalized to the nuclear staining.
Statistics
We used GraphPad Prism 7.0 software (GraphPad Software, CA, USA). Data were log transformed, and one-way ANOVA was used comparing groups with the experimental control. Graphs show the mean ± SEM.
Cell Culture
The following cell lines were a gift from S. Gutkind (University of California San Diego [UCSD]): WSU-HN12, WSU-HN6, WSU-HN8. They were cultured in DMEM (11965-092; Thermo Fisher Scientific) with 10% FBS, in 5% CO2, at 37°C.
WST-1 Proliferation Assay
Cells were seeded at 300 cells/well on 96-well dish, allowed to adhere overnight, then serum starved for 24 hr. Cells were then stimulated with Nrtn (0.01 and 1 ng/mL) (R&D Systems, Minneapolis, MN, USA), diluted in 0.1% BSA in PBS. Cell proliferation was assayed in both serum-free and 1% FBS with a WST-1 colorimetric cell proliferation assay according to the manufacturer’s instructions (05015944001; Sigma, St. Louis, MO, USA).
Author Contributions
Conceptualization, M.P.H., J.N.A.F., and I.M.A.L.; Investigation, J.N.A.F., C.Z., I.M.A.L., C.M.G., A.P.C., J.M.S., and V.N.P.; Writing, J.N.A.F., I.M.A.L., V.N.P., and M.P.H.; Supervision, V.N.P. and M.P.H.
Acknowledgments
We would like to give thanks to Drs. Wendy Knosp, Alex Chibly, and Belinda Hauser for review and discussions. J.N.A.F. was supported by the Dental Clinical Research Fellowship Program at NIDCR, NIH. This work was supported by the Division of Intramural Research of the National Institute of Dental and Craniofacial Research at the NIH, Department of Health and Human Services.
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.02.008.
Supplemental Information
References
- 1.Vigneswaran N., Williams M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. North Am. 2014;26:123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijers O.B., Levendag P.C., Braaksma M.M., Boonzaaijer M., Visch L.L., Schmitz P.I. Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck. 2002;24:737–747. doi: 10.1002/hed.10129. [DOI] [PubMed] [Google Scholar]
- 3.Dirix P., Nuyts S., Van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer. 2006;107:2525–2534. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- 4.Vissink A., Mitchell J.B., Baum B.J., Limesand K.H., Jensen S.B., Fox P.C., Elting L.S., Langendijk J.A., Coppes R.P., Reyland M.E. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:983–991. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knox S.M., Lombaert I.M., Reed X., Vitale-Cross L., Gutkind J.S., Hoffman M.P. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi J., Airaksinen M.S. GDNF family signalling in exocrine tissues: distinct roles for GDNF and neurturin in parasympathetic neuron development. Adv. Exp. Med. Biol. 2002;506(Pt A):19–26. doi: 10.1007/978-1-4615-0717-8_2. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto H., Heuckeroth R.O., Golden J.P., Johnson E.M., Milbrandt J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development. 2000;127:4877–4889. doi: 10.1242/dev.127.22.4877. [DOI] [PubMed] [Google Scholar]
- 8.Heuckeroth R.O., Enomoto H., Grider J.R., Golden J.P., Hanke J.A., Jackman A., Molliver D.C., Bardgett M.E., Snider W.D., Johnson E.M., Jr., Milbrandt J. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- 9.Knox S.M., Lombaert I.M., Haddox C.L., Abrams S.R., Cotrim A., Wilson A.J., Hoffman M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proctor G.B., Carpenter G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Shai E., Falk H., Honigman A., Panet A., Palmon A. Gene transfer mediated by different viral vectors following direct cannulation of mouse submandibular salivary glands. Eur. J. Oral Sci. 2002;110:254–260. doi: 10.1034/j.1600-0722.2002.21200.x. [DOI] [PubMed] [Google Scholar]
- 12.Vitolo J.M., Baum B.J. The use of gene transfer for the protection and repair of salivary glands. Oral Dis. 2002;8:183–191. doi: 10.1034/j.1601-0825.2002.02865.x. [DOI] [PubMed] [Google Scholar]
- 13.Zheng C., Baum B.J. Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Mol. Ther. 2005;12:528–536. doi: 10.1016/j.ymthe.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Zheng C., Vitolo J.M., Zhang W., Mineshiba F., Chiorini J.A., Baum B.J. Extended transgene expression from a nonintegrating adenoviral vector containing retroviral elements. Mol. Ther. 2008;16:1089–1097. doi: 10.1038/mt.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum B.J., Alevizos I., Zheng C., Cotrim A.P., Liu S., McCullagh L., Goldsmith C.M., Burbelo P.D., Citrin D.E., Mitchell J.B. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA. 2012;109:19403–19407. doi: 10.1073/pnas.1210662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alevizos I., Zheng C., Cotrim A.P., Liu S., McCullagh L., Billings M.E., Goldsmith C.M., Tandon M., Helmerhorst E.J., Catalán M.A. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther. 2017;24:176–186. doi: 10.1038/gt.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks W.J., Jr., Bartus R.T., Siffert J., Davis C.S., Lozano A., Boulis N., Vitek J., Stacy M., Turner D., Verhagen L. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 18.Grundmann O., Fillinger J.L., Victory K.R., Burd R., Limesand K.H. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer. 2010;10:417. doi: 10.1186/1471-2407-10-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teos L.Y., Zheng C.Y., Liu X., Swaim W.D., Goldsmith C.M., Cotrim A.P., Baum B.J., Ambudkar I.S. Adenovirus-mediated hAQP1 expression in irradiated mouse salivary glands causes recovery of saliva secretion by enhancing acinar cell volume decrease. Gene Ther. 2016;23:572–579. doi: 10.1038/gt.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lombaert I.M., Brunsting J.F., Wierenga P.K., Kampinga H.H., de Haan G., Coppes R.P. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells. 2008;26:2595–2601. doi: 10.1634/stemcells.2007-1034. [DOI] [PubMed] [Google Scholar]
- 21.Baum B.J., Wellner R.B., Zheng C. Gene transfer to salivary glands. Int. Rev. Cytol. 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- 22.Parsons S.M. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 2000;14:2423–2434. doi: 10.1096/fj.00-0203rev. [DOI] [PubMed] [Google Scholar]
- 23.Guidine P.A., Rezende G.H., Queiroz C.M., Mello L.E., Prado V.F., Prado M.A., Pereira G.S., Moraes M.F. Vesicular acetylcholine transporter knock-down mice are more susceptible to pilocarpine induced status epilepticus. Neurosci. Lett. 2008;436:201–204. doi: 10.1016/j.neulet.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Martin D., Abba M.C., Molinolo A.A., Vitale-Cross L., Wang Z., Zaida M., Delic N.C., Samuels Y., Lyons J.G., Gutkind J.S. The head and neck cancer cell oncogenome: a platform for the development of precision molecular therapies. Oncotarget. 2014;5:8906–8923. doi: 10.18632/oncotarget.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen S.B., Pedersen A.M., Vissink A., Andersen E., Brown C.G., Davies A.N., Dutilh J., Fulton J.S., Jankovic L., Lopes N.N., Salivary Gland Hypofunction/Xerostomia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support. Care Cancer. 2010;18:1039–1060. doi: 10.1007/s00520-010-0827-8. [DOI] [PubMed] [Google Scholar]
- 26.Takakura K., Takaki S., Takeda I., Hanaue N., Kizu Y., Tonogi M., Yamane G.Y. Effect of cevimeline on radiation-induced salivary gland dysfunction and AQP5 in submandibular gland in mice. Bull. Tokyo Dent. Coll. 2007;48:47–56. doi: 10.2209/tdcpublication.48.47. [DOI] [PubMed] [Google Scholar]
- 27.Johnson J.T., Ferretti G.A., Nethery W.J., Valdez I.H., Fox P.C., Ng D., Muscoplat C.C., Gallagher S.C. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N. Engl. J. Med. 1993;329:390–395. doi: 10.1056/NEJM199308053290603. [DOI] [PubMed] [Google Scholar]
- 28.Daniels T.E., Wu A.J. Xerostomia—clinical evaluation and treatment in general practice. J. Calif. Dent. Assoc. 2000;28:933–941. [PubMed] [Google Scholar]
- 29.Shiboski C.H., Hodgson T.A., Ship J.A., Schiødt M. Management of salivary hypofunction during and after radiotherapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007;103(Suppl) doi: 10.1016/j.tripleo.2006.11.013. S66.e1–19. [DOI] [PubMed] [Google Scholar]
- 30.Burlage F.R., Roesink J.M., Kampinga H.H., Coppes R.P., Terhaard C., Langendijk J.A., van Luijk P., Stokman M.A., Vissink A. Protection of salivary function by concomitant pilocarpine during radiotherapy: a double-blind, randomized, placebo-controlled study. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:14–22. doi: 10.1016/j.ijrobp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Coppes R.P., Roffel A.F., Zeilstra L.J., Vissink A., Konings A.W. Early radiation effects on muscarinic receptor-induced secretory responsiveness of the parotid gland in the freely moving rat. Radiat. Res. 2000;153:339–346. doi: 10.1667/0033-7587(2000)153[0339:ereomr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Coppes R.P., Zeilstra L.J., Kampinga H.H., Konings A.W. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br. J. Cancer. 2001;85:1055–1063. doi: 10.1054/bjoc.2001.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang B., Li Y.J., Zhao X.B., Zou Y., Yu Z.G., Zhao Y.M., Zhang F.Y. Mechanism of the protective effect of phenylephrine pretreatment against irradiation-induced damage in the submandibular gland. Exp. Ther. Med. 2013;5:875–879. doi: 10.3892/etm.2012.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delporte C., O’Connell B.C., He X., Lancaster H.E., O’Connell A.C., Agre P., Baum B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prado V.F., Martins-Silva C., de Castro B.M., Lima R.F., Barros D.M., Amaral E., Ramsey A.J., Sotnikova T.D., Ramirez M.R., Kim H.G. Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron. 2006;51:601–612. doi: 10.1016/j.neuron.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Soltoff S.P., Hedden L. Isoproterenol and cAMP block ERK phosphorylation and enhance [Ca2+]i increases and oxygen consumption by muscarinic receptor stimulation in rat parotid and submandibular acinar cells. J. Biol. Chem. 2010;285:13337–13348. doi: 10.1074/jbc.M110.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg Z., Myers C., Heim V.M., Lathrop C.A., Rebustini I.T., Stewart J.S., Larsen M., Hoffman M.P. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–1234. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- 38.Rebustini I.T., Myers C., Lassiter K.S., Surmak A., Szabova L., Holmbeck K., Pedchenko V., Hudson B.G., Hoffman M.P. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev. Cell. 2009;17:482–493. doi: 10.1016/j.devcel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adesanya M.R., Redman R.S., Baum B.J., O’Connell B.C. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum. Gene Ther. 1996;7:1085–1093. doi: 10.1089/hum.1996.7.9-1085. [DOI] [PubMed] [Google Scholar]
- 40.Muanza T.M., Cotrim A.P., McAuliffe M., Sowers A.L., Baum B.J., Cook J.A., Feldchtein F., Amazeen P., Coleman C.N., Mitchell J.B. Evaluation of radiation-induced oral mucositis by optical coherence tomography. Clin. Cancer Res. 2005;11:5121–5127. doi: 10.1158/1078-0432.CCR-05-0403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.