Abstract
Following spinal cord injury, severe deficits result from damages to ascending and descending tracts, such as the corticospinal tract (CST) which is highly relevant for the motor execution in humans. Unfortunately, no curative treatment is available and intensive efforts are deployed in animal models, such as the CST transection model, to identify interventions providing functional regeneration after spinal cord injury.
The CatWalk XT is a system for multi-parameter gait analysis of voluntary locomotion. In this study, the performance of the CatWalk XT for monitoring of functional deficits associated with dorsal CST lesion in rats was compared to skilled locomotion tests. Motor deficits associated with dorsal CST transection could be reliably monitored over seven weeks based on skilled locomotion testing, i.e. Horizontal Ladder Walk and Grid Walk. The collateral lesion to the overlaying gracile and cuneate funiculi occurring during dorsal CST transection resulted in slight hyposensitivity and proprioceptive deficit, which likely contributed to the lowered performance in skilled locomotion. In contrast, parameters of voluntary locomotion were not significantly affected by dorsal CST transection. Finally, an abnormal adduction reflex was detected immediately after lesion of the CST and could be conveniently used to confirm successful CST lesion in rats of experimental groups.
The functional relevance of the dorsal CST in locomotion of rats is not as prominent as compared to in humans and thus challenging the motor execution is mandatory to reliably investigate CST function. A detailed analysis of voluntary walking using the CatWalk XT is not adequate to detect deficits following dorsal CST lesion in rats.
Keyword: Neuroscience
1. Introduction
Spinal cord injury (SCI) has devastating consequences for affected individuals and families, and constitutes furthermore a socio-economic burden. More than half of all insults to the spinal cord affect the cervical level and lead to complete or incomplete tetraplegia, sensory disturbances and autonomic dysfunctions affecting ultimately almost every organ system [1, 2]. Incomplete tetraplegia is the most common clinical presentation among SCI patients [2]. Unfortunately, no effective curative treatment options for SCI are available and intensive efforts are being deployed in animal models, in particular in rats, to identify novel therapeutic approaches providing functional regeneration following traumatic SCI.
A central aspect of the pathophysiology of SCI is the disruption of descending and ascending spinal tracts, such as the corticospinal tract (CST), which is essential for fine and skilled motor control. Due to functional importance of the CST for motor control performance in humans, the development of regenerative strategies for this tract remains a research priority. Within the human spinal cord, the CST is predominantly located in the lateral white matter [3, 4]. However, in rodents, most of the fibers from the CST are located in the ventral part of the dorsal column, below the gracile and cuneate funiculi (Fig. 1A). This rodent-specific neuroanatomical tract distribution provides an easy access for the targeted disruption of approximately 90–95% of the descending corticospinal projections in the form of a bilateral dorsal funiculotomy [5, 6, 7].
Fig. 1.
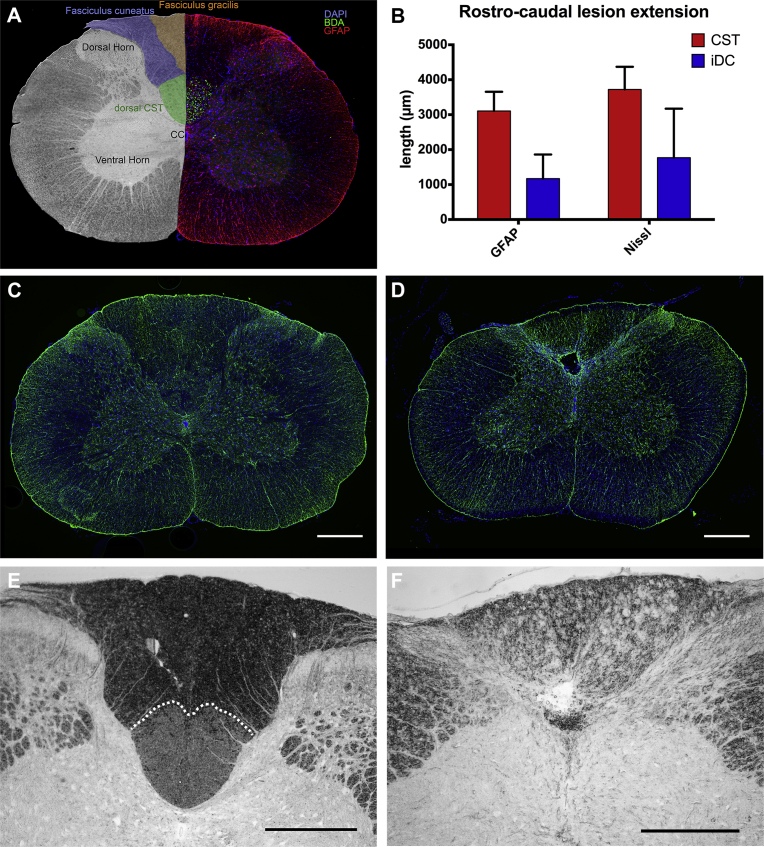
A) Coronal section of the spinal cord at the C4 level. Left, the position of the dorsal CST (green) as well as the cuneate (blue) and gracile fasciculi (orange). Right, immunodetection of anterogradely BDA-labeled fibers (green) revealed the position of the dorsal CST within the dorsal funiculus. Immunodetection of GFAP is shown in red, nuclei counterstaining in blue was performed using DAPI. B) Average rostro-caudal extent of the lesion detected in the spinal cord parenchyma based on Nissl staining and GFAP-immunodetection. Representative immunodetection of GFAP on coronal sections at C4 level of a rat from the (C) sham group or (D) dorsal CST lesion group slightly caudal of the lesion. Representative inverted dark-field micrographs revealing the dCST within the dorsal column of a sham (E, dotted line) and its disappearance following complete transection (F). Scale bars = 500 μm.
The transection model of the dorsal column in rodents offers the possibility to study the regeneration potential of the dorsal CST specifically without interfering with other descending spinal cord tracts. In addition, this model is not associated with massive secondary damages at the lesion site such as observed in most SCI models, e.g. contusion injury [8]. Although the pathophysiology of axonal injury and regeneration shares common aspects with the human situation, functional deficits resulting from the transection of the dorsal CST in rodents remain subtle. The fact that the dorsal CST projects mostly into the dorsal and medial laminae [5] and has only indirect contacts with the spinal motor neurons may explain the discrepancy between the extent of the CST lesion and the low magnitude of functional deficit. Moreover, locomotion in quadrupeds may have a lower requirement for a supraspinal control. Hence, only specific and sensitive tests addressing skilled motor control can detect the functional deficits resulting from dorsal CST lesion in rats. Importantly, the transection of the dorsal column not only lesions the CST, but also disrupts the overlaying sensory ascending tracts forming the gracile and cuneate funiculi, which are responsible for proprioception and the perception of fine pressure and vibration. Therefore, the net functional outcomes following a dorsal column transection in rodents will comprise a motor and a sensory component. The subtleness and the duality of the functional deficits make the study of dorsal CST functional regeneration and the interpretation of various outcome measures in the dorsal column transection models particularly challenging.
The selection of relevant functional readouts following dorsal CST transection in rodents continues to be a subject of discussion. Nevertheless, a robust test is mandatory for the assessment of deficits and functional recovery. Unfortunately, testing of motor function in rodents remains challenging, since motor control is mediated by an adaptive network of descending tracts with overlapping functions [9]. Moreover, functional regeneration addressed by various behavioral testing can be achieved through structural restoration, but also via compensation and/or changes in movement execution strategy. Considering the necessity of reliable pre-clinical validation for promising treatment options pointing at the horizon, we investigate the efficiency of the CatWalk XT, a gait analysis system for voluntary locomotion, and compared its performance to some locomotion readouts currently available for their capacity to evaluate motor deficits and eventually functional regeneration following dorsal CST lesion in adult rats.
2. Materials and methods
2.1. Animal subjects
This study was carried out on female Fisher-344 rats (bodyweight 169 ± 8 g; n = 20). All experiments were performed in accordance with the Directive 2010/63/EU of the European Parliament and of the Council and were approved by the local animal health commission. Rats were acquired from Charles River Laboratories (Sulzfeld, Germany) and were housed in groups of five in standard conditions with a 12-hours light-dark cycle. Food and water was provided ad libitum. Directly after surgery, rats were provided with higher caloric food (Ssniff R-Z autoclavable, Ssniff Spezialdiäten GmbH, Germany) to prevent weight loss and to promote recovery from surgery.
2.2. Lesion model and surgical procedures
Rats were randomly allocated in two groups: (i) a lesion group undergoing a bilateral transection of the dorsal column at the fourth cervical segment (n = 10) and (ii) a sham group, which was only laminectomized at cervical level 4 (n = 10).
For surgery, rats were deeply anaesthetized using an intramuscular injection of 46.5 mg/kg bodyweight (bw) ketamine (Narketan® 10%, Vétoquinol, Austria), 2.3 mg/kg bw xylazine (Rompun®, Bayer Austria GmbH, Austria) and 0.46 mg/kg bw acepromazine (Vanastress®, Vana GmbH, Austria). To prevent hypothermia rats were placed on a homeostatic heating pad with body temperature monitoring via rectal sensor. The dorsal spine of the rat was exposed and a laminectomy at cervical level 4 was performed to expose the spinal cord. Using a blunt tungsten wire-knife device (M122, David Kopf Instruments, USA) the dorsal column was precisely transected bilaterally (2.5 mm width, 1.1 mm depth) previously described (Fig. 1D) [6, 10, 11, 12]. To prevent infections after surgery, animals were treated with 10 mg/kg bw Enrofloxacin (Baytril®, 2.5% injection solution, Bayer Austria GmbH, Austria) daily for 5 consecutive days starting peri-operatively. Analgesia was provided by daily subcutaneous administration of 1.25 mg/kg bw Meloxicam (Metacam®, Boehringer Ingelheim Vetmedica GmbH, Germany) for 5 consecutive days after surgery. In the first two days after surgery, 0.02 mg/kg bw buprenorphine (Bupaq®, Richter Pharma AG, Austria) was injected subcutaneously (s.c) twice a day. Upon signs of dehydration, 1–2 mL of 0.9% NaCl solution was injected subcutaneously. In this SCI model, bladder function is sufficient and does not require manual voiding.
At the end of the follow-up period, the CST of 2 sham rats was anterogradely labeled by the injection of three-hundred nanoliters of a 10% solution of biotinylated-dextran amine (BDA; 10,000 MW; Molecular Probes) into 18 sites of the sensorimotor cortex per hemisphere as previously described [6]. The surgical procedures were performed under the same conditions as described above and the rats were perfused for histological analysis two weeks later, i.e. 9 weeks after laminectomy.
2.3. Motor and sensory tests
At least two weeks of training prior to the surgery were taken to familiarize the rats with the different tests employed. Additionally, the last training pre-surgery served as baseline performance of the rats.
2.3.1. Horizontal Ladder Walk and Grid Walk
The “Horizontal Ladder Walk” assesses skilled walking, limb placement and limb coordination. In this test, rats walk across a ladder with uniform rung pattern (1 m length; 3 mm rung diameter, 30 mm rung spacing) laid horizontally [13]. Similar to the Horizontal Ladder Walk, the “Grid Walk” (wire caliber 1 mm, mesh: 25 × 25 mm) was performed as described in [14, 15] and assesses forelimb placement.
For both tests, rats were trained for at least 2 weeks prior to injury to voluntarily cross the ladder/grid from a neutral cage to reach their home cage with littermates as positive reinforcement. For each rat, the 2 weeks training phase comprised 4 training sessions of 5 runs per session. Testing post-surgery was performed in the first week at least 24 hours after the last application of buprenorphine, and thereafter testing was carried out weekly. Total left and right forelimb correct steps and foot-faults were counted during the 5 runs of one session by one observer on each side of the apparatus. The percentage of correct steps was calculated for each rat and averages compared between groups.
2.3.2. CatWalk
Gait analyses of voluntary locomotion were performed using the CatWalk XT system (Noldus Information Technology, Netherlands). Every session consisted of six valid runs defined as a transit across the recording window with a variation of the walking speed of less than 60%. Sessions were performed two times per week prior to surgery and thereafter twice weekly for seven consecutive weeks. Each paw was individually documented and recorded parameters (see Tables 1, 2, 3, 4, 5, 6, and 7) were compared using a nonparametric test for longitudinal data optimized for small sample sizes in multifactorial experiments. For comparison of the three experimental groups, left and right feet as well as the two time points per week were pooled for analyses.
Table 1.
Analysis of gait parameters during voluntary locomotion after lesion (week 1).
| Parameter | adjusted p-value | 1 week |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sham |
CST |
iDC |
||||||||
| 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | ||
| Stand Front (s) | 0,88 | 0,22 | 0,25 | 0,27 | 0,22 | 0,24 | 0,26 | 0,20 | 0,21 | 0,25 |
| Stand Hind (s) | 0,66 | 0,23 | 0,26 | 0,29 | 0,21 | 0,23 | 0,25 | 0,22 | 0,23 | 0,24 |
| Stride length Front (cm) | 0,62 | 12,47 | 12,85 | 13,66 | 13,66 | 14,26 | 14,94 | 12,33 | 12,48 | 13,45 |
| Stride length Hind (cm) | 0,42 | 12,43 | 12,86 | 13,34 | 13,43 | 14,39 | 14,91 | 12,26 | 12,52 | 13,98 |
| Swing Front (s) | 0,77 | 0,12 | 0,14 | 0,15 | 0,13 | 0,14 | 0,14 | 0,13 | 0,14 | 0,15 |
| Swing Hind (s) | 0,91 | 0,13 | 0,13 | 0,16 | 0,13 | 0,14 | 0,14 | 0,13 | 0,13 | 0,15 |
| Swing speed Front (cm/s) | 0,66 | 88,01 | 97,03 | 104,20 | 113,00 | 113,60 | 124,20 | 90,79 | 97,43 | 114,40 |
| Swing speed Hind (cm/s) | 0,90 | 93,95 | 100,50 | 110,10 | 98,34 | 101,20 | 105,50 | 82,61 | 99,06 | 108,30 |
| Step cycle mean (s) | 0,90 | 0,36 | 0,38 | 0,44 | 0,34 | 0,37 | 0,40 | 0,33 | 0,34 | 0,40 |
| Print position left (cm) | 0,51 | 0,45 | 0,74 | 0,91 | −0,02 | 0,20 | 0,34 | −0,20 | 0,13 | 0,42 |
| Print position right (cm) | 0,88 | 0,16 | 0,49 | 0,87 | −0,63 | 0,28 | 0,51 | −0,27 | 0,23 | 0,64 |
| Base of support front (cm) | 0,91 | 1,49 | 1,74 | 1,86 | 1,68 | 1,81 | 2,02 | 1,52 | 1,65 | 1,99 |
| Base of support hind (cm) | 0,13 | 1,98 | 2,27 | 3,06 | 1,76 | 1,90 | 2,43 | 1,75 | 1,93 | 2,12 |
| Regularity index (%) | 0,90 | 97,92 | 99,07 | 100,00 | 98,33 | 98,33 | 99,54 | 99,07 | 99,07 | 99,07 |
Stand or Stance phase: duration of contact of a paw with the glass plate; Stride Length: distance between successive placements of the same paw; Swing: duration of no contact of a paw with the glass plate; Swing Speed: speed of the paw during Swing; Step Cycle: time between two consecutive initial contacts of the same paw; Print Positions: distance between the position of the hind paw and the position of the previously placed front paw on the same side of the body (ipsilateral) and in the same Step Cycle; Base of Support (BOS): average width between either the front paws or the hind paws; Regularity Index: number of normal step sequence patterns relative to the total number of paw placements. Two-factorial nonparametric analysis of the data did not reveal a significant difference in any of the parameters between sham, dCST and iDC group. Data are provided as median and interquartile range. Table 1 provides the adjusted p values for the 7-week period.
Table 2.
Analysis of gait parameters during voluntary locomotion after lesion (week 2).
| Parameter | 2 weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham |
CST |
iDC |
|||||||
| 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | |
| Stand Front (s) | 0,20 | 0,22 | 0,23 | 0,17 | 0,21 | 0,22 | 0,18 | 0,20 | 0,22 |
| Stand Hind (s) | 0,19 | 0,20 | 0,24 | 0,15 | 0,19 | 0,22 | 0,17 | 0,19 | 0,24 |
| Stride length Front (cm) | 12,75 | 13,58 | 13,78 | 13,05 | 14,17 | 14,63 | 12,84 | 14,34 | 15,15 |
| Stride length Hind (cm) | 12,55 | 13,62 | 13,85 | 13,28 | 14,39 | 14,92 | 13,18 | 14,58 | 15,34 |
| Swing Front (s) | 0,12 | 0,15 | 0,15 | 0,14 | 0,15 | 0,16 | 0,14 | 0,15 | 0,15 |
| Swing Hind (s) | 0,12 | 0,14 | 0,15 | 0,14 | 0,15 | 0,16 | 0,14 | 0,15 | 0,15 |
| Swing speed Front (cm/s) | 86,58 | 95,25 | 111,80 | 99,17 | 103,30 | 108,00 | 91,68 | 106,90 | 118,20 |
| Swing speed Hind (cm/s) | 89,51 | 97,02 | 113,10 | 87,41 | 93,01 | 96,36 | 90,78 | 102,90 | 107,40 |
| Step cycle mean (s) | 0,31 | 0,35 | 0,38 | 0,31 | 0,35 | 0,36 | 0,31 | 0,35 | 0,38 |
| Print position left (cm) | 0,10 | 0,47 | 0,86 | 0,11 | 0,30 | 0,53 | −0,73 | 0,14 | 0,77 |
| Print position right (cm) | −0,13 | 0,23 | 0,76 | −0,12 | 0,31 | 0,67 | −0,36 | 0,25 | 1,16 |
| Base of support front (cm) | 1,54 | 1,77 | 1,88 | 1,62 | 1,74 | 1,86 | 1,52 | 1,79 | 2,02 |
| Base of support hind (cm) | 1,70 | 2,10 | 2,49 | 1,53 | 1,94 | 2,34 | 1,70 | 1,88 | 1,98 |
| Regularity index (%) | 97,87 | 100,00 | 100,00 | 99,54 | 100,00 | 100,00 | 96,07 | 98,33 | 99,54 |
Stand or Stance phase: duration of contact of a paw with the glass plate; Stride Length: distance between successive placements of the same paw; Swing: duration of no contact of a paw with the glass plate; Swing Speed: speed of the paw during Swing; Step Cycle: time between two consecutive initial contacts of the same paw; Print Positions: distance between the position of the hind paw and the position of the previously placed front paw on the same side of the body (ipsilateral) and in the same Step Cycle; Base of Support (BOS): average width between either the front paws or the hind paws; Regularity Index: number of normal step sequence patterns relative to the total number of paw placements. Two-factorial nonparametric analysis of the data did not reveal a significant difference in any of the parameters between sham, dCST and iDC group. Data are provided as median and interquartile range. Table 1 provides the adjusted p values for the 7-week period.
Table 3.
Analysis of gait parameters during voluntary locomotion after lesion (week 3).
| Parameter | 3 weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham |
CST |
iDC |
|||||||
| 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | |
| Stand Front (s) | 0,20 | 0,22 | 0,27 | 0,18 | 0,21 | 0,23 | 0,18 | 0,22 | 0,25 |
| Stand Hind (s) | 0,20 | 0,21 | 0,28 | 0,16 | 0,19 | 0,22 | 0,16 | 0,19 | 0,26 |
| Stride length Front (cm) | 13,41 | 14,49 | 14,78 | 13,97 | 14,35 | 15,43 | 13,09 | 14,05 | 14,99 |
| Stride length Hind (cm) | 12,85 | 13,97 | 15,04 | 13,20 | 14,60 | 15,73 | 12,81 | 14,65 | 14,97 |
| Swing Front (s) | 0,13 | 0,15 | 0,15 | 0,15 | 0,15 | 0,16 | 0,13 | 0,15 | 0,16 |
| Swing Hind (s) | 0,13 | 0,14 | 0,15 | 0,14 | 0,16 | 0,16 | 0,14 | 0,15 | 0,17 |
| Swing speed Front (cm/s) | 87,55 | 103,50 | 118,00 | 97,18 | 114,20 | 115,80 | 83,42 | 104,10 | 123,10 |
| Swing speed Hind (cm/s) | 86,31 | 103,50 | 116,80 | 80,48 | 94,33 | 98,33 | 81,12 | 102,00 | 108,90 |
| Step cycle mean (s) | 0,34 | 0,38 | 0,43 | 0,32 | 0,35 | 0,38 | 0,30 | 0,36 | 0,42 |
| Print position left (cm) | −0,09 | 0,73 | 0,98 | 0,06 | 0,24 | 0,32 | −0,51 | 0,05 | 0,86 |
| Print position right (cm) | −0,32 | 0,45 | 0,95 | −0,59 | 0,00 | 0,40 | −0,34 | 0,15 | 1,25 |
| Base of support front (cm) | 1,58 | 1,81 | 1,90 | 1,71 | 1,86 | 1,87 | 1,48 | 1,65 | 1,82 |
| Base of support hind (cm) | 1,69 | 2,15 | 2,56 | 1,64 | 1,77 | 2,09 | 1,51 | 1,67 | 1,76 |
| Regularity index (%) | 97,31 | 100,00 | 100,00 | 98,70 | 99,07 | 100,00 | 98,24 | 98,33 | 100,00 |
Stand or Stance phase: duration of contact of a paw with the glass plate; Stride Length: distance between successive placements of the same paw; Swing: duration of no contact of a paw with the glass plate; Swing Speed: speed of the paw during Swing; Step Cycle: time between two consecutive initial contacts of the same paw; Print Positions: distance between the position of the hind paw and the position of the previously placed front paw on the same side of the body (ipsilateral) and in the same Step Cycle; Base of Support (BOS): average width between either the front paws or the hind paws; Regularity Index: number of normal step sequence patterns relative to the total number of paw placements. Two-factorial nonparametric analysis of the data did not reveal a significant difference in any of the parameters between sham, dCST and iDC group. Data are provided as median and interquartile range. Table 1 provides the adjusted p values for the 7-week period.
Table 4.
Analysis of gait parameters during voluntary locomotion after lesion (week 4).
| Parameter | 4 weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham |
CST |
iDC |
|||||||
| 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | |
| Stand Front (s) | 0,17 | 0,22 | 0,25 | 0,19 | 0,20 | 0,22 | 0,18 | 0,20 | 0,24 |
| Stand Hind (s) | 0,17 | 0,21 | 0,24 | 0,17 | 0,19 | 0,22 | 0,17 | 0,20 | 0,22 |
| Stride length Front (cm) | 14,39 | 14,65 | 15,12 | 14,51 | 14,90 | 15,20 | 14,15 | 14,83 | 15,41 |
| Stride length Hind (cm) | 13,89 | 14,50 | 15,25 | 14,19 | 14,97 | 15,17 | 14,22 | 14,89 | 15,33 |
| Swing Front (s) | 0,13 | 0,15 | 0,16 | 0,15 | 0,15 | 0,15 | 0,13 | 0,15 | 0,16 |
| Swing Hind (s) | 0,13 | 0,15 | 0,16 | 0,14 | 0,15 | 0,16 | 0,13 | 0,14 | 0,16 |
| Swing speed Front (cm/s) | 90,94 | 113,00 | 119,20 | 101,20 | 116,20 | 121,50 | 94,04 | 117,80 | 122,00 |
| Swing speed Hind (cm/s) | 87,86 | 102,90 | 115,20 | 89,73 | 93,00 | 103,90 | 87,33 | 103,10 | 112,90 |
| Step cycle mean (s) | 0,30 | 0,36 | 0,40 | 0,33 | 0,35 | 0,37 | 0,31 | 0,34 | 0,40 |
| Print position left (cm) | 0,31 | 0,70 | 0,97 | 0,18 | 0,49 | 0,53 | −0,39 | 0,31 | 0,86 |
| Print position right (cm) | −0,17 | 0,65 | 0,78 | 0,07 | 0,31 | 0,60 | −0,19 | 0,48 | 1,30 |
| Base of support front (cm) | 1,52 | 1,66 | 1,84 | 1,65 | 1,74 | 1,91 | 1,60 | 1,72 | 1,89 |
| Base of support hind (cm) | 1,86 | 2,18 | 2,47 | 1,69 | 1,87 | 2,38 | 1,53 | 1,69 | 1,96 |
| Regularity index (%) | 97,50 | 100,00 | 100,00 | 97,41 | 99,07 | 100,00 | 97,78 | 99,07 | 100,00 |
Stand or Stance phase: duration of contact of a paw with the glass plate; Stride Length: distance between successive placements of the same paw; Swing: duration of no contact of a paw with the glass plate; Swing Speed: speed of the paw during Swing; Step Cycle: time between two consecutive initial contacts of the same paw; Print Positions: distance between the position of the hind paw and the position of the previously placed front paw on the same side of the body (ipsilateral) and in the same Step Cycle; Base of Support (BOS): average width between either the front paws or the hind paws; Regularity Index: number of normal step sequence patterns relative to the total number of paw placements. Two-factorial nonparametric analysis of the data did not reveal a significant difference in any of the parameters between sham, dCST and iDC group. Data are provided as median and interquartile range. Table 1 provides the adjusted p values for the 7-week period.
Table 5.
Analysis of gait parameters during voluntary locomotion after lesion (week 5).
| Parameter | 5 weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham |
CST |
iDC |
|||||||
| 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | |
| Stand Front (s) | 0,18 | 0,20 | 0,26 | 0,18 | 0,22 | 0,23 | 0,18 | 0,21 | 0,24 |
| Stand Hind (s) | 0,17 | 0,18 | 0,24 | 0,15 | 0,18 | 0,22 | 0,16 | 0,18 | 0,22 |
| Stride length Front (cm) | 14,34 | 15,03 | 15,75 | 14,88 | 15,12 | 16,21 | 14,72 | 15,08 | 15,63 |
| Stride length Hind (cm) | 13,72 | 14,57 | 15,86 | 14,49 | 15,67 | 16,53 | 14,68 | 15,20 | 15,71 |
| Swing Front (s) | 0,12 | 0,15 | 0,15 | 0,14 | 0,15 | 0,16 | 0,14 | 0,15 | 0,17 |
| Swing Hind (s) | 0,13 | 0,15 | 0,16 | 0,14 | 0,15 | 0,17 | 0,14 | 0,15 | 0,17 |
| Swing speed Front (cm/s) | 94,84 | 105,00 | 122,30 | 96,74 | 120,80 | 128,90 | 97,68 | 111,40 | 121,60 |
| Swing speed Hind (cm/s) | 93,67 | 101,10 | 121,00 | 84,43 | 102,70 | 112,00 | 85,23 | 103,00 | 106,80 |
| Step cycle mean (s) | 0,30 | 0,35 | 0,41 | 0,31 | 0,36 | 0,37 | 0,31 | 0,35 | 0,40 |
| Print position left (cm) | 0,03 | 0,60 | 0,84 | −0,26 | 0,40 | 0,52 | −0,57 | 0,16 | 0,73 |
| Print position right (cm) | −0,06 | 0,33 | 0,97 | −0,31 | −0,02 | 0,38 | −0,21 | 0,10 | 0,93 |
| Base of support front (cm) | 1,45 | 1,75 | 1,96 | 1,49 | 1,70 | 1,97 | 1,52 | 1,53 | 1,74 |
| Base of support hind (cm) | 1,79 | 2,10 | 2,36 | 1,64 | 1,69 | 2,04 | 1,33 | 1,51 | 1,91 |
| Regularity index (%) | 100,00 | 100,00 | 100,00 | 98,70 | 100,00 | 100,00 | 98,70 | 99,07 | 100,00 |
Stand or Stance phase: duration of contact of a paw with the glass plate; Stride Length: distance between successive placements of the same paw; Swing: duration of no contact of a paw with the glass plate; Swing Speed: speed of the paw during Swing; Step Cycle: time between two consecutive initial contacts of the same paw; Print Positions: distance between the position of the hind paw and the position of the previously placed front paw on the same side of the body (ipsilateral) and in the same Step Cycle; Base of Support (BOS): average width between either the front paws or the hind paws; Regularity Index: number of normal step sequence patterns relative to the total number of paw placements. Two-factorial nonparametric analysis of the data did not reveal a significant difference in any of the parameters between sham, dCST and iDC group. Data are provided as median and interquartile range. Table 1 provides the adjusted p values for the 7-week period.
Table 6.
Analysis of gait parameters during voluntary locomotion after lesion (week 6).
| Parameter | 6 weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham |
CST |
iDC |
|||||||
| 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | |
| Stand Front (s) | 0,16 | 0,22 | 0,26 | 0,16 | 0,22 | 0,25 | 0,18 | 0,21 | 0,22 |
| Stand Hind (s) | 0,16 | 0,22 | 0,24 | 0,14 | 0,21 | 0,23 | 0,16 | 0,20 | 0,21 |
| Stride length Front (cm) | 14,17 | 15,79 | 16,13 | 14,56 | 15,29 | 15,54 | 15,00 | 15,42 | 16,28 |
| Stride length Hind (cm) | 13,56 | 15,32 | 16,09 | 14,61 | 15,50 | 15,99 | 14,68 | 15,27 | 17,06 |
| Swing Front (s) | 0,14 | 0,15 | 0,16 | 0,14 | 0,16 | 0,16 | 0,13 | 0,15 | 0,16 |
| Swing Hind (s) | 0,13 | 0,16 | 0,16 | 0,13 | 0,15 | 0,16 | 0,13 | 0,15 | 0,16 |
| Swing speed Front (cm/s) | 98,59 | 102,80 | 124,30 | 111,30 | 118,30 | 133,40 | 107,60 | 114,20 | 132,40 |
| Swing speed Hind (cm/s) | 89,14 | 95,86 | 113,80 | 91,79 | 95,94 | 113,40 | 93,02 | 97,45 | 126,90 |
| Step cycle mean (s) | 0,30 | 0,36 | 0,40 | 0,28 | 0,37 | 0,40 | 0,31 | 0,35 | 0,36 |
| Print position left (cm) | −0,19 | 0,38 | 0,96 | −0,04 | 0,38 | 0,73 | −0,31 | 0,49 | 0,72 |
| Print position right (cm) | −0,40 | 0,17 | 1,03 | 0,01 | 0,32 | 0,42 | −0,10 | 0,28 | 1,15 |
| Base of support front (cm) | 1,44 | 1,81 | 2,04 | 1,64 | 1,83 | 1,99 | 1,52 | 1,75 | 1,83 |
| Base of support hind (cm) | 1,94 | 2,03 | 2,49 | 1,64 | 1,93 | 2,21 | 1,52 | 1,64 | 1,98 |
| Regularity index (%) | 97,87 | 99,07 | 99,54 | 97,94 | 98,33 | 100,00 | 97,28 | 100,00 | 100,00 |
Stand or Stance phase: duration of contact of a paw with the glass plate; Stride Length: distance between successive placements of the same paw; Swing: duration of no contact of a paw with the glass plate; Swing Speed: speed of the paw during Swing; Step Cycle: time between two consecutive initial contacts of the same paw; Print Positions: distance between the position of the hind paw and the position of the previously placed front paw on the same side of the body (ipsilateral) and in the same Step Cycle; Base of Support (BOS): average width between either the front paws or the hind paws; Regularity Index: number of normal step sequence patterns relative to the total number of paw placements. Two-factorial nonparametric analysis of the data did not reveal a significant difference in any of the parameters between sham, dCST and iDC group. Data are provided as median and interquartile range. Table 1 provides the adjusted p values for the 7-week period.
Table 7.
Analysis of gait parameters during voluntary locomotion after lesion (week 7).
| Parameter | 7 weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham |
CST |
iDC |
|||||||
| 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | 25-percentile | Median | 75-percentile | |
| Stand Front (s) | 0,16 | 0,20 | 0,26 | 0,18 | 0,21 | 0,23 | 0,16 | 0,21 | 0,24 |
| Stand Hind (s) | 0,15 | 0,19 | 0,23 | 0,15 | 0,17 | 0,18 | 0,18 | 0,22 | 0,23 |
| Stride length Front (cm) | 13,64 | 15,12 | 16,23 | 15,53 | 16,52 | 16,71 | 15,04 | 15,29 | 15,74 |
| Stride length Hind (cm) | 13,59 | 15,15 | 15,92 | 15,74 | 15,95 | 16,64 | 14,07 | 14,53 | 16,55 |
| Swing Front (s) | 0,13 | 0,14 | 0,16 | 0,15 | 0,15 | 0,16 | 0,13 | 0,14 | 0,17 |
| Swing Hind (s) | 0,13 | 0,15 | 0,17 | 0,14 | 0,15 | 0,16 | 0,14 | 0,15 | 0,17 |
| Swing speed Front (cm/s) | 97,49 | 111,50 | 123,70 | 122,30 | 126,10 | 130,90 | 95,80 | 105,40 | 143,10 |
| Swing speed Hind (cm/s) | 81,40 | 106,00 | 120,30 | 95,50 | 99,20 | 103,40 | 80,43 | 97,97 | 118,90 |
| Step cycle mean (s) | 0,30 | 0,34 | 0,40 | 0,31 | 0,34 | 0,37 | 0,28 | 0,35 | 0,39 |
| Print position left (cm) | 0,20 | 0,71 | 0,86 | 0,27 | 0,40 | 0,58 | −0,34 | 0,06 | 0,72 |
| Print position right (cm) | −0,11 | 0,33 | 0,90 | 0,02 | 0,21 | 0,89 | −0,03 | 0,20 | 1,27 |
| Base of support front (cm) | 1,21 | 1,83 | 1,94 | 1,49 | 1,72 | 1,88 | 1,38 | 1,47 | 1,77 |
| Base of support hind (cm) | 1,63 | 1,94 | 2,17 | 1,37 | 1,58 | 1,69 | 1,32 | 1,60 | 1,68 |
| Regularity index (%) | 100,00 | 100,00 | 100,00 | 96,67 | 98,15 | 100,00 | 100,00 | 100,00 | 100,00 |
Stand or Stance phase: duration of contact of a paw with the glass plate; Stride Length: distance between successive placements of the same paw; Swing: duration of no contact of a paw with the glass plate; Swing Speed: speed of the paw during Swing; Step Cycle: time between two consecutive initial contacts of the same paw; Print Positions: distance between the position of the hind paw and the position of the previously placed front paw on the same side of the body (ipsilateral) and in the same Step Cycle; Base of Support (BOS): average width between either the front paws or the hind paws; Regularity Index: number of normal step sequence patterns relative to the total number of paw placements. Two-factorial nonparametric analysis of the data did not reveal a significant difference in any of the parameters between sham, dCST and iDC group. Data are provided as median and interquartile range. Table 1 provides the adjusted p values for the 7-week period.
2.3.3. Von Frey's hair test
An electronic version of von Frey hair test (Ugo Basile, Gemonio, Varese, Italy) was used to assess abnormal nociception, hypo- or hypersensitivity and allodynia. The rats were placed in a Plexiglas box with a grid floor. Using the thin filament of the probe, pressure was applied on the center of the plantar surface of each paw until the rats withdrew their paw. The maximal pressure applied until paw withdrawal was recorded. Each paw was tested five times during one session and the mean force measured before withdrawal of 5 measurements was used for statistical analysis. The electronic von Frey's hair test was performed in the fifth week after surgery.
2.3.4. Sticker test
The sticker test is used to test superficial and deep sensation in the dorsal part of the front paws. For the test, a sticker (Avery, 6 mm) was placed on one of the front paws (as previously described by [15, 16]. The latency between the application of the sticker and perception of the foreign object as revealed by the first attend to remove the sticker or by shaking of the paw was recorded. Each front paw was tested separately for three times with a delay of several minutes between each trial. For statistical analysis, the values of the shortest latency out of the three trials were used. This test was performed weekly.
2.4. Histology
For histological analysis, rats were transcardially perfused with 0.9% NaCl for 5 minutes followed by 0.1 M phosphate-buffered 4% paraformaldehyde (PFA) pH 7.4 for 10 min. Whole vertebral columns were dissected and post-fixed in 4% PFA solution overnight at 4 °C and stored in PBS with 0.05% sodium azide at 4 °C until further processing. Before cutting, spinal cords were dissected from the vertebral column and transferred at 4 °C in a 0.1 M phosphate-buffered solution with 15% sucrose and then with 30% sucrose for at least 24 hours each, and finally transferred to a 1:1 mix of 30% sucrose solution and TissueTek O.C.T Compound (Sakura Finetek USA, Inc.), overnight. Spinal cords were then embedded in TissueTek using Cryomolds (Sakura Finetek USA, Inc.) and subsequently frozen in 2-methylbutane over liquid nitrogen. Spinal cords were cut in 15 μm coronal sections using a Leica 1950 Cryostat and collected into 1/10 series for stereological analysis. Slices were immediately mounted on Superfrost Plus Slides (Thermo Scientific) and stored at −20 °C until further processing.
For immunohistological analysis sections were washed with PBS + 0.1% Tween-20 (Sigma-Aldrich), and blocking and antibody dilution were performed using a PBS solution containing 1% bovine serum albumin fraction (Sigma-Aldrich), 0.2% fish skin gelatin (Sigma-Aldrich) and 0.1% Tween-20 (Sigma-Aldrich). Then, a guinea-pig anti-GFAP antibody (Progen, 1:500) was applied overnight at 4 °C. A donkey anti-guinea-pig Alexa Fluor 647 (Dianova, 1:1000) was applied as secondary antibody for 4 h at room temperature. For BDA detection, Alexa Fluor 568 conjugated streptavidin (Invitrogen, 1:400) was applied simultaneously to the secondary antibody incubation. Nuclei were stained using 4′6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, 0.5 μg/mL). Finally, sections were mounted using Prolong Gold Antifade (Life Technologies).
Additionally, a Nissl staining was performed on the following section series as described in [6]. Differentiation was stopped with absolute ethanol and sections were then transferred to Neoclear (Merck) and mounted using Neomount (Merck).
The dorsal column and the border of the dorsal CST were visualized by dark field phase contrast on dark-field microscopy (Fig. 1E and F). The percentage of transected dorsal CST was determined based on the CST area remaining after surgery at the level of the lesion's epicenter and the average CST area detected at the same level in the sham group.
2.5. Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc.), Excel 2010 (Microsoft) and “R” (RCoreTeam (2017). R: A Language and Environment for Statistical Computing. R foundation for Statistical Computing. https://R-project.org/). Testing for normality of the data was performed using the Kolmogorov-Smirnov Test, a test optimized for small sample sizes. Parametric data were analyzed with one-way ANOVA with Tukey post-hoc tests and are provided as mean ± standard deviation. Nonparametric data were analyzed using two factorial analysis of the data with the R nparLD package, which is optimized for nonparametric analysis of longitudinal data in factorial experiments with ANOVA-like evaluation and Bonferroni post-hoc tests and are provided as median (interquartile range - IQR) [17]. A p-value ≤0.05 was considered significant. Graphical representation of the data is provided as box-whisker-plot. To control for false discovery rate in the non-parametric analyses, p-values were adjusted using the FDR-method in “R”.
3. Results
3.1. Histological evaluation of the spinal cord lesions
The rostro-caudal extent of lesions was determined by histological analyses (Fig. 1). One rat of the sham group (laminectomy only, n = 10) was excluded due to the presence of a superficial spinal cord lesion. A total of 10 rats underwent a transection of the dorsal column, of which 5 rats presented a completed lesion (dCST lesion). In this group, the lesion transected 99.2 ± 1.2% of the dCST and most of the ventral ascending sensory fibers (n = 5) (Fig. 1E, F). The remaining 5 rats had either incomplete dorsal CST transection or misplaced lesion and were designated as the incomplete dorsal column lesion group (iDC lesion). Of the latter, one rat had a misplaced lesion, 2 rats had a lesion restricted to the ascending sensory tracts, and 2 more rats had only 33.2% and 68.4% of the dorsal CST transected, respectively. The immunohistological detection of GFAP (Fig. 1C–D) revealed a rostro-caudal extent of astrogliosis of 3105.0 ± 550.1 μm (n = 5) in the dCST-group and 1170.0 ± 690.7 μm (n = 5) in the iDC-group seven weeks after transection, whereas the examination of Nissl staining revealed an average lesion spreading over 3720.0 ± 648.7 μm (n = 5) in the dCST-group and 1770.0 ± 1401 μm (n = 5) in the iDC–group (Fig. 1B).
3.2. Postural reflexes
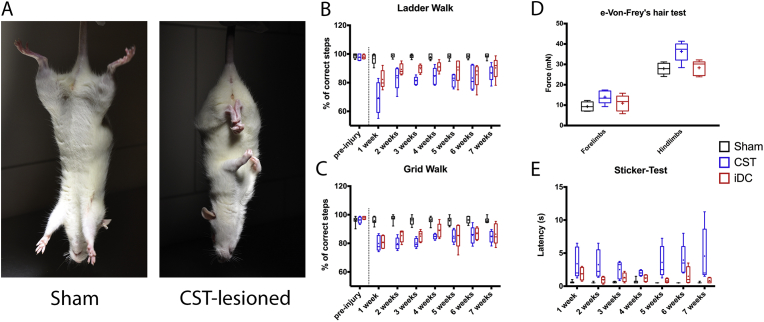
The so-called “scissoring” reflex results from an involuntary adduction of the limbs due to the denervation of the CST. Rats of the lesion group in which the CST was damaged showed overt adduction and “scissoring” of the limbs immediately after the surgery and this abnormal reflex remained for up to seven weeks (Fig. 2A). In contrast, scissoring was neither observed from rats of the sham group, nor from the three rats of the iDC lesion group which retained an intact CST.
Fig. 2.
A) Comparison of the postural reflexes observed in rats of the sham and lesion group upon suspension by the tail. The lesion of the dorsal CST was associated with abnormal adduction reflexes also referred to as the “scissoring” reflex. Skilled walking performance was assessed as the percentage of correct forepaw stepping during the (B) Horizontal Ladder Walk and (C) the Grid Walk. Transection of the dorsal CST was associated with a permanent motor deficit over seven weeks. D) Threshold of withdrawal upon point pressure on the plantar surface of the paws was measured with the electronic von Frey hair test. Five weeks after dCST lesion, a hyposensitivity in hind paws was detected and a slight, although not statistically significant, hyposensitivity in the fore paws as well. Rats with incomplete lesion showed neither hyper nor hyposensitivity. E) Sensory function of the forepaws was also evaluated based on the latency to detect a small sticker following application. Transection of the dorsal funiculus led to an increase of the latency of detection in both lesion groups.
3.3. Motor tests
3.3.1. Skilled walking
The CST is important for the fine motor control and has been assessed by two skilled walking tests, i.e. with the “Horizontal Ladder Walk” and with the “Grid Walk” (Fig. 2B–C). The baseline performance in correct steps was recorded during the last training pre-surgery. As expected, no significant differences were observed pre-surgery between the sham and lesion groups (99.4% (IQR: 97.3–100.0%) sham vs 97.7% (IQR: 96.3–100.0%) lesion and 96.5% (IQR: 95.5–97.5%) sham vs 97.3% (IQR: 96.1–98.8%) lesion for the Horizontal Ladder Walk and the Grid Walk respectively; sham n = 10, lesion n = 10). One week after surgery, the frequency of correct steps in both tests significantly dropped in CST-lesioned rats (68.9% (IQR: 59.2–79.9%) Horizontal Ladder Walk and 77.7% (IQR: 75.3–85.5%) Grid Walk, n = 5) as well as in rats with an incomplete lesion of the dorsal column (79.9% (IQR: 77.4–88.3%) Horizontal Ladder Walk and 80.5% (IQR: 76.5–85.4%) Gridwalk, n = 5) as compared to rats of the sham group (98.7% (IQR: 93.6–99.5%) Horizontal Ladder Walk, n = 9; p < 0.0001 with both lesioned groups and 95.4% (IQR: 94.7–98.4%) Grid Walk, n = 9; p < 0.0001 with both lesioned groups) (Fig. 2B–C). No difference between the lesion groups were observed in a pairwise comparison in both skilled tests. The performance of the lesioned rats plateaued from the second week post-contusion until the end of the experiment.
3.3.2. Voluntary walking (CatWalk)
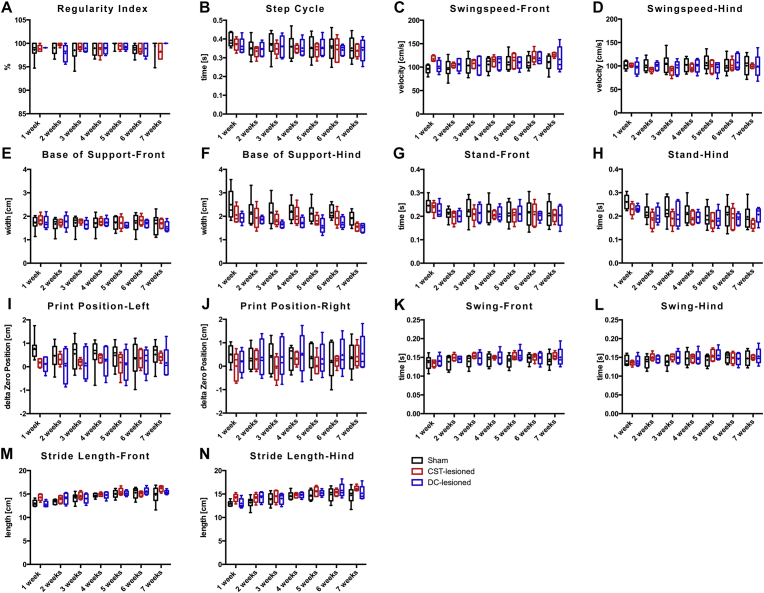
During voluntary walking analysis, rats freely transit across a glass plate without any constrains or challenges. Numerous gait parameters were recorded using a CatWalk XT apparatus (Noldus). Comparative analysis of 8 principal gait parameters (i.e. base of support, regularity index, step cycle, print position, stand, swing, swing speed, stride length; Tables 1, 2, 3, 4, 5, 6, and 7, Fig. 3) between rats of the sham and the lesion groups did not reveal differences reaching a statistical significance during voluntary and unchallenged walking.
Fig. 3.
Gait analysis during voluntary locomotion by using the Catwalk XT system: A-N) Box-Whisker-plot representations of the gait parameters over the follow up period. No statistical differences were detected between the sham and the lesion groups (dCST and iDC) over the 7-week follow-up period.
3.4. Sensory tests
3.4.1. Electronic von Frey's hair test
The von Frey's hair test was performed on each paw separately of rats from the sham and lesioned groups to detect potential changes in pressure perception, e.g. sensory deficit or a decrease of the nocifensive threshold due to the appearance of allodynia (Fig. 2D). No differences were detected between right and the left paws and therefore values were pooled together. For the hind paws, the pressure required before the rats perceived and withdrew their foot was significantly higher in the CST-lesioned group than for the sham group and rats with the smaller incomplete lesion of the dorsal column (27.9 ± 2.8 mN sham n = 5; 36.3 ± 4.9 mN CST-lesioned n = 5; 28.32 ± 3.6 mN iDC-Lesion n = 5; p = 0.0041 Sham:CST p < 0.01, Sham:DC = ns, CST:DC p < 0.05 (Tukey's post-hoc)). Similarly, a higher, but not statistically significant, threshold of plantar pressure was required for the forelimbs of the CST-lesioned group (9.3 ± 2.3 mN sham n = 5; 13.9 ± 3.2 mN CST-lesioned n = 5; 10.98 ± 3.9 mN iDC-lesion n = 5; p = 0.0700).
3.4.2. Sticker test
The sticker test assesses the fine sensory perception of rats. In this test, the latency for awareness and first removal attempt of a sticker placed on the dorsal side of a front paw have been recorded. One week following lesion of the dorsal CST, the latency in both lesion groups was significantly longer as compared to the sham group (Fig. 2E). However, no difference was observed between CST-lesion and iDC-lesion group. The longer latency in the lesioned groups remained significant over the 7 weeks post-surgery. In contrast, no significant differences in the sham group were detected pre and post-surgery. The median latency pre-surgery for all rats was 0.75 s (IQR: 0.5–1.0 s).
4. Discussion
Translation of therapeutic strategies from basic science to clinical practice is challenging in the SCI field [18]. Hence, appropriate behavioral tests to detect deficits and recovery are crucial in order to develop and conduct reliable preclinical trials. Clinical studies, which remain time consuming and expensive, profit from clear preclinical read-outs. This study revisited the functional impact of dorsal CST dysfunction in a rat model, since the CST tract is of paramount importance for the control of skilled movements in human.
Following dorsal CST transection, rats showed upon suspension by the tail a pronounced adduction and extension reflexes in the fore and hind limbs, the so-called “scissoring” reflex (Fig. 2A). This phenomenon has also been described in rats following ablation of their motor cortex or in decorticated rats [19]. Strikingly, this reflex was not observed in rats with lesions of the dorsal column leaving the dorsal CST intact and point towards a specificity of this reflex for the CST. Indeed, the two rats bearing an incomplete lesion of the dorsal CST also showed some degree of scissoring. In the clinical practice, this type of reflex can also be seen in patients with disturbed CST function, such as cerebral palsy. Our observation indicates that the “scissoring” reflex is a valuable noninvasive tool that can be used acutely to validate lesion of the dorsal CST in rats before the initiation of treatment paradigms.
The role of the CST input in voluntary locomotion is fundamentally different in rat as compared to human. Two anatomical differences are determinant in this respect. First, rats are quadrupeds and therefore have a different requirement of supraspinal control to maintain body stability during locomotion. Moreover, innervation of alpha motor neurons residing in the spinal ventral horn may involve a greater contribution of the rubrospinal and reticulospinal tracts inputs in the rats, as compared to humans [20, 21, 22]. Furthermore, the fraction of CST fibers having a monosynaptic connection to alpha motor neurons is significantly lower in rats than human, and these are strictly located in the lateral and ventral white matter tracts of the spinal cord in rodents [5, 23]. Together, these differences may allow for a greater compensation capacity of the motor control network following disruption of the dorsal CST in rats as compared to the human.
Disturbances in voluntary locomotion were scrutinized using a CatWalk XT system, which allows for an unbiased collection of multiple locomotion parameters simultaneously [24, 25]. The CatWalk has been previously shown to detect subtle, but significant changes, in the stride length of mice following unilateral pyramidotomy at the medulla level, although no overt deficit in over-ground locomotion were detected [26]. Similarly, following a dorsal hemi-transection in rats, a lesion affecting more spinal tracts than the selective dorsal CST transection performed in this study, a decrease in stride length and regularity index were also observed in the early phase after lesion [24]. In contrast, Hendricks and colleagues reported that following selective transection of the dorsal CST at the thoracic level 8, CatWalk analyses did not reveal any significant differences as compared to the sham group, whereas rats with lesions involving dorsal CST and the rubrospinal tract showed differences in the regularity index and the base of support [27]. Similarly, in the current study involving a transection of the dorsal CST at a higher level (i.e. cervical level 4), none of the locomotion parameters analyzed in the lesioned groups presented significant differences, as compared to the sham group, when considering the whole follow-up period of 7 weeks. These parameters included stride length, regularity index and base of support (the complete data set is represented in Tables 1, 2, 3, 4, 5, 6, and 7). Some parameters significantly changed over time: base of support (hindlimbs), stand (hindlimbs), stride length (fore and hindlimbs), swing (fore and hindlimbs) and swing speed (forelimbs). However, these changes observed over time took place regardless of group belonging, which suggests a training effect rather than a treatment effect. Taken together, these observations substantiate that the relevance of the dorsal CST during unchallenged locomotion in rats is minor and can be efficiently compensated by local spinal circuits and/or other descending tracts [28, 29].
“Horizontal Ladder Walk” and the “Grid Walk” test were used to assess skilled walking, which is assumed to rely more strongly on CST function. In agreement with previous studies [14, 15, 30], transection of the CST provoked functional deficits persisting over the entire follow-up period for both tests (Fig. 2). Deficits were also observed in rats bearing a lesion of the dorsal column with incomplete or absence of dorsal CST transection. While not statistically significant different from the dCST lesion group, the iDC group had a slightly milder impairment. Although our data underscore the pivotal role of the dorsal CST for the skilled locomotion, lesion restricted of the gracile and cuneate fasciculi have also been shown to cause sensorimotor deficits. Therefore, the contribution of damages to these ascending sensory pathways, which is inevitable following dorsal CST transection, should not be neglected nor underestimated [15, 31, 32].
In addition to classical locomotion-based functional analysis, the food pellet-reaching test has been established to assess CST functionality in rat SCI models. The food pellet-reaching test assesses skilled and goal-orientated behaviors that can be sub-divided in various components, up to eleven, and comprises: aim, pronation, grasp, supination [33, 34]. Cervical unilateral hemisection and bilateral dorsal column transection were reported to result in a significant and permanent decrease of pellet retrieval success [32, 33]. Transection of the dorsal CST also leads to decrease in pellet-reaching performance. However, the long term outcome appears to be dependent on the level of the lesion, as a C3 transection of the complete dorsal columns was reported to lead to a transient deficit, whereas transection at the cervical level 2 has been associated with only partial recovery [6, 32]. Assessing and comparing the performance for the pellet reaching test is moreover complicated by adaptive grasping strategies acquired by the lesioned rats [9, 33, 35] and the differences in reaching characteristics between different rat strains [36, 37]. Finally, the food pellet-reaching test has been shown to bear a strong rubrospinal tract component and was for all these reasons not included in this study [22, 35].
Hypersensitivity and allodynia can become severe debilitating issues after SCI and therefore sensory monitoring is an essential aspect to be considered throughout the development of new treatment strategies for SCI. Monitoring for neuropathic pain is crucial following SCI as this sensory deficit is observed in about 80% of patients following spinal cord injury, and in addition, regenerative therapies could either diminish or even exacerbate this symptom through sprouting and plasticity [38]. Our measurements using the von Frey hair test during the follow-up period substantiated that the near to complete dorsal column transection occurring during the dorsal CST transection causes hyposensitivity rather than a lower nocifensive threshold over time. However, the lesions leaving parts of or the complete dorsal CST intact were smaller in size and did neither cause significant hypo or hypersensitivity in the paws (Fig. 2D). A hyposensitivity following transection of the dorsal CST was also suggested by the longer latency in the “sticker test”, although the variability in this test appeared to be substantial (Fig. 2E).
In summary, pre-clinical research in the SCI field requires reliable tests to monitor the CST function due to its paramount importance in motor execution in human. This study underscores the importance of challenging motor functions to reveal the functional deficits resulting from dorsal CST transection in rats. Hence, even sophisticated analyses addressing voluntary walking, such as provided by the CatWalk, did not reveal motor deficit. In contrast, during the Horizontal Ladder Walk and Grid Walk tests, rats require continuous feedback of the visual and proprioceptive input in order to successfully execute stepping and therefore these two tests were sensitive to dorsal CST lesion. “Scissoring” reflex was found to be reliable to assess the lesion of the dorsal CST acutely after surgery and can allow for immediate validation of rats for the experimental groups. Finally, one should keep in mind that recovery following traumatic spinal cord injury will always involve functional restoration and functional compensation and adaptation. The extent of these processes are species specific and it is still largely unknown which animal readouts can predict functional outcome in humans at best.
Declarations
Author contribution statement
Lara Bieler, Lukas Grassner: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Pia Zaunmair, Christina Kreutzer, Lukas Lampe, Julia Marschallinger: Performed the experiments.
Eugen Trinka, Ludwig Aigner: Conceived and designed the experiments; Wrote the paper.
Sebastien Couillard-Despres: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the FWF Special Research Program (SFB) F44 (F4413-B23) “Cell Signaling in Chronic CNS Disorders”, by the FWF Hertha-Firnberg Postdoctoral programme n° T736-B24, n° HEALTH-F2-2011-279288 (IDEA), n° FP7-REGPOT-316120 (GlowBrain), and the research support fund of the Paracelsus Medical University PMU-FFF R-13/05/054-GRA; E-15/21/109-COU; E-15/22/113-COS.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to Georg Zimmermann (University Clinic of Neurology and Spinal Cord Injury and Tissue Regeneration Center Salzburg (SCI-TReCS), Paracelsus Medical University, Salzburg, Austria) for his support in statistical analysis.
References
- 1.Montoto-Marqués A., Ferreiro-Velasco M.E., Salvador-de la Barrera S., Balboa-Barreiro V., Rodriguez-Sotillo A., Meijide-Failde R. Epidemiology of traumatic spinal cord injury in Galicia, Spain: trends over a 20-year period. Spinal Cord. 2017;55:588–594. doi: 10.1038/sc.2017.13. [DOI] [PubMed] [Google Scholar]
- 2.Spinal cord injury (SCI) 2016 facts and figures at a glance. J. Spinal Cord Med. 2016;39:493–494. doi: 10.1080/10790268.2016.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charles W., Paxinos G., Kayalioglu G., editors. The Spinal Cord, First. Academic Press, Elsevier; Amsterdam: 2009. [Google Scholar]
- 4.Lemon R.N. Stroke recovery. Curr. Biol. 1993;3:463–465. doi: 10.1016/0960-9822(93)90358-u. [DOI] [PubMed] [Google Scholar]
- 5.Bareyre F.M., Kerschensteiner M., Misgeld T., Sanes J.R. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat. Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 6.Weidner N., Ner A., Salimi N., Tuszynski M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown L.T. Projections and termination of the corticospinal tract in rodents. Exp. Brain Res. 1971;13:432–450. doi: 10.1007/BF00234340. [DOI] [PubMed] [Google Scholar]
- 8.Nardone R., Florea C., Höller Y., Brigo F., Versace V., Lochner P. Rodent, Large Animal and Non-human Primate Models of Spinal Cord Injury. Zoology (Jena) 2017 doi: 10.1016/j.zool.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Fouad K., Hurd C., Magnuson D.S.K. Functional testing in animal models of spinal cord injury: not as straight forward as one would think. Front. Integr. Neurosci. 2013;7 doi: 10.3389/fnint.2013.00085. Art 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weidner N., Grill R.J., Tuszynski M.H. Elimination of basal lamina and the collagen “scar” after spinal cord injury fails to augment corticospinal tract regeneration. Exp. Neurol. 1999;160:40–50. doi: 10.1006/exnr.1999.7200. [DOI] [PubMed] [Google Scholar]
- 11.Vroemen M., Aigner L., Winkler J., Weidner N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur. J. Neurosci. 2003;18:743–751. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- 12.Sandner B., Rivera F.J., Caioni M., Nicholson L., Eckstein V., Bogdahn U. Bone morphogenetic proteins prevent bone marrow stromal cell-mediated oligodendroglial differentiation of transplanted adult neural progenitor cells in the injured spinal cord. Stem Cell Res. 2013;11:1–14. doi: 10.1016/j.scr.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Metz G.A., Whishaw I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Meth. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 14.Sharp K.G., Yee K.M., Stiles T.L., Aguilar R.M., Steward O. A re-assessment of the effects of treatment with a non-steroidal anti-inflammatory (ibuprofen) on promoting axon regeneration via RhoA inhibition after spinal cord injury. Exp. Neurol. 2013;248:321–337. doi: 10.1016/j.expneurol.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Onifer S.M., Zhang Y.P., Burke D.A., Brooks D.L., Decker J.A., McClure N.J. Adult rat forelimb dysfunction after dorsal cervical spinal cord injury. Exp. Neurol. 2005;192:25–38. doi: 10.1016/j.expneurol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Bradbury E.J., Moon L.D.F., Popat R.J., King V.R., Bennett G.S., Patel P.N. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi K., Gel Y.R., Brunner E., Konietschke F. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J. Stat. Software. 2012;50:1–23. [Google Scholar]
- 18.Kwon B.K., Hillyer J., Tetzlaff W. Translational research in spinal cord injury: a survey of opinion from the SCI community. J. Neurotrauma. 2010;27:21–33. doi: 10.1089/neu.2009.1048. [DOI] [PubMed] [Google Scholar]
- 19.Kolb B., Whishaw I.Q. Dissociation of the contributions of the prefrontal, motor, and parietal cortex to the control of movement in the rat: an experimental review. Can. J. Psychol. 1983;37:211–232. doi: 10.1037/h0080724. [DOI] [PubMed] [Google Scholar]
- 20.Massion J. Red nucleus: past and future. Behav. Brain Res. 1988;28:1–8. doi: 10.1016/0166-4328(88)90071-x. [DOI] [PubMed] [Google Scholar]
- 21.Küchler M., Fouad K., Weinmann O., Schwab M.E., Raineteau O. Red nucleus projections to distinct motor neuron pools in the rat spinal cord. J. Comp. Neurol. 2002;448:349–359. doi: 10.1002/cne.10259. [DOI] [PubMed] [Google Scholar]
- 22.Whishaw I.Q., Gorny B., Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav. Brain Res. 1998;93:167–183. doi: 10.1016/s0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 23.Kuypers H.G.J.M. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2011. Anatomy of the Descending Pathways. [Google Scholar]
- 24.Hamers F.P., Lankhorst A.J., van Laar T.J., Veldhuis W.B., Gispen W.H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma. 2001;18:187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- 25.Hamers F.P.T., Koopmans G.C., Joosten E.A.J. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- 26.Starkey M.L., Barritt A.W., Yip P.K., Davies M., Hamers F.P.T., McMahon S.B. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp. Neurol. 2005;195:524–539. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks W.T.J., Eggers R., Ruitenberg M.J., Blits B., Hamers F.P.T., Verhaagen J. Profound differences in spontaneous long-term functional recovery after defined spinal tract lesions in the rat. J. Neurotrauma. 2006;23:18–35. doi: 10.1089/neu.2006.23.18. [DOI] [PubMed] [Google Scholar]
- 28.Muir G.D., Whishaw I.Q. Complete locomotor recovery following corticospinal tract lesions: measurement of ground reaction forces during overground locomotion in rats. Behav. Brain Res. 1999;103:45–53. doi: 10.1016/s0166-4328(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 29.Webb A.A., Muir G.D. Unilateral dorsal column and rubrospinal tract injuries affect overground locomotion in the unrestrained rat. Eur. J. Neurosci. 2003;18:412–422. doi: 10.1046/j.1460-9568.2003.02768.x. [DOI] [PubMed] [Google Scholar]
- 30.Kanagal S.G., Muir G.D. Effects of combined dorsolateral and dorsal funicular lesions on sensorimotor behaviour in rats. Exp. Neurol. 2008;214:229–239. doi: 10.1016/j.expneurol.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Nathan P.W., Smith M.C., Cook A.W. Sensory effects in man of lesions of the posterior columns and of some other afferent pathways. Brain. 1986;109(Pt 5):1003–1041. doi: 10.1093/brain/109.5.1003. [DOI] [PubMed] [Google Scholar]
- 32.Kanagal S.G., Muir G.D. Bilateral dorsal funicular lesions alter sensorimotor behaviour in rats. Exp. Neurol. 2007;205:513–524. doi: 10.1016/j.expneurol.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Anderson K.D., Gunawan A., Steward O. Quantitative assessment of forelimb motor function after cervical spinal cord injury in rats: relationship to the corticospinal tract. Exp. Neurol. 2005;194:161–174. doi: 10.1016/j.expneurol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Metz G.A., Whishaw I.Q. Skilled reaching an action pattern: stability in rat (Rattus norvegicus) grasping movements as a function of changing food pellet size. Behav. Brain Res. 2000;116:111–122. doi: 10.1016/s0166-4328(00)00245-x. [DOI] [PubMed] [Google Scholar]
- 35.Hurd C., Weishaupt N., Fouad K. Anatomical correlates of recovery in single pellet reaching in spinal cord injured rats. Exp. Neurol. 2013;247:605–614. doi: 10.1016/j.expneurol.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Whishaw I.Q., Gorny B., Foroud A., Kleim J.A. Long-Evans and Sprague-Dawley rats have similar skilled reaching success and limb representations in motor cortex but different movements: some cautionary insights into the selection of rat strains for neurobiological motor research. Behav. Brain Res. 2003;145:221–232. doi: 10.1016/s0166-4328(03)00143-8. [DOI] [PubMed] [Google Scholar]
- 37.VandenBerg P.M., Hogg T.M., Kleim J.A., Whishaw I.Q. Long-Evans rats have a larger cortical topographic representation of movement than Fischer-344 rats: a microstimulation study of motor cortex in naïve and skilled reaching-trained rats. Brain Res. Bull. 2002;59:197–203. doi: 10.1016/s0361-9230(02)00865-1. [DOI] [PubMed] [Google Scholar]
- 38.Kramer J.L.K., Minhas N.K., Jutzeler C.R., Erskine E.L.K.S., Liu L.J.W., Ramer M.S. Neuropathic pain following traumatic spinal cord injury: models, measurement, and mechanisms. J. Neurosci. Res. 2017;95:1295–1306. doi: 10.1002/jnr.23881. [DOI] [PubMed] [Google Scholar]