Abstract
Background
Vaccine-derived polioviruses (VDPV) outbreaks typically occur in areas of low poliovirus immunity. Madagascar successfully eradicated wild poliovirus in 1997; however, multiple VDPV outbreaks have occurred since then, and numerous vaccination campaigns have been carried out to control the VDPV outbreaks. We conducted a survey of poliovirus neutralizing antibodies among Malagasy children to assess performance of vaccination campaigns and estimate the risk of future VDPV outbreaks.
Methods
This was a random community survey in children aged 6–11 months, 36–59 months and 5–14 years of age in high risk areas of Madagascar (Mahajanga, Toliara, Antsalova, and Midongy-atsimo); and in a reference area (Antananarivo). After obtaining informed consent, basic demographic and vaccination history, 2 mL of peripheral blood were collected. Neutralizing antibodies against all three poliovirus serotypes were detected by using a standard microneutralization assay.
Results
There were 1500 children enrolled and 1496 (>99%) provided sufficient quantity of blood for analysis. Seroprevalence for poliovirus type 1 (PV1) was >90% in all age groups and study areas. PV2 seroprevalence ranged between 75–100%; it was lowest in the youngest age group in Midongy and Toliara. PV3 seroprevalence ranged between 79–100%. Seroprevalence in the reference area was not significantly different from polio high risk sites.
Discussion
Madagascar achieved high population immunity. In order to preserve these gains, routine immunization needs to be strengthened. Currently, the risk of new VDPV emergences in Madagascar appears low.
Keywords: Immunology, Public health, Infectious disease, Vaccines
1. Background
Poliomyelitis cases caused by wild polioviruses have declined from hundreds of thousands in 1980s to 21 reported globally in 2017, all caused by wild poliovirus type 1 (WPV1); WPV2 has been certified as eradicated, and last WPV3 was detected in 2012 [1]. In Madagascar, the last poliomyelitis case caused by WPV was detected in 1997 [2].
In addition to wild polioviruses, the viruses emerging from the use of oral poliovirus vaccines (OPV), referred to as vaccine-derived polioviruses (VDPVs) may, in rare circumstances, lead to outbreaks of paralytic poliomyelitis [3, 4, 5]. Madagascar experienced an unusually high number of independent emergences of VDPVs of different serotypes in the last decade [6, 7, 8, 9]. The last such outbreak was reported in 2014–2015, involving 11 circulating VDPV (cVDPV) type 1 paralytic cases [7]. As a response to the VDPV outbreaks, the government of Madagascar together with partners of the Global Polio Eradication Initiative (GPEI) implemented a large number of supplementary vaccination campaigns with oral poliovirus vaccine (OPV) targeting children below 5 or 15 years of age. Nine campaigns were conducted in Madagascar to control the latest VDPV outbreak; some of the campaigns were nation-wide, while others focused only on high-risk areas. In addition to supplementary vaccination campaigns, routine immunization of young infants with poliovirus vaccines provides protection against circulation of wild and vaccine-derived polioviruses. The latest available estimates of routine immunization coverage (2016) with the third dose of OPV in Madagascar was 90%; it was 65% with the newly introduced inactivated poliovirus vaccine (IPV); however, large differences in routine immunization coverage within the country likely exist [10, 11]. The routine immunization schedule in Madagascar includes bivalent OPV vaccine administered at birth, 6, 10, and 14 weeks of age; and one does of IPV administered at 14 weeks of age. IPV was introduced into routine immunization schedule in Madagascar in 2015.
VDPV outbreaks usually occur in areas of low population immunity to polioviruses; in addition, there have been suggestions that the presence of other enteroviruses in the environment, particularly the high prevalence of species C was linked to emergence of VPDVs in Madagascar [6].
Seroprevalence surveys have been used as a tool to evaluate polio program performance and to identify population immunity gaps. They have been routinely carried out in Nigeria, India, Pakistan and other areas [12, 13, 14, 15, 16, 17, 18, 19] and led to changes in polio eradication strategies in these countries.
To better understand the underlying population immunity, and to assess risk of future outbreaks of VDPVs, we conducted a population-based seroprevalence survey of anti-polio antibodies in those areas of Madagascar that had experienced multiple VDPV outbreaks in the recent past and compared the results with the reference zone of the capital city, Antananarivo.
2. Methods
This was a community-based, cross-sectional survey conducted in five study sites of Madagascar: Mahajanga, Toliara, Antsalova, and Midongy-atsimo (all considered as high risk areas for VDPV outbreaks because of their history of past VDPV emergences, low performance of the routine vaccination program, or low performance of poliovirus surveillance), and Antananarivo (considered as a reference zone because of standard immunization program performance).
Children in each study site were randomly selected from three age groups: 6–11 months; 36–59 months; and 5–14 years. A sample size of 100 children in each age group and each study site was calculated to be sufficient to detect, at the 95% confidence level, a seroprevalence point estimate with a precision of approximately ±5% assuming ≥90% seroprevalence and the proportion of non-consenting parents ≤10%. The selection of children was based on existing heath center records; it was carried out using simple random sampling from lists of eligible children for each age group in each health center within the study sites.
A simple demographic questionnaire including poliovirus vaccination history was administered and children's height was recorded. Chronic malnutrition was defined as height for age z-score <−2 standard deviations from mean z-score. Trained phlebotomists drew 2 mL of peripheral blood. The blood specimens were allowed to clot, and sera were separated and transported to Pasteur Institute in Antananarivo where they were stored at −20 °C until shipment to the Centers for Disease Control and Prevention (CDC) in Atlanta, USA. The sera were tested for the presence of poliovirus neutralizing antibodies at CDC using standard neutralization assays [20]. Seropositivity was defined as reciprocal titers of poliovirus neutralizing antibodies ≥8. Highest reported antibody titer was 1:1448.
Analysis was carried out using EpiInfo 7 package. Proportions and 95% confidence intervals were calculated for seroprevalence data; and median titers and 95% intervals were calculated using the “boot-strap” method. Univariate analysis of risk factors was carried out using chi-square comparisons.
The study was carried out between May and September 2016; the protocol was reviewed and approved by the National Ethics Committee of the Ministry of Public Health in Madagascar (Agreement N° 21-MSANP/CE) as well as by the Ethics Review Committee of the World Health Organization, Geneva, Switzerland.
3. Results
In total, 1500 children were enrolled. There were no non-consenting parents. Sera were collected from all children; however, the quantity of sera in 4 of 1500 (<1%) children was insufficient for the performance of the neutralization assay. We present results obtained from 1496 children in whom the sera were analysed (Table 1).
Table 1.
Demographic indicators of the study population.
| Antananarivo |
Antsalova |
Mahajanga |
Midongy-atsimo |
Toliara |
TOTAL |
|
|---|---|---|---|---|---|---|
| n = 299 | n = 300 | n = 298 | n = 299 | n = 300 | N = 1496 | |
| Gender | ||||||
| Female | 49% | 58% | 53% | 54% | 55% | 54% |
| Age group distribution (n) | ||||||
| 6–11 mo | 99 | 100 | 100 | 99 | 101 | 499 |
| 36–59 mo | 99 | 100 | 98 | 100 | 94 | 491 |
| 5–14 yrs | 101 | 100 | 100 | 100 | 105 | 506 |
| Median age (in month) | ||||||
| 6–11 mo | 11 | 11 | 9 | 10 | 10 | 10 |
| 36–59 mo | 51 | 48 | 44 | 48 | 54 | 48 |
| 5–14 yrs | 124 | 108 | 130 | 120 | 127 | 121 |
| Vaccination history of OPV 3 (% documented by date in vaccination card) | 19% | 0% | 5% | 8% | 1% | 7% |
| Chronic malnutrition (%) | 34% | 12% | 15% | 39% | 31% | 26% |
OPV3- third dose of oral poliovirus vaccine.
In the 6–11 months age group, the majority of enrolled children were ≥10 months old (Table 1). Documented coverage with the third dose of OPV obtained through routine immunization varied significantly among study sites (p < 0.001); it was the highest in the reference area (Antananarivo – 19%) and the lowest in Antsalova (0%) where no children were able to produce a vaccination card. Chronic malnutrition varied significantly among study sites and ranged between 12–39% (p < 0.001, Table 1).
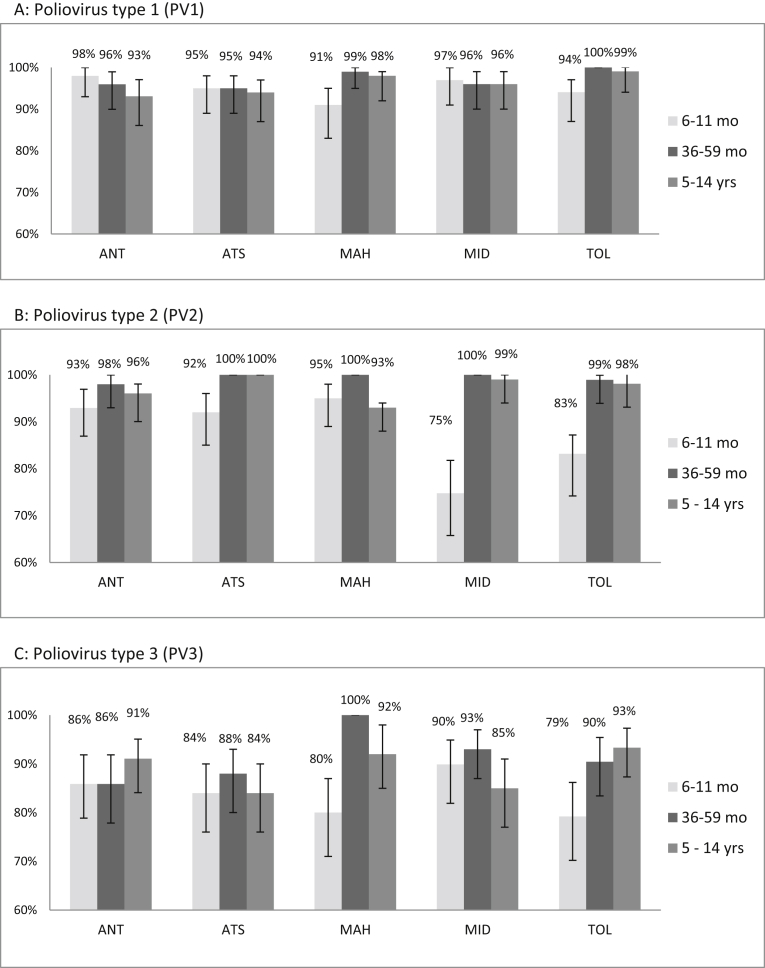
Seroprevalence for poliovirus type 1 (PV1) was >90% in all areas and age groups. In Mahajanga and Toliara, the PV1 seroprevalence was significantly lower in the youngest age group than in the older age groups (p < 0.05). There were no other significant differences in PV1 seroprevalence neither among study areas nor among age groups (Fig. 1A).
Fig. 1.
Prevalence of anti-polio antibodies in selected areas of Madagascar. ANT: Antananarivo, ATS: Antsalova, MAH: Mahajanga, MID: Midongy-atsimo, TOL: Toliara.
Seroprevalence for poliovirus type 2 (PV2) ranged between 75–100% (Fig. 1B). In the youngest age group, the PV2 seroprevalence was significantly lower in Midongy-atsimo (75%) and Toliara (83%) than in the other areas (p < 0.001). Overall, the 36–59 months age group had the highest PV2 seroprevalence when compared with the other age groups (99% vs 92%, p < 0.001).
PV3 seroprevalence ranged between 79–100% (Fig. 1C). In Mahajanga and Toliara, the youngest age group had lower PV3 seroprevalence than the other age groups (p < 0.05).
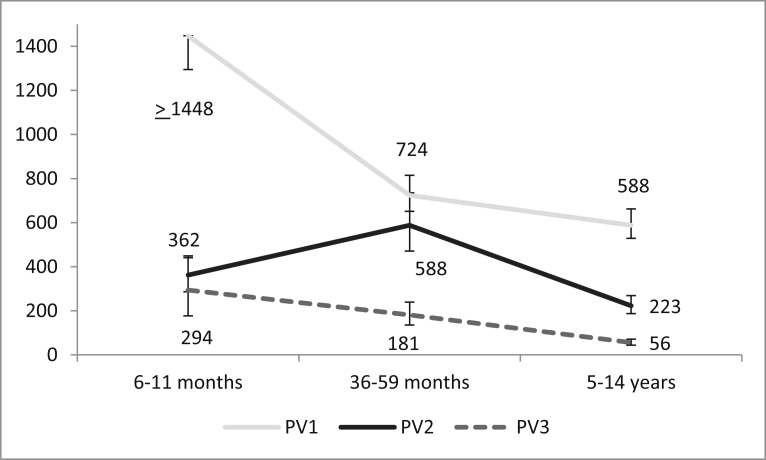
Median reciprocal titers were higher in the youngest age group than in the older age groups for serotypes 1 and 3 (p < 0.05); the titer for PV2 was the highest in the middle age group (588), followed by the youngest age group (362) and the oldest age group (223). Median titers were highest for PV1, followed by PV2 and PV3 (Fig. 2).
Fig. 2.
Median Reciprocal titers of anti-polio antibodies (PV1-3: poliovirus type 1–3) in selected areas of Madagascar. [95% confidence interval shown by error bars].
We analysed risk factors for seronegativity. There were 257/1496 (17%) children in our sample that did not have antibodies against at least one poliovirus serotype. Of the youngest age group, 128/499 (26%) children were seronegative for at least one serotype, of the middle age group it was 49/491 (10%) in the; and 80/506 (16%) in the oldest age group (p < 0.001). There was no statistical difference in the proportion of seronegative children among the study sites (p = 0.2). There was no statistical difference in documented history of OPV received as part of routine immunization program (p = 0.7 for comparison of children divided into groups based on number of documented OPV doses received). There were 182/1091 (17%) seronegatives among normally nourished children compared to 73/389 (19%) among chronically malnourished (p = 0.4).
4. Discussion
Madagascar was able to achieve uniformly high seroprevalence rates with the caveat of low PV2 rates in the youngest children in two study sites. The response with multiple vaccination campaigns using bivalent and trivalent OPV vaccines to the VDPV1 outbreak in 2014–16 resulted in high seroprevalence to PV1 in all areas and age groups, exceeding 90%. We do not assume that cVDPV1 outbreak itself contributed to any detectable increase in population immunity because its scope was limited. PV2 seroprevalence was overall high except in the youngest age groups in Midongy-atsimo and Toliara. In our study, the reference area of Antananarivo did not significantly differ from the identified polio high risk areas for PV1 or PV3; for PV2 it was significantly lower in Midongy-atsimo and Toliara in the youngest age group.
We observed declining antibody titers with age with the exception of PV2 which had the highest titer in the middle age group; probably a result of repeated vaccination campaigns targeting that age group. In our study, unlike in a previous study from Pakistan, we have not observed chronic malnutrition to be a clear risk factor for seronegativity [21]. The rates of chronic malnutrition in Madagascar are considered among the highest in the world (estimated at about 50% of under-five population); and our findings confirmed this trend [22].
Our study had some limitations. IPV history was not reported, and OPV history was unreliable due to absence of vaccination cards in the vast majority of children. In addition, we were unable to collect reliable data on campaign vaccination history. Further, the selection of children for the study was based on available health center registries. While considered exhaustive, this method may have introduced some selection bias as those not being included in the registries were likely under-vaccinated.
The risk of new VDPV emergence in Madagascar is currently low due to overall high population immunity with the exception of <85% serological protection against PV2 in in Midongy-atsimo and Toliara in the youngest age group. Rather than strong performance of the routine immunization program, it was likely the conduct of multiple vaccination campaigns that contributed to the high population immunity. The effect of these campaigns will be short-lived unless routine immunization is strengthened and continues to provide adequate poliovirus protection to the youngest cohorts of the Malagasy children. In addition, the predominant absence of vaccination cards needs to be addressed by the immunization program.
Declarations
Author contribution statement
Richter Razafindratsimandresy, Ondrej Mach, Jean-Michel Heraud, Barivola Bernardson, William Weldon, Steven Oberste, Roland Sutter: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the World Health Organization.
Competing interest statement
The authors declare no conflict of interest. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of CDC and other contributing agencies.
Additional information
No additional information is available for this paper.
Acknowledgements
We wish to acknowledge the Ministry of Health in Madagascar for their support, the staff from the national reference laboratory for poliomyelitis at the Institut Pasteur de Madagascar for their intense field work and blood collection, especially Fitahiana Rakotoarison, Seta Andriamamonjy and Jean-Pierre Ravalohery. We also thank the following CDC staff for performing the neutralization testing: Deborah Moore, Yiting Zhang, Sharla McDonald, William Hendley, Patricia Mitchell, and Mario Nicolas.
References
- 1.Cases of Wild Poliovirus by Country and Year. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpolioviruslist.aspx. (Accessed 12 February 2018).
- 2.UNICEF. UNICEF Corrects Report of Polio in Madagascar. Available at: https://www.unicef.org/media/media_60247.html. (Accessed 16 June 2017).
- 3.Burns C.C., Shaw J., Jorba J., Bukbuk D., Adu F., Gumede N. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J. Virol. 2013;87:4907–4922. doi: 10.1128/JVI.02954-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minor P. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine. 2009;27:2649–2652. doi: 10.1016/j.vaccine.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 5.Kew O.M., Sutter R.W., de Gourville E.M., Dowdle W.R., Pallansch M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 6.Rakoto-Andrianarivelo M., Guillot S., Iber J., Balanant J., Blondel B., Riquet F. Co-circulation and evolution of polioviruses and species C enteroviruses in a district of Madagascar. PLoS Pathog. 2007;3:e191. doi: 10.1371/journal.ppat.0030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales M., Nnadi C.D., Tangermann R.H., Wassilak S.G. Notes from the field: circulating vaccine-derived poliovirus outbreaks - five countries, 2014-2015. MMWR Morb. Mortal. Wkly. Rep. 2016;65:128–129. doi: 10.15585/mmwr.mm6505a5. [DOI] [PubMed] [Google Scholar]
- 8.Rakoto-Andrianarivelo M., Gumede N., Jegouic S., Balanant J., Andriamamonjy S.N., Rabemanantsoa S. Reemergence of recombinant vaccine-derived poliovirus outbreak in Madagascar. J. Infect. Dis. 2008;197:1427–1435. doi: 10.1086/587694. [DOI] [PubMed] [Google Scholar]
- 9.Diop O.M., Burns C.C., Sutter R.W., Wassilak S.G., Kew O.M. Update on vaccine-derived polioviruses - Worldwide, January 2014-March 2015. MMWR Morb. Mortal. Wkly. Rep. 2015;64:640–646. [PMC free article] [PubMed] [Google Scholar]
- 10.WHO and UNICEF Estimates of National Immunization Coverage. Available at: http://www.who.int/immunization/monitoring_surveillance/data/mdg.pdf. (Accessed 12 February 2017).
- 11.IVB/WHO. Data, Statistics and Graphics. Available at: http://www.who.int/immunization/monitoring_surveillance/data/en/. (Accessed 10 July 2017).
- 12.Bahl S., Estivariz C.F., Sutter R.W., Sarkar B.K., Verma H., Jain V. Cross-sectional serologic assessment of immunity to poliovirus infection in high-risk areas of northern India. J. Infect. Dis. 2014;210(Suppl. 1):S243–S251. doi: 10.1093/infdis/jit492. [DOI] [PubMed] [Google Scholar]
- 13.Bahl S., Gary H.E., Jr., Jafari H., Sarkar B.K., Pathyarch S.K., Sethi R. An acute flaccid paralysis surveillance-based serosurvey of poliovirus antibodies in Western Uttar Pradesh, India. J. Infect. Dis. 2014;210(Suppl. 1):S234–S242. doi: 10.1093/infdis/jiu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshpande J.M., Bahl S., Sarkar B.K., Estivariz C.F., Sharma S., Wolff C. Assessing population immunity in a persistently high-risk area for wild poliovirus transmission in India: a serological study in Moradabad, Western Uttar Pradesh. J. Infect. Dis. 2014;210(Suppl. 1):S225–S233. doi: 10.1093/infdis/jiu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamage D., Palihawadana P., Mach O., Weldon W.C., Oberste S.M., Sutter R.W. Achieving high seroprevalence against polioviruses in Sri Lanka-results from a serological survey, 2014. J. Epidemiol. Glob. Health. 2015;5:S67–S71. doi: 10.1016/j.jegh.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baba M.M., Haruna B.A., Ogunmola O., Ambe J.P., Shidali N.N., Oderinde B. A survey for neutralizing antibodies to the three types of poliovirus among children in Maiduguri, Nigeria. J. Med. Virol. 2012;84:691–696. doi: 10.1002/jmv.23228. [DOI] [PubMed] [Google Scholar]
- 17.Giwa F.J., Olayinka A.T., Ogunshola F.T. Seroprevalence of poliovirus antibodies amongst children in Zaria, Northern Nigeria. Vaccine. 2012;30:6759–6765. doi: 10.1016/j.vaccine.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Craig K.T., Verma H., Iliyasu Z., Mkanda P., Touray K., Johnson T. Role of serial polio seroprevalence studies in guiding implementation of the polio eradication initiative in Kano, Nigeria: 2011-2014. J. Infect. Dis. 2016;213(Suppl. 3):S124–S130. doi: 10.1093/infdis/jiv774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliyasu Z., Verma H., Craig K.T., Nwaze E., Ahmad-Shehu A., Jibir B.W. Poliovirus seroprevalence before and after interruption of poliovirus transmission in Kano State, Nigeria. Vaccine. 2016;34:5125–5131. doi: 10.1016/j.vaccine.2016.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weldon W.C., Oberste M.S., Pallansch M.A. Standardized methods for detection of poliovirus antibodies. Methods Mol. Biol. 2016;1387:145–176. doi: 10.1007/978-1-4939-3292-4_8. [DOI] [PubMed] [Google Scholar]
- 21.Saleem A.F., Mach O., Quadri F., Khan A., Bhatti Z., Rehman N.U. Immunogenicity of poliovirus vaccines in chronically malnourished infants: a randomized controlled trial in Pakistan. Vaccine. 2015;33:2757–2763. doi: 10.1016/j.vaccine.2015.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UNICEF. Health and Nutrition, Madagascar. Available at: https://www.unicef.org/madagascar/5557_6446.html. (Accessed 6 February 2018).