Abstract
To elucidate the nature of plant response to infection and transformation by Agrobacterium tumefaciens, we compared the cDNA-amplified fragment length polymorphism (AFLP) pattern of Agrobacterium- and mock-inoculated Ageratum conyzoides plant cell cultures. From 16,000 cDNA fragments analyzed, 251 (1.6%) were differentially regulated (0.5% down-regulated) 48 h after cocultivation with Agrobacterium. From 75 strongly regulated fragments, 56 were already regulated 24 h after cocultivation. Sequence similarities were obtained for 20 of these fragments, and reverse transcription–PCR analysis was carried out with seven to confirm their cDNA-AFLP differential pattern. Their sequence similarities suggest a role for these genes in signal perception, transduction, and plant defense. Reverse transcription–PCR analysis indicated that four genes involved in defense response are regulated in a similar manner by nonpathogenic bacteria, whereas one gene putatively involved in signal transduction appeared to respond more strongly to Agrobacterium. A nodulin-like gene was regulated only by Agrobacterium. These results demonstrate a rapid plant cell response to Agrobacterium infection, which overlaps a general response to bacteria but also has Agrobacterium-specific features.
Agrobacterium tumefaciens infects and transfers a piece of its tumor-inducing plasmid, the transferred DNA (T-DNA), to most dicotyledonous plants, thereby modifying their genome and inducing a hyperplastic response that results in a crown gall. This is the only verified example of natural interkingdom DNA transfer and, as a consequence, Agrobacterium is widely used to genetically engineer plants and to generate insertional disruptions in genes, facilitating functional genomics of plants (1). Its uses may broaden, as Agrobacterium is capable of transferring its T-DNA to fungi (2, 3) and even human cells (4). Despite these extensive applications and biological significance, very little is known about the events that take place in the host cell during genetic transformation by Agrobacterium.
In contrast to our lack of knowledge regarding the host partner, the molecular events that occur within the bacterial partner during the interaction have been intensively studied. The mechanism of T-DNA transfer is adapted from bacterial conjugation (5) and involves a number of virulence genes encoded mostly in the tumor-inducing plasmid but also in the bacterial chromosome (6). Their expression is induced by signal molecules secreted from wounded plants (7) and results in the formation and export of the T-DNA (8). What host components are involved in recognition, transfer, and integration of the T-DNA into the host genome remain largely unknown. Recently, a number of studies have begun to unravel some of the host factors that may play a role in these processes (9). A plant cyclophilin (10) and a plant karyopherin α protein (11) were identified by an interaction screen, and a plant histone H2A was identified by mutational analysis (12). Additionally, a plant DNA-ligase was reported to determine T-DNA integration efficiency by an in vitro assay (13).
So far, no attempt has been made to systematically explore the host gene expression response to Agrobacterium. In addition to identifying factors that might be relevant for transformation, a study of changes in gene expression should help elucidate the general response of the plant to Agrobacterium infection. This information could be compared with the responses of plants to other pathogens and symbionts. The various studies of interactions with pathogens (14–16) and symbionts (17–19), such as Rhizobium (20–22), have demonstrated that host defense responses are both induced and repressed, and host components are subverted for the benefit of the microbe.
The relationship of Agrobacterium to host plants is unique among plant pathogens. Agrobacterium does not induce the hypersensitive response (23), even though the bacterium introduces several proteins into the host cell. Agrobacterium is closely related to symbiotic bacteria from the genus Rhizobium (7). Interestingly, the peptide flagellin from Agrobacterium tumefaciens and from Rhizobium meliloti is unable to induce defense-related responses in plants, whereas the corresponding peptide from several other bacteria, including Pseudomonas species and Escherichia coli, acts as a potent elicitor (24). Taken together, these lines of evidence raise the question of whether Agrobacterium is capable of altering plant gene expression and, more specifically, whether it can alter the expression of plant defense-related genes. Here we report the use of a differential screen, the cDNA–amplified fragment length polymorphism (AFLP) (25), to examine the initial response of gene expression in plant cells exposed to Agrobacterium. We show that a number of plant transcripts have their expression altered at 24 and 48 h after interaction with Agrobacterium, and that the proteins encoded by these genes have a putative role in plant signal transduction and in defense response.
Materials and Methods
Plant and Cell Cultures Conditions and Treatments.
Ageratum conyzoides cell cultures (26) were maintained in 100 ml of an organic Murashige and Skoog (MS) minimal organics medium (GIBCO/BRL) containing 1 μM naphthaleneacetic acid. The cultures were shaken at 140 rpm at room temperature, and 10 ml were subcultured every 2 weeks. Tobacco BY-2 cell cultures (27) were maintained under the same conditions, except that 2,4-dichlorophenoxyacetic acid (0.25 μg/ml) was used as the growth regulator, and 5 ml of cells were subcultured every 2 weeks. The plant cells were inoculated with A. tumefaciens 2–3 days after subculture. An overnight culture of Agrobacterium was transferred to induction broth (28) containing 100 μM acetosyringone (AS), and the bacteria were induced for 16 h at 30°C. Just before inoculation, the cells were centrifuged and resuspended in MS medium to OD600 = 1.0. Each 100 ml of plant cells received AS (100 μM) and 1 ml of the bacterial suspension. The mock-inoculated controls received AS and 1 ml of MS medium. Arabidopsis thaliana (Columbia ecotype) leaf tissue was inoculated by vacuum infiltration, as described (29), and root tissue was cocultivated with Agrobacterium, as described (30). The A. tumefaciens strain EHA105 (pBISN1) used in this study is a nononcogenic hypervirulent strain (31), harboring a binary plasmid containing a β-glucuronidase (GUS)–intron construct that allows expression in plants but not in bacteria. The GUS gene is under the control of a “superpromoter” (32). Inoculation of Ageratum cells with E. coli DH5α was performed in the same manner as described for Agrobacterium, except that the E. coli cells were grown in LB medium at 37°C without AS.
GUS Colorimetric (Histochemical) and Fluorometric Analysis.
Plant cells or tissue were incubated at 37°C overnight in GUS-staining solution (0.5 mg/ml of bromo-chloro-indolyl glucuronic acid (X-Gluc/10 mM EDTA/0.5 mM ferricyanide/0.5 mM ferrocyanide, in 0.1 M phosphate buffer, pH 7.0). GUS activity was detected by a fluorogenic assay, as described (33).
RNA Extraction.
Total RNA was extracted from Ageratum and BY-2 cells and from Arabidopsis tissues at different times after inoculation with Agrobacterium by using the Trizol reagent (GIBCO/Life Technologies, Grand Island, NY). The procedure was done according to instructions from the manufacturer with the following modifications for the plant cell suspensions: the RNA was precipitated in 0.2 volume of 1 M acetic acid and 0.7 volume of 100% ethanol, at −20°C overnight. The pellet was washed twice in 3 M sodium acetate, pH 5.5, and once in 70% ethanol before being resuspended in diethyl pyrocarbonate-treated water.
cDNA-AFLP Procedure.
mRNA was isolated from Ageratum total RNA by using biotinylated oligo(dT) and streptavidin magnetic beads (PolyAtract IV, Promega). The sequence of adapters and primers, cDNA synthesis, template preparation, and analysis of the products by PAGE were done as described (25), except that the preamplification cycle consisted of 1 min at 94°C, 1 min at 56°C, and 2 min at 72°C, and the TaqI primer instead of the AseI primer was end-labeled with radioactivity. cDNA fragments were visualized by autoradiography after positionally marking gel and film.
Isolation and Sequencing of Fragments.
The film and gel were aligned, and the bands of interest were cut out from the gel with a razor blade. The gel slices were then hydrated in 100 μl of water and incubated at 95°C for 15 min. The eluted cDNA was amplified with the same primers and under the same conditions as for the cDNA-AFLP analysis, except that the PCR cycle consisted of 3 min at 96°C, 30 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min 30 sec at 72°C, and one final extension step of 10 min at 72°C. The fragments were sequenced by using Big Dye Terminator technology (Perkin–Elmer Applied Biosystems) and an automated sequencer and their homology determined by comparison with the database by using blast at National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/) and at The Arabidopsis Information Resource (TAIR) (http://www.arabidopsis.org/Blast/).
Reverse Transcription (RT)-PCR Analyses.
Equal amounts of RNA were treated with DNase, and cDNA was synthesized by using reverse transcriptase and random hexamers. The cDNA was amplified in a multiplex PCR reaction containing primers specific for the gene of interest and primers for two constitutive control genes, actin (left primer: CAGCAACTGGGATGATATGG; right primer: ATTTCGCTTTCAGCAGTGGT) and putative glycerol 3-phosphate dehydrogenase (left primer: TCACCCATATCAAGGCTCAG; right primer: GGGTACCTAATCGGGCAACT). For the GUS expression analysis, the control primers consisted of either cyclophilin (right primer: CACGACCTGCCCAAACAC; left primer: AAAACCCCTTCACTTCAA) or actin and the GUS primers consisted of the following: left primer: TATCAGCGCGAAGTCTTTATACC and right primer: CAGTTGCAACCACCTGTTGAT. The PCR cycle was described previously (except that for fragment number 325, the number of PCR cycles was 25 instead of 30), and the products were analyzed by agarose gel electrophoresis.
Results
A Plant Cell Culture Highly Competent for Agrobacterium Transformation.
To facilitate the identification of genes differentially expressed in response to Agrobacterium transformation, a system where a high percentage of host cells are competent for transformation is essential to prevent signal dilution. To identify changes in gene expression during the early events of transformation, possibly before or during the first integration events, selection for transformed cells was not applied in this study. The model plant A. thaliana would be a natural choice for the present work but, without selection, the efficiency of T-DNA transfer and expression in Arabidopsis root cells is less than 5%, whereas stable transformation is less than 0.5% (34). This response is likely to be not unique to Arabidopsis plants and represents a major limitation to our study. In addition, it is critical that the system be highly reproducible, which argues against using intact plants, where the location of transiently transformed cells is not predictable (34). Therefore, we explored the use of plant cell suspension cultures, which should overcome these limitations.
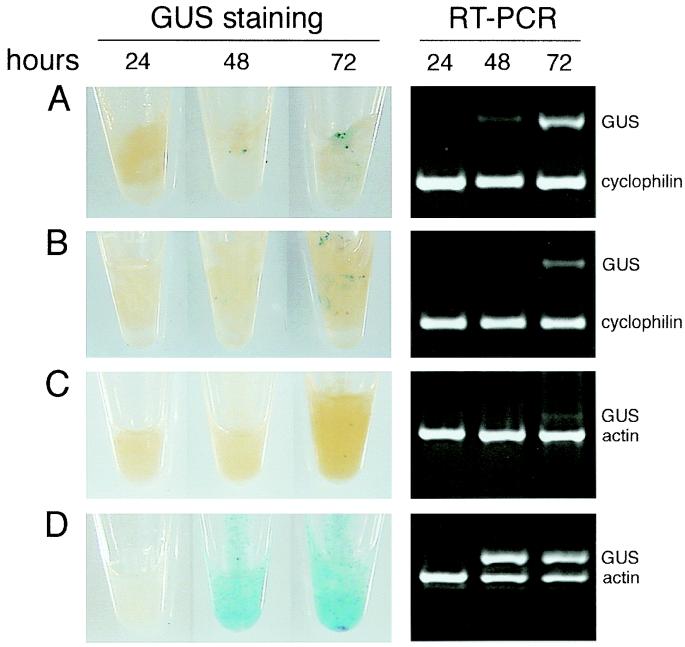
We compared two available suspension cultures that are rapidly growing and consist of very small clumps of cells: BY-2, from Nicotiana tabacum (27), and a suspension culture from A. conyzoides (26). Kanzaki et al. reported that transient transformation and expression of the GUS reporter gene in Ageratum cells was nearly 100 times higher than in BY-2 cells (26). We confirmed these results. We transformed Ageratum and tobacco BY-2 cell suspension cultures and Arabidopsis plants with A. tumefaciens strain EHA105 (pBISN1) containing a GUS–intron construct that allows expression in plants but not in the bacteria (32). The data in Fig. 1 confirm the high transformation competence of these cells compared with BY-2 cells and with Arabidopsis root and leaf tissue. GUS expression was analyzed by a colorimetric assay (staining) and by RT-PCR detection of the GUS transcript at 24, 48, and 72 h after transformation. GUS enzymatic activity measured by a fluorometric assay (data not shown) also agrees with these data and with data obtained by Kanzaki et al (26). Therefore, the plant cell culture from Ageratum represents the best system for the present study and any other type of study where reproducible high levels of transformation are required.
Figure 1.
GUS expression analysis. A. thaliana root (A) and leaf tissue (B), tobacco BY-2 cells (C), and A. conyzoides cells (D) at 24, 48, and 72 h after inoculation with Agrobacterium containing a GUS–intron construct. Primers for constitutive control genes were used in the RT-PCR analysis: cyclophilin primers for Arabidopsis and actin primers for BY-2 and Ageratum.
Analysis of Alterations in Ageratum Plant Cell Gene Expression 48 h After Agrobacterium Inoculation.
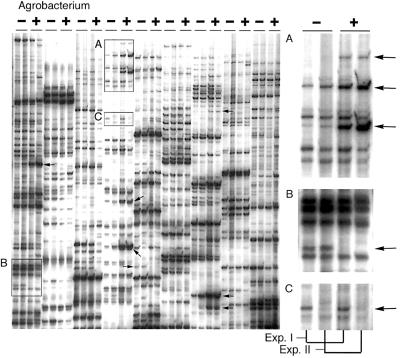
The data in Fig. 1 illustrate that high levels of GUS expression in Ageratum cells are observed at 48 h after inoculation. Therefore, we chose this time point to first analyze changes in gene expression by AFLP. Ageratum cells were cocultivated with Agrobacterium strain EHA105 (pBISN1) and, after 48 h, these cells as well as cells from mock-inoculated controls were harvested for RNA extraction and compared by cDNA-AFLP analysis. cDNAs from control and treated samples of two independent assays were amplified by using all possible 256 primer combinations. A section of a typical AFLP gel obtained is shown in Fig. 2. A total of 16,000 cDNA fragments were displayed, and 251 were differentially regulated by the treatment (Table 1). Even though most of the bands displayed were reproducible between the two independent experiments, ≈1% of all bands were specific to either experiment (Fig. 2C). We believe that these signals represent intrinsically variable genes, which can be identified by experimental replication. Only bands reproducibly altered by the treatment in two experiments were studied further.
Figure 2.
A. conyzoides cDNA-AFLP display after inoculation with Agrobacterium for 48 h. A. conyzoides cDNAs from mock-inoculated (−) and Agrobacterium-inoculated (+) cells from two independent experiments (Experiments I and II) were amplified with different primer combinations. Each set of four lanes represents amplification by one particular primer combination. Arrows indicate differential bands. (A) Enlarged view of A with arrows indicating bands induced by the treatment. (B) Enlarged view of B with arrow indicating a band repressed by the treatment. (C) Enlarged view of C with arrow indicating a nonreproducible (experiment-specific) band.
Table 1.
Overall results of cDNA-AFLP analysis
| Expression profile at 48 hours | Number | % |
| Bands or cDNA fragments displayed | 16,000 | 100 |
| Differential fragments | 251 | 1.6 |
| Up-regulated | 179 | 1.1 |
| Down-regulated | 72 | 0.5 |
| Nonreproducible (“noisy”) fragments | 165 | 1 |
| Expression profile at 24 hours (75 selected fragments differentially regulated at 48 hours) | ||
| Fragments tested | 75 | |
| Fragments regulated at 24 hours | 56 | |
| Up-regulated | 39 | |
| Down-regulated | 17 | |
| Sequence analysis of 56 fragments regulated at 24 and 48 hours | ||
| DNA sequence data obtained | 50 | |
| Similarity with the database (Table 2) | 20 |
Analysis of Fragments Altered at Both 24 and 48 h After Agrobacterium Inoculation.
To identify factors regulated earlier than 48 h after contact with Agrobacterium, the expression of 75 selected differential fragments was analyzed by AFLP 24 h after inoculation. GUS expression was not evident at this time (Fig. 1). These 75 fragments all showed a strong differential expression pattern at 48 h. Among them, 56 showed the same differential expression pattern at 24 h after inoculation (Table 1). Sections from AFLP gels resulting from this analysis are shown in Fig. 3. Bands were observed that were altered at 24 but not at 48 h. These fragments would potentially represent interesting candidates to be analyzed as well. However, this class of fragments was not followed further in this study. All 56 fragments altered at both 24 and 48 h after inoculation were isolated from the AFLP gels, reamplified by PCR, and sequenced.
Figure 3.
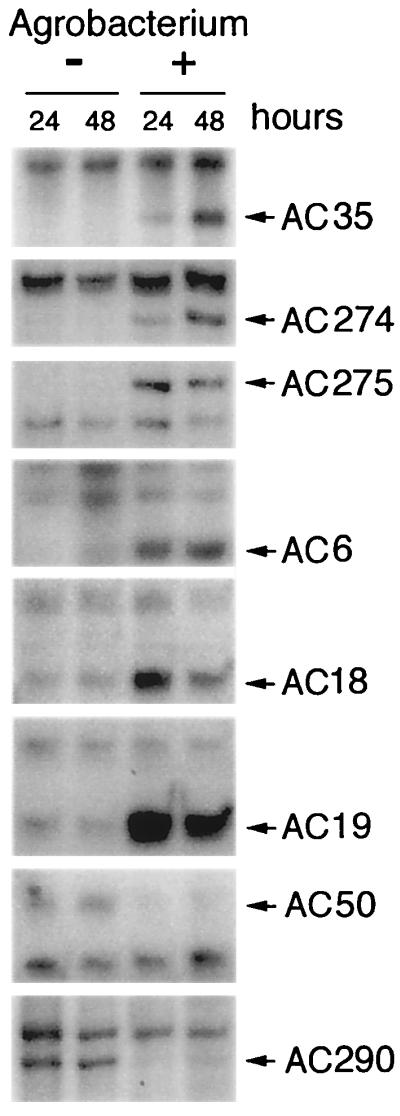
cDNA-AFLP fragments regulated at 24 and 48 h after inoculation with Agrobacterium. A. conyzoides cDNAs from mock- (−) and Agrobacterium-inoculated (+) cells were amplified with primer combinations known to result in differential fragments at the 48-h time point. Differential fragments are indicated by their numbers, as listed in Table 2. Fragments that are induced (AC6, 18, 19, 35, 274, and 275) and repressed (AC50 and 290) at both time points are shown.
Compilation of Sequences from Differentially Expressed cDNA Fragments.
DNA sequence data were obtained for 50 of the 56 fragments isolated. Sequence similarity to known genes was found for 20 fragments by blast searches (E-value cutoff = 5e−4). Their length, pattern of expression, and homology to known proteins are shown in Table 2. Four fragments are likely involved in signal perception/transduction, including homologues to a zinc-finger protein (AC18), a putative nodulin (AC168), a putative receptor kinase (AC181), and a lectin-like protein kinase (AC321). These four fragments were up-regulated, except AC181, which was down-regulated. Eight fragments are likely involved in disease and stress response, including a homologue to a pathogenesis-related (PR) protein (AC365), factors involved in biosynthesis of phenylpropanoids (AC121 and AC290), a peroxidase precursor (AC275), and factors involved in response to wounding (AC174 and AC315), starvation (AC178), and drought (AC274). These eight fragments were up-regulated, except AC290, which was down-regulated.
Table 2.
Homologies of AFLP fragments to sequences in the databases
| AFLP fragment | GeneBank accession no. | Length, bp | Change | Homology* | blastx score |
|---|---|---|---|---|---|
| AC18 | BI397491 | 210 | Up | ZPT2-14 (zinc-finger protein) from Petunia × hybrida (AB006601) | 2e-11 |
| AC19 | BI397492 | 180 | Up | Putative anion exchange protein from Arabidopsis (AC007236) | 1e-19 |
| AC35 | BI397493 | 230 | Up | Hypothetical protein; ferric reductase-like transmembrane component (At1g01590) | 4e-9 |
| AC50 | BI397494 | 210 | Down | Lysophospholipase isolog from Arabidopsis (U95973) | 8e-12 |
| AC79 | BI397495 | 260 | Down | Putative protein, similar to F-box protein Fb12; Homo sapiens (AT5g01720). F-box proteins are involved in protein degradation (ubiquitination). | 2e-17 |
| AC95 | BI397496 | 420 | Up | Member of the PF|0093 glyoxalase family from Arabidopsis (AC007591) | 2e-15 |
| AC121 | BI397497 | 500 | Up | Putative glucosyltransferase from Arabidopsis (AC006248); involved in phenylpropanoid metabolism; salicylic acid induced. | 8e-34 |
| AC167 | BI397498 | 420 | Up | Unknown protein similar to glucosaminyl (N-acetyl) transferase; H. sapiens (At1g71070) | 2e-12 |
| AC168 | BI397499 | 180 | Up | Putative nodulin from Oryza sativa (AP002747) | 1e-5 |
| AC174 | BI397500 | 230 | Up | Strong similarity to extracellular dermal glycoprotein precursor from Daucus carota (AC005278). Involved in response to wounding. | 5e-9 |
| AC178 | BI397501 | 400 | Up | Intracellular ribonuclease LX precursor; RNAseLX (P80196); starvation induced. | 9e-37 |
| AC181 | BI397502 | 300 | Down | Hypothetical protein F23E13.70; putative receptor protein kinase from Arabidopsis (AL022141). Similar 4e−43 to Xa21, a receptor kinase disease resistance protein from rice. | 2e-13 |
| AC274 | BI397503 | 300 | Up | Probable short-chain alcohol dehydrogenase CPRD12, drought-inducible, from cowpea (D88121). S-locus specific stigma protein from sporophytic self-incomp. Ipomoea trifida | 2e-9 |
| AC275 | BI397504 | 270 | Up | Bacterial-induced peroxidase precursor from G. hirsutum (AF155124). | 2e-18 |
| AC282 | BI397505 | 300 | Up | Putative hydroxymethylglutaryl-CoA lyase from Arabidopsis (AC005168). | 2e-21 |
| AC290 | BI397506 | 340 | Down | Cytochrome P450 monooxygenase, putative similar to cytochrome P450 from P. sativum (AT3g25180). Methyl jasmonate-inducible; involved in biosynthesis of phenylpropanoids. | 5e-4 |
| AC315 | BI397507 | 650 | Up | Elicitor inducible protein from N. tabacum (AB040410). Similar 4e−43 to potato wound-induced protein. | 8e-44 |
| AC321 | BI397508 | 260 | Up | Lectin-like protein kinase from P. nigra (AB030083). | 2e-33 |
| AC325 | BI397509 | 290 | Up | Phosphate induced-1-like protein from Arabidopsis (AB008268). | 7e-26 |
| AC365 | BI397510 | 200 | Up | NtPRp27 from N. tabacum (AB024600); induced by virus, mechanical wounding, and drought treatment. | 2e-24 |
Database is nonredudant (all organisms), except fragments 35, 79, 167, and 290, for which the database is Arabidopsis.
The role for the remaining eight fragments in Agrobacterium plant interaction is unclear; their sequence homology suggests that they are related to metabolism [glyoxalase (AC95), glucosaminyl N-acetyl transferase (AC167), hydroxymethyl glutaryl-CoA lyase (AC282)], membrane transport/synthesis/degradation (anion exchange protein (AC19), ferric reductase-like transmembrane component (AC35), and lysophospholipase (AC50)], degradation of proteins through the ubiquitination pathway [F-box protein (AC79)] and possibly regulation of the cell cycle [phosphate-induced protein (AC325)] (35). These eight fragments were up-regulated, except AC50 and AC79, which were down-regulated.
RT-PCR Confirms the Differential Expression Pattern for Seven Selected Fragments.
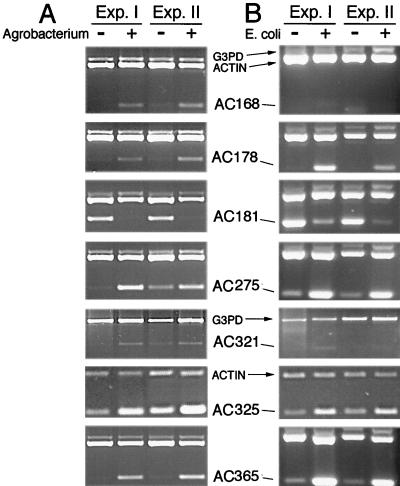
To verify the expression pattern, primers specific for seven of the sequenced fragments were designed and used for RT-PCR analysis. The proteins encoded by these fragments include the homologues to a nodulin-like protein (AC168), a starvation-induced ribonuclease (AC178), a putative receptor kinase (AC181), a bacterial-induced peroxidase precursor (AC275), a lectin-like protein kinase (AC321), a phosphate-induced protein (AC325), and a PR protein NtPRp27 (AC365). These fragments were up-regulated in the AFLP analysis, except AC181, which was down-regulated. Control primers for constitutively expressed genes were also used in this analysis to normalize for amounts of starting cDNA. Degenerate primers for plant actins were used to clone an Ageratum-specific actin for which primers were designed. In addition, we isolated, sequenced, and designed primers for a putative glycerol 3-phosphate dehydrogenase that experimentally showed constitutive expression pattern in the AFLP analysis. cDNAs from control cells and from cells treated with Agrobacterium for 48 h were amplified by one of the seven gene-specific primers in a multiplex PCR reaction containing also primers for the constitutive genes, actin, and putative glycerol 3-phosphate dehydrogenase (G3PD). Fragment AC321 represented a gene expressed at low levels, and therefore only the control primers for G3PD were used, as G3PD was expressed at a lower level than actin (actin was saturated at the cDNA concentrations used in these reactions). The opposite was true for fragment AC325, which was expressed at high levels. Therefore, only actin was used as a control (G3PD expression was undetectable at the cDNA concentrations used in these reactions). We found that 30 cycles of PCR provided the sensitivity needed to detect the differences in expression for most fragments. However, 25 cycles were used for fragment AC325. The products were analyzed by agarose gel electrophoresis, and the results are shown in Fig. 4A. The analysis was repeated in four independent experiments (two of those are shown in Fig. 4A), confirming the differential expression pattern for all seven fragments.
Figure 4.
RT-PCR analysis of seven cDNA-AFLP fragments. A. conyzoides cDNAs from mock- (−) and bacterial-inoculated (+, A and B) cells were amplified with primers specific for each fragment and for two control genes, actin (lower band) and putative G3PD. Fragments AC321 and AC325 were amplified with G3PD alone and actin alone, respectively. (A) One cDNA is repressed (AC181), and six cDNAs are induced (AC168, 178, 275, 321, 325, and 365) by Agrobacterium. The analysis was repeated in four independent experiments, of which two are shown. (B) One cDNA is repressed (AC181), four cDNAs are induced (AC178, 275, 325, and 365), and two cDNAs are not altered by E. coli. The analysis was repeated in two independent experiments.
The Specificity of the Responses to Agrobacterium.
The seven selected fragments were then further analyzed by RT-PCR to determine whether their pattern of expression represented a response specific to Agrobacterium infection or whether some of these transcripts, particularly the ones involved in defense response, could also be regulated by another type of stress, for example, exposure to nonpathogenic bacteria. We performed RT-PCR analysis with cDNA from plant cells exposed to E. coli cells for 48 h. The results show that genes induced by Agrobacterium that are involved in defense or stress response, such as the starvation-induced ribonuclease (AC178), the bacterial-induced peroxidase precursor (AC275), and the PR protein NtPRp27 (AC365), are also induced by E. coli (Fig. 4B). The gene encoding a receptor kinase homologue (AC181) is repressed by both bacteria. The lectin-like kinase gene (AC321) was induced reproducibly by Agrobacterium but in only one experiment by E. coli (Fig. 4B). This gene is expressed at a very low level, and the nonreproducible behavior may reflect quantitative differences in mRNA accumulation between treatments. However, the gene encoding a nodulin-like protein (AC168) is induced by Agrobacterium but not by E. coli. Additionally, by using AFLP-cDNA from plant cells exposed to E. coli for 48 h, we analyzed expression of the 20 genes represented by the fragments listed in Table 2, confirming the RT-PCR results for the seven fragments listed above (at least one Agrobacterium-specific gene and five genes commonly regulated by E. coli). Among the remaining 13 fragments, at least three more (AC35, AC121, and AC274) appeared to be regulated by Agrobacterium but not by E. coli (data not shown).
Discussion
To understand how plants respond to A. tumefaciens infection, we have taken a broad approach and screened changes in gene expression in response to virulent Agrobacterium as compared with mock-inoculated controls. Such screen should identify possible responses, from preattachment phase, to cell attachment, to T-DNA transfer. To avoid plant responses induced by hormonal perturbations, we used an Agrobacterium strain whose modified T-DNA contained a reporter gene but lacked all wild-type T-DNA genes. Using the cDNA-AFLP technique, we identified several cDNAs whose expression was altered. This study represents, to our knowledge, the first demonstration that plants can modulate their gene expression in response to Agrobacterium exposure, and that Agrobacterium can trigger the plant defense machinery. Additionally, we showed that many of these cDNAs are also induced by a nonpathogen such as E. coli. However, some genes, such as one encoding a nodulin-like protein, can be uniquely regulated by A. tumefaciens. The identification of this gene as a nodulin suggests a possible commonality with the interaction between Rhizobium and legumes. The induction of common defense responses by Agrobacterium, which was somewhat unexpected given the noninducing properties of its flagellin (24), suggests that Agrobacterium may have nonflagellin elicitors. Because defense responses were also induced by E. coli, which does not bind to the plant cell, we can conclude that the responses observed do not require binding and should involve a diffusible elicitor.
In the detection of changes in gene expression, the cDNA-AFLP technique has significant advantages over differential display [DD (36)] and microarrays (37). Because longer primers allow the use of more stringent annealing conditions, cDNA-AFLP is more reproducible than DD. In contrast to microarray analysis, cDNA library can be performed in the absence of DNA sequence data. This characteristic allowed us to exploit the high transformation rate of Ageratum, which was not available in the plant model system Arabidopsis. Additionally, cDNA-AFLP analysis requires small amounts of RNA when compared with the amounts needed for microarrays, and it also provides the opportunity for automation with the use of fluorescent dyes in a manner similar to the microarray technology (38).
A high percentage of the differentially regulated cDNAs identified in this study has homology to genes commonly regulated during plant defense/stress responses, such as the gene encoding the PR protein NtPRp27 from tobacco. PR proteins are markers of induced defense responses, and some have a defined role in pathogenesis, such as degradation of the cell wall of microbes (39). Included in the class of defense response are proteins involved in biosynthesis of phenylpropanoids, such as a glucosyltransferase from Arabidopsis and a cytochrome P450 monooxygenase from Pisum sativum. Phenylpropanoids are secondary plant metabolites that can have antimicrobial activity and also serve in signaling and chemotaxis to both pathogenic and symbiotic microorganisms (40). Plant defense responses also include the synthesis of reactive oxygen species and the crosslinking of the cell wall, reactions that involve peroxidases (41). One of the fragments isolated is homologous to a bacterial-induced gene encoding a peroxidase precursor from Gossypium hirsutum. Interestingly, Agrobacterium appears to have evolved ways to counteract the production of reactive oxygen species by the plant. Inactivation of an Agrobacterium catalase, which converts certain reactive oxygen species to nontoxic products, attenuates the ability of this mutant to cause tumors on plants (42). It is very likely that Agrobacterium evolved ways to cope with other plant defense responses, because it can infect and live in close association with a wide variety of plants.
Another fragment isolated in this study encodes a product similar to the receptor kinase Xa21, a disease-resistance protein from rice. Interestingly, we found that this putative receptor kinase is repressed by Agrobacterium as well as by E. coli infection. Generally, disease resistance genes of the receptor kinase type are either constitutively expressed (43) or induced by the infection of the recognized pathogen (44) during an incompatible interaction leading to resistance. This difference, observed during the interaction with Agrobacterium or with E. coli, raises the possibility that surveillance receptors for specific pathogens may be suppressed during compatible or nonspecific interactions.
We have identified one cDNA that is induced only by Agrobacterium and not by E. coli and another that may respond quantitatively more to Agrobacterium. They encode, respectively, products homologous to a nodulin-like protein and a lectin-like protein kinase. Nodulins form a group of proteins induced in the root nodule of leguminous plants in response to Rhizobium infection or to oligosaccharides (nod factors) produced by Rhizobium. Even though the function of most nodulins is still unclear, some may be involved in cell division/differentiation and are thought to regulate the organogenesis of the nodule (45, 46). Lectins are carbohydrate-binding proteins whose function in plants is also unclear, although they have been implicated in cell–cell recognition processes. Kinases containing a lectin domain form a new class of kinases in plants, largely undescribed, but with potential important roles in cellular communication and in the transduction of oligosaccharides signals (47), perhaps including nod factors from rhizobia. The finding of these two cDNAs reflects the relatedness of Agrobacterium and Rhizobium and suggests that these two bacteria may produce compounds that are recognized by plants in a similar manner, although the final outcome of the two interactions is strikingly different.
In conclusion, we have demonstrated altered gene regulation in A. conyzoides plant cells in response to Agrobacterium infection. We have shown that Agrobacterium induced responses that are commonly triggered by abiotic stress and by nonpathogenic bacteria, as well as responses that appear unique to Agrobacterium and perhaps are shared with its close relative, Rhizobium. Differential screens that compare challenge by a nonpathogen to challenge by Agrobacterium should facilitate the identification of genes responding to Agrobacterium-specific stimuli, such as binding to plant cell walls and T-DNA entry in the plant cell. Agrobacterium-specific genes can be further analyzed by comparing plant responses to wild-type Agrobacterium with the ones generated by exposure to avirulent mutants, defective at different steps of the interaction and transformation process. Such studies should further dissect the interaction of plant cells with this unusual plant pathogen.
Acknowledgments
We thank Dr. Hiroshi Kanzaki (Faculty of Agriculture, Okayama University, Japan) for supplying the A. conyzoides cell culture, and Drs. Lishan Chen and Derek Wood (Dept. of Microbiology, University of Washington) for providing A. tumefaciens strains and valuable comments. This work was supported by National Science Foundation Grant 9723735, by Public Health Service Grant GM32618, and by a scholarship from CNPq, Brazil (to R.F.D.).
Abbreviations
- AFLP
amplified fragment length polymorphism
- GUS
β-glucuronidase
- RT-PCR
reverse transcription PCR
- PR
pathogenesis-related
- G3PD
glycerol 3-phosphate dehydrogenase
- T-DNA
transferred DNA of A. tumefaciens
Footnotes
References
- 1.Azpiroz-Leehan R, Feldmann K A. Trends Genet. 1997;13:152–156. doi: 10.1016/s0168-9525(97)01094-9. [DOI] [PubMed] [Google Scholar]
- 2.Bundock P, den Dulk-Ras A, Beijersbergen A, Hooykaas P J. EMBO J. 1995;14:3206–3214. doi: 10.1002/j.1460-2075.1995.tb07323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piers K L, Heath J D, Liang X, Stephens K M, Nester E W. Proc Natl Acad Sci USA. 1996;93:1613–1618. doi: 10.1073/pnas.93.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunik T, Tzfira T, Kapulnik Y, Gafni Y, Dingwall C, Citovsky V. Proc Natl Acad Sci USA. 2001;98:1871–1876. doi: 10.1073/pnas.041327598. . (First Published January 30, 2001; 10.1073/pnas.041327598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie P J, Vogel J P. Trends Microbiol. 2000;8:354–360. doi: 10.1016/s0966-842x(00)01792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kado C I. Genet Eng. 1998;20:1–24. doi: 10.1007/978-1-4899-1739-3_1. [DOI] [PubMed] [Google Scholar]
- 7.Winans S C. Microbiol Rev. 1992;56:12–31. doi: 10.1128/mr.56.1.12-31.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheng J, Citovsky V. Plant Cell. 1996;8:1699–1710. doi: 10.1105/tpc.8.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelvin S B. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:223–256. doi: 10.1146/annurev.arplant.51.1.223. [DOI] [PubMed] [Google Scholar]
- 10.Deng W, Chen L, Wood D W, Metcalfe T, Liang X, Gordon M P, Comai L, Nester E W. Proc Natl Acad Sci USA. 1998;95:7040–7045. doi: 10.1073/pnas.95.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballas N, Citovsky V. Proc Natl Acad Sci USA. 1997;94:10723–10728. doi: 10.1073/pnas.94.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mysore K S, Nam J, Gelvin S B. Proc Natl Acad Sci USA. 2000;97:948–953. doi: 10.1073/pnas.97.2.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziemienowicz A, Tinland B, Bryant J, Gloeckler V, Hohn B. Mol Cell Biol. 2000;20:6317–6322. doi: 10.1128/mcb.20.17.6317-6322.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durrant W E, Rowland O, Piedras P, Hammond-Kosack K E, Jones J D. Plant Cell. 2000;12:963–977. doi: 10.1105/tpc.12.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk P M, Kazan K, Wilson I, Anderson J P, Richmond T, Somerville S C, Manners J M. Proc Natl Acad Sci USA. 2000;97:11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salzer P, Bonanomi A, Beyer K, Vogeli-Lange R, Aeschbacher R A, Lange J, Wiemken A, Kim D, Cook D R, Boller T. Mol Plant–Microbe Interact. 2000;13:763–777. doi: 10.1094/MPMI.2000.13.7.763. [DOI] [PubMed] [Google Scholar]
- 17.Lambais M R, Mehdy M C. Mol Plant–Microbe Interact. 1993;6:75–83. [Google Scholar]
- 18.Ruiz-Lozano J M, Roussel H, Gianinazzi S, Gianinazzi-Pearson V. Mol Plant–Microbe Interact. 1999;12:976–984. [Google Scholar]
- 19.Sikorski M M, Biesiadka J, Kasperska A E, Kopcinska J, Lotocka B, Golinowski W, Legocki A B. Plant Sci. 1999;149:125–137. [Google Scholar]
- 20.Krause A, T, L V, Broughton W J. Mol Plant–Microbe Interact. 1997;10:388–393. doi: 10.1094/MPMI.1997.10.3.388. [DOI] [PubMed] [Google Scholar]
- 21.Arsenijevic-Maksimovic I, Broughton W J, Krause A. Mol Plant–Microbe Interact. 1997;10:95–101. doi: 10.1094/MPMI.1997.10.1.95. [DOI] [PubMed] [Google Scholar]
- 22.Crockard M A, Bjourson A J, Cooper J E. Mol Plant–Microbe Interact. 1999;12:825–828. doi: 10.1094/MPMI.1999.12.9.825. [DOI] [PubMed] [Google Scholar]
- 23.Robinette D, Matthysse A G. J Bacteriol. 1990;172:5742–5749. doi: 10.1128/jb.172.10.5742-5749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felix G, Duran J D, Volko S, Boller T. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 25.Bachem C W, van der Hoeven R S, de Bruijn S M, Vreugdenhil D, Zabeau M, Visser R G. Plant J. 1996;9:745–53. doi: 10.1046/j.1365-313x.1996.9050745.x. [DOI] [PubMed] [Google Scholar]
- 26.Kanzaki H, Kagemori T, Asano S, Kawazu K. Biosci Biotechnol Biochem. 1998;62:2328–2333. doi: 10.1271/bbb.62.2328. [DOI] [PubMed] [Google Scholar]
- 27.Nagata T, Nemoto Y, Hasezawa S. Int Rev Cytol. 1992;132:1–30. [Google Scholar]
- 28.Cangelosi G A, Best E A, Martinetti G, Nester E W. Methods Enzymol. 1991;204:384–397. doi: 10.1016/0076-6879(91)04020-o. [DOI] [PubMed] [Google Scholar]
- 29.Bechtold N, Ellis J G, Pelletier G. C R Acad Sci Paris, Sci la Vie/Life Sci. 1993;316:1194–1199. [Google Scholar]
- 30.Akama K, Shiraishi H, Ohta S, Nakamura K, Okada K, Shimura Y. Plant Cell Rep. 1992;12:7–11. doi: 10.1007/BF00232413. [DOI] [PubMed] [Google Scholar]
- 31.Hood E E, Gelvin S B, Melchers L S, Hoekema A. Transgenic Res. 1993;2:208–218. [Google Scholar]
- 32.Narasimhulu S B, Deng X B, Sarria R, Gelvin S B. Plant Cell. 1996;8:873–886. doi: 10.1105/tpc.8.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jefferson R A. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- 34.De Buck S, De Wilde C, Van Montagu M, Depicker A. Mol Plant–Microbe Interact. 2000;13:658–665. doi: 10.1094/MPMI.2000.13.6.658. [DOI] [PubMed] [Google Scholar]
- 35.Sano T, Kuraya Y, Amino S, Nagata T. Plant Cell Physiol. 1999;40:1–8. doi: 10.1093/oxfordjournals.pcp.a029464. [DOI] [PubMed] [Google Scholar]
- 36.Liang P, Pardee A B. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 37.Schena M, Shalon D, Davis R W, Brown P O. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 38.Cho Y-j, Meade J D, Walden J C, Chen X, Guo Z, Liang P. BioTechniques. 2001;30:562–572. doi: 10.2144/01303rr01. [DOI] [PubMed] [Google Scholar]
- 39.Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, Legrand M, Fritig B. Biochimie. 1993;75:687–706. doi: 10.1016/0300-9084(93)90100-7. [DOI] [PubMed] [Google Scholar]
- 40.Paiva N L. J Plant Growth Regul. 2000;19:131–143. doi: 10.1007/s003440000016. [DOI] [PubMed] [Google Scholar]
- 41.Lamb C, Dixon R A. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 42.Xu X Q, Pan S Q. Mol Microbiol. 2000;35:407–414. doi: 10.1046/j.1365-2958.2000.01709.x. [DOI] [PubMed] [Google Scholar]
- 43.Century K S, Lagman R A, Adkisson M, Morlan J, Tobias R, Schwartz K, Smith A, Love J, Ronald P C, Whalen M C. Plant J. 1999;20:231–236. doi: 10.1046/j.1365-313x.1999.00589.x. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura S, Yamanouchi U, Katayose Y, Toki S, Wang Z X, Kono I, Kurata N, Yano M, Iwata N, Sasaki T. Proc Natl Acad Sci USA. 1998;95:1663–1668. doi: 10.1073/pnas.95.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long S R. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stougaard J. Plant Physiol. 2000;124:531–540. doi: 10.1104/pp.124.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herve C, Dabos P, Galaud J P, Rouge P, Lescure B. J Mol Biol. 1996;258:778–788. doi: 10.1006/jmbi.1996.0286. [DOI] [PubMed] [Google Scholar]