Abstract
Drug-resistant spinal tuberculosis (TB) is an emerging health problem in both developing and developed countries. In this review article, we aim to define management protocols for suspicion, diagnosis, and treatment of such patients. Spinal TB is a deep-seated paucibacillary lesion, and the demonstration of acid-fast bacilli on Ziehl-Neelsen staining is possible only in 10%–30% of cases. Drug resistance is suspected in patients showing the failure of clinicoradiological improvement or appearance of a fresh lesion of osteoarticular TB while on anti tubercular therapy (ATT) for a minimum period of 5 months. The conventional culture of Mycobacterium tuberculosis remains the gold standard for both bacteriological diagnosis and drug sensitivity testing (DST); however, the high turn around time of 2–6 weeks for detection with added 3 weeks for DST is a major limitation. To overcome this problem, rapid culture methods and molecular methods have been introduced. From a public health perspective, reducing the period between diagnosis and treatment initiation has direct benefits for both the patient and the community. For all patients of drug-resistant spinal TB, a complete Drug-O-Gram should be prepared which includes details of all drugs, their doses, and duration. Patients with confirmed multidrug-resistant TB strains should receive a regimen with at least five effective drugs, including pyrazinamide and one injectable. Patients with resistance to additional antitubercular drugs should receive individualized ATT as per their DST results.
Keywords: Drug resistant, Gene-Xpert, line probe assay, multidrug-resistant tuberculosis, spinal tuberculosis
MeSH terms: Multidrug resistance, drug resistance, bacterial, tuberculosis, Pott disease
Introduction
Drug resistant spinal tuberculosis is an emerging health problem in both developing and developed countries. Drug resistant spinal tuberculosis poses a unique set of challenges vis-à-vis drug resistant pulmonary tuberculosis. Drug sensitivity testing (DST) is easier to perform in patients of pulmonary tuberculosis due to ready availability of sputum samples unlike spinal tuberculosis which is complicated by the inherent difficulty in procurement of tissue or pus samples. Secondly spinal tuberculosis is a pauci-bacillary disease hence the probability of mycobacterial growth and culture sensitivity testing is bleak even in patients where adequate samples are obtained.1 The diagnosis of drug resistant spinal tuberculosis is often delayed resulting in development of spinal deformity, neurological complications. Lastly while well defined management protocols exist for the diagnosis and management of drug resistant pulmonary tuberculosis, the same cannot be said for spinal tuberculosis. Thus in this review article we aim to address the following gaps in the current knowledge: When should drug resistance be suspected in a case of spinal tuberculosis (Presumptive Drug Resistance)? What should be the investigative and management protocol for patients of drug resistant spinal tuberculosis? What are the drugs regimen, dosage and the duration of anti tubercular therapy (ATT) in confirmed drug resistant cases of spinal TB?What should be the management protocol in patients where drug resistance is not demonstrated on culture and sensitivity reports?
Methods
We conducted a systematic review of literature on drug resistant cases of spinal tuberculosis. PubMed and Cochrane Library were accessed and articles written in the English language, published between 1991-2015 containing the key words “drug resistance, MDR-TB, spinal tuberculosis, multidrug resistance were included in the search. 104 articles containing the specified key words were found. 3 articles were found to be duplicated in the search and were excluded. 44 articles primarily discussed pulmonary tuberculosis and not included in the article. Full text was obtained for 57 articles. Following independent detailed reading of the full text by the authors 35 articles were excluded due to duplication of data and lack of well-defined inclusion and exclusion criteria. A total of 22 articles were included in the present study which comprised of 6 review articles, 5 prospective studies, 2 retrospective studies, 6 cross sectional studies and 3 editorials [Figure 1].
Figure 1.
PRISMA diagram showing data retrieval and analysis
Discussion
Definitions of drug-resistant tuberculosis
Bacilli demonstrating resistance to a single anti tubercular agent are termed Mono drug resistance. Resistance to both Isoniazid and rifampicin is termed as MDR and extensively drug-resistant tuberculosis (XDR-TB) is defined as resistance to INH and rifampicin along with resistance to any fluoroquinolone and at least one injectable second line anti-tuberculosis drug. Bacteria demonstrating resistance to all known anti tubercular drugs are termed as total drug resistant.2
Primary resistance is that which has not resulted from the treatment of the patient with the drug concerned. It includes resistance in wild strains that have never come into contact with the drug (natural resistance) and the resistance occurring as a result of exposure of the strain to the drug but in another patient. The term “acquired resistance” has often been used with the implication that resistance has developed due to exposure of the strain to anti-tuberculosis drugs and the consequent selecting out of resistant mutant bacilli.3
The main factors contributing to the development of drug resistance
Inadequate and incomplete treatment: Multidrug resistance is rarely innate and is usually the result of inappropriate drug therapy. Johnson et al. (2003) in a study of 109 culture positive patients of pulmonary tuberculosis found a high incidence of drug resistance in previous treatment defaulters while only 4 of the 27 (14.8%) new cases had MDR-TB4
Non-adherence to the treatment: Noncompliance with anti tubercular therapy is an important cause of development of drug resistance, particularly in patients following alternate day regimens where they tend to miss doses. Short course chemotherapy with drug resistant strains of the bacilli may create even more resistance to the drugs in use, which has been called the amplifier effect5
Genetic predisposition: Park et al. (2002) found that the susceptibility to MDR-TB is strongly associated with HLA-DRB1*08032-DQB1*0601 haplotypes.6 Sharma S.K et al. (2003) reported that patients having HLA-DRB1*13 and HLA-DRB1*14 have a two fold risk of developing MDR-TB7
Coinfection with HIV positive: Maurya et al. reported HIV and MDR-TB co-infection rates to be 13.1%.8 Similar results were also obtained by Gandhi et al. and WHO/IUATLD Study (31.6%).9
Molecular Basis of Drug Resistance
DNA sequencing of the mycobacterial genome has resulted in the identification of the various genes responsible for drug resistance. These specific DNA sequences are used for the identification of drug resistant mycobacterial strains via molecular methods i.e., Line Probe assay (LPA) and Gene-Xpert testing. A summary of genes involved in drug resistance is provided in Table 1.
Table 1.
Molecular Basis of Drug Resistance
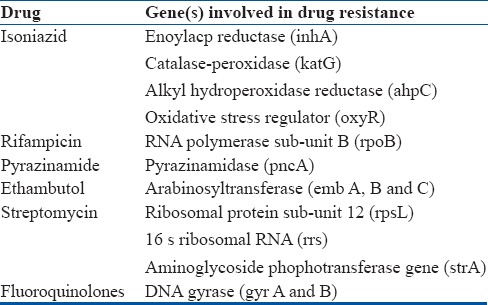
Suspected Drug-Resistant Tuberculosis (Presumptive Drug Resistance)
In pulmonary tuberculosis when a case continues to remain sputum positive under CAT I or RNTCP DOTS treatment at 5 months or under CAT II at 4 months, it is labelled as a suspected case of drug resistant tuberculosis. History of previous drug treatment or repeated defaulters or a patient who has converted to sputum negative and then again becomes sputum positive also raises suspicion for MDR-TB cases.2
Spinal TB is a deep seated paucibacillary lesion, the diagnosis is often clinicoradiological including MRI. Bacteriological and histological diagnosis requires invasive procedure (guided biopsy) to procure tissue for bacteriological and histological diagnosis. Most of the time spinal lesions can be treated by clinico-imaging and histological diagnosis. The demonstration of acid-fast bacilli on ZN staining is possible in 10-30% cases.10 It is practically impossible to perform repeated sampling from spinal lesion to suspect drug resistance. Thus the clinical criteria for suspicion of multidrug-resistant tuberculosis are defined.
Tuli et al. suggested the clinical criteria to suspect drug resistant cases of spinal tuberculosis, which include patients of spinal tuberculosis on ATT for 5 months or more showing:11
Poor clinical and radiological response OR
Appearance of a fresh lesion of osteoarticular tuberculosis OR
Deterioration of spinal deformity OR
Appearance of discharging sinus OR
Wound dehiscence of previously operated scar.
Jaggi and Jain have conducted a study on tissue sampling (unpublished thesis data) on the above criteria and found that poor clinical and radiological response or appearance of a fresh lesion of osteo-articular tuberculosis while on anti tubercular therapy are reliable indicators to suspect drug resistance. (Presumptive Drug Resistance)
Epidemiology of Multidrug-Resistant-Tuberculosis
WHO reported the median prevalence of primary and acquired MDR-pulmonary TB to be 3.4% and 25%, respectively.12 In India, prevalence of primary MDR-TB in newly diagnosed cases has been observed to be 3.4 per cent or less.5 The prevalence is found to be at a low level in most of the country where it has been studied. Data from studies conducted by National Institute for Research in Tuberculosis (NIRT), have found MDR-TB levels of 1% to 3% in new cases and around 12% in re-treatment cases.13
Studies on drug resistant spinal tuberculosis have been primarily from India and China, with these countries accounting for more than 50% of all incident cases. 5 studies reported patient data on drug resistant spinal tuberculosis of which 4 studies included all patients with histological or bacteriological confirmation of tuberculosis. Following inclusion all patients subsequently underwent drug sensitivity testing. Li L et al. reported 249 patients of histologically proven spinal tuberculosis of which 127 (51%) produced a positive culture. 39 of the 127 culture positive patients (30.7%) were found to have documented drug resistance. 4 of 39 patients were excluded from the study due to loss of followup and non-completion of treatment. 12/35 patients were found to have multidrug resistant tuberculosis (MDR-TB), 16/35 patients were found to have mono-drug resistance while the remaining 7 patients were found to have resistance to additional anti tubercular drugs. The rates of resistance to isoniazid were 54.3%, 48.6% for rifampicin and 34.3% for streptomycin amongst the demonstrable drug resistant cases.1 Pawar UM et al. evaluated 238 cases of histologically proven spinal tuberculosis and found 28 patients (11.7%) to have multidrug resistant strains. 25 of these 28 patients were included in their study and evaluated for response to second line ATT. The authors haven’t reported on mono-drug resistance and resistance to other anti tubercular drugs.14 Mohan K et al. analysed 686 culture positive patients of spinal tuberculosis and found 111 patients (16.2%) to have drug resistant strains to atleast one anti tubercular drug. 87 of 111 patients were found to be multidrug resistant, 3 patients were diagnosed as XDR strains while the remaining 21 patients were found to have mono-drug resistant strains. Prevalence of resistance to isoniazid was 15.0% (93/686), to rifampicin was 13.5% (93/686) and 11.2% (77/686) for streptomycin.15 Xu Lan et al. (2013) evaluated 152 patients of histologically proven spinal tuberculosis and reported bacteriological culture positivity in 76 patients. 23 of 76 patients (30.3%) were found to have drug resistant tuberculosis.16 Thus the prevalence of drug resistant spinal tuberculosis in native population varies from 11.7% to 30.7% of culture positive patients.
Jain et al. conducted a study on therapeutically refractory patients of spinal tuberculosis where 15 patients were included on the basis of suspected drug resistance. Bacteriological culture was possible in 3 of 15 patients (20%) and these patients were taken up for drug sensitivity testing. DST results revealed multidrug resistance in 2 of 3 patients (66.6%) while the remaining 1 patient was found to be sensitive to all first and second line anti tubercular drugs. However, all patients enrolled as therapeutically refractory cases were labelled as clinically drug resistant and were treated with second line ATT.17
The reported rates of drug resistance vary considerably in studies that have been conducted by various authors. However, these results may not reflect the true percentage of drug resistance as bacteriological culture and subsequent drug sensitivity testing were only possible in 50% of the cases. A summary of the studies performed on drug resistant spinal tuberculosis is presented in Table 2.
Table 2.
Studies performed on drug resistant spinal tuberculosis.

Diagnosis of Drug-Resistant Tuberculosis
Conventional culture of Mycobacterium tuberculosis remains the gold standard for both bacteriological diagnosis and drug sensitivity testing (DST) however the high turn around time (TAT) of 2-6 weeks for detection with added 3 weeks for DST is a major limitation.18 Traditionally, Lowenstein-Jensen (LJ) culture uses three methods for DST. While absolute concentration method and resistance-ratio method determines the minimum inhibitory concentration (MIC), proportion method determines the critical proportion for sensitive or resistant strains.
To overcome this problem rapid culture methods have been introduced with reduced TAT of 31 days with Nitrate reduction assay indicating both INH and RIF resistance, Thin layer agar (TLA) culture also detects the same but within 11 days and with better sensitivity. BACTEC-46 has a TAT of 10 days while BACTEC-MGIT-96 and Septi-Chek AFB are comparable for detection of AFB (around 13 days) but Septi-Chek is better for simultaneous detection of NTM (non-tuberculous mycobacteria). Time to detection along with DST is up to 48 hours using mycobacterio phages (Fast Plaque Assay) with a sensitivity of 50 mycobacteria/ml.19
Considerable advancement has been made in the last few years to resolve the basis of resistance against INH and RIF. Rapid identification, which is essential for earlier treatment initiation, improved patient outcomes, and more effective public health interventions, relies on nucleic acid amplification techniques. In 2008 World Health Organization (WHO) endorsed GenoTypeMTBDRplus (version 1.0) molecular line probe assay (LPA), which is a rapid detection procedure of Mycobacterium tuberculosis complex (MTB) and also serves to detect mutations in the resistance specific genes conferring resistance against RIF and INH in AFB smear-positive sputum specimens.20 The GeneXpert MTB/RIF assay was also implemented by WHO in 2010 as a novel integrated diagnostic device for the diagnosis of tuberculosis and rapid detection of RIF resistance in clinical specimens. It has revolutionized TB control by contributing to the rapid diagnosis of TB disease and drug resistance in less than 2 hours.21 RIF resistance is a predictor of MDR-TB as, in most instances, resistance to RIF co-exists with resistance to INH thus allowing TB patients to start on effective treatment much sooner than waiting for results from other types of DST. The test appeared to be as sensitive (>95%) as culture with smear-positive specimens but less sensitive (55%) with smear-negative pulmonary and extrapulmonary specimens that include low numbers of bacilli. While comparing the two, Xpert has been shown to demonstrate more accuracy in the detection of RIF susceptibility compared with DRplus.22
Barnard et al. (2008) found a sensitivity and specificity of 98.9% and 99.4%, respectively for detection of RIF resistance; sensitivity was 94.2% with specificity of 99.7% for detection of INH resistance. Results were interpretable for 97% of the specimens within 1 or 2 days. They demonstrated that the strip assay is rapid and accurate for the detection of mutations found in MDR-TB strains, providing an excellent platform for development to detect XDR-TB strains.23
In a prospective study conducted by Held et al.(2014), 69 cases of spinal Tuberculosis in a tertiary care hospital showed the Gene-Xpert test had a sensitivity of 95.6% and a specificity of 96.2% for spinal tuberculosis. The results of the Gene-Xpert test were available within 48 hours as compared to a median of 35 days. All cases of MDR-TB were diagnosed accurately by the test with the MDR-TB rate being 5.8%. They recommend Gene-Xpert test for the initial diagnosis of spinal tuberculosis.24
Rufai S.B et al. compared LPA and Gene-Xpert results with MGIT 960 data and found 100% concordance between MGIT and LPA results, where as 64.4% concordance was noted with Gene-Xpert testing.25 Singhal et al. compared LPA results with MGIT 960 and found concordance rates to be 96.6% and 84.7% for RIF and INH resistance respectively.26 Madhuri et al. (2015) in a study of 687 suspected pulmonary tuberculosis also recommended that LPA as an excellent diagnostic tool for early and accurate diagnosis of MDR TB.27
Hybridisation on DNA Chips can also be used for rapid detection of mutations responsible for drug resistance. The overall specificity is 100% and 95% for INH and RIF resistance respectively.
Clinical impact of Drug Sensitivity Testing
From a public health perspective, reducing the period between diagnosis and treatment initiation by the introduction of the LPA has direct benefits for both the patient and the community. Xu lan et al.(2013) reported the mean delay in diagnosis for spinal tuberculosis (interval between onset of symptoms and establishment of spinal tuberculosis clinically) was 8.52+/- 6.15 months and the mean delay in diagnosis of drug resistant tuberculosis (interval between diagnosis of spinal tuberculosis and DST results) a further 8.25+/- 2.76 months.16 Li L et al. reported the mean delay in diagnosis of drug resistant spinal tuberculosis to be 8.43+/- 2.12 months. They recommend that Drug sensitivity testing should be carried out on all initial and re-treatment cases of spinal tuberculosis.1
Pawar U.M (2009) reported the loss of 7 or more months of appropriate second line ATT has several potentially critical consequences, i.e., patients with MDR-TB may have a progressive disease and associated morbidities, potential chance of development of XDR-TB pathogens and lastly ongoing disease transmission is likely to occur in patients with concomitant active pulmonary tuberculosis.14
Management of Multidrug-Resistant-Tuberculosis: Treatment Principles
Delay in the diagnosis of spinal tuberculosis is well known. There is a mean delay of 6-8 months before the diagnosis of spinal tuberculosis is made, particularly in endemic countries. Drug resistant spinal tuberculosis is suspected when the patient is not responding to ATT for minimum period of 5-6 months which leads to a further delay in diagnosing drug resistant cases of spinal tuberculosis. Ideally all patients of spinal tuberculosis should be treated after obtaining drug sensitivity reports at the first instance. However, in a high disease load country as ours, with limited resources and lack of universal access to drug sensitivity testing, patients are often treated on clinico-imaging including MRI findings and patients are investigated for drug resistance only when failure of treatment is suspected. (Presumptive drug resistance).
It is not uncommon to find a patient having failure of treatment where no bacteriological growth is detected i.e., culture negative. In such patients if adequate clinical suspicion is present and histological examination is suggestive of tubercular pathology, they can be labelled as clinically drug resistant cases and may be treated as multidrug resistant spinal tuberculosis.
For all patients of drug resistant spinal tuberculosis, a complete drug-o-gram should be prepared which includes details of all drugs, their doses, and duration. This helps the treating doctor to know which drugs have never been used for the treatment in the past for particular patient. In each patient, efforts should be made to obtain bacteriological culture and subsequent drug sensitivity testing results, which would serve as a guide for subsequent ATT. The tissue sample should be obtained through percutaneous aspiration or through surgical debridement. Since spinal tuberculosis is a deep seated pauci-bacillary lesion it is suggested that wherever possible surgical debridement should be performed to obtain adequate tissue and pus samples for histopathology and drug sensitivity testing. Surgical debridement has the added advantage of reducing the bacterial load of the lesion.
Conventional bacteriological microscopy and cultures have limited sensitivity, specificity and a delayed diagnosis. Culture in BACTEC radioactive liquid medium and genotypic analysis involving amplification by polymerase chain reaction followed by post-amplification analysis of mutation, have reduced the TAT to days rather than weeks or months. The conventional assay however, must still be performed as it offers drug sensitivity testing for second line anti tubercular drugs and offers the clinician the ability to differentiate between active and inactive tubercular lesions.
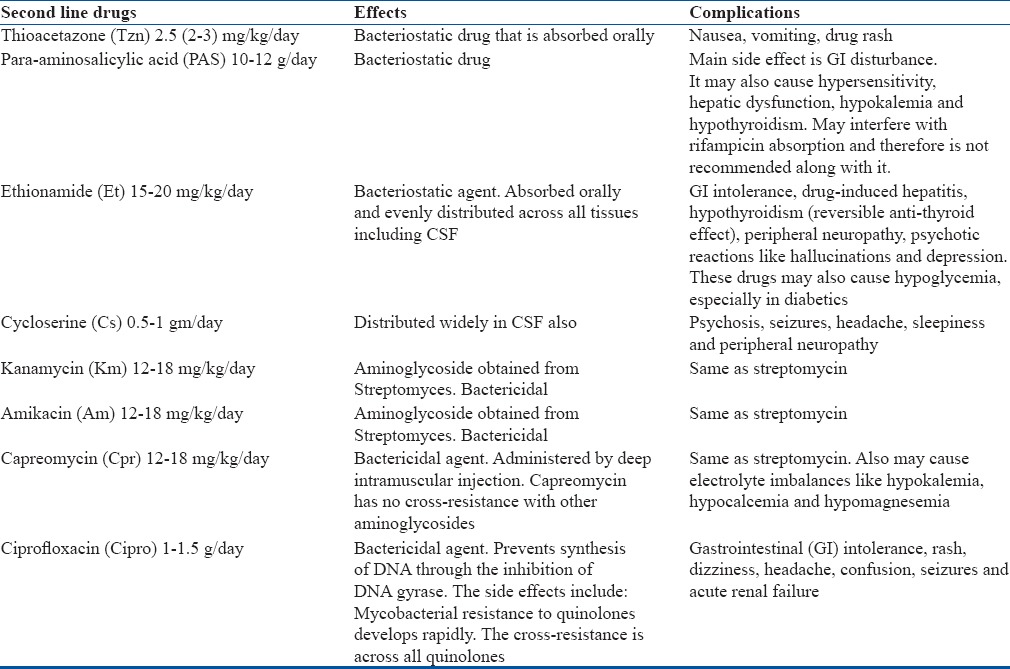
The 2011 WHO guidelines for multi drug resistant pulmonary tuberculosis recommend a minimum of four second line anti tubercular drugs based on individual drug sensitivity pattern for 8 months of intensive phase with a total duration of 20 months.28 The practice of adding a single new drug to a failing regimen should be avoided. Second line drugs are potentially more toxic than primary drugs and a physician experienced in the treatment of MDR-TB must be included in the management of the patient. The second line drugs are best given in once a day dosing. Details of the common complications of second line agents are outlined in Table 3. The adverse drug reactions and hepatic side effects should be monitored diligently. An injectable aminoglycoside (Kanamycin/Amikacin) should be added for a minimum period of 6 months.
Table 3.
Complications of Second Line Anti tubercular Drugs
The current WHO guidelines for management of drug resistant tuberculosis (2016) are defined in Table 4 which state that patients with confirmed MDR-TB strains should receive a regimen with atleast 5 effective drugs, including pyrazinamide and 4 core second line drugs – one chosen from group A, one from group B and at least 2 from group C. Patients with resistance to additional anti tubercular drugs should receive individualized ATT as per their DST results.29
Table 4.
Recommended Medicines for MDR-TB

WHO currently recommends individual tailor made regimens for each patient of drug resistant tuberculosis as per their individual DST results however broad guidelines of anti tubercular drug combinations are presented in Table 5.
Table 5.
Recommended Anti tubercular Drug combinations

Second common mistake commonly practiced across the globe is to preserve the best drugs according to sensitivity for future use. The best way of achieving the cure is to hit the lesion hard by exposing it to the most sensitive drug at the first instance.
Monitoring the Response to Treatment
WHO guidelines for drug resistant pulmonary tuberculosis recommend performing monthly sputum smear microscopy and cultures to assess the response to therapy and for documentation of healed status; however, repeated sampling and cultures is impossible in spinal tuberculosis. The authors recommend clinical evaluation and laboratory evaluation in the form of Liver Function Testing (LFT) and Kidney Function Testing (KFT) every month for the first 6 months. Radiographic evaluation (X-rays) and a follow up ESR every 3 months should be obtained to evaluate the response to treatment. MRI evaluation at 12 months and subsequently at 6 month intervals should be performed till the lesion has healed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Li L, Zhang Z, Luo F, Xu J, Cheng P, Wu Z, et al. Management of drug-resistant spinal tuberculosis with a combination of surgery and individualised chemotherapy: a retrospective analysis of thirty-five patients. IntOrthop. 2012;36:277–83. doi: 10.1007/s00264-011-1398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Multidrug and extensively drug-resistant TB (M/XDR-TB): global report on surveillance and response 2010 [Google Scholar]
- 3.Paramasivan CN, Venkataraman P. Drug resistance in tuberculosis in India. Indian J Med Res. 2004;120:377–86. [PubMed] [Google Scholar]
- 4.Johnson J, Kagal A, Bharadwaj R. Factors associated with drug resistance in pulmonary tuberculosis. Indian J Chest Dis Allied Sci. 2003;45:105–9. [PubMed] [Google Scholar]
- 5.Sharma SK, Mohan A. Multidrug-resistant tuberculosis. Indian J Med Res. 2004;120:354–76. [PubMed] [Google Scholar]
- 6.Park MH, Song EY, Park HJ, Kwon SY, Han SK, Shim YS. HLA-DRB1 and DQB1 gene polymorphism is associated with multidrug-resistant tuberculosis in Korean patients. Hum Immunol. 2002;63:S33. [Google Scholar]
- 7.Sharma SK, Turaga KK, Balamurugan A, Saha PK, Pandey RM, Jain NK, et al. Clinical and genetic risk factors for the development of multidrug-resistant tuberculosis in non-HIV infected at a tertiary care center in India: a case-control study. Infect Genet Evol. 2003;3:183–8. doi: 10.1016/s1567-1348(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 8.Maurya AK, Kant S, Nag VL, Kushwaha RA, Dhole TN. Trends of anti-tuberculosis drug resistance pattern in new cases and previously treated cases of extrapulmonary tuberculosis cases in referral hospitals in northern India. J Postgrad Med. 2012;58(3):185–9. doi: 10.4103/0022-3859.101379. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender R, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 10.Tuli SM. Tuberculosis of the spine: A historical review. ClinOrthop. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- 11.Tuli SM. Challenge of therapeutically refractory and multidrug resistant tuberculosis in orthopaedic practice. Indian J Orthop. 2002;36:211–3. [Google Scholar]
- 12.Prasad R. Management of multidrug resistant tuberculosis: practitioners view point. Indian J Tuberc. 2007;54:3–11. [PubMed] [Google Scholar]
- 13.Tuli S. Tuberculosis of the skeletal system. 4th edition. Jaypee Brothers; 2004. [Google Scholar]
- 14.Pawar UM, Kundnani V, Agashe V, Nene A, Nene A. Multidrug-resistant tuberculosis of the spine--is it the beginning of the end. A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine. 2009;34(22):E806–10. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]
- 15.Mohan K, Rawall S, Pawar UM, Sadani M, Nagad P, Nene A, et al. Drug resistance patterns in 111 cases of drug-resistant tuberculosis spine. Eur Spine J. 2013;22:647–52. doi: 10.1007/s00586-012-2154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L1, Jian-Zhong X, Xue-Mei L, Bao-Feng G. Drug susceptibility testing guided treatment for drug-resistant spinal tuberculosis: a retrospective analysis of 19 patients? Int Surg. 2013;98(2):175–80. doi: 10.9738/INTSURG-D-12-00004.1. doi: 10.9738/INTSURG-D-12-00004.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain AK, Dhammi IK, Modi P, Kumar J, Sreenivasan R, Saini NS. Tuberculosis spine: Therapeutically refractory disease. Indian J Orthop. 2012;46:171–8. doi: 10.4103/0019-5413.93685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent TK, Kubica GP. Public health mycobacteriology. A guide for the level III laboratory. Atlanta, Center for Disease Control. 1985. [Last accessed on 2016 Mar 15]. Available from http://wwwnc.cdc.gov/eid/content/12/5/pdfs/v12-n5.pdf .
- 19.Lee CN, Heifets LB. Determination of minimal inhibitory concentrations of antituberculosis drugs by radiometric and conventional methods. Am Rev Respir Dis. 1987;136:349–52. doi: 10.1164/ajrccm/136.2.349. [DOI] [PubMed] [Google Scholar]
- 20.Geneva, Switzerland: World Health Organization; 2008. World Health Organization. Molecular line probe assays for rapid screening of patients at risk of multidrug resistant tuberculosis (MDR-TB) [Google Scholar]
- 21.Geneva, Switzerland: World Health Organization; 2011. World Health Organization. Rapid implementation of the Xpert MTB/RIF diagnostic test: technical and operational ‘how to’ practical considerations. [Google Scholar]
- 22.Rahman A, MahfuzaSahrin, Sadia Afrin, Keith Earley, Shahriar Ahmed, Rahman S. M. Mazidur. SayeraBanuComparison of Xpert MTB/RIF Assay and GenoTypeMTBDRplus DNA Probes for Detection of Mutations Associated with Rifampicin Resistance in Mycobacterium tuberculosis. PLoS One. 2016;11(4):e0152694. doi: 10.1371/journal.pone.0152694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnard M, Albert H, Coetzee G, O’brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J RespirCrit Care Med. 2008;177:787–92. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 24.Held M, Laubscher M, Zar HJ, Dunn RN. GeneXpert polymerase chain reaction for spinal tuberculosis: an accurate and rapid diagnostic test. Bone Joint J. 2014;96-B:1366–9. doi: 10.1302/0301-620X.96B10.34048. [DOI] [PubMed] [Google Scholar]
- 25.Rufai SB, Kumar P, Singh A, Prajapati S, Balooni V, Singh S. Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis. J ClinMicrobiol. 2014;52:1846–52. doi: 10.1128/JCM.03005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singhal R, Arora J, Lal P, Bhalla M, Myneeedu VP, Behera D. Comparison of line probe assay with liquid culture for rapid detection of multidrug resistance in Mycobacterium tuberculosis. Indian J Med Res. 2012;136(6):1044–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Madhuri K1, Deshpande S1, Dharmashale S1, Bharadwaj R1. Utility of Line Probe Assay for the Early Detection of Multidrug-Resistant Pulmonary Tuberculosis. J Glob Infect Dis. 2015;7(2):60–5. doi: 10.4103/0974-777X.157237. doi: 10.4103/0974-777X.157237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. [Last accessed on 2017 Apr 23]. Available from: http://apps.who.int/iris/bitstream/10665/44597/1/97892415015 83_eng.pdf .
- 29. [Last accessed on 2017 Apr 23]. Available from: http://www.who.int/tb/areas-of-work/drugresistant-tb/MDRTBguidelines2016.pdf .



