Abstract
Bitter taste receptors (T2Rs) are G-protein coupled transmembrane proteins initially identified in the gustatory system as sensors for the taste of bitter. Recent evidence on expression of these receptors outside gustatory tissues suggested alternative functions, and there is growing interest of their potential role in cancer biology. In this study, we report for the first time, expression and functionality of the bitter receptor family member T2R10 in both human pancreatic ductal adenocarcinoma (PDAC) tissue and PDAC derived cell lines. Caffeine, a known ligand for T2R10, rendered the tumor cells more susceptible to two standard chemotherapeutics, Gemcitabine and 5-Fluoruracil. Knocking down T2R10 in the cell line BxPC-3 reduced the caffeine-induced effect. As possible underlying mechanism, we found that caffeine via triggering T2R10 inhibited Akt phosphorylation and subsequently downregulated expression of ABCG2, the so-called multi-drug resistance protein that participates in rendering cells resistant to a variety of chemotherapeutics. In conclusion, T2R10 is expressed in pancreatic cancer and it downmodulates the chemoresistance of the tumor cells.
Keywords: Pancreatic cancer, bitter receptors, chemoresistance, caffeine
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer deaths with a 5-year survival rate of approximately 7% 1. Due to late occurrence of symptoms and thus often progressed disease, only a minor percentage of PDAC patients is suitable for potential curative surgery 2. Therefore, chemotherapy often remains the last treatment option 2. However, its effectiveness is limited because pancreatic cancer exhibits numerous mechanisms of resistance towards a wide variety of drugs 3, 4. Thus, understanding resistance on a molecular basis is imperative for the development of novel therapeutic targets to restore drug efficacy and to overcome chemoresistance. A plethora of synthetic and natural compounds has been tested so far, often in combination with conventional chemotherapy, to improve chemotherapeutic regimen for malignancies, including PDAC 5-9. In this context, bitter tasting substances and the family of their corresponding receptors came into focus.
Bitter taste receptors (T2R) are classical G-protein coupled seven transmembrane-domain receptors that respond to ligation with various bitter compounds of foods 10-13, such as sinigrin in vegetables 14, caffeine and epigallocatechin-3-gallate in green tea 15, of drugs such as erythromycin 12, or metabolites of the microbiome 16. In humans, the family of T2R is composed of 25 functional receptors 11. Based on initial description of these receptors in the oral cavity, it was originally assumed that they mediate exclusively the perception of bitter taste. However, recent studies described the presence of these receptors in non-gustatory cells, including airway epithelia 17, breast epithelia 18, gastro-intestinal cells 19, placental 20, brain 21, or myeloid cells 16, suggesting functions apart from sensing bitter. Sensing bacterial-derived products and thus participating in the local host response has been suggested for receptors expressed on epithelial cells 17, 22 or myeloid cells 16. Recent reports described a functional role of T2R10 as bronchodilator and vasomodulator in smooth muscle cells, because activation by bitter agonists causes changes in Ca2+ oscillations that resulted in relaxation of smooth muscle cells 23-26. Moreover, a potential role in cancer biology has been shown recently for the family member T2R38 in pancreatic cancer; its stimulation upregulates the multi-drug-resistance-protein ABCB1, a major player in the induction of chemoresistance 27. On the other hand, bitter compounds can also display anticancer or chemotherapy enhancing activities, whereby the exact mechanisms are often still unknown 28-32. Most of the bitter receptors are promiscuous, and - by not yet well-understood mechanisms - can be triggered by different chemically not related substances. One of those receptors is T2R10, which reacts with caffeine, coumarin, erythromycin, and haloperidol 12, but its role beyond sensing bitter is unexplored. A cancer-related expression of T2R10 has been shown in breast cancer cell lines, although a function has not been described yet 18.
The aim of the present study was to evaluate the expression of T2R10 receptor in PDAC tissue and in tumor derived cell lines (AsPC-1, BxPC-3, Capan-1, COLO-357, MiaPaCa-2, SU.86.86, PANC-1, and T3M4), and to investigate a functional role of this receptor in the context of chemoresistance.
Materials and Methods
Chemicals, Reagents, and Antibodies
Caffeine (Sigma Aldrich, St. Louis, MO, USA) was dissolved in distilled water, Gemcitabine (Eli Lilly & Co., Indianapolis, IN, USA) in phosphate buffered saline (PBS; Sigma Aldrich), and 5-Fluorouracil (5-FU; Sigma Aldrich) in Dimethylsulfoxide (DMSO; Sigma Aldrich). For Western blot analysis the following antibodies were used: anti-ABCG2 (EPR2099, abcam); anti-Phospho-Akt Ser473 (D9E, Cell Signaling, Cambridge, UK), anti-pan-Akt (C67E7, Cell Signaling); anti-GAPDH (14C10, Cell signaling); anti-T2R10 (orb 164582; Biorbyt, Cambridge, UK); anti-tubulin (sc-58886, Santa Cruz); goat anti-rabbit IgG-horse radish peroxidase (HRP; Santa Cruz) as secondary antibody. For flow cytometry, a Fc Blocking Reagent (human, Miltenyi Biotec, Bergisch-Gladbach, Germany) was used; anti-T2R10 (ab138285, abcam, Cambridge, UK), a rabbit polyclonal isotype control (abcam), and anti-rabbit-IgG conjugated with phycoerythrin (PE) (Jackson Immunoresearch, West Grove, PA, USA).
Cell Culture
The pancreatic cancer cells AsPC-1, BxPC-3, Capan-1, COLO-357, MiaPaCa-2, SU.86.86, PANC-1, and T3M4 were obtained from ATCC (Rockville, MD, USA). All cells were cultured in RPMI 1640 supplemented with 10% calf serum and 1% penicillin-streptomycin all from Life Technologies (Darmstadt, Germany). Cells were maintained in a humidified atmosphere with 5% CO2 at 37°C. Accutase (Life Technologies) was used for detaching cells.
Patients and Biopsies
Pancreatic tissue samples were obtained from the Pancobank of the European Pancreas Center, Department of Surgery, University Hospital Heidelberg or the tissue bank of the National Center for Tumor Diseases (NCT, Heidelberg, Germany) in accordance with the regulations of the tissue bank. The histological examination of formalin fixed, paraffin embedded and H&E stained pancreatic tissue sections was performed at the Pathology Department University of Heidelberg. The study was approved by the ethics committee of the University of Heidelberg (approval votes no. 301/2001, 159/2002, 206/2005), and a written informed consent was obtained from all patients. Patient demographics and clinical data are summarized in table 1.
Table 1.
Patients data
| diagnosis | pancreatic ductal adenocarcinoma | number of patients (62) |
|---|---|---|
| gender (female : male) | 30 : 32 | |
| age (years) | 39-83 (mean: 65.5; median: 65.5) | |
| localization of the tumor | head | 47 |
| body | 14 | |
| tail | 1 | |
| tumor size *pT | pT1 (smaller 2 cm): | 9 |
| pT2 (between 2 and 4 cm): | 31 | |
| pT3 (larger than 4 cm): | 22 | |
| lymph node metastases *pN | pN0: no regional lymph node metastases | 7 |
| pN1: metastases in 1 to 3 regional lymph nodes | 27 | |
| pN2: metastases in 4 or more regional lymph nodes | 28 | |
| *pM | pM0: no distant metastases | 54 |
| pM1: distant metastases | 8 | |
|
histological grading (growth pattern) |
G1: well differentiated | 4 |
| G2: moderately differentiated | 39 | |
| G3: poorly differentiated | 18 | |
| one patient excluded because of neoadjuvant therapy | ||
| neoadjuvant chemotherapy | received | 1 |
| not received | 61 | |
| resection margin | more than 1 mm from resection margin | 10 |
| less than 1 mm from resection margin | 46 | |
| no data | 6 |
*Pathological evaluation according to the guidelines of the Union for International Cancer Control 2017
Tissue staining
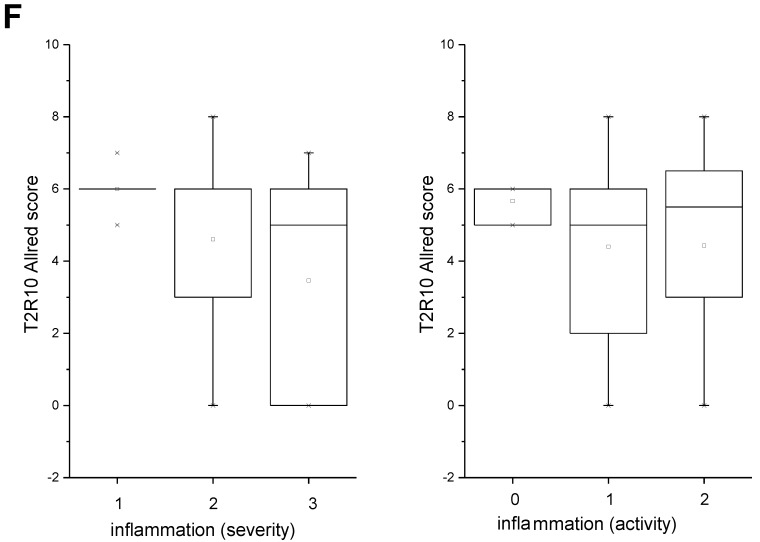
After deparaffinization and rehydration of the tissue in xylene and graded alcohols, an anti-human T2R10 antibody (Thermo Scientific, 1:400, retrieval condition: citrate buffer, pH 6.0) was incubated for 1 hour at room temperature. As secondary antibody the DCS Detection Line Super Vision Red2 system (Innovative Diagnostik-Systeme, Hamburg, Germany) was used, followed by the color reaction with liquid permanent red (Zytomed, Berlin, Germany) and counter stain with hematoxylin. Presence of T2R10 was evaluated using an established scoring system in which the sum of the staining intensity (0: absent; 1: weak; 2: moderate; 3 strong) and distribution (0: absent; 1: 0 - 1 %; 2: > 1 - 10%; 3: > 10 - 33%; 4: > 33 - 66 %; 5: > 66 - 100%) was calculated (Allred 33). The intratumor inflammation was determined on whole tumor sections by evaluating the accumulation of inflammatory cells (lymphocytes, plasma cells, macrophages). Severity was determined as absent (score 0), mild (score 1), moderate (score 2), and severe (score 3). Activity of inflammation was judged by the density of neutrophils, and scored as absent (score 0), mild (score 1), and moderate to severe (score 2), using an established scoring system for pancreatic cancer 34. Infiltrated T cells were identified by expression of CD3 (antibody obtained from Thermo Scientific, Darmstadt, Germany).
Gene expression Analysis
The mRNA data of T2R10 expression in PDAC tumors (n=84) was obtained from the database of the Heidelberg Institute of Personalized Oncology (HiPO) biobank. Tumor samples with reads per kilobase per million mapped reads (rpkm) ≥ 1 for T2R10 were defined as T2R10 positive 35. Follow-up data were available in 46 cases. Kaplan-Meier analysis was used to determine survival and differences between patients with T2R10 negative (<1.0 rpkm) (n=8) and T2R10 positive PDAC tumors (≥1.0rpkm) (n=38) were calculated using Rank correlation.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated using Magna-Pure LC RNA Isolation Kit High Performance (Roche lifescience, Indianapolis, USA) according to the manufacturer's instructions. cDNA was then generated with the 1st Strand cDNA Synthesis Kit from Roche Diagnostics and used as a template for polymerase chain reaction (PCR). The PCR was carried out in a 25 µl reaction mixture containing 12.5 µl Read Tag Mix Ready Use (Sigma Aldrich), 0.4 µM Primer, 8 µl nuclease-free water (Sigma Aldrich) and 2 µl cDNA. T2R10-Primer sequences were as follows: forward GACTTGTAAACTGCATTGACTGTGCC, and reverse AAAGAGGCTTGCTTTAGCTTGCTG. For GAPDH (glycerin aldehyde-3-phosphate dehydrogenase) used as control the forward primer was GCCAAAAGGGTCATCATCTC and reverse primer GTAGAGGCAGGGATGATGTTC. The PCR reaction consisted of 10 minutes initial denaturation at 94°C following for 50 cycles of 30 seconds denaturation (94°C), 30 seconds annealing (60°C), 30 seconds extension (72°C) and a final extension for 2 minutes at 72°C. Products were separated on a 1.5% agarose gel stained with ethidium bromide. Fusion-SL (Vilber, Eberhardzell, Germany) was used for capturing gel images under UV light. For control, reactions were performed using water instead the sample, or without master mix (polymerase, primer).
Flow Cytometry
For flow cytometry analyses ~1x106 cells were harvested with Accutase (Life technologies, Darmstadt, Germany) and resuspended in 100 µl flow cytometry buffer (FACS buffer: PBS; 0.5% FCS) followed by fixation in ice-cold Methanol (80%) for 20 minutes at 4°C. Then, cells were washed with flow cytometry buffer and permeabilized with PBS plus 1% Tween 20 (Gerbu Biotechnik, Heidelberg, Germany) for 20 minutes at room temperature. After washing with FACS-buffer containing 0.1% Tween 20, cells were pre-incubated with 10 µl Fc Blocking Reagent (Becton and Dickinson, Heidelberg, Germany) for 15 minutes. Then the primary antibody against T2R10 (ab138285, abcam, Cambridge, UK) (20 ng/ml) and IgG isotype control (20 ng/ml) were incubated for 35 minutes, respectively. Cells were washed and incubated with a PE-labeled secondary antibody (5 ng/ml) for 20 minutes protected from light. Finally, cells were washed and resuspended in 300 µl buffer. All incubation steps were performed at room temperature. BD FACS Canto 2 was used for detection. Results were analyzed with FlowJo 10.0.8 software (Tree Star, Ashland, OR, USA). The mean fluorescence intensity (MFI) was used to show expression. In the knock down experiments, the MFI of cells treated with siRNA negative control was defined as 100%. Knockdown efficacy was expressed as percentage decrease of MFI.
Transfection
siRNA Select against T2R10 and siRNA negative control Select 1 were purchased from Invitrogen (Carlsbad, CA, USA). Reverse transfection with 10 nM siRNA in the presence of Hiperfect (Qiagen, Hilden, Germany) was performed according to the manufacturer's instructions. 200,000 cells were seeded and cultured for 48 hours before performing a retransfection. Another 48 hours later siRNA knock down efficacy of T2R10 was assessed by flow cytometry.
Drug Treatment
For Western blot (WB) analysis and crystal violet assays 200,000 cells in 2 ml medium were seeded in 6-well plates and for MTS assays 5,000 cells with 100 µl were seeded in 96-well plates. Cells were cultured for 96 hours and treated according to the protocol shown in supplementary Fig. 1A. Caffeine (dotted lines) was added to the medium at 12, 24, 48, 72, and 84 hours after seeding, each time at a concentration of 100 µM or 200 µM, respectively. These concentrations were not toxic for the tumor cells. A single dose of chemotherapeutic drug (solid line) was administered 24 hours after seeding. BxPC-3 cells were treated with either 5 nM Gemcitabine or 500 nM 5-FU and PANC-1 cells with 500 nM Gemcitabine or 2 µM 5-FU. Sensitivity to drugs (chemoresponse assays) and cell harvesting for further protein analyses were performed 72 h post drug exposure. For T2R10 knockdown experiments, cells were reversely transfected with siRNA, with treatment starting point delayed by 12 hours. These cells were cultured for a total of 108 hours as shown in supplementary Fig. 1B. T2R10 knockdown cells used for protein lysates were retransfected with siRNA 48 hours after seeding as shown in supplementary Fig 1C.
Chemoresponse assays
Two ml medium containing 200,000 cells per well were seeded in 6-well plates and treated with either caffeine, Gemcitabine, 5-FU, or in combination according to the scheme presented above in supplementary Fig 1A. BxPC-3 cells were treated with 5 nM Gemcitabine or 500 nM 5-FU, and PANC-1 cells with 500 nM Gemcitabine or 2 µM 5-FU, respectively. Five applications of either 100 µM or 200 µM caffeine were added to the medium. On day 0 and day 3, a crystal violet assay was performed. 1 ml of 0.05% crystal violet in 20% ethanol was added to each well. Cells were stained for 20 min at room temperature. Crystal violet was rinsed using cold tap water and plates were dried overnight. The next day, 1 ml of methanol per well was added to elute the stained crystals from the cells. Plates were placed on a horizontal shaker for 20 min and absorbance was measured at λ = 595 nm. Untreated cells were used for normalization. The optical densities 36 were normalized to day 0 (0% growth) and day three (100% growth).
To test for cytotoxicity, the IC50 was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assays. 5,000 cells were seeded in 100 µl medium per 96-well. MTS measurements were performed on day 0 and day 3. 10 µl of MTS CellTiter 96 Aqueous One Solution (Promega, Wisconsin, USA) was added to each well. After 2 hours incubation at 37°C absorbance was measured at 490 nm using Synergy HTX Multi-Mode-Reader (Biothek, Wörrstadt, Germany). For normalization optical density values 36 obtained on day 0 were defined as 0% growth. ODs of cells not treated with chemotherapy were measured on day 3 were defined as 100% growth. This was done separately for the control (PBS) and caffeine treatment group. To assess synergism, the combined drug intoxication (CDI) was calculated 37.
Western Blot Analysis
After the respective treatments (Supplementary Fig. 1A, 1C), cells were washed twice with PBS (Sigma Aldrich), transferred in RIPA lysis buffer (Santa Cruz) and sonicated (Bandelin Berlin, Germany) for 45 seconds on ice, to support cell lysis, DNA fragmentation and cell membrane disintegration. Protein concentration was measured using BCA Protein Assay Kit 38 according to the manufacturer's instructions. Samples were mixed with Sample Buffer and Reducing Agent (Invitrogen) and separated on a 4-12% Bis-Tris-Gel (Invitrogen). After blotting, the membranes were blocked in 5% milk powder dissolved in Tris-buffered saline with 0.1% Tween20 (TBST) for one hour at room temperature. Primary antibodies were incubated overnight at 4°C. The next day membranes were washed three times with TBST and once with TBS, for 10 minutes and subsequently incubated with secondary antibodies conjugated to horseradish peroxidase for one hour at 4°C. After washing three times, the horseradish peroxidase reaction was visualized with ECL solution (SuperSignal West Dura Extended Duration Substrate, Thermo Fischer Scientific). WB images were captured using Fusion-SL (Vilber). Band intensities were quantified with Image J Software (NIH, Bethesda, USA). They were normalized to GAPDH in the case of ABCG2 and to pan-Akt for phosphorylated-Akt. Protein levels in the control group were defined as 100%. For each experimental condition, at least three blots were done.
Statistical Analysis
IC50 doses were calculated using Graph Pad Prism Software 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Statistical significance of data obtained from proliferation assays was tested by running extra-sum-of-squares F tests and One-way-Anova followed by Dunnett post-hoc- tests. Statistical analysis of expression data was performed with the appropriate tests for the clinical correlations (Mann-Whitney-U) and Log-Rank-survival test.
Results
T2R10 is expressed in human PDAC tissue and human pancreatic cancer cell lines
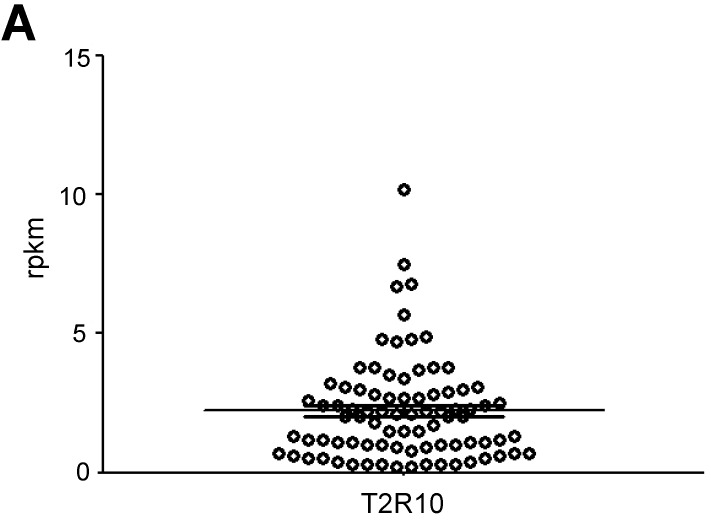
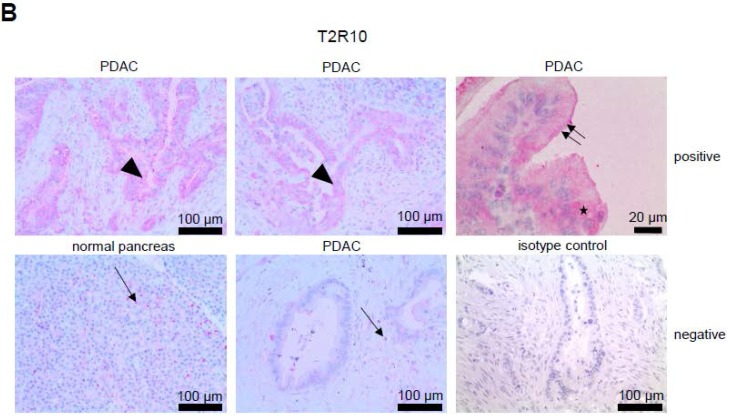
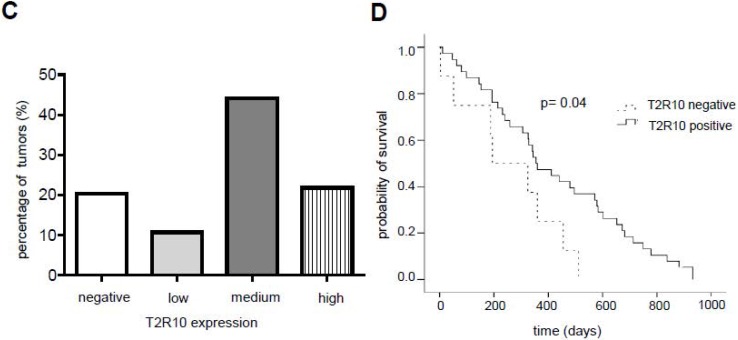
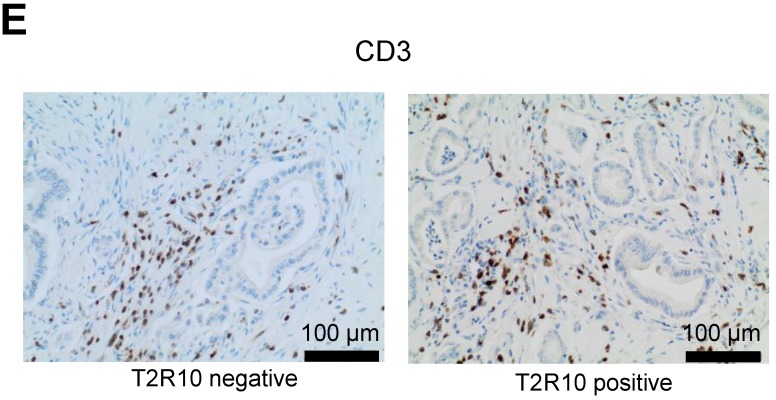
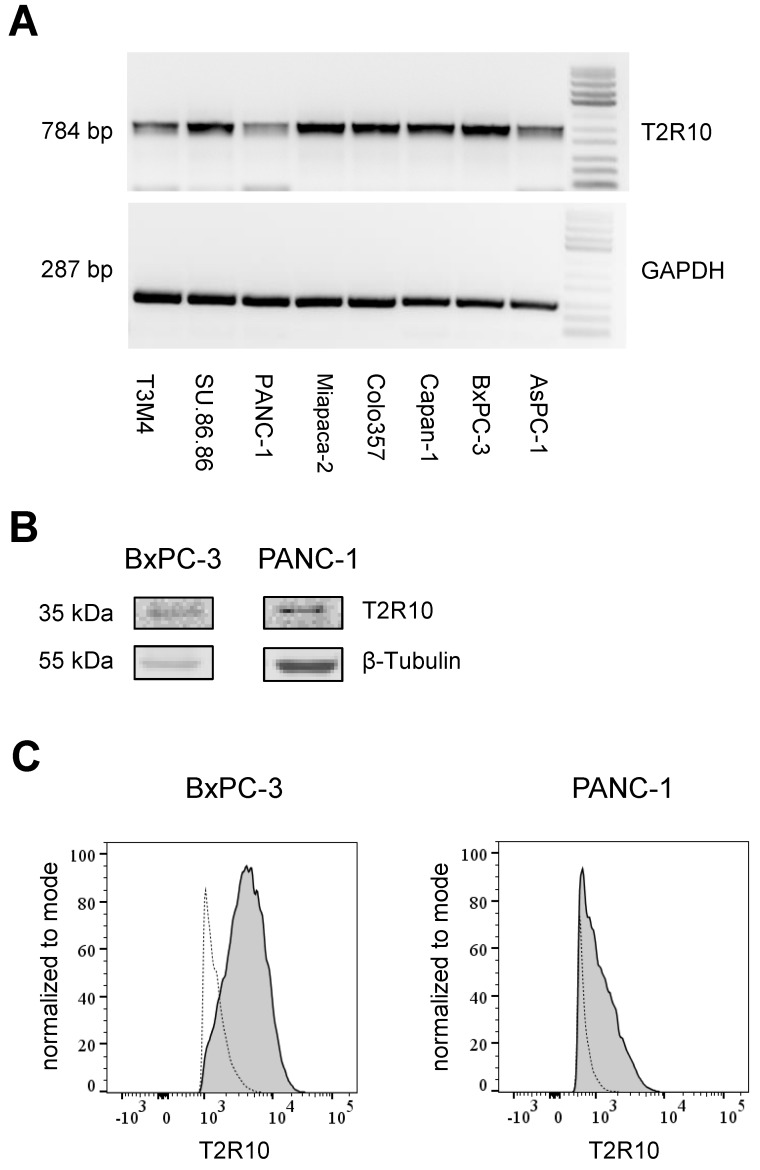
T2R10 is expressed in primary human pancreatic tumor tissues. According to the data provided by the HiPO biobank, T2R10 mRNA was found in 63 of 84 tumors (75%) (Fig. 1A). For detection of T2R10 on protein level, a second cohort of 62 tissue samples from patients with pancreatic ductal adenocarcinoma was analyzed by immunohistochemistry (data of patients summarized in table 1). Here, 79% of the tumors expressed T2R10. Low expression was seen in 11.3 % of the patients, medium expression in 45.2 %, and high expression in 22.6 % (examples in Fig. 1B; data summarized in Fig. 1C). T2R10 was located on the cell surface and in the cytoplasm as well. T2R10 expression did not correlate to pathological parameters including TNM stage, histopathological grading, and R-status. There was, however, a trend towards higher survival rate in patients with high T2R10 expression (Fig. 1D). Moderate to severe intratumor inflammation was seen in the majority of patients, as was infiltration of T cells (example in Fig. 1E). T2R10 expression did not correlate with severity nor activity of inflammation, although there might be a trend towards reduced expression of T2R10 in specimen with high inflammatory activity (Fig. 1F). Normal pancreatic tissue reveals single cellular weak T2R10 staining in acinar cells and ductal epithelial cells. Eight human pancreatic cancer cell lines, Aspc-1, BxPC-3, Capan-1, COLO-357, Miapaca-2, PANC-1, Su.86.86, and T3M4 were tested for expression of T2R10 by mRNA by reverse transcription polymerase chain reaction. All cell lines showed T2R10 transcripts (Fig. 2A). For further studies two of these cell lines, BxPC-3, expressing high T2R10, and PANC-1 with low T2R10 (Fig. 2B and 2C) were selected. BxPC-3 and PANC-1 differed with regard to their sensitivity towards chemotherapy, with PANC-1 being more resistant 39, and in our hands a 100-times higher concentration of Gemcitabine was required to cause cell death in ranges similar to BxPC3.
Figure 1.
T2R10 expression in PDAC tissue. A RNA Sequencing data showing T2R10 transcripts in PDAC tumor samples of 84 patients (Data are presented as quantified transcript levels in reads per kilobase of exon model per million mapped reads (rpkm). B Representative tissue specimen of PDAC tumors from three patients (upper panel) and of normal pancreas. T2R10 was detected in tumor cells (arrowheads) and immune cells (arrows). High magnification (upper panel, right) shows expression of T2R10 on the cell membrane and in the cytoplasm (asterisk). C Tumor samples (n=62) were categorized according to their T2R10 expression using an immunoreactivity score; negative= score 0, low= score 2-3, medium= score 4-6, high= score 7-8. D Kaplan-Meier survival analysis of PDAC patients with T2R10 negative (n=8) and T2R10 positive PDAC tumors (n=38) E Infiltrated T cells, identified by expression of CD3 are shown; F Inflammation severity scores (0-3) and activity scores (0-2) were related to T2R10 expression, the latter quantified using Allred score. The groups did not differ from each other.
Figure 2.
T2R10 expression in tumor derived cell lines. A Agarose gel electrophoresis analysis of PCR products. T2R10 mRNA was determined in eight pancreatic tumor cell lines. B T2R10 expression was assessed by Western blotting the cell lines BxPC3 and PANC-1 C Flow cytometry analysis was used to detect T2R10 surface expression in BxPC-3 and PANC-1 cell lines (bold line indicates binding of anti-T2R10, dotted line is IgG isotype control).
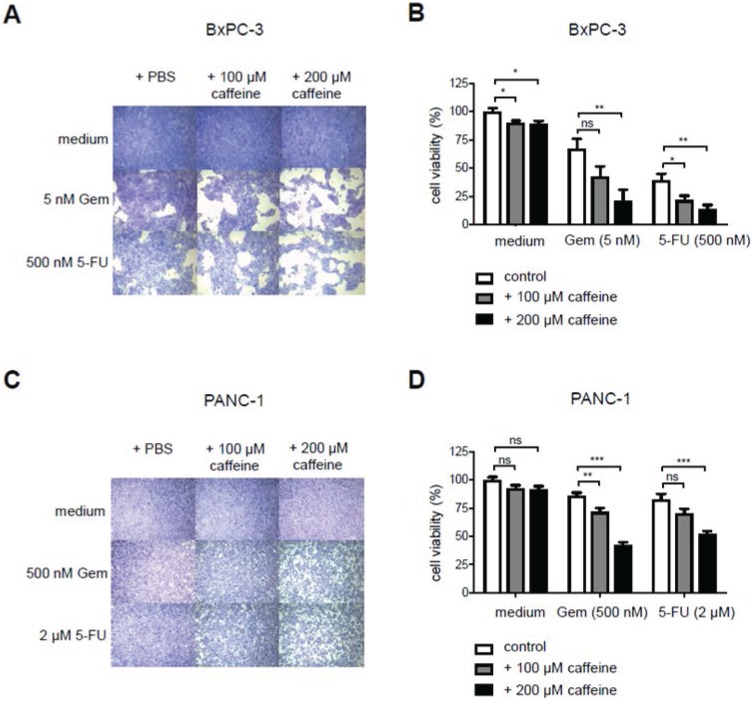
Caffeine increases Gemcitabine or 5-FU-induced cell death
To test for T2R10-mediated effects on the chemoresponse of BxPC-3 and PANC-1, caffeine, a known and widely used T2R10 agonist 12, was used, and sensitivity of the tumor cells towards either Gemcitabine or 5-FU was tested (protocol in supplementary Fig. 1A). Caffeine alone reduced the viability of the cells slightly. Hundred µM caffeine, however, enhanced the 5-FU-induced cell death by 17% (p<0.05) in BxPC-3 (Figs. 3A,B), and the Gemcitabine-induced cell death by 14% (p<0.01) in PANC-1 cells (Figs. 3C,D). When 200 µM caffeine was added to BxPC-3, cell viability in the combinational treatment subset was reduced by 46% as compared to Gemcitabine alone (p< 0.01) and 25% (p<0.01) compared to 5-FU alone (Figs. 3 A,B). Similarly, in PANC-1, cells treated with Gemcitabine and 200 µM caffeine or 5-FU and 200 µM caffeine showed a significant decrease in cell viability (34% for Gemcitabine; p< 0.001) and 31% for 5-FU (p< 0.001) (Figs. 3C,D), when compared to cells exposed to chemotherapeutic treatment only.
Figure 3.
Caffeine increases chemo-responsiveness in pancreatic tumor cells. A Crystal violet staining of BxPC3 exposed to either PBS, caffeine, gemcitabine (Gem), 5-Fluorouracil (5-FU), or combined treatment. B shows the quantification of these data (open bar: PBS; grey bar 100 µM caffeine; black bar 200 µM caffeine). C,D show the same experimental set up for PANC-1. (Values were normalized to the control group treated with PBS. Data are presented as mean +/- SEM of three independent experiments, each performed in triplicates. Statistical significance was assessed with one-way Anova analysis followed by Dunnett post-hoc test. (*p<0.05, **p<0.01, ***p<0.001)
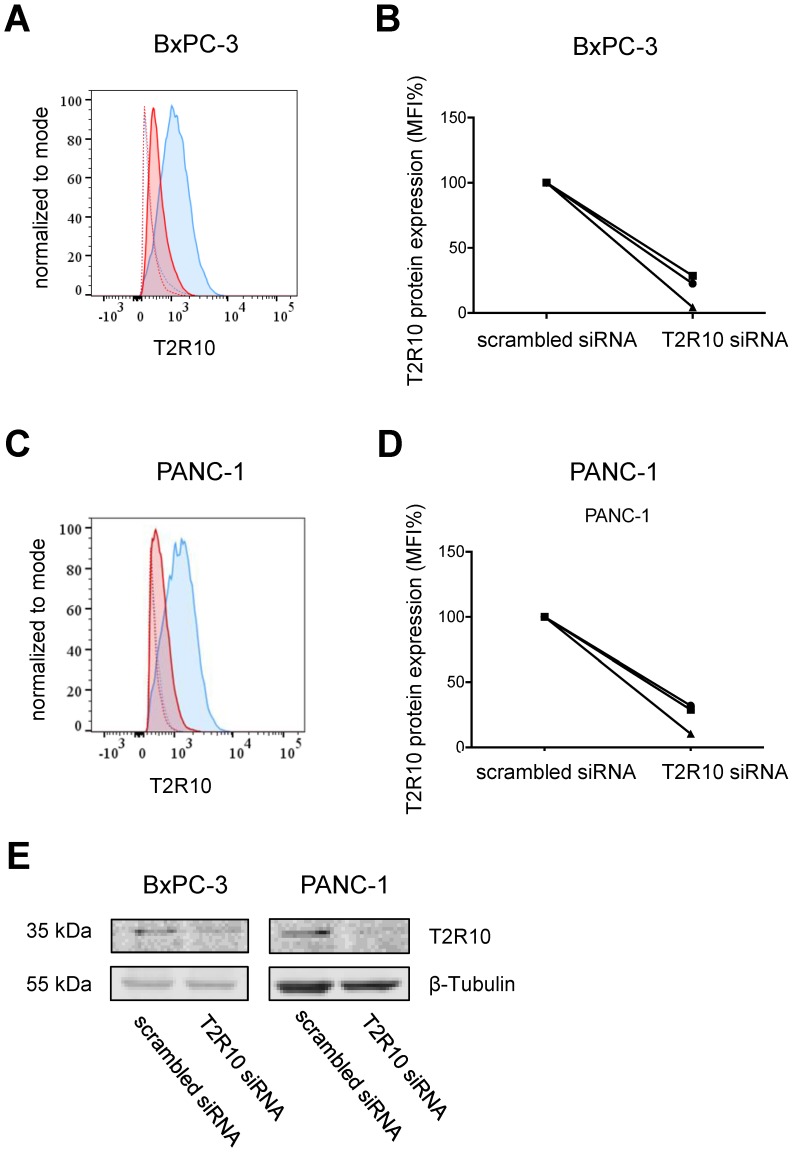
To test whether the caffeine mediated-effect was dependent on T2R10, the receptor was knocked-down by transient transfection with siRNA. As measured by flow cytometry, protein expression was reduced on average by 85% in BxPC-3 cells, and 80% in PANC-1 cells when compared to cells treated with scrambled siRNA (mean of three experiments; Figs. 4A-D). Reduced expression of T2R10 could be confirmed by Western blotting (Fig. 4E).
Figure 4.
siRNA knockdown of T2R10. Flow cytometry analysis was used to determine surface expression of T2R10 in BxPC-3 (A) and PANC-1 cells (C) transfected with either scrambled siRNA (blue line) or siRNA targeted against T2R10 (red line). Thick lines represent antibody binding to T2R10 and dotted lines isotype control. B,D Quantification of the mean fluorescence intensity (MFI) indicates a reduction of T2R10 expression in BxPC-3 and PANC-1 (three independent experiments are shown; MFI for cells treated with scrambled siRNA was set as 100%). E Western blot analysis confirmed silencing of T2R10 on protein level
Cells were then treated with either Gemcitabine or 5-FU at various concentrations, and caffeine (200 µM) or PBS (control) (supplementary Fig. 1). In BxPC-3 cells, the treatment with 200 µM caffeine reduced the IC50 of Gemcitabine and 5-FU by 77% (p < 0.05) and 53% (p < 0.01), respectively (Figs. 5A,B), but in T2R10 knock-downs only by 37%, suggesting that the caffeine effect was at least in part mediated by T2R10. As shown before, PANC-1 cells became more susceptible to either Gemcitabine (63%; p < 0.01) or 5-FU (37%; p < 0.01) (Fig. 5C,D). Silencing T2R10, however, had no apparent effect. For both cell lines BxPC-3 and PANC-1, a synergistic effect of caffeine was detected (Table 3 and 4).
Figure 5.
Combined treatment with caffeine reduces IC50 of Gemcitabine and 5-FU. BxPC-3 (left panel) and PANC-1 (right panel) were treated with various concentrations of Gemcitabine (upper panel) or 5-FU (lower panel), with or without caffeine (200 µM).Compared are cells silenced for T2R10 expression with those transfected with scrambled siRNA. MTS assay was used to determine the number of viable cells after treatment. Values were normalized to the groups treated neither with Gemcitabine nor 5-FU. Each data point represents the mean +/- SEM of at least three independent experiments, each of them performed in triplicates.
Table 3.
Synergistic effect of caffeine and gemcitabine or 5-FU-induced cytotoxicity in BxPC-3 cells
| CDI value | CDI value | |||||
|---|---|---|---|---|---|---|
| Gem (nM) | scrambled siRNA | T2R10 siRNA | 5-FU (nM) | scrambled siRNA | T2R10 siRNA | |
| 1 | 0.95 | 1.02 | 125 | 0.93 | 1.03 | |
| 2 | 0.95 | 0.97 | 250 | 0.93 | 1.00 | |
| 3 | 0.93 | 0.92 | 500 | 0.91 | 0.94 | |
| 4 | 0.85 | 0.94 | 1000 | 0.89 | 0.94 | |
| 5 | 0.76 | 0.85 | 2000 | 0.85 | 0.90 | |
| 6 | 0.66 | 0.82 | 3000 | 0.86 | 0.92 | |
| 7 | 0.73 | 0.79 | 4000 | 0.86 | 0.88 | |
| 8 | 0.73 | 0.75 | 5000 | 0.87 | 0.89 | |
Table 4.
Synergistic effect of caffeine and gemcitabine or 5-FU-induced cytotoxicity in PANC-1 cells
| CDI value | CDI value | |||||
|---|---|---|---|---|---|---|
| Gem (nM) | scrambled siRNA | T2R10 siRNA | 5-FU (nM) | scrambled siRNA | T2R10 siRNA | |
| 1 | 1.09 | 1.00 | 1 | 1.02 | 1.04 | |
| 10 | 1.03 | 1.02 | 10 | 1.04 | 1.07 | |
| 100 | 0.94 | 0.88 | 100 | 1.03 | 1.05 | |
| 1000 | 0.94 | 0.90 | 1000 | 1.00 | 1.02 | |
| 5000 | 0.95 | 0.89 | 2500 | 0.96 | 1.00 | |
| 10000 | 0.95 | 0.93 | 5000 | 0.93 | 0.95 | |
| 50000 | 0.96 | 0.91 | 10000 | 0.94 | 0.96 | |
| 100000 | 0.93 | 0.88 | 100000 | 0.92 | 0.91 | |
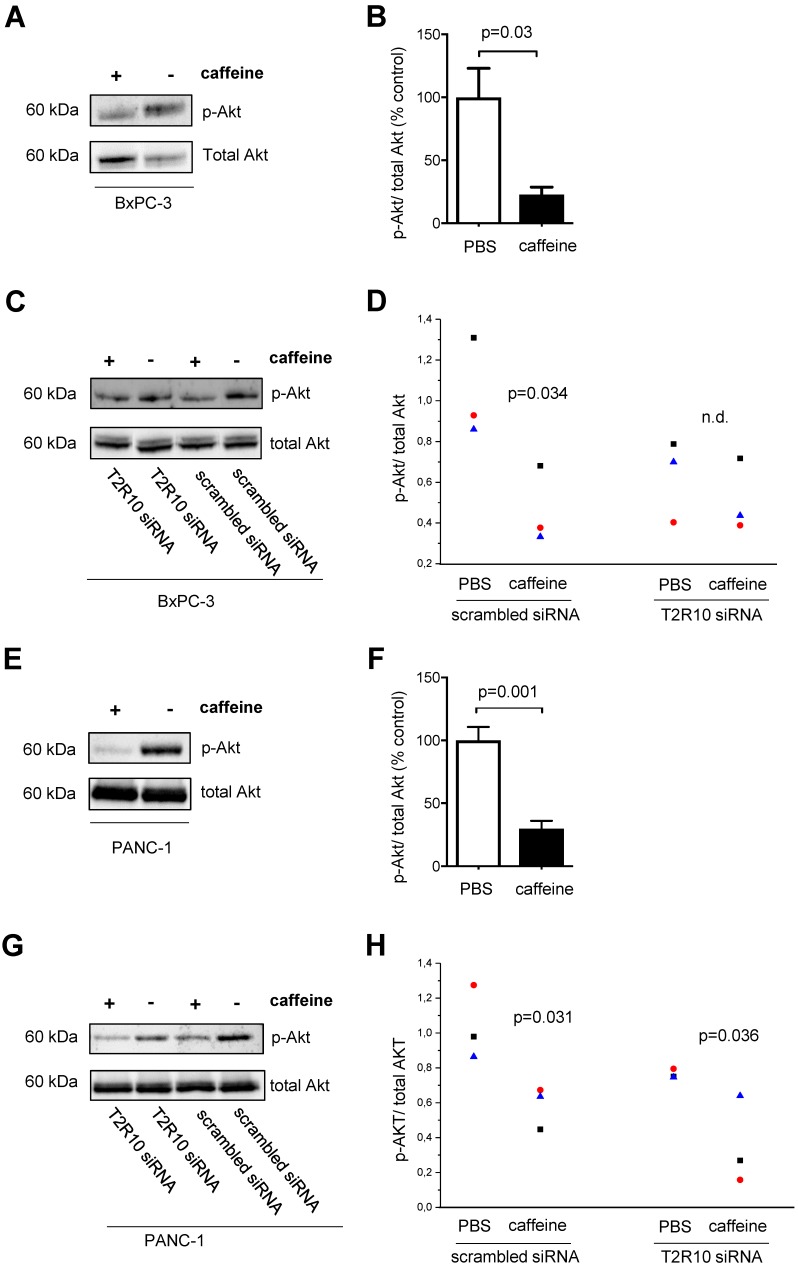
Caffeine treatment inhibits Akt phosphorylation at Ser473
T2R10 agonists such as chloroquine and quinine have been shown to inhibit Akt phosphorylation 40. Because activation of Akt plays a crucial role in the chemoresponse of pancreatic tumor cells 41-43, we tested whether caffeine reduced Akt-phosphorylation in BxPC-3 and PANC-1 cells. By Western blotting, the ratio of phosphorylated (activated) Akt to total Akt was determined. In both, BxPC-3 and PANC-1, caffeine significantly suppressed the phosphorylation of Akt compared to the control group (PBS) on average of 77% and 70% respectively (Figs. 6A,B and 6E,F). Silencing of T2R10 prevented the caffeine- mediated inhibition of Akt phosphorylation in BxPC-3 (Figs. 6C,D), whereas for PANC-1 still an effect, though somewhat reduced was seen (Figs. 6G,H).
Figure 6.
Caffeine inhibits Akt-phosphorylation. BxPC-3 (upper panel) and PANC-1 (lower panel) cells were grown in the presence of serum for 96 hours or for 108 hours in the case of siRNA transfected cells. During this incubation period, five doses of 200 µM caffeine were accumulatively added to the medium according to the scheme shown in supplementary Fig. 1A and 1C. Akt activation was measured as ratio between phosphorylated Akt (pAkt) and total Akt. A and E show Western blots and B and F the respective quantification (summary of three experiments). C and G show Western blots of cells silenced for expression of T2R10 (T2R10 siRNA) or for comparison cells treated with scrambled siRNA, all cultivated either in the presence of absence of caffeine. D and H show the respective quantification data (summary of three experiments as indicated by the different symbols (statistical differences between the groups were calculated by ANOVA).
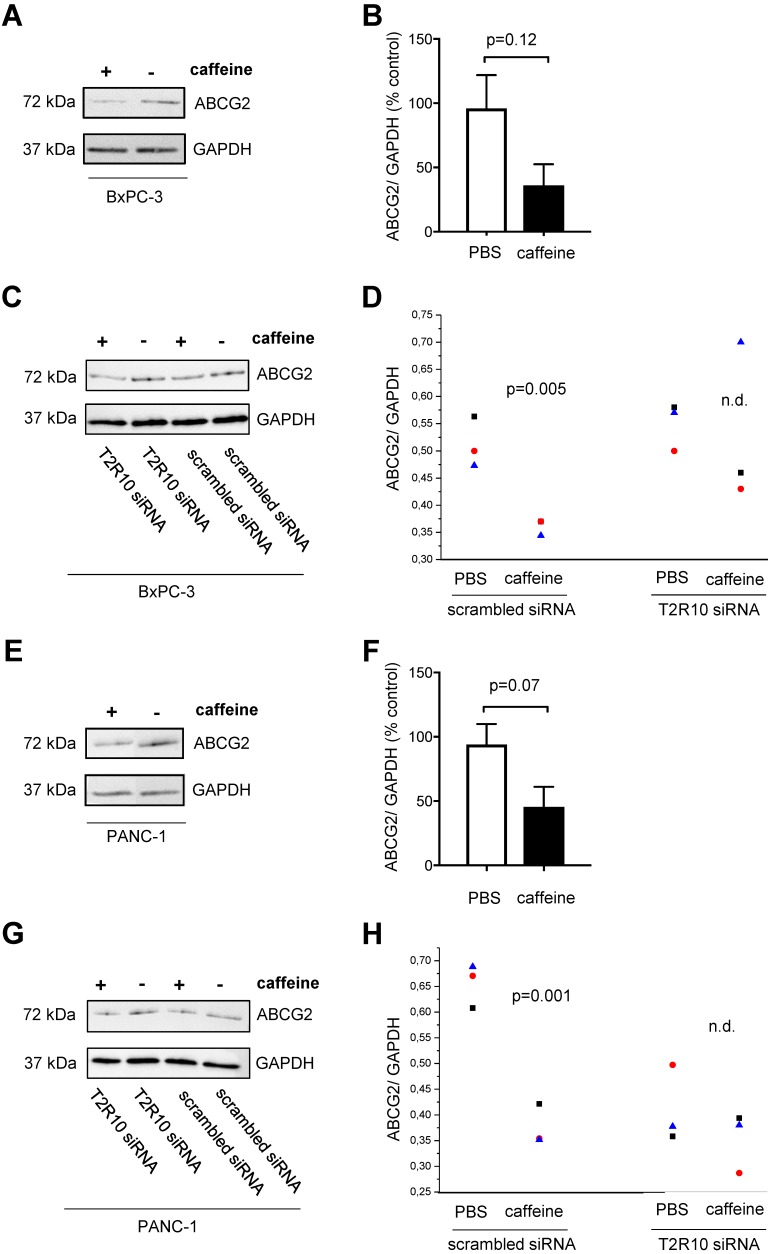
Caffeine downregulates ABCG2 protein expression
One of the downstream targets of Akt is ABCG2 44, 45, which is crucially involved in multidrug resistance 46-49. Following exposure to caffeine, ABCG2 protein expression was reduced in both cell lines (data summarized in Fig. 7). Again, silencing of T2R10 abrogated the effect of caffeine.
Figure 7.
Caffeine down-regulates ABCG2 on protein expression. BxPC-3 (upper panel) and PANC-1 (lower panel) were grown in the presence of serum for 96 hours or 108 hours in the case of siRNA transfected cells. During this incubation period, five doses of 200 µM caffeine were accumulatively added to the medium according to the scheme shown in supplementary Fig. 1A and 1C. Expression of ABCG2 was measured by Western blotting (A,E) and quantified as ratio of ABCG2 to GAPDH (B,F; summary of three experiments). C,G show cells silenced for expression of T2R10 (T2R10 siRNA) and for comparison cells treated with scrambled siRNA cultivated with or without caffeine. D,E Data from three independent Western blots are shown (groups were compared using ANOVA).
Discussion
Despite an increasing understanding of the molecular mechanisms underlying pancreatic cancer, the therapeutic options are still limited. Hence, also unconventional or unattended targets come into the focus of research, among those bitter-tasting food compounds, such as bitter melon extract, epigallocatechin-3-gallate, or caffeine, which have some anticancer activities 28-32. All these substances act through specialized bitter receptors, and enhance the chemotoxicity of anticancer drugs, whereby the exact molecular mechanisms have not been fully elucidated as yet 28-32. Because chemoresistance to numerous chemotherapeutics is a major problem in the therapy of pancreatic cancer 3, 4, we addressed the question whether pancreatic cancer cells expressed a functional bitter receptor for caffeine, and whether signaling via this receptor would affect chemoresistance of a given tumor cell. Caffeine was chosen, because epidemiologic data suggested a protective effect, e.g. for liver cancer 50.
We found expression of T2R10 in the majority of human pancreatic cancer samples (79 %), whereas T2R10 was basically not detected in normal pancreatic tissue. Expression varied among patients. Although survival of patients with tumors with high T2R10 expression was prolonged, there was no correlation to clinical data. A trend towards lower expression of T2R10 was seen in patients with high inflammatory score, which would be in line with the observation that a high intratumor inflammatory reaction is associated with reduced survival in PDAC 51. Whether there is a direct link between inflammation and T2R10 expression cannot be decided as yet, because there is, so far, no evidence for regulation of T2R10 expression by inflammatory mediators.
To assess a possible function of T2R10, pancreatic cancer cell lines were used. All cell lines tested expressed T2R10, though to a varying degree. Of these, two cell lines were chosen, namely BxPC3, which showed high surface expression of T2R10, and PANC-1, which according to our flow cytometry data expressed little T2R10. These cell lines also differ with regard to their susceptibility towards the used chemotherapeutics, still for both a pronounced effect of caffeine was seen, and caffeine and the chemotherapeutics act in a synergistic manner (see tables 3 and 4). To assess the participation of T2R10, receptor expression was silenced by siRNA. On average, there was a 70 % downregulation of surface expression. In BxPC-3 cells, silencing of T2R10 reduced the caffeine-induced effect, indicative of a participation of T2R10. In PANC-1, silencing of T2R10 had no substantial effect, suggesting an alternative caffeine signaling pathway.
A known downstream target of T2R10 is Akt 40 that regulates numerous cell functions, including cell survival, proliferation and metabolism (reviewed in 52-55). In our experiments, caffeine reduced the abundance of activated Akt, and subsequently, the expression of its downstream target ABCG2, in BxPC3 in a T2R10-dependent manner; in PANC-1 the caffeine effect was at least in part independent of T2R10. ABCG2 is a transmembrane drug-efflux pump that transports toxic xenobiotic substances out of cells thereby making them less susceptible to drugs (reviewed in 46-49). Effects of bitter substances such as bitter melon extract and xanthines on ABCG2 have been described before 28, 30, as have its effects on chemoresistance to both Gemcitabine and 5-FU in pancreatic tumor cells (MiaPaCa2, ASPC-1) 56. Furthermore, high ABCG2 expression correlates with poor chemoresponse, and low overall survival in PDAC patients 57, 58. The experiments with silenced T2R10 indicated that it participates in signaling pathways regulating chemoresistance of pancreatic tumor cells.
The fact that in PANC-1 caffeine-induced effects were also seen in cells with reduced T2R10 expression suggested involvement of other receptors or alternative triggering mechanisms of caffeine. Possibly, caffeine does not exclusively act through T2R10, but uses also other bitter receptors. It is quite common that bitter tasting substances engage with more than one receptor, and the receptors are promiscuous as well. For caffeine, interactions with T2R7, T2R43, and T2R46 on other cells have been described 12. Our own, not yet published data, showed expression of T2R43 and T2R46 in PDAC, supporting the notion of multiple receptor engagement. Though not tested rigorously, PANC-1 expressed less T2R10 than BxPC-3, suggesting that receptors other than T2Rs mediated the caffeine effect. On the other hand, also direct effects of caffeine are possible, because among others, it acts as a non-selective inhibitor of phosphodiesterases 59, 60, which leads to increasing levels of intracellular cyclic adenosine nucleotide monophosphate (cAMP) 61, a key second messenger involved in regulation of cellular growth in pancreatic cancer cells 62-64. Moreover, amphiphilic bitter substances, such as caffeine, in high doses stimulate G-proteins directly, in addition to its activation via the G-protein-linked T2R receptors 65. Since mechanisms of chemoresistance are usually multifactorial, the observed increase in drug susceptibility could be the result of a summation of several different pathways affected by caffeine.
In conclusion, we have shown for the first time expression of the bitter receptor T2R10 in PDAC, and its involvement in signaling pathways relevant to chemoresistance. Our data support the concept that triggering bitter receptor T2R10 on pancreatic tumor cells is a means to overcome chemoresistance.
Supplementary Material
Supplementary figure 1.
Table 2.
Effect of caffeine on Gemcitabine and 5-FU-induced cytotoxicity
| IC50* (nM) | IC50 (nM) | |||||
|---|---|---|---|---|---|---|
| Gemcitabine | Gemcitabine + caffeine | decrease in % | 5-FU | 5-FU + caffeine | decrease in % | |
| BxPC-3 scrambled siRNA | 1.7 | 0.4 | 77%* | 639 | 337 | 53%** |
| BxPC-3 T2R10 siRNA | 2.4 | 1.5 | 38% | 1024 | 641 | 37% |
| PANC-1 scrambled siRNA | 344,205 | 127,747 | 63%** | 6264 | 3922 | 37%** |
| PANC-1 T2R10 siRNA | 183,289 | 55,561 | 70%*** | 5918 | 3910 | 34%** |
*IC50 was calculated on the basis of MTS-derived data (mean of three independent experiment). Compared were cells treated with Gemcitabine alone or Gemcitabine plus caffeine; or 5-FU or 5-FU plus caffeine, respectively. As statistical method the extra sum of squares test was used (* p<0.05; ** p<0.01; ***p<0.001).
Acknowledgments
We thank the DKFZ-Heidelberg Center for Personalized Oncology (DKFZ-HIPO) for technical support and funding through HiPO015. We thank Karin Rebholz, Kathrin Schneider, Melanie Heiss, and Markus Herbst for excellent technical support. The work of MMG was funded by the German Research Foundation (DFG; GA 1818/ 2-1).
Pancreatic tissue samples were obtained from the Pancobank of the European Pancreas Center, Department of Surgery, University Hospital Heidelberg or the tissue bank of the National Center for Tumor Diseases (NCT, Heidelberg, Germany) in accordance with the regulations of the tissue bank.
Author Contributions
L.S. designed and performed the majority of experiments and analyzed data. N.G. performed experiments and analyzed data. T.H. and O.S. collected tissue samples and clinical data and helped with data analysis. P.S. and K.F. analyzed data and helped preparing the manuscript. M.M.G. designed the study, supervised the experiments, analyzed data, and wrote the manuscript. All authors read the manuscript and contributed their suggestions.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Spadi R, Brusa F, Ponzetti A, Chiappino I, Birocco N, Ciuffreda L. et al. Current therapeutic strategies for advanced pancreatic cancer: A review for clinicians. World J Clin Oncol. 2016;7:27–43. doi: 10.5306/wjco.v7.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chand S, O'Hayer K, Blanco FF, Winter JM, Brody JR. The Landscape of Pancreatic Cancer Therapeutic Resistance Mechanisms. International journal of biological sciences. 2016;12:273–82. doi: 10.7150/ijbs.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G. et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review) Oncology reports. 2010;23:1183–92. doi: 10.3892/or_00000749. [DOI] [PubMed] [Google Scholar]
- 5.Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK. et al. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. International journal of cancer Journal international du cancer. 2010;127:257–68. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S. et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer research. 2009;69:5575–83. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Wang Z, Kong D, Sarkar FH. 3,3'-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer research. 2009;69:5592–600. doi: 10.1158/0008-5472.CAN-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Zhang JG, Zhao G, Qin Q, Wang B, Liu L, Liu Y. et al. Nicotinamide prohibits proliferation and enhances chemosensitivity of pancreatic cancer cells through deregulating SIRT1 and Ras/Akt pathways. Pancreatotomy: official journal of the International Association of Pancreatology. 2013;13:140–6. doi: 10.1016/j.pan.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 9.El-Rayes BF, Ali S, Ali IF, Philip PA, Abbruzzese J, Sarkar FH. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-kappaB. Cancer research. 2006;66:10553–9. doi: 10.1158/0008-5472.CAN-06-2333. [DOI] [PubMed] [Google Scholar]
- 10.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 11.Behrens M, Meyerhof W. Bitter taste receptors and human bitter taste perception. Cellular and molecular life sciences: CMLS. 2006;63:1501–9. doi: 10.1007/s00018-006-6113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B. et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–70. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 13.Behrens M, Meyerhof W. Bitter taste receptor research comes of age: from characterization to modulation of TAS2Rs. Seminars in cell & developmental biology. 2013;24:215–21. doi: 10.1016/j.semcdb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Behrens M, Gunn HC, Ramos PC, Meyerhof W, Wooding SP. Genetic, functional, and phenotypic diversity in TAS2R38-mediated bitter taste perception. Chemical senses. 2013;38:475–84. doi: 10.1093/chemse/bjt016. [DOI] [PubMed] [Google Scholar]
- 15.Narukawa M, Noga C, Ueno Y, Sato T, Misaka T, Watanabe T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochemical and biophysical research communications. 2011;405:620–5. doi: 10.1016/j.bbrc.2011.01.079. [DOI] [PubMed] [Google Scholar]
- 16.Gaida MM, Dapunt U, Hansch GM. Sensing developing biofilms: the bitter receptor T2R38 on myeloid cells. Pathogens and disease; 2016. p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science (New York, NY) 2009;325:1131–4. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh N, Chakraborty R, Bhullar RP, Chelikani P. Differential expression of bitter taste receptors in non-cancerous breast epithelial and breast cancer cells. Biochemical and biophysical research communications. 2014;446:499–503. doi: 10.1016/j.bbrc.2014.02.140. [DOI] [PubMed] [Google Scholar]
- 19.Latorre R, Huynh J, Mazzoni M, Gupta A, Bonora E, Clavenzani P. et al. Expression of the Bitter Taste Receptor, T2R38, in Enteroendocrine Cells of the Colonic Mucosa of Overweight/Obese vs. Lean Subjects. PloS one. 2016;11:e0147468. doi: 10.1371/journal.pone.0147468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfle U, Elsholz FA, Kersten A, Haarhaus B, Schumacher U, Schempp CM. Expression and Functional Activity of the Human Bitter Taste Receptor TAS2R38 in Human Placental Tissues and JEG-3 Cells. Molecules; 2016. p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochemical and biophysical research communications. 2011;406:146–51. doi: 10.1016/j.bbrc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L. et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. The Journal of clinical investigation. 2014;124:1393–405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manson ML, Safholm J, Al-Ameri M, Bergman P, Orre AC, Sward K. et al. Bitter taste receptor agonists mediate relaxation of human and rodent vascular smooth muscle. European journal of pharmacology. 2014;740:302–11. doi: 10.1016/j.ejphar.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Jing F, Liu M, Yang N, Liu Y, Li X, Li J. Relaxant effect of chloroquine in rat ileum: possible involvement of nitric oxide and BKCa. The Journal of pharmacy and pharmacology. 2013;65:847–54. doi: 10.1111/jphp.12041. [DOI] [PubMed] [Google Scholar]
- 25.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS biology. 2013;11:e1001501. doi: 10.1371/journal.pbio.1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan X, Sanderson MJ. Bitter tasting compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. British journal of pharmacology. 2014;171:646–62. doi: 10.1111/bph.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaida MM, Mayer C, Dapunt U, Stegmaier S, Schirmacher P, Wabnitz GH, Expression of the bitter receptor T2R38 in pancreatic cancer: localization in lipid droplets and activation by a bacteria-derived quorum-sensing molecule. Oncotarget; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwatra D, Venugopal A, Standing D, Ponnurangam S, Dhar A, Mitra A. et al. Bitter melon extracts enhance the activity of chemotherapeutic agents through the modulation of multiple drug resistance. Journal of pharmaceutical sciences. 2013;102:4444–54. doi: 10.1002/jps.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyn-Cook BD, Rogers T, Yan Y, Blann EB, Kadlubar FF, Hammons GJ. Chemopreventive effects of tea extracts and various components on human pancreatic and prostate tumor cells in vitro. Nutrition and cancer. 1999;35:80–6. doi: 10.1207/S1532791480-86. [DOI] [PubMed] [Google Scholar]
- 30.Ding R, Shi J, Pabon K, Scotto KW. Xanthines down-regulate the drug transporter ABCG2 and reverse multidrug resistance. Molecular pharmacology. 2012;81:328–37. doi: 10.1124/mol.111.075556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawano Y, Nagata M, Kohno T, Ichimiya A, Iwakiri T, Okumura M. et al. Caffeine increases the antitumor effect of Cisplatin in human hepatocellular carcinoma cells. Biological & pharmaceutical bulletin. 2012;35:400–7. doi: 10.1248/bpb.35.400. [DOI] [PubMed] [Google Scholar]
- 32.Mei Y, Wei D, Liu J. Reversal of cancer multidrug resistance by tea polyphenol in KB cells. Journal of chemotherapy. 2003;15:260–5. doi: 10.1179/joc.2003.15.3.260. [DOI] [PubMed] [Google Scholar]
- 33.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 1998;11:155–68. [PubMed] [Google Scholar]
- 34.Gaida MM, Welsch T, Herpel E, Tschaharganeh DF, Fischer L, Schirmacher P. et al. MHC class II expression in pancreatic tumors: a link to intratumoral inflammation. Virchows Arch. 2012;460:47–60. doi: 10.1007/s00428-011-1175-x. [DOI] [PubMed] [Google Scholar]
- 35.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 36.Morgan RT, Woods LK, Moore GE, Quinn LA, McGavran L, Gordon SG. Human cell line (COLO 357) of metastatic pancreatic adenocarcinoma. International journal of cancer Journal international du cancer. 1980;25:591–8. doi: 10.1002/ijc.2910250507. [DOI] [PubMed] [Google Scholar]
- 37.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 38.Thermo Fisher Scientific. User Guide: Pierce BCA Protein Assay Rockford, IL, USA.
- 39.Kim Y, Han D, Min H, Jin J, Yi EC, Kim Y. Comparative proteomic profiling of pancreatic ductal adenocarcinoma cell lines. Molecules and cells. 2014;37:888–98. doi: 10.14348/molcells.2014.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma P, Panebra A, Pera T, Tiegs BC, Hershfeld A, Kenyon LC. et al. Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells. American journal of physiology Lung cellular and molecular physiology. 2016;310:L365–76. doi: 10.1152/ajplung.00373.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fahy BN, Schlieman M, Virudachalam S, Bold RJ. AKT inhibition is associated with chemosensitisation in the pancreatic cancer cell line MIA-PaCa-2. British journal of cancer. 2003;89:391–7. doi: 10.1038/sj.bjc.6601037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2002;5:234–48. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 43.Ng SS, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7:3269–75. [PubMed] [Google Scholar]
- 44.Huang FF, Zhang L, Wu DS, Yuan XY, Yu YH, Zhao XL. et al. PTEN regulates BCRP/ABCG2 and the side population through the PI3K/Akt pathway in chronic myeloid leukemia. PLoS One. 2014;9:e88298. doi: 10.1371/journal.pone.0088298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW. et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell stem cell. 2009;4:226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen JD, Schinkel AH. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2) Molecular cancer therapeutics. 2002;1:427–34. [PubMed] [Google Scholar]
- 47.Natarajan K, Xie Y, Baer MR, Ross DD. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochemical pharmacology. 2012;83:1084–103. doi: 10.1016/j.bcp.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mo W, Zhang JT. Human ABCG2: structure, function, and its role in multidrug resistance. International journal of biochemistry and molecular biology. 2012;3:1–27. [PMC free article] [PubMed] [Google Scholar]
- 49.Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–58. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 50.Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 2015;148:118–25. doi: 10.1053/j.gastro.2014.10.005. quiz e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam MS, Gaida MM, Bergmann F, Lasitschka F, Giese T, Giese NA. et al. Selective inhibition of the p38 alternative activation pathway in infiltrating T cells inhibits pancreatic cancer progression. Nature medicine. 2015;21:1337–43. doi: 10.1038/nm.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. British journal of clinical pharmacology; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 54.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang SJ, Hsu SY, Deng CR, Huang HC, Liu SL, Shi GY. et al. Dextromethorphan attenuates LPS-induced adhesion molecule expression in human endothelial cells. Microcirculation. 2013;20:190–201. doi: 10.1111/micc.12024. [DOI] [PubMed] [Google Scholar]
- 56.Hamada S, Satoh K, Hirota M, Kanno A, Umino J, Ito H. et al. The homeobox gene MSX2 determines chemosensitivity of pancreatic cancer cells via the regulation of transporter gene ABCG2. J Cell Physiol. 2012;227:729–38. doi: 10.1002/jcp.22781. [DOI] [PubMed] [Google Scholar]
- 57.Lee SH, Kim H, Hwang JH, Lee HS, Cho JY, Yoon YS. et al. Breast cancer resistance protein expression is associated with early recurrence and decreased survival in resectable pancreatic cancer patients. Pathology international. 2012;62:167–75. doi: 10.1111/j.1440-1827.2011.02772.x. [DOI] [PubMed] [Google Scholar]
- 58.Yuan Y, Yang Z, Miao X, Li D, Liu Z, Zou Q. The clinical significance of FRAT1 and ABCG2 expression in pancreatic ductal adenocarcinoma. Tumour Biol. 2015;36:9961–8. doi: 10.1007/s13277-015-3752-0. [DOI] [PubMed] [Google Scholar]
- 59.Wells JN, Miller JR. Methylxanthine inhibitors of phosphodiesterases. Methods in enzymology. 1988;159:489–96. doi: 10.1016/0076-6879(88)59048-1. [DOI] [PubMed] [Google Scholar]
- 60.Sawynok J, Yaksh TL. Caffeine as an Analgesic Adjuvant - a Review of Pharmacology and Mechanisms of Action. Pharmacol Rev. 1993;45:43–85. [PubMed] [Google Scholar]
- 61.Sutherland EW, Rall TW. Fractionation and Characterization of a Cyclic Adenine Ribonucleotide Formed by Tissue Particles. Journal of Biological Chemistry. 1958;232:1077–91. [PubMed] [Google Scholar]
- 62.Cho-Chung YS, Pepe S, Clair T, Budillon A, Nesterova M. cAMP-dependent protein kinase: role in normal and malignant growth. Crit Rev Oncol Hematol. 1995;21:33–61. doi: 10.1016/1040-8428(94)00166-9. [DOI] [PubMed] [Google Scholar]
- 63.Zhao H, Wei R, Wang L, Tian Q, Tao M, Ke J. et al. Activation of glucagon-like peptide-1 receptor inhibits growth and promotes apoptosis of human pancreatic cancer cells in a cAMP-dependent manner. Am J Physiol Endocrinol Metab. 2014;306:E1431–41. doi: 10.1152/ajpendo.00017.2014. [DOI] [PubMed] [Google Scholar]
- 64.Lorenz R, Aleksic T, Wagner M, Adler G, Weber CK. The cAMP/Epac1/Rap1 pathway in pancreatic carcinoma. Pancreas. 2008;37:102–3. doi: 10.1097/MPA.0b013e318160748f. [DOI] [PubMed] [Google Scholar]
- 65.Naim M, Seifert R, Nurnberg B, Grunbaum L, Schultz G. Some taste substances are direct activators of G-proteins. The Biochemical journal. 1994;297( Pt 3):451–4. doi: 10.1042/bj2970451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1.