Abstract
At mucosal sites such as the intestine, the immune system launches robust immunity against invading pathogens while maintaining a state of tolerance to commensal flora and ingested food antigens. The molecular mechanisms underlying this phenomenon remain poorly understood. Here, we report that signaling by GPR81, a receptor for lactate, in colonic DCs and macrophages plays an important role in suppressing colonic inflammation and restoring colonic homeostasis. Genetic deletion of GPR81 in mice led to increased Th1/Th17 cell differentiation and reduced regulatory T cell differentiation, resulting in enhanced susceptibility to colonic inflammation. This was due to increased production of pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) and decreased expression of immune regulatory factors (IL-10, RA and IDO) by intestinal APCs lacking GPR81. Consistent with these findings, pharmacological activation of GPR81 decreased inflammatory cytokine expression and ameliorated colonic inflammation. Taken together, these findings identify a new and important role for the GPR81 signaling pathway in regulating immune tolerance and colonic inflammation. Thus, manipulation of the GPR81 pathway could provide novel opportunities for enhancing regulatory responses and treating colonic inflammation.
Introduction
GPR81 is a cell-surface G-protein coupled receptor with high homology to GPR109a and GPR109b (1, 2). GPR81 is expressed at a relatively high level in fat cells and at lower levels in brain, intestine, kidney and many other tissues (3–5). Recent studies have shown that GPR81 is activated by lactate (3). Commensal bacteria in the gut ferment dietary fibers into their metabolites such as lactate and other short-chain fatty acids (SCFA), mainly acetate, propionate and butyrate (6, 7). Interestingly, the colon also contains high levels of lactate (10 mM) which serves as a substrate for butyrate-producing bacteria (8). Lactate is mostly produced from fermented food by lactic acid-producing bacteria and from dietary fibers by bifidobacteria (9, 10). Recent studies have highlighted the importance of GPR109a and GPR43, receptors that recognize short-chain fatty acids, in regulating intestinal inflammation and oral tolerance to ingested antigens (11–13). The most widely studied function of GPR81 is its ability to protect tissues from injury as observed in mouse models of hepatic, pancreatic and brain injury (14, 15). However, the role of GPR81 in regulating intestinal inflammation and immune homeostasis is unknown.
In the intestine, antigen-presenting cells (APCs) such as dendritic cells (DCs) and macrophages play a critical role in controlling the delicate balance between regulatory and inflammatory responses (16, 17). They regulate immune tolerance through induction of regulatory T cells while limiting the differentiation of pathological Th1/Th17 cells in the gut (18–20). However, the receptors and signaling networks that program intestinal APCs to a regulatory versus an inflammatory state remain poorly understood. Previous studies have shown that lactate can suppress the activation and maturation of DCs and macrophages (15, 21–23). These APCs show markedly reduced levels of inflammatory cytokines in response to LPS. In addition, lactate treatment protects mice against trinitrobenzenesulfonic acid-induced colitis (24). However, the underlying molecular mechanisms remain poorly understood. Whether GPR81 can modulate immune responses in the gut remains unexplored. This is particularly relevant in the colon as gastrointestinal mucosa is exposed to high concentrations of lactate in the lumen (10 mM) (9, 25). We thus investigated whether GPR81 impacts immune-homeostasis in the intestine.
In the current study, we show that GPR81-mediated signaling in colonic DCs and macrophages plays an important role in suppressing colonic inflammation and in restoring gut homeostasis. Our data show that the GPR81 pathway in colonic DCs and macrophages is critical for inducing immune regulatory factors and suppressing the expression of inflammatory cytokines. This is critical for driving regulatory T cell differentiation while limiting pathological Th1/Th17 cell differentiation in the colon. Furthermore, genetic deletion of GPR81 in mice enhances susceptibility to colonic inflammation. These results reveal a novel mechanism by which colonic APCs control colonic inflammation and commensal homeostasis via GPR81 signaling, and that lactate, a dietary constituent and a bacterial metabolite, serves as a signaling molecule in this phenomenon.
Methods
Mice
C57BL/6 (B6), CD45.1 (B6) and Rag2−/− B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred on-site. GPR81−/− mice (26), kindly provided by Dr. Stefan Offermanns (Max-Planck-Institute for Heart and Lung Research, Germany) were on a B6 (>10 generation) background. GPR81−/− and B6 mice were crossed and the resultant GPR81+/− B6 mice interbred to obtain GPR81+/+ (WT) and GPR81−/− littermates, which were then caged together upon weaning. Rag2−/− (B6) and GPR81−/− (B6) mice were crossed to obtain Rag2−/−/GPR81−/− mice. All experiments were carried out with age-matched controls unless specified otherwise. Both male and female mice were used and were between 8–14 weeks of age at the time of experiments. All mice were housed under specific pathogen-free conditions in facilities of the Laboratory Animal Services of Augusta University. Animal care protocols were approved by the Institutional Animal Care and Use Committee of Augusta University.
Antibodies and reagents
Antibodies against mouse CD3 (145-2C11), CD4 (GK1.5), CD45 (30-F11), Foxp3 (FJK-16s), IL-10 (JES5-16E3), CD11c (N418), CD11b (M1/70), I-Ab (25-9-17), CD90.1 (HIS51), V alpha 2 TCR (B20.1), V beta 5.1/5.2 TCR (MR9-4), IFN-γ (XMG1.2), IL-22 (1H8PWSR) and IL17A (17B7) were purchased from eBioscience and Biolegend. GPR81 and actin antibodies were purchased from Sigma.
CD45RBhigh CD4+ T cell transfer
CD4+ T cells from WT mouse spleen and lymph nodes (inguinal and axillary) were first enriched using CD4-specific microbeads and MACS column (Miltenyi Biotech; Auburn, CA). CD4+ T cell subsets were then further purified by FACS-sorting to collect two different populations of cells, CD4+ CD45RBhigh CD25− cells and CD4+ CD25+ cells. Approximately 3×105 CD4+ CD45RBhigh CD25− cells were injected i.p. into the indicated recipient Rag2−/− or Rag2−/−/GPR81−/− mice. Mice were then monitored for body weight twice a week. In some experiments, Rag2−/− or Rag2−/−/GPR81−/− mice were treated orally with the GPR81 agonist 3Cl-5OH-BA (Calbiochem) at indicated time points.
Induction of DSS-induced colonic inflammation
WT (B6), GPR81−/− (B6), Rag2−/− (B6) or Rag2−/−/GPR81−/− (B6) mice were fed with 2.5% (w/v) DSS in their drinking water for 6 days and sacrificed at day 9. Mice were monitored for weight changes, diarrhea and rectal bleeding as previously described (11, 27). Diarrhea was scored as (0) normal stool; (1) soft but formed pellet; (2) very soft pellet; (3) diarrhea (no pellet), or; (4) dysenteric diarrhea. Rectal bleeding was recorded as (0) no bleeding; (2) presence of occult blood in stool, or; (4) gross macroscopic bleeding.
Isolation of intestinal APCs and lymphocytes
APCs and lymphocytes from colons were isolated as described in our previous study (28). Briefly, mice were euthanized and the colon was washed, cleaned of fat tissue and longitudinally cut and suspended in 1× HBSS with 20 mM HEPES, 1 mM DTT and 5 mM EDTA for 30 min at 37°C with shaking (150 r.p.m) to remove epithelial cells. After that, pieces of colon were digested with collagenase VIII (Sigma) (0.3 mg/ml in RPMI with DNAse I (0.1mg/ml) and 2% FCS) for 30 min at 37 °C with shaking (150 r.p.m.). Tissue was processed through a 100-μm-cell strainer, and the resulting suspension was pelleted. For lymphocyte isolation, cells derived following collagenase digestion were resuspended in 7 ml of 40% Percoll and layered on top of 2 ml of 70% Percoll (GE Amersham). After centrifugation for 15 min at 1,500 r.p.m without brakes, the middle layer was removed, washed in 2% FBS in RPMI and the lymphocytes were obtained. Isolated lymphocytes were cultured with phorbol myristate acetate (50 ng/ml) plus ionomycin (750 ng/ml) in the presence of GolgiStop and Golgiplug for 5 h. Cells were fixed and stained for CD4, IL-10, IFN-γ and IL-17. For LP DCs and macrophages, the collagenase-digested cells were filtered through a 100-μm strainer and pelleted and stained. For cell sorting, APCs in this preparation were enriched with CD11c+ and CD11b+ magnetic beads according to the manufacturer’s instructions (Miltenyi Biotec) and sorted on FACS Aria at the Augusta University flow cytometer core.
In vitro lymphocyte co-culture
FACS-sorted colonic dendritic cells (CD45+I-Ab+CD11c+ CD64−) or macrophage (CD45+I-Ab+CD11b+ CD64+) cells (1×105) were cultured with naïve CD4+CD25−CD62L+ OT-II CD4+ transgenic T cells (1×105) and OVA peptide (ISQVHAAHAEINEAGR; 1 μg/ml) in a total volume of 200 μl RPMI complete medium. The culture supernatants were analyzed after 96 h and cells were harvested and restimulated for 6 h with plate-bound antibodies against CD3 (5 μg/ml; 145.2C11 from Becton Dickinson) and CD28 (2 μg/ml; 37.51 from Becton Dickinson) in the presence of GolgiStop and Golgiplug for intracellular cytokine detection (IL-17A, IL-10 and IFN-γ).
OT-II CD4+ T cell adoptive transfer
Naïve CD4+CD25− T cells were isolated from the spleen and lymph nodes of Rag1−/− OT-II Thy1.1 transgenic mice and 5×106 cells were intravenously transferred into WT and GPR81−/− mice. Mice received 1 mg of OVA (Sigma) dissolved in 0.2 ml of PBS by gavage on 5 consecutive days after transfer.
Bone marrow chimeras
WT (CD45.1) or GPR81−/− (CD45.2) mice were irradiated (900 rads) and injected intravenously with donor bone marrow cells (2 × 106 cells/mouse). Reconstitution was confirmed by staining for the donor-specific CD45 allele (CD45.1 versus CD45.2) in blood. Two months after reconstitution, mice were used for DSS-induced inflammation experiments.
Ex vivo colon culture and ELISAs
Colons were isolated from mice, opened longitudinally and washed three times with sterile HBSS with 100U/ml penicillin G and 100 μg/ml streptomycin (Gibco, Carlsbad, CA), and ~1cm sections were cultured in 500 μl RPMI supplemented with 2% FBS, L-glutamine, penicillin/streptomycin and 100 μg/ml gentamicin (Sigma-Aldrich) for 24 hours at 37°C, 5% CO2. After 48 hours, supernatants were removed and cleared of debris by centrifugation and the cytokine concentrations determined by ELISA. IL-17, IL-6, IL-12 (p40), IL-12 (p70), IL-10, TNF-α, IFN-γ and IL-1β in culture supernatants were quantitated using ELISA kits purchased from eBioscience and Bio-Legend.
Histopathology and immunohistochemistry
Sections (5 μm) from formalin-fixed and paraffin-embedded colons were placed onto glass slides. H&E-stained sections were blindly scored for severity of colonic inflammation as described previously (29). The parameters used were (a) lamina propria (LP) inflammation (0–3); (b) goblet cell loss (0–2); (c) abnormal crypts (0–3); (d) crypt abscesses (0–1); (e) mucosal erosion or ulceration (0–1) and (f) submucosal spread to transmural inflammation (0–4). The individual scores from each parameter were summed to derive histological score for colonic inflammation (maximum score 14).
Measurement of intestinal permeability
Mice were given fluorescein isothiocyanate (FITC)-dextran by oral gavage at a dose of 0.5 mg/g of body weight (MW 4000; FD4, Sigma-Aldrich). Serum was collected four hours later and fluorescence intensity was determined (excitation, 492 nm; emission, 525 nm; BioTek). FITC-dextran concentrations were determined using a standard curve generated by serial dilutions of FITC-dextran.
Myeloperoxidase activity
Pieces of colon (100 mg weight) were homogenized in phosphate buffer (20 mM; pH 7.4) and centrifuged. The pellet was re-suspended in phosphate buffer (50 mM; pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma). The sample was freeze-thawed and sonicated, followed by warming to 60°C for 2 hr. and subsequent centrifugation. Redox reactions of 3,3′,5,5′-tetramethylbenzidine (Sigma) with the supernatant was used to determine myeloperoxidase (MPO) activity. The reaction was terminated with 2N HCl and absorbance was read at 450 nm.
Real-time PCR
Total mRNA was isolated from the colon or an indicated cell type using the Omega Total RNA Kit according to the manufacturer’s protocol. cDNA was generated using the RNA to cDNA Ecodry Premix Kit (Clontech) according to the manufacturer’s protocol. cDNA was used as a template for quantitative real-time PCR using SYBR Green Master Mix (Roche) and gene-specific primers (30, 31). PCR analysis was performed using a MyiQ5 ICycler (BioRad). Gene expression was normalized relative to Gapdh.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software. An unpaired one-tailed Student’s t test was used to determine statistical significance for mRNA expression levels, Treg percentages and cytokines released by various cell types between different groups. A P value less than 0.05 (*) was considered to be significant, a P value less than 0.01 (**) was considered to be very significant, and a P value less than 0.001 (***) was considered to be extremely significant.
Results
GPR81 deficiency enhances susceptibility to DSS-induced colonic inflammation
We hypothesized that GPR81 has a regulatory role in the colon. In order to examine the role of GPR81 in colitis, we challenged GPR81−/− and littermate control (WT) mice with 2.5% dextran sulfate sodium (DSS), an experimental model of tissue injury and intestinal inflammation. GPR81−/− mice were more susceptible to DSS-induced inflammation and showed more severe weight loss and a significant reduction in colon length compared to DSS-treated WT mice (Fig. 1A, B). Myeloperoxidase activity, a hallmark of colonic inflammation, was markedly increased in colons of GPR81−/− mice after DSS treatment (Fig. 1C). Loss of intestinal permeability is an important feature during the course of DSS-induced colitis, so we tested the gut epithelial barrier integrity by orally feeding the DSS-treated GPR81−/− and WT mice with FITC-dextran. We observed an increase in FITC-dextran in the serum of DSS-treated GPR81−/− mice, indicating severely impaired epithelial barrier integrity (Fig. 1D). Consistent with enhanced gut inflammation, histopathological analysis of colons of DSS-treated GPR81−/− mice showed extensive damage to the mucosa with epithelial erosion, loss of crypts and infiltration of immune cells compared to the colons of DSS-treated WT mice (Fig. 1E, F). Colons from untreated WT and GPR81−/− mice showed no morphological signs of damage or inflammation (data not shown).
Figure 1. GPR81−/− mice exhibit increased susceptibility to DSS-induced colonic inflammation.
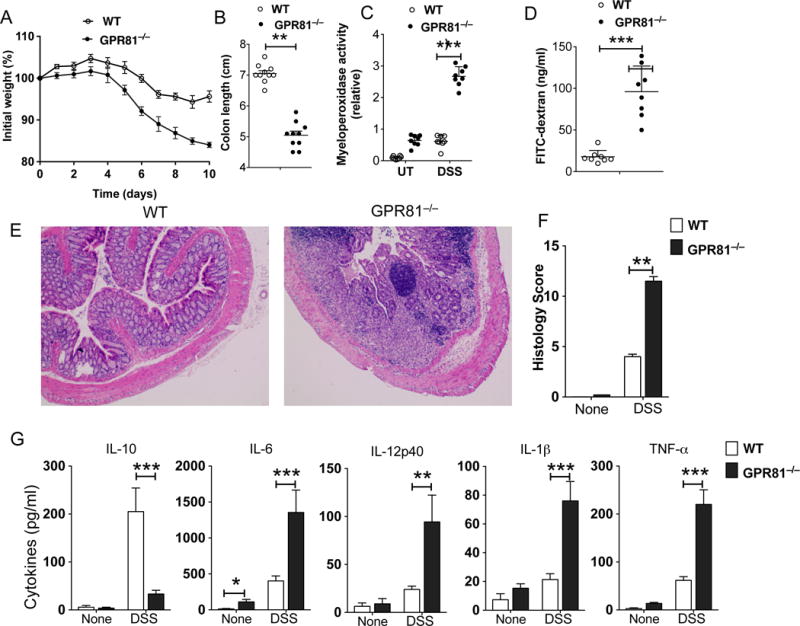
(A-G) GPR81−/− and GPR81+/+ littermate control (WT) mice were treated with 2.5% DSS in drinking water for 6 days and at day 9 the colons of mice were analyzed for inflammation. (A, B) Changes in body weight and colon length (day 9) of WT and GPR81−/− mice. (C) Myeloperoxidase activity in colon. (D) Mice were fed with FITC-dextran on day 9, and 4 h later FITC-dextran was quantified in serum (n=5). (E) Representative colonic tissue sections of DSS-treated WT and GPR81−/− mice (Hematoxylin and eosin staining with original magnification, 100X). (F) Histopathological score (inflammation + epithelial damage) of colons was graded following analysis of H&E-stained cross sections of colons of DSS-treated WT and GPR81−/− mice. (G) Excised colon samples were cultured for 2 days ex vivo, and the secreted IL-6, TNF-α, IL-1β, IL-12p40 and IL-10 cytokine levels in the culture supernatants were quantified by ELISA. Data are representative of three separate experiments (n>5). **p<0.01; ***p<0.001. Error bars indicate mean ±SEM.
GPR81 is expressed by both non-hematopoietic cells as well as immune cells such as macrophages and neutrophils (3–5, 15, 23). Next, we sought to address the role of GPR81 in hematopoietic versus non-hematopoietic cells in the settings of DSS-induced colitis. We generated bone marrow (BM) chimeric mice in which donors and/or recipients were either WT (CD45.1) or GPR81−/− (CD45.2). We assessed such chimeric mice 8 weeks after transplantation by surface staining of peripheral blood and bone marrow (BM)-derived cells with CD45.1- and CD45.2-specific antibodies, and the mice were found to be fully chimerized (data not shown). WT hosts that received GPR81−/− BM exhibited significantly greater DSS-induced weight loss than corresponding recipients of WT-BM cells (Supplementary Fig. 1A). In contrast, GPR81−/− hosts that received WT-BM showed weight loss comparable to WT hosts that received WT-BM (Supplementary Fig. 1A). These results suggest that Gpr81 expression in hematopoietic cells is critical for limiting colonic inflammation, whereas GPR81 expression in non-hematopoietic cells is dispensable in this model of intestinal inflammation.
To assess the relative contribution of GPR81 in adaptive immune cells versus innate immune cells in limiting intestinal inflammation, we generated Rag2−/−/GPR81−/− mice by crossing GPR81−/− mice to Rag2−/− mice. We then conducted DSS challenges in Rag2−/−/GPR81−/− and Rag2−/− mice. Rag2−/−/GPR81−/− mice were more susceptible to DSS and showed more severe weight loss with significant reduction in colon length compared to DSS-treated littermate controls (Supplementary Fig 1B, C). These results suggest that GPR81 expression in innate immune cells is critical for protecting mice from DSS-induced intestinal inflammation.
In DSS-induced intestinal inflammation, inflammatory cytokines produced by innate immune cells present in the gut microenvironment promote colitis (32). Thus, we analyzed the expression levels of various inflammatory and anti-inflammatory cytokines in the colons of WT and GPR81−/− mice treated with or without DSS. Colon explant culture showed that explants from GPR81-deficient mice released lower levels of immune regulatory cytokine IL-10 and higher levels of inflammatory cytokines, such as IL-6, IL-12p40, IL-1β and TNF-α, compared to explants from WT mice both under homeostatic conditions and upon DSS treatment (Fig 1G). Collectively, these results indicate that the absence of GPR81 leads to an imbalance in the expression of pro-inflammatory versus immune regulatory cytokines in the intestine.
GPR81 deficiency enhances susceptibility in the T-cell transfer model of colitis
Since DSS-induced colitis appears to be independent of T and B cell responses (32), it is not yet clear if and how GPR81 signaling shapes T cell responses or limits T cell-dependent colitis. Thus, we next tested whether GPR81 regulates intestinal inflammation using a well-characterized CD45RBhiCD4+ T cell-transfer colitis model in which colitis is caused by disruption of T cell homeostasis with uncontrolled Th1 and Th17 responses to the commensal microbiota (33, 34). Wild-type CD45RBhiCD4+ T-cells were adoptively transferred into Rag2−/− and Rag2−/−/GPR81−/− mice, and mice were then monitored for clinical signs of colitis. When compared to Rag2−/− mice, Rag2−/−/GPR81−/− mice showed increased colitis severity as revealed by weight loss, severe destruction of colonic tissues and histology score (Fig 2 A, B, C). Consistent with these observations, the percentages of IFNγ+ and IL-17A+ CD4+ T cells were markedly increased in the colon of Rag2−/−/GPR81−/− mice compared to those of control mice (Fig 2 D, E). In agreement with these results, colons of Rag2−/−/GPR81−/− colitic mice produced higher levels of IL-17A and IFN-γ ex vivo compared to control colons (Fig 2F). In contrast, adoptive transfer of CD45RBhiCD4+ T cells isolated from WT or GPR81−/− mice into Rag2−/− mice resulted in similar levels of disease severity, suggesting that GPR81 expression in T cells is dispensable in this model of intestinal inflammation (Supplementary Figure 1D). Collectively, these results suggest that GPR81-signaling in innate immune cells, but not in T cells, plays a key role in regulating intestinal inflammation.
Figure 2. The absence of GPR81 signaling exacerbates susceptibility to T cell-mediated colitis.
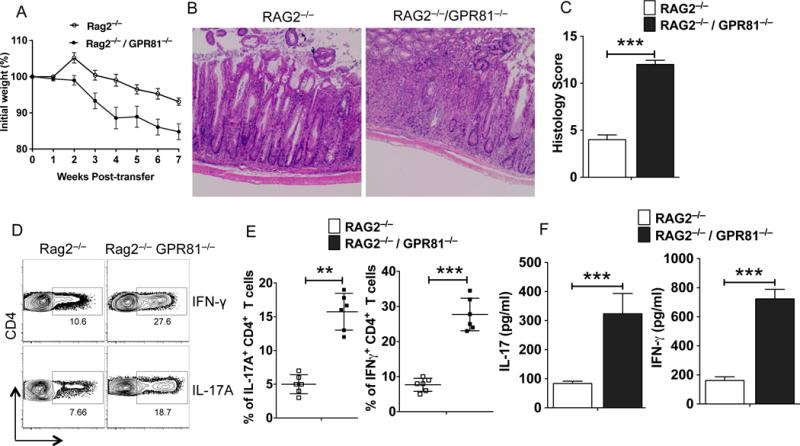
(A) Percent weight change compared to initial weight for Rag2−/− and Rag2−/−/GPR81−/− mice at indicated time points post-CD4+CD25−CD45RBhi T cell adoptive transfer. (B) Representative colonic tissue sections of Rag2−/− and Rag2−/−/GPR81−/− mice 6 weeks post-naïve CD4+ T cell transfer (Hematoxylin and eosin staining with original magnification, 100X). (C) Colonic histopathology scores of Rag2−/− and Rag2−/−/GPR81−/− mice 6 weeks post-naïve CD4+ T cell transfer. (D, E) Representative FACS plots and frequencies of IL-17A+ and IFN-γ+ CD4+ T cells in the colons of Rag2−/− and Rag2−/−/GPR81−/− mice 6 weeks post-transfer of naïve CD4+ T cells. (F) Excised colon samples were cultured for 48 hrs ex vivo, and the secreted IL-17A and IFN-γ cytokine levels in the culture supernatants were quantified by ELISA. Data are representative of two independent experiments. The error bars indicate mean ± SEM of 6–8 mice/group. *p<0.05; **p<0.01; ***p<0.001.
GPR81-mediated signals impart a regulatory phenotype on colonic antigen presenting cells
A recent study showed that GPR81 is highly expressed by antigen presenting cells in the liver and is critical for limiting inflammation in the liver (15). So, we investigated GPR81 expression in DCs and macrophages isolated from the spleen, lung, small intestine lamina propria (SI-LP) and colon. We isolated intestinal DCs and macrophages based on surface expression of CD45, MHCII, CD11c, CD11b, CD64 and F4/80(35–37). DCs (CD45+ I-Ab+ CD11c+ CD64− F4/80−) and macrophages (CD45+ I-Ab+ CD11b+ CD64+ F4/80+) isolated from SI-LP and colon expressed markedly higher levels of GPR81 compared to splenic DCs and macrophages (Supplementary Figure 2A, B). Like intestinal APCs, GPR81 was also highly expressed in DCs and macrophages isolated from lung (Supplementary Figure 2A). However, GPR81 is expressed at relatively lower levels in other immune cells such as CD4+ T cells, CD8+ T cells and B cells (Supplementary Figure 2A). These results suggest GPR81 is highly expressed in mucosal APCs compared to other immune cell types.
Intestinal DCs and macrophages play a critical role in maintaining balance between regulatory and effector T cells in the intestine (17, 20, 38–40). The above results prompted us to assess whether GPR81-mediated signals regulate APC function through the expression of inflammatory and anti-inflammatory cytokine factors. Thus, we quantified the expression levels of immune regulatory factors and inflammatory cytokines in FACS-sorted colonic DCs and macrophages FACS-sorted from GPR81−/− and WT mice. Colonic DCs and macrophages isolated from GPR81−/− mice expressed markedly higher levels of inflammatory factors such as IL-6, IL-1β, TNF-α and IL-12p40 compared to WT colonic DCs and macrophages (Fig. 3A). In contrast, GPR81−/− colonic DCs and macrophages expressed markedly lower mRNA levels of immune regulatory factors such as Aldh1, IDO1 and IL-10 compared to WT DCs and macrophages (Fig. 3B). Consistent with these results, colonic DCs and macrophages from GPR81−/− mice produced markedly lower levels of IL-10 and higher levels of cytokines such as IL-6, IL-1β and IL-12 (Fig. 3C). Collectively, these results suggest that GPR81-signaling in intestinal APCs imparts an immune regulatory phenotype and limits the expression of inflammatory cytokines.
Figure 3. GPR81 signaling imparts an anti-inflammatory phenotype on colonic DCs and macrophages.
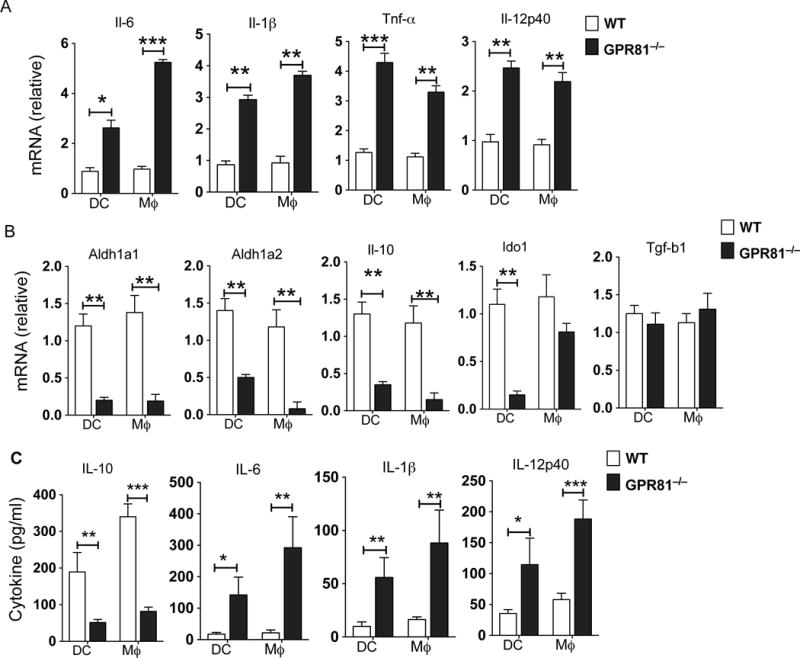
(A, B) Quantitative real-time PCR analysis of Il6, Tnfa, Il1b, Il12p40, Il10, Aldh1a1, Aldh1a2, Ido1 and Tgfb1mRNA expression in colonic DCs and macrophages sorted from WT and GPR81−/− mice. Data are presented as fold change relative to WT. (C) ELISA of IL-6, IL-1β, IL-12p40 and IL-10 in the culture supernatants of colonic DCs and macrophages isolated from WT and GPR81−/− mice and cultured for 24 h. Data are from of two separate experiments that were then pooled. The error bars indicate mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
GPR81-signaling regulates the balance between regulatory T cell and Th1/Th17 cell frequency in the colon
The balance between regulatory and effector T cells is critical for gut homeostasis(41–43). T cell priming and differentiation happens in the draining lymph nodes, whereas the activation and expansion of effector T cells happens in the gut lamina propria (44–46). Intestinal APCs control both T cell differentiation and expansion through the secretion of various inflammatory and anti-inflammatory cytokines (17, 18, 40, 43). The results presented above show an imbalance in production of anti-inflammatory molecules versus inflammatory molecules by GPR81-deficient colonic APCs. Thus, we tested the ability of colonic DCs and macrophages isolated from GPR81−/− mice and WT mice to promote the differentiation of naïve OT-II CD4 cells into Treg/Th1/Th17 cells. Colonic DCs and macrophages deficient in GPR81 are more potent in inducing IFNγ- and IL-17-producing T cells compared to those of WT colonic DCs and macrophages (Fig. 4 A, B). In contrast, GPR81-deficient colonic DCs and macrophages were less potent in inducing Foxp3+ Tregs and IL-10+ regulatory T (Tr1) cells compared to that of WT colonic DCs and macrophages (Fig. 4 A, Supplementary Fig. 3A).
Figure 4. GPR81 signaling in intestinal APCs suppresses Th1/Th17 cell differentiation and induces regulatory T cell differentiation.
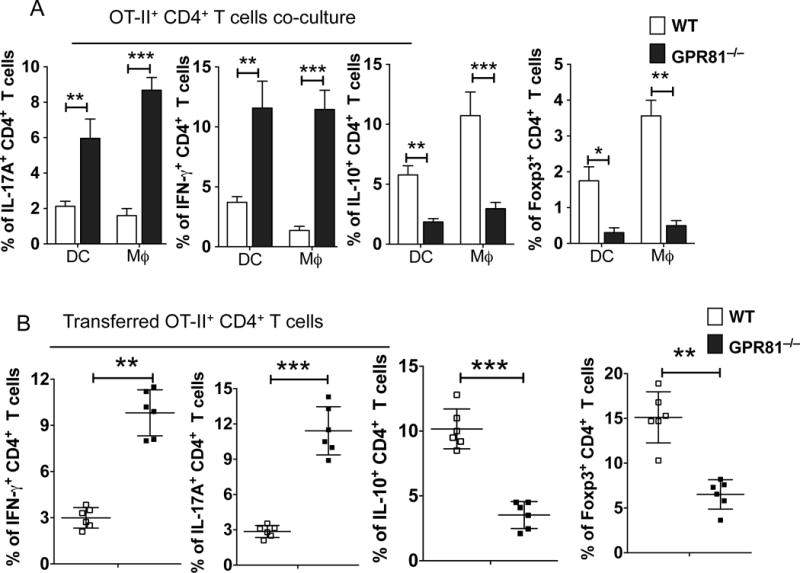
(A) Cumulative frequencies of IL-17A+, IFN-γ+, IL-10+ and Foxp3+ OT-II CD4+ T cells after culturing with colonic DCs and macrophages isolated from GPR81−/− and WT mice. (B) Cumulative frequencies of adoptively transferred naïve OT-II CD4+ T cells positive for IFN-γ, IL-17A, IL-10 and Foxp3 isolated from colons of WT and GPR81−/− mice treated orally with OVA protein. Data are representative of two independent experiments (n>5). The error bars indicate mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
Oral administration of a nominal protein antigen results in conversion and expansion of antigen-specific Tregs in the small and large intestine(46–48). So, we asked whether deficiency in GPR81 affects the generation of Treg/Th1/Th17 cells in the gut in response to oral administration of ovalbumin (OVA). We adoptively transferred naïve OT-II Thy1.1+ cells into GPR81−/− and WT mice and fed them with OVA, the cognate Ag for OT-II cells, for 5 days. Intracellular cytokine analysis on day 6 post-transfer showed a significant increase in naïve OT-II Thy1.1 CD4+ T cell differentiation towards Th1 and Th17 cells in the colon of GPR81−/− mice compared to the colon of WT mice (Fig. 4B, Supplementary Fig. 3B). Further characterization of transferred OT-II T cells showed a marked decrease in the differentiation of Foxp3+ Tregs and IL-10-producing Tr1 cells in the colon of GPR81−/− mice compared to that seen in the colon of WT mice (Fig. 4B, Supplementary Fig. 3B).
Next, we determined the frequencies of endogenous CD4+ T cells that are positive for IL-17, IFN-γ and IL-10 in the colon of GPR81−/− and WT mice. GPR81−/− mice harbored higher frequencies of colonic CD4+ T cells producing the inflammatory cytokines IL-17 and IFN-γ compared to WT mice (Supplementary Fig. 4A, B). In contrast, the frequency of CD4+ T cells expressing IL-10 were markedly reduced in the colon of GPR81−/− mice compared to WT mice (Supplementary Fig. 4A, B). Interestingly, there were no significant differences in the frequencies of Th1 and Th17 cells in the spleens of GPR81−/− and WT mice (data not shown), suggesting that the increase in the numbers of Th1 and Th17 cells in GPR81−/− mice is specific to colonic LP. Thus, GPR81 in the colon is critical for maintaining the balance between regulatory T cells and CD4+ T effector populations.
Pharmacological activation of GPR81 suppresses colonic inflammation
Next, we examined the effects of pharmacological activation of GPR81 in the Rag-deficient T-cell-transfer model of colitis by treating mice with GPR81-specific agonist (3-chloro-5-hydroxybenzoic acid) (49), with treatments starting post-T cell transfer. As expected, control Rag2−/− mice adoptively transferred with naïve T cells showed rapid body weight loss around 4 weeks post-T cell transfer (Fig. 5A). In contrast, GPR81 agonist treatment significantly delayed disease onset and reduced disease severity (Fig. 5B, C). In line with these observations, the percentages of IFNγ+ and IL-17A+ CD4+ T cells, as well as IL-17A and IFN-γ cytokine levels, were markedly reduced in GPR81-agonist treated mice as compared to control mice (Fig. 5D, E, F). In contrast, we observed a significant increase in IL-10+ CD4+ Tr1 cells, as well as colonic IL-10 cytokine levels, in response to GPR81-agonist treatment (Fig. 5D, E, F). On the contrary, adoptive transfer of CD45RBhiCD4+ T cells isolated from WT mice into Rag2−/−/GPR81−/− mice resulted in similar levels of disease severity with or without GPR81-agonist treatment (Supplementary Fig. 4C). Collectively, these results suggest that activation of the GPR81 pathway suppresses intestinal inflammation by promoting various innate immune regulatory functions of APCs and supporting the induction of IL-10-producing Tr1 vs. Th1/Th17 cell differentiation.
Figure 5. Pharmacological activation of GPR81 ameliorates mucosal inflammation.
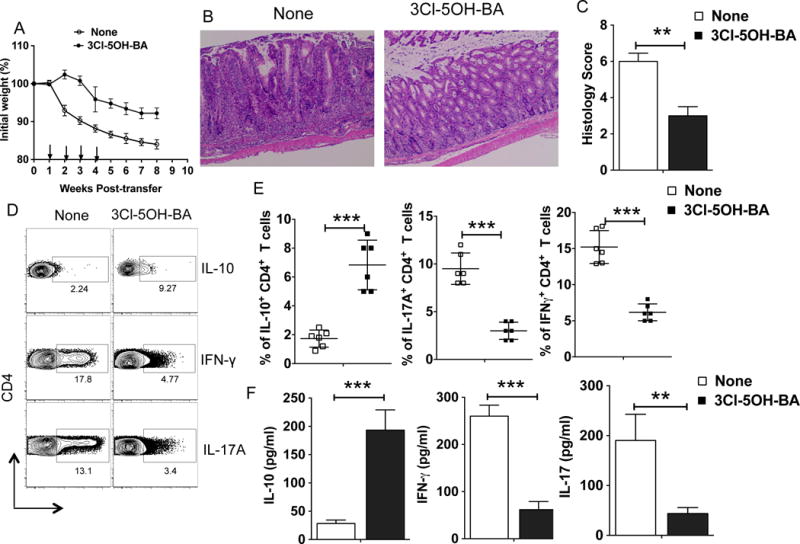
(A-E) CD45RBhiCD4+ T cells isolated from WT mice were adoptively transferred into Rag2−/− mice. Animals were treated with GPR81 agonist orally (3-chloro-5-hydroxybenzoic acid (3Cl-5OH-BA); 10 mg/kg; on Weeks 1, 2, 3 and 4) and monitored over a period of time for percent weight loss compared to initial weight. (A) Percent weight change for Rag2−/− mice treated with 3Cl-5OH-BA compared with untreated mice at various weeks post-naïve CD4+ T cell adoptive transfer. (B, C) Representative colon histology (H&E staining with original 10X magnification) and histology scores of Rag2−/− mice 6 weeks post-naïve CD4+ T cell transfer treated with or without 3Cl-5OH-BA treatment. (D, E) Representative FACS plots and cumulative frequencies of colonic IL-17A+, IFN-γ+ and IL-10+ CD4+ T cells from Rag2−/− mice treated with 3Cl-5OH-BA treatment compared with untreated mice on week 6 post-naïve CD4+ T cell adoptive transfer. (F) Excised colon samples in panel C were cultured for 2 days ex vivo, and then the secreted IL-17A, IFN-γ and IL-10 cytokine amounts in the culture supernatants were quantified by ELISA. Data are representative of three independent experiments (n>6). The error bars indicate mean ± SEM. **p<0.01; ***p<0.001.
Discussion
The immune system in the intestine maintains a state of tolerance to commensal flora and ingested food antigens while mounting robust responses against pathogens (17, 39). Intestinal DCs and macrophages are important in this process but the mechanisms underlying this phenomenon remain poorly understood. The current study demonstrates for the first time an essential role for GPR81 in intestinal APCs in the suppression of inflammation and restoration of homeostasis in the gut. A key mechanism contributing to this role of GPR81 involves the induction of immune regulatory genes (IL-10, RA and IDO) with suppression of the expression of inflammatory cytokines in DCs and macrophages, resulting in the maintenance of the balance between the regulatory T cell and Th1/Th17 cell frequencies in the colon. Accordingly, GPR81−/− mice were highly susceptible to intestinal inflammation. Conversely, 3-chloro-5-hydroxybenzoic acid, a pharmacological GPR81 agonist, suppressed colitis by limiting Th1/Th17 cell differentiation and enhancing regulatory T cell differentiation. Collectively, these findings support the hypothesis that GPR81 has an immune regulatory role in the colon. Hence, this pathway could be a new target for colitis therapy.
GPR81 is expressed at higher levels in fat cells but at lower levels in secondary lymphoid tissues, gut, brain, kidney and many other tissues (3, 4, 23). Recent studies have shown that GPR81 is highly expressed in peritoneal macrophages(15, 23). Our study shows that intestinal DCs and macrophages express higher levels of GPR81 compared to splenic DCs and macrophages. Like gut DCs, DCs isolated from the mesenteric lymph node (MLN) and lung express relatively higher levels of GPR81 compared to T and B cells. These results suggest that GPR81 expression in mucosal DCs and macrophages may be regulated by the tissue microenvironment. An important unresolved question is how GPR81 expression is regulated in the gut microenvironment. A previous study has shown that PPARγ transcriptionally regulates GPR81 expression in adipocytes(50). Dietary lipids and their metabolites are widely present in the intestine and are known to activate members of the peroxisome proliferative-activated receptor (PPAR) family of transcription factors(51–53). Intestinal APCs express high levels of PPAR isoforms and the PPAR pathway is highly active in intestinal DCs and macrophages (54–56). It is possible that PPARγ-mediated signaling might regulate GPR81 expression in gut APCs. However, further studies are warranted to confirm a direct link between PPARγ and GPR81-expression in the gut.
Lactate activates GPR81 in its physiological concentration range of 1–20 mM (3). The gastrointestinal mucosa is exposed to high levels of lactate (~10 mM) from diet and bacterial fermentation (8). So, it is conceivable that lactate concentrations in the colon are sufficient to activate GPR81. Past studies have shown that lactate can modulate the innate immune functions of DCs and macrophages (15, 21–23). Lactate was also shown to suppress LPS-induced inflammatory cytokines in both a GPR81-dependent and -independent manner (15, 23, 57). Our study shows that treatment with 3-chloro-5-hydroxybenzoic acid can protect mice from intestinal inflammation by inducing regulatory responses and suppressing inflammatory responses. Interestingly, GPR81, together with another receptor such as GPR109a and GPR43, appears to play a similar but non-redundant role by regulating innate immune functions of intestinal APCs and the differentiation of Treg/Th1/Th17 cells, resulting in suppression of intestinal inflammation.
In the intestine, T cell priming and differentiation happens in the draining lymph nodes, whereas the activation and expansion of effector T cells occurs in the lamina propria (44–46). A delicate balance between regulatory T cells versus pathological effector T cells underlies disease progression in many inflammatory diseases, including IBD (17, 20, 38, 39). Intestinal DCs and macrophages play a critical role in controlling this balance between tolerance and inflammation (17, 20, 38, 39). These cells suppress inflammation by expressing immune regulatory factors such as retinoic acid (RA), IL-10 and IDO that are critical for driving regulatory T cell differentiation while limiting the differentiation and expansion of pathological Th1/Th17 cells in the gut. Our study shows that GPR81-signaling in intestinal APCs is critical for the induction of immune regulatory factors and suppression of inflammatory cytokines. Genetic deletion of GPR81 in mice resulted in reduced numbers of Tr1 cells and Foxp3+ Tregs with a concomitant increase in the frequencies of Th1/Th17 cells in the colon. This imbalance in T cell subsets was due to increased expression of inflammatory cytokines by colonic APCs that drive Th1/Th17 cell differentiation. Though not analyzed in the present study, GPR81-mediated signals in intestinal APCs might modulate inflammatory responses in the intestine by regulating the suppressive function of Tregs.
Microflora in the gut play a critical role in the induction of CD4+ effector T cells and regulatory T cells (42, 58). Loss of immune tolerance to commensal microflora or commensal dysbiosis results in host susceptibility to colonic inflammation. So, the observed increase in inflammatory cytokine levels and Th1/Th17 cells in the colon of GPR81−/− mice might be due to the loss of immune tolerance to commensal flora or alterations in the composition of gut flora. In line with this observation, recent studies have shown that GPR81-mediated signals can suppress the expression of inflammatory cytokines produced in response to TLR-stimulation (15). Our study also shows that GPR81−/− mice is susceptible to both DSS-induced colitis as well as T cell-transfer colitis, demonstrating an important role for this receptor in suppressing intestinal inflammation. A similar protective role for GPR81 was observed in hepatic, pancreatic and ischemic brain injury (14, 15). Likewise, a recent study has shown that GPR81-mediated signaling suppresses uterine inflammation during labor(57). However, it remains to be determined how GPR81-mediated signaling in intestinal APCs regulates the expression of immune regulatory versus pro-inflammatory factors.
In summary, our study reveals a novel role for GPR81 in DCs and macrophages in regulating immune homeostasis and inflammation in the intestine. Additionally, pharmacological activation of GPR81 suppressed intestinal inflammation by inducing regulatory T cell responses and limiting pathological Th1/Th17 responses. Furthermore, deletion of GPR81 resulted in increased expression of inflammatory cytokines that drive Th1/Th17 responses and intestinal inflammation. These findings have important implications for prevention as well as treatment of inflammatory bowel disease.
Supplementary Material
Acknowledgments
We thank Dr. Stefan Offermanns (Max-Planck-Institute for Heart and Lung Research, Germany) for kindly providing GPR81−/− mice. We thank Jeanene Pihkala and Ningchun Xu for technical help with FACS sorting and analysis; Janice Randall with mouse husbandry, as well as our colleagues in the Augusta University Cancer Immunology, Inflammation and Tolerance program for constructive comments on various aspects of this study.
This work was supported by National Institutes of Health Grants DK097271 (to SM).
Footnotes
Conflict of Interest: Authors disclose no conflict of interest.
References
- 1.Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, Lewis T, Evans JF, George SR, O’Dowd BF. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene. 2001;275:83–91. doi: 10.1016/s0378-1119(01)00651-5. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed K, Tunaru S, Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 2009;30:557–562. doi: 10.1016/j.tips.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, Mazur C, Kamme F, Lovenberg TW. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 4.Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen J, Bergersen LH. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J Neurosci Res. 2015;93:1045–1055. doi: 10.1002/jnr.23593. [DOI] [PubMed] [Google Scholar]
- 5.Ge H, Weiszmann J, Reagan JD, Gupte J, Baribault H, Gyuris T, Chen JL, Tian H, Li Y. Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J Lipid Res. 2008;49:797–803. doi: 10.1194/jlr.M700513-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Garrote GL, Abraham AG, Rumbo M. Is lactate an undervalued functional component of fermented food products? Front Microbiol. 2015;6:629. doi: 10.3389/fmicb.2015.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada T, Fukuda S, Hase K, Nishiumi S, Izumi Y, Yoshida M, Hagiwara T, Kawashima R, Yamazaki M, Oshio T, Otsubo T, Inagaki-Ohara K, Kakimoto K, Higuchi K, Kawamura YI, Ohno H, Dohi T. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat Commun. 2013;4:1654. doi: 10.1038/ncomms2668. [DOI] [PubMed] [Google Scholar]
- 10.Bourriaud C, Robins RJ, Martin L, Kozlowski F, Tenailleau E, Cherbut C, Michel C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J Appl Microbiol. 2005;99:201–212. doi: 10.1111/j.1365-2672.2005.02605.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Z, Jiang L, Yuan Y, Deng T, Zheng YR, Zhao YY, Li WL, Wu JY, Gao JQ, Hu WW, Zhang XN, Chen Z. Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury. CNS Neurosci Ther. 2015;21:271–279. doi: 10.1111/cns.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763–1774. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asquith M, Powrie F. An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med. 2010;207:1573–1577. doi: 10.1084/jem.20101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 18.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rescigno M. Dendritic cells in oral tolerance in the gut. Cell Microbiol. 2011;13:1312–1318. doi: 10.1111/j.1462-5822.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 20.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 22.Nasi A, Rethi B. Disarmed by density: A glycolytic break for immunostimulatory dendritic cells? Oncoimmunology. 2013;2:e26744. doi: 10.4161/onci.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Errea A, Cayet D, Marchetti P, Tang C, Kluza J, Offermanns S, Sirard JC, Rumbo M. Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner. PLoS One. 2016;11:e0163694. doi: 10.1371/journal.pone.0163694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iraporda C, Romanin DE, Bengoa AA, Errea AJ, Cayet D, Foligne B, Sirard JC, Garrote GL, Abraham AG, Rumbo M. Local Treatment with Lactate Prevents Intestinal Inflammation in the TNBS-Induced Colitis Model. Front Immunol. 2016;7:651. doi: 10.3389/fimmu.2016.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. 2015;74:13–22. doi: 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J, Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Manicassamy S, Manoharan I. Mouse models of acute and chronic colitis. Methods in molecular biology. 2014;1194:437–448. doi: 10.1007/978-1-4939-1215-5_25. [DOI] [PubMed] [Google Scholar]
- 28.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlick KP, Ostanin DV, Furr KL, Laroux FS, Brown CM, Gray L, Kevil CG, Grisham MB. Role of T-cell-associated lymphocyte function-associated antigen-1 in the pathogenesis of experimental colitis. International immunology. 2006;18:389–398. doi: 10.1093/intimm/dxh378. [DOI] [PubMed] [Google Scholar]
- 30.Suryawanshi A, Manoharan I, Hong Y, Swafford D, Majumdar T, Taketo MM, Manicassamy B, Koni PA, Thangaraju M, Sun Z, Mellor AL, Munn DH, Manicassamy S. Canonical wnt signaling in dendritic cells regulates Th1/Th17 responses and suppresses autoimmune neuroinflammation. J Immunol. 2015;194:3295–3304. doi: 10.4049/jimmunol.1402691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 33.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. The Journal of experimental medicine. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. The Journal of experimental medicine. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harusato A, Flannigan KL, Geem D, Denning TL. Phenotypic and functional profiling of mouse intestinal antigen presenting cells. J Immunol Methods. 2015;421:20–26. doi: 10.1016/j.jim.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 37.De Calisto J, Villablanca EJ, Mora JR. FcgammaRI (CD64): an identity card for intestinal macrophages. Eur J Immunol. 2012;42:3136–3140. doi: 10.1002/eji.201243061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 39.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison OJ, Powrie FM. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Spencer SP, Belkaid Y. Dietary and commensal derived nutrients: shaping mucosal and systemic immunity. Current opinion in immunology. 2012;24:379–384. doi: 10.1016/j.coi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houston SA, Cerovic V, Thomson C, Brewer J, Mowat AM, Milling S. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol. 2016;9:468–478. doi: 10.1038/mi.2015.77. [DOI] [PubMed] [Google Scholar]
- 45.Veenbergen S, van Berkel LA, du Pre MF, He J, Karrich JJ, Costes LM, Luk F, Simons-Oosterhuis Y, Raatgeep HC, Cerovic V, Cupedo T, Mowat AM, Kelsall BL, Samsom JN. Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103(+) dendritic cells. Mucosal Immunol. 2016;9:894–906. doi: 10.1038/mi.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen LP, Pan J, Dinh TT, Hadeiba H, O’Hara E, 3rd, Ebtikar A, Hertweck A, Gokmen MR, Lord GM, Jenner RG, Butcher EC, Habtezion A. Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat Immunol. 2015;16:207–213. doi: 10.1038/ni.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dvorak CA, Liu C, Shelton J, Kuei C, Sutton SW, Lovenberg TW, Carruthers NI. Identification of Hydroxybenzoic Acids as Selective Lactate Receptor (GPR81) Agonists with Antilipolytic Effects. ACS Med Chem Lett. 2012;3:637–639. doi: 10.1021/ml3000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeninga EH, Bugge A, Nielsen R, Kersten S, Hamers N, Dani C, Wabitsch M, Berger R, Stunnenberg HG, Mandrup S, Kalkhoven E. Peroxisome proliferator-activated receptor gamma regulates expression of the anti-lipolytic G-protein-coupled receptor 81 (GPR81/Gpr81) J Biol Chem. 2009;284:26385–26393. doi: 10.1074/jbc.M109.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viladomiu M, Hontecillas R, Yuan L, Lu P, Bassaganya-Riera J. Nutritional protective mechanisms against gut inflammation. The Journal of nutritional biochemistry. 2013;24:929–939. doi: 10.1016/j.jnutbio.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bassaganya-Riera J, Hontecillas R. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease. Current opinion in clinical nutrition and metabolic care. 2010;13:569–573. doi: 10.1097/MCO.0b013e32833b648e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutrition journal. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nature reviews Immunology. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 55.Bertin B, Dubuquoy L, Colombel JF, Desreumaux P. PPAR-gamma in ulcerative colitis: a novel target for intervention. Current drug targets. 2013;14:1501–1507. doi: 10.2174/13894501113149990162. [DOI] [PubMed] [Google Scholar]
- 56.Manoharan I, Suryawanshi A, Hong Y, Ranganathan P, Shanmugam A, Ahmad S, Swafford D, Manicassamy B, Ramesh G, Koni PA, Thangaraju M, Manicassamy S. Homeostatic PPARalpha Signaling Limits Inflammatory Responses to Commensal Microbiota in the Intestine. J Immunol. 2016;196:4739–4749. doi: 10.4049/jimmunol.1501489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madaan A, Nadeau-Vallee M, Rivera JC, Obari D, Hou X, Sierra EM, Girard S, Olson DM, Chemtob S. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1) Am J Obstet Gynecol. 2017;216:60 e61–60 e17. doi: 10.1016/j.ajog.2016.09.072. [DOI] [PubMed] [Google Scholar]
- 58.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


