Abstract
The IL-6/JAK/STAT3 pathway is aberrantly hyperactivated in many types of cancer, and such hyperactivation is generally associated with a poor clinical prognosis. In the tumour microenvironment, IL-6/JAK/STAT3 signalling acts to drive the proliferation, survival, invasiveness, and metastasis of tumour cells, while strongly suppressing the antitumour immune response. Thus, treatments that target the IL-6/JAK/STAT3 pathway in patients with cancer are poised to provide therapeutic benefit by directly inhibiting tumour cell growth and by stimulating antitumour immunity. Agents targeting IL-6, the IL-6 receptor, or JAKs have already received FDA approval for the treatment of inflammatory conditions or myeloproliferative neoplasms and for the management of certain adverse effects of chimeric antigen receptor T cells, and are being further evaluated in patients with haematopoietic malignancies and in those with solid tumours. Novel inhibitors of the IL-6/JAK/STAT3 pathway, including STAT3-selective inhibitors, are currently in development. Herein, we review the role of IL-6/JAK/STAT3 signalling in the tumour microenvironment and the status of preclinical and clinical investigations of agents targeting this pathway. We also discuss the potential of combining IL-6/JAK/STAT3 inhibitors with currently approved therapeutic agents directed against immune-checkpoint inhibitors.
The IL-6/JAK/STAT3 pathway has a key role in the growth and development of many human cancers. Elevated levels of IL-6 are observed in chronic inflammatory conditions, such as rheumatoid arthritis and inflammatory bowel disease, and in a large number of patients with haematopoietic malignancies or solid tumours1. In the pathogenesis of cancer, elevated levels of IL-6 stimulate hyperactivation of JAK/STAT3 signalling, which is often associated with poor patient outcomes2–5. Furthermore, the genes encoding JAK enzymes, particularly JAK2, are frequently mutated in myeloproliferative neoplasms, leading to constitutive activation of JAK/STAT3 signalling. Hyperactivation of STAT3 signalling occurs in the majority of human cancers and also correlates with a poor prognosis. STAT3 hyperactivation in tumour cells can occur as a result of elevated IL-6 levels in the serum and/or in the tumour microenvironment, owing to signals from other growth factors and/or their receptors, activation by non-receptor tyrosine kinases (such as SRC and BCR–ABL1), or loss-of-function mutations affecting negative regulators of STAT3. These negative regulators include members of the protein inhibitor of activated STAT (PIAS) and suppressor of cytokine signalling (SOCS) families as well as several cellular phosphatases (tyrosine-protein phosphatase non-receptor type 6 (SHP1; also known as PTPN6), tyrosine-protein phosphatase non-receptor type 11 (SHP2), dual specificity protein phosphatase 22 (DUSP22), receptor-type tyrosine-protein phosphatase-δ (PTPRD), receptor-type tyrosine-protein phosphatase T (PTPRT), tyrosine-protein phosphatase non-receptor type 2 (PTPN2) and tyrosine-protein phosphatase non-receptor type 1 (PTPN1))6–11. Aberrant expression of microRNAs (miRNAs) that regulate STAT3 expression can also contribute to elevated STAT3 activity in tumours.
IL-6 is produced by multiple cell types located within the tumour microenvironment, including tumour-infiltrating immune cells, stromal cells, and the tumour cells themselves1,12–15. IL-6 acts directly on tumour cells to induce the expression of STAT3 target genes, which encode proteins that then drive tumour proliferation (such as cyclin D1) and/or survival (such as BCL2-like protein 1 (BCL-xL)). The ability of STAT3 to promote IL6 gene expression then results in a feedforward autocrine feedback loop16. STAT3 also induces the expression of factors that promote angiogenesis, such as VEGF; invasiveness and/or metastasis, such as matrix metalloproteinases (MMPs); and immunosuppression, such as IL-10 and TGFβ (in addition to VEGF and IL-6)14,17,18.
In addition to direct effects on tumour cells, IL-6 and JAK/STAT3 signalling can have a profound effect on tumour-infiltrating immune cells. STAT3 is often hyperactivated in tumour-infiltrating immune cells and exerts negative regulatory effects on neutrophils, natural killer (NK) cells, effector T cells, and dendritic cells (DCs), suggesting that STAT3 activation in immune cells likely leads to downmodulation of antitumour immunity19–29. At the same time, STAT3 positively regulates regulatory T (Treg) cells and myeloid-derived suppressor cell (MDSC) populations17,19. Collectively, these effects contribute to a highly immunosuppressive tumour microenvironment.
The understanding that IL-6/JAK/STAT3 signalling promotes tumour growth and progression while severely hindering antitumour immunity has stimulated the search for clinical agents that can effectively inhibit this pathway. Siltuximab and tocilizumab are antibodies that target IL-6 and the IL-6 receptor-α (subsequently referred to as IL-6R), respectively, and have been approved by the FDA for the treatment of multicentric Castleman disease (siltuximab), arthritis (tocilizumab), and chimeric antigen receptor (CAR) T cell-induced cytokine-release syndrome (tocilizumab). Similarly, tofacitinib is a small-molecule tyrosine kinase inhibitor that primarily targets JAK1 and JAK3 and has been approved by the FDA for the treatment of arthritis, whereas ruxolitinib is a small-molecule inhibitor of JAK1 and JAK2 and is approved for use in patients with myelofibrosis or polycythaemia vera. Clinical evaluations of these agents in patients with haematopoietic or solid tumours are currently ongoing. Moreover, a large number of novel IL-6, IL-6R, JAK, and STAT3 inhibitors are currently the subject of preclinical and/or clinical investigations. In this Review, we summarize our current understanding of the role of IL-6/JAK/STAT3 signalling in cancer and in antitumour immunity, and the progress being made towards the development of clinical agents targeting this vital signalling pathway. Perspective is offered on the prospect of combining IL-6/JAK/STAT3 inhibitors with antibodies targeting the immune-checkpoint proteins programmed cell death protein 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), and cytotoxic T lymphocyte protein 4 (CTLA-4).
The IL-6/JAK/STAT3 signalling pathway
IL-6
Chronic inflammation promotes the development and progression of tumours. IL-6 can be expressed at high levels in the tumour microenvironment and is a major mediator of inflammation. In addition, IL-6 can directly stimulate the proliferation, survival, and invasiveness of tumour cells. IL-6 also induces the production of pro-inflammatory and angiogenesis-promoting factors, including IL-1β, IL-8, C-C motif chemokine (CCL)2, CCL3, CCL5, GM-CSF, and VEGF, which act in an autocrine and/or paracrine fashion on immune and non-immune cells within the tumour microenvironment30. Together, these effects underscore the important role that IL-6 has in a variety of different cancers as well as the prognostic value of circulating IL-6 levels in patients with this disease.
The IL-6 protein is 21–28 kDa in size, depending on the extent of glycosylation. A viral form of IL-6, encoded by human herpesvirus 8, has approximately 25% homology with human IL-6 (REFS 31,32). IL-6 was first identified as a factor capable of promoting B cell development and regulating the acute-phase immune response33,34. Loss of IL-6 signalling reduces the effectiveness of both innate and adaptive immune responses to invading microorganisms and parasites35. Notably, IL-6-deficient mice are resistant to both antigen-induced and collagen-induced arthritis and to multicentric Castleman disease36–38. Elevated levels of IL-6 are seen in patients with arthritis or Castleman disease, and this observation has stimulated the successful clinical application of inhibitors of IL-6 signalling in the treatment of patients with these conditions39,40.
IL-6 is produced in the tumour by infiltrating immune cells, by the tumour cells themselves, and by stromal cells. Thus, tumour-associated macrophages, granulocytes, and fibroblasts, as well as cancer cells, are all primary sources of IL-6 (REFS 1,12–14). Adipocytes, T cells, and MDSCs also contribute to the elevated levels of IL-6 seen in tumours1,14,15. Nuclear factor-κB (NF-κB) is a key transcription factor that drives the expression of IL-6 (REF. 41). Notably, hyperactivation of NF-κB is commonly observed in many human cancers. Hyperactivation of STAT3 in tumour cells also induces the production of IL-6, thus generating a positive-feedback loop16.
The IL-6R
IL-6 signalling is mediated by two different pathways: the classic signalling pathway and the trans-signalling pathway (FIG. 1). The classic signalling pathway involves binding of IL-6 to IL-6R on the cell surface and the subsequent interaction of this complex with the membrane-spanning protein IL-6 receptor subunit-β (gp130; also known as IL-6Rβ) to initiate intracellular signalling. In the trans-signalling pathway, IL-6 binds to a secreted form of the IL-6R (sIL-6R), followed by interaction of the IL-6–sIL-6R complex with gp130. Each pathway regulates distinct biological effects of IL-6. Classic signalling is particularly important for the acute-phase immunological response, haematopoiesis, and central homeostatic processes35. Trans-signalling has a key role in the tumour microenvironment, acting to control the recruitment of leukocytes and the inflammatory activation of tumour-associated stromal cells35,42.
Fig. 1. IL-6 signalling pathways.
a | In the classic IL-6 signalling pathway, IL-6 binds to the membrane-bound IL-6 receptor-α (subsequently referred to as IL-6R), thus inducing formation of a heterohexameric complex consisting of two molecules each of IL-6, IL-6R, and the IL-6 receptor subunit-β (gp130). Formation of this complex results in activation of the JAK/STAT3 signalling pathway, leading to the transcription of STAT3 target genes. The IL-6/IL-6R/gp130 complex can also activate the PI3K/AKT/mTOR (mechanistic target of rapamycin) and RAS/RAF/MEK/ERK pathways (not pictured). b | In the IL-6 trans-signalling pathway, soluble IL-6R (sIL-6R) binds to IL-6. sIL-6R can be generated by alternative splicing of IL6R mRNA or cleavage of membrane-bound IL-6R by disintegrin and metalloproteinase domain-containing protein 170(ADAM10) or ADAM17. When IL-6 binds with sIL-6R, this complex is then able to bind to and induce the dimerization of gp130, leading to the activation of downstream signalling pathways (as described above for classic IL-6 signalling pathways). gp130 is ubiquitously expressed, although the IL-6R is expressed in only a limited number of cell types. Trans-signalling via sIL-6R enables IL-6 to act on cells with limited or absent IL-6R expression. IL-6 trans-signalling can be negatively regulated by soluble gp130 (sgp130), which is generated by alternative splicing. This molecule competes with membrane-bound gp130 for binding to the IL-6–sIL-6R complex, thereby inhibiting IL-6 trans-signalling but not the classic IL-6 signalling pathway.
During classic signalling, IL-6 binds to the IL-6R, which is a single membrane-spanning protein of 80 kDa with a short cytoplasmic domain that lacks signalling capacity. Expression of IL-6R is largely restricted to specific subsets of leukocytes, megakaryocytes, hepatocytes, and certain barrier epithelial cells, thus limiting classic IL-6 signalling to these cell types43,44. Intracellular signalling is initiated by the formation of the heterohexameric complex, consisting of IL-6, IL-6R, and gp130, followed by the recruitment of cellular signalling proteins, including JAKs and STAT3 (REF. 45)(FIG. 1). Unlike IL-6R, gp130 is expressed on most cell types and is a shared co-receptor for other cytokines and growth factors, including IL-11, IL-27, LIF, oncostatin M, CNTF, CT1 (also known as CTF1), neuropoietin, humanin, and cardiotrophin-like cytokine6.
The discovery of trans-signalling was aided by the detection of sIL-6R in serum and urine samples46,47. The findings of subsequent studies demonstrated that sIL-6R are generated by proteolytic cleavage of the IL-6R by the disintegrin and metalloproteinase domain-containing protein 17 (ADAM17) or ADAM10 proteases or by alternative splicing48,49. The IL-6–sIL-6R complex retains the capacity to bind and activate signalling via gp130 (FIG. 1). Importantly, because gp130 is ubiquitously expressed, cells that do not express the IL-6R can respond to IL-6 through the trans-signalling pathway. Thus, shedding of sIL-6R by tumour-infiltrating neutrophils, monocytes, and T cells provides an opportunity for IL-6 to activate signalling in tumour and stromal cells with low levels of IL-6R expression and in those that do not express IL-6R35.
Alternative splicing can generate four different secreted forms of gp130 (sgp130)35,50. Secreted sgp130 proteins bind to the IL-6–sIL-6R complex and selectively inhibit trans-signalling51 (FIG. 1). This effect seems to be specific to IL-6 signalling as sgp130 has not been reported to affect signalling by other cytokines that activate signalling pathways involving gp130 (REF. 52). The inhibitory activity of sgp130 has provided the basis for the development of agents and strategies designed to selectively inhibit IL-6-induced trans-signalling in patients.
JAKs
IL-6 signalling, via either the classic or trans-signalling pathways, involves the engagement of gp130, which leads to activation of gp130-associated JAKs. Four mammalian JAKs have been identified, JAK1, JAK2, JAK3, and TYK2, all of which are expressed in human cells, share considerable structural similarity, and have molecular masses ranging from 120 to 140 kDa. JAK1, JAK2, and TYK2 are widely expressed, while expression of JAK3 is largely restricted to cells of a haematopoietic origin53. The carboxyl-terminal regions of JAKs contain a JAK homology (JH)1 domain, in which the tyrosine kinase domain is located. The JH1 domain is preceded by a pseudokinase domain (JH2), which interacts with the JH1 domain to restrain kinase activity in unstimulated cells6. Engagement of gp130 by the IL-6–IL-6R complex results in selective activation of JAK1, JAK2, and/or TYK2 via associations of these enzymes with membrane proximal domains (Box domains 1 and 2) in the gp130 protein54 (FIG. 2). These interactions disrupt JH2-mediated inhibition of JH1 kinase activity, resulting in reciprocal transphosphorylation and full activation of JAKs. The activated JAK enzymes subsequently phosphorylate multiple tyrosine residues in the cytoplasmic region of gp130, which then serve as docking sites for proteins initiating the PI3K/AKT, RAS/RAF/MEK/MAPK, and JAK/STAT3 signalling pathways.
Fig. 2. Signalling downstream of the IL-6 receptor.
JAK proteins bind to the Box 1 and Box 2 domains in the intracellular portion of IL-6 receptor subunit-β (gp130). This leads to JAK-mediated phosphorylation of gp130 at several tyrosine residues, including four C-terminal residues that serve as docking sites for STAT3. Once bound to gp130, STAT3 is phosphorylated by JAKs at tyrosine 705, leading to STAT3 dimerization and nuclear translocation, followed by STAT3-mediated transcription of target genes. The figure shows signalling initiated by classic signalling. Trans-signalling pathways initiate downstream signalling in the same fashion. Tyrosine phosphorylation of STAT3 can also be induced by other oncogenic proteins, including SRC and BCR–ABL1. IL-6/JAK/STAT3 signalling is negatively regulated by a number of mechanisms. Suppressor of cytokine signalling (SOCS) 1 and SOCS3 bind to and inhibit the kinase activity of JAKs. SOCS3 is a STAT3 target gene; following transcription, SOCS3 then acts as a component of a negative-feedback loop that maintains tight regulation of this pathway. The following phosphatases also have a role in the negative regulation of this pathway: tyrosine-protein phosphatase non-receptor type 6 (SHP1; also known as PTPN6); tyrosine-protein phosphatase non-receptor type 11 (SHP2); dual specificity protein phosphatase 22 (DUSP22); receptor-type tyrosine-protein phosphatase-δ (PTPRD); receptor-type tyrosine-protein phosphatase T (PTPRT); tyrosine-protein phosphatase non-receptor type 1 (PTPN1); tyrosine-protein phosphatase non-receptor type 2 (PTPN2). Protein inhibitor of activated STAT3 (PIAS3), an E3 SUMO protein ligase, as well as PDZ and LIM domain protein 2 (PDLIM2), an ubiquitin E3 ligase, are additional endogenous proteins that inhibit STAT3 by mediating STAT3 degradation. The expression of PIAS3 and PDLIM2 can be inhibited by oncogenic microRNAs (miRNAs): miR-18a targets PIAS3, whereas miR-221 and miR-222 target PDLIM2. Another miRNA, miR-551b-3p, promotes STAT3 gene expression. Other miRNAs act to negatively regulate the IL-6/JAK/STAT3 pathway: expression of IL6R is inhibited by miR-218 and miR-34a, while STAT3 expression is inhibited by miR-17-5p, miR-20a, and miR-124.
STAT3
The JAK/STAT3 signalling pathway has a prominent role in mediating many of the effects of IL-6 on tumour cell proliferation, survival, invasion, and metastasis as well as suppressive effects on antitumour immunity. Among the seven members of the STAT protein family (STATs 1–4, 5A, 5B, and 6), STAT3 is the most strongly associated with the promotion of tumour growth and immunosuppression17,55 and is the only family member whose genetic deletion results in embryonic lethality56,57. In the inactive state, STAT3 exists as a monomer located in the cytoplasm. Following JAK-mediated tyrosine phosphorylation of growth factor receptors such as gp130, the SRC homology domain 2 (SH2) domain of STAT3 recognizes and binds to these phosphotyrosine docking sites, placing STAT3 within close proximity of active JAK enzymes, which subsequently phosphorylate STAT3 at Tyr705 (FIG. 2). Phosphorylation of Tyr705 results in SH2-domain-mediated, head-to-tail dimerization of the STAT3 protein and translocation of the STAT3 dimer to the nucleus via an importin-α–importin-β1-dependent mechanism58. In the nucleus, STAT3 binds to consensus response elements in the promoters of target genes, thus inducing the transcription of a broad panel of genes encoding regulators of cellular proliferation (such as cyclin D1 and MYC) and survival (such as BCL-xL and survivin) as well as angiogenesis-promoting (such as VEGF) and immunosuppressive growth factors and cytokines (such as IL-6)14,17. The ability to phosphorylate and/or activate STAT3 is not limited to JAKs, as SRC and BCR–ABL1 tyrosine kinases have also been shown to directly activate STAT3 (REFS 59,60). Additional post-translational modifications that might affect the function and/or stability of STAT3 have been reported, including phosphorylation of Ser727, and ubiquitylation, sumoylation, acetylation, or methylation of other residues61,62. Phosphorylation of Ser727, although less well studied than Tyr705 phosphorylation, can be mediated by several kinases, including JNK and other MAPKs and protein kinase C, and generally promotes STAT3 activity.
Stimulation of nonmalignant cells with IL-6 or other cytokines results in transient phosphorylation and/or activation of STAT3. In these cells, STAT3 activation can be controlled by at least three different classes of negative regulators: PIAS proteins, SOCS proteins (such as suppressor of cytokine signalling (SOCS) 1, 2, and 3), and several cellular phosphatases (SHP1, SHP2, DUSP22, PTPRD, PTPRT, and PTPN1 and 2)8,56,63,64. As described in a subsequent section, inhibition or reduced expression of these negative regulators can lead to constitutive activation of STAT3, an effect that is often observed in patients with cancer.
Signalling via the IL-6/JAK/STAT3 pathway is also regulated via a complex interplay with cellular miRNAs. Several miRNAs have been shown to dampen IL-6/JAK/STAT3 signalling by reducing expression of the components of this pathway, either in tumour cells or in tumour-infiltrating immune cells (FIG. 2). For example, miR-17-5p, miR-20a, and miR-124 reduce STAT3 expression, and miR-34a and miR-218 suppress IL-6R expression65–68. However, several miRNAs directly induce STAT3 upregulation (miR-551b-3p) or act to reduce expression of negative regulators of STAT3 (miR-18a, miR-221, and miR-222)69–71. Regarding the latter activity, miRNA-18a negatively regulates expression of E3 SUMO protein ligase PIAS3, while miR-221 and miR-222 suppress expression of PDZ and LIM domain protein 2 (PDLIM2), an E3 ubiquitin ligase that promotes polyubiquitylation and subsequent proteasomal degradation of STAT3. Altered expression of miRNAs that regulate cellular levels of STAT3 is likely to have a role in the hyperactivation of STAT3 signalling in cancer.
IL-6, JAKs, and STAT3 in cancer
IL-6, IL-6R, and gp130
IL-6 acts to recruit immune cells within the tumour microenvironment, therefore stimulating the production of additional pro-inflammatory cytokines. Thus, IL-6 serves as a key link between chronic inflammation and tumour progression. Similar to patients with arthritis and those with Castleman disease, or following infection, elevated IL-6 levels are observed in patients with a variety of different forms of cancer1. In particular, elevated circulating levels of IL-6 have been reported in patients with breast72, cervical73, colorectal74, oesophageal75, head-and-neck76,77, ovarian78,79, pancreatic80, prostate81, and renal82 cancers as well as in those with non-small-cell lung cancer (NSCLC)83 or multiple myeloma5. Furthermore, circulating IL-6 levels are increased by surgery84 and chemoradiation85, and are reported to be increased in patients with recurrent tumours1. Elevated serum IL-6 levels are also observed in patients with inflammatory bowel disease, and IL-6 levels generally correlate with tumour size, stage, and metastasis in patients with colorectal cancer86. In those with breast cancer, the highest levels of IL-6 are detected at the leading edges of the tumours, and IL-6 levels have been shown to be closely correlated with advanced-stage disease, as indicated by the number of tumour-positive lymph nodes16. Importantly, circulating IL-6 levels have been shown to be prognostic indicators of survival73,76,78,79,83,86,87 as well as predictors of a response to therapy75,85,88 in several different types of cancer.
Data from preclinical studies have confirmed that IL-6 signalling has important roles in tumour development. Findings from preclinical models and patient samples demonstrate that IL-6 promotes the development of breast16,89, colorectal90–92, lung (NSCLC)93, pancreatic94, and skin30 cancers. In breast cancer, IL-6 induces Notch 3 activation, thus promoting self-renewal of tumour stem cells89, and exogenous expression of IL-6 has been shown to promote breast cancer metastasis16. IL-6 signalling also stimulates epithelial-to-mesenchymal transition in breast95 and head-and-neck96 cancers. IL-6 signalling is also required for the survival of nonmalignant intestinal epithelial cells as well as the development of colitis-associated and sporadic forms of colorectal cancer92,97.
To date, no clinically relevant genomic alterations in the genes encoding IL-6, IL-6R, or gp130 have been detected in the tumour types analysed by The Cancer Genome Atlas (TCGA)98,99. However, activating mutations in gp130 have been shown to occur in approximately 60% of surgical specimens from patients with inflammatory hepatocellular adenomas100. Additionally, a single-nucleotide polymorphism (-174G>C) in the promoter region of the IL6 gene has been shown to result in increased expression of this cytokine101. Epigenetic alterations have a prominent role in aberrant activation of the IL-6/IL-6R/JAK/STAT3 pathway in cancer, and changes in the expression and/or activation of transcription factors might have a prominent role in the elevated expression of IL-6 in cancer.
JAKs
JAKs mediate the activation of STAT3 in both nonmalignant and malignant cells exposed to IL-6. The anti-inflammatory effects of JAK inhibitors, which have been broadly investigated, are largely attributed to inhibition of STAT3 and/or STAT5 activation. Much of the interest in the role of JAKs in cancer and the clinical application of JAK inhibitors has focused on haematological malignancies and conditions involving chronic inflammation6,102–106. For example, the JAK2V617F mutation is found in >95% of patients with polycythaemia vera and results in a JAK2 enzyme that is constitutively active independent of cytokine signalling102,106–108. JAK2V617F is also observed in patients with essential thrombocythemia and in those with primary myelofibrosis. Additional JAK2 mutations occur in B cell acute lymphoblastic leukaemia (B-ALL), Down syndrome ALL, Hodgkin lymphoma, and B cell lymphoma105,106. In paediatric T cell acute lymphoblastic leukaemia (T-ALL), a chromosomal translocation results in the constitutively active transcription factor ETV6 (TEL)–JAK2 fusion protein109. Moreover, mutations leading to dysregulation of JAK1 activity have been reported in B-ALL, adult T-ALL, and essential thrombocythemia106. Similarly, JAK3 mutations are also found in patients with B-ALL, adult T-ALL, and essential thrombocythemia106. Mutations in the genes encoding JAK enzymes seem to be much less common in solid tumours. However, JAK1 is mutated in 3.8% and 11.5% of hepatocellular carcinomas and endometrial cancers, respectively, including the presence of activating mutations98,99,110.
STAT3
Aberrantly elevated STAT3 activity has been estimated to occur in >70% of human cancers111,112. Malignancies in which hyperactivation of STAT3 has been reported include acute myeloid leukaemia (AML), multiple myeloma, and solid tumours of the bladder, bone, breast, brain, cervix, colon, oesophagus, head-and-neck, kidney, liver, lung, ovary, pancreas, prostate, stomach, and uterus113–125. The levels of phosphorylated and/or activated STAT3 have been shown to correlate with a poor clinical prognosis in several of these cancers. Exogenous expression of a constitutively active form of STAT3 confers anchorage-independent growth and tumorigenic capacity on fibroblasts, thus demonstrating the oncogenic activity of the STAT3 protein126.
Preclinical studies aimed at modulating the expression and/or function of STAT3 have demonstrated important roles of STAT3 in the development and/or progression of multiple cancers, including bladder, colorectal, head-and-neck, lung, pancreatic, prostate, and skin cancers127–133. STAT3 also promotes resistance to conventional chemotherapy and radiation therapy as well as to targeted therapies, such as cetuximab134,135. Indeed, activation of STAT3 via a positive-feedback loop constitutes a primary mechanism of resistance in drug-treated, oncogene-addicted cells136. These preclinical data suggest that agents and approaches that block the activity of STAT3 in tumour cells could have substantial additional value in preventing or reversing anticancer drug resistance.
The effects of STAT3 activation on the growth of tumour cells are mediated, in large part, by the STAT3-mediated induction of key target genes that regulate cellular proliferation and metabolism, suppression of apoptosis, and responses to hypoxia14. Activated STAT3 also induces the expression of VEGF and selected MMPs, which promote angiogenesis and invasiveness and/or metastasis, respectively18. In addition, STAT3 binds to the IL6 promoter, generating a positive-feedback loop, leading to increased IL-6 expression16. Both VEGF and IL-6 can also have immunosuppressive effects, which might facilitate immune evasion by tumour cells harbouring hyperactivated STAT3. The tumour-promoting effects of STAT3 might also be mediated by induction of miR-21 and miR-181b-1, which suppress the expression of the tumour suppressors PTEN and ubiquitin carboxyl-terminal hydrolase CYLD, respectively137.
Emerging evidence indicates that STAT3 is also hyperactivated in tumour-infiltrating immune cells and might have profound effects on antitumour immunity19,20. Conditional deletion of the Stat3 gene in murine haematopoietic cells has revealed several potent immunosuppressive effects of STAT3, including negative regulation of neutrophil and NK cell function, induction of PD-1 expression, inhibition of effector T cell function, inhibition of DC maturation and function, and expansion of Treg cell and MDSC numbers in the tumour microenvironment19,21–29,138. STAT3-mediated induction of immunosuppressive factors in the tumour microenvironment, including IL-6, IL-10, TGFβ, and VEGF, stimulates positive-feedback amplification of STAT3 activation in both tumour cells and tumour-infiltrating immune cells17,20,29,139,140. The end result of this complex crosstalk between cancer and immune cells in the tumour microenvironment is increased tumour cell growth and survival, with diminished antitumour immunity. Collectively, these observations suggest that selectively targeting STAT3 in cancer could provide multiple benefits: inhibition of cell-autonomous effects on tumour cell growth and metastasis; inhibition of cell-autonomous immunosuppressive effects in infiltrating immune cells; and inhibition of immunosuppressive crosstalk between tumour cells and tumour-infiltrating immune cells.
STAT3 mutations are rare and primarily restricted to patients with haematological malignancies. Mutations in the exon encoding the STAT3 SH2 domain were originally identified by Koskela et al.141 in 31 of a cohort of 77 patients with large granular lymphocytic (LGL) leukaemia. Additional activating mutations in the SH2 domain have been reported in NK and T cell lymphomas142. Activating mutations outside of the SH2 domain have also been identified by Andersson et al.143 in patients with LGL leukaemia.
Hyperactivation of STAT3 in tumours can occur through a variety of mechanisms. Elevated expression of IL-6 and increased stimulation of IL-6R commonly result in hyperactivation of JAK/STAT3 signalling in tumours. Autocrine stimulation of growth factor receptors, such as EGFR, can also lead to induction of STAT3 signalling. In certain malignancies, such as NSCLC, EGFR is overexpressed or mutated to a constitutively active form, and a similar situation can occur with JAK enzymes144. Furthermore, many tumours harbour hyperactivation of SRC, which can promote STAT3 phosphorylation and/or activation145. Thus, therapies targeting EGFR or SRC, in addition to those targeting components of the IL-6 pathway, could potentially be used to downmodulate STAT3 activation and signalling.
Alterations in proteins that negatively regulate STAT3 in nonmalignant cells can also contribute to aberrant activation of STAT3. Loss of SOCS1, and/or 3 expression, often owing to promoter hypermethylation, occurs in multiple forms of cancer, including tumours of the brain, cervix, colon, head and neck, liver, lung, ovary, pancreas, and prostate as well as in melanoma6. Inhibition or mutation of the SHP1 and SHP2 phosphatases, in addition to loss of expression of PIAS family members, has also been reported6,7. Mutations in the genes encoding PTPRT and PTPRD have been found in 5.6% and 3.7%, respectively, of head-and-neck cancers, with promoter methylation contributing further to loss of expression8–11.
Hyperactivation of STAT3 is primarily associated with the promotion of tumour growth, although a growing body of evidence indicates that activated STAT3 might have tumour-suppressive functions in certain settings. STAT3 is reported to be a negative regulator of the development and progression of KRAS-induced lung cancer in Apc-mutant mice146–148. Levels of activated STAT3 have been positively correlated with a better prognosis in patients with nasopharyngeal carcinoma, colorectal carcinoma, or leiomyosarcoma149–151. Thus, the eventual clinical application of STAT3 inhibitors requires careful consideration of the role of STAT3 activation in each specific cancer.
Targeting the IL-6/JAK/STAT3 pathway
Inhibitors of IL-6 and IL-6Rs
Three main approaches to inhibition of IL-6-mediated signalling at the ligand and/or receptor level are currently in use: directly targeting IL-6 with antibodies, such as siltuximab; targeting the IL-6R with antibodies, such as tocilizumab; and targeting the IL-6–sIL-6R complex using fusion proteins incorporating sgp130. Direct targeting of IL-6 and IL-6 receptors inhibits both classic and trans-signalling, while targeting the IL-6–sIL-6R complex with sgp130 fusion proteins selectively inhibits trans-signalling.
Siltuximab, a chimeric mouse–human antibody, is currently the most extensively developed clinical agent targeting IL-6 (FIG. 3; TABLE 1). Following positive results of several clinical trials, siltuximab was granted FDA approval in 2014 for the treatment of multicentric Castleman disease152–154. The findings of preclinical studies indicate that siltuximab increases the activity of melphalan in in vitro models of multiple myeloma155, although the addition of siltuximab to bortezomib-based or melphalan-based regimens did not improve overall response rates or progression-free survival rates in clinical trials156–160. In preclinical models of solid tumours, siltuximab demonstrated antitumour efficacy against ovarian161, prostate162, and lung163 cancers. Analyses of tumour material from phase I–II studies involving patients with prostate cancer have shown that siltuximab decreases levels of activated STAT3 and MAPKs and results in a serum PSA-defined response rate of 3.8%, with no significant improvements in patient outcomes164–166. Stabilized disease was obtained in >50% of patients with metastatic renal cell carcinoma receiving siltuximab in a phase I–II clinical trial167. However, no clinical activity was observed in phase I–II trials involving patients with advanced-stage cancer (including colorectal, head-and-neck, lung (NSCLC), ovarian, or pancreatic tumours)168. Thus, while promising results have been obtained in preclinical studies, evidence demonstrating activity of siltuximab against solid tumours in clinical trials has been largely limited. These findings highlight the possibility that targeting IL-6 alone in unselected patient populations is unlikely to have a marked effect on the outcomes of patients with solid tumours. Overcoming this obstacle will require the development of effective combination therapies as well as the identification of reliable and robust biomarkers that are predictive of a response. Additional anti-IL-6 antibodies currently in preclinical development in cancer include sirukumab, olokizumab, clazakizumab, and MEDI5117 (REFS 169–171).
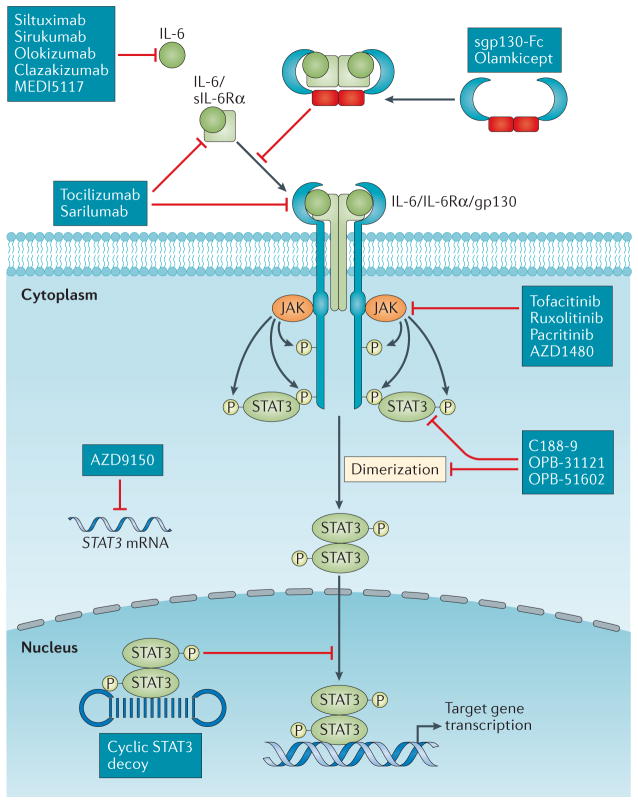
Fig. 3. Inhibitors of the IL-6/JAK/STAT3 signalling pathway.
Various targeted agents that inhibit different nodes of the IL-6 signalling pathway have been developed. Siltuximab, sirukumab, olokizumab, clazakizumab, and MEDI5117 are anti-IL-6 monoclonal antibodies. Tocilizumab and sarilumab are monoclonal antibodies that target IL-6R. These antibodies inhibit both the classic and trans-signalling pathways. By contrast, the gp130–Fc fusion protein olamkicept inhibits IL-6 trans-signalling but not the classic signalling pathway. Tofacitinib, ruxolitinib, pacritinib, and AZD1480 are small-molecule tyrosine kinase inhibitors that target JAKs, preventing phosphorylation of STAT3. C188-9, OPB-31121, OPB-51602, and other Src homology domain 2 (SH2) domain inhibitors interfere with STAT3 dimerization. The STAT3 antisense oligonucleotide AZD9150 binds to and causes the destruction of STAT3 mRNA, thus decreasing STAT3 expression. The cyclic STAT3 decoy contains a nucleotide sequence derived from the promoter of the STAT3 target gene FOS. This decoy competitively inhibits STAT3 binding to genomic response elements in the promoter regions of target genes.
Table 1.
Anti-IL-6 or anti-IL-6-receptor antibodies in clinical trials
| Inhibitor (type of agent) | Indication | Study phase | NCT identifier | Trial results | Refs |
|---|---|---|---|---|---|
| Siltuximab (anti-IL-6 mAb) | Multiple myeloma, B cell non-Hodgkin lymphoma, Castleman disease | I | NCT00412321 | No DLTs observed; recommended dose for future studies determined | 154 |
| Multiple myeloma (smoldering or indolent) | I | NCT01219010 | 10% M-protein response, 30% minor M-protein response; acceptable safety profile | NA | |
| Multiple myeloma | I | NCT01309412 | Study terminated owing to safety concerns | 156 | |
| Multiple myeloma | I/II | NCT01531998 | 90.9% ORR (PR) in combination with RVD; MTD determined | 160 | |
| Multiple myeloma | II | NCT00401843 | No increase in PFS or OS compared with bortezomib alone | 157 | |
| Multiple myeloma (high-risk smoldering) | II | NCT01484275 | NA | NA | |
| Multiple myeloma | II | NCT00402181 | No response to single-agent siltuximab; 17% ORR in combination with dexamethasone in patients with dexamethasone-refractory disease | 158 | |
| Multiple myeloma | II | NCT00911859 | Addition of siltuximab to VMP did not improve the number of patients having a CR, PFS, or OS but did improve the number of patients with a VGPR | 159 | |
| Myelodysplastic syndromes | II | NCT01513317 | Study terminated owing to a lack of efficacy | NA | |
| Prostate cancer | I | 2047 SN:218/4.2 (Innsbruck Medical University) | No siltuximab-related adverse events observed | 164 | |
| Metastatic, hormone-refractory prostate cancer | I | NCT00401765 | 62.2% of patients had a PSA-defined response; 89.7% of patients discontinued treatment prior to completion of all 14 cycles | NA | |
| Metastatic, hormone-refractory prostate cancer | II | NCT00385827 | Study terminated owing to a lack of efficacy; well tolerated in combination with MP | 166 | |
| Metastatic, hormone-refractory prostate cancer | II | NCT00433446 | Minimal clinical activity despite evidence of a reduction in IL-6 levels (decrease in serum CRP levels) | 165 | |
| Solid tumours | I/II | NCT00841191 | No clinical activity observed but well tolerated as monotherapy; recommended phase II dose determined | 168 | |
| Metastatic renal cell carcinoma | I/II | NCT00265135 | SD in >50% of patients; no DLTs observed | 167 | |
| Tocilizumab (anti-IL-6 receptor mAb) | B cell chronic lymphocytic leukaemia | I | NCT02336048 | NA | NA |
| Metastatic HER2+ breast cancer | I | NCT03135171 | NA | NA | |
| Ovarian cancer | I/II | NCT01637532 | Immunological changes consistent with decreased levels of immunosuppression; no DLTs observed | 174 | |
| Pancreatic cancer | II | NCT02767557 | NA | NA |
CR, complete response; CRP, C-reactive protein; DLT, dose-limiting toxicity; mAb, monoclonal antibody; MP, mitoxantrone plus prednisone; MTD, maximum-tolerated dose; NA, not available; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; PSA, prostate-specific antigen; RVD, lenalidomide plus bortezomib and dexamethasone; SD, stable disease; VGPR, very good partial response; VMP, bortezomib plus melphalan and prednisone.
Tocilizumab is a humanized monoclonal antibody that recognizes IL-6R and disrupts both classic and trans-signalling (FIG. 3; TABLE 1). Tocilizumab has been approved by the FDA for use in adult patients with rheumatoid arthritis and in patients with systemic juvenile idiopathic arthritis and for the management of cytokine-release syndrome in adult or paediatric patients with B-ALL receiving treatment with CAR T cells. The findings of preclinical studies suggest that tocilizumab is effective against ovarian172, pancreatic173, and colitis-associated colorectal cancers92. The findings of a phase I clinical trial demonstrated the combination of tocilizumab with carboplatin and/or doxorubicin to be feasible and safe in patients with ovarian cancer174. Early phase trials exploring the safety and effectiveness of tocilizumab in patients with B cell chronic lymphocytic leukaemia (B-CLL) and in those with breast or pancreatic cancers are currently ongoing (NCT02336048, NCT03135171, and NCT02767557). Another monoclonal antibody that targets the IL-6R, sarilumab, is currently in preclinical development.
Selective inhibition of trans-signalling might be of particular value in patients whose tumours have either very limited or no IL-6R expression. Trans-signalling can be selectively inhibited by proteins incorporating the sgp130 sequence, which bind with and inhibit the IL-6–IL-6R complex51. An sgp130-Fc fusion protein has been shown, in preclinical models, to inhibit the development and/or progression of KRAS-driven NSCLC, pancreatic cancer, and colitis-associated premalignant colorectal cancer173,175,176. Olamkicept, an sgp130-Fc fusion protein, is currently in phase I–Ib testing in patients with rheumatoid arthritis and in those with inflammatory bowel diseases. In addition to activating STAT3, IL-6 can also activate STAT1 via gp130 (REFS 177,178). STAT1 is known to have tumour-suppressive properties. Thus, targeted inhibition of IL-6 signalling in patients with cancer has the potential downside of reducing STAT1 activity in tumour cells.
JAK inhibitors
Tofacitinib, ruxolitinib, and pacritinib are currently the most extensively investigated clinical inhibitors of JAKs, with several other agents currently in preclinical development (FIG. 3). To date, the clinical application of JAK inhibitors has focused heavily on conditions involving chronic inflammation and myeloproliferative neoplasms, with less evaluation of these agents in patients with solid tumours.
Tofacitinib is an orally administered JAK inhibitor that selectively inhibits JAK1 and JAK3, with a lower affinity for JAK2 (REF. 179). Tofacitinib has been approved by the FDA for the treatment of rheumatoid arthritis180–183. Tofacitinib is also undergoing clinical evaluation as a treatment of other inflammatory conditions, including chronic plaque psoriasis184, ulcerative colitis185, and Crohn’s disease186. Ruxolitinib is an orally administered JAK inhibitor with selectivity for JAK1 and JAK2 and has received FDA approval for the treatment of patients with intermediate-risk or high-risk myelofibrosis and for patients with hydroxyurea-resistant or intolerant polycythaemia vera105. The FDA approval of ruxolitinib for myelofibrosis was based on data from the COMFORT-1 and COMFORT-2 trials, which compared the efficacy of ruxolitinib with that of placebo or the best available therapy187–189. Ruxolitinib was found to induce a marked reduction in spleen volumes and total symptom scores in both trials, although myelosuppression and anaemia were frequent dose-limiting events. Given the importance of JAK2 for haematopoiesis190, the myelosuppression associated with ruxolitinib is not surprising. Interestingly, the effectiveness of ruxolitinib in patients with myelofibrosis is not dependent on the presence of JAK2V617F mutations191. The FDA approval of ruxolitinib as a treatment of polycythaemia vera was based on findings of the RESPONSE clinical trial, in which ruxolitinib had superior efficacy to that of the standard-of-care approach (including hydroxyurea, interferons or pegylated interferons, pipobroman, anagrelide, or immunomodulators such as lenalidomide or thalidomide)192,193. Evaluations of the efficacy of ruxolitinib in patients with essential thrombocythemia in later-phase clinical trials are currently ongoing.
The dose-limiting myelosuppression that frequently accompanies treatment with ruxolitinib has stimulated the search for JAK inhibitors with a reduced risk of adverse events. Pacritinib is an orally administered, selective JAK2 and FLT3 inhibitor classified as lacking in myelosuppressive activity. Clinical evaluations of the efficacy of pacritinib in the PERSIST-1 trial in patients with myelofibrosis revealed favourable levels of activity compared with that of the best available therapy (ruxolitinib not included), even in patients with thrombocytopenia194,195. However, accrual to the subsequent PERSIST-2 trial, which was designed to evaluate the efficacy of pacritinib in patients with myelofibrosis with thrombocytopenia, was temporarily halted by the FDA owing to the emergence of severe cardiac and haemorrhagic adverse events. This trial was terminated, but pacritinib is now being investigated in a phase II clinical trial in patients with myelofibrosis who hab been previously treated with ruxolitinib (NCT03165734).
Clinical investigation of the efficacy of JAK inhibitors in patients with solid tumours is currently limited. In preclinical studies, JAK inhibition has been shown to curtail the in vivo growth of a broad variety of solid tumours, including brain, breast, colorectal, gastric, head-and-neck, liver, lung, pancreatic, and ovarian cancers14,196–200. Many of the early preclinical studies used the JAK1 and JAK2 inhibitor AZD1480. However, phase I testing in patients with solid tumours revealed a major risk of neurological adverse events following treatment with AZD1480, including anxiety, ataxia, behavioural changes, hallucinations, and memory loss, resulting in the discontinuation of further testing of this agent201. Phase I evaluations of the safety and tolerability of ruxolitinib in children with recurrent and/or refractory solid tumours have revealed acceptable levels of tolerability and have enabled the identification of a recommended dose for further testing in phase II studies202. Data from a phase II study involving adults with metastatic pancreatic cancer have shown that the combination of ruxolitinib and capecitabine is well tolerated and might lead to improved survival outcomes203. Additional early phase trials exploring the safety and effectiveness of ruxolitinib in patients with breast, colorectal, head-and-neck, lung, ovarian, pancreatic, or prostate cancers are currently ongoing (for example, NCT02066532, NCT03153982, NCT02713386, and NCT03274778).
STAT3 inhibitors
STAT3 has established tumour-promoting properties, is overexpressed and/or hyperactivated in the majority of human cancers, and is commonly associated with a poor prognosis; therefore, considerable effort has gone into identifying and developing STAT3 inhibitors that can be applied in the clinic. However, given the status of STAT3 as an intracellular transcription factor that, therefore, lacks enzymatic activity, it has often been considered an ‘undruggable’ target, and the development of possible inhibitors has been difficult204. Nonetheless, several compounds that inhibit either the function or expression of STAT3 have now reached clinical trials (FIG. 3; TABLE 2).
Table 2.
STAT3 inhibitors in clinical trials
| Inhibitor | Indication | Study phase | NCT identifier | Trial results | Refs |
|---|---|---|---|---|---|
| AZD9150 (STAT3 antisense oligonucleotide) | DLBCL | I | NCT02549651 | NA | NA |
| Advanced-stage and/or metastatic hepatocellular carcinoma | I | NCT01839604 | NA | NA | |
| Advanced-stage solid tumours, metastatic HNSCC | I/II | NCT02499328 | NA | NA | |
| Advanced-stage cancers, DLBCL, advanced-stage lymphomas | I/II | NCT01563302 | SD or PR in 44% of patients; durable PR in 2 of 6 patients with DLBCL; MTD determined | 240 | |
| Advanced-stage pancreatic cancer, NSCLC, and CRC | II | NCT02983578 | NA | NA | |
| C188-9 (STAT3 SH2 domain binder) | Advanced-stage cancers | I | NCT03195699 | NA | NA |
| OPB-31121 (STAT3 SH2 domain binder) | Solid tumours | I | NCT00955812 | No objective responses; MTD determined | 225 |
| Advanced-stage solid tumours | I | NCT00657176 | SD in 8 of 18 evaluable patients; MTD determined | 226 | |
| Hepatocellular carcinoma | I/II | NCT01406574 | SD in 6 of 23 patients; authors described antitumour activity as insufficient, thus precluding further clinical development | 227 | |
| OPB 51602 (STAT3 SH2 domain binder) | Multiple myeloma, NHL, AML, ALL, and CML | I | NCT01344876 | No clear therapeutic response; MTD and recommended dose determined | 229 |
| Nasopharyngeal carcinoma | I | NCT02058017 | Study terminated owing to the emergence of intolerable lactic and metabolic acidosis | NA | |
| Advanced-stage cancers | I | NCT01423903 | NA | NA | |
| Advanced-stage solid tumours | I | NCT01184807 | PR in 2 of 37 evaluable patients (both with EGFR–TKI -refractory NSCLC); MTD and recommended phase II dose determined | 228 | |
| STAT3 decoy oligonucleotide (STAT3 response element from FOS) | HNSCC | 0 | NCT00696176 | Decreased STAT3 target gene expression in tumours following intratumoural injection; no toxicities reported | 238 |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CML, chronic myeloid leukaemia; CRC, colorectal cancer; DLBCL, diffuse large B cell lymphoma; HNSCC, head and neck squamous cell carcinoma; MTD, maximum-tolerated dose; NA, not available; NHL, non-Hodgkin lymphoma; NSCLC, non-small-cell lung cancer; PR, partial response; SD, stable disease; SH2, SRC homology domain 2; STAT3, signal transducer and activator of transcription 3; TKI, tyrosine kinase inhibitor.
The first direct inhibitors of STAT3 to be developed were based on tyrosine-phosphorylated peptides (PY*LKTK) and peptidomimetics (ISS-610, PM-73G), which are capable of binding to the SH2 domain of STAT3, and disrupt STAT3 dimerization and DNA-binding activity205–208. These agents have demonstrated pro-apoptotic and antitumour activity against cancer cells with STAT3 hyperactivation, although they are limited in potency, cellular uptake, stability, and potential immunogenicity and have primarily been used as research tools. A number of non-peptide SH2 domain inhibitors have also been identified and have been shown to inhibit the growth of cells and/or tumours with elevated levels of activated STAT3, including STA-21, LLL-3, STATTIC, WP1066, S3I-201, BP-1-102, STX-0119, and HJC0123 (REFS 209–221). Of these compounds, BP1-102, STX-0119, and HJC0123 are orally bioavailable in preclinical models.
The nonpeptide STAT3–SH2 domain antagonists OPB-31121, OPB-51602, and C188-9 have all been evaluated in early phase clinical trials222–230. Phase I studies exploring the safety and tolerability of orally administered OPB-31121 in patients with hepatocellular carcinoma or other advanced-stage solid tumours have been completed, and maximum-tolerated doses have been established225–227. Evidence of antitumour activity was reported by Oh et al.226, although peripheral sensory neuropathy was observed in the study conducted by Okusaka et al.227. OPB-51602 can also be orally administered and has been evaluated in phase I studies involving patients with relapsed and/or refractory haematological malignancies and treatment-refractory solid tumours228,229. Possible antitumour activity was seen in patients with NSCLC, although both studies revealed tolerability-related difficulties, including peripheral neuropathy and drug-induced pneumonitis. C188-9 is a high-affinity STAT3 inhibitor (Kd ~5 nM) that can also be orally administered. It inhibits the growth of radioresistant head-and-neck cancer xenografts and is currently being evaluated in a phase I study involving patients with advanced-stage solid tumours230.
An alternative method of inhibiting STAT3 function involves competitive inhibition of the interactions between STAT3 and promoter elements in target genes. A 15-bp double-stranded decoy oligonucleotide corresponding to the STAT3 response element in the FOS promoter has been shown to competitively inhibit STAT3 binding to DNA and to suppress the tumour growth of preclinical models of brain, breast, head-and-neck, lung, ovarian, and skin cancers as well as AML129,231–237. A phase 0 study involving intratumoural injection of this linear STAT3 decoy oligonucleotide in patients with head-and-neck cancer has demonstrated downregulation of STAT3 target genes238. A cyclic version of the STAT3 decoy has subsequently been generated238. The cyclic STAT3 decoy has increased heat and nuclease resistance, antitumour activity against xenograft tumour models following intravenous administration, and no apparent toxicities when administered at high doses238,239.
Inhibition of STAT3 expression using antisense oligonucleotides provides another distinctly different approach to inhibition of cellular STAT3 activity. AZD9150 is a second-generation STAT3 antisense oligonucleotide that is optimized from previous iterations by incorporating 2′–4′ constrained ethyl-modified residues240. Preclinical testing has revealed a lack of end-organ damage and other adverse events following AZD9150 administration241. Notably, intravenous delivery of AZD9150 has been shown to inhibit the growth of lymphoma and NSCLC xenografts and to increase the sensitivity of cell-line models of neuroblastoma to cisplatin240,242. Moreover, clinical evaluations of AZD9150 have revealed activity against treatment-refractory lymphoma and NSCLC, with a maximum-tolerated dose of 3 mg/kg (REF. 240). The major toxicities associated with AZD9150 in previous studies proved modest and primarily involved rapid induction of thrombocytopenia in two of the nine patients treated with 4 mg/kg doses. The generally favourable safety profile and preliminary evidence suggesting efficacy in patients support the further evaluation of AZD9150 in clinical trials. A further challenge facing the clinical implementation of both AZD9150 and the cyclic STAT3 decoy involves the delivery of nucleotide-based agents owing to the high molecular weight of both molecules.
Immunotherapy combinations
The targeted inhibition of immune checkpoints using monoclonal antibodies has led to dramatic improvements in the treatment of patients with advanced-stage cancer. Ipilimumab inhibits CTLA-4 activation, while pembrolizumab and nivolumab inhibit PD-1 signalling. Both CTLA-4 and PD-1 are inhibitory cell-surface receptors that act to restrain T cell-mediated immune responses. PD-L1 activates PD-1 and is commonly expressed on the surface of tumour cells or tumour-infiltrating immune cells. Collectively, these antibodies have received FDA approval for the treatment of a diverse range of cancers, including melanoma, Hodgkin lymphoma, bladder and head-and-neck cancers, and NSCLC. Given the early successes observed with immune-checkpoint inhibition, broader clinical application of these and similar antibodies targeting immune-checkpoint proteins is likely. Inhibition of IL-6/JAK/STAT3 signalling can also affect the tumour microenvironment and has implications for antitumour immunity; therefore, determining whether co-targeting of immune checkpoints and the IL-6/JAK/STAT3 signalling pathway might be beneficial is important. Early indications suggest that inhibition of IL-6/JAK/STAT3 signalling will be useful in combating the various adverse inflammatory effects resulting from treatment with immune-checkpoint inhibitors. Moreover, preclinical evidence is emerging that inhibition of IL-6/JAK/STAT3 signalling might augment the antitumour efficacy of immune-checkpoint inhibitors.
Treatment of patients with cancer with immune-checkpoint inhibitors can stimulate the production of IL-6 (REFS 243–245). The elevation of serum IL-6 levels in these patients is typically manifested as inflammatory conditions, such as psoriasiform dermatitis, arthritis, and Crohn’s disease243,244,246,247. Importantly, treatment with tocilizumab has been shown to resolve these conditions and to enable patients to continue to receive immune-checkpoint inhibitors245–247.
The findings of numerous studies have shown that signalling via the IL-6/JAK/STAT3 pathway induces the expression of PD-1 and/or PD-L1 (REFS 248–252). Inhibition of IL-6/JAK/STAT3 signalling downregulates PD-1 and/or PD-L1 expression and might, hypothetically, be expected to have one of two possible effects on the efficacy of immune-checkpoint inhibition. Targeting IL-6/JAK/STAT3 signalling might be expected to improve the effectiveness of pembrolizumab and nivolumab as a result of direct inhibitory effects on tumour cells as well as effects on immune cells in the tumour microenvironment. Alternatively, downregulation of PD-1 and PD-L1 by inhibitors of IL-6/JAK/STAT3 signalling could result in the attenuation of the activity of pembrolizumab and/or nivolumab by reducing the expression of the proteins targeted by these antibodies. Substantial preclinical and clinical research will be required to address this important issue, although preliminary studies in preclinical models suggest a clinical benefit from the combination of agents targeting the IL-6/JAK/STAT3 pathway with immune-checkpoint inhibition. Co-targeting of IL-6 and PD-L1 leads to enhanced inhibition of tumour growth in mouse models of both pancreatic ductal and hepatocellular carcinomas253,254. Treatment with ruxolitinib has been shown to overcome resistance to anti-PD-1 antibodies in mice with pancreatic orthotopic tumours255. Clearly, further investigations involving these combination approaches are warranted.
Future directions
The IL-6/JAK/STAT3 pathway is hyperactivated in many patients with cancer, and the findings of numerous studies involving preclinical in vitro and in vivo models demonstrate that targeting individual nodes in this pathway can have antitumour effects. In addition to the importance of IL-6/JAK/STAT signalling in tumour cells, activation of this pathway has also been implicated in suppressing antitumour immune responses in the tumour microenvironment. Thus, therapies that target this pathway are likely to benefit patients with cancer, both by inhibition of tumour cell growth and through stimulation of antitumour immunity. Agents that target individual nodes, including IL-6, IL-6R, and JAKs, are all approved by the FDA for the treatment of inflammatory conditions or myeloproliferative neoplasms. Many of these agents are also currently under active investigation as treatments of other haematopoietic malignancies and solid tumours. Novel inhibitors of the IL-6/JAK/STAT3 pathway, including STAT3-selective agents, are also being developed, and early phase clinical trials are currently ongoing.
Predictive biomarkers, beyond pathway hyperactivation, are needed in order to rationally incorporate IL-6/JAK/STAT3-targeting agents into multimodality treatment plans, including combinations with chemotherapy, radiotherapy, and immune-checkpoint inhibitors. In addition, as the therapeutic armamentarium of these agents increases, comparative evaluations, which are currently lacking, will be needed in both preclinical and clinical settings. The search for biomarkers that predict a response should include the evaluation of miRNAs, which are currently not widely explored. Moreover, molecular targeting or exogenous expression of specific miRNAs might prove to be a fruitful means of suppressing signalling via the IL-6/JAK/STAT3 pathway. Interestingly, a mimic of miR-34, which represses the expression of IL-6R, has entered phase I evaluations in patients with solid tumours. However, the pursuit of miRNAs as targets or therapeutic agents will be challenged by the broad effects of miRNAs on protein expression. Thus, the effects of miRNA mimics or inhibitors are unlikely to be highly selective for IL-6/JAK/STAT3 signalling pathways.
The clinical use of IL-6/JAK/STAT3-targeting agents will benefit from a deeper understanding and consideration of the genomic profile of patients’ tumours. Information regarding the identity of oncogenic drivers will also be useful in guiding the development of personalized combination therapies. For example, mutations in the PIK3CA gene are common in a wide variety of cancers and lead to activation of the PI3K signalling pathway. The PI3K pathway promotes tumour growth independently of JAK–STAT3; therefore, inhibitors of JAK–STAT3 signalling are likely to be ineffective as monotherapies in tumours with PIK3CA-activating mutations. Instead, the presence of PIK3CA mutations in concert with STAT3 hyperactivation suggests that a therapeutic regimen comprising a JAK–STAT3 inhibitor, in combination with an inhibitor of the PI3K signalling pathway, will be an effective approach.
Conclusions
In summary, targeting the IL-6/JAK/STAT3 signalling axis, which has already been shown to be beneficial in the treatment of certain cancers in human patients, holds considerable promise for the suppression of tumour growth and the restoration of antitumour immunity. The identification of predictive biomarkers and the development of rational combination therapies based on the immune and genomic profiles of tumours is required in order to maximize the broad utility and efficacies of agents targeting the IL-6/JAK/STAT3 signalling pathway and their use in precision medicine for patients with cancer.
Key points.
The IL-6/JAK/STAT3 signalling pathway is aberrantly hyperactivated in patients with chronic inflammatory conditions and in those with haematopoietic malignancies or solid tumours
Multiple cell types in the tumour microenvironment produce IL-6, leading to activation of JAK/STAT3 signalling in both tumour cells and tumour-infiltrating immune cells, which can promote tumour-cell proliferation, survival, invasiveness, and metastasis
STAT3 is hyperactivated in tumour-infiltrating immune cells and acts to negatively regulate neutrophils, natural killer cells, effector T cells, and dendritic cells while positively regulating populations of myeloid-derived suppressor cells and regulatory T cells
Targeting components of the IL-6/JAK/STAT3 signalling pathway can inhibit tumour cell growth and relieve immunosuppression in the tumour microenvironment
Inhibitors of IL-6, the IL-6 receptor, or JAKs have all received FDA approval for various malignancies, and other novel inhibitors of the IL-6/JAK/STAT3 signalling pathway are currently in clinical and/or preclinical development
Investigations of the efficacy of IL-6/JAK/STAT3 inhibitors, in combination with immune-checkpoint inhibitors, are warranted
Acknowledgments
The work of the authors is supported by grants from the US NIH (R01 DE24728 and P50 CA097190 to D.E.J., F31 DE026951 to R.A.O., R01 DE023685 and P50 CA097190 to J.R.G.) and the American Cancer Society (CRP-13-308-06-COUN to J.R.G.).
Footnotes
Author contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 2.Kusaba T, et al. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep. 2006;15:1445–1451. [PubMed] [Google Scholar]
- 3.Chen Y, et al. STAT3, a poor survival predicator, is associated with lymph node metastasis from breast cancer. J Breast Cancer. 2013;16:40–49. doi: 10.4048/jbc.2013.16.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macha MA, et al. Prognostic significance of nuclear pSTAT3 in oral cancer. Head Neck. 2011;33:482–489. doi: 10.1002/hed.21468. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig H, Nachbaur DM, Fritz E, Krainer M, Huber H. Interleukin-6 is a prognostic factor in multiple myeloma. Blood. 1991;77:2794–2795. [PubMed] [Google Scholar]
- 6.Buchert M, Burns CJ, Ernst M. Targeting JAK kinase in solid tumors: emerging opportunities and challenges. Oncogene. 2016;35:939–951. doi: 10.1038/onc.2015.150. [DOI] [PubMed] [Google Scholar]
- 7.Tartaglia M, et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34:148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci USA. 2007;104:4060–4064. doi: 10.1073/pnas.0611665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyser ND, et al. Frequent promoter hypermethylation of PTPRT increases STAT3 activation and sensitivity to STAT3 inhibition in head and neck cancer. Oncogene. 2016;35:1163–1169. doi: 10.1038/onc.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyser ND, et al. Loss-of-function PTPRD mutations lead to increased STAT3 activation and sensitivity to STAT3 inhibition in head and neck cancer. PLoS ONE. 2015;10:e0135750. doi: 10.1371/journal.pone.0135750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lui VW, et al. Frequent mutation of receptor protein tyrosine phosphatases provides a mechanism for STAT3 hyperactivation in head and neck cancer. Proc Natl Acad Sci USA. 2014;111:1114–1119. doi: 10.1073/pnas.1319551111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasaki T, et al. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469–478. doi: 10.1038/bjc.2013.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bournazou E, Bromberg J. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT. 2013;2:e23828. doi: 10.4161/jkst.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang Q, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Jove R. The STATs of cancer — new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 19.Kortylewski M, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 21.Harris TJ, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann A, et al. Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res. 2010;70:7455–7464. doi: 10.1158/0008-5472.CAN-10-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kujawski M, et al. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 2010;70:9599–9610. doi: 10.1158/0008-5472.CAN-10-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel AM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwata-Kajihara T, et al. Enhanced cancer immunotherapy using STAT3-depleted dendritic cells with high Th1-inducing ability and resistance to cancer cell-derived inhibitory factors. J Immunol. 2011;187:27–36. doi: 10.4049/jimmunol.1002067. [DOI] [PubMed] [Google Scholar]
- 26.Gotthardt D, et al. Loss of STAT3 in murine NK cells enhances NK cell-dependent tumor surveillance. Blood. 2014;124:2370–2379. doi: 10.1182/blood-2014-03-564450. [DOI] [PubMed] [Google Scholar]
- 27.Hossain DM, et al. Leukemia cell-targeted STAT3 silencing and TLR9 triggering generate systemic antitumor immunity. Blood. 2014;123:15–25. doi: 10.1182/blood-2013-07-517987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kortylewski M, Yu H. Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol. 2008;20:228–233. doi: 10.1016/j.coi.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Pal SK, Reckamp K, Figlin RA, Yu H. STAT3: a target to enhance antitumor immune response. Curr Top Microbiol Immunol. 2011;344:41–59. doi: 10.1007/82_2010_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lederle W, et al. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer. 2011;128:2803–2814. doi: 10.1002/ijc.25621. [DOI] [PubMed] [Google Scholar]
- 31.Mullberg J, et al. IL-6 receptor independent stimulation of human gp130 by viral IL-6. J Immunol. 2000;164:4672–4677. doi: 10.4049/jimmunol.164.9.4672. [DOI] [PubMed] [Google Scholar]
- 32.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Hirano T, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 34.Kopf M, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 35.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 36.Ohshima S, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222–8226. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonzi T, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Screpanti I, et al. Inactivation of the IL-6 gene prevents development of multicentric Castleman’s disease in C/EBP beta-deficient mice. J Exp Med. 1996;184:1561–1566. doi: 10.1084/jem.184.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garnero P, Thompson E, Woodworth T, Smolen JS. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum. 2010;62:33–43. doi: 10.1002/art.25053. [DOI] [PubMed] [Google Scholar]
- 40.Nishimoto N, et al. Improvement in Castleman’s disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56–61. [PubMed] [Google Scholar]
- 41.Yoon S, et al. NF-kappaB and STAT3 cooperatively induce IL6 in starved cancer cells. Oncogene. 2012;31:3467–3481. doi: 10.1038/onc.2011.517. [DOI] [PubMed] [Google Scholar]
- 42.Campbell IL, et al. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J Neurosci. 2014;34:2503–2513. doi: 10.1523/JNEUROSCI.2830-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 44.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 45.Skiniotis G, Boulanger MJ, Garcia KC, Walz T. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat Struct Mol Biol. 2005;12:545–551. doi: 10.1038/nsmb941. [DOI] [PubMed] [Google Scholar]
- 46.Honda M, et al. Human soluble IL-6 receptor: its detection and enhanced release by HIV infection. J Immunol. 1992;148:2175–2180. [PubMed] [Google Scholar]
- 47.Novick D, Engelmann H, Wallach D, Rubinstein M. Soluble cytokine receptors are present in normal human urine. J Exp Med. 1989;170:1409–1414. doi: 10.1084/jem.170.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baran P, Nitz R, Grotzinger J, Scheller J, Garbers C. Minimal interleukin 6 (IL-6) receptor stalk composition for IL-6 receptor shedding and IL-6 classic signaling. J Biol Chem. 2013;288:14756–14768. doi: 10.1074/jbc.M113.466169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 50.Diamant M, et al. Cloning and expression of an alternatively spliced mRNA encoding a soluble form of the human interleukin-6 signal transducer gp130. FEBS Lett. 1997;412:379–384. doi: 10.1016/s0014-5793(97)00750-3. [DOI] [PubMed] [Google Scholar]
- 51.Jostock T, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 52.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haan C, Kreis S, Margue C, Behrmann I. Jaks and cytokine receptors — an intimate relationship. Biochem Pharmacol. 2006;72:1538–1546. doi: 10.1016/j.bcp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dorritie KA, McCubrey JA, Johnson DE. STAT transcription factors in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia. 2014;28:248–257. doi: 10.1038/leu.2013.192. [DOI] [PubMed] [Google Scholar]
- 57.Takeda K, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cimica V, Chen HC, Iyer JK, Reich NC. Dynamics of the STAT3 transcription factor: nuclear import dependent on Ran and importin-beta1. PLoS ONE. 2011;6:e20188. doi: 10.1371/journal.pone.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benekli M, Baumann H, Wetzler M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J Clin Oncol. 2009;27:4422–4432. doi: 10.1200/JCO.2008.21.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ilaria RL, Jr, Van Etten RA. P210 and P190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 61.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim E, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23:839–852. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sekine Y, et al. Regulation of STAT3-mediated signaling by LMW-DSP2. Oncogene. 2006;25:5801–5806. doi: 10.1038/sj.onc.1209578. [DOI] [PubMed] [Google Scholar]
- 64.Kim DJ, Tremblay ML, Digiovanni J. Protein tyrosine phosphatases, TC-PTP, SHP1, and SHP2, cooperate in rapid dephosphorylation of Stat3 in keratinocytes following UVB irradiation. PLoS ONE. 2010;5:e10290. doi: 10.1371/journal.pone.0010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang M, et al. Both miR-17-5p and miR-20a alleviate suppressive potential of myeloid-derived suppressor cells by modulating STAT3 expression. J Immunol. 2011;186:4716–4724. doi: 10.4049/jimmunol.1002989. [DOI] [PubMed] [Google Scholar]
- 66.Rokavec M, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei J, et al. miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res. 2013;73:3913–3926. doi: 10.1158/0008-5472.CAN-12-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Y, et al. MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol Cancer. 2017;16:141. doi: 10.1186/s12943-017-0710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaluvally-Raghavan P, et al. Direct upregulation of STAT3 by microRNA-551b-3p deregulates growth and metastasis of ovarian cancer. Cell Rep. 2016;15:1493–1504. doi: 10.1016/j.celrep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu W, et al. MicroRNA-18a modulates STAT3 activity through negative regulation of PIAS3 during gastric adenocarcinogenesis. Br J Cancer. 2013;108:653–661. doi: 10.1038/bjc.2012.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S, et al. microRNA 221- and 222-mediated feedback loop maintains constitutive activation of NFkappaB and STAT3 in colorectal cancer cells. Gastroenterology. 2014;147:847–859.e11. doi: 10.1053/j.gastro.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. 2013;138:657–664. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 73.Kotowicz B, Fuksiewicz M, Jonska-Gmyrek J, Bidzinski M, Kowalska M. The assessment of the prognostic value of tumor markers and cytokines as SCCAg, CYFRA 21.1, IL-6, VEGF and sTNF receptors in patients with squamous cell cervical cancer, particularly with early stage of the disease. Tumour Biol. 2016;37:1271–1278. doi: 10.1007/s13277-015-3914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83:222–226. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- 75.Chen MF, et al. IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol Cancer. 2013;12:26. doi: 10.1186/1476-4598-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jinno T, et al. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncol Rep. 2015;33:2161–2168. doi: 10.3892/or.2015.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riedel F, et al. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25:2761–2765. [PubMed] [Google Scholar]
- 78.Maccio A, Madeddu C. The role of interleukin-6 in the evolution of ovarian cancer: clinical and prognostic implications—a review. J Mol Med. 2013;91:1355–1368. doi: 10.1007/s00109-013-1080-7. [DOI] [PubMed] [Google Scholar]
- 79.Sanguinete MMM, et al. Serum IL-6 and IL-8 correlate with prognostic factors in ovarian cancer. Immunol Invest. 2017;46:677–688. doi: 10.1080/08820139.2017.1360342. [DOI] [PubMed] [Google Scholar]
- 80.Miura T, et al. Characterization of patients with advanced pancreatic cancer and high serum interleukin-6 levels. Pancreas. 2015;44:756–763. doi: 10.1097/MPA.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 81.Culig Z, Puhr M. Interleukin-6: a multifunctional targetable cytokine in human prostate cancer. Mol Cell Endocrinol. 2012;360:52–58. doi: 10.1016/j.mce.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Altundag O, Altundag K, Gunduz E. Interleukin-6 and C-reactive protein in metastatic renal cell carcinoma. J Clin Oncol. 2005;23:1044. doi: 10.1200/JCO.2005.05.155. [DOI] [PubMed] [Google Scholar]
- 83.Chang CH, et al. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer. 2013;132:1977–1985. doi: 10.1002/ijc.27892. [DOI] [PubMed] [Google Scholar]
- 84.Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157:362–380. doi: 10.1016/j.surg.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Wu CT, Chen MF, Chen WC, Hsieh CC. The role of IL-6 in the radiation response of prostate cancer. Radiat Oncol. 2013;8:159. doi: 10.1186/1748-717X-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients — a summary of published results. Int J Colorectal Dis. 2010;25:135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 87.Duffy SA, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 88.Gao J, Zhao S, Halstensen TS. Increased interleukin-6 expression is associated with poor prognosis and acquired cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep. 2016;35:3265–3274. doi: 10.3892/or.2016.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sansone P, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Becker C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 transsignaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 91.Becker C, et al. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- 92.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao SP, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lesina M, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 95.Sullivan NJ, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baltgalvis KA, et al. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–R401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 98.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rebouissou S, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fishman D, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 103.Jones AV, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–2168. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 104.Walters DK, et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10:65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 105.Bose P, Verstovsek S. JAK2 inhibitors for myeloproliferative neoplasms: what is next? Blood. 2017;130:115–125. doi: 10.1182/blood-2017-04-742288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Senkevitch E, Durum S. The promise of Janus kinase inhibitors in the treatment of hematological malignancies. Cytokine. 2017;98:33–41. doi: 10.1016/j.cyto.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 108.James C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 109.Lacronique V, et al. TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 110.Kan Z, et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23:1422–1433. doi: 10.1101/gr.154492.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007;251:199–210. doi: 10.1016/j.canlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 112.Roeser JC, Leach SD, McAllister F. Emerging strategies for cancer immunoprevention. Oncogene. 2015;34:6029–6039. doi: 10.1038/onc.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen CL, et al. Signal transducer and activator of transcription 3 activation is associated with bladder cancer cell growth and survival. Mol Cancer. 2008;7:78. doi: 10.1186/1476-4598-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sonnenblick A, et al. Tissue microarray-based study of patients with lymph node-positive breast cancer shows tyrosine phosphorylation of signal transducer and activator of transcription 3 (tyrosine705-STAT3) is a marker of good prognosis. Clin Transl Oncol. 2012;14:232–236. doi: 10.1007/s12094-012-0789-z. [DOI] [PubMed] [Google Scholar]
- 115.Schaefer LK, Ren Z, Fuller GN, Schaefer TS. Constitutive activation of Stat3alpha in brain tumors: localization to tumor endothelial cells and activation by the endothelial tyrosine kinase receptor (VEGFR-2) Oncogene. 2002;21:2058–2065. doi: 10.1038/sj.onc.1205263. [DOI] [PubMed] [Google Scholar]
- 116.Takemoto S, et al. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer. 2009;101:967–972. doi: 10.1038/sj.bjc.6605212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang HF, et al. The opposing function of STAT3 as an oncoprotein and tumor suppressor is dictated by the expression status of STAT3beta in esophageal squamous cell carcinoma. Clin Cancer Res. 2016;22:691–703. doi: 10.1158/1078-0432.CCR-15-1253. [DOI] [PubMed] [Google Scholar]
- 118.Geiger JL, Grandis JR, Bauman JE. The STAT3 pathway as a therapeutic target in head and neck cancer: barriers and innovations. Oral Oncol. 2016;56:84–92. doi: 10.1016/j.oraloncology.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li S, et al. Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS ONE. 2013;8:e81657. doi: 10.1371/journal.pone.0081657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y, Qu A, Wang H. Signal transducer and activator of transcription 4 in liver diseases. Int J Biol Sci. 2015;11:448–455. doi: 10.7150/ijbs.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suh YA, Jo SY, Lee HY, Lee C. Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. Int J Oncol. 2015;46:1405–1411. doi: 10.3892/ijo.2014.2808. [DOI] [PubMed] [Google Scholar]
- 122.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101:176–193. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Z, et al. Activation of STAT3 in human gastric cancer cells via interleukin (IL)-6-type cytokine signaling correlates with clinical implications. PLoS ONE. 2013;8:e75788. doi: 10.1371/journal.pone.0075788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Subramaniam KS, et al. Cancer-associated fibroblasts promote endometrial cancer growth via activation of interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res. 2016;6:200–213. [PMC free article] [PubMed] [Google Scholar]
- 125.Bar-Natan M, Nelson EA, Xiang M, Frank DA. STAT signaling in the pathogenesis and treatment of myeloid malignancies. JAKSTAT. 2012;1:55–64. doi: 10.4161/jkst.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bromberg JF, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 127.Ho PL, Lay EJ, Jian W, Parra D, Chan KS. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 2012;72:3135–3142. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kryczek I, et al. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase. doi: 10.1016/j.immuni.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leong PL, et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA. 2003;100:4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Y, et al. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 131.Fukuda A, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdulghani J, et al. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172:1717–1728. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim DJ, Angel JM, Sano S, DiGiovanni J. Constitutive activation and targeted disruption of signal transducer and activator of transcription 3 (Stat3) in mouse epidermis reveal its critical role in UVB-induced skin carcinogenesis. Oncogene. 2009;28:950–960. doi: 10.1038/onc.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spitzner M, et al. STAT3: a novel molecular mediator of resistance to chemoradiotherapy. Cancers. 2014;6:1986–2011. doi: 10.3390/cancers6041986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sen M, et al. Targeting Stat3 abrogates EGFR inhibitor resistance in cancer. Clin Cancer Res. 2012;18:4986–4996. doi: 10.1158/1078-0432.CCR-12-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee HJ, et al. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26:207–221. doi: 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 137.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li HS, et al. Bypassing STAT3-mediated inhibition of the transcriptional regulator ID2 improves the antitumor efficacy of dendritic cells. Sci Signal. 2016;9:ra94. doi: 10.1126/scisignal.aaf3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang T, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 140.Kujawski M, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koskela HL, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905–1913. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kucuk C, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun. 2015;6:6025. doi: 10.1038/ncomms7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Andersson E, et al. Activating somatic mutations outside the SH2-domain of STAT3 in LGL leukemia. Leukemia. 2016;30:1204–1208. doi: 10.1038/leu.2015.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grandis JR, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor-mediated cell growth In vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ozawa Y, et al. Src family kinases promote AML cell survival through activation of signal transducers and activators of transcription (STAT) Leuk Res. 2008;32:893–903. doi: 10.1016/j.leukres.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 146.Grabner B, et al. Disruption of STAT3 signalling promotes KRAS-induced lung tumorigenesis. Nat Commun. 2015;6:6285. doi: 10.1038/ncomms7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lei Y, et al. Hdac7 promotes lung tumorigenesis by inhibiting Stat3 activation. Mol Cancer. 2017;16:170. doi: 10.1186/s12943-017-0736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Musteanu M, et al. Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology. 2010;138:1003–1011.e5. doi: 10.1053/j.gastro.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 149.Hsiao JR, et al. Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlates with better prognosis. Br J Cancer. 2003;89:344–349. doi: 10.1038/sj.bjc.6601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gordziel C, Bratsch J, Moriggl R, Knosel T, Friedrich K. Both STAT1 and STAT3 are favourable prognostic determinants in colorectal carcinoma. Br J Cancer. 2013;109:138–146. doi: 10.1038/bjc.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Setsu N, et al. Phosphorylation of signal transducer and activator of transcription 3 in soft tissue leiomyosarcoma is associated with a better prognosis. Int J Cancer. 2013;132:109–115. doi: 10.1002/ijc.27655. [DOI] [PubMed] [Google Scholar]
- 152.van Rhee F, et al. Siltuximab, a novel anti-interleukin- 6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28:3701–3708. doi: 10.1200/JCO.2009.27.2377. [DOI] [PubMed] [Google Scholar]
- 153.van Rhee F, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15:966–974. doi: 10.1016/S1470-2045(14)70319-5. [DOI] [PubMed] [Google Scholar]
- 154.Kurzrock R, et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res. 2013;19:3659–3670. doi: 10.1158/1078-0432.CCR-12-3349. [DOI] [PubMed] [Google Scholar]
- 155.Hunsucker SA, et al. Blockade of interleukin-6 signalling with siltuximab enhances melphalan cytotoxicity in preclinical models of multiple myeloma. Br J Haematol. 2011;152:579–592. doi: 10.1111/j.1365-2141.2010.08533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suzuki K, et al. Phase 1 study in Japan of siltuximab, an anti-IL-6 monoclonal antibody, in relapsed/refractory multiple myeloma. Int J Hematol. 2015;101:286–294. doi: 10.1007/s12185-015-1743-y. [DOI] [PubMed] [Google Scholar]
- 157.Orlowski RZ, et al. A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am J Hematol. 2015;90:42–49. doi: 10.1002/ajh.23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Voorhees PM, et al. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013;161:357–366. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.San-Miguel J, et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood. 2014;123:4136–4142. doi: 10.1182/blood-2013-12-546374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shah JJ, et al. Siltuximab (CNTO 328) with lenalidomide, bortezomib and dexamethasone in newly-diagnosed, previously untreated multiple myeloma: an open-label phase I trial. Blood Cancer J. 2016;6:e396. doi: 10.1038/bcj.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Coward J, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–6096. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cavarretta IT, et al. Mcl-1 is regulated by IL-6 and mediates the survival activity of the cytokine in a model of late stage prostate carcinoma. Adv Exp Med Biol. 2008;617:547–555. doi: 10.1007/978-0-387-69080-3_56. [DOI] [PubMed] [Google Scholar]
- 163.Song L, et al. Antitumor efficacy of the anti-interleukin- 6 (IL-6) antibody siltuximab in mouse xenograft models of lung cancer. J Thorac Oncol. 2014;9:974–982. doi: 10.1097/JTO.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Karkera J, et al. The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. Prostate. 2011;71:1455–1465. doi: 10.1002/pros.21362. [DOI] [PubMed] [Google Scholar]
- 165.Dorff TB, et al. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res. 2010;16:3028–3034. doi: 10.1158/1078-0432.CCR-09-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fizazi K, et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48:85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 167.Rossi JF, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Angevin E, et al. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2192–2204. doi: 10.1158/1078-0432.CCR-13-2200. [DOI] [PubMed] [Google Scholar]
- 169.Liu X, Jones GW, Choy EH, Jones SA. The biology behind interleukin-6 targeted interventions. Curr Opin Rheumatol. 2016;28:152–160. doi: 10.1097/BOR.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 170.Finkel KA, et al. IL-6 inhibition with MEDI5117 decreases the fraction of head and neck cancer stem cells and prevents tumor recurrence. Neoplasia. 2016;18:273–281. doi: 10.1016/j.neo.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhong H, et al. Novel IL6 antibody sensitizes multiple tumor types to chemotherapy including trastuzumab-resistant tumors. Cancer Res. 2016;76:480–490. doi: 10.1158/0008-5472.CAN-15-0883. [DOI] [PubMed] [Google Scholar]
- 172.Yanaihara N, et al. Antitumor effects of interleukin-6 (IL-6)/interleukin-6 receptor (IL-6R) signaling pathway inhibition in clear cell carcinoma of the ovary. Mol Carcinog. 2016;55:832–841. doi: 10.1002/mc.22325. [DOI] [PubMed] [Google Scholar]
- 173.Goumas FA, et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int J Cancer. 2015;137:1035–1046. doi: 10.1002/ijc.29445. [DOI] [PubMed] [Google Scholar]
- 174.Dijkgraaf EM, et al. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-alpha2b in patients with recurrent epithelial ovarian cancer. Ann Oncol. 2015;26:2141–2149. doi: 10.1093/annonc/mdv309. [DOI] [PubMed] [Google Scholar]
- 175.Matsumoto S, et al. Essential roles of IL-6 transsignaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184:1543–1551. doi: 10.4049/jimmunol.0801217. [DOI] [PubMed] [Google Scholar]
- 176.Brooks GD, et al. IL6 trans-signaling promotes KRAS-driven lung carcinogenesis. Cancer Res. 2016;76:866–876. doi: 10.1158/0008-5472.CAN-15-2388. [DOI] [PubMed] [Google Scholar]
- 177.Hemmann U, et al. Differential activation of acute phase response factor/Stat3 and Stat1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. II Src homology SH2 domains define the specificity of stat factor activation. J Biol Chem. 1996;271:12999–13007. doi: 10.1074/jbc.271.22.12999. [DOI] [PubMed] [Google Scholar]
- 178.Gerhartz C, et al. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. I Definition of a novel phosphotyrosine motif mediating STAT1 activation. J Biol Chem. 1996;271:12991–12998. doi: 10.1074/jbc.271.22.12991. [DOI] [PubMed] [Google Scholar]
- 179.Meyer DM, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm. 2010;7:41. doi: 10.1186/1476-9255-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fleischmann R, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 181.van Vollenhoven RF, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 182.Lee EB, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 183.Hodge JA, et al. The mechanism of action of tofacitinib — an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:318–328. [PubMed] [Google Scholar]
- 184.Bachelez H, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386:552–561. doi: 10.1016/S0140-6736(14)62113-9. [DOI] [PubMed] [Google Scholar]
- 185.Sandborn WJ, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 186.Panes J, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049–1059. doi: 10.1136/gutjnl-2016-312735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Verstovsek S, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Harrison C, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 189.Harrison CN, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib versus best available therapy for myelofibrosis. Leukemia. 2016;30:1701–1707. doi: 10.1038/leu.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Neubauer H, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 191.Verstovsek S, et al. The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo-controlled, Phase III study in patients with myelofibrosis. Br J Haematol. 2013;161:508–516. doi: 10.1111/bjh.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vannucchi AM, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372:426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Passamonti F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 2017;18:88–99. doi: 10.1016/S1470-2045(16)30558-7. [DOI] [PubMed] [Google Scholar]
- 194.Komrokji RS, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood. 2015;125:2649–2655. doi: 10.1182/blood-2013-02-484832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Verstovsek S, et al. Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies. J Hematol Oncol. 2016;9:137. doi: 10.1186/s13045-016-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hedvat M, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xin H, et al. Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Res. 2011;71:6601–6610. doi: 10.1158/0008-5472.CAN-11-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sen M, et al. JAK kinase inhibition abrogates STAT3 activation and head and neck squamous cell carcinoma tumor growth. Neoplasia. 2015;17:256–264. doi: 10.1016/j.neo.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee JH, et al. The Janus kinases inhibitor AZD1480 attenuates growth of small cell lung cancers in vitro and in vivo. Clin Cancer Res. 2013;19:6777–6786. doi: 10.1158/1078-0432.CCR-13-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tavallai M, Booth L, Roberts JL, Poklepovic A, Dent P. Rationally repurposing ruxolitinib (Jakafi ®) as a solid tumor therapeutic. Front Oncol. 2016;6:142. doi: 10.3389/fonc.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Plimack ER, et al. AZD1480: a phase I study of a novel JAK2 inhibitor in solid tumors. Oncologist. 2013;18:819–820. doi: 10.1634/theoncologist.2013-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Loh ML, et al. A phase 1 dosing study of ruxolitinib in children with relapsed or refractory solid tumors, leukemias, or myeloproliferative neoplasms: a Children’s Oncology Group phase 1 consortium study (ADVL1011) Pediatr Blood Cancer. 2015;62:1717–1724. doi: 10.1002/pbc.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hurwitz HI, et al. Double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol. 2015;33:4039–4047. doi: 10.1200/JCO.2015.61.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wong ALA, et al. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin Investig Drugs. 2017;26:883–887. doi: 10.1080/13543784.2017.1351941. [DOI] [PubMed] [Google Scholar]
- 205.Turkson J, et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–45455. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 206.Turkson J, et al. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol Cancer Ther. 2004;3:261–269. [PubMed] [Google Scholar]
- 207.Mandal PK, et al. Potent and selective phosphopeptide mimetic prodrugs targeted to the Src homology 2 (SH2) domain of signal transducer and activator of transcription 3. J Med Chem. 2011;54:3549–3563. doi: 10.1021/jm2000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Auzenne EJ, et al. A phosphopeptide mimetic prodrug targeting the SH2 domain of Stat3 inhibits tumor growth and angiogenesis. J Exp Ther Oncol. 2012;10:155–162. [PMC free article] [PubMed] [Google Scholar]
- 209.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 210.Siddiquee K, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Song H, Wang R, Wang S, Lin J. A low-molecular- weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci USA. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang X, et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci USA. 2012;109:9623–9628. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen CL, et al. Signal transducer and activator of transcription 3 is involved in cell growth and survival of human rhabdomyosarcoma and osteosarcoma cells. BMC Cancer. 2007;7:111. doi: 10.1186/1471-2407-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fuh B, et al. LLL-3 inhibits STAT3 activity, suppresses glioblastoma cell growth and prolongs survival in a mouse glioblastoma model. Br J Cancer. 2009;100:106–112. doi: 10.1038/sj.bjc.6604793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hussain SF, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–9636. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 216.Pan Y, Zhou F, Zhang R, Claret FX. Stat3 inhibitor Stattic exhibits potent antitumor activity and induces chemo- and radio-sensitivity in nasopharyngeal carcinoma. PLoS ONE. 2013;8:e54565. doi: 10.1371/journal.pone.0054565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang X, et al. A novel inhibitor of STAT3 homodimerization selectively suppresses STAT3 activity and malignant transformation. Cancer Res. 2013;73:1922–1933. doi: 10.1158/0008-5472.CAN-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang X, et al. A novel small-molecule disrupts Stat3 SH2 domain-phosphotyrosine interactions and Stat3-dependent tumor processes. Biochem Pharmacol. 2010;79:1398–1409. doi: 10.1016/j.bcp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ashizawa T, et al. Antitumor activity of a novel small molecule STAT3 inhibitor against a human lymphoma cell line with high STAT3 activation. Int J Oncol. 2011;38:1245–1252. doi: 10.3892/ijo.2011.957. [DOI] [PubMed] [Google Scholar]
- 220.Matsuno K, et al. Identification of a new series of STAT3 inhibitors by virtual screening. ACS Med Chem Lett. 2010;1:371–375. doi: 10.1021/ml1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen H, et al. Fragment-based drug design and identification of HJC0123, a novel orally bioavailable STAT3 inhibitor for cancer therapy. Eur J Med Chem. 2013;62:498–507. doi: 10.1016/j.ejmech.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brambilla L, et al. Hitting the right spot: mechanism of action of OPB-31121, a novel and potent inhibitor of the signal transducer and activator of transcription 3 (STAT3) Mol Oncol. 2015;9:1194–1206. doi: 10.1016/j.molonc.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hayakawa F, et al. A novel STAT inhibitor, OPB-31121, has a significant antitumor effect on leukemia with STAT-addictive oncokinases. Blood Cancer J. 2013;3:e166. doi: 10.1038/bcj.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kim MJ, et al. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. 2013;335:145–152. doi: 10.1016/j.canlet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 225.Bendell JC, et al. Phase 1, open-label, dose-escalation, and pharmacokinetic study of STAT3 inhibitor OPB-31121 in subjects with advanced solid tumors. Cancer Chemother Pharmacol. 2014;74:125–130. doi: 10.1007/s00280-014-2480-2. [DOI] [PubMed] [Google Scholar]
- 226.Oh DY, et al. Phase I study of OPB-31121, an oral STAT3 inhibitor, in patients with advanced solid tumors. Cancer Res Treat. 2015;47:607–615. doi: 10.4143/crt.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Okusaka T, et al. Phase 1 and pharmacological trial of OPB-31121, a signal transducer and activator of transcription-3 inhibitor, in patients with advanced hepatocellular carcinoma. Hepatol Res. 2015;45:1283–1291. doi: 10.1111/hepr.12504. [DOI] [PubMed] [Google Scholar]
- 228.Wong AL, et al. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann Oncol. 2015;26:998–1005. doi: 10.1093/annonc/mdv026. [DOI] [PubMed] [Google Scholar]
- 229.Ogura M, et al. Phase I study of OPB-51602, an oral inhibitor of signal transducer and activator of transcription 3, in patients with relapsed/refractory hematological malignancies. Cancer Sci. 2015;106:896–901. doi: 10.1111/cas.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bharadwaj U, et al. Small-molecule inhibition of STAT3 in radioresistant head and neck squamous cell carcinoma. Oncotarget. 2016;7:26307–26330. doi: 10.18632/oncotarget.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xi S, Gooding WE, Grandis JR. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: implications for cancer therapy. Oncogene. 2005;24:970–979. doi: 10.1038/sj.onc.1208316. [DOI] [PubMed] [Google Scholar]
- 232.Shen J, Li R, Li G. Inhibitory effects of decoy-ODN targeting activated STAT3 on human glioma growth in vivo. In Vivo. 2009;23:237–243. [PubMed] [Google Scholar]
- 233.Sun Z, Yao Z, Liu S, Tang H, Yan X. An oligonucleotide decoy for Stat3 activates the immune response of macrophages to breast cancer. Immunobiology. 2006;211:199–209. doi: 10.1016/j.imbio.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 234.Zhang X, Zhang J, Wang L, Wei H, Tian Z. Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human lung cancer in xenograft mice. BMC Cancer. 2007;7:149. doi: 10.1186/1471-2407-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang X, et al. Inhibitory effects of STAT3 decoy oligodeoxynucleotides on human epithelial ovarian cancer cell growth in vivo. Int J Mol Med. 2013;32:623–628. doi: 10.3892/ijmm.2013.1431. [DOI] [PubMed] [Google Scholar]
- 236.Chan KS, et al. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang Q, et al. Serum-resistant CpG-STAT3 decoy for targeting survival and immune checkpoint signaling in acute myeloid leukemia. Blood. 2016;127:1687–1700. doi: 10.1182/blood-2015-08-665604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sen M, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sen M, et al. Systemic administration of a cyclic signal transducer and activator of transcription 3 (STAT3) decoy oligonucleotide inhibits tumor growth without inducing toxicological effects. Mol Med. 2014;20:46–56. doi: 10.2119/molmed.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hong D, et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med. 2015;7:314ra185. doi: 10.1126/scitranslmed.aac5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burel SA, et al. Preclinical evaluation of the toxicological effects of a novel constrained ethyl modified antisense compound targeting signal transducer and activator of transcription 3 in mice and cynomolgus monkeys. Nucleic Acid Ther. 2013;23:213–227. doi: 10.1089/nat.2013.0422. [DOI] [PubMed] [Google Scholar]
- 242.Odate S, et al. Inhibition of STAT3 with the generation 2.5 antisense oligonucleotide, AZD9150, decreases neuroblastoma tumorigenicity and increases chemosensitivity. Clin Cancer Res. 2017;23:1771–1784. doi: 10.1158/1078-0432.CCR-16-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Okiyama N, Tanaka R. Varied immuno-related adverse events induced by immune-check point inhibitors — Nivolumab-associated psoriasiform dermatitis related with increased serum level of interleukin-6 [Japanese] Nihon Rinsho Meneki Gakkai Kaishi. 2017;40:95–101. doi: 10.2177/jsci.40.95. [DOI] [PubMed] [Google Scholar]
- 244.Tanaka R, et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-alpha is a biomarker of nivolumab recativity. J Dermatol Sci. 2017;86:71–73. doi: 10.1016/j.jdermsci.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 245.Rotz SJ, et al. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64:e26642. doi: 10.1002/pbc.26642. [DOI] [PubMed] [Google Scholar]
- 246.Kim ST, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis. 2017;76:2061–2064. doi: 10.1136/annrheumdis-2017-211560. [DOI] [PubMed] [Google Scholar]
- 247.Uemura M, et al. Selective inhibition of autoimmune exacerbation while preserving the anti-tumor clinical benefit using IL-6 blockade in a patient with advanced melanoma and Crohn’s disease: a case report. J Hematol Oncol. 2016;9:81. doi: 10.1186/s13045-016-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Austin JW, Lu P, Majumder P, Ahmed R, Boss JM. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J Immunol. 2014;192:4876–4886. doi: 10.4049/jimmunol.1302750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Thorn M, et al. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther. 2016;23:188–198. doi: 10.1038/cgt.2016.19. [DOI] [PubMed] [Google Scholar]
- 250.Zhang N, et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int J Oncol. 2016;49:1360–1368. doi: 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- 251.Bu LL, et al. STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC. J Dent Res. 2017;96:1027–1034. doi: 10.1177/0022034517712435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Atsaves V, et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia. 2017;31:1633–1637. doi: 10.1038/leu.2017.103. [DOI] [PubMed] [Google Scholar]
- 253.Mace TA, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67:320–332. doi: 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liu H, Shen J, Lu K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun. 2017;486:239–244. doi: 10.1016/j.bbrc.2017.02.128. [DOI] [PubMed] [Google Scholar]
- 255.Lu C, Talukder A, Savage NM, Singh N, Liu K. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology. 2017;6:e1291106. doi: 10.1080/2162402X.2017.1291106. [DOI] [PMC free article] [PubMed] [Google Scholar]