Abstract
Abstract
Current treatments for allergic asthma primarily ameliorate symptoms rather than inhibit disease progression. Regulating the excessive T helper type 2 (Th2) responses may prevent asthma exacerbation. In this study, we investigated the protective effects of Ad5-gsgAM, an adenovirus vector carrying two mycobacterial antigens Ag85A and Mtb32, against allergic asthma. Using an ovalbumin (OVA)-induced asthmatic mouse model, we found that Ad5-gsgAM elicited much more Th1-biased CD4+T and CD8+T cells than bacillus Calmette-Guérin (BCG). After OVA challenge, Ad5-gsgAM-immunized mice showed significantly lowered airway inflammation in comparison with mice immunized with or without BCG. Total serum immunoglobulin E and pulmonary inducible-nitric-oxide-synthase were efficiently reduced. The cytokine profiles in bronchial-alveolar-lavage-fluids (BALFs) were also modulated, as evidenced by the increased level of interferon-γ (IFN-γ) and the decreased level of interleukin (IL)-4, IL-5, and IL-13. Anti-inflammatory cytokine IL-10 was sharply increased, whereas pro-inflammatory cytokine IL-33 was significantly decreased. Importantly, exogenous IL-33 abrogated the protective effects of Ad5-gsgAM, revealing that the suppression of IL-33/ST2 axis substantially contributed to protection against allergic inflammation. Moreover, regulatory T cells were essential for regulating aberrant Th2 responses as well as IL-33/ST2 axis. These results suggested that modulating the IL-33/ST2 axis via adenovirus-vectored mycobacterial antigen vaccination may provide clinical benefits in allergic inflammatory airways disease.
Key messages
•Ad5-gsgAM elicits Th1 responses and suppresses Th2-mediated allergic asthma in mice.
•Ad5-gsgAM inhibits IL-33/ST2 axis by reducing IL-33 secretion but not ILC2 recruiting.
•Treg is essential for modulating Th2 responses and IL-33/ST2 axis by Ad5-gsgAM.
Electronic supplementary material
The online version of this article (10.1007/s00109-017-1614-5) contains supplementary material, which is available to authorized users.
Keywords: Adenoviral vector, Allergic asthma, Cytokines, Immunization, Mycobacterial antigens
Introduction
Asthma is a complex syndrome characterized by airway hyperresponsiveness (AHR), airflow obstruction, and airway infiltration of inflammatory cells, which affects 5–16% of people worldwide [1]. The treatments available mainly depend on inhaled corticosteroids and sometimes in combination with long-acting beta agonists [1]. These treatments, which are usually administrated as life-long daily medications, only control symptoms but not inhibit disease progression [1]. Long-term use of these drugs leads to resistance and side effects, especially in patients with severe asthma. Recently, blocking antibodies against immunoglobulin E (IgE), interleukin (IL)-4, IL-5, IL-13, and IL-17 have emerged as adjunct therapies [2, 3]. However, a single antibody only shows moderate, if any, benefits in patients with severe asthma or in particular subgroups [3]. Adverse effects such as immuno-suppression are also observed [2, 4, 5]. Novel approaches, which can reduce and even prevent asthma exacerbation, are urgently needed.
T helper type 2 (Th2) cytokines including IL-4, IL-5, and IL-13 play crucial roles in the trigger and progression of allergic asthma, whereas Th1 cytokines such as interferon-γ (IFN-γ) and anti-inflammatory cytokine IL-10 ameliorate aberrant Th2 responses [6]. Th1-biased immune responses elicited by bacillus Calmette-Guérin (BCG) regulate excessive Th2 responses and alleviate airway inflammation in asthmatic animal models [7–9]. However, whether BCG confers protective effects in asthmatic patients remains controversial [10, 11]. Although BCG vaccination is inversely correlated to atopic diseases according to several epidemiological studies [12, 13], its benefits for asthma are absent in several clinical trials [10, 11]. The underlying mechanism remains unclear, but the variable capacity of different BCG strains in inducing Th1 responses may be an explanation [14, 15]. Mycobacterial antigens such as Ag85B or Ag85A-IL-17A fusion delivered as DNA or purified proteins were explored as immuno-therapeutics for allergic asthma [16, 17]. Nevertheless, the capacity of plasmid DNA or purified protein in inducing Th1-biased responses is relatively limited [18]. Alternative strategies, such as utilizing adenoviral vector to carry mycobacterial antigens, may generate enhanced Th1 responses and thereby provide consistent protection.
Replication-incompetent adenoviral vectors are able to elicit high levels of Th1 and CD8+T cell responses. Currently, adenoviral-vectored tuberculosis (TB) vaccines have shown great promise in preclinical trials and are undergoing clinical trials [19, 20]. Previously, we reported a recombinant adenovirus type 5 carrying two immuno-dominant mycobacterial antigens Ag85A and Mtb32 (Ad5-gsgAM), which induced robust systemic and pulmonary cellular responses [19]. Interestingly, Ag85A mainly raised IFN-γ-producing CD4+T cells, whereas Mtb32 predominantly elicited CD8+T cells which also secreted IFN-γ [19]. Both Th1 CD4+T cells and type 1 (Tc1) CD8+T cells may contribute to the suppression of Th2 responses, eosinophilia, as well as IgE production [21]. The cytokines secreted by these cells, especially IFN-γ, also participate in controlling Th2 responses and allergy [22]. We proposed that, if Ad5-gsgAM generates high levels of Th1- and Tc1-biased responses following allergen exposure, it may modulate Th2 responses and efficiently suppress allergic asthma.
In this study, we evaluated the protective effects of Ad5-gsgAM in an ovalbumin (OVA)-induced asthmatic mouse model. The effects of Ad5-gsgAM immunization on AHR, pulmonary inflammation, and Th1/Th2 responses in the airway were studied. The underlying mechanisms were also analyzed.
Materials and methods
Animals and asthma models
Six-week-old female C57BL/6 mice were purchased and housed in a specific pathogen-free facility in the Experimental Animals Center of Guangzhou Institutes of Biomedicine and Health (GIBH). The protocols of animal experiments were approved by the Institutional Animal Care and Use Committee of GIBH (Permit No. 2013026).
The asthma model was established as described previously [8, 16]. In brief, mice were sensitized by three intraperitoneal (I.P.) injections at weekly intervals with 50 μg of chicken OVA (Grade V, Sigma-Aldrich, St. Louis, MO) in 2 mg of alum (Sigma-Aldrich, St. Louis, MO). Three weeks after the final sensitization, the mice were challenged with aerosolized OVA (2% in saline) for 40 min for three consecutive days. One day after the last challenge, the mice were evaluated for AHR and then sacrificed.
Replication-incompetent adenovirus and immunization
Ad5 empty vector (Ad5) and Ad5-gsgAM were described in our previous study [19]. One week after the final OVA sensitization, the mice were immunized with saline (termed OVA mice), Ad5 (2.5 × 109 viral particles per mouse; termed OVA/Ad5 mice), or Ad5-gsgAM (2.5 × 109 viral particles per mouse; termed OVA/gsgAM mice) by intramuscular (I.M.) inoculation (n = 8 to 10 mice per group). One week later, the animals were intranasally (I.N.) boosted with the respective agents mentioned above. As for BCG (2 × 105 colony forming units per mouse; termed OVA/BCG mice) immunization, twice subcutaneous (S.C.) administrations were performed at similar time points to Ad5-gsgAM immunization. Additional mice treated with saline at each time point were used as healthy controls (termed Saline mice). To investigate the effects of Ag5-gsgAM on IL-33/ST2 axis, OVA/gsgAM mice were I.N. inoculated with recombinant mouse IL-33 (mIL-33; R&D Systems, Abingdon, UK) at 0.5 μg per mouse at 6 and 2 days before challenge. To investigate the roles of regulatory T cells (Tregs), OVA/gsgAM mice were I.P. inoculated with rat anti-mouse CD25 monoclonal antibody (PC61, 200 μg per mouse; ThermoFisher Scientific, Waltham, MA) for depletion of CD25+ Tregs at 6 and 2 days before challenge. Additional mice treated with rat anti-mouse IgG1 (R&D Systems, Abingdon, UK) were used as isotype controls. Each animal experiment was performed three times independently.
Analysis of airway allergic inflammation
The measurement of AHR, the collection and analysis of cells in the BALFs, and the examination of lung tissue sections are described in Supporting Information.
Enzyme-linked immunosorbent assay
The IgE concentration in the sera and the cytokine concentrations in the sera or BALFs were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. ELISA kits for murine IgE, IL-4, IL-5, and IL-10 were purchased from BD Bioscience (San Diego, CA). ELISA kits for murine IL-13, IL-33, ST2, IFN-γ, and TNF-α were purchased from R&D Systems (Abingdon, UK).
Enzyme-linked immunospot assay
IFN-γ- and IL-4-releasing cells are examined using enzyme-linked immunospot assay (ELISpot) assays as described in Supporting Information.
Immunohistochemistry
The immunostaining of iNOS was performed as previously reported [23]. In brief, the lung tissue sections were deparaffinized for 20 min in xylene, dehydrated for 10 min in 100% ethanol, and washed with PBS for 10 min. The endogenous peroxidase activity was then inhibited with 0.3% H2O2 for 15 min. Finally, the sections were incubated with a rat anti-iNOS antibody (Abcam, UK) overnight at 4 °C, developed with a goat anti-rat IgG, and revealed using immuno-peroxidase kit (Santa Cruz, Dallas, TX). The quantification of iNOS-expressing cells was described in Supporting Information.
Intracellular cytokine staining (ICS) and flow cytometry
Intracellular cytokine staining (ICS) assays were performed as previously described [19]. In brief, splenocytes and lung lymphocytes were isolated and seeded into 96-well plates, incubated with peptide pools of Ag85A or Mtb32 (2 μg/ml per peptide) or with 40 ng/ml Phorbol 12-myristate-13-acetate (PMA) and 1000 ng/ml ionomycin (Sigma-Aldrich, St. Louis, MO). One hour later, brefeldin A (10 μg/ml, BD Biosciences, San Diego, CA) was added and the PMA+ionomycin-stimulated cells were incubated for additional 5 h, whereas the peptide pool-stimulated cells were incubated for additional 10 h. The cells were harvested and stained with surface antibodies (CD3-PerCP, CD4-FITC, CD8-APC; BD Biosciences, San Diego, CA) for 1 h and were then washed, permeabilized, and stained with intracellular antibodies (IFN-γ-PE; BD Biosciences, San Diego, CA). Finally, the cells were detected with a BD Accuri™ C6 instrument.
To assess Tregs in the spleen and mediastinal lymph nodes (MLN), lymphocytes were isolated and stained with surface antibodies (CD3-Pacific Blue, CD4-FITC, CD25-APC; BD Biosciences, San Diego, CA) for 1 h and then washed, permeabilized, and stained with intracellular antibody (FoxP3-PE; BD Biosciences, San Diego, CA). The cells were detected with a BD Accuri™ C6 instrument.
Western blot analysis
The expression of iNOS in the lung tissues was evaluated through Western blot analysis as described in Supporting Information.
Data analysis and statistics
The flow cytometry data were collected and analyzed using the FlowJo software (version 7.6.2; Tree Star, Inc., Ashland, OR). The gel graphs were analyzed using ImageJ software (NIH, Bethesda, MD). The data were presented as the mean ± standard error of mean (SEM). The statistical comparisons between groups were analyzed by one-way analysis of variance (ANOVA), and Bonferroni post hoc tests were performed when multiple groups were compared. All the calculations were conducted with SPSS version 13.0 (SPSS Inc., Chicago, IL), and P values < 0.05 were considered statistically significant.
Results
Ad5-gsgAM immunization elicits robust Th1 CD4+ and Tc1 CD8+ T cell responses in OVA-sensitized mice
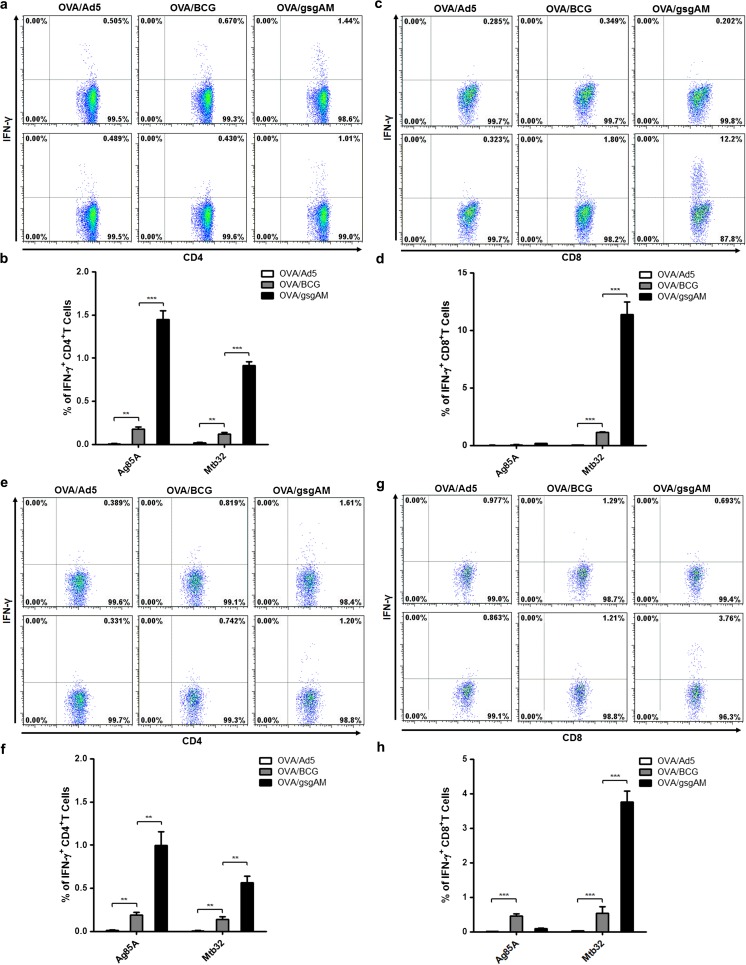
To assess the cellular responses elicited by Ad5-gsgAM following allergen sensitization, we administered Ad5-gsgAM, BCG, or Ad5 to OVA-sensitized C57BL/6 mice (Fig. S1a, b). Both BCG and Ad5-gsgAM elicited significant Ag85A-specific and Mtb32-specific IFN-γ+CD4+T and Mtb32-specific IFN-γ+CD8+T cell responses in the spleen (Fig. 1a–d). Notably, the frequencies of IFN-γ+CD4+T and IFN-γ+CD8+T cells were much higher in Ad5-gsgAM-immunized mice than in BCG-immunized mice (Fig. 1a–d), suggesting that Ag85A and Mtb32 have strong immunogenicity when harbored in an adenovirus vector. Similar to what we observed in non-asthmatic mice [19], Ag85A predominantly induced Th1 CD4+T cell responses, whereas Mtb32 mainly generated CD8+T cell responses (Fig. 1a–d). Pulmonary cellular responses exhibited similar trends as splenic ones (Fig. 1e–h). Moreover, when stimulated with PMA and ionomycin, much more IFN-γ-producing CD4+T and CD8+T cells were observed in Ad5-gsgAM-immunized mice than in BCG- or Ad5-immunized mice (Fig. S1c–f). Together, Ad5-gsgAM elicits much stronger Th1 CD4+T and Tc1 CD8+T cell responses than BCG in OVA-sensitized mice.
Fig. 1.
Ad5-gsgAM immunization generates robust antigen-specific T cell responses in OVA-sensitized mice. OVA-sensitized mice were immunized as depicted in Fig. S1. One week after the final immunization, lymphocytes were isolated from the spleens and lungs of different groups of mice and were stimulated with peptide pools of Ag85A or Mtb32. Unstimulated lymphocytes from each group were used as controls. Then, the cells were stained with CD3-PerCP, CD4-FITC, CD8-APC, and IFN-γ-PE and subjected to FACS analysis. a Representative dot plots of Ag85A-specific (upper panel) and Mtb32-specific (bottom panel) CD4+T cells secreting IFN-γ in the spleens. b The percentages of antigen-specific IFN-γ+CD4+T cells in total CD4+T cells in the spleens. c Representative dot plots of Ag85A-specific (upper panel) and Mtb32-specific (bottom panel) CD8+T cells secreting IFN-γ in the spleens. d The percentages of antigen-specific IFN-γ+CD8+T cells in total CD8+T cells in the spleens. e Representative dot plots of Ag85A-specific (upper panel) and Mtb32-specific (bottom panel) CD4+T cells secreting IFN-γ in the lungs. f The percentages of antigen-specific IFN-γ+CD4+T cells in total CD4+T cells in the lungs. g Representative dot plots of Ag85A-specific (upper panel) and Mtb32-specific (bottom panel) CD8+T cells secreting IFN-γ in the lungs. h The percentages of antigen-specific IFN-γ+CD8+T cells in total CD8+T cells in the lungs. Data are presented as mean ± standard error of the mean (SEM, n = 5 mice per group). **P < 0.01, ***P < 0.001. Similar results were obtained from two additional experiments
Ad5-gsgAM immunization reduces AHR, eosinophilia, and total serum IgE in OVA-induced asthmatic mice
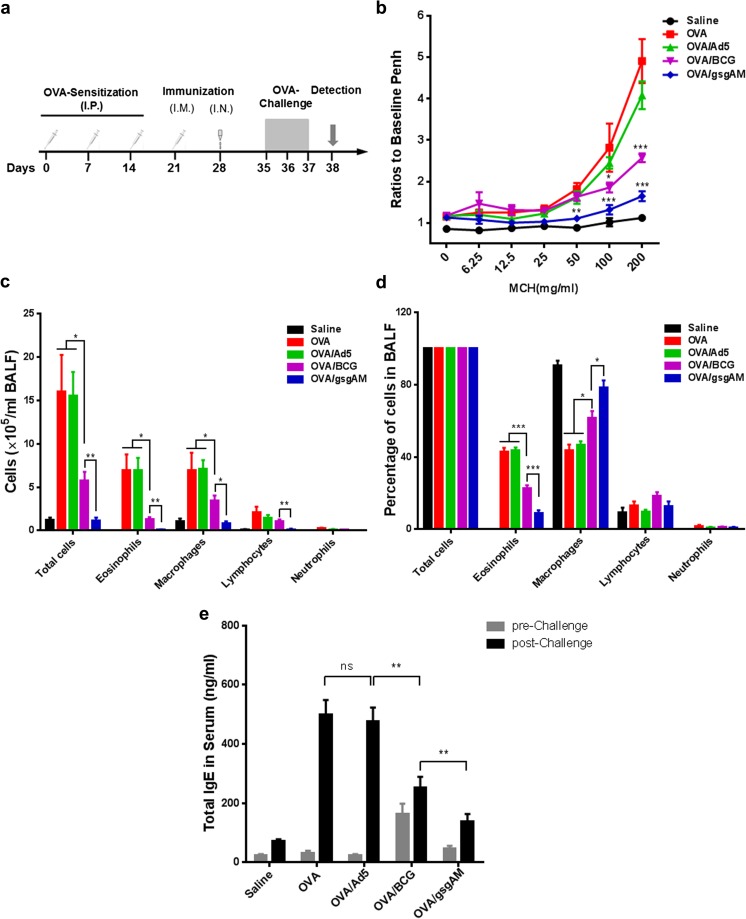
We administrated Ad5-gsgAM, BCG, or Ad5 to OVA-sensitized mice before OVA challenge (Fig. 2a). OVA/gsgAM but not OVA/Ad5 or OVA/BCG mice showed significantly lower Penh values in response to 50 mg/ml aerosolized MCh, in comparison with OVA mice (Fig. 2b). Ad5-gsgAM immunization led to a sharp reduction at both 100 and 200 mg/ml of MCh in comparison with Ad5 immunization, whereas BCG immunization only resulted in a moderate decrease of Penh values at 100 mg/ml of MCh and a significant decrease at 200 mg/ml of MCh (Fig. 2b). Thus, Ad5-gsgAM inhibits AHR more efficiently than BCG.
Fig. 2.
The effects of Ad5-gsgAM immunization on AHR, inflammatory cell infiltration, and total serum IgE. One week after sensitization with saline or OVA, mice were immunized with saline, Ad5, BCG, or Ad5-gsgAM and then challenged with OVA for three consecutive days. Twenty-four hours after the final challenge, they were subjected to whole-body plethysmograph analysis followed by sacrifice and lavage. a The schedules of sensitization, immunization, challenge, and detection. b The AHR in response to increased doses of inhaled MCh. c The absolute numbers of total cells, eosinophils, macrophages, lymphocytes, and neutrophils in the BALFs were counted using H&E staining. d The percentages of total cells, eosinophils, macrophages, lymphocytes, and neutrophils in the BALFs. e The concentrations of total serum IgE before and after challenge were assessed by ELISA. Data are presented as the mean ± SEM (n = 5 mice per group). Representative results from one of three independent experiments are shown. *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significance
Next, we analyzed the number and type of cells in the BALFs. Both Ad5-gsgAM and BCG significantly reduced the numbers of total cells, eosinophils, and macrophages as compared to Ad5 (Fig. 2c). Notably, Ad5-gsgAM immunization resulted in a greater reduction than BCG immunization (Fig. 2c), suggesting that Ad5-gsgAM has greater potency in suppressing inflammatory cell infiltration. The percentage of eosinophils was also much lower in OVA/gsgAM mice than in other experimental mice (Fig. 2d). Intriguingly, in the BALFs of OVA/gsgAM mice, macrophages were the dominant cells, similar to that of Saline mice (Fig. 2d). Moreover, Ad5-gsgAM but not BCG immunization decreased the number of lymphocytes (Fig. 2c). Together, Ad5-gsgAM potentially inhibits the infiltration of inflammatory cells.
We also examined the total serum IgE. Before OVA challenge, comparable serum IgE was detected for all groups but OVA/BCG mice, for which we observed a higher IgE level (Fig. 2e). After challenge, OVA and OVA/Ad5 but not OVA/BCG mice showed enhancement in IgE levels, whereas OVA/gsgAM mice displayed a significantly lower level of serum IgE than OVA/BCG mice (Fig. 2e), suggesting that Ad5-gsgAM suppresses the production of IgE more efficiently than BCG.
Ad5-gsgAM immunization ameliorates pulmonary inflammation and mucus over-production
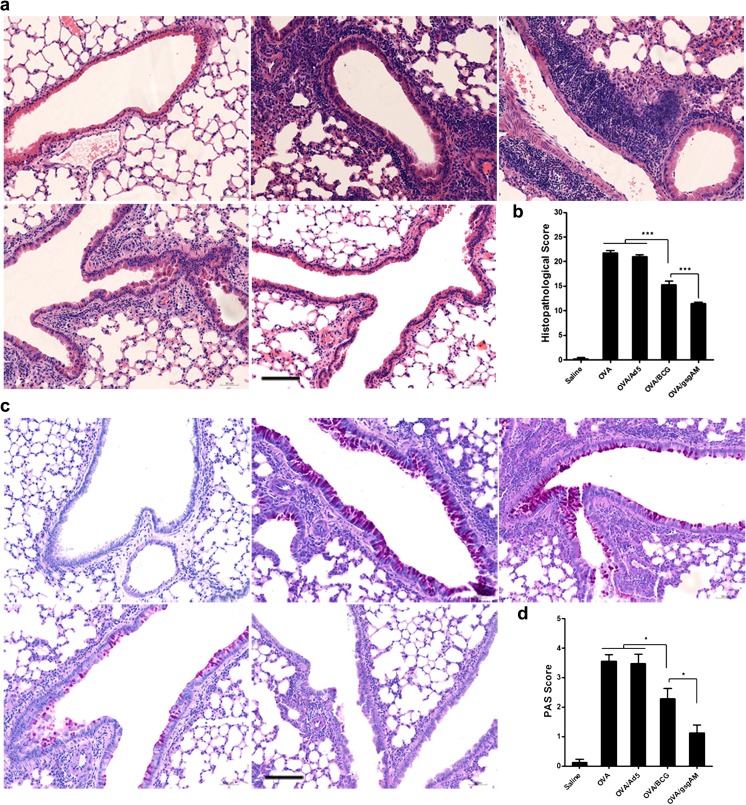
We then analyzed the lung tissue sections using H&E and PAS staining. The peribronchial and perivascular inflammation in OVA/gsgAM and OVA/BCG mice was attenuated as compared to OVA or OVA/Ad5 mice (Fig. 3a, b). Importantly, Ad5-gsgAM immunization showed a further alleviation in comparison with BCG immunization (Fig. 3a, b). Consistent with these observations, Ad5-gsgAM immunization remarkably reduced mucus-secreting goblet cells, whereas BCG immunization only achieved moderate reduction (Fig. 3c, d). These results suggested that Ad5-gsgAM substantially reduces airway inflammation and mucus over-production upon allergen challenge.
Fig. 3.
The effects of Ad5-gsgAM immunization on airway inflammation and goblet cell hyperplasia. Twenty-four hours after the final challenge, mouse lung tissue sections were subjected to histological examination. a Representative photomicrographs of H&E-stained lung sections from each group of mice (upper left panel: Saline mice; upper middle panel: OVA mice; upper right panel: OVA/Ad5 mice; bottom left panel: OVA/BCG mice; bottom middle panel: OVA/gsgAM mice). Bar = 100 μm. b Quantitative assessment of the peribronchial and perivascular inflammation. c Representative photomicrographs of PAS-stained lung sections from each group of mice (upper left panel: Saline mice; upper middle panel: OVA mice; upper right panel: OVA/Ad5 mice; bottom left panel: OVA/BCG mice; bottom middle panel: OVA/gsgAM mice). Bar = 100 μm. d Quantitative assessment of goblet cell hyperplasia. Data are presented as the mean ± SEM (n = 5 mice per group). Representative results from one of three independent experiments are shown. *P < 0.05, ***P < 0.001
Ad5-gsgAM immunization suppresses the expression of iNOS in the airway
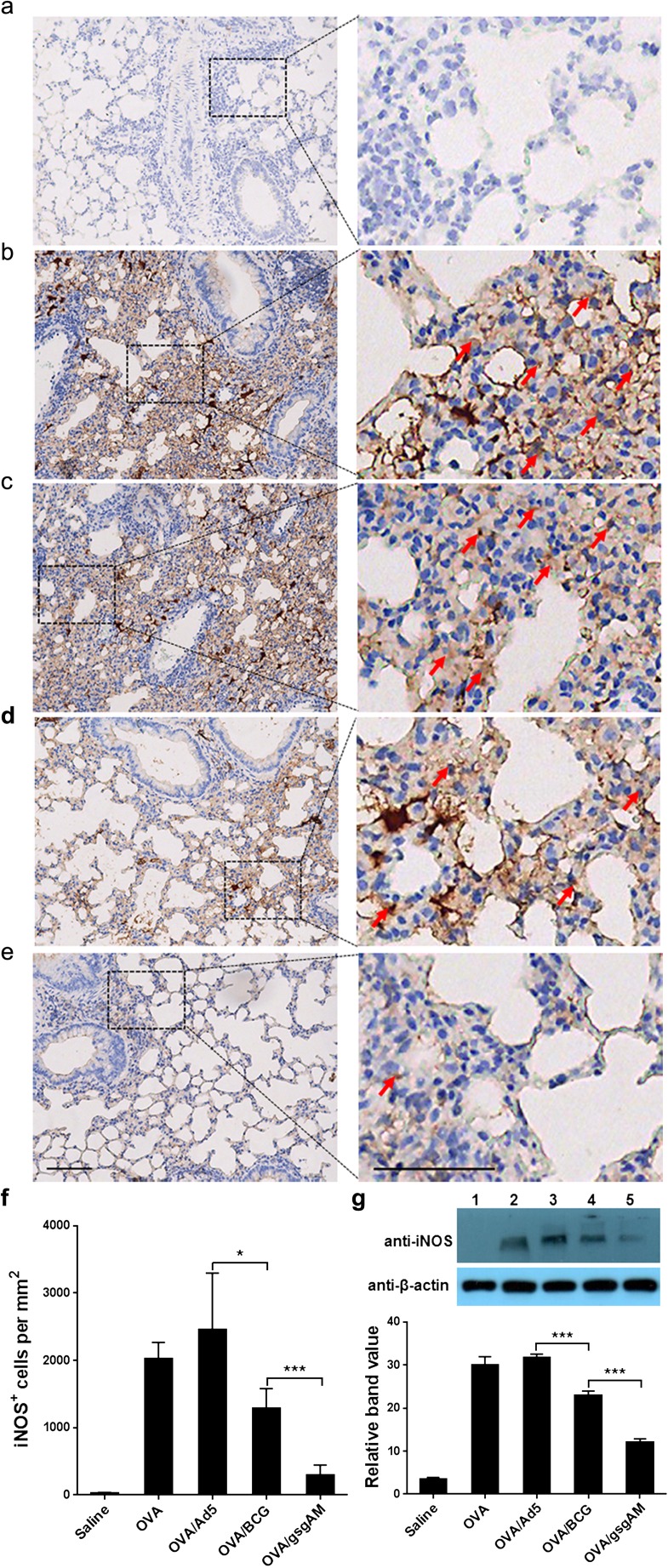
Because NO has been reported to promote the pathophysiology of asthma [24], we analyzed whether Ad5-gsgAM immunization affected the expression of iNOS. A large number of iNOS-expressing cells were observed in OVA-challenged mice treated with or without Ad5 but not in healthy control mice (Fig. 4a–c, f). Ad5-gsgAM significantly decreased the number of iNOS-expressing cells, whereas BCG resulted in a moderate reduction (Fig. 4d–f). Consistently, the amount of iNOS in OVA/gsgAM mice was reduced in comparison with OVA, OVA/Ad5, and even OVA/BCG mice (Fig. 4g), revealing that Ad5-gsgAM inhibits the expression of iNOS with greater efficiency than BCG.
Fig. 4.
The expression of iNOS in the lungs of the experimental and control mice. Twenty-four hours after the final challenge, mouse lung tissue sections were immuno-stained with anti-iNOS antibodies. a–e Representative photomicrographs of immuno-stained lung sections from Saline (a), OVA (b), OVA/Ad5 (c), OVA/BCG (d), and OVA/gsgAM (e) mice were shown. The iNOS-expressing cells are marked by red arrows. Bar = 100 μm. f Quantitative analysis of the iNOS-expressing cells in the lung tissue sections from each group of mice. Three sections of each mouse were analyzed. The iNOS-expressing cells in five random fields of each section were counted and calculated as cell numbers per square millimeter. Data are presented as the mean ± SEM (n = 4 to 6 mice per group). Representative results from one of three independent experiments are shown. *P < 0.05, ***P < 0.001. g The concentration of iNOS in the lung tissue homogenates pooled from five mice per group were examined with Western blot analysis (upper panel) and then quantified using ImageJ software (bottom panel). One representative gel from three independent experiments was shown. Lane 1: Saline mice; Lane 2: OVA mice; Lane 3: OVA/Ad5 mice; Lane 4: OVA/BCG mice; Lane 5: OVA/gsgAM mice. The density value of each band was read out and the net band value was obtained by deducting the background from the band value. The relative band value was calculated as the ratio of the net band value of iNOS vs that of β-actin. Data are presented as the mean ± SEM (n = 5 mice per group). Representative results from one of three independent experiments are shown. *P < 0.05, ***P < 0.001
Ad5-gsgAM immunization modulates the excessive pulmonary Th2 cytokines and suppresses IL-33/ST2 axis
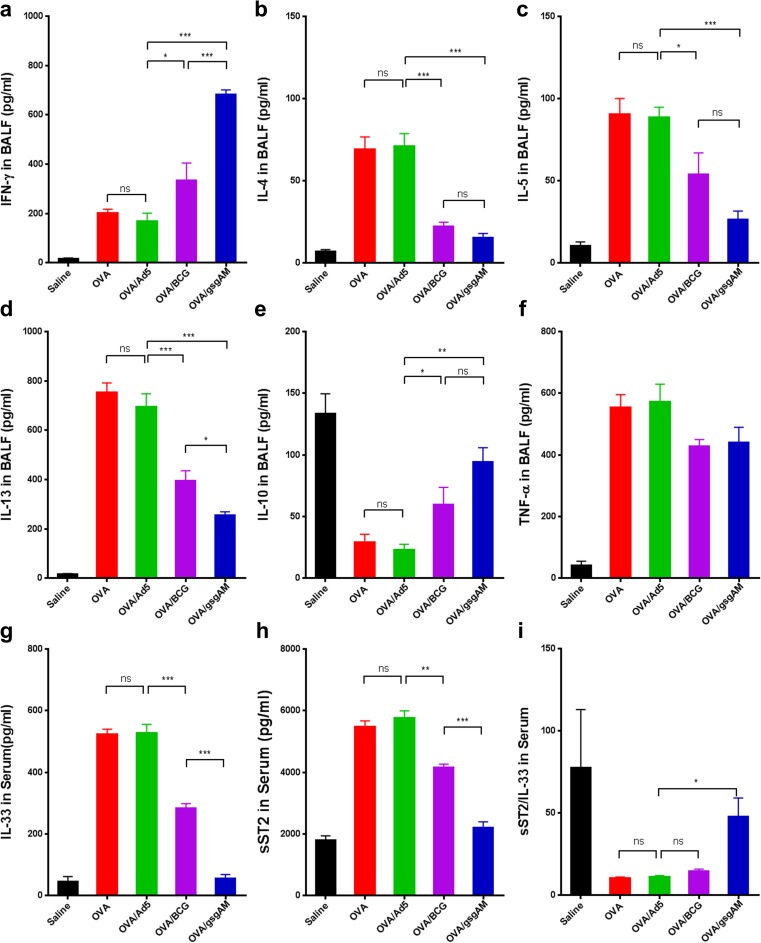
To determine whether Ad5-gsgAM immunization modulates Th1/Th2 immune responses, we assessed the cytokine profiles in the BALFs. OVA/gsgAM mice showed significantly elevated level of IFN-γ compared to OVA/BCG mice (Fig. 5a), and both were significantly higher in comparison with OVA/Ad5 mice (Fig. 5a), suggesting that Ad5-gsgAM immunization enhances pulmonary Th1 cytokines. On the other hand, both Ad5-gsgAM and BCG immunization sharply reduced the contents of IL-4, IL-5, and IL-13 (Fig. 5b–d). Notably, Ad5-gsgAM immunization resulted in significantly lower level of IL-13 in comparison with BCG immunization (Fig. 5d). IL-10, an anti-inflammatory cytokine mainly secreted by Tregs and monocytes [25], was significantly elevated in OVA/gsgAM and OVA/BCG mice (Fig. 5e). TNF-α, however, were comparable in experimental animals (Fig. 5f). Together, Ad5-gsgAM enhances Th1 and anti-inflammatory cytokines but decreases Th2 cytokines in the airway.
Fig. 5.
Profiling of cytokines in the BALFs and serums. Twenty-four hours after the final challenge, the cytokines in the BALFs and serums of different group of mice were evaluated by ELISA. a–f The concentrations of IFN-γ (a), IL-4(b), IL-5 (c), IL-13 (d), IL-10 (e), and TNF-α (f) in the BALFs. g–i The contents of IL-33 (g) and sST2 (f) in the serums were assessed by ELISA and the ratios (i) of sST2 to IL-33 were calculated and shown. Data are presented as the mean ± SEM (n = 5 mice per group). Representative results from one of three independent experiments are shown. *P < 0.05, **P < 0.01, ***P < 0.001. ns, no significance
IL-33/ST2 axis promotes the expression of pro-inflammatory cytokines and accumulation of inflammatory cells [26]. We therefore investigated whether Ad5-gsgAM affected this pathway. Both Ad5-gsgAM and BCG decreased serum IL-33 as compared to Ad5 (Fig. 5g), with OVA/gsgAM mice displaying a further reduction than OVA/BCG mice (Fig. 5g). OVA/gsgAM and OVA/BCG mice also showed significantly lower levels of soluble ST2 (sST2) (Fig. 5h). Interestingly, the ratio of sST2 to IL-33 was higher in OVA/gsgAM mice than in OVA or OVA/Ad5 mice (Fig. 5i). Because sST2 competitively blocks the binding of IL-33 to ST2 [27], Ad5-gsgAM immunization efficiently modulates IL-33/ST2 axis.
CD4+T and CD8+T cells elicited by Ad5-gsgAM suppress airway inflammation.
To confirm the protective effects of Ad5-gsgAM-induced T cell responses, we performed adoptive transfer assays. CD4+T and CD8+T cells were isolated from the spleens and lungs of Ad5-gsgAM- or Ad5-immunized mice. One day before challenge, OVA-sensitized mice were inoculated with splenic and lung T cells through intravenous and intranasal routes, respectively (Fig. S2a). Both CD4+T and CD8+T cells from Ad5-gsgAM- but not Ad5-immunized mice significantly reduced eosinophilia (Fig. S2b, c). The suppressive efficacy of CD4+T cells is comparable to CD8+T cells. Consistently, IL-5, IL-13, IL-33, as well as sST2 were reduced, as observed in OVA/gsgAM mice (Fig. S2d–g). Therefore, CD4+T and CD8+T cells induced by Ad5-gsgAM have protective effects against allergic inflammation.
Exogenous IL-33 abolishes the anti-asthmatic effects of Ad5-gsgAM.
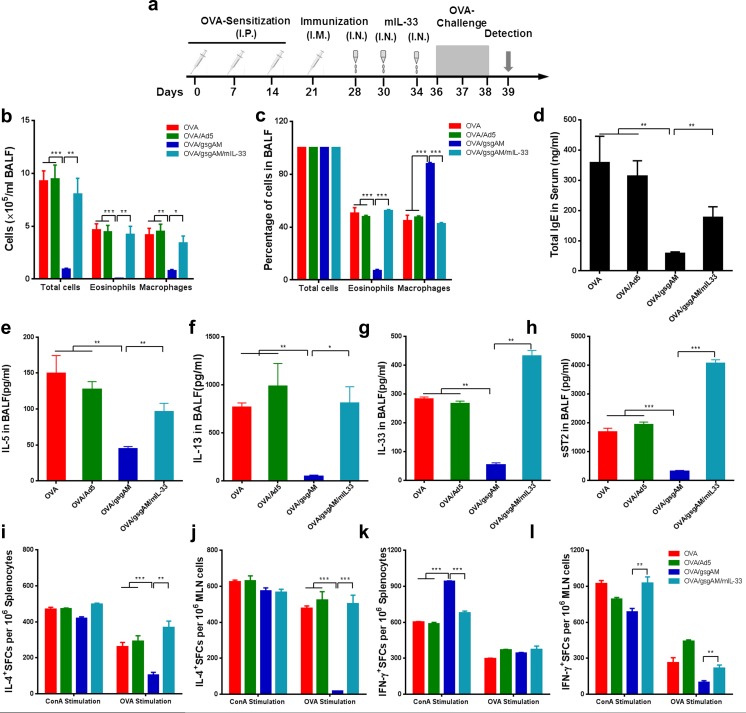
To further investigate the modulation of IL-33/ST2 axis by Ad5-gsgAM, we administrated mIL-33 to Ad5-gsgAM-immunized mice prior to OVA challenge (Fig. 6a). Exogenous mIL-33 significantly elevated inflammatory cell infiltration into airways (Fig. 6b, c). In addition, mIL-33 partially restored the total serum IgE (Fig. 6d). The modulation of aberrant Th2 cytokines by Ad5-gsgAM also disappeared in the presence of mIL-33, as IL-5 and IL-13 in the BALFs substantially increased (Fig. 6e–g). Notably, exposure to mIL-33 induced a higher level of sST2 in the BALFs (Fig. 6h). Therefore, exogenous inoculation with mIL-33 abolishes the protective effects of Ad5-gsgAM.
Fig. 6.
Exogenous IL-33 reverses the suppressive effects of Ad5-gsgAM on asthma. Six-week-old mice were sensitized with OVA, immunized with Ad5 or Ad5-gsgAM, inoculated with saline or mouse IL-33 (termed as OVA/gsgAM/mIL-33), and then challenged with OVA. Twenty-four hours after the final challenge, the mice were sacrificed. a Schedules of OVA sensitization, immunization, IL-33 inoculation, OVA challenge, and detection. b The absolute numbers of total cells, eosinophils, and macrophages in the BALFs were counted based on H&E staining. c The percentages of total cells, eosinophils, and macrophages in the BALFs. d The contents of IgE in the serums of the experimental mice were determined by ELISA. e–h The contents of IL-5 (e), IL-13 (f), IL-33 (g), and sST2 (h) in the BALFs were determined by ELISA. i The IL-4-secreting cells in the splenocytes. j The IL-4-secreting cells in the MLN lymphocytes. k The IFN-γ-secreting cells in the splenocytes. l The IFN-γ-secreting cells in the MLN lymphocytes. Lymphocytes from the spleens and MLNs were stimulated with 10 μg/ml ConA or 10 μg/ml OVA. After a 24-h incubation for IFN-γ or a 48-h incubation for IL-4 detection, the IL-4- or IFN-γ-secreting cells were assessed by ELISpot. Data are presented as the mean ± SEM (n = 5 mice per group). Representative results from one of three independent experiments are shown. *P < 0.05, **P < 0.01, ***P < 0.001
We also assessed the alterations of cellular responses after mIL-33 inoculation. Comparable IL-4-producing cells were observed in the spleen and MLN when stimulated with ConA (Fig. 6i, j). However, mIL-33 significantly enhanced OVA-specific IL-4-secreting cells, both in the spleen and MLN (Fig. 6i, j). Meanwhile, mIL-33 reduced ConA-stimulated IFN-γ-secreting cells in the spleen (Fig. 6k) but enhanced ConA- and OVA-stimulated IFN-γ-secreting cells in the MLN (Fig. 6l). These results suggested that exogenous mIL-33 alters the IL-4+ and IFN-γ+ cellular responses both in the spleen and in the airway.
We then examined group 2 innate lymphoid cells (ILC2s), one of the target cells of IL-33, in the airways of asthmatic mice treated with Ad5-gsgAM or Ad5. The frequency of ILC2 is comparable in these two groups of mice (Fig. S3), suggesting that Ad5-gsgAM treatment does not decrease the number of pulmonary ILC2s. Thus, Ad5-gsgAM modulates IL-33/ST2 pathways more likely through decreasing the production of IL-33 but not the recruitment of ILC2.
Suppression of pulmonary inflammation by Ad5-gsgAM is dependent on regulatory T cells
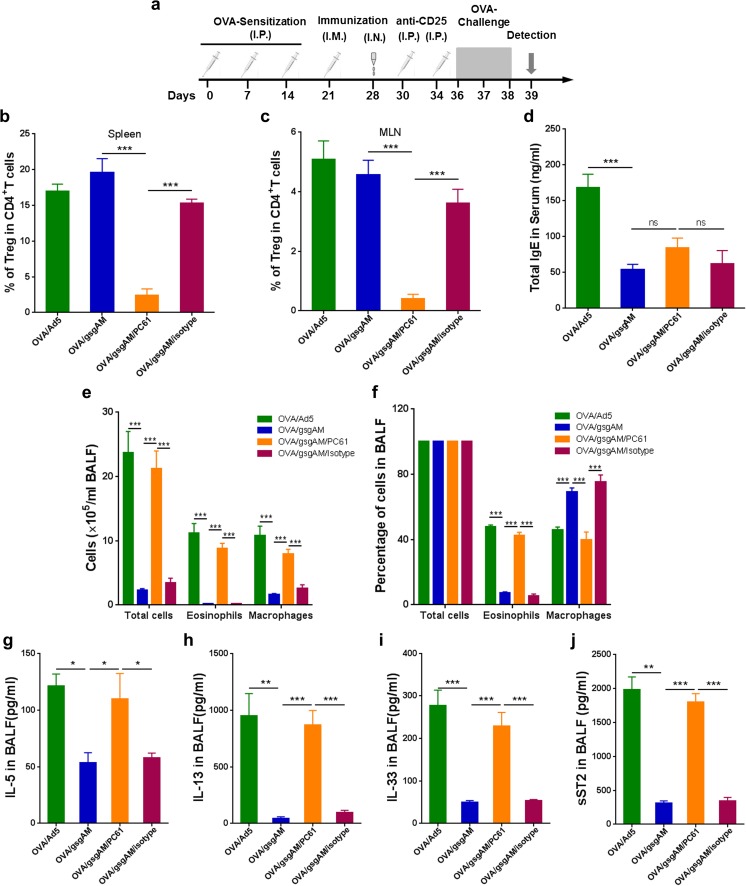
To investigate whether Tregs participant in the protective effects of Ad5-gsgAM, we depleted CD4+CD25+FoxP3+ cells with anti-CD25 antibody PC61 before challenge (Fig. 7a). Most Tregs were depleted in PC61-treated but not isotype-treated mice (Fig. 7b, c). Treg depletion did not reverse the suppression of serum IgE by Ad5-gsgAM (Fig. 7d) but enhanced inflammatory cells, especially eosinophils, in the BALFs (Fig. 7e, f), implying that Tregs were essential for suppressing inflammatory cell infiltration but not IgE production. IL-5 and IL-13 were significantly elevated (Fig. 7g, h). The content of IL-33 and sST2 was also sharply increased in Treg-depleted animals (Fig. 7i, j), suggesting that Tregs were important for the modulation of IL-33/ST2 axis. Together, Tregs are essential for suppressing airway inflammation by Ad5-gsgAM, but there are other pathways participating in the inhibition of IgE production.
Fig. 7.
Treg is essential for the suppressive effects of Ad5-gsgAM on asthma. Six-week-old mice were sensitized with OVA, immunized with Ad5 or Ad5-gsgAM, inoculated with anti-CD25 antibody PC61 or isotype control (termed as OVA/gsgAM/PC61 or OVA/gsgAM/isotype, respectively), and then challenged with OVA. Twenty-four hours after the final challenge, the mice were sacrificed. a Schedules of OVA sensitization, immunization, antibody inoculation, OVA challenge, and detection. b, c The frequency of CD4+CD25+FoxP3+ cells in the spleen (b) and MLN (c) were examined by flow cytometry. d The contents of IgE in the serums were determined by ELISA. e The absolute numbers of total cells, eosinophils, and macrophages in the BALFs were counted based on H&E staining. f The percentages of total cells, eosinophils, and macrophages in the BALFs. g–j The contents of IL-5 (g), IL-13 (h), IL-33 (i), and sST2 (j) in the BALFs were determined by ELISA. Data are presented as the mean ± SEM (n = 5 to 7 mice per group). Representative results from one of three independent experiments are shown. *P < 0.05, **P < 0.01, ***P < 0.001. ns, no significance
Discussion
To our knowledge, we are the first to adopt Ad5, the most potent vector for generating T cell responses [28], as the carrier of mycobacterial antigens for the purpose of preventing allergic asthma. Ad5-gsgAM elicits much stronger T cell responses, including Th1 CD4+T and Tc1 CD8+T cells, than BCG (Figs. 1and S1). After OVA challenge, Ad5-gsgAM provides efficient protection against allergic asthma (Figs. 2 and 3). Ad5-gsgAM not only inhibits iNOS expression and modulates excessive Th2 responses (Figs. 4 and 5) but also suppresses the IL-33/ST2 axis through inhibiting IL-33 production (Figs. 6, S2, and S3). Moreover, Tregs are essential for the protective effects of Ad5-gsgAM (Fig. 7). Our results support that Ad5-gsgAM is worthy for further exploration as an alternative immunotherapy against asthma.
Ad5-gsgAM has at least three advantages over other candidates such as BCG and mycobacterial proteins: (i) Ag85A and Mtb32 harbored in Ad5 vector have strong immunogenicity (Figs. 1 and S1). Both Th1 CD4+T and Tc1 CD8+T cells induced by Ag85A and Mtb32 may downregulate Th2 responses and have benefits for allergic asthma (Fig. S2) [21]. More than 90% decrease of BALF cells was observed in Ad5-gsgAM-immunized mice (Fig. 2), whereas treatment with pMG-Ad85B or Ag85a-IL-17A only achieved 37% or nearly 40% decrease of BALF cells, respectively [16, 17], demonstrating the robustness of Ad5-gsgAM. Thus, Ad5-gsgAM may evade the variant efficiency of different BCG strains and provide consistent protection [14, 15]. (ii) Incorporating two immuno-dominant antigens may cover more T cell epitopes than those containing only a single antigen and thereby facilitates to generate immune responses in genetically heterologous individuals [29]. (iii) Recombinant Ad5 vectors, including those carrying mycobacterial antigens, have good safety profiles [18, 20], which may allay the safety concerns of BCG, especially for immuno-suppressed individuals.
In accordance with the inhibition of airway inflammation, Ad5-gsgAM suppresses the production of total serum IgE (Fig. 2), similar to another study using Ag85B protein [30]. Surprisingly, BCG immunization elicits significant serum IgE before OVA challenge (Fig. 2). Actually, an early study showed that BCG elicited higher level of total IgE than a modified BCG in mice [31]. Individuals exposed to Mycobacterium tuberculosis or Mycobacterium avium Subsp. Paratuberculosis infection generated IgE responses [32, 33]. Although mycobacterial antigen is generally considered to downregulate IgE production [34], our and others’ results implied that some mycobacterium including BCG could elicit IgE responses. Therefore, Ad5-gsgAM may be better than BCG in controlling IgE production.
iNOS and its product nitric oxide (NO) play important roles in tissue damage during airway inflammation [35]. Selective inhibitors of iNOS reduce the influx of inflammatory cells in animals [23]. Although these inhibitors have shown minimal benefits for asthma in clinical trials, knocking-out all of the NOS isoforms decreases airway inflammation and reduces Th2 cytokines such as IL-4, IL-5, and IL-13 in asthmatic mice [24]. Thus, the suppression of iNOS by Ad5-gsgAM may contribute to the inhibition on airway inflammation and Th2 responses (Fig. 4).
The mechanism by which BCG or mycobacterial antigens alleviate asthma remains unclear. According to the “hygiene hypothesis,” Th1 responses antagonize excessive Th2 responses and prevent the onset of allergic asthma [6]. IFN-γ+T cells other than Tregs contribute to the protective effects in asthmatic mice receiving neonatal BCG immunization [36]. However, other studies indicate that IL-10-secreting Tregs generated by BCG or freeze-dried BCG are important for the suppression of allergic inflammation [37]. We showed that Ad5-gsgAM-induced CD4+T and CD8+T cells were protective against airway inflammation (Fig. S2). We also showed that Tregs were essential for the modulation of Th2 responses and airway inflammation, because IL-10 in the airways were sharply increased in OVA/gsgAM mice (Fig. 5), whereas Tregs depletion reversed the inhibition of airway inflammation (Fig. 7). Therefore, multiple pathways, including Th1 responses and Tregs, participate in the modulation of Th2 responses by Ad5-gsgAM.
Recently, the importance of IL-33/ST2 axis has been recognized in the trigger and maintenance of allergic asthma [38]. IL-33 is an “alarmin” and releases upon cell injury [39, 40], whereas ST2 has two isoforms, the full-length transmembrane ST2 and the truncated soluble sST2 [41]. The interaction of IL-33 with ST2 activates ILC2, memory Th2, and mast cells, which secrete IL-5 and IL-13 and facilitate pulmonary eosinophilic inflammation [26, 42]. sST2 is a decoy receptor of IL-33 and competitively inhibits IL-33/ST2 pathway [41]. The ratio of IL-33 vs sST2 reflects the bioavailability of circulating IL-33 and the activity of IL-33/ST2 pathway [43–45]. Ad5-gsgAM efficiently reduced serum and pulmonary IL-33, and elevated the ratio of sST2 vs IL-33 to a similar level to healthy control mice, reflecting an efficient suppression on IL-33/ST2 pathway (Figs. 5 and 6). Exogenous mIL-33 abolished the protective effects of Ad5-gsgAM, consistent with others’ results [46]. Interestingly, exogenous IL-33 induced large amounts of sST2 (Fig. 6), possibly because sST2 could be synthesized by mast cells when activated by IL-33 [44]. The reduction of serum and pulmonary IL-33 in Ad5-gsgAM-treated mice may limit the secretion of sST2 (Figs. 5 and 6). However, Ad5-gsgAM did not reduce the frequency of pulmonary ILC2 (Fig. S3). Therefore, Ad5-gsgAM modulates IL-33/ST2 axis more likely through reducing IL-33 production but not decreasing pulmonary ILC2.
Several limitations still existed in this study. Firstly, we used non-invasive whole-body plethysmography (Penh) to measure AHR (Fig. 2). Compared to lung resistance (RL) measurement, Penh has significant advantages such as no need of anesthesia and surgery and thereby avoids the influence on physiological parameters of experimental animals [47]. However, the sensitivity of Penh in analyzing pulmonary mechanics is relatively limited [48, 49]. Both Penh and RL detected AHR in severe asthma, but only RL detected AHR in mild asthma [47]. Nevertheless, Ad5-gsgAM significantly decreased AHR when measured by Penh, consistent to the results of serum IgE and airway inflammation (Figs. 2 and 3). Therefore, although more accurate results may be dependent on RL measurements, Penh detection may also give insightful results. Secondly, further studies are needed to dissect the link between innate and adaptive immunity elicited by Ad5-gsgAM during the protection against allergic asthma. Thirdly, the function mechanisms of Ad5-gsgAM other than modulation of IL-33/ST2 axis need to be clarified using IL-33−/− or ST2−/− mice, which is unavailable in our laboratory.
In summary, we demonstrated that Ad5-gsgAM generates robust Th1 and Tc1 responses and inhibits airway inflammation in an OVA-induced asthmatic mouse model. The modulation of excessive Th2 responses and IL-33/ST2 pathway and the suppression of iNOS expression contribute to the protective effects. Tregs are essential for the protective effects conferred by Ad5-gsgAM. Our results provide new insights for the design of alternative therapeutic vaccines against allergic asthma.
Electronic supplementary material
(PDF 218 kb)
(PDF 158 kb)
(PDF 112 kb)
(PDF 130 kb)
Acknowledgments
We thank Zheyu Cai at the Experimental Animal Center of GIBH for his technical assistance. This study was supported by the National Natural Science Foundation of China (Nos. 91442102, 31200693, 31470892, and 31300764), the Guangzhou Health Care and Cooperative Innovation Major Project (Nos. 201508020252 and 201604020006), the Pearl River S&T Nova Program of Guangzhou (No. 201506010076), and a grant from the CAS Youth Innovation Promotion Association (No. 2014328).
Contribution of authors
YZ, YF and LL performed the experiments, analyzed the data, and contributed to the writing of this manuscript. XY, JW, QW, PL, NL, XZ, and XG contributed to the collection and analysis of data. CL, FL, BS, KL, ZS, and NZ revised the manuscript. LC and LF provided the conception and design of the study, wrote, and revised the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Yiling Zhang, Ying Feng and Liang Li contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s00109-017-1614-5) contains supplementary material, which is available to authorized users.
Contributor Information
Ling Chen, Phone: +86-20-32015289, Email: chen_ling@gibh.ac.cn.
Liqiang Feng, Phone: +86-20-32015289, Email: feng_liqiang@gibh.ac.cn.
References
- 1.Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ. 2014;349(nov24 8):g5517. doi: 10.1136/bmj.g5517. [DOI] [PubMed] [Google Scholar]
- 2.Chung KF. New treatments for severe treatment-resistant asthma: targeting the right patient. Lancet Respir Med. 2013;1(8):639–652. doi: 10.1016/S2213-2600(13)70128-0. [DOI] [PubMed] [Google Scholar]
- 3.Nixon J, Newbold P, Mustelin T, Anderson GP, Kolbeck R. Monoclonal antibody therapy for the treatment of asthma and chronic obstructive pulmonary disease with eosinophilic inflammation. Pharmacol Ther. 2017;169:57–77. doi: 10.1016/j.pharmthera.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Yalcin AD, Bisgin A, Cetinkaya R, Yildirim M, Gorczynski RM. Clinical course and side effects of anti-IgE monoclonal antibody in patients with severe persistent asthma. Clin Lab. 2013;59(1-2):71–77. doi: 10.7754/clin.lab.2012.120406. [DOI] [PubMed] [Google Scholar]
- 5.Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012;11(12):958–972. doi: 10.1038/nrd3792. [DOI] [PubMed] [Google Scholar]
- 6.Bosnjak B, Stelzmueller B, Erb KJ, Epstein MM. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res. 2011;12(1):114. doi: 10.1186/1465-9921-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nahori MA, Lagranderie M, Lefort J, Thouron F, Joseph D, Winter N, Gicquel B, Lapa e Silva JR, Vargaftig BB (2001) Effects of Mycobacterium bovis BCG on the development of allergic inflammation and bronchial hyperresponsiveness in hyper-IgE BP2 mice vaccinated as newborns. Vaccine 19: 1484–1495, 11-12, DOI: [DOI] [PubMed]
- 8.Shen H, Huang H, Wang J, Ye S, Li W, Wang K, Zhang G, Wang P. Neonatal vaccination with Bacillus Calmette-Guerin elicits long-term protection in mouse-allergic responses. Allergy. 2008;63(5):555–563. doi: 10.1111/j.1398-9995.2008.01637.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang G, Wang P, Qiu Z, Qin X, Lin X, Li N, Huang H, Liu H, Hua W, Chen Z, Zhao H, Li W, Shen H. Distant lymph nodes serve as pools of Th1 cells induced by neonatal BCG vaccination for the prevention of asthma in mice. Allergy. 2013;68(3):330–338. doi: 10.1111/all.12099. [DOI] [PubMed] [Google Scholar]
- 10.El-Zein M, Parent ME, Benedetti A, Rousseau MC. Does BCG vaccination protect against the development of childhood asthma? A systematic review and meta-analysis of epidemiological studies. Int J Epidemiol. 2010;39(2):469–486. doi: 10.1093/ije/dyp307. [DOI] [PubMed] [Google Scholar]
- 11.Park SS, Heo EY, Kim DK, Chung HS, Lee CH. The association of BCG vaccination with atopy and asthma in adults. Int J Med Sci. 2015;12(8):668–673. doi: 10.7150/ijms.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275(5296):77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 13.Strannegard IL, Larsson LO, Wennergren G, Strannegard O. Prevalence of allergy in children in relation to prior BCG vaccination and infection with atypical mycobacteria. Allergy. 1998;53(3):249–254. doi: 10.1111/j.1398-9995.1998.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi IS, Lin XH, Koh YA, Koh YI, Lee HC. Strain-dependent suppressive effects of BCG vaccination on asthmatic reactions in BALB/c mice. Ann Allergy Asthma Immunol. 2005;95(6):571–578. doi: 10.1016/S1081-1206(10)61021-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, HW R, Chen FZ, Jin CY, Sun RF, Fan XY, Guo M, Mai JT, WX X, Lin QX, et al. Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Mol Ther. 2016;24(2):398–405. doi: 10.1038/mt.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Xu J, Cai C, Gao X, Li L, Zhong N. Ag85B DNA vaccine suppresses airway inflammation in a murine model of asthma. Respir Res. 2009;10(1):51. doi: 10.1186/1465-9921-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin R, Guo S, Wang MY, Li YH, LX W, Ma H, Lowrie DB, Fan XY, Zhang JH. Administration of mycobacterial Ag85A and IL-17A fusion protein attenuates airway inflammation in a murine model of asthma. Int Immunopharmacol. 2013;17(4):1067–1074. doi: 10.1016/j.intimp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Asmuth DM, Brown EL, DiNubile MJ, Sun X, del Rio C, Harro C, Keefer MC, Kublin JG, Dubey SA, Kierstead LS, Casimiro DR, Shiver JW, Robertson MN, Quirk EK, Mehrotra DV. Comparative cell-mediated immunogenicity of DNA/DNA, DNA/adenovirus type 5 (Ad5), or Ad5/Ad5 HIV-1 clade B gag vaccine prime-boost regimens. J Infect Dis. 2010;201(1):132–141. doi: 10.1086/648591. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Feng L, Li L, Wang D, Li C, Sun C, Li P, Zheng X, Liu Y, Yang W, Niu X, Zhong N, Chen L. Effects of the fusion design and immunization route on the immunogenicity of Ag85A-Mtb32 in adenoviral vectored tuberculosis vaccine. Hum Vaccin Immunother. 2015;11(7):1803–1813. doi: 10.1080/21645515.2015.1042193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smaill F, Jeyanathan M, Smieja M, Medina MF, Thanthrige-Don N, Zganiacz A, Yin C, Heriazon A, Damjanovic D, Puri L, et al. (2013) A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med 5: 205ra134 [DOI] [PubMed]
- 21.Betts RJ, Kemeny DM. CD8+ T cells in asthma: friend or foe? Pharmacol Ther. 2009;121(2):123–131. doi: 10.1016/j.pharmthera.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C, Provost K, Niu N, Homer R, Cohn L. IFN-gamma acts on the airway epithelium to inhibit local and systemic pathology in allergic airway disease. J Immunol. 2011;187(7):3815–3820. doi: 10.4049/jimmunol.1100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trifilieff A, Fujitani Y, Mentz F, Dugas B, Fuentes M, Bertrand C. Inducible nitric oxide synthase inhibitors suppress airway inflammation in mice through down-regulation of chemokine expression. J Immunol. 2000;165(3):1526–1533. doi: 10.4049/jimmunol.165.3.1526. [DOI] [PubMed] [Google Scholar]
- 24.Akata K, Yatera K, Wang KY, Naito K, Ogoshi T, Noguchi S, Kido T, Toyohira Y, Shimokawa H, Yanagihara N, Tsutsui M, Mukae H. Decreased bronchial eosinophilic inflammation and mucus hypersecretion in asthmatic mice lacking all nitric oxide synthase isoforms. Lung. 2016;194(1):121–124. doi: 10.1007/s00408-015-9833-4. [DOI] [PubMed] [Google Scholar]
- 25.Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest. 2014;124(11):4678–4680. doi: 10.1172/JCI78891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endo Y, Hirahara K, Iinuma T, Shinoda K, Tumes DJ, Asou HK, Matsugae N, Obata-Ninomiya K, Yamamoto H, Motohashi S, Oboki K, Nakae S, Saito H, Okamoto Y, Nakayama T. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity. 2015;42(2):294–308. doi: 10.1016/j.immuni.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282(36):26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert SC. T-cell-inducing vaccines—what's the future. Immunology. 2012;135(1):19–26. doi: 10.1111/j.1365-2567.2011.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soria-Guerra RE, Nieto-Gomez R, Govea-Alonso DO, Rosales-Mendoza S. An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J Biomed Inform. 2015;53:405–414. doi: 10.1016/j.jbi.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Tsujimura Y, Inada H, Yoneda M, Fujita T, Matsuo K, Yasutomi Y. Effects of mycobacteria major secretion protein, Ag85B, on allergic inflammation in the lung. PLoS One. 2014;9(9):e106807. doi: 10.1371/journal.pone.0106807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar M, Behera AK, Matsuse H, Lockey RF, Mohapatra SS. A recombinant BCG vaccine generates a Th1-like response and inhibits IgE synthesis in BALB/c mice. Immunology. 1999;97(3):515–521. doi: 10.1046/j.1365-2567.1999.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araujo Z, Giampietro F, Rivas-Santiago B, Luna-Herrera J, Wide A, Clark W, de Waard JH. Patients exposed to Mycobacterium tuberculosis infection with a prominent IgE response. Arch Med Res. 2012;43(3):225–232. doi: 10.1016/j.arcmed.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Cossu D, Otsubo S, Otsubo Y, Eda S, Suzuki T, Iwao Y, Kuribayashi T, Yamamoto S, Sechi LA, Momotani E (2017) Mycobacterium avium Subsp. paratuberculosis induces specific IgE production in Japanese people with allergies. Int J Inflam 2017: 7959154 [DOI] [PMC free article] [PubMed]
- 34.Kim YJ, Kim HJ, Kang MJ, HS Y, Seo JH, Kim HY, Park SJ, Lee YC, Hong SJ. Bacillus Calmette-Guerin suppresses asthmatic responses via CD4(+)CD25(+) regulatory T cells and dendritic cells. Allergy Asthma Immunol Res. 2014;6(3):201–207. doi: 10.4168/aair.2014.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naura AS, Zerfaoui M, Kim H, Abd Elmageed ZY, Rodriguez PC, Hans CP, Ju J, Errami Y, Park J, Ochoa AC, Boulares AH. Requirement for inducible nitric oxide synthase in chronic allergen exposure-induced pulmonary fibrosis but not inflammation. J Immunol. 2010;185(5):3076–3085. doi: 10.4049/jimmunol.0904214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Zhang G, Qin X, Qiu Z, Li N, Chen Z, Li W, Shen H. Inhibition of allergen-induced airway remodeling by neonatal bacillus Calmette-Guerin vaccination is associated with interferon-gamma-producing T cells but not regulatory T cells in mice. Ann Allergy Asthma Immunol. 2011;107(2):163–170. doi: 10.1016/j.anai.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Lagranderie M, Guyonvarc'h PM. The interplay between bacillus Calmette-Guerin and Treg cells and its role to prevent or cure inflammatory diseases. Expert Rev Clin Immunol. 2014;10(6):741–745. doi: 10.1586/1744666X.2014.909286. [DOI] [PubMed] [Google Scholar]
- 38.Makrinioti H, Toussaint M, Jackson DJ, Walton RP, Johnston SL. Role of interleukin 33 in respiratory allergy and asthma. Lancet Respir Med. 2014;2:226–237. doi: 10.1016/S2213-2600(13)70261-3. [DOI] [PubMed] [Google Scholar]
- 39.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125(3):752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 40.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134(583–592):e586. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475. doi: 10.3389/fimmu.2017.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188(3):1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin H, Li XY, Liu T, Yuan BH, Zhang BB, SL H, HB G, Jin XB, Zhu JY. Adenovirus-mediated delivery of soluble ST2 attenuates ovalbumin-induced allergic asthma in mice. Clin Exp Immunol. 2012;170(1):1–9. doi: 10.1111/j.1365-2249.2012.04629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandara G, Beaven MA, Olivera A, Gilfillan AM, Metcalfe DD. Activated mast cells synthesize and release soluble ST2-a decoy receptor for IL-33. Eur J Immunol. 2015;45(11):3034–3044. doi: 10.1002/eji.201545501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohto-Ozaki H, Kuroiwa K, Mato N, Matsuyama Y, Hayakawa M, Tamemoto H, Tominaga S. Characterization of ST2 transgenic mice with resistance to IL-33. Eur J Immunol. 2010;40(9):2632–2642. doi: 10.1002/eji.200940291. [DOI] [PubMed] [Google Scholar]
- 46.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185(6):3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 47.Verheijden KAT, Henricks PAJ, Redegeld FA, Garssen J, Folkerts G. Measurement of airway function using invasive and non-invasive methods in mild and severe models for allergic airway inflammation in mice. Front Pharmacol. 2014;5:190. doi: 10.3389/fphar.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol. 2004;97(1):286–292. doi: 10.1152/japplphysiol.00821.2003. [DOI] [PubMed] [Google Scholar]
- 49.Bates J, Irvin C, Brusasco V, Drazen J, Fredberg J, Loring S, Eidelman D, Ludwig M, Macklem P, Martin J, Milic-Emili J, Hantos Z, Hyatt R, Lai-Fook S, Leff A, Solway J, Lutchen K, Suki B, Mitzner W, Paré P, Pride N, Sly P. The use and misuse of Penh in animal models of lung disease. Am J Resp Cell Mol. 2004;31(3):373–374. doi: 10.1165/ajrcmb.31.3.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 218 kb)
(PDF 158 kb)
(PDF 112 kb)
(PDF 130 kb)