‘Life is not measured by the number of breaths we take, but by the ones we analyze’
As the headspace of the blood, our exhaled breath contains a vast array of substances and molecules that hold great promise for monitoring our health and for the diagnosis and management of various lung and systemic diseases [1]. With recent advances in technology, essentially anything in the blood that is potentially volatile or has a volatile metabolite can be measured in exhaled breath [2]. This includes substances we produce endogenously as part of our normal (or disease-related) metabolism whether this is local in the lung or systemic in origin [3–6]. Since we are constantly inhaling air from our environment as we breathe in the ambient air, exhaled breath can also reflect our environmental exposure(s) [7]. Furthermore, our breath contains volatile compounds produced by our ‘internal environment’: the bacteria in our gut and mouth [3]. Add to all of those volatile byproducts generated from our diet, medications, drugs, or toxins that we are exposed to and you get a very rich matrix that has great potential to revolutionize and personalize medicine [2, 3]. But in order to unlock the great potential of this highly complex resource, we need to find ways to understand its complexity and control or account for the sources of ambiguity.
Tackling such a monumental task requires transdisciplinary collaborations and partnerships among all the stakeholders and any and all other disciplines that can inform the field. This includes, but is not limited to, medical professionals (physicians, researchers, biologists, etc), scientists (chemists, physicists, biochemists, statisticians, etc), engineers, commercial and industrial partners, and regulatory agencies, among others. This critical need for a collaborative approach was the most important conclusion of the Breath Analysis Summit held at the Cleveland Clinic in 2007 [1]. The Summit focused on medical applications brought together industry executives and entrepreneurs with scientists and clinicians to discuss key trends, future directions, and upcoming technologies in breath analysis and medicine. It was very clear at the Summit that the major limitation in the breath analysis field at the time was not a lack of technology or the ability to measure biomarkers in the breath [2] but rather the fact that while scientist, physicians, and industry are all making progress in their own domains, there was very little meaningful and early interaction between these groups. Most interactions were afterthoughts rather than deliberately planned collaborations. Fortunately, not only did the Summit identify the critical need for a collaborative approach, but it also resulted in the forging of several such collaborations and attracted several new talents into the field.
One of several such successful outcomes was the establishment of collaboration between the Cleveland Clinic, NASA, OSU, and CWRU that attracted significant funding from the State of Ohio and established partnerships with several local, national and international institutions and industries all focused on the medical applications of exhaled breath analysis [8–10].
An additional measure of success for the Cleveland Breath Summit was the interest the Summit generated in breath analysis among other disciplines and societies like engineering. In this issue of Journal of Breath Research, we present selected papers from the first ever breath analysis meeting to be organized by Engineering Conferences International (ECI). This is a major milestone for breath analysis and for engineering. The meeting ‘Exhaled Breath Analysis Conference: From Sensors to Devices and Applications’, held in Barga, Italy, in 2010, brought together participants from across the spectrum of science, medicine and engineering in a week long conference that identified and discussed the recent advances and current challenges for breath analysis from all of these different perspectives. The compendium of publications in this issue will give the reader a glimpse of what occurred in that groundbreaking meeting.
Some of the recurring themes from prior meetings included the spectrum of breath analysis applications (medical, environmental, intelligence, etc), standardization of sample collection, the use of novel methods (to present, analyze, and report data), intellectual property issues (including funding and commercialization), and interaction with regulatory agencies. The exhaled nitric oxide story remains the main model to follow from discovery [11], to understanding of the biology [12–14], to standardization of methods [15, 16], to realizing the strengths [17, 18] and limitations [6], to approval by the regulatory agencies [19], to acceptance in clinical application and practice [20].
New themes also emerged including the importance of early and frequent communication among collaborators, the importance of getting early input from potential end-users (regarding needs and expectations), and the critical need to study and understand the biologic relevance of the compounds present in exhaled breath. This is relevant in the discovery phase (can help predict what compounds may be important and relevant to look for in the breath) but it is certainly crucial for the validation and application phase.
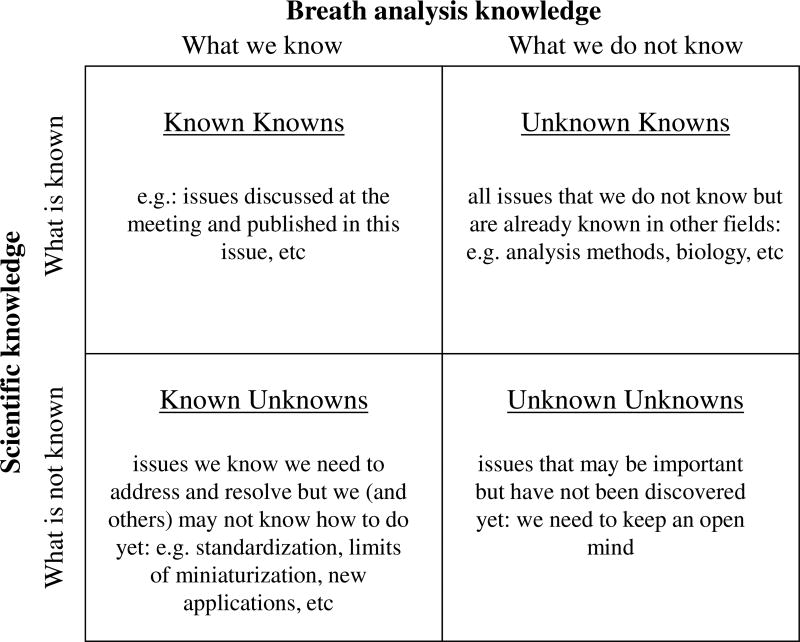
Finally, it was recognized that we need to understand the limitation of our own knowledge and to keep an open mind for new collaborations and new methods of measurement and analysis. For while we obviously know what we know (known knowns) and we may be aware of some of what we do not know (known unknowns), we need people from other disciplines to enlighten us about what they know that we do not (unknown knowns), and we will always be limited by what we do not even know that we do not know (unknown unknowns) (figure 1).
Figure 1.
Knowledge matrix. See text for explanation.
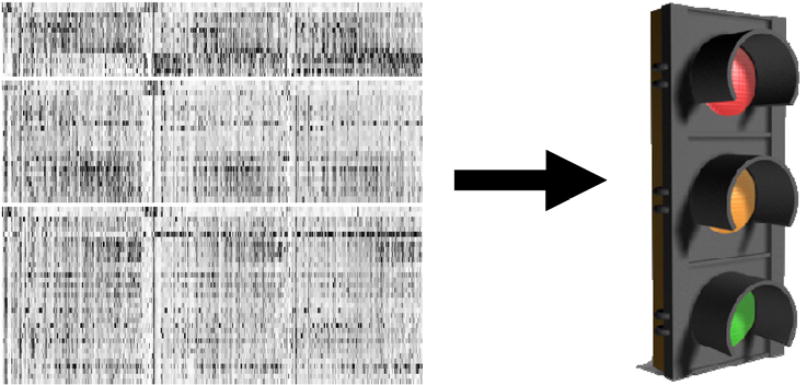
The only way for us to keep moving forward and meet the great challenges that face the breath analysis field today is to focus on what we know now and keep adding to the pool of knowledge by forging strong transdisciplinary collaborations with the many other relevant disciplines in the basic and applied sciences. This will help us handle the complex reality of our field and allow us to deliver the simple useful solutions expected by end users (figure 2) and thus fulfill the great potential and promise of breath analysis.
Figure 2.
Major challenge for breath analysis is to understand and handle complexity while delivering simplicity. A heat map of mass spectrometry data on the left will not likely be very helpful for end users (testing for a disease in the clinic for example). To be useful, this complex array of data needs to be simplified in a way that allows for decision making similar to the way traffic lights are used.
Yaron Bogin, Gaspard Pardon, Andras Bikov, Yoav Broza, Charlene Bayer, Noboru Suzuki, Anil Modak, Joachim Pleil*, Claudio Loccioni, Makoto Sawano, Koichiro Nagamine, Anton Amann*, Phillip Trefz, Andru Prescod, Elena Shnayder, Wolfram Miekisch, Simonetta Capone, Herman Bieber (ECI), Gagan Jodhani, Michael Epton*, Troy Hibbard, Gary Hunter*, David Grove, Bo Li, Ingrid Kohl, Jane Hill, Ann-Charotte Almstrand, Marco Righettoni, Malina Storer, Anna-Carin Olin, Sean Ellwood, Raed Dweik* (Chair), Jack Dummer, Terence Risby*, Sven Kelling, Trevor Smith, Kelly Paschke, Simona Cristescu, Jens Herbig, Stefano Ferretti, Tim Mottin, Jonathan Beauchamp, Petra Neff, Olavi Vaittinen, Joanne Shorter, Cristina Davis*, Michael Madden, Charles, Forbes, Dietmar Hein, Prabir Dutta*, Stephen Fowler, Julian King, Karl Unterkofler, Antoaneta Kazandzieva, Veronika Ruzsanyi (organizing committee*)
Acknowledgments
Dr Dweik is supported by the following grants: HL081064, HL107147, HL095181 and RR026231 from the National Institutes of Health (NIH), and BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development (ODOD).
References
- 1.Dweik RA, Amann A. Exhaled breath analysis: the new frontier in medical testing. J. Breath Res. 2008;2:030301. doi: 10.1088/1752-7163/2/3/030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mashir A, Dweik RA. Exhaled breath analysis: the new interface between medicine and engineering. Adv. Powder Technol. 2009;20:420–5. doi: 10.1016/j.apt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paschke KM, Mashir A, Dweik RA. Clinical applications of breath testing. F 1000 Med. Rep. 2010;2:56. doi: 10.3410/M2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grob NM, Aytekin M, Dweik RA. Biomarkers in exhaled breath condensate: a review of collection, processing and analysis. J. Breath Res. 2008;2:037004. doi: 10.1088/1752-7155/2/3/037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grob NM, Dweik RA. Exhaled nitric oxide in asthma: progress since the introduction of standardized methodology. J. Breath Res. 2008;2:037002. doi: 10.1088/1752-7155/2/3/037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grob NM, Dweik RA. Exhaled nitric oxide in asthma. From diagnosis, to monitoring, to screening: are we there yet? Chest. 2008;133:837–9. doi: 10.1378/chest.07-2743. [DOI] [PubMed] [Google Scholar]
- 7.Pleil JD, Lindstrom AB. Exhaled human breath measurement method for assessing exposure to halogenated volatile organic compounds. Clin. Chem. 1997;43:723–30. [PubMed] [Google Scholar]
- 8.Hunter GW, Xu JC, Dungan LK, Ward BJ, Rowe S, Williams J, Makel DB, Liu C-C, Chang CW. Smart sensor systems for aerospace applications: from sensor development to application testing. ECS Trans. 2008;16:333–44. [Google Scholar]
- 9.Hunter GW, Dweik RA. Applied breath analysis: an overview of the challenges and opportunities in developing and testing sensor technology for human health monitoring in aerospace and clinical applications. J. Breath Res. 2008;2:037020. doi: 10.1088/1752-7155/2/3/037020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondal SP, Dutta PK, Hunter GW, Ward BJ, Laskowski D, Dweik RA. Development of high sensitivity potentiometric NOx sensor and its application to breath analysis. Sensors Actuators B. 2011;158:292–8. [Google Scholar]
- 11.Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem. Biophys. Res. Commun. 1991;181:852–7. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 12.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl Acad. Sci. USA. 2001;98:2622–7. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dweik RA, Laskowski D, Abu-Soud HM, Kaneko F, Hutte R, Stuehr DJ, Erzurum SC. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J. Clin. Invest. 1998;101:660–6. doi: 10.1172/JCI1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khatri SB, Hammel J, Kavuru MS, Erzurum SC, Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J. Appl. Physiol. 2003;95:436–40. doi: 10.1152/japplphysiol.01127.2002. 435 (discussion) [DOI] [PubMed] [Google Scholar]
- 15.Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am. J. Respir. Crit. Care Med. 1999;160:2104–17. doi: 10.1164/ajrccm.160.6.ats8-99. [DOI] [PubMed] [Google Scholar]
- 16.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 17.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med. 2005;352:2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 18.Dweik RA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am. J. Respir. Crit. Care Med. 2010;181:1033–41. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silkoff PE, Carlson M, Bourke T, Katial R, Ogren E, Szefler SJ. The Aerocrine exhaled nitric oxide monitoring system NIOX is cleared by the US Food and Drug Administration for monitoring therapy in asthma. J. Allergy Clin. Immunol. 2004;114:1241–56. doi: 10.1016/j.jaci.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Dweik RA, Boggs P, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]