Abstract
Neurodegenerative diseases are conventionally demarcated as disorders with selective loss of neurons. Conventional as well as newer molecules have been tested but they offer just symptomatic advantages along with abundant side effects. The discovery of more compelling molecules that can halt the pathology of these diseases will be considered as a miracle of present time. Several synthetic compounds are available but they may cause several other health issues. Therefore, natural molecules from the plants and other sources are being discovered to replace available medicines. In conventional medicational therapies, several plants have been reported to bestow remedial effects. Phytochemicals from medicinal plants can provide a better and safer alternative to synthetic molecules. Many phytochemicals have been identified that cure the human body from a number of diseases. The present article reviews the potential efficacy of plant-derived alkaloids, which possess potential therapeutic effects against several NDDs including Alzheimer's disease (AD), Huntington disease (HD), Parkinson's disease (PD), Epilepsy, Schizophrenia, and stroke. Alkaloids include isoquinoline, indole, pyrroloindole, oxindole, piperidine, pyridine, aporphine, vinca, β-carboline, methylxanthene, lycopodium, and erythrine byproducts. Alkaloids constitute positive roles in ameliorating pathophysiology of these illnesses by functioning as muscarinic and adenosine receptors agonists, anti-oxidant, anti-amyloid and MAO inhibitors, acetylcholinestrase and butyrylcholinesterase inhibitor, inhibitor of α-synuclein aggregation, dopaminergic and nicotine agonist, and NMDA antagonist.
Keywords: Neurodegenerative diseases, Phytochemicals, Plant derived alkaloids, acetylcholinestrase, butyrylcholinesterase, monoamine oxidase.
Introduction
Neurodegenerative diseases (NDDs) are the disorders of nervous system and are conventionally demarcated as a selective loss of neurons. Studies demonstrated that physicochemical properties of proteins are altered that cause deposition of these proteins in the human brain leading to neuronal degeneration 1. NDDs are cureless till date and are significantly increasing the mortality and morbidity rate in the developed countries and same scenario is expected in the developing countries as the information pile up 2,3. Neuropsychiatric and neurological disorders like schizophrenia, depression, anxiety, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), Amyotrophic Lateral Sclerosis (ALS), cerebrovascular impairment, seizure disorders and head injuries are the major health issues along with other systemic disorders like cardiovascular, respiratory, renal, gastrointestinal and many others in the 21st century 3. The available remedies against NDDs are solely based on the management of these diseases but actual treatment is yet to be explored. There are several NDDs with similar symptoms but have different mechanisms of pathogenesis 4-6.
AD is one of the major NDDs with the progressive deterioration of memory, learning and other cognitive functions and involves the formation of disorientated plaques, neurofibrillary masses that result in an extensive neuronal loss. Beta-amyloid peptide (Aβ) is the foremost element of senile plaques that is mainly involved in the developmental process of AD 7. Aggregation of Aβ leads to neuronal cell death by causing energy reduction, oxidative stress, inflammation and apoptosis 8. Brain sections with plaques usually demonstrate the decreased figure of synapses as well as the plaques associated neurites are normally scratched, which signifies that Aβ damages neurites and synapses. Whereas acetyl-cholinergic or glutamatergic neurons give the impression as of predominantly affected. Importantly, amyloid precursor protein (APP) outcomes as the creation and accretion of neurotoxic forms of Aβ 9. Aβ are generated by gamma-secretase-mediated sequential cleavages of APP and β-secretase (beta-site amyloid precursor protein cleaving enzyme, BACE) 10.
PD involves the dopaminergic neuronal loss in substantia nigra pars compacta (SNpc) and is second most common neurodegenerative disorder. It chiefly results in the tremor, rigidity, bradyphrenia, bradykinesia, postural instability and gait impairment 11-13. The adult hippocampal dentate gyrus (DG) receives inputs from dopaminergic neurons in SN. So, deterioration of dopaminergic neurons may directly affect adult hippocampal neurogenesis 14. Lewy bodies (LB) are associated with the pathology of PD and α-synuclein is the chief component of LB which become aggregated in PD 15.
HD is a progressive neurodegenerative disorder and is documented to have the frequency of 5-10 cases per 100,000 globally. It is an inherited disease of autosomal dominant trait 22 which involves neuronal cell death particularly in cerebral cortex and striatum. It mitigates the life expectancy and leads to the disability of the effected subject 16. HD is characterized by decline in cognitive and motor ability, psychiatric disturbance, chorea, suicide risk and depression which eventually lead to death 17,18. Onset age for HD is typically mid to late stage of life but infrequently juvenile onset is also observed 19. The gene which encodes for HD is huntingtin (Htt) that has variable length of polyglutamine (polyQ) tract. HD occurs due to CAG repeat expansion in Htt gene which results in the development mutant Htt protein with extended polyQ tract greater than 36Q in the N-terminus of Htt 20,21.
Stroke results in the abrupt loss in brain functioning because of cerebral blood supply disruption and are the second foremost cause of death. The symptoms usually last for at least for 24 hours or lead to the death 23. Stroke is an acute neurologic malfunctioning of vascular source with sudden (seconds) or may be at rapid (hours) occurrence of symptoms 24. Cerebral blood reduction and cerebrovascular diseases are mainly caused by embolism, focal hypoperfusion and thrombosis. At rest brain receives 20% of the blood, so it is intricately sensitive to ischemia. Even short-lived periods of ischemia to neurons can elicit a multifaceted events that may result in everlasting cerebral mutilation 24.
Almost 45% of the ischemic strokes are instigated by thrombus in large or small artery, 20 % of strokes are embolic in derivation. When plaques are produced along the injured vessel and intima is abraded, it can results in the formation of thrombosis in intracranial arteries 25. The injury of the endothelial consents platelets to adhere and aggregate, then coagulation starts, and thrombus progresses at the plaque site. As a result, intracranial and extra cranial systemic blood flow decreases, and function is maintained by collateral system 26. Studies suggest that during cerebral ischemia there is an activation of NMDA receptor which results in accumulation of ROS resulting in oxidation of vital cellular components. This also amends the signaling pathways which then lead to cellular damage and death 27.
Schizophrenia is a serious psychiatric disorder with symptoms of hallucinations, delusions and disorganized thinking. This is an illness with an unclear cause but the theories have suggested that demyelination of white matter is the etiology of schizophrenia 28. Some of the morphological brain anomalies perceived in schizophrenia patients includes loss of cortical gray matter 29 the minimized hippocampal volume, the amygdala, temporal, frontal lobes and enlarged ventricular regions 30. Schizophrenic pathophysiology involve reduced availability of ATP followed by mitochondrial dysfunction diminishing the activity of Na+/K+ ATPase maintaining the membrane potential leading to prolonged depolarization and increasing receptors activity by exuding magnesium from the N-methyl-D-aspartate receptors (NMDAR) 31.
Several approaches have been used to manage the NDDs and most of them exerts positive efficacy by improving health status but none of them has been proved as a proper remedy 32. Plant derived components or phytomedicines such as alkaloids and flavonoids have been used from the ancient times against NDDS. They have been potentially proved as the most effective management and therapeutic agents to minimize the crucial aspects of NDDs such as AD, PD, stroke and schizophrenia 33. Phytochemicals or the phytomedicines are the bioactive plant compounds found in vegetables, fruits, grains etc. and regular intake of fruits and vegetables can reduce the risks of various diseases that cause neuronal dysfunction. There are several phytochemicals which possess neuroprotective potential and alkaloids are one of the most reliable agent against NDDs 33.
Alkaloids are naturally occurring compounds containing carbon, hydrogen, nitrogen, and usually oxygen and are primarily found in plants, especially in certain flowering plants 34. A single plant species usually comprises of few kind of alkaloids but numerous families of plants such as Solanaceae (nightshades), Papaveraceae (poppies family), Ranunculaceae (buttercups) and Amaryllidaceae (amaryllis) are predominantly rich in several kinds of alkaloids 35. Figure 1 illustrates the role of alkaloids in a variety of NDDs.
FIGURE 1.
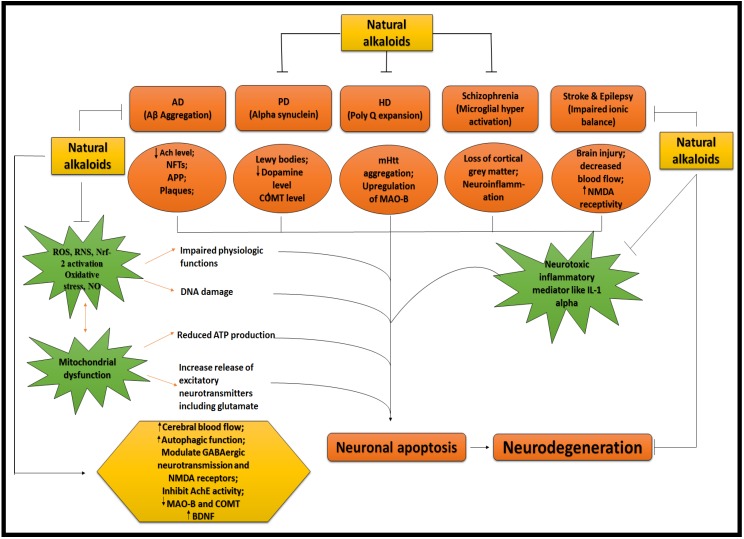
ALKALOIDS IN NEURODEGENERATIVE DISEASES. Because of the influential and multiple actions of alkaloids, they possess a variety of pharmacological potentials in modern medicine and the effects includes analgesic (e.g., morphine), anti-hyperglycemic (e.g., piperine), anticancer (e.g., berberine), antiarrhythmic (e.g., quinidine), antibacterial (e.g., ciprofloxacin). Some other alkaloids exhibit stimulant effects to CNS (e.g., cocaine, caffeine, and nicotine) as well as psychotropic effects (e.g., psilocin). Although alkaloids have an extensive history and numerous applications but only a few are promoted as active medicine 36. Alkaloids exert numerous neuro-protective activities in numerous diseases such as epilepsy, psychological disorders, cerebral ischemia, dementia and memory impairment, depression, anxiety, HD, PD, AD and many others 37. Alkaloids attenuates the development of NDDs through their vast mode of action, i.e., via inhibiting the activity of acetyl-cholinesterase (AChE) enzyme 38, by increasing the level of gamma-aminobutyric acid (GABA) 35, by acting as antagonist of NMDA 39 and many more as described in Fig 2.
Classification of alkaloids
Alkaloids are divided into several classes based on their sources, pharmacokinetics and chemical structures. A variety of pharmacological implications of alkaloids in several NDDs are summarized in Table 1.
Table 1.
Classes of Alkaloids, their source and target neurodegenerative diseases
| Class of alkaloids | Alkaloids | Plants source | Diseases | References |
|---|---|---|---|---|
| Isoquinoline alkaloids | Berberine | Hydrastis Canadensis, Coptis Chinensis, Berberis aquifolium, Berberis vulgaris, Berberis aristata | AD, PD, HD, and Epilepsy | 40-43 |
| Morphine | Papaver somniferum (opium poppy) | AD | 35 | |
| Montanine | Hippeastrum vittatum | Epilepsy | 44 | |
| Salsoline | Salsola oppositefolia | AD | 45 | |
| Galantamine | Narcissus tazetta, Galanthus nivalis, Leucojum aestivum | AD | 46 | |
| Indole alkaloids | Geissospermine | Geissospermum vellosii | AD | 33 |
| Pyrroloindole alkaloids | Physostigmine | Physostigma venosum | AD, PD | 45,47 |
|
Piperidine alkaloids |
Piperine | black pepper (Piper nigrum) and long pepper (Piper longum) | AD, PD, Epilepsy | 48-51 |
| Lobeline | Lobelia inflate | PD | 52 | |
| Aporphine alkaloids | Nantenine | fruit of Nandina domestica | Epilepsy | 53 |
|
Pyridine alkaloids |
Nicotine | Nicotiana tobaccum | AD | 28,33,54 |
| Arecoline | Areca catechu nut | Schizophrenia | 28,55 | |
| Methylxanthine derivatives | Caffeine | Coffea arabica | AD, PD | 33,56 |
| Vinca alkaloid | Vinpocetine | Vinca minor | Ischemia and hypoxia | 57,58 |
| Lycopodium alkaloid | Huperzine A |
Huperzia serrate | AD | 59,60 |
| Indole β-carboline | Harmine | Peganum harmala | AD | 33,61 |
| Erythrine byproducts | (+)-erythravine and (+)-11-α-hydroxyerythravine | Erythrina mulungu | Epilepsy | 62,63 |
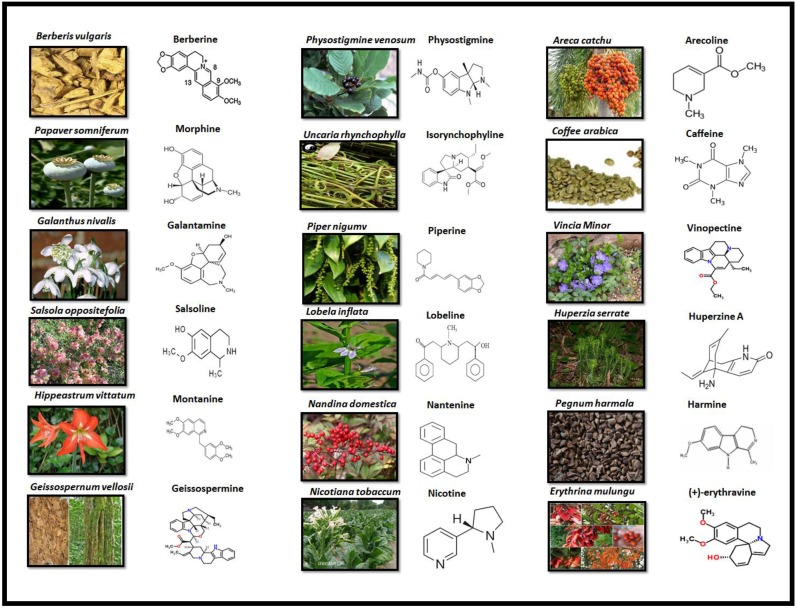
Alkaloids are elucidated with their availability within the specific part of different plants and their chemical structure in Fig 3.
FIGURE 3.
PLANT SOURCE AND CHEMICAL STRUCTURE OF ALKALOIDS
1. Berberine
Berberine (BBR) belongs to the isoquinoline class of alkaloids with a bitter taste and yellow colour, having medicinal use in Ayurvedic and Chinese medicines for about 3000 years. It can be isolated from several plants including Hydrastis Canadensis (Goldenseal), Berberis vulgaris (barberry), Coptis Chinensis (copies or golden thread) and Berberis aristata (tree turmeric) 7.
BBR has multiple pharmacological effects like anti-inflammatory, anti-hypertensive, anti-oxidant, anti-depressant, anti-cancer, anti-microbial, anti-diarrheal, cholesterol and glucose lowering properties 33. Studies reported that it is beneficial in a number of neuropsychiatric disorders and NDDs. It produces anxiolytic, antidepressant, anti-amnesic effects and exhibits a positive potential in the treatment of drug addiction 64. BBR possess therapeutic potential for diseases such as AD, PD, HD, cerebral ischemia and schizophrenia 7,65.
1.1 Therapeutic efficacy of BBR in AD
Studies have suggested that BBR may be of clinical significance for AD due to its potential in attenuating the Aβ 40. As the BACE-1 is the APP cleaving enzyme which initiates the Aβ production 66. BBR improved the behavioral impairment by preventing the hippocampal neurodegeneration and also reduced the activity of BACE-1 activity 67. Importantly, it also possesses monoamine oxidase (MAO) inhibiting property 68 as well as AChE inhibiting property as both are involved in the advancement of AD 69. Recently it has been illustrated in another literature that BBR attenuates the deposition of Aβ plaques and prevent the expression of BACE-1 70.
1.2 Therapeutic efficacy of BBR in PD
BBR enhances the motor stability and synchronization by prevention of neuronal damage of dopaminergic neurons. It also improves short-term memory by inhibiting apoptosis and improving neurogenesis in hippocampal dentate gyrus 71. It was found that BBR significantly prevented both balance and memory loss in PD and there was reduction in SN dopaminergic neuronal loss and decrease apoptosis in the hippocampus 41.
1.3 Therapeutic efficacy of BBR in HD
Currently, HD has no effective medicational therapy, but there are some plant-derived alkaloids, which may have potent effects against this disease. It has been demonstrated that one of the possible therapeutic targets for HD is autophagy 20. BBR up-regulates the autophagic function 72, which may also beneficial for clearing misfolded proteins in case of HD because misfolding of proteins is hallmark in manifestation of HD 73. It has also been reported that BBR reduces mutant Htt deposits and aggregation by activation of autophagic function which improves movement coordination and motor function 42.
1.4 Therapeutic efficacy of BBR in Epilepsy
Epilepsy is the neurological disorder, which is characterized by seizures. Although, several antiepileptic drugs (AEDs) are available but they affect the patients with copious side effects and numerous AEDs are seizures resistant. This enhances the interest of researchers to discover phytotherapy to attenuate events of epilepsy 44. Studies have suggested that extract from Berberis Vulgaris is beneficial in the treatment of epilepsy and convulsions 43. It has been illustrated that excitotoxicity of NMDAR is linked with pathology of epilepsy. BBR may provide therapeutic potential by averting the activation of excessive extrasynaptic NMDAR 74 and it has been reported that BBR modulates the neurotransmitter system and act as an antagonist of NMDAR 39. However, more work need to be done to explore the potential of BBR as an antagonist of NMDAR.
Toxicity of BBR
The recommended dose of BBR in Chinese medicine is 0.2-1.0 g/day 75. In some studies, up to 1.5 g/day has also been recommended in clinical conditions but it may generate adverse effects on gastrointestinal tract (GIT) 76. There are limited reports about adverse effects of BBR on GIT which include constipation and diarrhea. Neonatal hemolytic jaundice has also been observed due to intake of BBR during pregnancy 77. Its adverse effects can be reduced if it is administered along with absorption enhancer because it possesses low bioavailability due to its poor intestinal invasion 76.
2. Morphine
Morphine is an isoquinoline alkaloid which exerts persuasive narcotic and analgesic effects and is used in the attenuation of moderately serious to extreme pain. Morphine mediates its analgesic activity through the µ-opioid receptor (MOR) 78,79. Various experimental models revealed that morphine can exhibit advantageous role against neuronal system injuries 80.
2.1 Therapeutic efficacy of morphine in AD
Morphine plays a vibrant role in AD treatment via binding to MOR in CNS which increases the levels of GABA in synapse of brain 35 and also provide protection from oxidative stress mediated neurotoxicity 81. Morphine protects against intracellular Aβ (iAβ) venomousness in primary neuronal cultures of rats and human beings. It is capable to reverse the iAβ induced electrophysiological changes including capacitance and resting membrane potential 80. Administration of morphine in a dose dependent way reduced the Aβ tempted neurotoxicity via activation of MOR 82.
Toxicity of morphine
The significant dose for morphine is less than 200-300μg from a decade ago 83. The use of morphine is limited due to its side effects such as vomiting and nausea 84. Administration of morphine during lactation and during and before pregnancy results in pyramidal cell damage as well as impairs spatial memory 85.
3. Montanine
Montanine is an isoquinoline alkaloid belonging to Amaryllidaceae family which is isolated from an ornamental plant named Hippeastrum vittatum. It possesses anticonvulsant property 44. AChE inhibiting property of montanine in a dose dependent manner has also been reported 86. There are nine Amaryllidaceae species which possess AChE inhibiting properties and montanine is one of them. Montanine is found in bulbs of Hippeastrum vittatum 87.
3.1 Therapeutic potential of montanine in epilepsy
Studies have suggested that there is an important role of GABAergic neurotransmission in stress, anxiety, and epilepsy. GABAergic neurotransmission dysfunction has been reported in the patients of anxiety which is associated with epilepsy 88. It has been reported through experimental investigation that montanine protected against convulsions by modulating the neurotransmitter systems including GABAA receptors 44. It has been suggested that PTZ causes cognitive deficits linked with seizures 89.
Toxicity of montanine
The adverse health issue which is concerned with montanine is GIT disturbance 90. Although no latest information has been reported regarding montanine.
4. Salsoline
Salsoline is an isoquinoline alkaloid belonging to Chenopodiaceae family 91. The genus Salsola (Chenopodiaceae) encompasses about 120 species, which are wide- spread bushy plants, particularly in the deep grounds of the moderate and subtropical areas of several countries 91. It possesses neuroprotective potential against AD through its cholinesterase inhibitory action 92.
4.1 Therapeutic efficacy of salsoline in AD
It has been investigated that cholinergic transmission is reduced in case of AD which results in loss of cognitive function 93 which can be improved by AChE inhibitors 35. Salsoline possesses the property of inhibiting the AChE enzyme 45. It has been demonstrated that the AChE and BuChE inhibitors are beneficial in the treatment of AD 94. Studies revealed the action of three salsola species for the first time as AChE and BuChE inhibitors. Salsoline is characterized by a selective action against BuChE, thus targeting a novel approach for AD treatment 91. Hence, more work need to be done in this regard.
5. Galantamine
Galantamine is included in isoquinoline class of alkaloids which can be secluded from various plants such as Snowflake (Leucojum aestivum), Snowdrop (Galanthus nivalis) and from Daffodil (Narcissus tazetta) as well 35. Russian pharmacologist for the first time used Snowdrop in the treatment of poliomyelitis. For the treatment of AD, synthetic galantamine was first recognized in 2000 in Sweden. It was successively approved in the United States and the European Union 95.
5.1 Therapeutic efficacy of galantamine in AD
It has been suggested that AD is caused by the imbalance in cholinergic system as well as loss of levels of ACh in the brain due to over activation of AChE which might be accountable for the decline in cognition as the neuronal excitability is regulated by ACh 96. This might generate the hypothesis that AChE inhibitor could ameliorate the drawbacks of AD. It has been reported that galantamine impacts on central cholinergic pathways by inhibiting AChE 69. As galantamine is the AChE inhibitor (AChEI), thereby it ameliorates the cholinergic neurotransmission by reducing the ACh breakdown 46. In another literature, it is demonstrated that it binds to the AChE in the brain which in turn increases the level of ACh in synaptic cleft by reducing the catabolism of ACh 35. It has also been reported that it is potentially reversible and selective AChE inhibitor and several preclinical studies were also carried out to define its pharmacokinetics 33. Clinical trials have proved that galantamine (24mg/d) improved the cognition/perception in the patients of slight AD with no symptoms of hepatotoxicity 97. Strong suggestions indicated that functional nAChR (particularly the α7 subtype) attenuation/dysfunction in AD also decreases the learning and memory by blocking of nicotinic agonists 98,99. Galantamine allosterically modulate nAChR activity which potentiates nicotinic neurotransmission and enhance cognition and memory 100. Furthermore, it exhibits positive stimulation in hippocampal neurogenesis through a7 nicotinic ACh receptors 101. Many studies have revealed that galantamine inhibits Aβ accumulation and cytotoxicity which is also another hallmark in pathology of AD 102,103. Galantamine also protects the neurons against oxidative damage by scavenging ROS but more work need to be done in this context 102. So, all above shows that galantamine may decline the neurodegenerative insults in case of AD via neurogenesis and neuroprotection.
Toxicity of galantamine
It has been reported that galantamine at the dose of 26mg/kg is very effective in ameliorating the behavioral and cognitive abilities in AD. It also delays the formation of Aβ plaque 104. But it has also been considered that the long term use of galantamine can significantly attenuate the mortality 105. The adverse side effects which are linked with the galantamine intake are GIT disturbance, vomiting and nausea 106,107.
6. Geissospermine
It is an indole alkaloid belonging to Apocynaceae family which is isolated from Brazilian tree Geissospermum vellosii. It possesses strong anticholinesterase action as well as anti-inflammatory and antinociceptive properties 108. Aqueous infusions of its bark are frequently used by native societies for countless medicinal determinations. It is medically significant plant and exerts a round figure of medicinal potentials as anti-oxidant 109 as well as antibacterial and antimalarial 110,111.
6.1 Therapeutic efficacy of GSP in AD
As it is well-known that AChE leads to advancement of AD. It has been reported that GSP improves cholinergic transmission by its AChE inhibiting property 33. One of the investigation demonstrated that docking of the GSP into the dynamic place of AChE displayed particular restricting mode between particular groups of AChE residues and GSP. Some of the putative ligand/protein collaborations, for example, hydrogen bonds, and hydrophobic interactions were identified in the above complex 112. Hydrogen holding collaborations were identified with His440 and Ser200 that could propose the impact of catalytic triad for the AChE inhibiting mechanism through GSP which would help the sketch of new prospective Hurt preventers significant for the management of AD 112.
7. Physostigmine
Physostigmine is a pyrroloindole alkaloid which is also called eserine. It is the first natural alkaloid which was studied as AChE inhibitor 113. It belongs to the Leguminosae family and isolated from ripe dried seeds of Physostigma venosum . It has the ability to cross blood brain barrier (BBB) 45. Despite its property to cross the BBB, it has also unveiled narrow therapeutic index, short half- life and peripheral side effects 113.
7.1 Therapeutic efficacy of physostigmine in AD
It has been reported that physostigmine improves the cognitive abilities in normal and AD patients 45 and also has the efficacy to inhibit BChE enzyme which is also reported in etiology of AD but inhibition of BChE may cause some adverse effects like nausea and vomiting 113,114. Rivastigmine is reported as therapeutically successful synthetic analogue of physostigmine 33 possessing its advantageous clinical efficacy against AD which is recently investigated in patients of AD in Taiwan 94. Rivastigmine possess dual action, AChE as well as BuChE inhibiting properties which is useful in alleviating the symptoms of AD 115.
7.2 Therapeutic efficacy of physostigmine in PD
It has been shown that α-synuclein expression increased in PD. There is a great dearth of data regarding antiparkinson effect of physostigmine. However, it was described that phenserine is a physostigmine derivative which possess the efficacy to lower the α-synuclein expression in neural cell lines 47. Recently, no work has been done to explore the therapeutic potential of physostigmine to attenuate the symptoms of PD.
Toxicity of physostigmine
The dosage of physostigmine as 0.04 mg/kg considered to be the highest dose with no side effects 116. There are several side effects which are linked with the use of high dose of piperine including nausea, vomiting, diarrhea, headaches and dizziness 113. Some other reported adverse effects of physostigmine are enhanced blood pressure and heart rate 117.
8. Isorhynchophylline
Isorhynchophylline (IRN) belongs to tetracyclic oxindole class of alkaloids which is extracted from Uncaria rhynchophylla, a chinese herbal medicine which is commonly used in the treatment of neural associated disorders 118,119. It has been reported that it exert positive effects against neurological disorders such as dementia and amnesia 120 as well as ischemia, and epilepsy 35,121.Japanese neurotrophic medicine called Yokukansan also includes IRN as an important component 122.
8.1 Therapeutic efficacy of IRN in AD
Oxidative stress attenuates the body's antioxidant capability and enhances the level of reactive oxygen species (ROS). The brain in particular vulnerable to ROS which is also a main causative factor of abnormal aggregation of Aβ peptides that results in the progression of AD 123. It has been reported that a phytochemical, IRN is beneficial in the treatment of AD because of its neuroprotective efficacy against neurotoxicity caused by Aβ 33 by the suppression of cellular apoptosis induced by mitochondrial pathway and inhibition of oxidative stress 124,125. Experimentation revealed that IRN treatment expressively improves the cognitive shortfalls persuaded in the rats by Aβ25-35. Furthermore, IRN down-regulate the levels of the ratio of Bcl-2/Bax of protein by reducing the Aβ25-35 tempted neuronal apoptosis in hippocampus 125 as the enhanced level of Bcl-2 promote the survival of altered cells and increase the apoptosis 126.
8.2 Therapeutic efficacy of IRN in PD
Alpha-synuclein (α-Syn) masses are the chief constituents of Lewy bodies, which are specific pathological features in PD brain 127. IRN is an effective agent to treat PD 33 as it degrades the α-synuclein 128 and protects neuronal cells through the autophagy-lysosome pathway 129. Autophagy referred as self-eating machinery which is used to destroy and reprocess the long lived organelles and proteins for maintenance of cellular homeostasis 130.
9. Piperine
Piperine (PIP) is a chief alkaloid found in long pepper (Piper longum) and black pepper (Piper nigrum). It belongs to the Piperaceae family 131. It has been reported that piperine possesses a diversity of pharmacological effects which include anti-inflammatory, antifungal, insecticidal, antihypertensive, antipyretic, antitumor and analgesic effects as well as possess numerous neuroprotective properties such as antioxidant, antidepressant, anticonvulsant activities 132,133. The ethanolic extract of Piper longum fruits exert anti-snake venom activities as neurotoxins present in the snake venom cause deadly effects on CNS 134. Studies have suggested that PIP at all dosage expressively reduced memory impairment. Conversely, it was also found that low dose of PIP is able to increase neuronal density in hippocampus. However, further studies need to be done on its neurogenesis action in hippocampus resulting in memory impairment 50.
9.1 Therapeutic efficacy of PIP in Epilepsy
PIP modulates the neurotransmitter systems (serotonin, GABA, Norepinephrine) and delays the tonic-clonic seizures by elevating the cortical and hippocampal serotonin and GABA level 48. GABA receptors dysfunctions have been suggested to be the underlying cause of epilepsy 135. Electrophysiological studies revealed the basic mechanism through which PIP serves as an anti-convulsive agent because of its potential to antagonize sodium/calcium channel 48. Unfortunately, limited data describe the efficacy of PIP as an anti-convulsive agent. Studies need to be done to explore its potential therapeutic role for clinical medical use.
9.2 Therapeutic efficacy of PIP in AD
PIP promotes the cognitive enhancement via inhibition of AChE and β-secretase enzymes. It has been considered that PIP might cross BBB 136. Administration of Piper nigum L. methanolic extract proved to be beneficial in ameliorating cognition as well as attenuating the oxidative stress in hippocampus of Aβ1-42 rat model of AD 49. Most recently it has been demonstrated that if PIP (20 mg/kg) is administered in combination with quercetin (20, 40 and 80 mg/kg), it enhances the neuroprotective effect of quercetin as well as attenuates the cognitive deficits and oxidative stress as in case of AD 137.
9.3 Therapeutic efficacy of PIP in PD
MAOs are the mitochondrial bounded enzymes which control the concentration of neurotransmitter such as DA which depicts that they are targets for neurodegenerative disease such as PD 51. An important enzyme that metabolizes DA is MAO-B. It has been reported that piperine has the ability to inhibit MAO B enzyme which in turn enhance the DA level. PIP also exerts persuasive anti-depressant action which is beneficial in case of PD 50,51. Studies revealed that PIP has the efficacy to improve the coordination and balance in 6-OHDA induced parkinsonian rats. Furthermore, it has the potency to diminish inflammatory markers, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in Parkinson's rats which are 6-OHDA-induced. 138.
Toxicity of PIP
It has been reported that 330 mg/kg and 514 mg/kg are lethal doses of piperine in mice and rats respectively. 100 mg/kg dose of piperine for 7 days is suggested to be non-toxic 139.
10. Lobeline
Lobeline is a piperidine alkaloid isolated from Lobelia inflata and exhibits neuroprotective effects 33. It is a lipophilic alkaloidal component of Indian tobacco 140.
10.1 Therapeutic efficacy of lobeline in PD
Lobeline protects dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) which decreases nigral DA 140. It motivates the DA reverse transportation from synaptic vesicles and inhibits the uptake of DA into synaptic vesicles through the vesicular monoamine transporter 2 communication with it 52.
Toxicity of lobeline
Although the lobeline exerts neuroprotective effects but it also exerts numerous adverse effects at high doses including nausea, bradycardia, tingling and oral numbness 141.
11. Nantenine
Nantenine is an aporphine alkaloid which was first extracted from the Nandina domestica fruit 142. Nantenine exerts sedative and anticonvulsant effects 53.
11.1 Therapeutic efficacy of nantenine in Epilepsy
Calcium signaling causes hyper-excitability by calcium influx which modulate the neuronal activity 143. Nantenine is effective in epilepsy due to its ability to compansate neuronal hyper-excitability by decreasing calcium influx into the cell 44,144.
12. Nicotine
Nicotine is a pyridine alkaloid belonging to Solanaceae family and majorly found in Nicotiana tobaccum. It exhibits extensive pharmacological properties in central nervous system (CNS) as well as the peripheral nervous systems (PNS) mediated by the stimulation of nicotinic acetylcholine receptors (nAChRs) 145. Nicotine along with few of its derivatives is able to improve dopaminergic neuronal survival as well as synaptic plasticity. Moreover, It reduces neuroinflammation and oxidative stress to decrease the level of pathological condition 146. Cigarette smoking and tobacco usage have been reported to lower the prevalence of PD and AD 33.
12.1 Therapeutic efficacy of nicotine in AD
AD involves the reduction of cholinergic neurons and high affinity nAChR.Glutamate and several other excitatory amino acids induced neurotoxicity have been also associated with progression of AD 147. Nicotine elucidates its potential efficacy in promoting the neuroprotection in AD by significantly up regulating the α4 and α7 nAChRs level 148. It has been stated as to constrain the formation of Aβ-peptide by binding to α-helical structure and also improve the memory and learning mediated via neuropeptide Y (NPY1) receptors 33. An earlier study on animal model showed that nicotine is able to diminish AD pathology through one of its chief metabolite named as cotinine 149. Cotinine expresses its possessions as an alternative of nicotine and exerts potential advantageous properties against pathological mechanism of AD. Furthermore, it averts memory loss in a mouse model of AD by preventing the Aβ peptides accumulation 54.
Toxicity of nicotine
Despite the potential neuroprotective efficacy in NDDs, nicotine also portrays several toxicological effects 150. According to the standard data base and safety sheets its lethal dose is 30-60 mg for adults whereas values determined in laboratory animals ranges as 50 mg/kg in rats and 3.3 mg/kg in mice 151. Nicotine exerts its toxic effects through mitochondrial membrane disruption as well as by inducing the production of ROS. Furthermore, it causes the ER stress, which in turn leads to the protein degradation by activating the caspase-3 activity and finally induces the apoptosis 152.
13. Arecoline
Arecoline is a pyridine alkaloid belonging to the family Arecaceae and chiefly found in the fruit of the palm tree Areca catechu L 55. It has been reported to use as masticatory throughout different parts of Southeast Asia and Indian subcontinent due to its potential therapeutic efficacy 153. It directly stimulates the cholinergic activity and also elucidates the potential stimulation of CNS by binding to the M2 muscarinic receptors 154. Several scientific studies have been conducted on Areca nut which indicates its several pharmacological effects like anti-inflammatory, analgesic, and antioxidant effects and anti-schizophrenic 155. Communally, arecoline is a potential agent for treating some CNS disorders due to its efficacy to cross BBB 55.
13.1 Therapeutic efficacy of arecoline in schizophrenia
It has been proposed that Oligodendrocytes (OLs) majorly play a significant role in the schizophrenia pathogenesis 156. Arecoline is reported as a partial agonist of acetylcholine muscarinic receptor which is assumed to exert favorable effects against the schizophrenic symptoms 157. Arecoline possess its efficacy against schizophrenia by directly targeting the OLs and also prevents the demyelination of white matter. It enhances the social and cognitive activity as well as protects the myelin damage in cortex by facilitating oligodendrocyte precursor cells (OPC) differentiation through dephosphorylating the activated protein kinase AMPKα 28. Five subtypes (M1-M5) of muscarinic receptor are widely distributed in the CNS which are chiefly involved in a vast variety of functions including nociception, cognition and movement regulation 158.Some of the earlier studies reported that a significant decrease of M1 and M4 receptors expression in schizophrenic patients with in various regions of brain such as hippocampus 159, prefrontal cortices 160, anterior and posterior cingulate cortex and superior temporal gyrus 161. Arecoline exerts its potential efficacy against schizophrenia by promoting the body excitability through muscarinic receptors (M receptors) stimulation as well as expand memory and learning abilities 55.
Toxicity of Arecoline
As it is comprehensively mentioned that arecoline exerts potential benefits in NDDs. Minimum lethal dose of arecoline in animal models of horses, dogs and mice has been accounted as 1.4, 5 and 100 mg/kg respectively 162. It also exerts inflammatory response at the dose of 0.1 mM by producing and releasing the cytokines including prostaglandin E2 and IL-6 153. In an experimental study at mature mice model it tends to facilitates the production of ROS at the intra peritoneal dose of 10 mg/kg 163. Furthermore, it also exerts cytotoxic effects in neuronal, myoblasts and endothelial cells at the dose of 50 μM, 40μM, and 111 μg/mL in vitro 164,165.
14. Caffeine
Caffeine is a methylxanthine derivative which is isolated from coffea arabica plant and has been extensively consumed as psychoactive substance, being primarily disbursed in soft drinks, tea and coffee 33. Its brain stimulation activity primarily involved increased cortical activity, metabolism of cerebral energy, extracellular levels of acetylcholine which communally results in enhanced alertness 166. A study on animal model revealed that it possesses the ability to reverse the neurotoxicity by acting as antagonist of adenosine receptor 56. Furthermore, it may able to stimulate the behavioral functions like vigilance, attention, mood, and arousal. Caffeine also exhibits anti-oxidative and anti-inflammatory effects 35. One of the recent studies reports that coffee consumption reduces the risk of AD and PD development 33.
14.1 Therapeutic efficacy of caffeine in AD
Mutation in presenilins 1 and 2 encoding genes causes alteration in the activity of γ-secretase and produce Aβ42 isoform in case of AD 147. Caffeine reduced the Aβ deposition in cortex and hippocampus and suppresses the level of presinilin-1 (PS1), β- secretase 167. As the Caffeine exerts a potential efficacy to act as antagonist of adenosine receptor, thus it potentially reduces neurotoxicity in trial models of AD 56. Normally microtubules are stabilized by the association of an axonal protein named as tau, whereas misfolding, aggregation as well as abnormal phosphorylation of tau leads to neuronal cell death via the formation of neurofibrillary tangle 168. Caffeine exerts expressive efficacy to reduce tau phosphorylation in hippocampus and the corresponding proteolytic tau fragments 169. It also protects the basal forebrain neurons and cerebellar granule neurons from neurotoxicity triggered by Aβ 168. Daily consumption of caffeine at the dose of 300-400 mg 170 in tea, coffee and soft drinks have been reported to reduce cognitive impairment as well as the risk of AD development because of its psycho-stimulant possessions 171.
14.2 Therapeutic efficacy of caffeine in PD
Adenosine A2 receptor regulation has been concerned with the modulation of the pro-inflammatory or anti-inflammatory responses and is able to attenuate the locomotor dysfunction as well as tissue damage. Excitotoxicity induced neuronal death can be attenuated by blocking the A2 receptor pharmacologically. Caffeine portrays its activity as adenosine A2 receptor antagonist which results in enhanced loco motor activity in Parkinsonism 56. An augmented number of activated microglial cells had constantly been described in neuro-inflammatory development of PD. The activated microglial cells portray a deleterious influence on dopaminergic neurons 172. Caffeine has been stated to hold an ability of influencing dopaminergic neurotransmission as well as to attenuate neuronal cell death. Furthermore, it mediates dopamine receptor arbitrated behavioral responses, such as cognitive and locomotion functions 173. It directs the down-regulation of adenosine A2 receptors to counter the suppressive effect of adenosine on brain dopaminergic transmission 174. Hence it is stated that caffeine attenuates the pathological progressions of Parkinsonism via different mode of actions as mentioned earlier.
15. Vinpocetine
Vinpocetine is an alkaloid which is extracted from Vinca minor or commonly known as periwinkle plant 175. It was originally marketed under the trade name as Cavinton in 1978. Since the time, it has widely been used in the improvement of cognitive impairment in the conditions such as AD, stroke, memory disturbance as well as in the treatment of cerebrovascular disorders 176. Its biological activities include an increase in blood flow, glucose uptake, improvement in microcirculation, reduction in platelets accumulation 177. Its advantageous clinical outcome is based on its varied range of action such as anti-inflammatory, anti-oxidant, vasodilation and neuro-protective agent via different underlying mechanisms.one of the underlying mechanism of its vasodilation activity involves the enhanced glucose and oxygen uptake as well as elevated production of neuronal ATP 177.
15.1 Therapeutic efficacy of Vinpocetine in Ischemia and hypoxia
After ischemic stroke restoration of blood flow to the brain prevents additional widespread impairment but can lead to the reperfusion injury. The inflammatory reaction accounts for one of the many features involved in cerebral ischemia reperfusion damage 57. A protein complex known as necrosis factor-kappa Bis (NF-κB) is majorly involved in the continuation and amplification (cascade outcome) of the inflammatory response 178. Activated NF-κB stimulate other proteins like RNA polymerase as well as co-activators to enhance transcription of mRNA. Thus Vinpocetine efficiently exerts a potential to induce a wide range of cell factors like chemokine's and cytokines leading to the further activation of NF-κB 179. It halts the inflammatory response via defeating the NF-κB movement from the cytoplasm to the nucleus. Thus it diminishes the volume of cerebral infarction via delaying necrosis and ultimately act as anti- inflammatory agent 57. It has long been used in ischemic stroke patients due to its potential efficacy to cross BBB and potentially inhibit the Ca2+/calmodulin-dependent phosphodiesterase-1 and voltage-dependent Na+ channels 58.
Toxicity of Vinpocetein
The effective dose of vinpocetine against ischemia has been reported as (10 mg/kg) via intraperitoneal injection 57. It appears to be probably harmless and even no substantial destructive possessions were reported in a study of people with AD one year treatment at the dose of 60 mg per day. On contrary some of the side effects of vinpocetine include nausea, stomach pain, headache, nervousness, flushing of face and weakened immune system 175,180.
16. Huperzine A
Huperzine A is extracted from Chinese herb named Huperzia serrate which is commonly known as club moss and has been used for centuries to treat the inflammation, swelling, strains and fever 35. It also potentially improves the cognitive function by increasing ACh levels in the brain 59 due to an efficacy to act as a natural inhibitor of AChE 181. Its neuro-protective mechanism of action is similar to donepezil and galantamine 182 but it parades effective penetration to the BBB, higher oral bioavailability as well as longer duration of action 59. Different strategies have been done to develop the alternative sources of huperzine A because it occurs in slow growing members of Huperziaceae. This includes Phlegmariurus aquarrosus in vitro tissue culture 183 and extraction from endophytic fungi which is secluded from huperzine A manufacturing herb 184,185.
16.1 Therapeutic efficacy of huperzine A in AD
Huperzine A ameliorates the pathogenesis of AD through its neuro-protective property which majorly involves the mitochondrial protection from Aβ aggregation induced toxicity. In vitro as well as in vivo studies revealed that it acts as an inhibitor of Aβ and thus protects the mitochondrial damage to attenuate the oxidative stress 33. The chronology of Aβ induced neuron functional impairment is not utterly understood. However, a number of indications propose that Aβ causes extra-synaptic space glutamate level elevation that consequences in ligand gated excitatory ion channels NMDARs overstimulation and finally leading to cell death as well as synaptic loss 186,187. Huperzine A reduces the glutamate excite-toxicity via acting as NMDA receptor antagonist and minimize the level of synaptic loss along with neuronal cell death 188. As the brain derived neurotropic factor (BDNF) is crucially important in memory and learning process, because it regulates synaptic plasticity, neuronal differentiation, axonal sprouting, as well as long-term potentiation (LTP) 189. It has been revealed by voluminous number of studies that there is a reduced or diminished level of BDNF in patients with AD as well as with mild cognitive impairment (MCI) 189. Huperzine A potentially exerts neuroprotection activity via signaling and promoting the production of BDNF and pro- BDNF to minimize the cognitive deficits and learning impairment induced by reduced level of BDNF 190.Due to the potential neuroprotection possessions of huperzine A there is a need of further research to consider it as a drug for the treatment of AD.
Toxicity of Huperzine A
Although huperzine A has been stated as a potential fighter of cognitive decline but it could be a lethal agent at high dose. Studies clarified its lethal dose as >4mg/kg of bodyweight and even 3mg/kg for longer period like180 days in male rats whereas 1mg/kg dose has been postulated as lethal for female rats as 2-4mg/kg of bodyweight 191.
17. Harmine
Harmine is an indole β-carboline belonging to Nitrariaceae family which is isolated from Peganum harmala.It has a wide spectrum of pharmacological activities including antioxidant, antimicrobial, anti-inflammatory and neuro-protective properties 33. It portrays neuro protective efficacy because of AChE, MAO-A and MAO-B 192 and tyrosine- phosphorylation regulated kinase (DYRK1A) inhibition 61.
17.1 Therapeutic efficacy of Harmine in AD
AChE existence majorly contributes in the pathogenesis of AD which is more often associated with the formation of neurofibrillary tangles and Aβ plaques 147. Harmine has been reported to target the pathogenesis of AD because of its efficacy to potentially penetrate the BBB, the parenchyma cells of brain as well as constrain the activity of AChE 193 which is the key enzyme involved in the metabolism and breakdown of a neurotransmitter known as ACh 194. An experimental study suggested that harmine at the dose of 20 mg/kg could efficiently augment spatial cognition as well as amend compromised memory via cholinergic neurotransmission improvement 193. Another pathological feature in AD is the aggregation and accumulation of tau proteins as well as the overexpression of brain proliferative agent named as tyrosine-phosphorylation regulated kinase (DYRK1A) 195. Harmine facilitates the brain development and proliferation via inhibiting the DYRK1A 61 as well as to significantly condense the tau protein phosphorylation via inhibiting the DYRK1A-catalyzed phosphorylation of tau. These findings suggested that harmine may have auspicious satisfying assistances in the treatment of AD 33.
Toxicity of harmine
Harmine tends to exhibit cytotoxic effects at certain doses in human skin keratinocytes. Experimental concentration of harmine and the percentage level of cytotoxicity in keratinocytes have been elucidated as 10, 30, 60 and 100 µg/ml cause 19.0, 12.5, 11.7 and 11.0 % cytotoxicity. It may interact with DNA topoisomerase due to the presence of methoxyl group and results in the cytotoxicity 196.
18. (+)-erythravine and (+)-11-α-hydroxyerythravine
(+)-erythravine and (+)-11-α-hydroxyerythravine are the erythrine byproducts which are isolated from Erythrina mulungu flowers, commonly recognized as mulungu. It is chiefly found in several tropical regions and Brazilian cerrados 62. Several parts including bark, fruits, seeds and flowers have been used to treat central nervous system (CNS) disorders and insomnia. It exerts anti-inflammatory effects, antinociceptive 197,198, sedative, hypotonic, analgesic 62 and anticonvulsant effects 63.
18.1 Therapeutic efficacy of (+)-erythravine and (+)-11-α-hydroxyerythravine in epilepsy
Alterations in excitatory and inhibitory neurotransmitter pathways are major underlying mechanism involved in the onset as well as the conservation of epileptic seizures. Erythrine by products reduce the acetylcholine evoked currents by acting as an antagonist of neuronal nicotinic receptors 63. Anticonvulsant possessions of (+)-erythravine and (+)-11-α-hydroxyerythravine have been reported to inhibit the seizures induced by phenyl tetrazole (PTZ), kainic acid and bicuculline, NMDA and kainic acid respectively.Both are efficiently absorbed in the GIT and also able to cross the BBB, which is crucially relevant to the advancement of new drugs directing the CNS, as well as anti- leptic drugs 62.
Conclusion and future perspectives
There are several drugs, which have been used for NDDs till date, but they do not possess the efficacy to amend the disease progression, rather they exert copious side effects. Frequent disease amending strategies have been discovered in the recent years and numerous compounds are being explored under these strategies but none of them have successfully grasped the market. In this perspective, plant grounded drugs have also developed as an innovative acumen. Numerous natural alkaloids retain mounting effects in the treatment of several NDDs. Along with modulating neurotransmitter system, natural alkaloids also possess anti-amyloid, anti-inflammatory, and antioxidant properties as well as anti-depressive and anti-convulsing efficacy. Thus, natural alkaloids possess multiple mechanistic approaches in the treatment of NDDs. Alkaloids exert vast neuroprotective actions, but studies are needed on their toxic effects. There are few alkaloids which have been published with their toxic effects. Although many of the alkaloids with their adverse effects need to be reported. It has been suggested that the selection of natural alkaloids in the treatment of NDDs is safe as compared to synthetic drug. Numerous alkaloids and their derivatives have marvelous scope in the treatment of NDDs. But only few of them have prevalent clinical use. There is a vital requirement to design clinical trials for such compounds that are not even entered in the clinical trials till date, because the natural alkaloids are encouraging hope in slowing the development and progression of NDDs.
FIGURE 2.
NEUROPROTECTIVE EFFICACY OF ALKALOIDS. Ach = Acetylcholine; AchE = Acetylcholinestrase; APP = Amyoid precursor protein; COMT = Catechol-O-methyltransferase; MAO-B = Monoamine oxidase B; NMDA = N-methyl-D-aspartate; IL-1 = Interleukin 1; ROS = Reactive oxygen species; RNS = Reactive nitrogen species; NO = Nitric oxide; Nrf = Nuclear factor erthyroid; BDNF = Brain derived neurotropic factor
Acknowledgments
This study was supported by the Ministry of Science and Technology (2016YFE0128500), Jilin Provincial Science & Technology Department (20170204009YY, 20150101187JC, 20150414007GH), Changchun Science & Technology Department (17YJ006, 17YJ001), Jilin Province Development and Reform Commission (2016C047-3, 2016C048-3), Jilin Province Education Department (2015-526, 2015-551), University S & T Innovation Platform of Jilin Province for Economic Fungi (#2014B-1). National Natural Science Foundation of China (No. 30871301, 30700827), and the Program for Introducing Talents to Universities (No. B07017).
References
- 1.Kovacs GG. CURRENT CONCEPTS OF NEURODEGENERATIVE DISEASES. Cit EMJ Neurol. 2014;1(July):78–86. [Google Scholar]
- 2.Van Assche R, Temmerman L, Dias DA, Boughton B, Boonen K, Braeckman BP. et al. Metabolic profiling of a transgenic Caenorhabditis elegans Alzheimer model. Metabolomics. 2015;11(2):477–86. doi: 10.1007/s11306-014-0711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain G, Shahzad A, Anwar H, Sohail MU, Baig SM, Shabbir A, Neurological disorder burden in Faisalabad, Punjab-Pakistan: data from the major tertiary care centers of the city. Pakistan J Neurol Sci; 2017. p. 12. (3) [Google Scholar]
- 4.Kumar Gp, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev [Internet] 2012;6(12):81.. doi: 10.4103/0973-7847.99898. Available from: http://www.phcogrev.com/text.asp?2012/6/12/81/99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain G, Schmitt F, Henriques A, Lequeu T, Rene F, Bindler F. et al. Systemic down-regulation of delta-9 desaturase promotes muscle oxidative metabolism and accelerates muscle function recovery following nerve injury. PLoS One [Internet] 2013;8(6):e64525.. doi: 10.1371/journal.pone.0064525. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3681796&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain G, Schmitt F, Loeffler J-P, Gonzalez de Aguilar J-L. Fatting the brain: a brief of recent research. Front Cell Neurosci [Internet] 2013;7(September):144.. doi: 10.3389/fncel.2013.00144. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3766822&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt F, Hussain G, Dupuis L, Loeffler J-P, Henriques A. A plural role for lipids in motor neuron diseases: energy, signaling and structure. Front Cell Neurosci [Internet] 2014;8(February):25.. doi: 10.3389/fncel.2014.00025. Available from: http://journal.frontiersin.org/article/10.3389/fncel.2014.00025/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Z, Wang C, Yang W. Role of berberine in Alzheimer's disease. Neuropsychiatric Disease and Treatment. 2016;12:2509–20. doi: 10.2147/NDT.S114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon J, Seo YH, Lee JE, Seo EK, Li S, Guo Y. et al. Spiroindole Alkaloids and Spiroditerpenoids from Aspergillus duricaulis and Their Potential Neuroprotective Effects. J Nat Prod. 2015;78(11):2572–9. doi: 10.1021/acs.jnatprod.5b00508. [DOI] [PubMed] [Google Scholar]
- 10.Nhan HS, Chiang K, Koo EH. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathologica. 2015;129:1–19. doi: 10.1007/s00401-014-1347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baranello RJ, Bharani KL, Padmaraju V, Chopra N, Lahiri DK, Greig NH. et al. Amyloid-Beta Protein Clearance and Degradation (ABCD) Pathways and their Role in Alzheimer's Disease. Curr Alzheimer Res. 2015;12:32–46. doi: 10.2174/1567205012666141218140953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamagata K, Tomiyama H, Hatano T, Motoi Y, Abe O, Shimoji K. et al. A preliminary diffusional kurtosis imaging study of Parkinson disease: Comparison with conventional diffusion tensor imaging. Neuroradiology. 2014;56(3):251–8. doi: 10.1007/s00234-014-1327-1. [DOI] [PubMed] [Google Scholar]
- 13.Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson's disease in primary care: A case-control study. Lancet Neurol. 2015;14(1):57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 14.Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC. et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383(9923):1138–46. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 15.McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17(12):1658–60. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recasens A, Dehay B. Alpha-synuclein spreading in Parkinsonâ€TMs disease. Front Neuroanat; 2014. p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schapira AH V, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson's disease and Huntington's disease: Future therapeutic perspectives. Lancet. 2014;384(9942):545–55. doi: 10.1016/S0140-6736(14)61010-2. [DOI] [PubMed] [Google Scholar]
- 18.Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Huntington disease. Vol. 1, Nature Reviews Disease Primers; 2015. [DOI] [PubMed] [Google Scholar]
- 19.Sepers MD, Raymond LA. Mechanisms of synaptic dysfunction and excitotoxicity in Huntington's disease. Drug Discovery Today. 2014;19:990–6. doi: 10.1016/j.drudis.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Jiang W, Wei W, Gaertig MA, Li S, Li XJ. Therapeutic effect of berberine on Huntington's disease transgenic mouse model. PLoS One. 2015;10(7):1–16. doi: 10.1371/journal.pone.0134142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin DDO, Ladha S, Ehrnhoefer DE, Hayden MR. Autophagy in Huntington disease and huntingtin in autophagy. Trends Neurosci. 2015;38(1):26–35. doi: 10.1016/j.tins.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Vieweg S. The role of the polyglutamine and {N}-terminal domains in regulating the aggregation and structural properties of {H}untingtin {E}xon 1. [Lausanne]: EPFL; 2017. [Google Scholar]
- 23.Rawlins MD, Wexler NS, Wexler AR, Tabrizi SJ, Douglas I, Evans SJW, The prevalence of huntington's disease. Vol. 46, Neuroepidemiology; 2016. pp. 144–53. [DOI] [PubMed] [Google Scholar]
- 24.Kopyta I, Zimny M. Significant risk factors in the etiology of arterial ischemic stroke in children. CNS J. 2015;1(1):6–13. [Google Scholar]
- 25.Guo Y, Li P, Guo Q, Shang K, Yan D, Du S. et al. Pathophysiology and Biomarkers in Acute Ischemic Stroke - A Review. Trop J Pharm Res [Internet] 2014;12(6):1097.. Available from: http://www.ajol.info/index.php/tjpr/article/view/99924. [Google Scholar]
- 26.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, 2015 AHA/ASA Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment. Stroke; 2015. STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 27.Salinet ASM, Panerai RB, Robinson TG. The longitudinal evolution of cerebral blood flow regulation after acute ischaemic stroke. Cerebrovasc Dis Extra. 2014;4(2):186–97. doi: 10.1159/000366017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Reis C, Applegate R, Stier G, Martin R, Zhang JH. Ischemic conditioning-induced endogenous brain protection: Applications pre-, per- or post-stroke. Vol. 272, Experimental neurology; 2015. pp. 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adilijiang A, Guan T, He J, Hartle K, Wang W, Li X. The protective effects of areca catechu extract on cognition and social interaction deficits in a cuprizone-induced demyelination model. Evidence-based Complement Altern Med; 2015. p. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rački V, Petrić D, Kučić N, Gržeta N, Jurdana K, Rončević-Gržeta I. Cortical gray matter loss in schizophrenia: Could microglia be the culprit? Med Hypotheses. 2016;88:18–21. doi: 10.1016/j.mehy.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, Schizophrenia. Nat Rev Dis Prim; 2015. p. 1. [DOI] [PubMed] [Google Scholar]
- 32.Snyder MA, Gao W-J. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci; 2013. p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhury B, Saytode P, Shah V. NEURODEGENRATIVE DISORDERS: PAST, PRESENT AND FUTURE. Int J Appl Pharm Biotechnol. 2014;5(2):14–28. [Google Scholar]
- 34.Girdhar S, Girdhar A, Verma SK, Lather V, Pandita D. Plant derived alkaloids in major neurodegenerative diseases : from animal models to clinical trials. J Ayurvedic Herb Med. 2015;1(3):91–100. [Google Scholar]
- 35.Cushnie TPT, Cushnie B, Lamb AJ. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Vol. 44, International Journal of Antimicrobial Agents. Elsevier B.V. 2014. pp. 377–86. [DOI] [PubMed]
- 36.Ng YP, Or TCT, Ip NY. Plant alkaloids as drug leads for Alzheimer's disease. Neurochem Int. 2015 Oct;89:260–70. doi: 10.1016/j.neuint.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Amirkia V, Heinrich M. Alkaloids as drug leads - A predictive structural and biodiversity-based analysis. Phytochem Lett. 2014;10:xlviii–liiii. [Google Scholar]
- 38.Abhijit Dey AM. Plant-Derived Alkaloids: A Promising Window for Neuroprotective Drug Discovery. In: Discovery and Development of Neuroprotective Agents from Natural Products; 2017. pp. 237–320. [Google Scholar]
- 39.Cortes N, Posada-Duque RA, Alvarez R, Alzate F, Berkov S, Cardona-Gómez GP. et al. Neuroprotective activity and acetylcholinesterase inhibition of five Amaryllidaceae species: A comparative study. Life Sci. 2015;122:42–50. doi: 10.1016/j.lfs.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Imenshahidi M, Qaredashi R, Hashemzaei M, Hosseinzadeh H. Inhibitory effect of Berberis vulgaris aqueous extract on acquisition and reinstatement effects of morphine in conditioned place preferences (CPP) in mice. Jundishapur J Nat Pharm Prod; 2014. p. 9. (3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin Y, Khadka DB, Cho W-J. Pharmacological effects of berberine and its derivatives: a patent update. Expert Opin Ther Pat. 2016;26(2):229–43. doi: 10.1517/13543776.2016.1118060. [DOI] [PubMed] [Google Scholar]
- 42.Jiang WX, Li SH, Li XJ. Therapeutic potential of berberine against neurodegenerative diseases. Vol. 58, Science China Life Sciences; 2015. pp. 564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang W, Wei W, Gaertig MA, Li S, Li XJ. Therapeutic effect of berberine on Huntington's disease transgenic mouse model. PLoS One; 2015. p. 10. (7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mojarad TB, Roghani M. The anticonvulsant and antioxidant effects of berberine in kainate-induced temporal lobe epilepsy in rats. Basic Clin Neurosci. 2014;5(2):124–30. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu HL, Wan JB, Wang YT, Li BC, Xiang C, He J, Medicinal compounds with antiepileptic/anticonvulsant activities. Vol. 55, Epilepsia; 2014. pp. 3–16. [DOI] [PubMed] [Google Scholar]
- 46.Acqua SD. Plant-derived acetylcholinesterase inhibitory alkaloids for the treatment of Alzheimer ' s disease. Bot Targets Ther. 2013;3:19–28. [Google Scholar]
- 47.Kumar A, Singh A, Ekavali. A review on Alzheimer's disease pathophysiology and its management: An update. Vol. 67, Pharmacological Reports; 2015. pp. 195–203. [DOI] [PubMed] [Google Scholar]
- 48.Mikkilineni S, Cantuti-Castelvetri I, Cahill CM, Balliedier A, Greig NH, Rogers JT. The anticholinesterase phenserine and its enantiomer posiphen as 5′untranslated-region-directed translation blockers of the Parkinson's alpha synuclein expression. Parkinsons Dis. 2012;2012:5–8. doi: 10.1155/2012/142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishra A, Punia JK, Bladen C, Zamponi GW, Goel RK. Anticonvulsant mechanisms of piperine, a piperidine alkaloid. Channels. 2015;9(5):317–23. doi: 10.1080/19336950.2015.1092836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hritcu L, Noumedem JA, Cioanca O, Hancianu M, Kuete V, Mihasan M. Methanolic Extract of Piper nigrum Fruits Improves Memory Impairment by Decreasing Brain Oxidative Stress in Amyloid Beta(1-42) Rat Model of Alzheimer's Disease. Cell Mol Neurobiol. 2014;34(3):437–449. doi: 10.1007/s10571-014-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Baghdadi OB, Prater NI, Van Der Schyf CJ, Geldenhuys WJ. Inhibition of monoamine oxidase by derivatives of piperine, an alkaloid from the pepper plant Piper nigrum, for possible use in Parkinson's disease. Bioorganic Med Chem Lett. 2012;22(23):7183–8. doi: 10.1016/j.bmcl.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 52.Carradori S, Ascenzio MD, Chimenti P, Secci D, Bolasco A. Selective MAO-B inhibitors : a lesson from natural products. Mol Divers. 2014;18:219–43. doi: 10.1007/s11030-013-9490-6. [DOI] [PubMed] [Google Scholar]
- 53.Dimatelis JJ, Russell VA, Stein DJ, Daniels WM. The effects of lobeline and naltrexone on methamphetamine-induced place preference and striatal dopamine and serotonin levels in adolescent rats with a history of maternal separation. Vol. 27, Metabolic Brain Disease; 2012. pp. 351–61. [DOI] [PubMed] [Google Scholar]
- 54.Xiao F, Yan B, Chen L, Zhou D. Review of the use of botanicals for epilepsy in complementary medical systems - Traditional Chinese Medicine. Vol. 52, Epilepsy and Behavior; 2015. pp. 281–9. [DOI] [PubMed] [Google Scholar]
- 55.Grizzell JA, Patel S, Barreto GE, Echeverria V. Cotinine improves visual recognition memory and decreases cortical Tau phosphorylation in the Tg6799 mice. Prog Neuro-Psychopharmacology Biol Psychiatry. 2017;78:75–81. doi: 10.1016/j.pnpbp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y-J, Peng W, Hu M-B, Xu M, Wu C-J. The pharmacology, toxicology and potential applications of arecoline: a review. Pharm Biol. 2016;54(11):2753–60. doi: 10.3109/13880209.2016.1160251. [DOI] [PubMed] [Google Scholar]
- 57.Rivera-Oliver M, Díaz-Ríos M. Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: A review. Vol. 101, Life Sciences; 2014. pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Zhang K, Zhao L, Tang J, Gao L, Wei Z. Anti-inflammatory effects of vinpocetine on the functional expression of nuclear factor-kappa B and tumor necrosis factor-alpha in a rat model of cerebral ischemia-reperfusion injury. Neurosci Lett. 2014;566:247–51. doi: 10.1016/j.neulet.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F, Yan C, Wei C, Yao Y, Ma X, Gong Z, Vinpocetine Inhibits NF-κB-Dependent Inflammation in Acute Ischemic Stroke Patients. Transl Stroke Res; 2017. [DOI] [PubMed] [Google Scholar]
- 60.Xing S, Zhu C, Zhang R, An L. Huperzine A in the Treatment of Alzheimer's Disease and Vascular Dementia: A Meta-Analysis. Evidence-Based Complement Altern Med. 2014;2014:1–10. doi: 10.1155/2014/363985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yadav M, Parle M, Sharma N, Ghimire K, Khare N. Role of Bioactive Phytoconstituents from Several Traditional Herbs as Natural Neuroprotective Agents Role of Bioactive Phytoconstituents from Several Traditional Herbs as Natural Neuroprotective Agents. 2016;(August)
- 62.Dakic V, Maciel R de M, Drummond H, Nascimento JM, Trindade P, Rehen SK. Harmine stimulates proliferation of human neural progenitors. PeerJ. 2016;4:e2727. doi: 10.7717/peerj.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faggion SA, Cunha AOS, Fachim HA, Gavin AS, dos Santos WF, Pereira AMS. et al. Anticonvulsant profile of the alkaloids (+)-erythravine and (+)-11-α-hydroxy-erythravine isolated from the flowers of Erythrina mulungu Mart ex Benth (Leguminosae-Papilionaceae) Epilepsy Behav. 2011;20(3):441–6. doi: 10.1016/j.yebeh.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 64.Setti-Perdigão P, Serrano MAR, Flausino OA, Bolzani VS, Guimarães MZP, Castro NG. Erythrina mulungu alkaloids are potent inhibitors of neuronal nicotinic receptor currents in mammalian cells. PLoS One. 2013;8(12):8–13. doi: 10.1371/journal.pone.0082726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patil S, Tawari S, Mundhada D, Nadeem S. Protective effect of berberine, an isoquinoline alkaloid ameliorates ethanol-induced oxidative stress and memory dysfunction in rats. Pharmacol Biochem Behav. 2015;136:13–20. doi: 10.1016/j.pbb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Han AM, Heo H, Kwon YK. Berberine Promotes Axonal Regeneration in Injured Nerves of the Peripheral Nervous System. J Med Food [Internet] 2012;15(4):413–7. doi: 10.1089/jmf.2011.2029. Available from: http://online.liebertpub.com/doi/abs/10.1089/jmf.2011.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan R, Vassar R. Targeting the β secretase BACE1 for Alzheimer's disease therapy. Vol. 13, The Lancet Neurology; 2014. pp. 319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panahi N, Mahmoudian M, Mortazavi P, Hashjin GS. Effects of berberine on beta-secretase activity in a rabbit model of Alzheimer's disease. Arch Med Sci [Internet] 2013;9(1):146–50. doi: 10.5114/aoms.2013.33354. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23516061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathew B, Suresh J, Mathew GE, Parasuraman R, Abdulla N. Plant secondary metabolites- potent inhibitors of monoamine oxidase isoforms. Cent Nerv Syst Agents Med Chem. 2014;14(1):28–33. doi: 10.2174/1871524914666140826111930. [DOI] [PubMed] [Google Scholar]
- 70.Pohanka M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Vol. 15, International Journal of Molecular Sciences; 2014. pp. 9809–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer's disease. Exp Gerontol. 2017;91:25–33. doi: 10.1016/j.exger.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Kim M, Cho KIHO, Shin MS, Lee JM, Cho HS, Kim CJU. et al. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson's disease. Int J Mol Med. 2014;33(4):870–8. doi: 10.3892/ijmm.2014.1656. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Q, Bian H, Guo L, Zhu H. Pharmacologic preconditioning with berberine attenuating ischemia-induced apoptosis and promoting autophagy in neuron. Am J Transl Res. 2016;8(2):1197–207. [PMC free article] [PubMed] [Google Scholar]
- 74.Ciechanover A, Kwon YT. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med. 2015;47(3):e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82(2):279–93. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 76.Chen C, Yu Z, Li Y, Fichna J, Storr M. Effects of Berberine in the Gastrointestinal Tract — A Review of Actions and Therapeutic Implications. Am J Chin Med. 2014;42(5):1053–70. doi: 10.1142/S0192415X14500669. [DOI] [PubMed] [Google Scholar]
- 77.Godugu C, Patel AR, Doddapaneni R, Somagoni J, Singh M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS One; 2014. p. 9. (3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pang B, Zhao L, Zhou Q, Zhao T, Wang H, Gu C, Review Article Application of Berberine on Treating Type 2 Diabetes Mellitus. Int J Endocrinol; 2015. p. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaur R, Arora S. Alkaloids- Important Therapeutic Secondary Metabolites Of Plant Origin. J Crit Rev. 2015;2(3):1–8. [Google Scholar]
- 80.Ye D, Bu H, Guo G, Shu B, Wang W, Guan X. et al. Activation of CXCL10/CXCR3 signaling attenuates morphine analgesia: Involvement of Gi protein. J Mol Neurosci. 2014;53(4):571–9. doi: 10.1007/s12031-013-0223-1. [DOI] [PubMed] [Google Scholar]
- 81.Cui J, Wang Y, Dong Q, Wu S, Xiao X, Hu J. et al. Morphine Protects against Intracellular Amyloid Toxicity by Inducing Estradiol Release and Upregulation of Hsp70. J Neurosci [Internet] 2011;31(45):16227–40. doi: 10.1523/JNEUROSCI.3915-11.2011. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3915-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Almeida MB, Costa-Malaquias A, Nascimento JLM, Oliveira KR, Herculano AM, Crespo-López ME. Therapeutic concentration of morphine reduces oxidative stress in glioma cell line. Brazilian J Med Biol Res. 2014;47(5):398–402. doi: 10.1590/1414-431X20143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Wang YX, Liu T, Law PY, Loh HH, Qiu Y. et al. μ-Opioid Receptor Attenuates Aβ Oligomers-Induced Neurotoxicity Through mTOR Signaling. CNS Neurosci Ther. 2015;21(1):8–14. doi: 10.1111/cns.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berger JS, Gonzalez A, Hopkins A, Alshaeri T, Jeon D, Wang S, Dose-response of intrathecal morphine when administered with intravenous ketorolac for post-cesarean analgesia: a two-center, prospective, randomized, blinded trial. In: International Journal of Obstetric Anesthesia; 2016. pp. 3–11. [DOI] [PubMed] [Google Scholar]
- 85.Ding X, Jin S, Niu X, Wang T, Zhao X, Ren H. et al. Morphine with adjuvant ketamine versus higher dose of morphine alone for acute pain: A meta-analysis. Int J Clin Exp Med. 2014;7(9):2504–10. [PMC free article] [PubMed] [Google Scholar]
- 86.Ghafari S, Golalipour MJ. Prenatal morphine exposure reduces pyramidal neurons in CA1, CA2 and CA3 subfields of mice hippocampus. Iran J Basic Med Sci. 2014;17(3):155–61. [PMC free article] [PubMed] [Google Scholar]
- 87.Moraga F, Hormazábal E, Venthur H, Quiroz A, Mutis A. REVIEW ARTICLE ALKALOID DISCOVERY AS NATURAL ACETYLCHOLINESTERASE INHIBITORS, FROM NATURE TO MOLECULAR DOCKING. Rev Farm Chile. 2016;9(56):16–25. [Google Scholar]
- 88.de Andrade JP, Giordani RB, Torras-Claveria L, Pigni NB, Berkov S, Font-Bardia M. et al. The Brazilian Amaryllidaceae as a source of acetylcholinesterase inhibitory alkaloids. Phytochem Rev. 2016;15(1):147–60. [Google Scholar]
- 89.Rocha L, Alonso-Vanegas M, MartÃnez-Juárez IE, Orozco-Suárez S, Escalante-Santiago D, Feria-Romero IA, GABAergic Alterations in Neocortex of Patients with Pharmacoresistant Temporal Lobe Epilepsy Can Explain the Comorbidity of Anxiety and Depression: The Potential Impact of Clinical Factors. Front Cell Neurosci; 2015. p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Golechha M, Sarangal V, Bhatia J, Chaudhry U, Saluja D, Arya DS. Naringin ameliorates pentylenetetrazol-induced seizures and associated oxidative stress, inflammation, and cognitive impairment in rats: Possible mechanisms of neuroprotection. Epilepsy Behav. 2014;41:98–102. doi: 10.1016/j.yebeh.2014.09.058. [DOI] [PubMed] [Google Scholar]
- 91.Pagliosa LB, Monteiro SC, Silva KB, de Andrade JP, Dutilh J, Bastida J. et al. Effect of isoquinoline alkaloids from two Hippeastrum species on in vitro acetylcholinesterase activity. Phytomedicine. 2010;17(8-9):698–701. doi: 10.1016/j.phymed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Tundis R, Menichini F, Conforti F, Loizzo MR, Bonesi M, Statti G. et al. A potential role of alkaloid extracts from Salsola species (Chenopodiaceae) in the treatment of Alzheimer's disease. J Enzyme Inhib Med Chem. 2009;24(3):818–24. doi: 10.1080/14756360802399662. [DOI] [PubMed] [Google Scholar]
- 93.Orhan IE, Kucukboyaci N, Calis I, Cerón-Carrasco JP, den-Haan H, Peña-García J. et al. Acetylcholinesterase inhibitory assessment of isolated constituents from Salsola grandis Freitag, Vural & Adıgüzel and molecular modeling studies on N-acetyltryptophan. Phytochem Lett. 2017;20:373–8. [Google Scholar]
- 94.H. Ferreira-Vieira T, M. Guimaraes I, R. Silva F, M. Ribeiro F. Alzheimer's disease: Targeting the Cholinergic System. Curr Neuropharmacol. 2016;14(1):101–15. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen T-H, Chou M-C, Lai C-L, Wu S-J, Hsu C-L, Yang Y-H. Factors affecting therapeutic response to Rivastigmine in Alzheimer's disease patients in Taiwan. Kaohsiung J Med Sci. 2017;33(6):277–83. doi: 10.1016/j.kjms.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Kim JK, Park SU. Pharmacological aspects of galantamine for the treatment of Alzheimer's disease. Vol. 16, EXCLI Journal; 2017. pp. 35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lombardo S, Maskos U. Role of the nicotinic acetylcholine receptor in Alzheimer's disease pathology and treatment. Neuropharmacology. 2015;96(PB):255–62. doi: 10.1016/j.neuropharm.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 98.Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000 Jun;54(12):2261–8. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 99.Fuentealba J, Saez-Orellana F. Neuroactive alkaloids that modulate the neuronal nicotinic receptor and provide neuroprotection in an Alzheimer???s disease model: The case of teline monspessulana. Neural Regen Res. 2014;9(21):1880–1. doi: 10.4103/1673-5374.145349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Vol. 36, Trends in Pharmacological Sciences; 2015. pp. 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nikiforuk A, Kos T, Potasiewicz A, Popik P. Positive allosteric modulation of alpha 7 nicotinic acetylcholine receptors enhances recognition memory and cognitive flexibility in rats. Eur Neuropsychopharmacol. 2015;25(8):1300–13. doi: 10.1016/j.euroneuro.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 102.Kita Y, Ago Y, Higashino K, Asada K, Takano E, Takuma K. et al. Galantamine promotes adult hippocampal neurogenesis via M1 muscarinic and α7 nicotinic receptors in mice. Int J Neuropsychopharmacol [Internet] 2014;17(12):1957–68. doi: 10.1017/S1461145714000613. Available from: https://academic.oup.com/ijnp/article-lookup/doi/10.1017/S1461145714000613. [DOI] [PubMed] [Google Scholar]
- 103.Abd El-Wahab AE, Ghareeb DA, Sarhan EEM, Abu-Serie MM, El Demellawy MA. In vitro biological assessment of berberis vulgaris and its active constituent, berberine: Antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13(1):218. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castillo WO, Aristizabal-Pachon AF. Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer's disease. Vol. 12, Neural Regeneration Research; 2017. pp. 916–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhattacharya S, Haertel C, Maelicke A, Montag D. Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer's disease. PLoS One; 2014. p. 9. (2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hager K, Baseman AS, Nye JS, Brashear HR, Han J, Sano M. et al. Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer's disease. Neuropsychiatr Dis Treat. 2014;10:391–401. doi: 10.2147/NDT.S57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Woo FY, Basri M, Masoumi HRF, Ahmad MB, Ismail M. Formulation optimization of galantamine hydrobromide loaded gel drug reservoirs in transdermal patch for Alzheimer's disease. Int J Nanomedicine. 2015;10:3879–86. doi: 10.2147/IJN.S80253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caramelli P, Laks J, Palmini ALF, Nitrini R, Chaves MLF, Forlenza OV. et al. Effects of galantamine and galantamine combined with nimodipine on cognitive speed and quality of life in mixed dementia: a 24-week, randomized, placebo-controlled exploratory trial (the REMIX study) Arq Neuropsiquiatr. 2014;72(6):411–7. doi: 10.1590/0004-282x20140055. [DOI] [PubMed] [Google Scholar]
- 109.Lima JA, Costa TWR, Silva LL, Miranda ALP, Pinto AC. Antinociceptive and anti-inflammatory effects of a Geissospermum Vellosii stem bark fraction. An Acad Bras Cienc. 2016;88(1):237–48. doi: 10.1590/0001-3765201520140374. [DOI] [PubMed] [Google Scholar]
- 110.Sajkowska-Kozielewicz JJ, Kozielewicz P, Barnes NM, Wawer I, Paradowska K. Antioxidant, Cytotoxic, and Antiproliferative Activities and Total Polyphenol Contents of the Extracts of Geissospermum reticulatum Bark. Oxid Med Cell Longev; 2016. p. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reina M, Ruiz-Mesia W, López-Rodríguez M, Ruiz-Mesia L, González-Coloma A, Martínez-Díaz R. Indole alkaloids from Geissospermum reticulatum. J Nat Prod. 2012;75(5):928–34. doi: 10.1021/np300067m. [DOI] [PubMed] [Google Scholar]
- 112.Vital MJS, Carneiro ALB, Rocha LF, das Neves Amorim RC, Camargo MRM, Pohlit AM. Chemical composition, ethnopharmacology and biological activity of Geissospermum Allemão species (Apocynaceae Juss.) Rev Fitos Eletrônica. 2015;8(2):137–46. [Google Scholar]
- 113.Araújo JQ, Lima JA, Pinto ADC, De Alencastro RB, Albuquerque MG. Docking of the alkaloid geissospermine into acetylcholinesterase: A natural scaffold targeting the treatment of Alzheimer's disease. J Mol Model. 2011;17(6):1401–12. doi: 10.1007/s00894-010-0841-2. [DOI] [PubMed] [Google Scholar]
- 114.Shaikh S, Verma A, Siddiqui S, Ahmad SS, Rizvi SMD, Shakil S. et al. Current acetylcholinesterase-inhibitors: A neuroinformatics perspective. CNS Neurol Disord - Drug Targets. 2014;13(3):391–401. doi: 10.2174/18715273113126660166. [DOI] [PubMed] [Google Scholar]
- 115.Orhan G, Orhan I, Subutay-Oztekin N, Ak F, Sener B. Contemporary Anticholinesterase Pharmaceuticals of Natural Origin and Their Synthetic Analogues for the Treatment of Alzheimers Disease. Recent Pat CNS Drug Discov [Internet] 2009;4(1):43–51. doi: 10.2174/157488909787002582. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1574-8898&volume=4&issue=1&spage=43. [DOI] [PubMed] [Google Scholar]
- 116.Mohamed LA, Keller JN, Kaddoumi A. Biochimica et Biophysica Acta Role of P-glycoprotein in mediating rivastigmine effect on amyloid- β brain load and related pathology in Alzheimer â€TM s disease mouse model. BBA - Mol Basis Dis. 2016;1862(4):778–87. doi: 10.1016/j.bbadis.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wiese AJ, Brosnan RJ, Barter LS. Effects of acetylcholinesterase inhibition on quality of recovery from isoflurane-induced anesthesia in horses. Am J Vet Res. 2014;75(3):223–30. doi: 10.2460/ajvr.75.3.223. [DOI] [PubMed] [Google Scholar]
- 118.Tie H-T, Su G-Z, He K, Liang S-R, Yuan H-W, Mou J-H. Efficacy and safety of ondansetron in preventing postanesthesia shivering: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2014;14:12. doi: 10.1186/1471-2253-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wenger E, McDermott R, Synder W. Cultivating communities of pratice a guide to managing knowledge. J Knowl Manag Pract. 2002;24(2):304. [Google Scholar]
- 120.Guo H, Zhang X, Cui Y, Deng W, Xu D, Han H. et al. Isorhynchophylline protects against pulmonary arterial hypertension and suppresses PASMCs proliferation. Biochem Biophys Res Commun. 2014;450(1):729–34. doi: 10.1016/j.bbrc.2014.06.044. [DOI] [PubMed] [Google Scholar]
- 121.Zhou JY, Zhou SW. Isorhynchophylline: A plant alkaloid with therapeutic potential for cardiovascular and central nervous system diseases. Vol. 83, Fitoterapia; 2012. pp. 617–26. [DOI] [PubMed] [Google Scholar]
- 122.Ho TY, Tang NY, Hsiang CY, Hsieh CL. Uncaria rhynchophylla and rhynchophylline improved kainic acid-induced epileptic seizures via IL-1β and brain-derived neurotrophic factor. Phytomedicine. 2014;21(6):893–900. doi: 10.1016/j.phymed.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 123.Tsuji M, Takeuchi T, Miyagawa K, Ishii D, Imai T, Takeda K. et al. Yokukansan, a traditional Japanese herbal medicine, alleviates the emotional abnormality induced by maladaptation to stress in mice. Phytomedicine. 2014;21(3):363–71. doi: 10.1016/j.phymed.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 124.WEN-JUAN HUANG, XIA ZHANG, W-WC. Role of oxidative stress in Alzheimer's disease. Biomed Rep; 2016. pp. 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP. Protective effect of isorhynchophylline against β-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32(3):353–60. doi: 10.1007/s10571-011-9763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xian YF, Mao QQ, Wu JCY, Su ZR, Chen JN, Lai XP. et al. Isorhynchophylline treatment improves the amyloid-β-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. J Alzheimer's Dis. 2014;39(2):331–46. doi: 10.3233/JAD-131457. [DOI] [PubMed] [Google Scholar]
- 127.Akl H, Vervloessem T, Kiviluoto S, Bittremieux M, Parys JB, De Smedt H, A dual role for the anti-apoptotic Bcl-2 protein in cancer: Mitochondria versus endoplasmic reticulum. Vol. 1843, Biochimica et Biophysica Acta - Molecular Cell Research; 2014. pp. 2240–52. [DOI] [PubMed] [Google Scholar]
- 128.Cooper JM, Wiklander PBO, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M. et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord. 2014;29(12):1476–85. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang H, Bai L, He J, Zhong L, Duan X, Ouyang L. et al. Recent advances in discovery and development of natural products as source for anti-Parkinson's disease lead compounds. Eur J Med Chem. 2017;141:257–72. doi: 10.1016/j.ejmech.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 130.Lu J-H, Tan J-Q, Durairajan SSK, Liu L-F, Zhang Z-H, Ma L. et al. Isorhynchophylline, a natural alkaloid, promotes the degradation of alpha-synuclein in neuronal cells via inducing autophagy. Autophagy. 2012;8(1):98–108. doi: 10.4161/auto.8.1.18313. [DOI] [PubMed] [Google Scholar]
- 131.Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M. et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 132.Yeggoni DP, Rachamallu A, Kallubai M, Subramanyam R. Cytotoxicity and comparative binding mechanism of piperine with human serum albumin and α-1-acid glycoprotein. J Biomol Struct Dyn. 2015;33(6):1336–51. doi: 10.1080/07391102.2014.947326. [DOI] [PubMed] [Google Scholar]
- 133.Damanhouri ZA. A Review on Therapeutic Potential of Piper nigrum L. (Black Pepper): The King of Spices. Med Aromat Plants; 2014. p. 3. (3) [Google Scholar]
- 134.Vasavirama K, Upender M. Piperine: A valuable alkaloid from piper species. Vol. 6, International Journal of Pharmacy and Pharmaceutical Sciences; 2014. pp. 34–8. [Google Scholar]
- 135.Goswami PK, Samant M, Srivastava RS. Snake venom, anti-snake venom & potential of snake venom. Int J Pharm Pharm Sci. 2014;6(5):4–7. [Google Scholar]
- 136.Hirose S. Mutant GABAA receptor subunits in genetic (idiopathic) epilepsy. In: Genetics of Epilepsy; 2014. pp. 55–85. [DOI] [PubMed] [Google Scholar]
- 137.Murata K, Matsumura S, Yoshioka Y, Ueno Y, Matsuda H. Screening of β-secretase and acetylcholinesterase inhibitors from plant resources. J Nat Med. 2015;69(1):123–129. doi: 10.1007/s11418-014-0859-3. [DOI] [PubMed] [Google Scholar]
- 138.Rinwa P, Kumar A. Quercetin along with piperine prevents cognitive dysfunction, oxidative stress and neuro-inflammation associated with mouse model of chronic unpredictable stress. Arch Pharm Res. 2017;40(10):1166–75. doi: 10.1007/s12272-013-0205-4. [DOI] [PubMed] [Google Scholar]
- 139.Shrivastava P, Vaibhav K, Tabassum R, Khan A, Ishrat T, Khan MM. et al. Anti-apoptotic and Anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson's Rat model. J Nutr Biochem. 2013;24(4):680–7. doi: 10.1016/j.jnutbio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 140.Shailendra Wadhwa, Sarita Singhal SR. Bioavailability Enhancement by Piperine: A Review. Asian J Biomed Pharm Sci; 2014. pp. 1–8. [Google Scholar]
- 141.Li CY, Zhao LM, Shi XW, Zhang JD. Lobeline shows protective effects against MPTP-induced dopaminergic neuron death and attenuates behavior deficits in animalsz. Exp Ther Med. 2014;7(2):375–8. doi: 10.3892/etm.2013.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martin CA, Nuzzo PA, Ranseen JD, Kleven MS, Guenthner G, Williams Y, Lobeline Effects on Cognitive Performance in Adult ADHD. J Atten Disord; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Angeles ML, Kapadia N, Harding W. Aporphine Alkaloids as Ligands for Serotonin Receptors. Med Chem (Los Angeles) 2016;6(4):241–9. [Google Scholar]
- 144.Steinlein OK. Calcium signaling and epilepsy. Vol. 357, Cell and Tissue Research; 2014. pp. 385–93. [DOI] [PubMed] [Google Scholar]
- 145.Ribeiro RA, Leite JR. Nantenine alkaloid presents anticonvulsant effect on two classical animal models. Phytomedicine. 2003;10(6-7):563–8. doi: 10.1078/094471103322331557. [DOI] [PubMed] [Google Scholar]
- 146.Kharkwal G, Brami-Cherrier K, Lizardi-Ortiz JE, Nelson AB, Ramos M, Del Barrio D. et al. Parkinsonism Driven by Antipsychotics Originates from Dopaminergic Control of Striatal Cholinergic Interneurons. Neuron. 2016;91(1):67–78. doi: 10.1016/j.neuron.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barreto G, Iarkov A, Moran V. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson's disease. Front Aging Neurosci [Internet]; 2014. pp. 1–13. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4288130/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fargo K. Alzheimer's Association Report: 2014 Alzheimers disease facts and figures. Alzheimer's Dement; 2014. p. 10. (2) [DOI] [PubMed] [Google Scholar]
- 149.Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: Role of α4 and α7 receptors in neuroprotection. In: Journal of Molecular Neuroscience; 2010. pp. 211–6. [DOI] [PubMed] [Google Scholar]
- 150.Kim BG, Jee ÆSW, Lee ÆSH, Sin ÆJS, Bae ÆCJ, Lee BC, Nicotine Leads to Improvements in Behavioral Impairment and an Increase in the Nicotine Acetylcholine Receptor in Transgenic Mice. 2008;1783-8. [DOI] [PubMed]
- 151.Levy DT, Cummings KM, Villanti AC, Niaura R, Abrams DB, Fong GT. et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112(1):8–17. doi: 10.1111/add.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Archives of Toxicology. 2014;88(1):5–7. doi: 10.1007/s00204-013-1127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pelissier-Rota MA, Pelosi L, Meresse P, Jacquier-Sarlin MR. Nicotine-induced cellular stresses and autophagy in human cancer colon cells: A supportive effect on cell homeostasis via up-regulation of Cox-2 and PGE<inf>2</inf> production. Int J Biochem Cell Biol. 2015;65:239–56. doi: 10.1016/j.biocel.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 154.Bhat SK, Ashwin D, Sarpangala M. Contamination and Adulteration in Arecanut ( Areca Catechu L.) And Its Chewing Foms : The Less Focused Subject by Health Researchers. 2017;11(1):7-12.
- 155.Carruthers SP, Gurvich CT, Rossell SL. The muscarinic system, cognition and schizophrenia. Neurosci Biobehav Rev. 2015;55:393–402. doi: 10.1016/j.neubiorev.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 156.Coppola M, Mondola R. Potential action of betel alkaloids on positive and negative symptoms of schizophrenia: A review. Vol. 66, Nordic Journal of Psychiatry; 2012. pp. 73–8. [DOI] [PubMed] [Google Scholar]
- 157.Woo TUW. Neurobiology of schizophrenia onset. Curr Top Behav Neurosci. 2014;16:267–95. doi: 10.1007/7854_2013_243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bales A, Peterson MJ, Ojha S, Upadhaya K, Adhikari B, Barrett B. Associations between betel nut (Areca catechu) and symptoms of schizophrenia among patients in Nepal: A longitudinal study. Psychiatry Res. 2009;169(3):203–11. doi: 10.1016/j.psychres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 159.Thal DM, Sun B, Feng D, Nawaratne V, Leach K, Felder CC. et al. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature. 2016;531(7594):335–40. doi: 10.1038/nature17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry. 2000;48(5):381–8. doi: 10.1016/s0006-3223(00)00918-5. [DOI] [PubMed] [Google Scholar]
- 161.Cotter D. Reduced Neuronal Size and Glial Cell Density in Area 9 of the Dorsolateral Prefrontal Cortex in Subjects with Major Depressive Disorder. Cereb Cortex. 2002;12(4):386–94. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 162.Deng C, Huang X-F. Decreased density of muscarinic receptors in the superior temporal gyrusin schizophrenia. J Neurosci Res. 2005;81(6):883–90. doi: 10.1002/jnr.20600. [DOI] [PubMed] [Google Scholar]
- 163.Peng W, Liu Y-J, Wu N, Sun T, He X-Y, Gao Y-X. et al. Areca catechu L. (Arecaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Ethnopharmacol. 2015;164(Supplement C):340–56. doi: 10.1016/j.jep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 164.Zhou J, Sun Q, Yang Z, Zhang J. The Hepatotoxicity and Testicular Toxicity Induced by Arecoline in Mice and Protective Effects of Vitamins C and E. Korean J Physiol Pharmacol. 2014;18(2):143. doi: 10.4196/kjpp.2014.18.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng S-J, Ko H-H, Cheng S-L, Lee J-J, Chen H-M, Chang H-H. et al. Arecoline-stimulated placenta growth factor production in gingival epithelial cells: modulation by curcumin. Oral Dis. 2013;19(5):513–8. doi: 10.1111/odi.12034. [DOI] [PubMed] [Google Scholar]
- 166.Ullah M, Cox S, Kelly E, Boadle R, Zoellner H. Arecoline is cytotoxic for human endothelial cells. J Oral Pathol Med. 2014;43(10):761–9. doi: 10.1111/jop.12186. [DOI] [PubMed] [Google Scholar]
- 167.McIntire LK, McKinley RA, Goodyear C, Nelson J. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul. 2014;7(4):499–507. doi: 10.1016/j.brs.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 168.Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A. et al. Caffeine reverses cognitive impairment and decreases brain amyloid-β levels in aged alzheimer's disease mice. J Alzheimer's Dis. 2009;17(3):661–80. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 169.Collin L, Bohrmann B, Göpfert U, Oroszlan-Szovik K, Ozmen L, Grüninger F. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer's disease. Brain. 2014;137(10):2834–46. doi: 10.1093/brain/awu213. [DOI] [PubMed] [Google Scholar]
- 170.Laurent C, Eddarkaoui S, Derisbourg M, Leboucher A, Demeyer D, Carrier S. et al. Beneficial effects of caffeine in a transgenic model of Alzheimer's disease-like tau pathology. Neurobiol Aging. 2014;35(9):2079–90. doi: 10.1016/j.neurobiolaging.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 171.Nehlig A. Effects of coffee/caffeine on brain health and disease: What should I tell my patients? Pract Neurol. 2016;16(2):89–95. doi: 10.1136/practneurol-2015-001162. [DOI] [PubMed] [Google Scholar]
- 172.Ritchie K, Carrière I, de Mendonça A, Portet F, Dartigues JF, Rouaud O. et al. The neuroprotective effects of caffeine: a prospective population study (the Three City Study) Neurology. 2017;23:272–90. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- 173.More SV, Kumar H, Kim IS, Song SY, Choi DK. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson's disease. Vol. 2013, Mediators of Inflammation; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rivera-oliver M, Manuel D, Díaz-ríos M. Address for correspondence : NU SC. Life Sci; 2014. [Google Scholar]
- 175.Li X, Li W, Liu G, Shen X, Tang Y. Association between cigarette smoking and Parkinson's disease: A meta-analysis. Arch Gerontol Geriatr. 2015;61(3):510–6. doi: 10.1016/j.archger.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 176.Avula B, Chittiboyina AG, Sagi S, Wang YH, Wang M, Khan IA. et al. Identification and quantification of vinpocetine and picamilon in dietary supplements sold in the United States. Drug Test Anal. 2016;8(3-4):334–43. doi: 10.1002/dta.1853. [DOI] [PubMed] [Google Scholar]
- 177.Ruiz-Miyazawa KW, Zarpelon AC, Pinho-Ribeiro FA, Pav?o-De-Souza GF, Casagrande R, Verri WA. Vinpocetine reduces carrageenan-induced inflammatory hyperalgesia in mice by inhibiting oxidative stress, cytokine production and NF-?B activation in the paw and spinal cord. PLoS One; 2015. p. 10. (3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang J, Dong T, Zhang Y, Lu Z, Zhai K, Liu X. Effects of vinpocetine and ozagrel on behavioral recovery of rats after global brain ischemia. J Clin Neurosci. 2014;21(4):661–3. doi: 10.1016/j.jocn.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 179.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12(1):86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.National Toxicology Program. Chemical Information Review Document for Vinpocetine. 2013;(42971):260.
- 182.Ratia M, Giménez-Llort L, Camps P, Muñoz-Torrero D, Pérez B, Clos M V. et al. Huprine X and huperzine a improve cognition and regulate some neurochemical processes related with Alzheimer's disease in triple transgenic mice (3xTg-AD) Neurodegener Dis. 2013;11(3):129–40. doi: 10.1159/000336427. [DOI] [PubMed] [Google Scholar]
- 183.Yang G, Wang Y, Tian J, Liu J-P. Huperzine A for Alzheimer's disease: a systematic review and meta-analysis of randomized clinical trials. PLoS One [Internet] 2013;8(9):e74916.. doi: 10.1371/journal.pone.0074916. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3781107&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ma X, Gang DR. In vitro production of huperzine A, a promising drug candidate for Alzheimer's disease. Phytochemistry. 2008;69(10):2022–8. doi: 10.1016/j.phytochem.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 185.Yan R, Zhang Z, Wang Y, Yang H, Zeng Q, Zhu D. Efficient strategy for maintaining and enhancing the huperzine a production of Shiraia sp. Slf14 through inducer elicitation. J Ind Microbiol Biotechnol. 2014;41(7):1175–9. doi: 10.1007/s10295-014-1461-0. [DOI] [PubMed] [Google Scholar]
- 186.Su J, Yang M. Huperzine A production by Paecilomyces tenuis YS-13, an endophytic fungus isolated from Huperzia serrata. Nat Prod Res. 2015;29(11):1035–41. doi: 10.1080/14786419.2014.980245. [DOI] [PubMed] [Google Scholar]
- 187.Anggono V, Tsai L, Götz J. Glutamate Receptors in Alzheimer's Disease: Mechanisms and Therapies. Neural Plast. 2016;2016:1–2. doi: 10.1155/2016/8256196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Peters C, Sepúlveda FJ, Fernández-Pérez EJ, Peoples RW, Aguayo LG. The Level of NMDA Receptor in the Membrane Modulates Amyloid-β Association and Perforation. J Alzheimer's Dis. 2016;53(1):197–207. doi: 10.3233/JAD-160170. [DOI] [PubMed] [Google Scholar]
- 189.Shao Z-Q. Comparison of the efficacy of four cholinesterase inhibitors in combination with memantine for the treatment of Alzheimer's disease. Int J Clin Exp Med. 2015;8(2):2944–8. [PMC free article] [PubMed] [Google Scholar]
- 190.Budni J, Bellettini-santos T, Mina F, Garcez ML. The involvement of BDNF, NGF and GDNF in aging and Alzheimer â€TM s disease. 2016;6(5):331-41. [DOI] [PMC free article] [PubMed]
- 191.Mao XY, Cao DF, Li X, Yin JY, Wang Z Bin, Zhang Y. et al. Huperzine a ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Int J Mol Sci. 2014;15(5):7667–83. doi: 10.3390/ijms15057667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ma T, Gong K, Yan Y, Zhang L, Tang P, Zhang X. et al. Huperzine A promotes hippocampal neurogenesis in vitro and in vivo. Brain Res. 2013;1506:35–43. doi: 10.1016/j.brainres.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 193.Klein-Junior L, Santos Passos C, Moraes A, Wakui V, Konrath E, Nurisso A. et al. Indole Alkaloids and Semisynthetic Indole Derivatives as Multifunctional Scaffolds Aiming the Inhibition of Enzymes Related to Neurodegenerative Diseases - A Focus on Psychotria L. Genus. Curr Top Med Chem. 2014;14(8):1056–75. doi: 10.2174/1568026614666140324142409. [DOI] [PubMed] [Google Scholar]
- 194.He D, Wu H, Wei Y, Liu W, Huang F, Shi H. et al. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur J Pharmacol. 2015;768:96–107. doi: 10.1016/j.ejphar.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 195.Konrath EL, Passos CDS, Klein-Júnior LC, Henriques AT. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer's disease. J Pharm Pharmacol. 2013;65(12):1701–25. doi: 10.1111/jphp.12090. [DOI] [PubMed] [Google Scholar]
- 196.Qian W, Jin N, Shi J, Yin X, Jin X, Wang S. et al. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A) enhances tau expression. J Alzheimer's Dis. 2013;37(3):529–38. doi: 10.3233/JAD-130824. [DOI] [PubMed] [Google Scholar]
- 197.Chinwala Y, Bax L, Gallego A, Krishna K, Wright W. under the supervision of. 2016.
- 198.Vasconcelos SMM, Sales GTM, Lima N, Lobato R de FG, Macêdo DS, Barbosa-Filho JM. et al. Anti-inflammatory activities of the hydroalcoholic extracts from Erythrina velutina and E. Mulungu in mice. Brazilian J Pharmacogn. 2011;21(6):1155–8. [Google Scholar]
- 199.de Oliveira MSG, Aquino AB, da Silva DL, Aquino PG V, Santos MS, Porfírio APR. et al. Antinociceptive and anti-infl ammatory activity of hydroalcoholic extracts and fractions from Erythrina mulungu. Brazilian J Pharmacogn. 2011;22(1):157–61. [Google Scholar]