Abstract
Modern societies are replete with palatable food cues. A growing body of evidence suggests that food cue exposure activates conditioned appetitive physiological and psychological responses that may override current metabolic needs and existing eating goals, such as the desire to maintain a healthy diet. This conditioned response results in unhealthy dietary choices and is a contributing factor in the current obesity epidemic. Prime based obesity prevention measures such as health warnings at point-of-sale or on product packaging may have the potential to counteract the influence of the obesogenic environment at the crucial moment when people make food purchasing or consumption decisions. Existing research into the efficacy of these intervention strategies has predominantly employed self-report and population level measures, and little evidence exists to support the contention that these measures counteract food cue reactivity at the time of decision making. Using a dietary self-control priming paradigm, we demonstrated that brief exposure to food product health warnings enhanced dietary self-control. Further, we analysed electroencephalographic correlates of selective attention and food cue evoked craving (N1, P3, LPP) to show that health warning exposure reduced the automatic appetitive response towards palatable food cues. These findings contribute to existing evidence that exogenous information can successfully prime latent goals, and substantiate the notion that food product health warnings may provide a new avenue through which to curb excessive energy intake and reduce rising obesity rates.
Keywords: Health warnings, Dietary decision making, Self-control, Electroencephalogram, EEG, N1, P3, LPP
Highlights
-
•
Food product health warnings successfully promote dietary self-control.
-
•
ERP components P3 and LPP sensitive to food characteristics and health warnings
-
•
LPP amplitudes also directly predict dietary self-control.
-
•
Health warnings useful to reduce appetitive food responses in obesity prevention
Abbreviations
1. Introduction
The relationship between conscious intentions to alter behaviour and observed behavioural change is well documented (Sheeran et al., 2016). However, it is widely accepted that factors outside our consciously stated intentions can have a considerable influence on our behaviour (Inauen et al., 2016). For example, the highly palatable food cues that are ubiquitous in modern Western and Westernised societies are capable of triggering conditioned appetitive responses such as increased salivation (Rogers and Hill, 1989) and neural activity in gustatory and reward-relevant brain areas (Simmons, 2005). This collection of physiological and cognitive responses is experienced as craving, and has the potential to override homeostatic signalling of current metabolic needs and previous conscious decisions regarding eating behaviour, such as healthy eating goals (Bilman et al., 2017). As such, food cue reactivity has been linked to increased food consumption (Larsen et al., 2012), weight gain (Murdaugh et al., 2012) and may be exacerbating rising obesity rates (Morris et al., 2015).
The increase in environmental and habitual factors that bias people towards unhealthy eating has driven calls for prime-based intervention strategies that may cue latent health goals at the time of decision-making (Cohen and Lesser, 2016) and prevent momentary lapses in self-control. One such intervention that has received legislative attention is the proposal to require health warning messages on certain food products (Popova, 2016). Nonetheless, there is still insufficient research on whether health warnings can effectively prime healthy eating goals, and whether exposure to health warnings leads to detectable changes in neural processing of food items. The present study examined electroencephalographic (EEG) correlates of cognitive processes related to dietary self-control and food cue reactivity after priming with health warnings in order to test this idea.
Food cue reactivity research has provided evidence that the neural processing of food stimuli is influenced by choice-relevant stimulus characteristics and the individual's current motivational state (amongst other factors; Asmaro and Liotti, 2014). For example, early, automatic attentional processes reflected in the occipital N1 event-related potential (ERP) component responded to the energetic value of food (Toepel et al., 2009). This attentional filtering process is thought to reduce the deployment of neural resources for goal-irrelevant items and is therefore influenced by the match between stimulus characteristics and the current decision-making goal (Harris et al., 2013). Similarly, the parietal P3 and late positive potential (LPP) are mid- to late-latency ERP components that have been studied extensively in substance cue reactivity and have consistently been found to index the subjective cue-induced craving response (Littel et al., 2012). This craving response is subject to top-down cognitive regulation (Meule et al., 2013), making these ERP components a valuable neural index for the study of the ability of food product health warnings to counteract appetitive food cue reactivity.
We used a dietary self-control priming paradigm in which participants provided health and taste ratings of snack food items, and then made choices regarding which foods to eat at the experiment's conclusion, prior to- and post-exposure to health warning messages (Rosenblatt et al., submitted). Using participants' subjective health and taste ratings we classified trials into self-control trials (where health and taste ratings conflict) and non-self-control trials (no conflict between health and taste), allowing us to test the extent to which health warnings increase dietary self-control. We also tested whether observed changes in self-control are driven by differences in the perceived palatability or health qualities of food stimuli. Using ERPs, we additionally investigated whether neural attention regulation and craving responses to food stimuli were modulated accordingly, and whether these changes were predictive of observed changes in dietary self-control. We hypothesised that participants would display increased dietary self-control after viewing health warnings, and this change would be reflected in an up-regulation of attention towards healthy food items and/or a down regulation of attention towards unhealthy items. We further hypothesised that after viewing health warnings, brain signals correlating with subjective cue-induced craving would be increased in response to unpalatable/healthy food items and diminished in response to palatable/unhealthy food items. Lastly, we hypothesised that food-stimulus-evoked brain signals that were influenced by health warning exposure would be associated with successful dietary self-control.
2. Methods
2.1. Participants
Ninety-six right-handed English-speaking participants (M age = 22.64 years, SD = 4.94, M BMI = 21.54, SD = 3.09, BMI range = 16.65–34.71, 66 female) with normal or corrected-to-normal vision were recruited via advertisements. The behavioural data presented here have been used in analyses that have been reported elsewhere (Rosenblatt et al., under review). Exclusion criteria were any history of eating disorders, or any medical, ethical, religious or other belief or condition that prevented them from eating the food items presented in the study. Fourteen participants were excluded post-hoc due to persistent artifacts in the EEG data. Three participants were removed due to technical problems with data recording. The remaining 79 participants' (M age = 22.91 years, SD = 5.31, M BMI = 21.65, SD = 3.25, BMI range = 16.65–34.71, 52 female) data were used in the analyses. The University of Melbourne Human Research Ethics Committee approved all study procedures (No. 1443258), and participants gave written informed consent before participating.
2.2. Materials and stimuli
Snack food stimuli were 100 color pictures of snack foods presented on a white background. All food images measured 500 × 500 pixels, subtending at a visual angle of 13.4°×13.4°. Food stimuli were selected based on ratings provided by an independent sample of 259 participants (M age = 27.56 years, SD = 10.54, 183 female) and had been used in a previous study by our group (Rosenblatt et al., under review). Taste, health and familiarity ratings were recorded for 492 snack food items using 6-point scales. The 100 food items selected for the current study met the following criteria: they collectively encompassed the full range of health and taste ratings such that they could be divided into four groups defined by these attributes (healthy/tasty, healthy/not-tasty, unhealthy/tasty, and unhealthy/not-tasty); displayed a low degree of interdependence between taste and health ratings across items (r = 0.17); exhibited health and taste ratings that varied minimally across participants (SD < 1.5); and on average were familiar to participants (mean > 3 on a scale from 1 = “not at all familiar” to 6 = “extremely familiar”). The chosen food items included chips, chocolate bars, biscuits, nuts, fruits and vegetables.
Twenty five health warning (HW) messages were created based on the National Health and Medical Research Council Dietary Guidelines for Australians (NHMRC, 2013) and were consistent with current epidemiological evidence. As described in Rosenblatt et al. (under review), four variants were created for each health warning topic: text-only/positive message frame, text-only/negative framing, image-and-text/positive framing, and image-and-text/negative framing, and each participant was exposed to health warnings from one of these groups. In order to select the best exemplars of the health warnings that were created, a second independent sample of 95 participants (M age = 28.18 years, SD = 10.85, 73 female) provided perceived efficacy ratings of these sample health warnings on a nine-item scale adapted from existing health communication efficacy questionnaires (Hammond et al., 2007; Nonnemaker et al., 2015). The scale items assessed the extent to which participants thought each warning would motivate healthier dietary choices (e.g. “This health warning is effective”, “This health warning would prompt me to purchase a healthier snack”) and related constructs such as whether they believed the content of the warning or whether it was likely to capture their attention (e.g. “This health warning grabs my attention”, “This health warning is worth remembering”, “This health warning makes a strong argument for eating a healthy diet”). Participants responded to these questions on a 5-point scale ranging from “Strongly Disagree” to “Strongly Agree”. The 10 health warning messages that received the highest average perceived efficacy ratings across all four variants were selected for use in the experiment. Twenty control stimuli were created by randomly sorting the pixels of the chosen image-and-text HWs horizontally and replacing the text with pronounceable non-words of equivalent length (Rastle et al., 2002). Text-only health warnings were 500 × 200 pixels (subtending at a visual angle of 13.4°× 5.3°), while image-and-text health warnings were 500 × 500 pixels (13.4°× 13.4°).
For the purposes of conserving statistical power for the present analyses, all health warning variants were grouped together, resulting in one health warning group and one control group. This means that while different participants were exposed to different health warning variants (13 participants per variant on average), the health warning topic of the messages featured in these warnings was consistent for all participants. For more information regarding the characteristics of the health warning stimuli, see Rosenblatt et al. (under review). The experimental task was presented on a 1680 × 1050 pixel LCD monitor with a screen refresh rate of 60 Hz, with participants seated comfortably at a chin rest with their eyes 60 cm from the screen. MATLAB (R2014b; The MathWorks) and Psychtoolbox (Brainard, 1997) were used for stimulus presentation and data acquisition.
2.3. Procedure
Participants were asked to fast for at least 4 h prior to the experiment, to control for hunger disparities (cf. Harris et al., 2013). At the start of the session, participants rated their hunger on an eight-point scale (1 = not at all hungry, 8 = extremely hungry), and recorded the number of hours since their last meal.
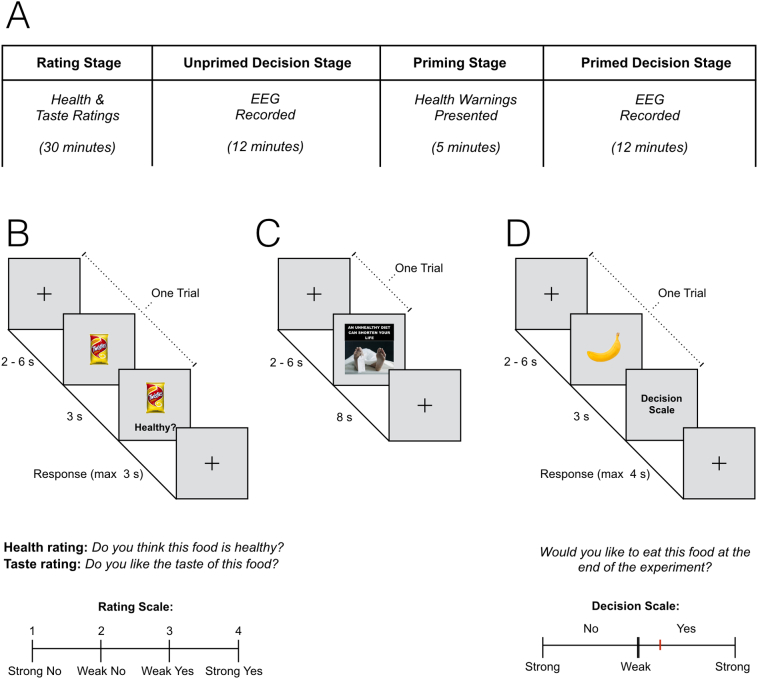
Participants then completed a computer-based experimental task consisting of four stages: a rating stage; an unprimed decision stage; a priming stage; and a primed decision stage (Fig. 1a). After completing each stage, participants were given a short break during which the next stage was explained. Participants were informed that, based on their choices during the decision stages, they would be given one food item to eat at the completion of the experiment. This incentivized participants to choose items reflecting their true preferences. EEG was recorded for the duration of the experiment.
Fig. 1.
Testing session procedure. (A) Participants completed an experimental task with four stages: the rating stage, the unprimed decision stage, the priming stage, and the primed decision stage. (B) One example rating stage trial. During this stage participants rated all food items on perceived health and taste. These ratings were used to allocate items to the unprimed and primed decision stages, ensuring that these stages featured a balanced set of stimuli. (C) One priming stage trial (health warning group, without attention check). Control group participants completed the same task during this stage, however they were shown scrambled health warning images. (D) One example decision stage trial. During these stages, participants chose items they would like to eat at the end of the experiment, while also indicating the strength of their choice. Participants completed the same task in unprimed and primed decision stage trials.
2.3.1. Rating stage
Each trial began with a central fixation cross that appeared on screen for two to 6 s (randomly jittered). Participants were then shown one of the 100 food items for 3 s. After this interval a response scale accompanied by one of two questions (“Do you think this food is healthy?” or “Do you like the taste of this food?”) appeared below the food item (which remained on screen) for a further 3 s. Participants responded during this interval via keyboard button press, using a four-point scale (1 = Strong No, 2 = Weak No, 3 = Weak Yes, 4 = Strong Yes), the ordering of which was counterbalanced from trial to trial. Participants completed 200 trials, which were presented in four blocks of 50 trials. This allowed participants to rate each food item on both attributes. Between each block participants took a self-paced break. The order in which food items were displayed and paired with attribute ratings was randomized. See Fig. 1b for task schematic.
Each participant's health and taste ratings were used to individually categorize each food item into one of five groups (which were specific to that participant): Healthy-Tasty (mean number of items in this group per participant = 28.74, SE = 0.88), Healthy-Not Tasty (mean item number = 19.42, SE = 0.8), Unhealthy-Tasty (mean item number = 24.3, SE = 1.1), Unhealthy-Not Tasty (mean item number 24.5, SE = 1.21), and undefined items (items where participants did not provide one or both ratings in the allotted time window; mean number of items in this group = 3.04 items, SE = 0.35). Fifty percent (±1 for health-taste groups with an odd number of items) of food items from each of the health-taste groupings were then randomly allocated to the unprimed and primed decision stages, ensuring that each decision stage had an even balance of items from each of these groups (χ2(3) = 0.314, p = 0.957) and featured 50 items total. Furthermore, the proportion of trials in each of these trial type groups did not differ significantly between condition (health warning group participants and control group participants; χ2(3) = 2.101, p = 0.552).
2.3.2. Unprimed decision stage
In each trial of the unprimed decision stage (50 trials total) a fixation cross was presented centrally for between two and 6 s, followed by a food item for 3 s. Subsequently, participants responded to the question “Would you like to eat this food at the end of the experiment?” using a continuous response scale (on screen for 4 s). Response options ranged from “Strong No” through “Weak No”, “Weak Yes”, and to “Strong Yes”. The ordering of the “Yes” and “No” options randomly reversed from trial to trial. Responses were made by moving a graphical slider using a computer mouse, and it was not possible to select the exact midpoint of the scale. This required participants to make a binary decision as to whether they wanted the displayed stimulus while also indicating the strength of their response. See Fig. 1d for decision stage task schematic.
2.3.3. Priming stage
In the 10 priming stage trials, participants fixated on a central cross (2–6 s, jittered) and were then centrally presented with each of the 10 HW messages (in different formats depending on HW group allocation) or 10 of the control images (control group) for 8 s. Each HW message was shown once, in individually randomized order. Participants were instructed to attend to and read the HW messages. To ensure that participants attended to HWs, and to prevent deliberate disengagement from viewing HWs (Maynard et al., 2014), an attention check was included on a random three trials during this stage. Participants were instructed that on a small proportion of trials a red border would appear around the HW, in this case they were to press the space bar (at which point the border would turn green). Failure to respond to two or more attention checks was grounds for removing the participant from subsequent analyses, however no participant failed more than one attention check. See Fig. 1c for task schematic.
2.3.4. Primed decision stage
The primed decision stage was identical to the unprimed decision stage (Fig. 1d), except that it featured the remaining 50 food items, which were now presented after exposure to the HWs.
At the completion of the experiment, one of the food items the participant had responded “Yes” to in either the unprimed or primed decision stages was randomly selected, and given to them for consumption. Finally, participants completed final questionnaires on dietary habits and demographic characteristics, were remunerated AUD$20 and verbally debriefed on the study's purpose.
2.4. Behavioural data analysis and measures
Independent samples t-tests for continuous variables, Mann-Whitney U tests for ordinal data, and chi-squared tests for proportions were conducted to check for differences in individual and demographic factors between HW groups. A p-value of <0.05 in these tests was used as grounds to include the corresponding variable as a covariate in subsequent analyses. All other (behavioural and EEG) analyses were conducted using linear mixed effects modeling in the lme4 package in R (Bates et al., 2015b). These models allowed us to control for participant- and stimulus- level variance simultaneously without data aggregation. They also tolerate the unequal trial numbers that are often necessitated in EEG research and exacerbated by our experimental design in which trial classification was dependent on subjective participant ratings and therefore could not be pre-determined. All linear mixed effects models investigating higher-order interaction effects also contain all main effects and lower-order interactions. All models employed the maximal random effects structure allowed by our data (Bates et al., 2015a), and Satterthwaite degrees of freedom approximation was used to calculate p-values.
Two outcome variables that may be informative about the success of our health warning stimuli for influencing dietary choice behavior were identified. First, to calculate a measure of primed and unprimed dietary self-control (DSC) for each participant, primed and unprimed decision stage trials were divided into self-control trials and non-self-control trials. Trials featuring food items that had been rated relatively healthy-not tasty or relatively unhealthy-tasty were considered self-control trials. Trials featuring healthy-tasty or unhealthy-not tasty items were considered non-self-control trials, as subjective health and taste attributes were not in conflict, preventing the need to exercise dietary self-control. For self-control trials, successful dietary self-control was shown when participants responded “Yes” to the question “Would you like to eat this item at the end of the experiment?” for healthy-not tasty items, or “No” for unhealthy-tasty items. Because the subjective health and taste attributes of the foods featured in these trials were in conflict, participants were required to exercise dietary self-control by trading off immediate hedonic (taste) rewards with long-term health consequences. Unprimed and primed DSC was then calculated by taking the continuous decision responses for healthy-not tasty trials, and the reverse coded continuous decision responses for unhealthy-tasty trials, during the unprimed and primed decision stages. The second informative measure was simply the raw tendency for participants to choose or reject any given item (which we refer to as the “dietary choice response”), and by entering health and taste ratings as covariates, it was possible to measure the extent to which these attributes were predictive of dietary choices under both priming conditions and in both experimental groups (in this case, a positive value corresponds to saying “yes” to a given item, and a negative value corresponds to a “no” response).
To confirm that health warnings improved dietary self-control, the main effects of HW condition (HW, control), experimental decision stage (unprimed, primed) and condition-by-stage interaction on DSC were assessed via linear mixed effects modeling, with participant- and stimulus-level variance modelled using random intercepts and random slopes within experiment stage. Note that these analyses aggregated behavioural data across health warning groups to match the approach of the EEG analyses (for a more detailed analysis of the behavioural data that takes into account sub-groups, see Rosenblatt et al., under review). Random intercepts for participants and stimuli, and random slopes for participants and stimuli within experimental stage were included as random effects. We next conducted two follow-up analyses to investigate the extent to which participants' dietary choice responses varied with the perceived health and taste attributes of food items, and whether the effect of these attributes on choice differed between the primed and unprimed decision stages in the control and HW conditions. To test this, we estimated a linear mixed effects regression featuring the fixed effects of health and taste attributes, experimental stage, and all two- and three-way interactions of these variables on dietary choice response (continuous yes/no responses) for the control and HW conditions. To control for participant and item level variance, random intercepts by participant and stimulus and random slopes of these variables within food attribute variables were included in the model. This allowed us to test whether any changes in self-control that we observed as a result of health warning exposure were driven by an increase in the influence of health attributes on choice, by a decrease in the influence of taste attributes on choice, or a combination of the two.
2.5. EEG data recording, pre-processing and analysis
The electroencephalogram (EEG) was recorded from 64 active Ag/AgCl scalp electrodes placed in accordance with the international 10–20 configuration, using a BioSemi Active Two system and ActiView acquisition software. EEG data processing and measurement was conducted using the EEGlab (Delorme and Makeig, 2004) toolbox and ERPlab (Luck, 2014) plugin in MATLAB (R2014b; The MathWorks). An implicit electrode reference was used during recording, and the data were re-referenced offline against the average of both mastoids. Vertical and horizontal electrooculogram were recorded from electrodes infraorbital and lateral to the left eye. The EEG was continuously recorded with a sampling rate of 512 Hz and was filtered online using a 0.1 to 70 Hz band-pass filter. A standard 50 Hz notch filter was applied to the data to remove power supply artifacts. The continuous EEG signal was separated into epochs that were time-locked to the onset of food image stimuli in the unprimed and primed decision stages of the experiment. Epochs were 1600 ms long, with a 100 ms pre-stimulus baseline period and a 1500 ms post-stimulus window. Epochs were manually screened for skin potential and muscle artifacts, and the clean data were then subjected to an independent component analysis to identify and remove eye movements and eye-blink artifacts using standard EEGlab routines. Data were then manually screened a second time to remove any remaining artifacts, and finally data were screened to automatically remove signals exceeding ± 200.
Existing approaches to spatially and temporally defining the ERP components relevant to the present study vary extensively in the literature (see Polich, 2007 for examples), with recent studies suggesting that reliability in ERP measurements can be increased by averaging across sensors (Huffmeijer et al., 2014). Thus, we identified regions of interest for each ERP component based on typical electrode sites featured in the image viewing literature (Olofsson et al., 2008). Measurement time windows were selected by consulting grand average ERP plots as well as existing research (Key et al., 2011). The target measures of interest were the N1, the P3, and the LPP. We quantified the N1 component as the mean amplitude between 125 and 200 ms, averaged across occipital sensors at, and adjacent to, the midline (Oz, O2, O1 and Iz). The P3 (250 ms–450 ms) and LPP (450 ms–750 ms) components were measured using the average amplitude across midline parietal electrodes (CPz, Pz, POz). These electrode sites and time windows are consistent with those typical across the field (Asmaro and Liotti, 2014; Carbine et al., 2017; Hume et al., 2015; Littel et al., 2012; Meule et al., 2013; Sarlo et al., 2013; Toepel et al., 2009).
All measurements of ERP components involved averaging amplitudes at the regions of interest and over the specified time windows on a trial-by-trial basis, as is standard in mixed-effects modeling (Bates et al., 2015b). To investigate whether HW exposure modulated early attentional processes (indexed by the N1) and food cue induced craving (indexed by the P3 and LPP) in accordance with food stimulus characteristics (perceived heath and taste), a separate linear mixed effects model was constructed for each ERP component, resulting in three models (one for each of the N1, P3, and LPP). These models included main effects for HW condition (HW group, control group), experimental decision stage (unprimed, primed), health and taste ratings, as well as all two-, three-, and four-way interactions between these variables. Health and taste ratings were mean-centered to reduce collinearity in interaction terms featuring these variables. These models also included random intercepts for participants and food stimuli as well as random slopes for these variables within experimental stage. These analyses were intended to investigate whether any observed increase in dietary-self-control displayed after HW exposure was driven by altered food cue reactivity. Specifically, we reasoned that increased self-control could be engendered by cognitive regulation of food stimuli in two ways: through increasing appetitive or attentional processes towards healthy foods, increasing the likelihood that these foods are chosen, or through down regulation of these processes to highly palatable foods, reducing the chance that these foods were selected. As a result, the most informative effects that we tested centred on the influence of the food stimulus attributes (health and taste perceptions) on the measured ERP components, as a function of HW group membership and experiment stage. The main effects of these factorial variables (HW group, experiment stage) are less informative as, for example, increased ERP component amplitudes towards healthy food but decreased ERP component amplitudes towards tasty foods are likely to cancel out at this level of analysis.
A final linear mixed effects model was constructed to investigate whether modulation in the N1, the P3, and the LPP was predictive of the successful enactment of dietary self-control. In this analysis, trial-by-trial measurements for each ERP were entered into the model as main effects, and intercepts for participants and stimuli were modelled as random effects.
3. Results
3.1. Sample demographics and dietary behaviour
Descriptive statistics for the HW groups and whole-sample demographic characteristics are summarized in the supplementary materials (Table S1). The two HW groups did not differ significantly on any of the demographic, weight, hunger or dietary consumption measures. HW group participants also did not differ significantly in terms of restrained, emotional, or external eating tendencies as measured using the Dutch Eating Behavior Questionnaire (van Strien et al., 1986). Therefore, these variables were not included in subsequent analyses.
3.2. Behavioural results
3.2.1. Health warnings and dietary self-control
The main effects of HW condition (β = 3.35, SE = 7.11, t(77) = 0.47, p = 0.639) and experiment stage (β = 0.37, SE = 4.12, t(80) = 0.09, p = 0.929) were both non-significant. However, the interaction of these two variables on DSC was significant (β = 11.1, SE = 4.97, t(76) = 2.23, p = 0.028), indicating that the DSC exhibited by the HW group and control group participants did not significantly differ during the unprimed decision stage, and that HW exposure resulted in increased DSC in the HW group but not the control group (Fig. 2).
Fig. 2.
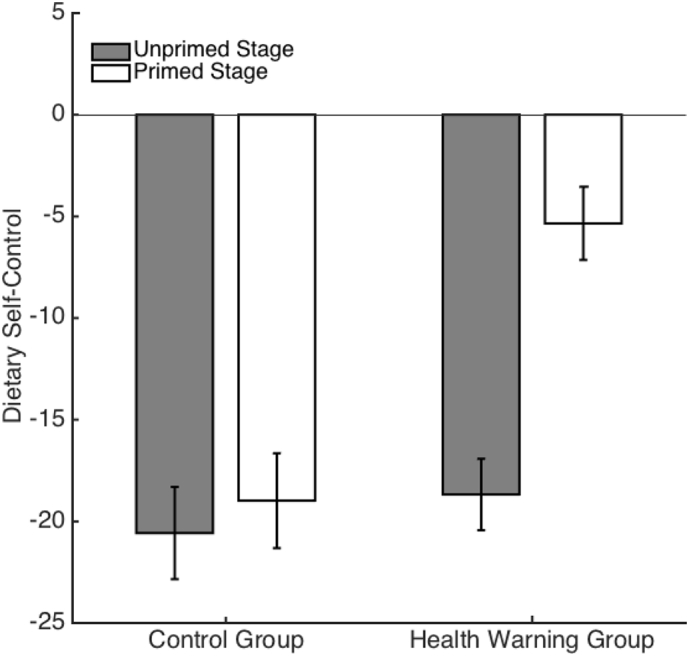
Mean dietary self-control (measured as proportion of healthy-not tasty items chosen relative to unhealthy-tasty items chosen) in the unprimed and primed decision stages for health warning group and control group participants. Error bars denote standard error.
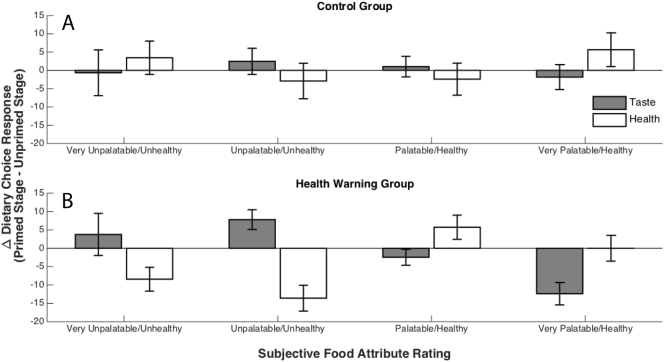
We next sought to test the extent to which health and taste attributes predicted dietary choice, and whether the influence of these food attributes was altered by health warning exposure. In the control group, the main effect of taste ratings on dietary choice response was significant (β = 42.06, SE = 2.74, t(35) = 15.35, p < 0.001), with more tasty items being more strongly desired. In the HW group, the main effects of taste ratings (β = 38.02, SE = 2.25, t(70) = 16.94, p < 0.001), health ratings (β = 5.33, SE = 2.45, t(65) = 2.18, p = 0.033), and stage (β = −4.7, SE = 1.22, t(4809) = −3.86, p < 0.001) were all significant. Most importantly, the two-way interactions of taste ratings by stage (β = −5.32, SE = 1.45, t(4652) = −3.67, p < 0.001) and health ratings by stage (β = 5.06, SE = 1.05, t(4817) = 4.82, p < 0.001) were both significant. No other significant main or interaction effects were identified. This pattern of results indicates that control group participants' choices depended almost exclusively on taste, and this remained true in the primed decision stage. HW group participants' choices were also largely driven by the taste attributes of food items in the unprimed decision stage. After exposure to HW messages, these participants displayed a reduction in the influence of taste on food choice and an increase in the influence of health, indicating that health warnings drove increases in dietary self-control through both an increased tendency to eat healthy food items and a repression of the desire to eat tasty items (Fig. 3). Lastly, HW group participants were less likely to choose to eat food items generally after exposure to HWs, possibly indicating that on average they had rated a greater proportion of the food items in the experiment as “unhealthy-tasty” or that, in addition to influencing the types of foods that participants chose to eat, HW exposure prompted a general reduction in participants' appetite.
Fig. 3.
Change in dietary choice response (positive values correspond with “yes” responses, negative values with “no” responses, to the question “Would you like to eat this food at the end of the experiment?”) between the unprimed and primed decision stages, as a function of stimulus health and taste attributes, for (A) control group participants, and (B) health warning group participants. In general, health warning group participants displayed an increased likelihood of choosing low taste and high health items and a decreased likelihood of choosing high taste and low health items after exposure to health warnings. Control group participants showed no clear systematic change in their dietary choices.
3.3. EEG results
Significant effects found in our mixed effects models are described below. See Table 1 for the full list of model estimates and significance values.
Table 1.
Model estimates and significance values summarising the influence of health warnings and perceived food attributes on ERP components.
| Outcome | Predictor | β (SE) | P |
|---|---|---|---|
| N1 | Intercept | 1.4 (0.71) | 0.051 |
| HW Condition | −0.04 (0.85) | 0.967 | |
| Stage | −0.1 (0.34) | 0.766 | |
| HW Condition × Stage | 0.21 (0.42) | 0.618 | |
| Taste Rating | −0.5 (0.29) | 0.085 | |
| Health Rating | 0.55 (0.22) | 0.012 | |
| HW Condition × Health Rating | 0.09 (0.24) | 0.728 | |
| Stage × Health Rating | −0.4 (0.28) | 0.161 | |
| HW Condition × Taste Rating | 0.6 (0.35) | 0.089 | |
| Stage × Taste Rating | 0.12 (0.4) | 0.756 | |
| Health Rating × Taste Rating | −0.32 (0.24) | 0.173 | |
| HW Condition × Stage × Health Rating | 0.1 (0.35) | 0.781 | |
| HW Condition × Stage × Taste Rating | −0.02 (0.49) | 0.962 | |
| HW Condition × Health Rating × Taste Rating | 0.41 (0.29) | 0.149 | |
| Stage × Health Rating × Taste Rating | 0.49 (0.33) | 0.13 | |
| HW Condition × Stage × Health Rating × Taste Rating | −0.38 (0.4) | 0.338 | |
| P3 | Intercept | 4.13 (0.66) | <0.001 |
| HW Condition | 1.49 (0.8) | 0.067 | |
| Stage | −0.48 (0.42) | 0.246 | |
| HW Condition × Stage | 0.8 (0.52) | 0.119 | |
| Taste Rating | −1.49 (0.37) | <0.001 | |
| Health Rating | 0.48 (2.62) | 0.069 | |
| HW Condition × Health Rating | 0.16 (0.31) | 0.615 | |
| Stage × Health Rating | −0.01 (0.36) | 0.986 | |
| HW Condition × Taste Rating | 1.8 (0.45) | <0.001 | |
| Stage × Taste Rating | 1.72 (0.51) | <0.001 | |
| Health Rating × Taste Rating | 0.39 (0.3) | 0.199 | |
| HW Condition × Stage × Health Rating | −0.47 (0.44) | 0.293 | |
| HW Condition × Stage × Taste Rating | −1.7 (0.62) | 0.006 | |
| HW Condition × Health Rating × Taste Rating | −0.55 (0.36) | 0.135 | |
| Stage × Health Rating × Taste Rating | −0.24 (0.41) | 0.568 | |
| HW Condition × Stage × Health Rating × Taste Rating | 0.31 (0.51) | 0.547 | |
| LPP | Intercept | 3.51 (0.65) | <0.001 |
| HW Condition | 0.74 (0.79) | 0.352 | |
| Stage | −0.45 (0.45) | 0.323 | |
| HW Condition × Stage | 0.41 (0.56) | 0.464 | |
| Taste Rating | −1.15 (0.4) | 0.003 | |
| Health Rating | 0.09 (0.28) | 0.752 | |
| HW Condition × Health Rating | 0.55 (0.34) | 0.1 | |
| Stage × Health Rating | 0.32 (0.39) | 0.406 | |
| HW Condition × Taste Rating | 1.66 (0.48) | <0.001 | |
| Stage × Taste Rating | 1.39 (0.54) | 0.011 | |
| Health Rating × Taste Rating | 0.12 (0.32) | 0.714 | |
| HW Condition × Stage × Health Rating | −0.82 (0.48) | 0.086 | |
| HW Condition × Stage × Taste Rating | −1.44 (0.67) | 0.03 | |
| HW Condition × Health Rating × Taste Rating | −0.21 (0.39) | 0.6 | |
| Stage × Health Rating × Taste Rating | −0.1 (0.45) | 0.826 | |
| HW Condition × Stage × Health Rating × Taste Rating | 0.04 (0.54) | 0.937 |
Coding: HW Group (Control = 0, Test = 1), Stage (Unprimed = 0, Primed = 1).
HW = Health Warning. Significant predictor variables highlighted in bold.
3.4. Modulation of ERP components in relation to health warnings and food stimulus attributes
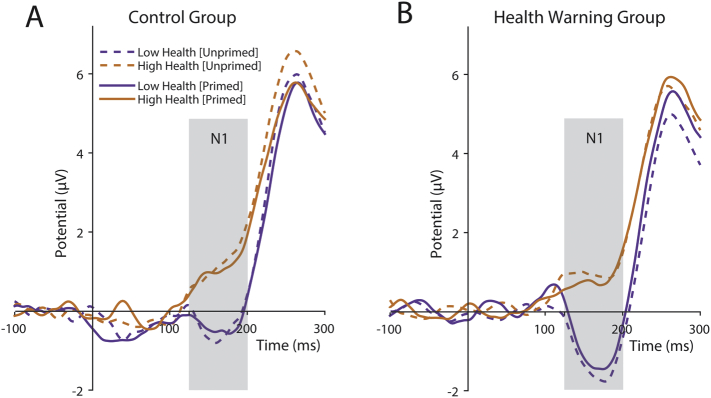
N1. We aimed to test which attributes of food stimuli engaged the deployment of attentional resources early in the cognitive decision process, as indexed by the N1, and whether this relationship changed after HW exposure. A significant main effect of health rating on N1 amplitude was found (β = 0.55, SE = 0.22, t(1463) = 2.51, p = 0.012). No other significant main or interaction effects were identified. These results indicate that less healthy food items elicited a greater (more negative) N1 response, and the absence of interactions, in particular with HW condition and experiment stage, shows that this pattern of results was not influenced by HW exposure (Fig. 4).
Fig. 4.
Grand average waveforms of the N1 ERP (125–200 ms) at occipital electrode cluster (Oz, O2, O1 and Iz) for (A) control group participants and (B) health warning group participants. Orange lines denote trials featuring food items perceived as healthy, purple lines denote trials featuring food items perceived as unhealthy. Dotted lines denote unprimed decision stage trials and solid lines denote primed decision stage trials. Positive amplitudes plotted upwards.
The next two analyses were intended to investigate whether the improved dietary-self-control displayed after HW exposure was driven by altered top-down food cue reactivity, as indexed by the P3 and the LPP.
3.4.1. P3
The main effect of taste rating (β = −1.49, SE = 0.37, t(6098) = 4.06, p < 0.001) on P3 amplitude was significant. No other significant main effects were detected. Significant two-way interaction effects of taste rating by HW group (β = 1.8, SE = 0.45, t(6555) = 4.02, p < 0.001) and taste rating by experiment stage (β = 1.72, SE = 0.51, t(7002) = 3.4, p < 0.001) were both found. We found no other significant two-way interaction effects. Crucially, the three-way interaction of taste rating, HW group, and experiment stage that was diagnostic for the effect of HW on a change in taste rating-dependent P3 amplitude between experimental phases was significant (β = −1.7, SE = 0.62, t(7001) = −2.74, p = 0.006). None of the remaining three- or four-way interaction effects reached significance.
3.4.2. LPP
We found a significant main effect of taste rating on LPP amplitude (β = −1.15, SE = 0.4, t(5973) = 2.92, p = 0.003), while no other main effects reached significance. The two-way interaction effects of taste rating and HW group (β = 1.66, SE = 0.48, t(6422) = 3.43, p < 0.001), and taste rating and stage (β = 1.39, SE = 0.54, t(6998) = 2.55, p = 0.011) were both significant. No further two-way interaction effects were significant. Again, the crucial three-way interaction of HW group, stage, and taste rating that indicated a health warning driven change in taste rating-dependent LPP amplitude between experimental conditions was also significant (β = −1.44, SE = 0.67, t(6993) = 2.17, p = 0.03). No other significant three- or four-way interaction effects were found.
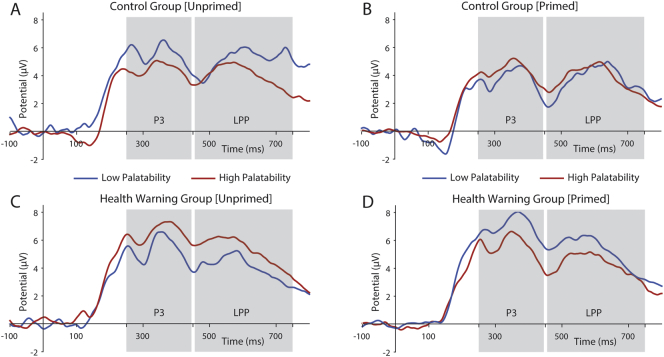
The pattern of results described here indicates that the influence of subjective taste on P3 and LPP amplitude was lower, on average, for the control group than the HW group during the first decision stage. Given that participants did not know at this stage that any health warnings would be shown, this is therefore most likely attributable to random variations or difference in health attributes for those items between groups. Importantly, however, our crucial measure was the comparison of the change in the amplitude of P3 and LPP components after HW priming between groups that does not depend on the absolute values of the first decision stage. Our results show that during the primed decision stage the control group's taste-rating dependent P3 and LPP amplitudes increased whereas the HW group's were attenuated (Fig. 5), demonstrating the effect of HWs at the neural level.
Fig. 5.
Grand average waveforms of the P3 (250–450 ms) and LPP (450–750 ms) ERPs at midline parietal electrodes (CPz, Pz and POz) for (A) control group participants during the unprimed decision stage, (B) control group participants during the primed decision stage, (C) health warning group participants during the unprimed decision stage, and (D) health warning group participants during the primed decision stage. Blue lines denote trials featuring food items perceived as tasty, red lines denote trials featuring food items perceived as not tasty. Positive amplitudes plotted upwards.
3.5. Predicting dietary self-control from ERP components
Finally, we ran a linear mixed effects regression of the ERP components on our measure of DSC to identify whether the HW induced changes in neural processing of food stimulus characteristics predicted successful dietary self-control. In this model, the main effects of N1 (β = 0.07, SE = 0.12, t(3148) = 0.56, p = 0.579) and P3 (β = 0.11, SE = 0.14, t(3125) = 0.79, p = 0.431) amplitude on DSC did not reach significance. However, the effect of LPP amplitude (β = −0.25, SE = 0.12, t(3121) = 2.04, p = 0.041) on DSC was significant. These results indicate that late timeframe neural correlates of food cue reactivity reflected in the LPP were related to dietary self-control, with reduced LPP amplitude predicting increased self-control success.
4. Discussion
The present study provides evidence that food product health warnings can effectively prime healthy eating behaviour at the time of decision making. We found that a single, relatively brief session of health warning exposure promoted increased dietary self-control. Our behavioural data showed that before health warning exposure dietary choices relied almost exclusively on the taste attributes of food stimuli. The subsequent increase in dietary self-control that we observed after health warning exposure was related to both an increased likelihood of choosing healthy food items and a reduction in choosing palatable foods. We sought to better understand this relationship by investigating how health warnings influence neural processes that have been studied in the context of dietary self-control and food cue reactivity. While we found that the amplitude of the occipital N1 ERP component varied with the perceived health qualities of food items, this activity was invariant to health warning exposure and was not predictive of successful dietary self-control. However, differences in later ERP components (the parietal P3 and LPP) were observed in response to the perceived taste qualities of food stimuli, and the direction of this relationship was modulated by health warning exposure. Specifically, there was a marked difference between health warning groups in the change of P3 and LPP amplitudes related to stimulus taste attributes from the unprimed to the primed decision stage.
This study failed to find a connection between N1 amplitude and dietary self-control. There is some existing evidence that early differences in attention modulation can be associated with behavioural effects in complex (multi-attribute) decision making tasks (Wichary et al., 2017), including evidence that the N1 indexes stimulus-attribute-triggered facilitation of task relevant processing (Slagter et al., 2016). Nonetheless, studies investigating the relationship between stimulus-evoked N1 activity and dietary choice remain equivocal. Our results are in contrast to those reported by Harris et al. (2013) who found that participants, when incentivised to exercise dietary self-control, displayed heightened N1 amplitudes during failed dietary self-control, but reduced N1 amplitudes during successful dietary self-control (both relative to trials in which self-control was not required). They argued that their results reflect a suppression of attentional resources when presented with tempting food items, preventing further cognitive analysis and reducing craving. However, in line with a study by Toepel et al. (2009), who found that N1 related activity is sensitive to the caloric content of food stimuli and that this activity was orthogonal to the task being performed, we observed greater (more negative) N1 amplitudes when participants were presented with food items they perceived to be unhealthy. This finding possibly indicates that our participants initially failed to automatically filter these items from their attention, with the low perceived healthiness of these items instead heightening their attentional salience. Thus, although our N1 results do not clearly speak to the influence of health warnings on cognitive self-control processes, our results join other studies in supporting the notion that early occipital ERP responses are, in some cases, invariant to subjective, task-related goals (Meule et al., 2013; Toepel et al., 2012). The literature is inconclusive about the nature of this relationship, and to this end it is worth noting that Harris et al. (2013) provided participants a financial incentive to lose weight, and perhaps this engendered stronger healthy eating goals than health warnings (as in this study) or simple instruction (Meule et al., 2013). Direct manipulation of the strength of high-level decision goals in future research may elucidate the seeming disparity in the time course of decision-relevant attention modulation recorded across the literature.
While early attentional processes were not influenced by health warning exposure in this study, the P3 and LPP ERP components showed differential modulation in response to food images after health warning presentation. These ERP components have been shown to correlate with reported liking, craving, and hunger, in response to food cues, an effect that was not observed in response to control image cues (Nijs et al., 2008, Nijs et al., 2009, Nijs et al., 2010a; Nijs et al., 2010b; Stockburger et al., 2009). Additionally, studies investigating cue reactivity across a number of behaviors including illicit substance use (Littel et al., 2012), smoking (Piasecki et al., 2017), and alcohol use (Bartholow et al., 2007) have shown that these components are reliable indices of cue-evoked craving. In the present study, the health warning group displayed decreased P3 and LPP amplitudes in response to palatable food items after exposure to health warnings. This finding is consistent with evidence from the tobacco control literature, which demonstrated that the P3 evoked by smoking cues was attenuated when the cue was preceded by an anti-smoking health warning (Wang et al., 2013). Similarly, in the domain of dietary self-control, some studies have shown that that the food cue evoked P3 and LPP can be modulated by cognitive regulation strategies, such as attempts to suppress cravings or reappraise the outcome of consuming a given food item (Meule et al., 2013; Sarlo et al., 2013; Svaldi et al., 2015). This also is fully in line with our finding that the LPP in particular directly predicted success in dietary self-control. Together, these findings support the idea that health warning exposure facilitated a top-down reduction of the subjective craving response evoked by appetitive food cues.
To better understand the cognitive mechanisms underlying the reduced craving response that we observed, this finding is now discussed in the broader context of research characterising the P3 and LPP. Evidence from the highly studied “oddball” paradigm (Squires et al., 1975), in which a heightened P3 is observed in response to target stimuli presented amongst other stimuli, even when the probability distribution of the two stimulus types is equal (Duncan-Johnson and Donchin, 1977) but not when distracted (Hillyard et al., 1973), supports the notion that the P3 reflects the allocation of capacity-limited resources towards motivationally salient stimuli. Furthermore, the affective picture viewing literature has consistently shown that highly arousing stimuli, including images that are emotionally evocative or feature intrinsically rewarding subjects (such as food), produce an increased P3 response (Johnston et al., 1986; Olofsson et al., 2008). A related pattern of results has been observed for the LPP (Schupp et al., 2004), however, in the case of the LPP this relationship appears to be independent of perceptual stimulus characteristics (Bradley et al., 2007), does not habituate over time (Codispoti et al., 2006), and has been additionally linked to encoding and maintenance in working memory (Dolcos and Cabeza, 2002). Thus, the cue-evoked P3 is associated with a phasic increase in attention towards stimuli that are motivationally relevant (both due to intrinsic stimulus characteristics and dependent on the current task or goal), while the LPP appears to track sustained attention towards, and processing of, these stimuli in working memory. In the context of these prior findings, the P3 and LPP results reported here are consistent with the notion that health warnings were able to make healthy eating goals momentarily active, decreasing the motivational salience of highly palatable foods, and potentially promoting a reduction of the sustained attention and maintenance in working memory directed at these stimuli, which in turn might have promoted healthy dietary choices.
Notably, compared to the health warning group, the control group displayed reduced P3 and LPP amplitudes towards highly palatable foods in the first stage of the experiment. Comparable findings have been reported in a selection of studies (Bloom et al., 2013; Wölfling et al., 2008). When analysed at an aggregate level, as in the meta-analysis by Littel et al. (2012), this result falls within the expected distribution of effects as a result of random variation. This explanation is further supported by the fact that at this first stage – preceding health warning presentation – both groups were indeed only exposed to identical experimental stimulation. Some differences in individual baseline levels of the P3 and LPP could also be driven by the unique and subjective health and taste combinations of the items in each group (which were not necessarily balanced in this analysis). Importantly, in the present study, we were primarily concerned with the change in P3 and LPP amplitudes between the unprimed and primed experimental stages regardless of individual absolute baseline levels. Hence, by comparing the change in ERP amplitudes across experimental stages between the control and health warning group we are able to isolate the influence of health warning exposure on these components and their correlated processes. Control group participants displayed increased parietal P3 and LPP amplitudes in response to subjective stimulus palatability in the second stage of the experiment relative to the first stage. It is plausible that this was driven by increases in factors that are known to produce enlarged P3 and LPP amplitudes, such as subjective craving and hunger (Stockburger et al., 2009). An increase in reported craving over the duration of the experiment is commonly found in studies where participants view appealing food images (e.g. Meule et al., 2012). These results indicate that health warning exposure not only counteracted this effect, but also reversed it, constituting strong evidence that health warnings successfully counteract food cue evoked craving at the time of decision making.
Taken together, these findings indicate compelling support for the efficacy of health warning messages on dietary choice at a neural level that warrants future experimentation. Given the epidemic obesity prevalence recorded across much of the globe (Ng et al., 2014), we chose to test healthy participants here to investigate the ability of health warnings to play a preventative role in combatting obesity. It is pertinent to extend these findings to other populations, in light of evidence that individual level characteristics such as body weight (Nijs et al., 2008, Nijs et al., 2010a; Simmank et al., 2015) or eating-disorder status (Hume et al., 2016; Svaldi et al., 2015) can produce differential craving, neural, and behavioural responses to food cues and obesity prevention messages. Furthermore, in this experiment participants only saw a small selection of health warnings for a brief period of time. If food product health warnings were introduced on product packaging or at point of sale, it is possible that repeated exposure over time would produce stronger healthy eating goals, which are more likely to be internalised. It is therefore possible that a stronger priming effect than reported here could be expected if health warnings were to be introduced as an obesity prevention measure, potentially accompanied by a greater modulation of neural correlates of craving. In contrast, there is evidence that the effectiveness of health warnings diminishes over time, most likely due to habituation, so regular refreshment with new warnings is required to prevent this (Hitchman et al., 2014). As this study constituted a single testing session it was not possible to test this idea, which remains an interesting topic for future research.
In conclusion, this study provides initial evidence that food product health warnings can increase dietary self-control by reducing conditioned appetitive food cue responses at the time of decision making. These findings contribute to the existing literature investigating how external information influences cognitive and behavioural responses to food cues and lend credence to the contention that prime-based intervention strategies may make an effective contribution to curbing habitual or non-conscious drivers of unhealthy dietary behaviour, promote self-control, and potentially reduce rising obesity rates.
Clinical trial registration
Australian New Zealand Clinical Trials Registry (ANZCTR) retrospective registration number: ACTRN12617000129381. Registered 24 January 2017.
Author contributions
D.R., S.B., C.M., M.W. and H.D. designed experiments. D.R., P.S. and A.N. collected data. D.R. conducted data analysis. D.R. wrote the initial draft of the paper. All authors were involved in the interpretation of the analyses and writing the paper. All authors approved the final version of the paper.
Acknowledgments
Acknowledgements
We thank Sophia Bock and Maja Brydevall for help with participant recruitment and data acquisition, and Daniel Bennett, Bowen Fung, and Katharina Voigt for helpful discussions.
Funding
This work was funded by a Cancer Council Victoria Centre for Behavioural Research in Cancer Post Graduate Cancer Research Scholarship awarded to D.R. and an Australian Research Council Discovery Early Career Researcher Award (DE140100350) awarded to S.B.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
This is an ongoing project and data will be made available at its completion. Prior to this, data and materials are available from the corresponding author on request.
Ethics approval and consent to participate
The University of Melbourne Human Research Ethics Committee approved all study procedures (reference number 1443258), and all participants provided written informed consent.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.03.004.
Appendix A. Supplementary material
Supplementary material
References
- Asmaro D., Liotti M. High-caloric and chocolate stimuli processing in healthy humans: an integration of functional imaging and electrophysiological findings. Nutrients. 2014;6(1):319–341. doi: 10.3390/nu6010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow B.D., Henry E.A., Lust S.A. Effects of alcohol sensitivity on P3 event-related potential reactivity to alcohol cues. Psychol. Addict. Behav. 2007;21(4):555–563. doi: 10.1037/0893-164X.21.4.555. [DOI] [PubMed] [Google Scholar]
- Bates D., Kliegl R., Vasishth S., Baayen H. 2015. Parsimonious Mixed Models. (arXiv:1506.04967) [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67(1) [Google Scholar]
- Bilman E., van Kleef E., van Trijp H. External cues challenging the internal appetite control system—overview and practical implications. Crit. Rev. Food Sci. Nutr. 2017;57(13):2825–2834. doi: 10.1080/10408398.2015.1073140. [DOI] [PubMed] [Google Scholar]
- Bloom E.L., Potts G.F., Evans D.E., Drobes D.J. Cue reactivity in smokers: an event-related potential study. Int. J. Psychophysiol. 2013;90(2):258–264. doi: 10.1016/j.ijpsycho.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Hamby S., Löw A., Lang P.J. Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology. 2007;44(3):364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Brainard D.H. The psychophysics toolbox. Spat. Vis. 1997;10:443–446. [PubMed] [Google Scholar]
- Carbine K.A., Christensen E., LeCheminant J.D., Bailey B.W., Tucker L.A., Larson M.J. Testing food-related inhibitory control to high- and low-calorie food stimuli: electrophysiological responses to high-calorie food stimuli predict calorie and carbohydrate intake. Psychophysiology. 2017;10(2):168. doi: 10.1111/psyp.12860. [DOI] [PubMed] [Google Scholar]
- Codispoti M., Ferrari V., Bradley M.M. Repetitive picture processing: autonomic and cortical correlates. Brain Res. 2006;1068(1):213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cohen D.A., Lesser L.I. Obesity prevention at the point of purchase. Obes. Rev. 2016;17(5):389–396. doi: 10.1111/obr.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dolcos F., Cabeza R. Event-related potentials of emotional memory: encoding pleasant, unpleasant, and neutral pictures. Cogn. Affect. Behav. Neurosci. 2002;2(3):252–263. doi: 10.3758/cabn.2.3.252. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson C.C., Donchin E. On quantifying surprise: the variation of event-related potentials with subjective probability. Psychophysiology. 1977;14(5):456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Hammond D., Fong G.T., Borland R., Cummings K.M., McNeill A., Driezen P. Text and graphic warnings on cigarette packages. Am. J. Prev. Med. 2007;32(3):202–209. doi: 10.1016/j.amepre.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A., Hare T., Rangel A. Temporally dissociable mechanisms of self-control: early attentional filtering versus late value modulation. J. Neurosci. 2013;33(48):18917–18931. doi: 10.1523/JNEUROSCI.5816-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard S.A., Hink R.F., Schwent V.L., Picton T.W. Electrical signs of selective attention in the human brain. Science. 1973;182(4108):177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hitchman S.C., Driezen P., Logel C., Hammond D., Fong G.T. Changes in effectiveness of cigarette health warnings over time in Canada and the United States, 2002–2011. Nicotine Tob. Res. 2014;16(5):536–543. doi: 10.1093/ntr/ntt196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffmeijer R., Bakermans-Kranenburg M.J., Alink L.R.A., van IJzendoorn M.H. Physiol. Behav. 2014;130(C):13–22. doi: 10.1016/j.physbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Hume D.J., Howells F.M., Rauch H.G.L., Kroff J., Lambert E.V. Electrophysiological indices of visual food cue-reactivity. Differences in obese, overweight and normal weight women. Appetite. 2015;85(C):126–137. doi: 10.1016/j.appet.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Hume D.J., Howells F.M., Rauch H.G.L., Kroff J., Lambert E.V. Healthy restrained eaters diminish consummatory food reward and inhibit prepotent feeding responses: an EEG study. Ment Health Fam Med. 2016;12:181–191. [Google Scholar]
- Inauen J., Shrout P.E., Bolger N., Stadler G., Scholz U. Mind the gap? An intensive longitudinal study of between-person and within-person intention-behavior relations. Ann. Behav. Med. 2016;50(4):516–522. doi: 10.1007/s12160-016-9776-x. [DOI] [PubMed] [Google Scholar]
- Johnston V.S., Miller D.R., Burleson M.H. Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology. 1986;23(6):684–694. doi: 10.1111/j.1469-8986.1986.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Key A.P.F., Charboneau E.J., Cowan R.L. Handbook of Behavior. Springer New York; New York, NY: 2011. Food perception in adults: neuroimaging findings; pp. 515–530. [Google Scholar]
- Larsen J.K., Hermans R.C.J., Engels R.C.M.E. Food intake in response to food-cue exposure. Examining the influence of duration of the cue exposure and trait impulsivity. Appetite. 2012;58(3):907–913. doi: 10.1016/j.appet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Littel M., Euser A.S., Munafò M.R., Franken I.H.A. Electrophysiological indices of biased cognitive processing of substance-related cues: a meta-analysis. Neurosci. Biobehav. Rev. 2012;36(8):1803–1816. doi: 10.1016/j.neubiorev.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Luck S.J. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard O.M., Attwood A., O'Brien L., Brooks S., Hedge C., Leonards U., Munafò M.R. Avoidance of cigarette pack health warnings among regular cigarette smokers. Drug Alcohol Depend. 2014;1(136):170–174. doi: 10.1016/j.drugalcdep.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A., Skirde A.K., Freund R., Vögele C., Kübler A. High-calorie food-cues impair working memory performance in high and low food cravers. Appetite. 2012;59(2):264–269. doi: 10.1016/j.appet.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Meule A., Kübler A., Blechert J. Time course of electrocortical food-cue responses during cognitive regulation of craving. Front. Psychol. 2013;30(4):669–681. doi: 10.3389/fpsyg.2013.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.J., Beilharz J.E., Maniam J., Reichelt A.C., Westbrook R.F. Neurosci. Biobehav. Rev. 2015;58(C):36–45. doi: 10.1016/j.neubiorev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Murdaugh D.L., Cox J.E., Cook E.W., III, Weller R.E. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2012;59(3):2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health and Medical Research Council . NHMRC; Canberra: 2013. Australian Dietary Guidelines; pp. 1–226. [Google Scholar]
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs I.M.T., Franken I.H.A., Muris P. Food cue-elicited brain potentials in obese and healthy-weight individuals. Eat. Behav. 2008;9(4):462–470. doi: 10.1016/j.eatbeh.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Nijs I.M.T., Franken I.H.A., Muris P. Enhanced processing of food-related pictures in female external eaters. Appetite. 2009;53(3):376–383. doi: 10.1016/j.appet.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Nijs I.M.T., Franken I.H.A., Muris P. Food-related Stroop interference in obese and normal-weight individuals: behavioral and electrophysiological indices. Eat. Behav. 2010;11(4):258–265. doi: 10.1016/j.eatbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Nijs I.M.T., Muris P., Euser A.S., Franken I.H.A. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54(2):243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Nonnemaker J.M., Choiniere C.J., Farrelly M.C., Kamyab K., Davis K.C. Reactions to graphic health warnings in the United States. Health Educ. Res. 2015;30(1):46–56. doi: 10.1093/her/cyu036. [DOI] [PubMed] [Google Scholar]
- Olofsson J.K., Nordin S., Sequeira H., Polich J. Affective picture processing: an integrative review of ERP findings. Biol. Psychol. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki T.M., Fleming K.A., Trela C.J., Bartholow B.D. P3 event-related potential reactivity to smoking cues: relations with craving, tobacco dependence and alcohol sensitivity in young adult smokers. Psychol. Addict. Behav. 2017;31(1):61–72. doi: 10.1037/adb0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L. Sugar-sweetened beverage warning labels: lessons learned from the tobacco industry. J. Calif. Dent. Assoc. 2016;44(10):633–640. [PMC free article] [PubMed] [Google Scholar]
- Rastle K., Harrington J., Coltheart M. 358,534 nonwords: the ARC nonword database. Q. J. Exp. Psychol. A Hum. Exp. Psychol. 2002;55(4):1339–1362. doi: 10.1080/02724980244000099. [DOI] [PubMed] [Google Scholar]
- Rogers P.J., Hill A.J. Breakdown of dietary restraint following mere exposure to food stimuli: interrelationships between restraint, hunger, salivation, and food intake. Addict. Behav. 1989;14:387–397. doi: 10.1016/0306-4603(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Rosenblatt D.H., Bode S., Dixon H., Murawski C., Sumerell P., Ng A. and Wakefield M., Health warnings promote healthier dietary decision making: effects of positive versus negative message framing and graphic versus text-based warnings, (under review). [DOI] [PubMed]
- Sarlo M., Übel S., Leutgeb V., Schienle A. Cognitive reappraisal fails when attempting to reduce the appetitive value of food: an ERP study. Biol. Psychol. 2013;94(3):507–512. doi: 10.1016/j.biopsycho.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Junghöfer M., Weike A.I., Hamm A.O. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41(3):441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Sheeran P., Maki A., Montanaro E., Avishai-Yitshak A., Bryan A., Klein W.M.P. The impact of changing attitudes, norms, and self-efficacy on health-related intentions and behavior: a meta-analysis. Health Psychol. 2016;35(11):1178–1188. doi: 10.1037/hea0000387. [DOI] [PubMed] [Google Scholar]
- Simmank J., Murawski C., Bode S., Horstmann A. Incidental rewarding cues influence economic decisions in people with obesity. Front. Behav. Neurosci. 2015;9(1073):836. doi: 10.3389/fnbeh.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons W.K. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb. Cortex. 2005;15(10):1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Slagter H.A., Prinssen S., Reteig L.C., Mazaheri A. Facilitation and inhibition in attention: functional dissociation of pre-stimulus alpha activity, P1, and N1 components. NeuroImage. 2016;125(C):25–35. doi: 10.1016/j.neuroimage.2015.09.058. [DOI] [PubMed] [Google Scholar]
- Squires N.K., Squires K.C., Hillyard S.A. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr. Clin. Neurophysiol. 1975;38(4):387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Stockburger J., Schmälzle R., Flaisch T., Bublatzky F., Schupp H.T. The impact of hunger on food cue processing: an event-related brain potential study. NeuroImage. 2009;47(4):1819–1829. doi: 10.1016/j.neuroimage.2009.04.071. [DOI] [PubMed] [Google Scholar]
- van Strien T., Frijters J.E.R., Bergers G., Defares P. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986;5(2):295–315. [Google Scholar]
- Svaldi J., Tuschen-Caffier B., Biehl S.C., Gschwendtner K., Wolz I., Naumann E. Effects of two cognitive regulation strategies on the processing of food cues in high restrained eaters. An event-related potential study. Appetite. 2015;92(C):269–277. doi: 10.1016/j.appet.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Toepel U., Knebel J.-F., Hudry J., le Coutre J., Murray M.M. The brain tracks the energetic value in food images. NeuroImage. 2009;44(3):967–974. doi: 10.1016/j.neuroimage.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Toepel U., Knebel J.-F., Hudry J., le Coutre J., Murray M.M. Gender and weight shape brain dynamics during food viewing. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.-L., Romer D., Elman I., Turetsky B.I., Gur R.C., Langleben D.D. Emotional graphic cigarette warning labels reduce the electrophysiological brain response to smoking cues. Addict. Biol. 2013;20(2):368–376. doi: 10.1111/adb.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichary S., Magnuski M., Oleksy T., Brzezicka A. Neural signatures of rational and heuristic choice strategies: a single trial ERP analysis. Front. Hum. Neurosci. 2017;11:491. doi: 10.3389/fnhum.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfling K., Flor H., Grüsser S.M. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur. J. Neurosci. 2008;27(4):976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
This is an ongoing project and data will be made available at its completion. Prior to this, data and materials are available from the corresponding author on request.