Abstract
The association of adiposity across the life span with cardiometabolic risk is not completely delineated. We used a group-based modeling approach to identify distinct trajectories of body shape from ages 5 years to 55 years among 84,792 women from the Nurses’ Health Study (1976–2010) and 37,706 men from the Health Professionals Follow-up Study (1986–2010) and assessed the associations between these trajectories and incidence of type 2 diabetes and cardiovascular disease (CVD) during a 17-year follow-up period. Compared with those who maintained leanness throughout the life span (“lean-stable” trajectory), participants who maintained a medium body shape (“medium-stable” trajectory) had somewhat increased risk. Those who started lean but had a moderate or marked increase in adiposity (“lean-moderate increase” and “lean-marked increase” trajectories) had even higher risk (e.g., for a “lean-marked increase” trajectory, the hazard ratio for diabetes was 8.11 (95% confidence interval (95% CI): 7.10, 9.27) in women and 2.36 (95% CI: 2.04, 2.74) in men; for CVD, it was 1.38 (95% CI: 1.25, 1.52) in women and 1.28 (95% CI: 1.16, 1.41) in men). Participants who started heavy and became heavier (a “heavy-increase” trajectory) had substantially elevated risk (for diabetes, the hazard ratio was 7.34 (95% CI: 6.40, 8.42) in women and 2.80 (95% CI: 2.37, 3.31) in men; for CVD, it was 1.55 (95% CI: 1.40, 1.71) in women and 1.35 (95% CI: 1.20, 1.53) in men). Our data showed that trajectories of body shape from ages 5 to 55 years were associated with subsequent risk of developing type 2 diabetes and CVD.
Keywords: adiposity, cardiovascular diseases, diabetes mellitus, obesity
Obesity has become an epidemic for both children and adults in the United States, where about one-third of children (persons aged 2–19 years) and 70% of adults are either overweight or obese (1). Obesity plays a vital role in the pathogenesis of numerous comorbid conditions (2, 3); for example, it is the single most important risk factor for type 2 diabetes (4) and is a strong risk factor for cardiovascular disease (CVD) (5). The costs of obesity and its associated comorbidity are escalating, in terms of both health-care expenditures and quality of life, underscoring the importance of implementing effective prevention strategies.
The epidemic of obesity began in the 1980s and had been growing substantially until recent years (6, 7). The impact of obesity on health outcomes evolves gradually over the life course (8). Prospective studies have suggested that adiposity in childhood and adolescence is positively associated with adulthood risk of CVD and cardiovascular death (9–12). One cohort study revealed that adiposity in both adolescence and adulthood was associated with increased risk of coronary heart disease (CHD), while the risk of diabetes was mainly associated with adiposity close to the time of diagnosis (13). However, these studies did not take into account the potential effects of intraindividual changes in adiposity over time, which is important because a change in adiposity may lead to subsequent changes in clinical outcomes (14). Cross-sectional analyses have indicated that adiposity or body weight trajectories from childhood to early adulthood are associated with cardiometabolic profile in early adulthood (15, 16). To our knowledge, no prospective study has reported on how group-based trajectories of body shape from childhood to middle age are related to subsequent risk of cardiometabolic disease.
Previously, we demonstrated that trajectories of body shape from childhood to midlife were associated with subsequent risk of cancer and mortality (17, 18). In the current study, we examined trajectory patterns of long-term change in body shape from childhood to midlife in relation to subsequent risk of diabetes and CVD among women and men from 2 large, well-established US cohorts. By classifying participants into distinct, mutually exclusive trajectory groups, our study allowed for scrutiny of the population heterogeneity in the change in body shape across the life span and direct comparison of the disease risks across these groups.
METHODS
Study populations
We included data from 2 ongoing US cohort studies: the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). The NHS began in 1976 with the enrollment of 121,700 female nurses aged 30–55 years. The HPFS began in 1986 with the enrollment of 51,529 male health professionals aged 40–75 years. Participants in both cohort studies completed a baseline questionnaire on medication use, lifestyle, and medical history and were followed biennially through validated questionnaires that obtained updated information on the aforementioned areas. Follow-up rates were approximately 90% in each 2-year cycle for both cohorts. Both studies have been described in detail elsewhere (19, 20).
The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health (Boston, Massachusetts). Informed consent was indicated by return of the baseline questionnaire.
Assessment of body shape
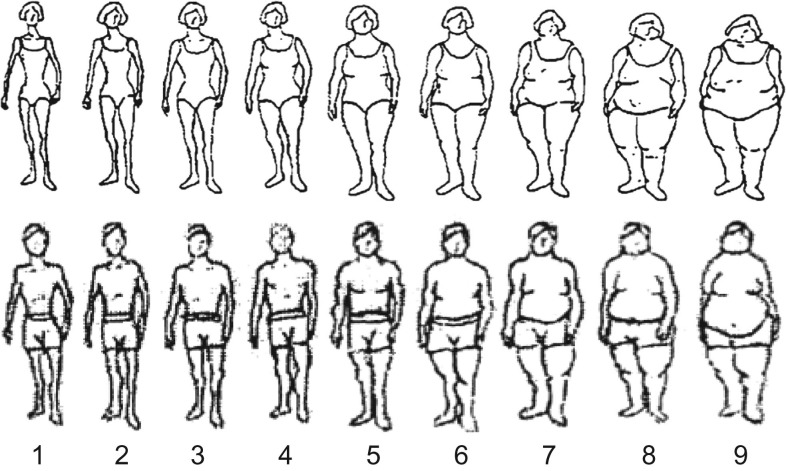
On the 1988 questionnaires, participants in both cohorts were asked to recall their body shape in early and middle life by choosing one of 9 pictorial body diagrams (somatotypes) (Figure 1) developed by Stunkard et al. (21). Participants were asked to select the diagram that best depicted their body outline at ages 5, 10, 20, 30, and 40 years. The validity of this measure of body shape was assessed among 181 participants aged 71–76 years in the Third Harvard Growth Study, where the participants’ recalled body shapes were compared with their measured body mass index (BMI; weight (kg)/height (m)2) at approximately the same ages (22). In women, the Pearson correlation coefficients were 0.60 for age 5 years, 0.65 for age 10 years, and 0.66 for age 20 years; in men, they were 0.36 for age 5 years, 0.66 for age 10 years, and 0.53 for age 20 years (22). We calculated BMI at ages 50 and 55 years using the reported body height and weight from updated follow-up questionnaires, and then converted BMI to the same scale as the somatotypes (see the Web Appendix, available at https://academic.oup.com/aje).
Figure 1.
Figure drawing used to assess body shape at ages 5, 10, 20, 30, and 40 years among women from the Nurses’ Health Study (1988) (A) and men from the Health Professionals Follow-up Study (1988) (B). (Reproduced from Stunkard et al. (21) with permission from Lippincott Williams & Wilkins, Philadelphia, Pennsylvania).
Ascertainment of outcomes and measurement of covariates
Participants with self-reported diagnoses of diabetes and CVD (including nonfatal and fatal myocardial infarction and stroke) on the main questionnaire were mailed a supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy (23). Information on potential confounders was collected via the biennial NHS and HPFS follow-up questionnaires. We generated an Alternate Healthy Eating Index score to evaluate diet quality, which has been significantly associated with CVD in our cohorts (24). Detailed information is presented in the Web Appendix.
Statistical analysis
We used a group-based trajectory modeling approach implemented in SAS Proc Traj (SAS Institute, Inc., Cary, North Carolina) to identify trajectory groups within each cohort that shared similar underlying trajectories of body shape from ages 5 to 55 years among 84,792 women from the NHS and 37,706 men from the HPFS who provided somatotype data for at least 4 different ages (25, 26). This method is designed to identify relatively homogeneous clusters of developmental trajectories within the population and has been successfully applied in large prospective cohort studies (17, 18, 27, 28). Participants were assigned to one of the trajectories to which they had the highest estimated probability of belonging. Based on the results from the trajectory analyses and the interpretability of the groups, we included 5 trajectories in subsequent analyses. More details about the trajectory analysis are provided in the Web Appendix. Web Figure 1 shows the process of participant selection.
We defined baseline as 1988 when body shape information was collected for the participants who were aged >55 years in 1988 and baseline as the year in which participants reached age 55 years for those who were aged ≤55 years in 1998, in order to avoid potential recall bias and survival bias. We calculated person-years from the baseline for each cohort to the date of incident diseases (type 2 diabetes or CVD, separately), death, or the end of follow-up (June 1, 2010, for NHS and January 31, 2010, for HPFS), whichever came first. We excluded persons who died or had a history of diagnosis of cancer, CVD, or diabetes (for diabetes analysis only) at baseline. We used Cox proportional hazards models to calculate univariable- and multivariable-adjusted hazard ratios and 95% confidence intervals for the risk of incident disease in relation to trajectory groups. We stratified on the age groups at the baseline of the cohort studies (i.e., 1976 in NHS and 1986 in HPFS). We adjusted for height, race, pack-years of smoking, regular aspirin use, menopausal hormone therapy (women only), physical activity, alcohol consumption, Alternate Healthy Eating Index score (possible range, 0–110; higher scores indicate better diet quality), and family history of disease in respective analysis (see details in the table footnotes). The proportional hazards assumption was evaluated with a likelihood ratio test comparing the models with and without an interaction term between age and each covariate (including trajectory groups), and there was no evidence suggesting that the proportional hazards assumption was violated (all P’s > 0.05 for the multivariable model).
Because smokers generally have lower adiposity and are at higher risk of chronic diseases, we examined effect modification by smoking and tested the statistical significance via a likelihood ratio test, by comparing the model with the product terms for the interaction between smoking (ever smokers vs. never smokers) and body shape trajectories (indicator variables for the 4 nonreference groups) with the model without these terms (i.e., degrees of freedom for the interaction = 4).
In a sensitivity analysis of CVD, we further adjusted for baseline hypertension, diabetes, and hypercholesterolemia. For CVD, we further analyzed the subtypes, including CHD (defined as nonfatal and fatal myocardial infarction) and stroke, separately. Other sensitivity analyses included 1) excluding persons who were underweight (BMI <17) in midlife to estimate the influence of underweight on our results; 2) excluding participants whose trajectory assignment probability was less than 0.80 to assess the robustness of the identified trajectories; 3) excluding the participants meeting either of the previous criteria; and 4) assessing whether the trajectory-disease associations were attenuated during the early 2000s (i.e., the end of follow-up was defined as 2000, 2005, and 2010 separately), a period in which there were significant changes in lifestyle and medication use (29).
Analyses were carried out with SAS software, version 9.3, at a 2-tailed α level of 0.05.
RESULTS
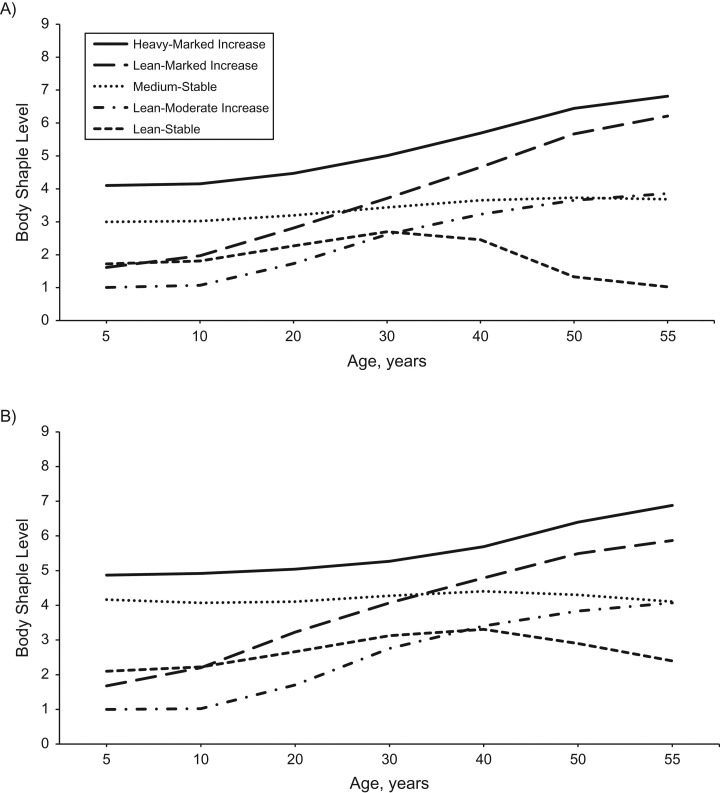
We identified 5 distinct trajectories of body shape from age 5 years to age 55 years (Figure 2): Approximately 15% of women and 17% of men had a “lean-stable” trajectory, where they maintained a lean body shape across the life span; 23% of women and 18% of men had a “lean-moderate increase” trajectory, where they started out lean and then experienced a moderate increase in body adiposity in adulthood; 18% of women and 35% of men had a “lean-marked increase” trajectory, where they started out lean and then gained a substantial amount of weight in adulthood; 30% of women and 17% of men had a “medium-stable” trajectory, where they maintained a medium body shape throughout life; and 13% of women and men had a “heavy-increase” trajectory, where they started out heavy and then gained additional weight during adulthood.
Figure 2.
Trajectories of body shape by age among women from the Nurses’ Health Study (1976–2010) (A) and men from the Health Professionals Follow-up Study (1986–2010) (B). We identified distinct trajectories of body fatness across the life span using a group-based modeling approach. In 1988, participants in both cohorts were asked to recall their body shapes in early and middle life by choosing one of 9 pictorial body diagrams (Figure 1) that best depicted their body outline at ages 5, 10, 20, 30, and 40 years. We calculated body mass index (weight (kg)/height (m)2) at ages 50 and 55 years and then converted body mass index to the same scale as that used at younger ages. Participants included prevalent cases of cancer, cardiovascular disease, and diabetes at age 55 years.
At baseline of the current study, compared with the participants in the “lean-stable” group, those in the other groups were more likely to be hypertensive, to be diabetic, to have elevated blood levels of cholesterol, and to have prevalent CVD in general (Tables 1 and 2). Compared with participants in the “stable” (“lean-stable” and “medium-stable”) groups, those in the “increase” (“lean-moderate increase,” “lean-marked increase,” and “heavy-increase”) groups were less likely to be physically active or to have a healthy diet (Tables 1 and 2).
Table 1.
Age-Standardized Characteristics of Female Participants at Age 55 Years, by Body Shape Trajectory Over the Life Course, Nurses’ Health Study (n = 84,792), 1976a
| Characteristic | Trajectory of Body Shape From Age 5 Years to Age 55 Years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lean-Stable (n = 12,861) | Medium-Stable (n = 25,652) | Lean-Moderate Increase (n = 19,324) | Lean-Marked Increase (n = 15,612) | Heavy-Increase (n = 11,343) | ||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Physical activity, MET-hours/week | 19.5 (22.0) | 17.0 (18.1) | 16.0 (18.4) | 13.0 (15.0) | 12.3 (13.7) | |||||
| Alcohol intake, g/day | 7.7 (10.6) | 7.1 (10.1) | 6.3 (9.4) | 4.6 (8.3) | 4.3 (8.1) | |||||
| Never smoker | 41.3 | 41.4 | 45.5 | 48.7 | 42.6 | |||||
| Pack-years of smoking | 14.7 (20.2) | 14.3 (19.7) | 12.7 (18.6) | 11.9 (18.7) | 14.5 (20.4) | |||||
| AHEI score | 44.3 (9.60) | 44.5 (9.0) | 42.9 (8.8) | 42.2 (8.4) | 42.8 (8.7) | |||||
| Current aspirin user | 41.7 | 46.1 | 45.8 | 53.1 | 55.9 | |||||
| Current MHT user | 44.2 | 41.1 | 38.8 | 31.3 | 31.0 | |||||
| Family history of diabetes | 23.4 | 27.3 | 30.1 | 36.6 | 36.8 | |||||
| Family history of CHD | 24.3 | 26.1 | 26.8 | 28.6 | 29.3 | |||||
| Hypertension | 20.4 | 27.3 | 32.2 | 49.3 | 53.8 | |||||
| Type 2 diabetes | 1.9 | 2.5 | 3.7 | 10.8 | 13.8 | |||||
| Hypercholesterolemia | 20.6 | 25.8 | 28.3 | 30.8 | 29.1 | |||||
| Cancer | 4.0 | 4.1 | 4.7 | 5.0 | 4.8 | |||||
| Cardiovascular disease | 4.0 | 4.7 | 5.7 | 9.1 | 9.1 | |||||
Abbreviations: AHEI, Alternate Healthy Eating Index; CHD, coronary heart disease; MET, metabolic equivalent of task; MHT, menopausal hormone therapy; SD, standard deviation.
a Data were standardized to the age distribution of the study population. Prevalent cases of chronic disease at baseline were included.
Table 2.
Age-Standardized Characteristics of Male Participants at Age 55 Years, by Body Shape Trajectory Over the Life Course, Health Professionals Follow-up Study (n = 37,636), 1986a
| Characteristic | Trajectory of Body Shape From Age 5 Years to Age 55 Years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lean-Stable (n = 6,378) | Medium-Stable (n = 6,298) | Lean-Moderate Increase (n = 6,927) | Lean-Marked Increase (n = 13,327) | Heavy-Increase (n = 4,706) | ||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Physical activity, MET-hours/week | 29.2 (28.9) | 31.5 (29.1) | 26.5 (26.1) | 25.9 (25.8) | 25.4 (26.1) | |||||
| Alcohol intake, g/day | 11.1 (14.7) | 11.5 (15.1) | 11.4 (14.6) | 11.6 (15.2) | 10.6 (14.1) | |||||
| Never smoker | 50.6 | 44.7 | 45.2 | 44.0 | 40.2 | |||||
| Pack-years of smoking | 11.6 (18.3) | 13.5 (19.3) | 13.4 (19.1) | 14.1 (19.5) | 16.5 (21.5) | |||||
| AHEI score | 42.1 (10.3) | 42.4 (9.8) | 41.0 (9.9) | 40.2 (9.6) | 41.2 (9.8) | |||||
| Current aspirin user | 44.6 | 49.4 | 50.1 | 51.4 | 54.7 | |||||
| Family history of diabetes | 13.5 | 15.0 | 15.8 | 16.8 | 20.1 | |||||
| Family history of CHD | 31.8 | 33.7 | 33.2 | 34.0 | 37.0 | |||||
| Hypertension | 17.2 | 19.9 | 23.6 | 28.9 | 32.3 | |||||
| Type 2 diabetes | 2.1 | 2.5 | 3.7 | 5.1 | 7.1 | |||||
| Hypercholesterolemia | 21.5 | 21.2 | 26.1 | 26.6 | 25.6 | |||||
| Cancer | 4.5 | 4.6 | 4.9 | 4.8 | 5.5 | |||||
| Cardiovascular disease | 7.6 | 8.9 | 9.2 | 11.1 | 12.5 | |||||
Abbreviations: AHEI, Alternate Healthy Eating Index; CHD, coronary heart disease; MET, metabolic equivalent of task; SD, standard deviation.
a Data were standardized to the age distribution of the study population. Prevalent cases of chronic disease at baseline were included.
Trajectory of body shape and type 2 diabetes risk
A total of 69,598 women and 30,910 men were included in the diabetes analysis, after exclusion of prevalent cases of cancer, diabetes, or CVD at baseline. We identified 6,250 incident cases of type 2 diabetes among women over a median follow-up period of 17 years (1,127,128 person-years in total) and 2,208 incident cases among men over a median follow-up period of 16 years (476,178 person-years in total) (Table 3).
Table 3.
Hazard Ratios for Type 2 Diabetes by Trajectory of Body Shape Over the Life Course (Ages 5–55 Years) Among Women (n = 69,598) From the Nurses’ Health Study (1976–2010) and Men (n = 30,910) From the Health Professionals Follow-up Study (1986–2010)a
| Body Shape Trajectory | No. of Participants | No. of Cases | Incidence Rateb | Age-Stratified HRc | 95% CI | Multivariate HRc,d | 95% CI |
|---|---|---|---|---|---|---|---|
| Women (NHS) | |||||||
| Lean-stable | 11,049 | 246 | 127.7 | 1.00 | Referent | 1.00 | Referent |
| Medium-stable | 21,961 | 1,036 | 282.5 | 2.21 | 1.92, 2.54 | 2.14 | 1.86, 2.46 |
| Lean-moderate increase | 16,043 | 1,458 | 541.9 | 4.26 | 3.72, 4.88 | 3.90 | 3.40, 4.46 |
| Lean-marked increase | 11,983 | 2,138 | 1,219.5 | 9.59 | 8.40, 10.95 | 8.11 | 7.10, 9.27 |
| Heavy-increase | 8,562 | 1,372 | 1,112.8 | 8.75 | 7.64, 10.03 | 7.34 | 6.40, 8.42 |
| Men (HPFS) | |||||||
| Lean-stable | 5,468 | 211 | 242.8 | 1.00 | Referent | 1.00 | Referent |
| Medium-stable | 5,385 | 220 | 262.5 | 1.08 | 0.89, 1.30 | 1.08 | 0.89, 1.30 |
| Lean-moderate increase | 5,646 | 402 | 430.7 | 1.77 | 1.50, 2.09 | 1.68 | 1.42, 1.98 |
| Lean-marked increase | 10,703 | 979 | 610.5 | 2.52 | 2.17, 2.92 | 2.36 | 2.04, 2.74 |
| Heavy-increase | 3,708 | 396 | 764.9 | 3.15 | 2.67, 3.73 | 2.80 | 2.37, 3.31 |
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; MET, metabolic equivalent of task; NHS, Nurses’ Health Study.
a Baseline participants with cancer, cardiovascular disease, or diabetes were excluded.
b Incidence rate per 100,000 person-years.
c HRs were estimated from a Cox proportional hazards model stratified on age group in 1976 (NHS) or 1986 (HPFS).
d Multivariable models adjusted for height (m; continuous), race (nonwhite or white), pack-years of smoking (women: 0, 1–5, 6–20, or >20; men: 0, 1–4, 5–25, 26–45, or >45), regular aspirin use (yes or no), menopausal hormone therapy (women only; past use, current use, or none), physical activity (MET-hours/week, in quintiles), alcohol consumption (women: 0–0.4, 0.5–1.9, 2.0–7.9, or ≥8.0 g/day; men: 0–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, or ≥30.0 g/day), Alternate Healthy Eating Index score (possible range, 0–110; in quintiles), and family history of diabetes (yes or no).
In the univariable analysis, compared with the “lean-stable” group (the reference group), participants in the other groups showed a generally increased risk of developing type 2 diabetes, except the “medium-stable” group in men. The results were moderately attenuated after adjustment for confounding factors, but the groups with increased adiposity still had an increased risk. In the greater adiposity groups, as compared with the “lean-stable” group, the hazard ratios for diabetes among women ranged from 2.14 (95% confidence interval (95% CI): 1.86, 2.46) in the “medium-stable” group to 8.11 (95% CI: 7.10, 9.27) in the “lean-marked increase” group, and those among men ranged from 1.08 (95% CI: 0.89, 1.30) in the “medium-stable” group to 2.80 (95% CI: 2.37, 3.31) in the “heavy-increase” group (Table 3).
We further examined whether smoking modified the association of body adiposity with diabetes risk (Web Table 1). In both women and men, trajectories of increasing adiposity were more strongly associated with increased diabetes risk among never smokers than among ever smokers, although the interaction test did not achieve statistical significance (in both sexes, P > 0.05).
The results remained essentially unchanged in the sensitivity analyses excluding participants who were underweight in midlife and/or excluding participants who had a <80% probability of belonging to a trajectory group (Web Table 2). Despite substantial lifestyle changes and increases in medication use since the early 2000s, the trajectory-diabetes association actually increased over the follow-up period (Web Table 3).
Trajectory of body shape and CVD
A total of 72,989 women and 31,970 men were included in the CVD (including CHD and stroke) analysis, after exclusion of persons with prevalent cancer or CVD at baseline. We observed 5,105 incident cases of CVD over a median follow-up period of 18 years (1,209,378 person-years in total) among women and 4,002 incident cases over a median of 16 years (496,651 person-years in total) among men (Table 4).
Table 4.
Hazard Ratios for Cardiovascular Disease by Trajectory of Body Shape Over the Life Course (Ages 5–55 Years) Among Women From the Nurses’ Health Study (1976–2010) and Men From the Health Professionals Follow-up Study (1986–2010)a
| Body Shape Trajectory | No. of Participants | No. of Cases | Incidence Rateb | Age-Stratified HRc | 95% CI | Multivariate HRc,d | 95% CI |
|---|---|---|---|---|---|---|---|
| Women (NHS) | |||||||
| Cardiovascular disease | |||||||
| Lean-stable | 11,183 | 742 | 384.3 | 1.00 | Referent | 1.00 | Referent |
| Medium-stable | 22,381 | 1,317 | 351.0 | 0.98 | 0.90, 1.07 | 0.97 | 0.89, 1.06 |
| Lean-moderate increase | 16,534 | 1,279 | 450.2 | 1.20 | 1.09, 1.31 | 1.18 | 1.08, 1.29 |
| Lean-marked increase | 13,201 | 979 | 468.8 | 1.45 | 1.32, 1.60 | 1.38 | 1.25, 1.52 |
| Heavy-increase | 9,690 | 788 | 531.9 | 1.69 | 1.53, 1.87 | 1.55 | 1.40, 1.71 |
| Coronary heart disease | |||||||
| Lean-stable | 11,199 | 351 | 179.6 | 1.00 | Referent | 1.00 | Referent |
| Medium-stable | 22,418 | 641 | 168.7 | 1.01 | 0.88, 1.14 | 0.99 | 0.87, 1.13 |
| Lean-moderate increase | 16,565 | 631 | 218.6 | 1.24 | 1.09, 1.41 | 1.21 | 1.06, 1.38 |
| Lean-marked increase | 13,245 | 528 | 248.5 | 1.62 | 1.42, 1.86 | 1.49 | 1.30, 1.71 |
| Heavy-increase | 9,717 | 448 | 297.6 | 1.99 | 1.73, 2.29 | 1.75 | 1.52, 2.02 |
| Stroke | |||||||
| Lean-stable | 11,192 | 424 | 217.1 | 1.00 | Referent | 1.00 | Referent |
| Medium-stable | 22,401 | 726 | 191.4 | 0.95 | 0.84, 1.07 | 0.95 | 0.84, 1.07 |
| Lean-moderate increase | 16,543 | 710 | 246.2 | 1.16 | 1.03, 1.31 | 1.16 | 1.03, 1.31 |
| Lean-marked increase | 13,240 | 504 | 237.0 | 1.31 | 1.15, 1.49 | 1.29 | 1.13, 1.47 |
| Heavy-increase | 9,714 | 371 | 245.8 | 1.40 | 1.22, 1.61 | 1.33 | 1.16, 1.54 |
| Men (HPFS) | |||||||
| Cardiovascular disease | |||||||
| Lean-stable | 5,559 | 607 | 700.7 | 1.00 | Referent | 1.00 | Referent |
| Medium-stable | 5,491 | 601 | 710.9 | 1.11 | 0.99, 1.24 | 1.10 | 0.98, 1.23 |
| Lean-moderate increase | 5,820 | 861 | 902.9 | 1.19 | 1.07, 1.32 | 1.16 | 1.05, 1.29 |
| Lean-marked increase | 11,162 | 1,470 | 878.6 | 1.32 | 1.20, 1.45 | 1.28 | 1.16, 1.41 |
| Heavy-increase | 3,938 | 463 | 831.9 | 1.43 | 1.27, 1.61 | 1.35 | 1.20, 1.53 |
| Coronary heart disease | |||||||
| Lean-stable | 5,564 | 420 | 478.1 | 1.00 | Referent | 1.00 | Referent |
| Medium-stable | 5,496 | 439 | 512.8 | 1.18 | 1.03, 1.35 | 1.17 | 1.02, 1.34 |
| Lean-moderate increase | 5,824 | 640 | 660.1 | 1.28 | 1.13, 1.45 | 1.25 | 1.10, 1.41 |
| Lean-marked increase | 11,170 | 1,116 | 657.3 | 1.45 | 1.30, 1.62 | 1.40 | 1.25, 1.57 |
| Heavy-increase | 3,942 | 354 | 627.1 | 1.58 | 1.37, 1.82 | 1.49 | 1.29, 1.72 |
| Stroke | |||||||
| Lean-stable | 5,567 | 220 | 247.4 | 1.00 | Referent | 1.00 | Referent |
| Medium-stable | 5,497 | 180 | 208.3 | 0.92 | 0.76, 1.12 | 0.92 | 0.75, 1.12 |
| Lean-moderate increase | 5,831 | 264 | 267.9 | 1.00 | 0.83, 1.19 | 0.98 | 0.82, 1.18 |
| Lean-marked increase | 11,183 | 419 | 241.9 | 1.03 | 0.87, 1.21 | 1.01 | 0.86, 1.19 |
| Heavy-increase | 3,951 | 121 | 210.9 | 1.03 | 0.83, 1.29 | 1.00 | 0.80, 1.25 |
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; MET, metabolic equivalent of task; NHS, Nurses’ Health Study.
a Baseline participants with cancer or cardiovascular disease were excluded.
b Incidence rate per 100,000 person-years.
c HRs were estimated from a Cox proportional hazards model stratified on age group in 1976 (NHS) or 1986 (HPFS).
d Multivariable models adjusted for height (m; continuous), race (nonwhite or white), pack-years of smoking (women: 0, 1–5, 6–20, or >20; men: 0, 1–4, 5–25, 26–45, or >45), regular aspirin use (yes or no), menopausal hormone therapy (women only; past use, current use, or none), physical activity (MET-hours/week, in quintiles), alcohol consumption (women: 0–0.4, 0.5–1.9, 2.0–7.9, or ≥8.0 g/day; men: 0–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, or ≥30.0 g/day), Alternate Healthy Eating Index score (possible range, 0–110; in quintiles), and family history of diabetes (yes or no).
Compared with those in the “lean-stable” group, participants in the groups with greater body adiposity showed an increased cardiovascular risk in general, except for women in the “medium-stable” group. In general, the associations of body shape trajectory with CVD risk were comparable between women and men (hazard ratios ranged from 0.97 (95% CI: 0.89, 1.06) in the “medium-stable” group in women to 1.55 (95% CI: 1.40, 1.71) in the “heavy-increase” group in women). Further adjustment for baseline hypertension, diabetes, and hypercholesterolemia only moderately attenuated the associations (data not shown).
In the multivariable model for CHD, consistently across men and women, participants in the “heavy-increase” group had the highest risk (hazard ratios were 1.75 (95% CI: 1.52, 2.02) for women and 1.49 (95% CI: 1.29, 1.72) for men) in comparison with the “lean-stable” group; those in the “lean-marked increase” group had the second-highest risk and those in the “lean-moderate increase” group had the third highest, followed by the “medium-stable” group. The results for stroke were similar to the CHD findings but showed weaker associations among women and failed to reach significance levels among men (Table 4).
The associations of body shape trajectories with cardiovascular risk among female never smokers were stronger than those among female ever smokers, although such associations seemed stronger among male ever smokers than among male never smokers in the “medium-stable” and “lean moderate-increase” groups (Web Table 1). Smoking significantly modified the association of body shape trajectory with cardiovascular risk (for overall interaction, P < 0.05 among women and men). Excluding participants who were underweight and/or had a low trajectory probability did not change the results materially (Web Table 2). The strength of the trajectory-CVD association increased gradually across the follow-up period (Web Table 3).
DISCUSSION
In 2 large cohorts of women and men, we identified 5 heterogeneous groups of body-adiposity trajectories over the course of 55 years from early childhood to middle adulthood. In general, women and men who maintained a lean body shape throughout the life span had the lowest risk, whereas those who had substantially increased body adiposity had the highest risk of developing diabetes and CVD. These findings extend our knowledge about obesity over the life course in relation to cardiometabolic disease, and they support the vital role of adiposity accumulation across the life span in disease pathogenesis.
Overweight and obesity pose a major challenge to chronic disease prevention and overall public health. In our study, most participants gained adiposity throughout early childhood and midlife. Changes of body adiposity in different life stages—for example, rapid accumulation of fat during childhood (30) and modest weight gain during adulthood (31–34)—have been associated with increased cardiometabolic risk. Interestingly, one study suggested that the potential health effects of childhood obesity can be offset by weight loss before or during adulthood (35). However, the cumulative effect of adiposity over time has yet to be thoroughly determined. In a study on the 1946 British birth cohort, Charakida et al. (36) retrospectively linked lifelong BMI patterns to carotid intima-media thickness and found that longer exposure to high adiposity in adulthood had a cumulative adverse effect on cardiovascular phenotype in later life. Thus far, prospective evidence on lifelong obesity and cardiometabolic risk is still lacking.
Overweight and obesity are the most important risk factors for type 2 diabetes (4). In our study, adiposity seemed to have a greater deleterious association with diabetes risk in women than in men. Women who either started out lean and markedly gained adiposity or started out heavy and gained further adiposity had an 8-fold increase in the risk of developing diabetes, compared with those who were lean all the time. Even women who maintained a medium body shape had more than double the risk of developing diabetes compared with women who maintained a lean body shape. A previous study showed that obese women had more than a 20-fold risk of diabetes, and even those who had a BMI within the normal range (23–24.9) had twice the risk of diabetes, compared with those who had a BMI less than 23 (37). Body shape trajectories in men were also significantly related to diabetes risk in our study, although the magnitude of such an association was weaker than that in women. In the current analysis, the difference in the strength of association between the sexes might partially reflect both the higher BMI in the greater body shape groups in women and the higher incidence rate in the lean-stable group in men; however, sex does play a role in obesity. A high body weight may implicate more adiposity in women and more muscularity in their male counterparts. Women are disproportionately affected by extreme obesity compared with men, regardless of age or race/ethnicity (38). However, previous studies suggested that adult men develop type 2 diabetes at lower BMI levels than women, because men have higher waist circumferences and are more insulin-resistant than women at equivalent BMIs (39, 40). In the present study, we measured body shape by means of pictorial body diagrams, which may be more reflective of body fat distribution than BMI measurement.
Consistent with previous studies showing direct associations between adiposity at different ages and CVD risk (5), our data showed that increased adiposity at most life stages was related to a higher risk of CVD, except for women who maintained medium adiposity. Increased BMI has been consistently related to a higher risk of both incident CHD and CHD mortality in 3 large meta-analyses (41–43), although there is less evidence for stroke. In our study, the association of body shape trajectories with CHD risk seemed stronger than that with stroke risk, and this is consistent with previous evidence from studies using individual adiposity measurements taken at different life stages (5, 44).
Smoking is a critical and complicated confounder of the association between adiposity and cardiometabolic diseases. Smokers tend to weigh less than nonsmokers because nicotine increases energy expenditure and could reduce appetite, but smokers have a much higher risk of disease development. This could combine to produce an artificially elevated disease risk among lean individuals (e.g., the reference group in our study population) and could partially explain in our study why the body shape trajectories were more strongly associated with cardiovascular risk in never smokers than in ever smokers among women. Smoking may also promote central fat accumulation (45–47), and smoking cessation is frequently followed by weight gain (48), which can further confound the association between body shape and cardiometabolic risk.
The strengths of this investigation include a novel statistical method for examining body shape trajectory patterns in 2 large, well-defined cohort studies with long follow-up periods. In our 2 independent cohorts, we observed similar body shape trajectories in both sexes. To our knowledge, this is the first study that has explored the influence of lifelong body shape trajectory on cardiometabolic risk among US women and men, and it provides a life-course perspective on adiposity and cardiometabolic disease prevention. Nevertheless, the findings of the current study should be interpreted in light of its limitations. First, because we used self-reported body shape to estimate adiposity, measurement errors were inevitable. However, due to the limited sample size in a previously published validation study (22), we were unable to correct the measurement error by regression calibration. Nevertheless, given the prospective study design, such misclassifications were likely to have been nondifferential and thus might have biased our relative risk estimates toward the null. Furthermore, the consistent results from the sensitivity analysis excluding participants with a relatively low probability for trajectory assignment (<80%) indicated that our findings were robust to modest trajectory misclassification. Second, we did not distinguish between intentional and unintentional weight changes. Nevertheless, we excluded patients with chronic diseases at baseline in the main analysis, and we excluded underweight participants from the sensitivity analysis. Such strategies may have partially accounted for unintentional weight change. Moreover, our study participants were predominantly white, and the generalizability of our findings to other populations may be limited. A previous study showed that the association between adiposity trajectory and mortality in older Japanese was different from that in the US population (28). More data on diabetes and CVD from other racial/ethnic groups are needed. Finally, we acknowledge the general limitations of the 2-step regression approach in our analysis, such as loss of efficiency and the fact that the residual variances in the first regression are carried into the second regression (49).
In conclusion, we identified 5 distinct trajectories of body shape across the life span and found distinct patterns of cardiometabolic risk across these trajectories. Our results suggest that increased body adiposity throughout the early and middle stages of life is associated with greater risk of cardiometabolic diseases in later adulthood, especially for persons who are heavy at a young age and gain further weight in adulthood. This study underscores the importance of early prevention of excess weight gain and obesity throughout the life span.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Yan Zheng, Mingyang Song, Edward L. Giovannucci, Frank B. Hu); State Key Laboratory of Genetic Engineering, School of Life Sciences, Fudan University, Shanghai, China (Yan Zheng); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (JoAnn E. Manson, Edward L. Giovannucci, Frank B. Hu); Clinical and Translational Epidemiology Unit and Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts (Mingyang Song); Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (JoAnn E. Manson); and Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (JoAnn E. Manson, Edward L. Giovannucci, Frank B. Hu).
This study was supported by the National Institutes of Health (grants UM1 CA186107, R01 HL034594, R01 HL088521, UM1 CA167552, and R01 HL35464). M.S. is a trainee at the Harvard Transdisciplinary Research Center on Energetics and Cancer. Y.Z. was supported by a fellowship from the American Diabetes Association (grant 7-12-MN-34).
The funders played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- HPFS
Health Professionals Follow-up Study
- NHS
Nurses’ Health Study
REFERENCES
- 1. American Heart Association Obesity information. http://www.heart.org/HEARTORG/GettingHealthy/WeightManagement/Obesity/Obesity-Information_UCM_307908_Article.jsp#.VjD8KCuwQwg. Updated October 18, 2016. Accessed November 16, 2016.
- 2. Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: World Health Organization; 2009. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. Accessed July 24, 2016. [Google Scholar]
- 4. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9(1):13–27. [DOI] [PubMed] [Google Scholar]
- 5. Hu FB. Obesity Epidemiology. New York, NY: Oxford University Press; 2008. [Google Scholar]
- 6. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 8. Preston SH, Mehta NK, Stokes A. Modeling obesity histories in cohort analyses of health and mortality. Epidemiology. 2013;24(1):158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Twig G, Yaniv G, Levine H, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016;374(25):2430–2440. [DOI] [PubMed] [Google Scholar]
- 10. Li L, Pinot de Moira A, Power C. Predicting cardiovascular disease risk factors in midadulthood from childhood body mass index: utility of different cutoffs for childhood body mass index. Am J Clin Nutr. 2011;93(6):1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lai CC, Sun D, Cen R, et al. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64(15):1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magnussen CG, Koskinen J, Chen W, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study. Circulation. 2010;122(16):1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364(14):1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Becque MD, Katch VL, Rocchini AP, et al. Coronary risk incidence of obese adolescents: reduction by exercise plus diet intervention. Pediatrics. 1988;81(5):605–612. [PubMed] [Google Scholar]
- 15. Araujo J, Severo M, Barros H, et al. Developmental trajectories of adiposity from birth until early adulthood and association with cardiometabolic risk factors. Int J Obes (Lond). 2015;39(10):1443–1449. [DOI] [PubMed] [Google Scholar]
- 16. Attard SM, Herring AH, Howard AG, et al. Longitudinal trajectories of BMI and cardiovascular disease risk: the National Longitudinal Study of Adolescent Health. Obesity (Silver Spring). 2013;21(11):2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song M, Hu FB, Wu K, et al. Trajectory of body shape in early and middle life and all cause and cause specific mortality: results from two prospective US cohort studies. BMJ. 2016;353:i2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. [DOI] [PubMed] [Google Scholar]
- 20. Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 21. Stunkard AJ, Sorenson T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness In: Kety SS, Rowland LP, Sidman RL, et al., eds. The Genetics of Neurological and Psychiatric Disorders. New York, NY: Raven Press; 1983:115–120. [PubMed] [Google Scholar]
- 22. Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. [DOI] [PubMed] [Google Scholar]
- 23. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97(1):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Method Res. 2001;29(3):374–393. [Google Scholar]
- 26. Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Method Res. 2007;35(4):542–571. [Google Scholar]
- 27. Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311(5):490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murayama H, Liang J, Bennett JM, et al. Trajectories of body mass index and their associations with mortality among older Japanese: do they differ from those of Western populations? Am J Epidemiol. 2015;182(7):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314(17):1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eriksson JG, Kajantie E, Lampl M, et al. Trajectories of body mass index amongst children who develop type 2 diabetes as adults. J Intern Med. 2015;278(2):219–226. [DOI] [PubMed] [Google Scholar]
- 31. Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–486. [DOI] [PubMed] [Google Scholar]
- 32. Koh-Banerjee P, Wang Y, Hu FB, et al. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159(12):1150–1159. [DOI] [PubMed] [Google Scholar]
- 33. Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the “normal” weight range. JAMA. 1995;273(6):461–465. [DOI] [PubMed] [Google Scholar]
- 34. Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141(12):1117–1127. [DOI] [PubMed] [Google Scholar]
- 35. Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. [DOI] [PubMed] [Google Scholar]
- 36. Charakida M, Khan T, Johnson W, et al. Lifelong patterns of BMI and cardiovascular phenotype in individuals aged 60–64 years in the 1946 British birth cohort study: an epidemiological study. Lancet Diabetes Endocrinol. 2014;2(8):648–654. [DOI] [PubMed] [Google Scholar]
- 37. Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. [DOI] [PubMed] [Google Scholar]
- 38. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Logue J, Walker JJ, Colhoun HM, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sattar N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract Res Clin Endocrinol Metab. 2013;27(4):501–507. [DOI] [PubMed] [Google Scholar]
- 41. Asia Pacific Cohort Studies Collaboration Body mass index and cardiovascular disease in the Asia-Pacific region: an overview of 33 cohorts involving 310 000 participants. Int J Epidemiol. 2004;33(4):751–758. [DOI] [PubMed] [Google Scholar]
- 42. McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15(2):87–97. [DOI] [PubMed] [Google Scholar]
- 43. Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167(16):1720–1728. [DOI] [PubMed] [Google Scholar]
- 44. He Y, Jiang B, Wang J, et al. BMI versus the metabolic syndrome in relation to cardiovascular risk in elderly Chinese individuals. Diabetes Care. 2007;30(8):2128–2134. [DOI] [PubMed] [Google Scholar]
- 45. Kim JH, Shim KW, Yoon YS, et al. Cigarette smoking increases abdominal and visceral obesity but not overall fatness: an observational study. PLoS One. 2012;7(9):e45815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lv J, Chen W, Sun D, et al. Gender-specific association between tobacco smoking and central obesity among 0.5 million Chinese people: the China Kadoorie Biobank Study. PLoS One. 2015;10(4):e0124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Canoy D, Wareham N, Luben R, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes Res. 2005;13(8):1466–1475. [DOI] [PubMed] [Google Scholar]
- 48. Aubin HJ, Farley A, Lycett D, et al. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Szpiro AA, Paciorek CJ. Measurement error in two-stage analyses, with application to air pollution epidemiology. Environmetrics. 2013;24(8):501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.