Abstract
Aims
Dysfunctional brain reward circuitry, particularly in the nucleus accumbens (NAcc), has been proposed as a risk factor for alcohol use disorder (AUD). This risk factor may be evident in binge drinkers (BD), who are at high risk for developing AUD. We examined whole-brain and NAcc reactivity to reward in BD compared to non-binge drinkers (NBD), hypothesizing that groups would differ in their neural reactivity and connectivity.
Methods
Healthy BD (N = 27) and NBD (N = 23)—none meeting AUD criteria—completed a reward-guessing game, the ‘Doors’ task, during functional magnetic resonance imaging. We conducted an exploratory whole-brain search for group differences, but given our a priori hypotheses, we also extracted activation from the NAcc to examine reactivity during reward (Win > Loss) and functional connectivity (FC) to the prefrontal cortex.
Results
Compared to NBD, BD exhibited greater activation in both the right and left NAcc during reward relative to loss. Additionally, NBD drinkers exhibited positive FC between the NAcc and dorsal anterior cingulate (dACC) whereas the BD showed negative FC between these regions. Furthermore, less NAcc–dACC FC was related to more past month alcohol use.
Conclusions
Our results provide preliminary evidence that BD exhibit greater NAcc activation during reward receipt relative to loss. This is consistent with the broader AUD literature and suggests aberrant neural reactivity may precede disorder onset. In addition, BD exhibited less NAcc–dACC FC, perhaps reflecting deficient regulation of activation to rewards compared to losses. This profile of reward brain circuitry could represent neural correlates of vulnerability for AUD.
Short summary
Healthy binge drinkers, at risk for alcohol use disorder, exhibited greater nucleus accumbens activation during reward relative to loss. In addition, binge drinkers exhibited reduced connectivity between the nucleus accumbens and dorsal anterior cingulate, which was associated with more past month alcohol use.
INTRODUCTION
Binge drinking is prevalent in the United States, and is characterized by the intake of large quantities of alcohol (at least 4–5 drinks) in a short period of time (NIAAA, 2004; Courtney and Polich, 2009). Importantly, binge drinking is a risk factor for developing alcohol use disorder (AUD) (Chassin et al., 2002) and is associated with poor psychosocial, cognitive and health outcomes (Jennison, 2004; Courtney and Polich, 2009; CDC, 2016). Given the substantial burden related to AUD (Rehm et al., 2009; Mokdad et al., 2016), it is critical to better understand the predictors of the disorder, including neural mechanisms associated with binge drinking. Such knowledge will help to target interventions and prevention strategies for those most at-risk of developing AUD.
Brain reward circuitry is implicated in AUD. Notably, alcohol acts in the striatum, including the nucleus accumbens (NAcc) (Volkow et al., 2007; Mitchell et al., 2012), which underlies the rewarding effect of alcohol, the development of incentive salience and drug-seeking behaviors (Koob and Volkow, 2016). Over time, repeated alcohol exposure causes neuroadaptations, dysregulating brain reward circuitry and resulting in compulsive and excessive alcohol use (Koob and Volkow, 2016). Indeed, individuals with AUD differ in their neural responses to reward compared to healthy controls, especially in the NAcc (Koob and Volkow, 2016). Some evidence demonstrates individuals with AUD show increased striatal activation to monetary reward (Bjork et al., 2008b) and alcohol-related cues (Braus et al., 2001; Myrick et al., 2004; Wrase et al., 2002, 2007) compared to healthy controls. However, other studies found no differences in striatal activation to monetary reward outcomes in abstinent individuals with AUD compared to healthy controls (Forbes et al., 2014) or in individuals with a family history of alcoholism (Andrews et al., 2011), indicating the data is mixed. A recent meta-analysis found individuals with substance use disorders (SUD) evidence blunted ventral striatum activation during reward anticipation and enhanced ventral striatum activation during reward outcome (Luijten et al., 2017).
In addition to findings of aberrant NAcc reactivity, evidence suggests dysfunctional connectivity with reward circuitry may contribute to the development of AUD (Sutherland et al., 2012). One study found abstinent individuals with AUD exhibit greater negative functional connectivity than healthy controls between the bilateral NAcc and areas of the prefrontal cortex (medial prefrontal cortex (mPFC), lateral orbitofrontal cortex (OFC), and dorsolateral prefrontal cortex (dlPFC)) during monetary reward receipt (Forbes et al., 2014). Additionally, accumulating evidence indicates resting-state connectivity is disrupted between the NAcc and areas of the prefrontal cortex (PFC) (Hong et al., 2009; Ma et al., 2010; Camchong et al., 2013; Cservenka et al., 2014; Wilcox et al., 2011; Motzkin et al., 2014) and structural connectivity is disrupted in reward regions (Squeglia et al., 2015) in at-risk youth and individuals with SUD. Some of these studies show decreased connectivity between the NAcc and the PFC (dorsal anterior cingulate (dACC), frontal operculum and dlPFC) (Hong et al., 2009; Ma et al., 2010; Motzkin et al., 2014; Wilcox et al., 2011), while one study show no group differences (Gu et al., 2010) among individuals with SUD and healthy controls. To date, however, few studies have examined whether neural abnormalities are observed within at-risk individuals.
There is some evidence that disrupted brain reward circuitry predates alcohol use and is a risk factor for alcohol initiation. Adolescents at risk for substance use show hyperactive brain reward circuitry to rewards (Bjork et al., 2010; Ivanov et al., 2012; Stice et al., 2013; Stice and Yokum, 2014). However, the specific reward regions implicated are mixed, as many studies did not look at the NAcc specifically, and other studies fail to detect these effects (Bjork et al., 2008a; Muller et al., 2015). In a combined fMRI and PET study of adolescents examining neural response to monetary reward outcome, at-risk adolescents had similar striatal BOLD response to monetary reward, but demonstrated more dopamine release in the NAcc during monetary reward compared to low risk individuals (Weiland et al., 2017). Further, greater BOLD activation and greater dopamine release in the NAcc were related to experiencing drunkenness at a younger age (Weiland et al., 2017). Together, these data suggest at-risk adolescents exhibit some disruption of NAcc reward circuitry and this disruption may be related to onset and/or chronicity of alcohol use.
Healthy, young adult binge drinkers (BD) offer a unique population to study whether brain reward circuitry is disrupted before the development of AUD. They are at-risk for AUD and for other poor health, psychosocial, and cognitive outcomes (Wechsler et al., 1994; Jennison, 2004). To our knowledge only one other study has examined neural activation to receipt of rewarding outcomes in BD, and this study was in adolescents (Cservenka et al., 2015). As such, the goal of the current study was to understand if reward brain circuitry is disrupted among young adult BD who are at-risk for AUD, but who have not yet developed the disorder. We hypothesized BD would display greater NAcc response and decreased NAcc-prefrontal connectivity to monetary reward relative to non-binge drinkers (NBD), in line with previous SUD studies. Given our a priori hypotheses, we first examined whole-brain group differences in activation during reward and then used a region-of-interest (ROI) approach to examine group differences in reactivity to reward within the NAcc. If our results revealed significant group differences in NAcc reactivity, we planned to examine group differences in functional connectivity (FC) between the NAcc and the prefrontal cortex during reward receipt.
METHODS
Design
These data were drawn from a larger study examining relationships between neural reactivity and subjective and objective responses to drugs and alcohol. For the parent study, participants first completed acute drug challenges of d-amphetamine or alcohol—data from these visits were not included in the current study. All participants then attended a separate visit at least a week later for an fMRI scan, during which they completed a reward-guessing game, the ‘Doors’ task.
Participants
Participants were right-handed, healthy young adults aged 21–31 recruited from nearby college campuses and surrounding communities through online and printed advertisements. Overall, 25 individuals who did not report any binge drinking episodes (i.e. 4+ drinks in a 2-h period for women or 5+ drinks in a 2-h period for men; NIAAA, 2004; Courtney and Polich, 2009) in the last month (NBD) and 29 individuals who reported at least 1 binge drinking episode in the past month (BD) were included. Inclusion criteria included body mass index between 19 and 26, at least a high school education, English fluency, no current or past year DSM-IV diagnosis, no lifetime history of SUD, no serious medical conditions, no night shift work, negative urine drug screen at fMRI visit and no contraindication for fMRI. Participants were excluded if they reported smoking >5 cigarettes per day, daily use of any medications other than birth control, or if they were pregnant, lactating or planning to become pregnant in the next 3 months.
Study procedure
Participants completed an initial screening and orientation visit during which they provided informed consent and completed the Timeline Followback interview (Sobell et al., 1986) to record alcohol use in the past month. Participants were also asked to report the frequency and amount of alcohol use during their period of heaviest drinking. Participants completed drug administration sessions for the parent study at the University of Chicago (UofC) and then attended a separate fMRI visit at the University of Illinois of Chicago (UIC) 1–2 weeks later. Before the scan participants were screened for MRI safety and provided breath and urine samples to test for recent alcohol and drug use. The Institutional Review Board at UofC and UIC approved the study and written informed consent was obtained. Participants were compensated for their participation.
Reward task and data acquisition
Participants completed a reward-guessing game, the ‘Doors’ task, during the scan. The Doors task provides an index of reactivity to monetary rewards and losses. Participants were told that behind one of the doors there was a monetary prize of $0.50 (‘↑’) while behind the other door there was a loss of $0.25 (‘↓’) and that they should use a button box to choose one of the two doors to either win or lose money for each trial (Supplemental Fig. 1). They were told they had a chance of winning between $0 and $15.00 at the end of the task depending on their performance. However, unbeknownst to participants, the task was rigged, so task behavior had no impact on actual outcomes and therefore was not analyzed or reported. The task consisted of 30 predetermined Wins and 30 Losses presented in a pseudorandom order over two runs. The task lasted for 15-min and is based on a task used in previous studies (Hajcak et al., 2006; Foti and Hajcak, 2009; Carlson et al., 2011) (see Supplement for more information).
Functional MRI data was collected using a 3 T GE magnetic resonance scanner at the UIC Center for Magnetic Resonance Research. Functional images were acquired using a gradient-echo echo-planar images (2 s TR, 25ms TE, 82° flip, 64 × 64 matrix, 200 mm FOV, 3 mm slice thickness, 0 mm gap, with 44 axial slices).
fMRI data analyses
All data were inspected and any individual with >2 mm displacement in any one direction were not included in the analysis resulting in four individuals being excluded (two NBD and two BD) and a total sample size of 50 (23 NBD and 27 BD). Remaining subjects met criteria for high quality and scan stability. There were no significant group differences in peak movements, mean movement, or variability during either run (P > 0.05). Preprocessing of fMRI data was conducted using Statistical Parametric Mapping software (SPM12, Wellcome Department of Imaging Neuro-Science, London, UK). Images were spatially realigned, slice-time corrected, warped to Montreal Neurological Institute (MNI) space using the participant’s mean functional image, resampled to 2 mm3 voxels, and smoothed (8 mm3 kernel). The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Condition effects were modeled with event-related regressors representing the occurrence of Win or Loss. Effects were estimated at each voxel, and for each subject. Individual contrast maps for Win trials (>Loss trials) were created for each person. Individual motion parameter files were included in the first levels models as regressors-of-no-interest.
To confirm that the task successfully activated reward-related regions, including the NAcc, during Win > Loss trials, we examined whole-brain task activation across all subjects. Due to concerns of high rates of false positives with lenient significance thresholds and following recent guidelines (Woo et al., 2014; Eklund et al., 2016), neural activity from task effects was considered significant if it exceeded correction of multiple comparisons across the entire brain (e.g. a whole-brain gray matter mask [volume=1,459,304 mm3]) as determined via simulation using the 3dClustSim utility (10,000 iterations); updated and ‘bug-free’ on December 2015; [https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html]; (Cox, 1996). Significance at corrected α < 0.05 and a voxel threshold of P < 0.001 yielded a minimum cluster size of at least 120 contiguous voxels (volume=960 mm3).
To test our hypotheses, we first conducted a whole-brain 2 (group) × 1 ANCOVA for Win > Loss, covarying for gender. We then conducted a planned ROI analysis in which BOLD signal activation during Win > Loss was extracted for each subject using left and right NAcc anatomical masks, defined via the AAL atlas and created using MARINA (http://www.bion.de/Marina.htm; (Walter et al., 2003). The parameter estimates/β-weights were extracted for each participant from NAcc ROIs representing BOLD signal response (parameter estimates, arbitrary units [a.u.]) averaged across all voxels within the anatomical masks. Group differences were compared in SPSS using two ANOVAs (one for left NAcc and one for right NAcc) with group (BD and NBD), gender (male and female) and the interaction of group and gender as independent variables and the extracted BOLD signals (β-weights) as the dependent variables.
Next, to examine NAcc functional coupling during Win > Loss, we used a generalized form of context-dependent psychophysiological interaction analyses (gPPI; http://brainmap.wisc.edu/PPI, (McLaren et al., 2012). The same anatomical left and right NAcc masks, described above, were used as the seeds-of-interest (SOIs). The de-convolved time series for each SOI were extracted for each subject to create the physiological variable. Condition onset times for Win, Loss and Fix events were separately convolved with the canonical hemodynamic response function for each condition, creating the psychological regressors. Finally, interaction terms (PPIs) were computed by multiplying the time series by the physiological variables. All physiological, psychological, and PPI terms were included as regressors in individual first-level models. Contrast images for Win > Loss were created for each subject and entered into second-level 2 (group) by 1 ANCOVAs, covarying for gender, for the left and right NAcc to determine whether there were group differences in NAcc FC. Given our a priori hypotheses, analyses were restricted to a predetermined anatomical mask consisting of the mPFC, OFC, anterior cingulate and dorsal cingulate; regions with known projections to the NAcc (Haber and Knutson, 2010; Britt et al., 2012) and implicated in reward (Haber and Knutson, 2010) and addiction (Goldstein and Volkow, 2011). To correct for multiple comparisons, joint height and extent thresholds were determined via Monte Carlo simulations (10,000 iterations) using the 3dClustSim software described above and at corrected α < 0.05 and a voxel threshold of P < 0.001 yielded a minimum cluster size of at least 21 contiguous voxels (volume=168 mm3) for both the right and left NAcc FC. To detect the direction of group effects for the PPI analyses, we extracted parameter estimates/β-weights representing connectivity strength averaged across all voxels within a 10 mm radius sphere surrounding the peak activation/connectivity clusters of each participant.
RESULTS
Group differences
As shown in Table 1, BD were younger than NBD, but the groups did not differ on gender, ethnicity or race. Groups also did not differ on prevalence or cigarette use rate or marijuana use frequency in the past month. However, as expected, BD drank significantly more total drinks within the past month and reported more drinks per week than NBD, highlighting meaningful differences in drinking behaviors across the two groups. Of note, four NBD (17%) reported having a period in their life that they met criteria for binge drinking; however, on average this period was 5 years ago (range: 2–10 years ago) and they reported not bingeing since then. Therefore, although the groups were defined by binge drinking in the past month, NBD’s current non-binge drinking pattern generally reflects their non-binge drinking pattern historically.
Table 1.
Participant characteristics
| Non-binge group, n = 23 | Binge group, n = 27 | P-value | |
|---|---|---|---|
| Age | 25.70 (2.95) | 24.00 (2.24) | 0.03 |
| Gender (% Female) | 43% | 44% | 0.59 |
| Ethnicity (% Hispanic) | 13% | 15% | 0.57 |
| Race | 0.16 | ||
| % Caucasian | 74% | 47% | |
| % More than 1 Race | 13% | 19% | |
| % African-American | 13% | 19% | |
| % Asian | 0% | 15% | |
| Number of binges in past month | – | 4.15 (2.63) | – |
| Average drinks per week | 3.14 (2.28) | 11.42 (6.51) | <0.001 |
| Total drinks in past month | 12.54 (9.12) | 45.67 (26.02) | <0.001 |
| % Smoked > 1 cigarette in past month | 26% | 33% | 0.76 |
| Average number of cigarettes per day | 0.81 (1.57) | 1.13 (2.15) | 0.76 |
| % Smoked Marijuana in past month | 30% | 48% | 0.14 |
| Frequency of Marijuana use* | 6.50 (9.42) | 4.27 (5.18) | 0.46 |
Note: All values are means and standard deviations unless otherwise noted; P < 0.05. *Frequency defined as number of occasions within the past 30 days.
Task activation in NAcc and whole-brain group differences during reward
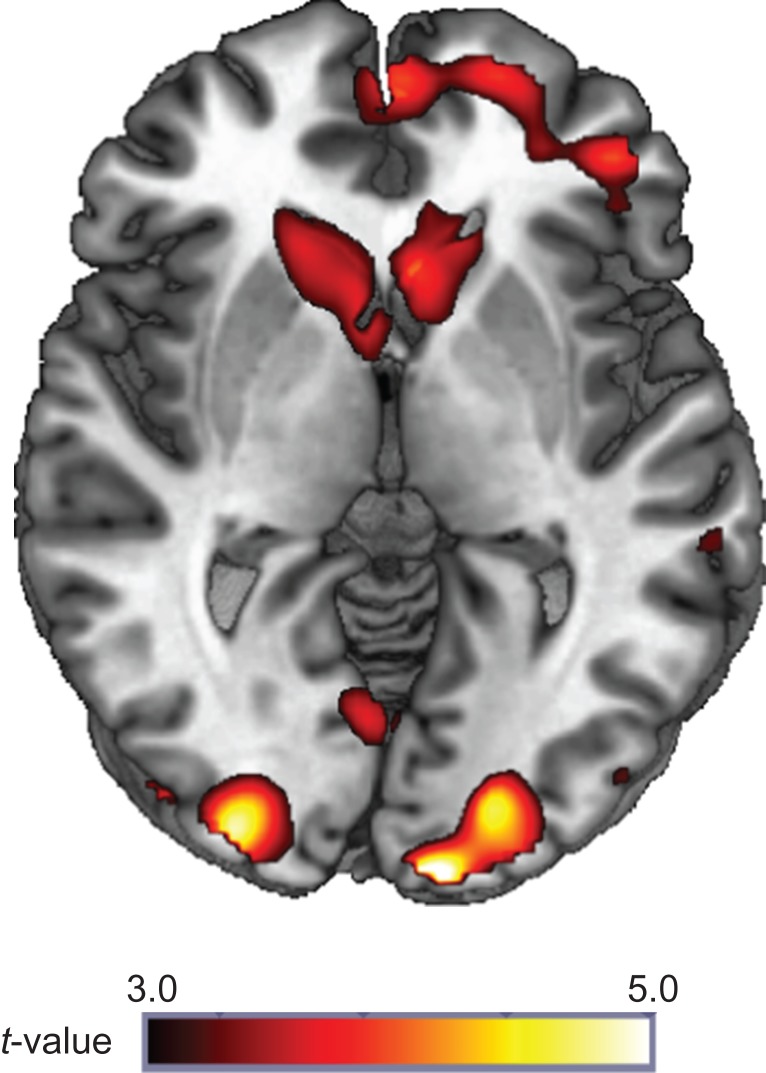
Results indicated that reward receipt relative to loss (Win > Loss) significantly activated a large contiguous cluster of frontal and mesolimbic reward regions, including bilateral NAcc, caudate and putamen (peak MNI [30, 30, 42], k = 13,611 voxels, Z = 5.02, P < 0.05, corrected). Mesolimbic activation during reward is illustrated in Fig. 1. All significant whole-brain peak clusters are shown in Table 2. Whole-brain search for group differences in activation during reward was not significant.
Fig. 1.
Whole-brain task activation during reward. Whole-brain task activation (P < 0.05, corrected) for all subjects to reward (Win > Loss trials).
Table 2.
Whole-brain task activation for reward
| Lobe | MNI coordinates | Z score | Voxels | ||
|---|---|---|---|---|---|
| x | y | z | (k) | ||
| Frontal/subcortical | |||||
| Contiguous cluster extending from the bilateral middle frontal gyrus to the caudate | 30 | 30 | 42 | 5.02 | 13,611 |
| Parietal/temporal | |||||
| L Angular gyrus extending to the middle temporal gyrus | −40 | −60 | 36 | 4.32 | 924 |
| Occipital | |||||
| L Cuneus, middle occipital gyrus | −8 | −102 | 4 | 5.09 | 513 |
| R Middle and interior occipital gyrus, R Cuneus | 30 | −94 | 4 | 4.87 | 1089 |
Note: Reporting of all significant peak voxels at P < 0.05 whole-brain corrected with a cluster size of > 120 contiguous voxels. L = Left; R = Right; MNI = Montreal Neurologic Institute.
NAcc ROI analyses
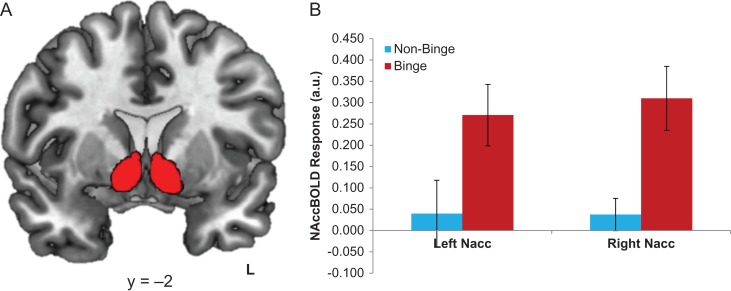
BD exhibited greater activation in both the left and right NAcc during reward receipt relative to loss compared to NBD (F(3,47) = 4.83, P = 0.03; F(3,47) = 6.07, P = 0.02; respectively) (Fig. 2).
Fig. 2.
Group differences in region-of-interest (ROI) NAcc BOLD reward reactivity. (A) Left and right NAcc ROI used (B) Extracted response (in β-weights/parameter estimates of activation) for each group from the left and right NAcc activation to reward (Win > Loss).
PPI analyses
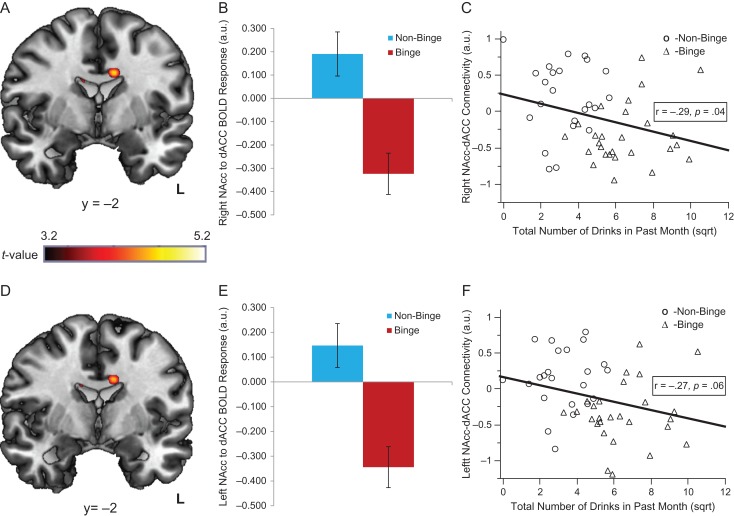
The gPPI FC analyses revealed BD and NBD displayed divergent patterns of NAcc FC during reward relative to loss (Fig. 3). Specifically, BD exhibited reduced, negative bilateral NAcc–dACC FC compared with NBD, who showed positive NAcc–dACC FC during reward (left: MNI peak [−10, −2, 32], k = 36 voxels, Z = 3.81, P < 0.05, corrected and MNI peak [10, −16, 28], k = 23 voxels, Z = 3.55, P < 0.05 corrected; right: MNI peak [−10, −2, 32], k = 40 voxels, Z = 3.85, P < 0.05, corrected and MNI peak [12, −22, 30], k = 51 voxels, Z = 3.71, P < 0.05 corrected; Fig. 3). There were no other significant findings within the PFC mask. Scatterplots depicting how groups differ on NAcc–dACC FC and NAcc activation are shown in Supplemental Fig. 3.
Fig. 3.
Group differences in NAcc–dACC functional connectivity during reward. (A and D) Activation in the dACC, where Binge drinkers displayed negative right NAcc to bilateral dACC functional connectivity (A) and negative left NAcc to bilateral dACC functional connectivity (D), while non-binge drinkers showed positive connectivity between these regions (P < 0.05, corrected). (B) Group differences in extracted right NAcc to dACC BOLD functional connectivity. (E) Group differences in extracted left NAcc to dACC BOLD functional connectivity. (C and F) The relationship between past month drinking and right and left NAcc–dACC BOLD functional connectivity, respectively.
Relationship between neural activation and drinking behavior
We ran correlations among extracted bilateral NAcc activation during reward relative to loss, extracted bilateral NAcc–dACC FC activation during reward relative to loss, and total number of drinks in the past month (square root transformed). Results indicated left and right NAcc activation during reward relative to loss was not significantly related to past month drinking (r = 0.18, P = 0.21; r = 0.21, P = 0.14, respectively). On the other hand, less right NAcc–dACC FC activation was associated with greater total drinks in the past month (r = −0.29, P = 0.04) and the relationship between less left NAcc–dACC FC activation and more total drinks in the past month trended toward significance (r = −0.27, P = 0.06) (Fig. 3).
Post-hoc analyses controlling for age and post-hoc analyses examining NAcc activation, as well as NAcc FC activation during Win > Fix and Loss > Fix can be found in the supplement.
DISCUSSION
Altered brain reward circuitry has been implicated in individuals with AUD, but little is known about whether these alterations predate AUD, or whether they are present in at-risk current drinkers, such as BD. In this study, we examined reactivity to reward within the NAcc, a key node in the brain reward circuitry, and FC with the NAcc during reward in healthy at-risk BD compared to NBD. We found preliminary evidence that BD had more activation in the left and right NAcc during reward receipt relative to loss than NBD. Post-hoc analyses showed that this was driven by the difference between Win and Loss events, as neither condition alone significantly differed by group. In addition, BD displayed negative bilateral NAcc–dACC FC, while NBD displayed positive bilateral NAcc–dACC FC during reward receipt. Post-hoc analyses demonstrated that in addition to the difference between Win and Loss events in FC, BD had less FC between the right NAcc and right dACC and between the right NAcc and right superior frontral gyrus during Win > Fix compared to NBD. Furthermore, NAcc–dACC FC during Win > Loss was related to past month drinking, such that less right NAcc–dACC FC was associated with greater total drinks in the past month and this effect trended toward significance for left NAcc–dACC FC. This extends previous findings of greater activation in the striatum during alcohol cues and monetary reward receipt (Braus et al., 2001; Myrick et al., 2004; Wrase et al., 2002, 2007) and greater negative FC between the NAcc and prefrontal regions during monetary reward receipt among individuals with AUD (Forbes et al., 2014). It is also consistent with lower resting-state FC between the NAcc and the dACC in individuals with SUD (Hong et al., 2009; Motzkin et al., 2014). Thus, we show similar patterns of neural reactivity to reward in healthy, non-dependent young adult BD, who are at-risk for AUD, and found initial evidence that patterns of neural activation were related to past month drinking.
It is important to note that our primary findings were based on an ROI approach and were not based on whole-brain corrected analysis. In fact, we did not find whole-brain corrected group differences in NAcc activation to reward receipt. This is not unusual, several previous studies use ROI approaches (Forbes et al., 2014), as current standards for whole-brain correction are conservative (Woo et al., 2014; Eklund et al., 2016) and often require large sample sizes to find effects. The present findings are therefore considered preliminary and require replication with larger samples.
Our results provide preliminary evidence that BD exhibit a stronger neural response to reward receipt relative to loss in the NAcc, a region implicated in the development and maintenance of AUD (Koob and Volkow, 2016). A larger difference in activation to rewards compared to losses may contribute to the continuation of risky drinking, eventually leading to AUD. Specifically, greater neural reactivity to rewards relative to losses may lead to increased hedonia and subjective pleasure from rewards (like alcohol), and/or insensitivity to losses resulting in excessive reward seeking and increased risk-taking to pursue rewards including alcohol consumption. Of note, several factors may moderate this effect, including genes, gender, sex-steroids, family history and personality traits (Andrews et al., 2011; Braams et al., 2016; Nikolova et al., 2013) and require further study.
We also observed inverse connectivity between the NAcc and the dACC, a region involved in cognitive control of behavior and emotion, in the two groups. BD showed negative NAcc–dACC FC, which may reflect deficient regulation of reward, as the NAcc and dACC circuit is implicated in affective regulation and the top-down inhibition of limbic regions (Haber and Knutson, 2010). Indeed, we found less NAcc–dACC connectivity is related to more drinks in the past month, suggesting NAcc–dACC connectivity plays an important role in modulating drinking behavior. Therefore, the dACC may have an impaired ability to modulate reward reactivity in the NAcc, leading to greater reward reactivity in the NAcc in BD. It is also possible that BD’s greater reward reactivity in the NAcc may overwhelm the circuit and thus the modulation of the NAcc by the dACC is not strong enough to counteract the NAcc response.
Among individuals with AUD, less fronto-striatal FC during response inhibition is related to greater severity of AUD, indicating that strength of fronto-striatal circuitry is an important factor in the progression of AUD (Courtney et al., 2013). Furthermore, previous evidence that reduced resting-state connectivity between the NAcc and dACC was associated with greater severity of nicotine dependence (Hong et al., 2009; Motzkin et al., 2014), indicates that NAcc–dACC connectivity may play an important role in addiction more generally and this may be especially true during reward and/or loss. It may be that decreased NAcc–dACC connectivity during reward receipt relative to loss reflects vulnerability for AUD, as BD may have difficulty appropriately engaging circuitry involved in reward and the regulation of behavior and emotion. It is also possible that dACC inhibitory inputs to the NAcc are a result of the neurotoxic effects of alcohol and this circuitry becomes more disrupted as alcohol use progresses.
To our knowledge, this is one of the first studies to examine disruptions in brain reward circuitry in non-dependent, healthy young adults at-risk for AUD. Our findings suggest disruptions in reward-related circuitry are present in healthy, high-risk individuals even before they develop AUD. However, the etiology of these disruptions is not known and it is possible the BD’s prior heavy alcohol use has caused alterations in their brain function. Thus, the observed findings may due to the neuroadaptations or neurotoxic effects of alcohol rather than being pre-existing risk factors. Future prospective studies following individuals who are initially alcohol naive will be critical in delineating the extent to which aberrant neural reward reactivity is a risk factor, disease marker or scar.
The study also has limitations. First, the sample size was relatively small, limiting statistical power to find subtle effects. In addition, the study focused on participants’ self-reported current drinking. Although lifetime SUD was exclusionary, other aspects of their prior substance use were not captured (e.g. age of first drink, extended periods of heavy drinking and lifetime drinks) and may have influenced the results. While the groups did not differ significantly in their use of other drugs (e.g. nicotine and marijuana), it is possible that BD differed on drug use measures not assessed here. Furthermore, we did not collect data on BOLD response to reward anticipation, making it difficult to compare our findings to other studies of AUD and risk for AUD (Heitzeg et al., 2015). Moreover, we did not collect self-report measures of task engagement, which could affect the results. We also did not collect family history of AUD, which has been shown to influence NAcc activation to rewards, NAcc connectivity, and structural connectivity of reward regions (Heitzeg et al., 2015). We would expect that BD are more likely to have a family history of AUD, but since this information was not captured, it is possible that the group differences we found may be more robust had we controlled for family history. Finally, gPPI analyses are correlational, so the directionality of NAcc–dACC connectivity cannot be determined.
CONCLUSIONS
Our study provides preliminary evidence that individuals at high risk for AUD display a neural pattern that mirrors individuals with active SUD such that they have greater NAcc reactivity, and reduced NAcc–dACC connectivity, to reward relative to loss. In addition, less NAcc–dACC connectivity was associated with more past month drinking. Therefore, individuals at high risk for AUD may have deficient regulation of their heightened responses to rewards relative to loss, which is related to their current drinking. When considered with previous studies, the current findings suggest that this profile of reward brain circuitry contributes to vulnerability for developing AUD and may therefore help to identify individual at-risk for AUD for intervention. The results also suggest that reactivity to natural reinforcers, as well as alcohol, may be an important therapeutic target within AUD prevention efforts.
Supplementary Material
ACKNOWLEDGEMENTS
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA or the National Institutes of Health.
SUPPLEMENTARY MATERIAL
Supplementary data are available at Alcohol And Alcoholism online.
FUNDING
This publication was funded by the National Institute on Drug Abuse (NIDA) (R01DA002812, PIs: HdW and KLP).
Conflict of Interest Statement
The authors declare no conflicts of interest.
REFERENCES
- Andrews MM, Meda SA, Thomas AD, et al. (2011) Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry 69:675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, et al. (2010) Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psychiatry 51:827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW (2008. a) Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction 103:1308–19. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW (2008. b) Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage 42:1609–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, Peper JS, Van Der Heide D, et al. (2016) Nucleus accumbens response to rewards and testosterone levels are related to alcohol use in adolescents and young adults. Dev Cogn Neurosci 17:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, et al. (2001) Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm (Vienna) 108:887–94. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, Mcdevitt RA, et al. (2012) Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76:790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, Fein G (2013) Resting-state synchrony in short-term versus long-term abstinent alcoholics. Alcohol Clin Exp Res 37:794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, et al. (2011) Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage 57:1608–16. [DOI] [PubMed] [Google Scholar]
- CDC 2016. Fact Sheet: Alcohol Use and Your Health [Online]. http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm.
- Chassin L, Pitts SC, Prost J (2002) Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. J Consult Clin Psychol 70:67–78. [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA (2013) Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol 18:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J (2009) Binge drinking in young adults: data, definitions, and determinants. Psychol Bull 135:142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–73. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, et al. (2014) Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res 221:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ (2015) Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev Cogn Neurosci 16:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 113:7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, et al. (2014) Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS One 9:e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G (2009) Depression and reduced sensitivity to non-rewards versus rewards: evidence from event-related potentials. Biol Psychol 81:1–8. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, et al. (2010) Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, et al. (2006) The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol 71:148–54. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, et al. (2015) Neuroimaging risk markers for substance abuse: recent findings on inhibitory control and reward system functioning. Curr Addict Rep 2:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, et al. (2009) Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Liu X, Shulz K, et al. (2012) Parental substance abuse and function of the motivation and behavioral inhibition systems in drug-naive youth. Psychiatry Res 201:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison KM. (2004) The short-term effects and unintended long-term consequences of binge drinking in college: a 10-year follow-up study. Am J Drug Alcohol Abuse 30:659–84. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kuhn S, et al. (2017) Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry 74:387–98. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, et al. (2010) Addiction related alteration in resting-state brain connectivity. Neuroimage 49:738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, et al. (2012) A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, O’neil JP, Janabi M, et al. (2012) Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med 4:116ra6. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Forouzanfar MH, Daoud F, et al. (2016) Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 387:2383–401. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Baskin-Sommers A, Newman JP, et al. (2014) Neural correlates of substance abuse: reduced functional connectivity between areas underlying reward and cognitive control. Hum Brain Mapp 35:4282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KU, Gan G, Banaschewski T, et al. (2015) No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls. Addict Biol 20:534–45. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, et al. (2004) Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology 29:393–402. [DOI] [PubMed] [Google Scholar]
- NIAAA 2004. NIAAA Council Approves Definition of Binge Drinking [Online]. https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf.
- Nikolova YS, Singhi EK, Drabant EM, et al. (2013) Reward-related ventral striatum reactivity mediates gender-specific effects of a galanin remote enhancer haplotype on problem drinking. Genes Brain Behav 12:516–24. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, et al. (2009) Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373:2223–33. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, et al. (1986) The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav 11:149–61. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Jacobus J, et al. (2015) Structural connectivity of neural reward networks in youth at risk for substance use disorders. Psychopharmacology (Berl) 232:2217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S (2014) Brain reward region responsivity of adolescents with and without parental substance use disorders. Psychol Addict Behav 28:805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS (2013) Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biol Psychiatry 73:869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Mchugh MJ, Pariyadath V, et al. (2012) Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage 62:2281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, et al. (2007) Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 27:12700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, et al. . 2003. MARINA: An easy to use tool for the creation of MAsks for Region of Interest Analyses. The 9th International Conference on Functional Mapping of the Human Brain. New York, New York.
- Wechsler H, Davenport A, Dowdall G, et al. (1994) Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. J Am Med Assoc 272:1672–7. [PubMed] [Google Scholar]
- Weiland BJ, Zucker RA, Zubieta JK, et al. (2017) Striatal dopaminergic reward response relates to age of first drunkenness and feedback response in at-risk youth. Addict Biol 22:502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, et al. (2011) Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend 115:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD (2014) Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Grusser SM, Klein S, et al. (2002) Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry 17:287–91. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, et al. (2007) Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35:787–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.