Abstract
Heart failure with preserved ejection fraction is often preceded by diastolic dysfunction (DD). Of several published DD criteria, it is unclear which, if any, are applicable to data obtained in epidemiologic cohorts. We evaluated the prevalence of DD using previously published definitions in a population-based study, the Coronary Artery Risk Development in Young Adults (CARDIA) Study, using data gathered in 2010–2011. Echocardiography was performed on 3,474 individuals (mean age = 50.2 years) at the CARDIA year 25 examination. Four published definitions of DD were studied. We calculated DD prevalence for each definition and determined the overlap between definitions. We used logistic regression to assess the strength of associations between demographic and clinical factors and the definitions of DD. Prevalence of DD ranged from 2% to 32% across the 4 definitions, with a minority of cases identified by more than 1 definition. Two definitions classified 38%–39% of the study sample as indeterminate for DD. Associations of risk factors with DD varied considerably, with male sex being associated positively with DD for one definition (odds ratio = 1.4, 95% confidence interval: 1.2, 1.6) and inversely for another (odds ratio = 0.7, 95% confidence interval: 0.6, 0.8). Prevalence of DD varies markedly in CARDIA by the definition applied. A uniform, reliable, and accurate definition of DD for epidemiologic studies is needed.
Keywords: diastolic dysfunction, echocardiography, epidemiologic methods, heart failure
Editor's note: An invited commentary on this article appears on page 1228, and the authors’ response appears on page 1231.
Heart failure currently affects about 5.7 million Americans, with 670,000 new cases and over 1 million hospitalizations annually (1). Approximately half of all heart failure cases occur with preserved ejection fraction (2). Heart failure with preserved ejection fraction develops from interplay among several mechanisms, including (but not limited to) left ventricular diastolic dysfunction, left ventricular systolic dysfunction, pulmonary hypertension, and extracardiac volume overload (3), but it is clear that diastolic dysfunction (DD) is a critical element underlying heart failure with preserved ejection fraction in many individuals (4). Preclinical DD (DD with normal ejection fraction and no symptoms of heart failure, i.e., stage B heart failure) is an independent predictor of incident clinical heart failure (5).
Since DD is a predictor of future heart failure, descriptive and analytical epidemiologic studies of DD will be essential to understanding the natural history of heart failure with preserved ejection fraction. However, epidemiologic studies of DD (6) to date have been hampered by a lack of consensus concerning appropriate echocardiographic definitions of DD to implement in analyses of population cohort studies. Therefore, we assessed 4 previously published echocardiographic definitions of DD (7–9) in the Coronary Artery Risk Development in Young Adults (CARDIA) cohort, where echocardiography was performed in the year 25 examination. We calculated DD prevalence by definition and examined concordance among definitions. Further, we used logistic regression to assess the strength of associations between known risk factors for heart failure and the various definitions of DD.
METHODS
Study population
CARDIA (10–12) is a prospective cohort study designed to investigate the development of cardiovascular risk factors and disease. In 1985–1986, 5,115 black and white men and women age 18–30 years were recruited from 4 urban US sites, including Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. CARDIA participants have been followed for nearly 30 years, with the collection of detailed demographic and clinical data. Participant data have been collected across 8 examination cycles, including baseline and at years 2, 5, 7, 10, 15, 20, and 25. Retention rates have been high, with 72% of survivors being examined at the year 25 examination and vital and clinical event status being known for about 90% of participants in the most recent 2 years of follow-up. All 3,474 individuals who attended the year 25 CARDIA examination and underwent echocardiography were included in the present analysis.
The study was approved by the institutional review boards at all study sites, and all participants provided written informed consent.
Demographic, anthropometric, and disease measures at the year 25 CARDIA visit
In CARDIA, race was determined by self-report at year 0 (baseline) and verified at year 2. Standardized protocols were used to measure height and weight (11). Body mass index was calculated as weight (kg) divided by squared height in meters (m2). Diabetes was defined as fasting glucose concentration ≥126 mg/dL, 2-hour glucose concentration (from an oral glucose tolerance test) ≥200 mg/dL, hemoglobin A1c level ≥6.5%, or use of glucose-lowering medication. Fasting plasma glucose was measured with the hexokinase method. Hemoglobin A1c was measured from a whole-blood aliquot by ion-exchange high-performance liquid chromatography (G7; Tosoh Biosciences Inc., South San Francisco, California). Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication. Trained technicians used an oscillometer (Omron, Kyoto, Japan) to measure blood pressure. Resting systolic and diastolic blood pressure were measured 3 times at 1-minute intervals. The average of the second and third measurements was used for analysis.
Echocardiographic measures
As described previously (13, 14), Doppler echocardiography and 2-dimensional guided M-mode echocardiography were performed using an Artida cardiac ultrasound scanner (Toshiba Medical Systems, Otawara, Japan) by trained sonographers using standardized protocols across all field centers at the year 25 CARDIA visit. At the reading center, experienced sonographers made measurements from digitized images using a standard software offline image analysis system (Digisonics, Houston, Texas). The full CARDIA echocardiography protocol can be seen online at the CARDIA Study website (http://cardia2.dopm.uab.edu/). Left atrial volume was measured from the apical 4-chamber view (15, 16) and was divided by body surface area per recommendations from the American Society of Echocardiography (ASE) (16). Peak velocities of the early phase (E) and late/atrial phase (A) of the mitral inflow and their ratio (E/A) were measured using pulse wave Doppler recordings of transmitral flow at the mitral valve leaflet tips. Using tissue Doppler imaging, early peak diastolic mitral annular velocities (e′) at the septal and lateral mitral annulus were measured (8). Average e′ was calculated from the average of the septal and lateral mitral annulus. E/e′ ratio was calculated (8). E-wave deceleration time was measured.
Definitions of DD
Different definitions for determination of dichotomous DD were applied to the year 25 CARDIA data; the precise implementation of these definitions is described in detail in Table 1. We chose initially to examine only 4 dichotomous definitions of DD (with mild, moderate, and severe DD being lumped together). The first algorithm, derived from the Umeå General Population Heart Study (17), was used in the Valsartan in Diastolic Dysfunction (VALIDD) Trial (7) and defines DD simply on the basis of lateral e′ velocities lower than age-specific cutoff values. For the second definition, Redfield et al. (9) used a number of different echocardiographic measures to categorize participants into multiple categories. Because 2 of the measures used to grade DD in the Redfield et al. paper (9) (pulmonary venous flow and mitral inflow during Valsalva maneuver) were not measured in CARDIA, we named our implementation of this DD algorithm the “(modified) Redfield definition.” We also examined 2 implementations of the ASE algorithm (8), one requiring both septal e′ velocity less than 8 cm/second and lateral e′ velocity less than 10 cm/second (first ASE definition (ASE 1)) and a second requiring either septal e′ velocity less than 8 cm/second or lateral e′ velocity less than 10 cm/second (second ASE definition (ASE 2)), recognizing that ASE guidelines mention the importance of averaging the septal and lateral annular measurements in some situations (8). Finally, we also then identified those individuals who would be classified as having grade III or severe DD based on the ASE (8) or Redfield (9) criteria using only cutpoints for E/A ratio, E/e′, and deceleration time. These represent modifications of both the ASE and Redfield criteria for grade III/severe DD, as we did not use all echocardiographic measures involved in the full definitions.
Table 1.
Implementation of Previously Published Algorithms for Diastolic Dysfunction in the CARDIA Study (Year 25 Visit), 2010–2011
| Algorithm (Reference No.(s)) | Classification | Criteria |
|---|---|---|
| VALIDD Trial (7, 17) | DD | Age 45–54 years and lateral e′ <10 cm/second; or age 55–65 years and lateral e′ <9 cm/second; or age >65 years and lateral e′ <8 cm/second |
| No DD | Anyone not meeting the criteria for VALIDD DD above | |
| ASE 1 (8) | DD | Lateral e′ <10 cm/second and septal e′ <8 cm/second and LAV ≥34 mL/m2 |
| No DD | Lateral e′ ≥10 cm/second and septal e′ ≥8 cm/second and LAV <34 mL/m2 | |
| Probably no DD | Lateral e′ ≥10 cm/second and septal e′ ≥8 cm/second and LAV ≥34 mL/m2 | |
| Indeterminate | Anyone not meeting any of the 3 categories of ASE 1 criteria above | |
| ASE 2 (8) | DD | Lateral e′ <10 cm/second or septal e′ <8 cm/second and LAV ≥34 mL/m2 |
| No DD | Lateral e′ ≥10 cm/second and septal e′ ≥8 cm/second | |
| Indeterminate | Anyone not meeting either of the 2 categories of ASE 2 criteria above | |
| (Modified) Redfield (9) | DD | E/A ratio <0.75 and E/e′ ratio <10 (mild DD) or 0.75 < E/A ratio < 1.5 and deceleration time >140 ms and E/e′ ratio ≥10 (moderate DD) or E/A ratio >1.5 and deceleration time <140 ms and E/e′ ratio ≥10 (severe DD) |
| No DD | 0.75 < E/A ratio < 1.5 and deceleration time >140 ms and E/e′ ratio <10 | |
| Indeterminate | Anyone not meeting either of the 2 categories of (modified) Redfield criteria above |
Abbreviations: ASE, American Society for Echocardiography; CARDIA, Coronary Artery Risk Development in Young Adults; DD, diastolic dysfunction; LAV, left atrial volume; VALIDD, Valsartan in Diastolic Dysfunction.
Statistical analyses
Analysis of variance and χ2 tests were used, as appropriate, to examine differences in participant characteristics, including indices of diastolic function, by sex/race subgroup. Prevalence estimates for DD were calculated by dividing the number of individuals classified into a given category of a definition by the total number of individuals with all echocardiographic measures available for the implementation of that definition. For Venn diagram analyses, all 3,474 individuals were included, since it was theoretically possible to be classified as having DD by one definition but have insufficient data available for classification by a second definition.
Multivariable association analyses of demographic and clinical factors with different definitions of DD were conducted using logistic regression implemented in SAS Proc Logistic (SAS Institute, Inc., Cary, North Carolina). Each definition of DD was regressed on age, sex, race, prevalent diabetes, prevalent hypertension, and body mass index (all modeled as binary variables) simultaneously. For these regression analyses, individuals with insufficient data or classified as indeterminate by any given definition were excluded from regression analyses for that definition. For the ASE 1 definition, individuals categorized as “probably no DD” were lumped with those categorized as “no DD” in the logistic regression. We considered associations with P values less than 0.05 to be significant.
RESULTS
Web Table 1 (available at http://aje.oxfordjournals.org/) presents the demographic and echocardiographic characteristics of the study sample overall and stratified by race and sex. At the year 25 CARDIA visit, participants were 43–55 years of age. As expected, there were statistically significant differences between sex/race subgroups for all demographic and echocardiographic variables (18). A large percentage (62.8%) of African-American females had a body mass index greater than or equal to 30; no other sex/race subgroup had more than 45% of individuals with a body mass index greater than or equal to 30. Annular velocities, whether septal or lateral, were consistently lower among African Americans. E/e′ ratios were consistently higher among African Americans; not surprisingly, left atrial volume indexes were also larger among African Americans.
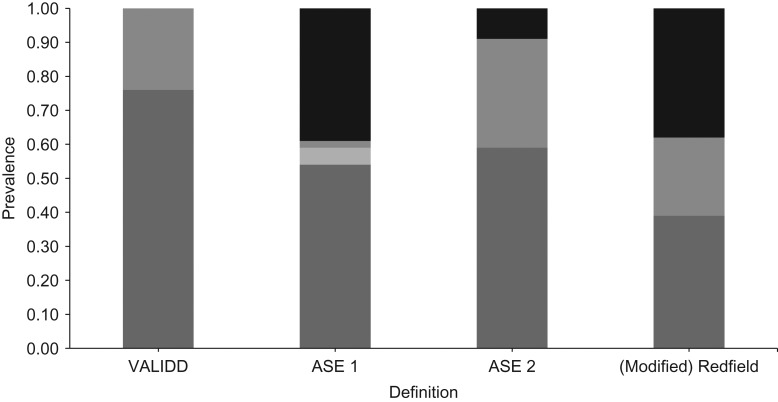
Figure 1 shows the prevalence of DD according to the 4 different definitions. The ASE 2 definition had the largest prevalence of DD (32%), whereas the ASE 1 definition had the lowest prevalence (2%). Both the (modified) Redfield and the ASE 1 definitions classified large percentages of the population as indeterminate with regard to DD (38% and 39%, respectively). The echocardiographic variable with the most missing values was E/e′ ratio, which was missing for 87 participants. Proportions of persons without sufficient echocardiographic data for classification ranged between 0.7% of the population for the (modified) Redfield definition and 2.5% of the population for the ASE definition.
Figure 1.
Prevalence of diastolic dysfunction according to different definitions in the Coronary Artery Risk Development in Young Adults Study (Year 25 Visit), 2010–2011. Light gray portions of columns, probably normal; medium gray portions, diastolic dysfunction; dark gray portions, normal; black portions, indeterminate. ASE, American Society for Echocardiography; VALIDD, Valsartan in Diastolic Dysfunction.
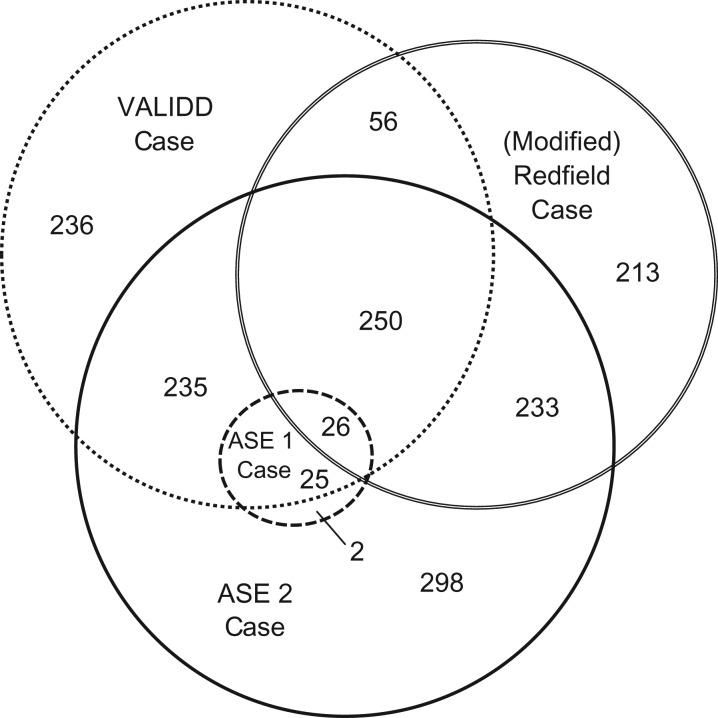
Figure 2 shows the overlap between different diagnoses of DD. The ASE 1 definition is represented as a circle within the ASE 2 definition, as the ASE 2 definition is a less stringent application of the criteria used in the ASE 1 definition. There is minimal overlap among the other definitions. Although the prevalence of nominal DD ranges only between 23% and 32% among the VALIDD, (modified) Redfield, and ASE 2 definitions, these definitions overlap for only 276 individuals (7.9% of the total study population). Thus, 700 individuals are classified as having DD by one of the definitions but not by the other two. These particular individuals are fairly equally spread across the VALDD, (modified) Redfield, and ASE 2 definitions.
Figure 2.
Overlap between 4 different definitions of diastolic dysfunction (DD) in the Coronary Artery Risk Development in Young Adults Study (Year 25 Visit), 2010–2011. A total of 1,900 participants did not meet any case definition. ASE, American Society for Echocardiography; VALIDD, Valsartan in Diastolic Dysfunction.
There was better concordance between the ASE definition of grade III DD (8) and the Redfield et al. definition (9) of severe DD when modifications to these definitions were applied using the prescribed cutoffs for E/A ratio, E/e′, and deceleration time, although the ASE definition proved far more stringent. Four individuals were classified as having both stage III heart failure and severe DD. Six individuals in the study sample (0.17%) were identified as having stage III heart failure by the ASE definition, and 35 individuals (1.0% of the entire study sample) were identified as having severe DD by the definition of Redfield et al. (9).
Table 2 shows the association of risk factors with the different definitions of DD, excluding persons found to be indeterminate for each definition. Because of this exclusion, the sample size varied considerably across analyses. The strength of the association for age (comparing persons aged ≥50 years with those aged <50 years) varies over 2-fold across the 4 definitions. The strength of the association for African-American race (compared with white) and for prevalent hypertension (compared with normotension) varied nearly 3-fold across the 4 definitions. Most strikingly, male sex was significantly and directly associated with DD as defined by the ASE 2 definition but significantly inversely associated with DD as defined by the (modified) Redfield definition.
Table 2.
Association of Selected Risk Factors With 4 Different Definitions of Diastolic Dysfunction (Multivariate Modela) in the CARDIA Study (Year 25 Visit), 2010–2011
| Risk Factor | Definition of DD | |||||||
|---|---|---|---|---|---|---|---|---|
| ASE 1b (n = 53 With DD; n = 1,968 Without DD) | ASE 2b (n = 1,034 With DD; n = 1,968 Without DD) | VALIDD Trial (n = 810 With DD; n = 2,505 Without DD) | (Modified) Redfieldb (n = 748 With DD; n = 1,304 Without DD) | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age ≥50 years vs. <50 years | 2.9 | 1.5, 5.5 | 2.0 | 1.7, 2.3 | 1.6 | 1.4, 2.0 | 1.3 | 1.0, 1.5 |
| Male sex vs. female sex | 1.4 | 0.8, 2.6 | 1.4 | 1.2, 1.6 | 1.0 | 0.8, 1.2 | 0.7 | 0.6, 0.8 |
| African-American race vs. white race | 5.2 | 2.5, 10.8 | 1.5 | 1.2, 1.8 | 1.5 | 1.3, 1.8 | 1.5 | 1.3, 1.9 |
| BMIc ≥30 vs. BMI <30 | 1.1 | 0.6, 2.1 | 1.6 | 1.4, 1.9 | 1.6 | 1.3, 1.9 | 1.3 | 1.0, 1.6 |
| Prevalent diabetes (yes vs. no) | 1.9 | 1.0, 3.8 | 1.8 | 1.5, 2.3 | 1.5 | 1.2, 1.8 | 2.1 | 1.6, 2.7 |
| Prevalent hypertension (yes vs. no) | 5.5 | 2.9, 10.6 | 2.1 | 1.8, 2.5 | 2.5 | 2.1, 3.0 | 1.6 | 1.3, 2.0 |
Abbreviations: ASE, American Society for Echocardiography; BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; DD, diastolic dysfunction; OR, odds ratio; VALIDD, Valsartan in Diastolic Dysfunction.
a The multivariate model included dichotomous variables for all risk factors listed in the table.
b Individuals found to be indeterminate for DD by this definition were excluded from analysis.
c Weight (kg)/height (m)2.
DISCUSSION
In this analysis of echocardiographic data obtained on 3,474 CARDIA participants with a mean age of 50 years, the prevalence of DD ranged widely across different diagnostic algorithms defining DD, from 2% for a narrow interpretation of the ASE definition (ASE 1) to 32% for a looser interpretation of the ASE definition (ASE 2). Although prevalence estimates for the (modified) Redfield, VALIDD, and ASE 2 definitions ranged only from 23% to 32%, overlap between the definitions was very poor, with only 8% of the study population identified as having DD by all 3 definitions. This paper clearly demonstrates for the first time that there is very poor overlap between existing definitions of DD. As studies examining predictors of and trends in DD proliferate, our documentation of this phenomenon will be helpful to readers in understanding the significant limitations of existing definitions, particularly when comparing studies using different definitions.
Given this poor overlap and the highly variable sample size entering analyses of different nominal DD variables due to large numbers of individuals being classified as indeterminate by some definitions, it was unsurprising that there was large variability in the strength of associations of proposed risk factors with the different definitions of DD. However, the finding that female sex was significantly and directly associated with DD by one definition and significantly inversely associated with DD by another definition further highlights the need for consensus criteria that are based on solid epidemiologic, physiological, and prognostic data.
It is difficult to compare the 4 prevalence estimates of DD presented in this paper with those previously published for other populations. A review by Wan et al. (6) identified 4 reports that have provided information about DD measures in populations without clinical disease; these estimates relied on a wide variety of methods. Examining the 4 papers in the review and 2 additional studies we identified (9, 19–23), we found reports of DD prevalence varying from 27% to 39.8% in populations with mean ages ranging from 48 years to 76 years. Results from our study suggest that previous variability in estimates could be explained simply by the use of different definitions.
One important issue highlighted in our analysis is that about 38% of individuals were classified as indeterminate using the ASE and (modified) Redfield definitions. This would be expected to apply to other epidemiologic studies as well. This high rate of indeterminacy for the Redfield definition resulted from the absence of pulmonary vein flow and Valsalva maneuver measurements and suggests, perhaps, that the Redfield definition should not be simplified. When a large percentage of individuals are unclassified by a definition, there are questions about the validity of prevalence estimates. Additionally, it complicates comparisons of association analyses (if, for example, one group includes “indeterminate” individuals in the “normal” group for association analyses and another does not) and concordance estimates across definitions. For meaningful analyses of population cohorts, DD definitions with only small percentages of individuals classified as indeterminate are necessary.
Integration of biomarkers such as B-type natriuretic peptide with echocardiographic systolic and diastolic function metrics should be considered in future attempts to create definitions of DD. Identifying the most useful combination of diastolic function measures will require 2 types of studies. Most important are those that link specific measures to outcomes. Currently the emphasis in the literature has been on older individuals, those with myocardial infarction, or those with existing heart failure (4, 5, 24–26). Given the age dependence of diastolic function measures, the generalizability of these studies to younger middle-aged individuals such as those participating in CARDIA may be limited. The second type of study would track diastolic function over a meaningful interval (at least 5 years) to determine tracking, factors that impact longitudinal change, and reproducibility of diastolic function assessment (27). The strength of tracking of an individual measure over time in a young cohort will determine the best measures to consider for event prediction many years before an event. Both types of studies should include a diversity of racial and ethnic groups, unlike many previous echocardiographic studies, which have focused primarily on Caucasians.
There were several strengths of our analysis. CARDIA has a large number of high-quality echocardiographic measurements obtained and read under stringent standardized conditions (14). With 3,474 individuals, this represents the largest study to date to have examined diastolic function in a population cohort study. We included data on large numbers of whites and African Americans and, for the first time, demonstrated how careful application of different DD definitions compares in a single population. The age range of CARDIA participants, 43–55 years, may be highly appropriate to the study of antecedents of heart failure. Weaknesses of the analysis include the absence of some measurements used in the original Redfield (9) definition of DD and inability to determine DD in an individualized manner as suggested by the ASE guidelines (8). The ASE guidelines state, “[A]ssessment should take into consideration patients’ ages and heart rates (mitral E, E/A ratio, and annular e′ decrease with increasing heart rate). Specifically, in older individuals without histories of cardiovascular disease, caution should be exercised before concluding that grade I DD is present” (8, p. 128). However, it is not possible to implement such individualization of case definition in secondary analysis of data generated in a cohort study. These weaknesses demonstrate complications of attempting to define DD in population-based studies.
In conclusion, published definitions of DD had poor agreement when applied to echocardiographic data gathered in the CARDIA population cohort study. Our results highlight the limitations of the currently published algorithms used to define DD and hopefully will promote further work to find better algorithms that uniformly define DD across populations. Until such time as an optimal definition of DD can be determined, researchers should be aware of these limitations and should provide detailed information on their implementation of DD definitions in research studies, as such information will be critical to interpretation of their results.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Laura J. Rasmussen-Torvik, Laura A. Colangelo, Donald M. Lloyd-Jones); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Joao A. C. Lima); Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (David R. Jacobs, Jr.); Department of Medicine and Public Health Sciences, School of Medicine, Wake Forest University, Winston-Salem, North Carolina (Carlos J. Rodriguez); A. I. DuPont Hospital for Children, Wilmington, Delaware (Samuel S. Gidding); and Division of Cardiology, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Sanjiv J. Shah, Donald M. Lloyd-Jones).
This work was supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intraagency agreement between the NIA and the NHLBI (agreement AG0005), all of which fund the Coronary Artery Risk Development in Young Adults Study.
Conflict of interest: none declared.
REFERENCES
- 1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115(12):1563–1570. [DOI] [PubMed] [Google Scholar]
- 3. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10(4):401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke MA, Katz DH, Beussink L, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7(2):288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306(8):856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63(5):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon SD, Janardhanan R, Verma A, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369(9579):2079–2087. [DOI] [PubMed] [Google Scholar]
- 8. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165–193. [DOI] [PubMed] [Google Scholar]
- 9. Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. [DOI] [PubMed] [Google Scholar]
- 10. Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12(1 suppl):1S–77S. [DOI] [PubMed] [Google Scholar]
- 11. Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 12. Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8(4 suppl):68S–73S. [DOI] [PubMed] [Google Scholar]
- 13. Kishi S, Armstrong AC, Gidding SS, et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail. 2014;2(5):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armstrong AC, Ricketts EP, Cox C, et al. Quality control and reproducibility in M-mode, two-dimensional, and speckle tracking echocardiography acquisition and analysis: the CARDIA study, year 25 examination experience. Echocardiography. 2015;32(8):1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1.e14–39.e14. [DOI] [PubMed] [Google Scholar]
- 17. Henein M, Lindqvist P, Francis D, et al. Tissue Doppler analysis of age-dependency in diastolic ventricular behaviour and filling: a cross-sectional study of healthy hearts (the Umeå General Population Heart Study). Eur Heart J. 2002;23(2):162–171. [DOI] [PubMed] [Google Scholar]
- 18. Gidding SS, Liu K, Colangelo LA, et al. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging. 2013;6(5):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abhayaratna WP, Marwick TH, Smith WT, et al. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92(9):1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lam CS, Lyass A, Kraigher-Krainer E, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mureddu GF, Agabiti N, Rizzello V, et al. Prevalence of preclinical and clinical heart failure in the elderly. A population-based study in central Italy. Eur J Heart Fail. 2012;14(7):718–729. [DOI] [PubMed] [Google Scholar]
- 22. Zhou J, Cui X, Jin X, et al. Association of renal biochemical parameters with left ventricular diastolic dysfunction in a community-based elderly population in China: a cross-sectional study. PLoS One. 2014;9(2):e88638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. AlJaroudi WA, Alraies MC, Halley C, et al. Incremental prognostic value of diastolic dysfunction in low risk patients undergoing echocardiography: beyond Framingham score. Int J Cardiovasc Imaging. 2013;29(7):1441–1450. [DOI] [PubMed] [Google Scholar]
- 24. Bella JN, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105(16):1928–1933. [DOI] [PubMed] [Google Scholar]
- 25. Meta-Analysis Research Group in Echocardiography (MeRGE) AMI Collaborators Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta-analysis: Meta-Analysis Research Group in Echocardiography Acute Myocardial Infarction. Circulation. 2008;117(20):2591–2598. [DOI] [PubMed] [Google Scholar]
- 26. Wang M, Yip G, Yu CM, et al. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. 2005;45(2):272–277. [DOI] [PubMed] [Google Scholar]
- 27. Borlaug BA, Redfield MM, Melenovsky V, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6(5):944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.