Abstract
Hepatitis B virus (HBV) infection is more common in African Americans than in white Americans. We compared the epidemiologic, clinical, and virological characteristics of US-born African Americans (USAAs) to those of foreign-born African Americans (FBAAs) with chronic hepatitis B. The adult cohort study of the Hepatitis B Research Network enrolls patients with HBV infection from 21 clinical sites in the United States and Canada. A total of 237 (15%) of the adult participants with chronic HBV infection that were enrolled from January 20, 2011, to October 2, 2013, were of African descent, including 57 USAAs and 180 FBAAs (76%). Compared with FBAAs, USAAs were older and more likely to have acquired HBV through sexual exposure, to be HBeAg-positive, to have higher HBV DNA levels, and to be infected with HBV genotype A2. FBAAs from West Africa were more likely to have elevated serum alanine aminotransferase (72% vs. 50%; P < 0.01) and higher HBV DNA levels (median, 3.2 log10 IU/mL vs. 2.8 log10 IU/mL; P = 0.03) compared with East African FBAAs. The predominant HBV genotype among West African FBAAs was E (67%), whereas genotypes A (78%) and D (16%) were common in East African FBAAs. Significant differences were found between USAAs and FBAAs, highlighting the need for tailored strategies for prevention and management of chronic HBV infection for African Americans.
Keywords: African American, genotype, hepatitis B e antigen, hepatitis B virus, mode of transmission
It is estimated that a third of the world's population has been exposed to hepatitis B virus (HBV) at some point in life. As many as 250 million people may have chronic HBV infection worldwide. Consequently, HBV is a leading cause of cirrhosis and hepatocellular carcinoma (HCC) globally (1, 2). Despite implementation of universal vaccination to prevent HBV infection in an increasing number of countries, hepatitis B remains a major global public health problem.
In the United States, HBV infection represents an area of health disparity. First, the burden of HBV infection is considerably higher among nonwhites than among whites (3, 4). Although HBV is commonly recognized as a health problem in Asian Americans, African Americans comprise another group with high HBV prevalence. National surveys have found that the prevalence of HBV in African Americans is 2–3 times higher than in white non-Hispanic Americans (5). Second, it is estimated that the majority of Americans with chronic HBV infection are foreign born (6). This is important because the proportion of foreign-born individuals in the United States is on the rise, with a current estimate of approximately 13% of the population being foreign born. The number of foreign-born Americans known to be infected with HBV has been estimated to be approximately 1.3 million (7). While the majority (n ~ 770,000) of foreign-born Americans with HBV comes from Asia, the next largest groups are from African (n ~ 150,000) and Caribbean (n ~ 160,000) countries, with the majority of Caribbean individuals with HBV infection being of African descent. The prevalence of HBV among recent immigrants from Africa may be similar, if not higher, than among recent immigrants from Asia (8, 9). Currently, the Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force recommend screening all foreign-born individuals originating from regions with HBV prevalence of 2% or higher (10, 11).
Globally, most published data about HBV infection are derived from patients of Asian descent, whereas HBV infection in Africa remains poorly studied. Africa as a whole has a high burden of HBV infection, with an estimated 50 million carriers. Within the continent, the prevalence of HBV infection shows regional variability—all sub-Saharan African countries are categorized as areas of high endemicity, whereas North African countries are of intermediate endemicity (12–15). The reported prevalence of HBV infection in sub-Saharan Africa varies from 9% in South Africa to 20% in the Democratic Republic of Congo (16, 17). Moreover, available data suggest that the epidemiology of HBV infection in Africa differs from that in Asia. For example, the predominant HBV genotypes in Africa are A, D, and E, compared with genotypes B and C in Asia (18–20). HCC often occurs at a younger age in Africans than in other racial/ethnic groups, although whether this is due to viral, host, or environmental factors remains to be determined (21, 22).
In recent decades, political unrest in many African countries has led to increased emigration to Europe and North America. In the United States, where foreign-born African individuals comprise a significant minority of recent arrivals, the epidemiologic, clinical, and virological profiles of foreign-born African Americans (FBAAs) infected with HBV have not been well studied. The primary aim of this study is to describe the demographic, epidemiologic, and clinical characteristics of US-born African Americans (USAAs) and FBAAs in the United States. We also compared the characteristics of FBAAs from East Africa versus West Africa. Last, we explored the association between the geographic origin of the participants and their HBV viral characteristics, HBV DNA concentrations, and serum alanine aminotransferase (ALT) activities.
METHODS
Data source
The Hepatitis B Research Network is a cooperative network funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to study the epidemiology, natural history, long-term outcome, and optimal therapy of chronic HBV infection among persons in North America. The Hepatitis B Research Network adult cohort study is an observational study that recruited hepatitis B surface antigen (HBsAg)-positive adults from 21 clinical sites, which included academic and community clinics in the United States and in Toronto, Canada. The primary aim of the study is to describe the characteristics of participants with chronic HBV infection from diverse regions and populations and to identify predictors of disease activation and progression. The details of the network and the adult cohort study have been described elsewhere (23).
Study participants underwent a baseline evaluation at the time of enrollment in cohort study and were followed prospectively at regular intervals. The baseline evaluation included demographic information, past medical and family history, physical examination, and laboratory data. Information was collected on risk factors for HBV infection, family history, comorbid conditions, health behavior, and socioeconomic status. Routine laboratory tests, such as measurement of serum aminotransferase activities, were performed locally. Virological assays—including HBV genotype, hepatitis B e antigen (HBeAg), and serum HBV DNA concentrations—were performed at central laboratories supporting the Hepatitis B Research Network.
Collection and analysis of data
Data on race and place of birth were based on self-reporting by the study participant. Participants of African descent were identified and categorized as USAAs or FBAAs. Because the majority of FBAAs originated from Africa, subsequent analysis considered differences in characteristics in FBAAs from countries on the Indian Ocean side (termed East Africa) and the Atlantic side (termed West Africa) of the continent. Because few patients came from land-locked countries or North Africa, participants from these regions were classified as coming from East or West Africa based on proximity and cultural relationship with either coast. The rationale for comparing participants from East versus West Africa was 1) historically, population migration patterns followed the coasts because of the difficulty and danger of transcontinental (East-West) passage, and 2) previous data showing differences in HBV characteristics (e.g., genotypes) between persons living in East and those in West Africa.
We compared risk factors, presumed sources of infection, laboratory data (including serum ALT, HBV DNA levels, and HBV genotype and subgenotype), and clinical phenotypes, as defined below, between USAAs and FBAAs. Serum HBV DNA levels were measured at a central virology laboratory at the University of Washington, using the real-time PCR assay, COBAS AmpliPrep/COBAS TaqMan HBV Test, v2.0 (Roche Molecular Diagnostics, Branchburg, New Jersey), with a lower limit of detection of 20 IU/mL. HBV genotype data were obtained from the Molecular Epidemiology and Bioinformatics Laboratory, Division of Viral Hepatitis, Centers for Disease Control and Prevention. Genotyping was done by nucleic acid mass spectrometry as previously described (24) and subgenotyping by sequencing a region of the S gene, at nt 217–658 of the HBV genome, followed by phylogenetic clustering with annotated reference strains (25). When these data from central laboratories were unavailable, historical data from local laboratories, if present, were used.
Clinical phenotypes were defined using the following criteria, where the upper limit of normal (ULN) of serum ALT was 30 U/L for men and 20 U/L for women. 1) Immune tolerant: HBeAg-positive, ALT below the ULN on ≥2 occasions at least 6 months apart, and HBV DNA levels >106 IU/mL; 2) HBeAg-positive chronic hepatitis: HBeAg-positive, ALT >2 × ULN on ≥2 occasions at least 6 months apart, and HBV DNA levels >104 IU/mL; 3) HBeAg-negative chronic hepatitis: HBeAg-negative, ALT ≥2 × ULN on ≥2 occasions at least 6 months apart, and HBV DNA levels ≥103 IU/mL; 4) Inactive carrier: HBeAg-negative, ALT below the ULN, and HBV DNA levels <103 IU/mL on ≥2 occasions at least 6 months apart; and 5) Indeterminate: not belonging in any of the above categories. Investigators assigned a phenotype for each patient on the day of enrollment.
For statistical analysis, we summarized categorical patient characteristics by the place of birth using frequencies and percentages. The χ2 test or its exact version was used to assess statistical significance. Continuous variables were summarized by medians and interquartile ranges. We used the Kruskal-Wallis test to compare the distributions of continuous characteristics across patient groups. In addition to these descriptive analyses, multivariable regression analyses were conducted to explore factors which might account for differences in the clinical profiles of USAAs and FBAAs. A multiple median regression model was created to model serum ALT after a log10 transformation. The log10 transformation was conducted due to the skewness of the ALT distribution. The following covariates were considered: age, sex, geographic origin, mode of transmission, HBV genotype, HBeAg status, and serum HBV DNA concentrations, and those with P values of ≤0.05 were retained in the final model by the stepwise selection method. All statistical analyses were performed in SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
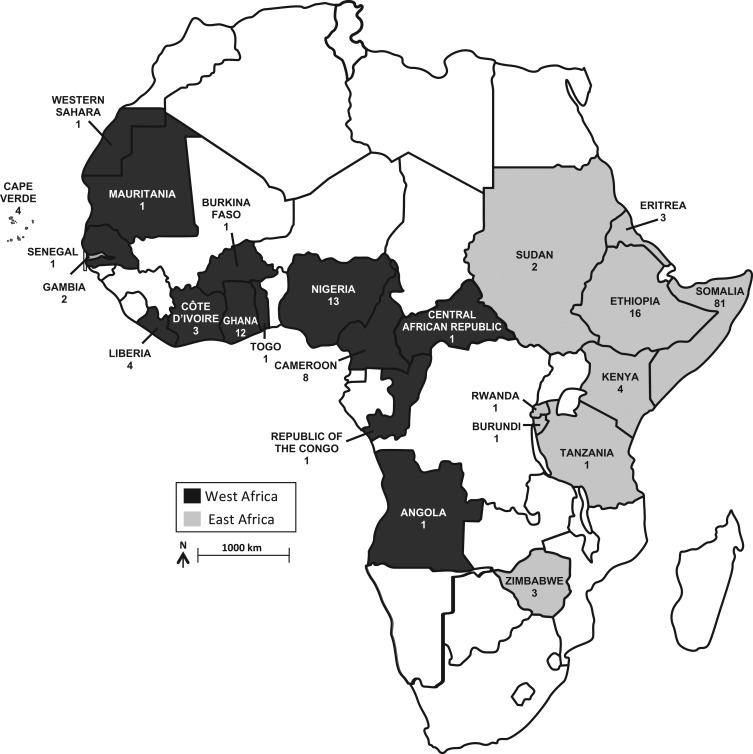
Of the consecutive adult participants with chronic HBV infection enrolled in the Hepatitis B Research Network cohort study from January 20, 2011, to October 2, 2013, a total of 237 (15%) were of African descent, including 57 USAAs (24% of the participants of African descent) and 180 FBAAs. FBAAs included 112 from East Africa, 54 from West Africa, and 14 from other regions of the world, including the Caribbean (n = 11), Europe (n = 1), and Asia (n = 2). Figure 1 summarizes the country of origin of the FBAA patients. Table 1 shows that USAAs, in comparison with all FBAAs, were older (median age = 47 years vs. 40 years; P = 0.004) and more likely to have sexual transmission (59% vs. 3%; P < 0.001) as the presumed source of HBV infection. They had a significantly more frequent history of sexually transmitted diseases (STDs) (45% vs. 8%; P < 0.001), same-sex sexual activities (10% vs. 1%; P = 0.009), illicit use of injection drugs (7% vs. 0%; P = 0.003) and intranasal drugs (16% vs. 0%; P < 0.001), and tattoos (19% vs. 8%; P = 0.02). Despite their older age, USAAs were more likely to be HBeAg-positive (19% vs. 9%; P = 0.04) and more often categorized as having HBeAg-positive chronic hepatitis, whereas FBAAs were more likely to be categorized as inactive carriers.
Figure 1.
Country of birth for foreign-born African Americans born in Africa, Hepatitis B Research Network Study, North America, 2011–2013. Among the 180 foreign-born African Americans, 112 were from East Africa, 54 from West Africa, and 14 from elsewhere.
Table 1.
Comparison of Selected Characteristics Among US-Born and Foreign-Born African Americans, Hepatitis B Research Network Study, North America, 2011–2013
| Characteristic | All | USAA | FBAA | P Valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Frequency | % | Sample Size | Frequency | % | Sample Size | Frequency | % | ||
| Age, yearsb | 237 | 43 (34–54) | 57 | 47 (40–56) | 180 | 40 (33–51) | 0.004 | |||
| Male sex | 237 | 131 | 55 | 57 | 27 | 47 | 180 | 104 | 58 | 0.168 |
| Presumed source | 178 | 32 | 146 | <0.001 | ||||||
| Vertical | 39 | 22 | 3 | 9 | 36 | 25 | ||||
| Sexual | 24 | 13 | 19 | 59 | 5 | 3 | ||||
| Other horizontal | 115 | 65 | 10 | 31 | 105 | 72 | ||||
| History of STDs | 211 | 36 | 17 | 51 | 23 | 45 | 160 | 13 | 8 | <0.001 |
| Sexual intercourse with same-sex partner | 220 | 7 | 3 | 52 | 5 | 10 | 168 | 2 | 1 | 0.009 |
| History of blood transfusion | 230 | 28 | 12 | 56 | 9 | 16 | 174 | 19 | 11 | 0.305 |
| History of illicit injection drug use | 236 | 4 | 2 | 57 | 4 | 7 | 179 | 0 | 0 | 0.003 |
| History of illicit intranasal drug use | 235 | 9 | 4 | 56 | 9 | 16 | 179 | 0 | 0 | <0.001 |
| Tattoos | 232 | 25 | 11 | 57 | 11 | 19 | 175 | 14 | 8 | 0.017 |
| Positive for HBeAg | 232 | 27 | 12 | 57 | 11 | 19 | 175 | 16 | 9 | 0.038 |
| Clinician-assigned phenotype | 237 | 57 | 180 | 0.011 | ||||||
| Immune tolerant | 5 | 2 | 0 | 0 | 5 | 3 | ||||
| HBeAg-positive, active | 19 | 8 | 10 | 18 | 9 | 5 | ||||
| HBeAg-negative, active | 35 | 15 | 8 | 14 | 27 | 15 | ||||
| Inactive carrier | 155 | 65 | 31 | 54 | 124 | 69 | ||||
| Indeterminate | 23 | 10 | 8 | 14 | 15 | 8 | ||||
Abbreviations: FBAA, foreign-born African Americans; HBeAg, hepatitis B e antigen; STD, sexually transmitted disease; USAA, US-born African Americans.
a The P values reflect the difference between USAAs and FBAAs.
b Age was summarized by median (interquartile range).
Table 2 compares characteristics of the 166 FBAAs from East and West Africa. West African FBAAs were younger and more likely to have acquired HBV by vertical transmission (51% vs. 14%). They had a less frequent history of blood transfusion (4% vs. 15%; P = 0.047). A majority of both groups (>88%) were HBeAg-negative; however, West African FBAAs were more likely to have HBeAg-negative chronic hepatitis (28%), whereas East African FBAAs were mostly characterized as inactive carriers (80%).
Table 2.
Comparison of Characteristics of Foreign-Born African American Participants From East Africa Versus West Africa, Hepatitis B Research Network Study, North America, 2011–2013
| Characteristic | All FBAA from Africa | From East Africa | From West Africa | P Valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Frequency | % | Sample Size | Frequency | % | Sample Sizes | Frequency | % | ||
| Age, yearsb | 166 | 40 (33–50) | 112 | 43 (33–55) | 54 | 39 (31–45) | 0.022 | |||
| Male sex | 166 | 96 | 58 | 112 | 60 | 54 | 54 | 36 | 67 | 0.110 |
| Presumed source | 137 | 98 | 39 | <0.001 | ||||||
| Vertical | 34 | 25 | 14 | 14 | 20 | 51 | ||||
| Sexual | 3 | 2 | 0 | 0 | 3 | 8 | ||||
| Other horizontal | 100 | 73 | 84 | 86 | 16 | 41 | ||||
| History of STDs | 148 | 11 | 7 | 97 | 7 | 7 | 51 | 4 | 8 | 1.000 |
| Sexual intercourse with same-sex partner | 157 | 2 | 1 | 105 | 2 | 2 | 52 | 0 | 0 | 1.000 |
| History of blood transfusion | 161 | 18 | 11 | 110 | 16 | 15 | 51 | 2 | 4 | 0.047 |
| Tattoos | 163 | 11 | 7 | 110 | 5 | 5 | 53 | 6 | 11 | 0.178 |
| Positive HBeAg | 161 | 13 | 8 | 107 | 7 | 7 | 54 | 6 | 11 | 0.363 |
| Clinician-assigned phenotype | 166 | 112 | 54 | <0.001 | ||||||
| Immune tolerant | 5 | 3 | 4 | 4 | 1 | 2 | ||||
| HBeAg-positive active | 6 | 4 | 2 | 2 | 4 | 7 | ||||
| HBeAg-negative active | 25 | 15 | 10 | 9 | 15 | 28 | ||||
| Inactive carrier | 117 | 70 | 90 | 80 | 27 | 50 | ||||
| Indeterminate | 13 | 8 | 6 | 5 | 7 | 13 | ||||
Abbreviations: FBAA, foreign-born African Americans; HBeAg, hepatitis B e antigen; STD, sexually transmitted disease.
a The P values reflect the difference between African Americans from East Africa and those from West Africa.
b Age was summarized by median (interquartile range).
Table 3 compares laboratory data from USAAs, East and West African FBAAs, and FBAAs from other parts of the world. For men, the median serum ALT was highest in USAAs, followed by the West African FBAAs. For women, the median ALT was highest among West African FBAAs. East African FBAAs were most likely to have normal serum ALT activities (50%) and to have the lowest median HBV DNA. Concomitant infection with hepatitis C or D virus was relatively uncommon in all groups.
Table 3.
Comparison of Laboratory Characteristics of African Americans According to Their Place of Birth, Hepatitis B Research Network Study, North America, 2011–2013
| Characteristic | All Participants | USAA | East African FBAA | West African FBAA | Other FBAA | P Valuea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Frequency | % | Sample Size | Frequency | % | Sample Size | Frequency | % | Sample Size | Frequency | % | Sample Size | Frequency | % | ||
| ALT, U/L | ||||||||||||||||
| Menb | 126 | 37 (26–63) | 26 | 55 (34–97) | 56 | 33 (23–41) | 36 | 46 (31–70) | 8 | 33 (24–69) | 0.001 | |||||
| Womenb | 102 | 21 (16–27) | 30 | 21 (18–27) | 48 | 19 (14–25) | 18 | 27 (20–43) | 6 | 24 (20–165) | 0.008 | |||||
| ALT, ULNc | 228 | 56 | 104 | 54 | 14 | 0.005 | ||||||||||
| ≤1.00 | 94 | 41 | 21 | 38 | 52 | 50 | 15 | 28 | 6 | 43 | ||||||
| 1.03–2.00 | 88 | 39 | 20 | 36 | 43 | 41 | 21 | 39 | 4 | 29 | ||||||
| ≥2.03 | 46 | 20 | 15 | 27 | 9 | 9 | 18 | 33 | 4 | 29 | ||||||
| HBV DNA (log10 IU/mL)b | 234 | 3.0 (2.0–3.9) | 56 | 3.4 (2.3–4.5) | 110 | 2.8 (1.9–3.4) | 54 | 3.2 (2.1–4.1) | 14 | 3.3(2.0–4.0) | 0.025 | |||||
| HBV DNA, IU/mL | 234 | 56 | 110 | 54 | 14 | 0.034 | ||||||||||
| <1,000 | 120 | 51 | 24 | 43 | 68 | 62 | 22 | 41 | 6 | 43 | ||||||
| 1,000–9,999 | 61 | 26 | 15 | 27 | 26 | 24 | 15 | 28 | 5 | 36 | ||||||
| 10,000–99,999 | 17 | 7 | 5 | 9 | 7 | 6 | 5 | 9 | 0 | 0 | ||||||
| 100,000–999,999 | 15 | 6 | 1 | 2 | 6 | 5 | 7 | 13 | 1 | 7 | ||||||
| 1,000,000–9,999,999 | 6 | 3 | 3 | 5 | 1 | 1 | 2 | 4 | 0 | 0 | ||||||
| ≥10,000,000 | 15 | 6 | 8 | 14 | 2 | 2 | 3 | 6 | 2 | 14 | ||||||
| Positive for anti-HDV | 166 | 11 | 7 | 50 | 3 | 6 | 60 | 3 | 5 | 45 | 5 | 11 | 11 | 0 | 0 | 0.470 |
| Positive for anti-HCV | 172 | 7 | 4 | 46 | 4 | 9 | 73 | 3 | 4 | 42 | 0 | 0 | 11 | 0 | 0 | 0.179 |
Abbreviations: ALT, alanine aminotransferase; anti-HCV, antibody to the hepatitis C virus; anti-HDV, antibody to the hepatitis D virus; FBAA, foreign-born African Americans; HBV, hepatitis B virus; ULN, upper limit of normal; USAA, US-born African Americans.
a The P values reflect the difference between USAAs, East African FBAAs, West African FBAAs, and other FBAAs.
b Continuous variables were summarized by median (interquartile range).
c ULN was 30 U/L for men and 20 U/L for women.
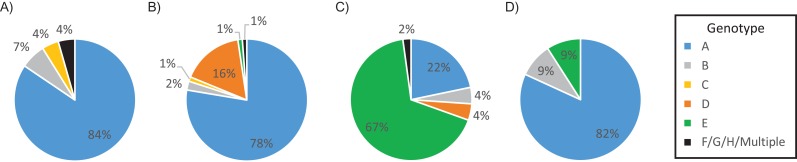
Figure 2 compares genotype distributions by the place of birth. Genotype A predominated in USAAs (84%). In East African FBAAs, genotype A was most common (78%), with a notable representation of genotype D (16%). The most prevalent genotype among West African FBAAs was genotype E (67%), followed by genotype A (22%). Genotype E was present in 1% of East African FBAAs and none of the USAAs. Subgenotype data were available for 171 patients (see Web Table 1, available at https://academic.oup.com/aje). Although genotype A was the most common genotype in both USAAs and East African FBAAs, the subtypes in the groups were different. East African FBAAs predominantly had subtype A1, whereas the vast majority (95%) of USAAs with genotype A had subtype A2. HBV DNA (IU/mL) level was lowest among African Americans with genotype D, but there was not a significant difference in HBV DNA across genotypes (P = 0.2; Web Figure 1).
Figure 2.
Genotype distribution by region of birth, Hepatitis B Research Network Study, North America, 2011–2013. The pies represent the United States (A), East Africa (B), West Africa (C), and other regions (D).
In univariable analyses correlating serum ALT activities with age, sex, geographic origin, mode of transmission, parenteral risk factors, HBV genotype, and HBeAg status, all of the variables, except history of intranasal drug use, previous diagnosis of STDs, and same-sex sexual activities, were significantly associated with ALT activities (Web Table 2). Multivariable regression modeling showed male sex, younger age, place of birth (not born in East Africa), and higher HBV DNA levels to be significantly associated with increased serum ALT activities (Table 4). All other factors, such as HBV genotype, were no longer significant after adjusting for the 4 selected factors.
Table 4.
Predictors of Serum ALT (U/L) Activities in a Multivariable Regression Modela, Hepatitis B Research Network Study, North America, 2011–2013
| Covariate | Estimate | 95% CI | P Value |
|---|---|---|---|
| Sex, male vs. female | 1.61 | 1.37, 1.89 | <0.001 |
| Age, per 5-year increase | 0.95 | 0.93, 0.98 | 0.002 |
| HBV DNA, IU/mL | |||
| 1,000–9,999 vs. <1,000 | 1.03 | 0.85, 1.23 | 0.779 |
| 10,000–99,999 vs. <1,000 | 1.05 | 0.78, 1.40 | 0.761 |
| 100,000–9,999,999 vs. <1,000 | 2.46 | 1.60, 3.80 | <0.001 |
| ≥10,000,000 vs. <1,000 | 3.12 | 2.24, 4.33 | <0.001 |
| Birthplace, East Africa vs. all otherb | 0.84 | 0.71, 1.00 | 0.046 |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HBV, hepatitis B virus.
a The predictors’ effects on ALT activities corresponded to multiplicative effects. For example, the median ALT among men was 1.61 times that of women, and each 5-year increase in age was associated with 0.95 times (i.e., −5%) change in median ALT when holding other factors constant.
b West Africa, the United States, and other non-African regions were combined based on preliminary regression results.
DISCUSSION
African Americans have a 2–3-fold higher prevalence of HBV infection than do white Americans, but there have been very few studies on the epidemiologic, clinical, and virological characteristics of HBV infection among African Americans. Moorman et al. found that the burden of viral hepatitis among blacks in the United States was high both in terms of morbidity and mortality rates (26); however, that study did not provide data on HBV genotype or country of birth, and clinical and laboratory data were collected retrospectively. Our study, which included more than 200 African Americans with chronic HBV infection enrolled from clinical sites distributed all over the United States, provided a unique opportunity to study the characteristics of HBV infection among African Americans. We found many differences in epidemiologic, virological, and clinical characteristics between US- and foreign-born participants of African descent with chronic HBV infection (27). FBAAs were also not homogeneous, and we observed significant differences between East and West African–born participants. USAAs, the majority of whom derive their ancestry from West Africa, shared some characteristics with West African FBAAs, such as high serum ALT and HBV DNA.
It is well known that in endemic areas, HBV is commonly acquired at an early age, either vertically at birth from an infected mother or horizontally during early childhood (28, 29). Although vertical transmission is thought to be the most common route of HBV transmission in Asia, our data and previous epidemiologic investigations have indicated that horizontal transmission plays a more important role in maintaining HBV endemicity in Africa. The low prevalence of HBeAg-positivity or high-level viremia, particularly in East African FBAAs, may in part explain the low frequency of vertical transmission, because the risk of vertical transmission is in direct correlation with the viral load of the mother at the time of delivery (30). For example, in Namibia, where only 15% of HBsAg-positive mothers are HBeAg-positive, only 1% of children under 6 months of age are HBsAg-positive; however, HBsAg prevalence reaches 13% in children aged more than 1 year, and this increases further with age (31). Similar findings have been reported in Somalia, Kenya, and Ethiopia (32–34).
Sexual transmission, an important route for HBV infection in adults, was the most common source of infection in USAAs. A history of STDs and/or same-sex sexual activities was reported at much higher frequencies in USAAs than in FBAAs. Nonetheless, venereal transmission of HBV remains an important mode of transmission in African communities. Certain social practices of the region may contribute to this phenomenon, including polygamy and forced marriage of young girls to older men (35, 36). A study in Mauritania linked the prevalence of HBV in pregnant women with risk factors such as age, marital status, polygamy, and the number of children. Other studies showed similar results in West Africa (37, 38).
The natural history of chronic HBV infection is complex, consisting of several clinical phenotypes or phases (39, 40). Most of the published data to date on the natural history of HBV infection were derived from studies of individuals of Asian descent, and it is unknown whether the same principles apply to persons of African descent who acquired infection at a later stage in life and are infected with different genotypes. In our study, there were significant differences in the HBV phenotypes among the different groups of African-American participants. USAAs were more likely to have HBeAg-positive active hepatitis B than were FBAAs. Among FBAAs, East Africans were more likely than West Africans to be inactive carriers. Whether these differences may be attributed to viral (e.g., HBV subgenotype) or host (e.g., age at time of infection, genetic predisposition) factors remain to be fully determined.
One of the most interesting findings in this study was the geographic distribution of HBV genotypes (41). Genotype A was most common among East African FBAAs and USAAs, while among West Africans FBAAs, genotype A was a minority type with genotype E being predominant. In light of the anthropological background that most USAAs are of West African ancestry, the discrepancy in HBV genotypes between modern-day West African FBAAs and USAAs raises an interesting epidemiologic question. Our data showed that USAA patients are predominantly infected with subtype A2, which is the European subtype, while East African FBAAs are predominantly infected with subtype A1. The fact that USAAs have a higher prevalence of sexual risk factors suggests that HBV infection in USAAs may have been acquired in adolescence or adulthood from the community at large. HCC has been reported to present at a younger age in Africans with chronic HBV infection, and HBV subtype A1 has been associated with a high risk of HCC in young noncirrhotic persons (42–44). Thus, while clinical characteristics of HBV infection in USAAs and FBAAs appeared to be distinct from each other, the extent to which HBV genotype and subtype drives these differences remains to be determined, given the close correlation between the subtype and geographic origin in our data. Further study is warranted as these data will influence decisions about the age at which surveillance for HCC should begin.
The predominance of genotype E in our West African FBAAs is consistent with previous epidemiologic surveys in the region, including Cote d'Ivoire, Cameroon, and Ghana. In contrast, the complete absence of genotype E among USAAs has been hypothesized to indicate that genotype E and, to a lesser extent, subtype A3 were disseminated in West Africa after the end of the transatlantic slave trade (45). However, the most recent data suggest that HBV genotypes and subtypes have been in existence for thousands of years during human migration (46). It is possible that HBV vaccination programs in all newborns in the United States since 1992 have prevented spread of HBV genotype E from West African FBAAs to USAAs in the last 2 decades (47, 48).
Although this study drew from the largest cohort of African Americans with HBV infection, the number of participants included was modest. Other limitations of the study include the fact that risk factors for HBV infection were as reported by the participant, and not directly observed, and that most patients were recruited at academic centers, potentially decreasing the generalizability of the data to the entire African-American population. Nonetheless, the data clearly show that HBV infection in African Americans is a heterogeneous entity with diversity in the mode of transmission, clinical phenotypes, and virological characteristics. The study also highlights the differences between East and West African FBAAs. While some of these differences may be driven by HBV genotype, the extent to which other epidemiologic and host-specific factors also contribute remains to be studied. We conclude that HBV infection in persons of African descent is not a monomorphic entity, and our results may be relevant not only to American patients but also to recent African immigrants living in Europe and other parts of the globe. Our data highlight that strategies for prevention and management of chronic HBV infection need to be tailored separately for FBAA and USAA communities.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Gastroenterology, Hepatology, and Nutrition, Department of Internal Medicine, School of Medicine, University of Minnesota, Minneapolis, Minnesota (Mohamed A. Hassan); Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, California (W. Ray Kim); Department of Biostatistics, School of Public Health, University of Texas Health Science Center at Houston, Houston, Texas (Ruosha Li); Medstar Georgetown Transplant Institute, Georgetown University, Washington DC (Coleman I. Smith); Division of Gastroenterology and Hepatology, Department of Medicine, School of Medicine, University of North Carolina, Chapel Hill, North Carolina (Michael W. Fried); Division of Gastroenterology, Hepatology, and Nutrition, Department of Internal Medicine, School of Medicine, Virginia Commonwealth University, Richmond, Virginia (Richard K. Sterling); National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland (Marc G. Ghany); Department of Biostatistics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania (Abdus S. Wahed); Division of Viral Hepatitis, Center for Disease Control and Prevention, Atlanta, Georgia (Lilia M. Ganova-Raeva); Division of Gastroenterology and Hepatology, Mayo Clinic College of Medicine and Science, Rochester, Minnesota (Lewis R. Roberts); and University of Michigan, Ann Arbor, Michigan (Anna S. F. Lok).
This Hepatitis B Research Network study was funded by U01 grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants DK082843 to L.R.R., DK082863 to A.S.F.L., DK082867 to M.W.F., DK082923 to R.K.S., and DK082864 to A.S.W and R.L.), an interagency agreement with NIDDK (grant A-DK-3002-001 to L.M. G.), and support from the intramural program, NIDDK, National Institutes of Health (NIH) to M.G.G.; the National Center for Advancing Translational Sciences (NCATS) (grant UL1TR000058 to R.K.S.); a Clinical and Translational Science Award (grants UL1TR001111 to M.W.F. and UL1RR024986 to A.S.L.); the NIDDK (grant K24 DK92336 to W.R.K.); and by Gilead Sciences, Inc., and Roche Molecular Systems via a Cooperative Research and Development Agreement through the NIDDK.
The Hepatitis B Research Network: Harvard Consortium: Daryl T-Y Lau, MD, MPH (Beth Israel Deaconess Medical Center, Boston, Massachusetts), Raymond T. Chung, MD (Massachusetts General Hospital, Boston Massachusetts). Saint Louis Midwest Hep B Consortium: Adrian M. Di Bisceglie, MD (Saint Louis University School of Medicine, St Louis, Missouri), Mauricio Lisker-Melman, MD (Washington University, St. Louis, Missouri). University of Toronto Consortium: Harry L. A. Janssen, MD, PhD (Toronto Western & General Hospitals, Toronto, Ontario), David K. Wong, MD (Toronto Western & General Hospitals, Toronto, Ontario), Joshua Juan, MD (Toronto Western & General Hospitals, Toronto, Ontario), Jordan Feld, MD, MPH (Toronto Western & General Hospitals, Toronto, Ontario), Colina Yim (Toronto Western & General Hospitals, Toronto, Ontario), Jenny Heathcote, MD (Toronto Western & General Hospitals, Toronto, Ontario). HBV CRN North Texas Consortium: William M. Lee, MD (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas), Robert Perrillo, MD (Baylor University Medical Center, Dallas, Texas), Son Do, MD (University of Texas Southwestern, Dallas, Texas). Los Angeles Hepatitis B Consortium: Steven-Huy B. Han, MD (David Geffen School of Medicine, UCLA, Los Angeles, California), Tram T. Tran, MD (Cedars Sinai Medical Center, Los Angeles, California). San Francisco Hepatitis B Research Group Consortium: Norah A. Terrault, MD, MPH (University of California-San Francisco), Mandana Khalili, MD, MAS (Department of Medicine, University of California- San Francisco, San Francisco, California), Stewart L. Cooper, MD (California Pacific Medical Center, Research Institute & Sutter Pacific Medical Foundation, Division of Hepatology, San Francisco, California). Michigan Hawaii Consortium: Robert J. Fontana, MD (University of Michigan, Ann Arbor, Michigan), Naoky Tsai, MD (University of Hawaii/Queen's Medical Center, Honolulu, Hawaii). Chapel Hill, NC Consortium: Keyur Patel, MD (Duke University Medical Center, Durham, North Carolina), Donna Evon, PhD (University of North Carolina at Chapel Hill, Chapel Hill, North Carolina). PNW/Alaska Clinical Center Consortium: Robert C. Carithers, MD (University of Washington Medical Center, Seattle, Washington), Margaret Shuhart, MD (Harborview Medical Center, Seattle, Washington), Kris V. Kowdley, MD (Virginia Mason Medical Center, Seattle, Washington), Chia C. Wang, MD (Harborview Medical Center, Seattle, Washington). Liver Diseases Branch, NIDDK: T. Jake Liang, MD (National Institutes of Health, Bethesda, Maryland). Immunology Center: Kyong-Mi Chang, MD (University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania), Jang-June Park, PhD (University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania). Data Coordinating Center: Steven Belle, PhD, MScHyg (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania). Central Pathology: David Kleiner, MD, PhD (Center for Cancer Research, National Cancer Institute, NIH, Bethesda, Maryland).
The Hepatitis B Research Network thanks the following: Harvard Consortium: Dr. Nezam Afdhal, Dr. Asad Javaid, Jianghe Niu, Johanna Han, Dr. Imad Nasser (Beth Israel Deaconess Medical Center, Boston, Massachusetts). Minnesota Alliance for Research in Chronic Hepatitis BAlisha C. Stahler, Linda Stadheim (Mayo Clinic Rochester, Rochester, Minnesota). Saint Louis Midwest Hep B Consortium: Debra L. King (Saint Louis University School of Medicine, St. Louis, Missouri), Rosemary A. Nagy (Washington University, St. Louis, Missouri). University of Toronto Consortium: Danie La (Toronto Western & General Hospitals, Toronto, Ontario), Lucie Liu (Toronto Western & General Hospitals, Toronto, Ontario). HBV CRN North Texas Consortium: Stacey Minshall (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas), Sheila Bass (University of Texas Southwestern, Dallas, Texas). Los Angeles Hepatitis B Consortium: Dr. Samuel French, Velma Peacock (David Geffen School of Med, UCLA, Los Angeles, California). San Francisco Hepatitis B Research Group Consortium: Ashley Ungermann, Claudia Ayala, Emma Olson, Ivy Lau (University of California-San Francisco), Veronika Podolskaya, Nata DeVole (California Pacific Medical Center, Research Institute). Michigan Hawaii Consortium: Dr. Barbara McKenna, Kelly Oberhelman, Sravanthi Kaza, B Pharm, Cassandra Rodd (University of Michigan, Ann Arbor, Michigan), Leslie Huddleston, Dr. Peter Poerzgen (University of Hawaii/Queen's Medical Center, Honolulu, Hawaii). Chapel Hill, NC Consortium: Dr. Jama M. Darling, Dr. A. Sidney Barritt, Tiffany Marsh, Vikki Metheny, Danielle Cardona (University of North Carolina at Chapel Hill, Chapel Hill, North Carolina). Virginia Commonwealth University Medical Center Dr. Velimir A. Luketic, Paula G. Smith, Charlotte Hofmann (Virginia Commonwealth University Health System, Richmond, Virginia). PNW/Alaska Clinical Center Consortium: Terri Mathisen, Susan Strom (University of Washington Medical Center, Seattle, Washington) Jody Mooney, Lupita Cardona-Gonzalez (Virginia Mason Medical Center, Seattle, Washington). Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH: Nancy Fryzek, Elenita Rivera, Nevitt Morris, Vanessa Haynes-Williams. Immunology Center: Mary E. Valiga, Keith Torrey, Danielle Levine, James Keith, Dr. Michael Betts (University of Pennsylvania, Philadelphia, Pennsylvania), Luis J. Montaner, DPhil (Wistar Institute, Philadelphia, Pennsylvania). Data Coordinating Center: Dr. Yona Cloonan, Dr. Michelle Danielson, Tamara Haller, Geoffrey Johnson, Stephanie Kelley, Sharon Lawlor, Manuel Lombardero, Joan M. MacGregor, Andrew Pelesko, Donna Stoliker, Barbara Walters, Ella Zadorozny (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania).
Conflicts of interest: W.R.K. is on the consulting/advisory board for Gilead Sciences, Inc. C.I.S. received research support from Gilead, Abbvie, Merck, Bristol-Myers Squibb, Salix, Lumena, and Janssen and is on the consulting/advisory board for Gilead, Abbvie, Bristol-Myers Squibb, and Janssen. C.I.S. has also received speaker fees from Gilead, Abbvie, Janssen, and Bayer. M.W.F. received research support from Abbvie, Bristol-Myers Squibb, Gilead, Genetech, Janssen, Merck, and Vertex and is on the consulting/advisory board for Abbvie, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Janssen, Merck, and Vertex. R.K.S. received research support from Roche/Genetech, Merck, Bayer, Bristol-Myers Squibb, Abbvie, Gilead, Vertex, and Boehringer-Ingelheim and is on the consulting/advisory board for Bristol-Myers Squibb, Gilead, Bayer, Salix, Abbvie, and Janssen. L.R.R. received research support from Wako Diagnostics, Inova Diagnostics, Gilead, BTG International, and ARIAD Pharmaceuticals and is on the consulting/advisory board for Gilead. A.S.F.L. received research support from Bristol-Myers Squibb and is on the consulting/advisory board for Gilead. The other authors report no conflicts.
REFERENCES
- 1. Custer B, Sullivan SD, Hazlet TK, et al. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38(10 suppl 3):S158–S168. [DOI] [PubMed] [Google Scholar]
- 2. Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346(22):1682–1683. [DOI] [PubMed] [Google Scholar]
- 3. McQuillan G, Townsend TR, Fields HA, et al. Seroepidemiology of hepatitis B virus infection in the United States. 1976–1980. Am J Med. 1989;87(3):5S–10S. [DOI] [PubMed] [Google Scholar]
- 4. Coleman PJ, McQuillan GM, Moyer LA, et al. Incidence of hepatitis B virus infection in the United States, 1976–1994: estimates from the National Health and Nutrition Examination Surveys. J Infect Dis. 1998;178(4):954–959. [DOI] [PubMed] [Google Scholar]
- 5. Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in US households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology. 2016;63(2):388–397. [DOI] [PubMed] [Google Scholar]
- 6. Ioannou GN. Hepatitis B virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011;154(5):319–328. [DOI] [PubMed] [Google Scholar]
- 7. Rossi C, Shrier I, Marshall L, et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PLoS One. 2012;7(9):e44611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowdley KV, Wang CC, Welch S, et al. Prevalence of chronic hepatitis B among foreign‐born persons living in the United States by country of origin. Hepatology. 2012;56(2):422–433. [DOI] [PubMed] [Google Scholar]
- 9. Ugwu C, Varkey P, Bagniewski S, et al. Sero-epidemiology of hepatitis B among new refugees to Minnesota. J Immigr Minor Health. 2008;10(5):469–474. [DOI] [PubMed] [Google Scholar]
- 10. LeFevre ML; US Preventive Services Task Force . Screening for hepatitis B virus infection in nonpregnant adolescents and adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(1):58–66. [DOI] [PubMed] [Google Scholar]
- 11. Weinbaum CM, Williams I, Mast EE, et al. Recommendations for Identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008; 57(RR-8):1–20. [PubMed] [Google Scholar]
- 12. Rein DB, Lesesne SB, O'Fallon A, et al. Prevalence of hepatitis B surface antigen among refugees entering the United States between 2006 and 2008. Hepatology. 2010;51(2):431–434. [DOI] [PubMed] [Google Scholar]
- 13. André F. Hepatitis B epidemiology in Asia, the middle East and Africa. Vaccine. 2000;18(suppl 1):S20–S22. [DOI] [PubMed] [Google Scholar]
- 14. Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. [DOI] [PubMed] [Google Scholar]
- 15. Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. [DOI] [PubMed] [Google Scholar]
- 16. Ezzikouri S, Pineau P, Benjelloun S. Hepatitis B virus in the Maghreb region: from epidemiology to prospective research. Liver Int. 2013;33(6):811–819. [DOI] [PubMed] [Google Scholar]
- 17. Kiire C. Hepatitis B infection in sub-Saharan Africa. The African Regional Study Group. Vaccine. 1990;8(suppl):S107–S112. [DOI] [PubMed] [Google Scholar]
- 18. Forbi JC, Ben-Ayed Y, Xia GL, et al. Disparate distribution of hepatitis B virus genotypes in four sub-Saharan African countries. J Clin Virol. 2013;58(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hübschen JM, Mbah PO, Forbi JC, et al. Detection of a new subgenotype of hepatitis B virus genotype A in Cameroon but not in neighbouring Nigeria. Clin Microbiol Infect. 2011;17(1):88–94. [DOI] [PubMed] [Google Scholar]
- 20. Forbi JC, Vaughan G, Purdy MA, et al. Epidemic history and evolutionary dynamics of hepatitis B virus infection in two remote communities in rural Nigeria. PLoS One. 2010;5(7):e11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology. 1988;94(2):439–442. [DOI] [PubMed] [Google Scholar]
- 22. Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghany M, Perrillo R, Li R, et al. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol. 2015;13(1):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ganova-Raeva L, Ramachandran S, Honisch C, et al. Robust hepatitis B virus genotyping by mass spectrometry. J Clin Microbiol. 2010;48(11):4161–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norder H, Couroucé AM, Coursaget P, et al. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47(6):289–309. [DOI] [PubMed] [Google Scholar]
- 26. Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care of chronic viral hepatitis: the Chronic Hepatitis Cohort Study. Clin Infect Dis. 2013;56(2):40–50. [DOI] [PubMed] [Google Scholar]
- 27. Fasano M, Saracino A, Carosi G, et al. Hepatitis B and immigrants: a SIMIT multicenter cross-sectional study. Infection. 2013;41(1):53–59. [DOI] [PubMed] [Google Scholar]
- 28. Kiire C. The epidemiology and prophylaxis of hepatitis B in sub-Saharan Africa: a view from tropical and subtropical Africa. Gut. 1996;38(suppl 2):S5–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whittle HC, McLauchlan K, Bradley AK, et al. Hepatitis B virus infection in two Gambian villages. Lancet. 1983;321(8335):1203–1206. [DOI] [PubMed] [Google Scholar]
- 30. Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190(9):489–492. [DOI] [PubMed] [Google Scholar]
- 31. Botha JF, Dusheiko GM, Ritchie M, et al. Hepatitis B virus carrier state in black children in Ovamboland: role of perinatal and horizontal infection. Lancet. 1984;323(8388):1210–1212. [DOI] [PubMed] [Google Scholar]
- 32. Bile KM, Aden A, Lindberg G, et al. Epidemiology of hepatitis B in Somalia: inference from a cross-sectional survey of serological markers. Trans R Soc Trop Med Hyg. 1987;81(5):824–828. [DOI] [PubMed] [Google Scholar]
- 33. Hyams KC, Okoth FA, Tukei PM, et al. Epidemiology of hepatitis B in eastern Kenya. J Med Virol. 1989;28(2):106–109. [DOI] [PubMed] [Google Scholar]
- 34. Tsega E, Tsega M, Mengesha B, et al. Transmission of hepatitis B virus infection in Ethiopia with emphasis on the importance of vertical transmission. Int J Epidemiol. 1988;17(4):874–879. [DOI] [PubMed] [Google Scholar]
- 35. Bile K, Abdirahman M, Mohamud O, et al. Late seroconversion to hepatitis B in a Somali village indicates the important role of venereal transmission. J Trop Med Hyg. 1991;94(6):367–373. [PubMed] [Google Scholar]
- 36. Wasley A, Grytdal S, Gallagher K. Surveillance for acute viral hepatitis—United States, 2006. MMWR Surveill Summ. 2008;57(2):1–24. [PubMed] [Google Scholar]
- 37. Mansour W, Malick FZ, Sidiya A, et al. Prevalence, risk factors, and molecular epidemiology of hepatitis B and hepatitis delta virus in pregnant women and in patients in Mauritania. J Med Virol. 2012;84(8):1186–1198. [DOI] [PubMed] [Google Scholar]
- 38. Candotti D, Danso K, Allain JP. Maternofetal transmission of hepatitis B virus genotype E in Ghana, West Africa. J Gen Virol. 2007;88(Pt 10):2686–2695. [DOI] [PubMed] [Google Scholar]
- 39. Kim BK, Revill PA, Ahn SH. HBV genotypes: relevance to natural history, pathogenesis and treatment of chronic hepatitis B. Antivir Ther. 2011;16(8):1169–1186. [DOI] [PubMed] [Google Scholar]
- 40. Hadziyannis SJ. Natural history of chronic hepatitis B in Euro-Mediterranean and African countries. J Hepatol. 2011;55(1):183–191. [DOI] [PubMed] [Google Scholar]
- 41. Kramvis A, Paraskevis D. Subgenotype A1 of HBV–tracing human migrations in and out of Africa. Antivir Ther. 2012;18(3 Pt B):513–521. [DOI] [PubMed] [Google Scholar]
- 42. Ochwoto M, Chauhan R, Gopalakrishnan D, et al. Genotyping and molecular characterization of hepatitis B virus in liver disease patients in Kenya. Infect Genet Evol. 2013;20:103–110. [DOI] [PubMed] [Google Scholar]
- 43. El Khouri M, dos Santos VA. Hepatitis B: epidemiological, immunological, and serological considerations emphasizing mutation. Rev Hosp Clin Fac Med Sao Paulo. 2004;59(4):216–224. [DOI] [PubMed] [Google Scholar]
- 44. Kramvis A, Kew MC. Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatol Res. 2007;37(s1):S9–S19. [DOI] [PubMed] [Google Scholar]
- 45. Andernach IE, Hunewald OE, Muller CP. Bayesian inference of the evolution of HBV/E. PLoS One. 2013;8(11):e81690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paraskevis D, Magiorkinis G, Magiorkinis E, et al. Dating the origin and dispersal of hepatitis B virus infection in humans and primates. Hepatology. 2013;57(3):908–916. [DOI] [PubMed] [Google Scholar]
- 47. Andernach IE, Nolte C, Pape JW, et al. Slave trade and hepatitis B virus genotypes and subgenotypes in Haiti and Africa. Emerg Infect Dis. 2009;15(8):1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kew MC, Kramvis A, Yu MC, et al. Increased hepatocarcinogenic potential of hepatitis B virus genotype A in Bantu‐speaking sub‐Saharan Africans. J Med Virol. 2005;75(4):513–521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.