Abstract
We examined associations between ambient air pollution and hepatic steatosis among 2,513 participants from the Framingham (Massachusetts) Offspring Study and Third Generation Cohort who underwent a computed tomography scan (2002–2005), after excluding men who reported >21 drinks/week and women who reported >14 drinks/week. We calculated each participant's residential-based distance to a major roadway and used a spatiotemporal model to estimate the annual mean concentrations of fine particulate matter. Liver attenuation was measured by computed tomography, and liver-to-phantom ratio (LPR) was calculated. Lower values of LPR represent more liver fat. We estimated differences in continuous LPR using linear regression models and prevalence ratios for presence of hepatic steatosis (LPR ≤ 0.33) using generalized linear models, adjusting for demographics, individual and area-level measures of socioeconomic position, and clinical and lifestyle factors. Participants who lived 58 m (25th percentile) from major roadways had lower LPR (β = −0.003, 95% confidence interval: −0.006, −0.001) and higher prevalence of hepatic steatosis (prevalence ratio = 1.16, 95% confidence interval: 1.05, 1.28) than those who lived 416 m (75th percentile) away. The 2003 annual average fine particulate matter concentration was not associated with liver-fat measurements. Our findings suggest that living closer to major roadways was associated with more liver fat.
Keywords: air pollution, computed tomography, fine particulate matter, hepatic steatosis, liver fat
Nonalcoholic fatty liver disease refers to the presence of hepatic steatosis among individuals in the absence of excessive alcohol use or other causes of secondary fat accumulation (1, 2). Hepatic steatosis is very common, with a global prevalence of approximately 25% (3), and is associated with a large clinical and economic burden. In the United States alone, over 64 million adults are estimated to have hepatic steatosis, and the annual direct and societal costs are estimated to be $292 billion (4). Hepatic steatosis is closely associated with insulin resistance and cardiovascular disease, and patients with nonalcoholic fatty liver disease may show a spectrum of alterations ranging from simple hepatic steatosis to steatohepatitis, cirrhosis, and hepatocellular carcinoma (1, 2). Risk factors for hepatic steatosis include older age, male sex, obesity, diabetes, hyperlipidemia, and lifestyle factors such as consumption of sugar-sweetened beverages (1, 2, 5).
In animal studies, higher exposure to fine particulate matter (particulate matter having an aerodynamic diameter of ≤2.5 μm (PM2.5)) has been associated with abdominal obesity (6, 7) and hepatic lipid accumulation (8–10). Air pollution–induced oxidative stress and systemic inflammation have been hypothesized as underlying mechanisms (11–13). Inhaled air pollutants could induce hepatotoxicity by promoting release of proinflammatory cytokines into circulation or by translocating into circulation through alveolar wall (14). Among C57BL/6 mice fed with a high-fat chow diet, exposure to PM2.5 for 6 weeks was associated with inflammation in Kupffer cells and greater hepatic steatosis progression compared with mice exposed to filtered air (9). Zheng et al. (10) found development of hepatic steatosis, inflammation, and fibrosis in the liver of C57BL/6 mice fed a regular chow diet after a 10-week exposure to PM2.5. Higher air pollution may be associated with more liver fat in humans (15). In a few human studies, investigators examined associations between air pollution and aminotransferase levels and reported mixed results (16–19). However, aminotransferase levels are not good measures of hepatic steatosis—the majority of participants with hepatic steatosis may have normal aminotransferase levels (20). Computed tomography is a practical and reliable, noninvasive method that assesses hepatic steatosis by measuring attenuation, a marker of fat accumulation (21). Currently there is a lack of data about the association between air pollution and computed tomography-based measures of liver fat.
We therefore examined the associations of residential ambient PM2.5 pollution and proximity to the major roadways with liver-fat attenuation, assessed by multidetector computed tomography (MDCT), among participants from the Framingham Offspring Study and the Framingham Third Generation Cohort. MDCT-based liver-fat measurement has been shown to have high reproducibility (22). Our results may provide insight into the hypothesized associations between ambient air pollution and hepatic steatosis in free-living human populations.
METHODS
Study population
We included participants from the Framingham Offspring Study examination 7 (1998–2001) and the Third Generation Cohort examination 1 (2002–2005), in Massachusetts. Selection criteria and study design of the 2 cohorts have been previously described (23, 24). Between 2002 and 2005, eligible participants from the 2 examinations were enrolled in the MDCT study (25). For inclusion in the MDCT study, men were aged ≥35 years, women were aged ≥40 years and not pregnant, and all participants weighed <350 lb (160 kg), to accommodate the physical constraints of the scanner (25). Abdominal MDCT scans were obtained between 2002 and 2005, and data on demographics, medical history, smoking history, and alcohol intake were collected by questionnaire at each examination visit (1998–2001 for the Offspring Study and 2002–2005 for the Third Generation Cohort). A total of 3,158 participants underwent abdominal MDCT scan and had valid liver-fat measurements. Physical examinations were performed following standardized protocols. After excluding 444 participants who did not have valid measurements of PM2.5 concentrations or distance to major roadways, we excluded 81 participants who had missing information on educational attainment, cigarette-smoking status, pack-years of smoking, or alcohol intake, and we further excluded men who reported >21 drinks/week and women who reported >14 drinks/week (n = 120), based on the American Association for the Study of Liver Diseases practice guideline and to be consistent with previous work in the Framingham Heart Study (5, 26), leaving a total of 2,513 participants in our analysis. All participants provided written informed consent, and institutional review boards at Beth Israel Deaconess Medical Center, Boston University Medical Center, and Massachusetts General Hospital approved the study.
PM2.5 assessment
Participants’ residential addresses were collected during the examination visit (1998–2001 for the Offspring Study and 2002–2005 for the Third Generation Cohort) and geocoded using ArcGIS software (Esri, Redlands, California). We then estimated residential ambient PM2.5 concentrations at a 1 × 1 km2 resolution using a spatiotemporal model.
Satellite-based aerosol optical depth (AOD) is a measure of light attenuation in the atmospheric column (27). We employed a novel hybrid spatiotemporal model that used AOD data from the Moderate Resolution Imaging Spectroradiometer (MODIS), spatial predictors, and temporal predictors (27). The hybrid model incorporated day-specific AOD calibration to ground level PM2.5, which allowed us to predict PM2.5 on days when AOD data were not available. We used advanced Multi-Angle Implementation of Atmospheric Correction (MAIAC) algorithms to achieve a higher resolution (1 × 1 km2) (27).
Briefly, we first fitted a model regressing monitor-based PM2.5 concentrations against satellite-based AOD product, adjusting for land-use terms and meteorological variables. We used inverse probability weighting to address nonrandom missingness of daily AOD data. Predictions from this model had an excellent mean out-of-sample R2 of 0.88 (year-to-year variation of 0.82–0.90 for the years 2003–2011) and an excellent fit when comparing predictions with observations (slope = 0.99, year-to-year variation of 0.98–1.01 for the years 2003–2011) (27). Second, we predicted grid cells that had only AOD data available using the above fitted model. Third, for grid cells/days that had missing AOD measurements, we imputed data using a generalized additive model with smoothing and a random intercept for each grid cell. The overall mean out-of-sample R2 for this stage of PM2.5 predictions was 0.88, with small year-to-year variation (0.84–0.91). Last, for each residential address, we regressed residuals (differences between monitor-based measurement and predicted values for each cell) against monitor-specific spatial and temporal variables to generate daily local predictions. The total PM2.5 daily concentration estimates were then calculated as the sum of grid and localized predictions. We used the average PM2.5 concentration from the same index year (2003) for all participants, similar to our previous work (28, 29).
Distance to major roadways
A major roadway was defined as a primary highway with limited access (A1), a primary road without limited access (A2), or a secondary or connecting road (A3). For each participant we calculated residential distance to major roadways. We restricted our analyses to participants who lived within 1,000 m of major roadways (n = 2,230) for proximity analyses, because distance may not be an informative surrogate of traffic-related air pollution in semirural or rural areas. As in our previous work (28, 29), we classified participants into 5 groups based on the distance of their residential address to major roadways (<50.0 m, 50.0–99.9 m, 100.0–199.9 m, 200.0–399.9 m, and 400.0–999.9 m) to reflect the typical decreasing pattern of traffic-related air pollutants with distance to road (30).
MDCT protocol and liver-fat attenuation
The attenuation of the liver on MDCT scan relative to the MDCT penetrance of a calibration control is a noninvasive method to assess liver fat (22, 31). Details of liver-fat assessment in the MDCT study have been previously described (22). Briefly, each participant underwent abdominal MDCT scan in the supine position. An 8-slice scanner (LightSpeed Ultra, General Electric, Milwaukee, Wisconsin) was used to obtain 25 contiguous 5-mm thick slices (120 kVp, 400 mA, gantry rotation time 500 ms, table feed 3:1) covering 125 mm above the level of the first sacral vertebra. The MDCT Hounsfield units were measured in 3 areas of the liver and 1 area from a white external phantom control (calibration control). We then calculated the liver-to-phantom ratio (LPR) by dividing the average Hounsfield units of the liver by the Hounsfield units of the phantom control (22). Lower values of LPR represent more liver fat. In a validation study with 100 MDCT participants, the MDCT-based LPR was shown to be reproducible, with high intraclass correlation of 0.99 for both intrareader and interreader comparisons (22).
We analyzed LPR as a continuous variable first, and we defined the presence of hepatic steatosis as having LPR ≤ 0.33. This cutpoint has been shown to have a sensitivity of 70% and specificity of 98% for detecting hepatic steatosis compared with using a liver-to-spleen ratio of 1.1 (the gold standard) (32).
Statistical methods
We used multivariable linear regression models to estimate differences in continuous LPR, and, to estimate prevalence ratios, we used multivariable generalized linear models with a log link function and Poisson error distribution and robust variance for a binary indicator of hepatic steatosis (33). Model assumptions were assessed by residual plots, and no severe violations were noted. Covariates were selected a priori based on subject-matter knowledge. We adjusted for age at MDCT scan, (age at MDCT scan)2, sex, cigarette-smoking status (current, former, or never), pack-years of smoking, alcohol intake (drinks/week; standardized to 0.5 oz (15 mL) of alcohol per drink) (34), educational attainment (high school or less, some college, college graduate), physical activity index (in tertiles) (35), usual occupation (laborer, sales/homemaker/clerical, professional/executive/supervisory/technical, and unspecified) (36), antihypertensive medication use, statin use, quartile of median household income in the participant's census tract in 2000, census tract median value of owner-occupied housing units, census tract population density (population/km2), cardiovascular disease, diabetes, and an exam identifier. Based on our previous work (37, 38) and our hypothesis about the dispersion pattern of air pollutants, residential distance to major roadways was loge transformed.
We conducted several sensitivity analyses. Because body mass index may be an intermediate in the association between air pollution and liver-fat accumulation, we did not include it in the primary model, but we added body mass index in a sensitivity analysis because it may be a confounder. Because cardiovascular disease and diabetes may identify subsets of the population with different susceptibility for the associations between air pollution and health outcomes (39, 40), we evaluated the robustness of our results in a sensitivity analysis excluding those participants. Because these conditions were relatively rare, we were unable to evaluate the associations restricting analyses to those with cardiovascular disease or diabetes. By definition, nonalcoholic fatty liver disease can be assessed only among individuals with relatively low levels of alcohol intake (1). To explore the influence of potential misclassification of alcohol consumption, we conducted sensitivity analyses that excluded men who reported drinking more than 14 servings of alcohol per week and women who reported drinking more than 7 servings of alcohol per week. We also explored whether associations differed by median age, sex, or a binary indicator of educational level (high school or less vs. some college or higher) by adding interaction terms to the model. To explore whether participants who had certain levels of liver fat were more affected by exposure, we applied quantile regression analyses (41, 42) and examined the associations at the 25th, 50th, and 75th percentiles of the distribution of LPR. Additionally, we evaluated the influence of choosing 2003 as the index year by using a 3-year average PM2.5 (2003–2005) concentration, and we explored the influence of time trend by additionally adjusting for date of MDCT scan (continuous variable), days between MDCT scan and examination visit, and the year of MDCT scan (categorical variable). We also assessed the influence of excluding participants who lived ≥1,000 m from the nearest major roadway by including them in the analyses. Some of the Offspring Study participants moved between examinations 6 and 7; to assess the influence of address changes, we conducted a sensitivity analysis excluding the participants who moved. Because the sample sizes for PM2.5-concentration and distance analyses were different, we additionally examined whether the associations differed if we restricted the analyses to those with valid measurements of both PM2.5 concentration and residential proximity. Last, we conducted a set of minimally adjusted analyses that accounted only for age and sex.
Results for the analyses based on residential-based, estimated annual average PM2.5 concentrations in 2003 were scaled by 1.4 μg/m3, which approximated the interquartile range. Results from the residential proximity analyses were scaled by a factor of −2, which approximated contrasting participants who lived 58 m (25th percentile of the distribution of distance to major roadways) from a major roadway to those who lived 416 m (75th percentile) away.
Scaled regression coefficients and prevalence ratios were reported with 95% confidence intervals. In evaluations of potential effect measure modification, a 2-tailed P value of <0.05 was considered statistically significant. Analyses were performed using Proc GENMOD, and Proc QUANTREG in SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina). Figures were plotted using Stata, version 13 (StataCorp LP, College Station, Texas).
RESULTS
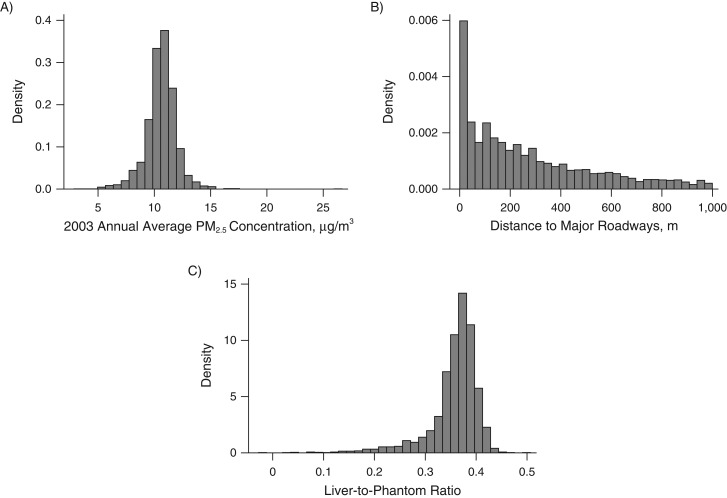
Table 1 shows the characteristics of the study population. The mean age of the study population at the time of MDCT study was 52.6 (standard deviation, 11.9) years; 52.7% were women. The residential-based estimated 2003 annual mean PM2.5 concentration was 10.6 μg/m3, which was lower than the current US Environmental Protection Agency's National Ambient Air Quality Standard (12.0 μg/m3). The distributions of 2003 annual average PM2.5, distance to major roadways, and LPR are shown in Figure 1. Of the whole study sample (n = 2,513), 17.4% (n = 436) had hepatic steatosis (LPR ≤ 0.33).
Table 1.
Characteristics of 2,513 Participants From the Multi-Detector Computed Tomography Studya, Boston, Massachusetts, 2002–2005.
| Characteristics | No. of Participants | % | Mean (SD) |
|---|---|---|---|
| Offspring Study cohort | 1,024 | 40.8 | |
| Age at the time of MDCT scan, years | 52.6 (11.9) | ||
| Women | 1,323 | 52.7 | |
| Alcohol consumption, drinks/weekb | 3.8 (4.6) | ||
| Current cigarette smoker | 307 | 12.2 | |
| Former cigarette smoker | 952 | 37.9 | |
| Education | |||
| High school or less | 580 | 23.1 | |
| Some college | 813 | 32.4 | |
| College graduate | 1,120 | 44.6 | |
| Antihypertensive medication use | 474 | 18.9 | |
| Statins use | 314 | 12.5 | |
| Cardiovascular disease | 174 | 6.9 | |
| Diabetes | 146 | 5.8 | |
| Liver-to-phantom ratio | 0.36 (0.05) | ||
| Hepatic steatosisc | 436 | 17.4 | |
| Annual average PM2.5 concentration (2003), μg/m3 | 10.6 (1.4) | ||
| Distance to major roadways, md | 271 (253) | ||
| Category of distance to major roadways, md | |||
| <50.0 | 514 | 20.5 | |
| 50.0–99.9 | 213 | 8.5 | |
| 100.0–199.9 | 396 | 15.8 | |
| 200.0–399.9 | 518 | 20.6 | |
| 400.0–999.9 | 589 | 23.4 |
Abbreviations: MDCT, multidetector computed tomography; PM2.5, particulate matter having an aerodynamic diameter of ≤2.5 μm; SD, standard deviation.
a All MDCT scans were conducted between 2002 and 2005. Age and cardiovascular events were updated to the date of MDCT scans; other baseline covariates were collected during 1998–2001 for participants from the Framingham Offspring Study examination 7 and during 2002–2005 for those from the Third Generation Cohort examination 1.
b Standardized to 0.5 oz (15 mL) of alcohol per drink.
c Defined as having a liver-to-phantom ratio of ≤0.33.
d We excluded 283 participants who lived more than 1,000 m from major roadways.
Figure 1.
Histograms of 2003 annual average PM2.5 concentrations (A), distance to major roadways (B), and liver-to-phantom ratio (C) in participants from the Multi-Detector Computed Tomography Study, Boston, Massachusetts, 2002–2005.
In the multivariable analyses (Table 2 and Table 3), participants who lived within 100 m of major roadways had more liver fat (lower LPR) than those who lived 400–1,000 m from major roadways. There was a log-linear relationship between distance to a major roadway and liver fat: Comparing participants who lived 58 m from major roadways with those who lived 416 m away, living closer to major roadways was associated with more liver fat (lower LPR) (multivariable-adjusted β = −0.003, 95% confidence interval (CI): −0.006, −0.001) (Table 2, model 3). We also observed higher prevalence of hepatic steatosis (LPR ≤ 0.33) among participants who lived closer to a major roadway (multivariable-adjusted prevalence ratio PR = 1.16, 95% CI: 1.05, 1.28) (Table 3, model 3). However, the 2003 annual PM2.5 concentration was not associated with LPR or hepatic steatosis (LPR ≤ 0.33) (Tables 2 and 3).
Table 2.
Associations of Distance to Major Roadways and 2003 Annual Average PM2.5 Concentration With Liver-to-Phantom Ratio Among Participants From the Multi-Detector Computed Tomography Study, Boston, Massachusetts, 2002–2005
| Exposure | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | |
| PM2.5 concentrationd | 0.001 | −0.001, 0.002 | 0.001 | −0.001, 0.003 | 0.000 | −0.002, 0.002 |
| Loge distancee | −0.004 | −0.006, −0.001 | −0.003 | −0.006, −0.001 | −0.003 | −0.006, −0.001 |
| Distance category, m | ||||||
| <50.0 | −0.005 | −0.011, 0.001 | −0.004 | −0.010, 0.002 | −0.004 | −0.010, 0.002 |
| 50.0–99.9 | −0.011 | −0.019, −0.003 | −0.010 | −0.018, −0.003 | −0.010 | −0.018, −0.003 |
| 100.0–199.9 | −0.003 | −0.009, 0.004 | −0.002 | −0.008, 0.004 | −0.002 | −0.008, 0.004 |
| 200.0–399.9 | −0.001 | −0.007, 0.005 | 0.000 | −0.006, 0.006 | 0.000 | −0.006, 0.005 |
Abbreviation: CI, confidence interval; MDCT, multidetector computed tomography; PM2.5, particulate matter having an aerodynamic diameter of ≤2.5 μm.
a Model 1 adjusted for age at MDCT scan, (age at MDCT scan)2, sex, and an exam identifier.
b Model 2 included the model 1 covariates plus cigarette-smoking status (current, former, or never), pack-years of smoking, alcohol intake, educational level, usual occupation, physical activity, antihypertensive medication use, statin use, quartile of median household income in the participant's census tract in 2000, median value of owner-occupied housing units in the census tract, and population density (population/km2) in the census tract.
c Model 3 included the model 2 covariates plus cardiovascular disease and diabetes.
d Scaled to be equivalent to per-1.4-μg/m3 increase in 2003 annual PM2.5 concentrations.
e Scaled to approximate comparing participants who lived 58 m (25th percentile) from the nearest major roadway with those who lived 416 m (75th percentile) from the nearest major roadway.
Table 3.
Associations of Distance to Major Roadways and 2003 Annual Average PM2.5 Concentration With Prevalence of Hepatic Steatosisa Among Participants From the Multi-Detector Computed Tomography Study, Boston, Massachusetts, 2002–2005
| Exposure | Model 1b | Model 2c | Model 3d | |||
|---|---|---|---|---|---|---|
| PR | 95% CI | PR | 95% CI | PR | 95% CI | |
| PM2.5 concentratione | 0.98 | 0.90, 1.05 | 0.96 | 0.89, 1.05 | 0.97 | 0.89, 1.06 |
| Loge distancef | 1.19 | 1.07, 1.32 | 1.16 | 1.05, 1.28 | 1.16 | 1.05, 1.28 |
| Distance category, m | ||||||
| <50.0 | 1.31 | 1.01, 1.69 | 1.20 | 0.93, 1.55 | 1.21 | 0.94, 1.55 |
| 50.0–99.9 | 1.48 | 1.08, 2.02 | 1.43 | 1.04, 1.96 | 1.44 | 1.05, 1.98 |
| 100.0–199.9 | 1.14 | 0.86, 1.52 | 1.08 | 0.81, 1.44 | 1.09 | 0.81, 1.46 |
| 200.0–399.9 | 1.00 | 0.76, 1.31 | 0.93 | 0.71, 1.23 | 0.95 | 0.72, 1.25 |
Abbreviation: CI, confidence interval; MDCT, multidetector computed tomography; PM2.5, particulate matter having an aerodynamic diameter of ≤2.5 μm; PR, prevalence ratio.
a Defined as a liver-to-phantom ratio of ≤0.33.
b Model 1 adjusted for age at MDCT scan, (age at MDCT scan)2, sex, and an exam identifier.
c Model 2 included the model 1 covariates plus cigarette-smoking status (current, former, or never), pack-years of smoking, alcohol intake, educational level, usual occupation, physical activity, antihypertensive medication use, statin use, quartile of median household income in the participant's census tract in 2000, median value of owner-occupied housing units in the census tract, and population density (population/km2) in the census tract.
d Model 3 included the model 2 covariates plus cardiovascular disease and diabetes.
e Scaled to be equivalent to per-1.4-μg/m3 increase in 2003 annual PM2.5 concentrations.
f Scaled to approximate comparing participants who lived 58 m (25th percentile) from the nearest major roadway with those who lived 416 m (75th percentile) from the nearest major roadway.
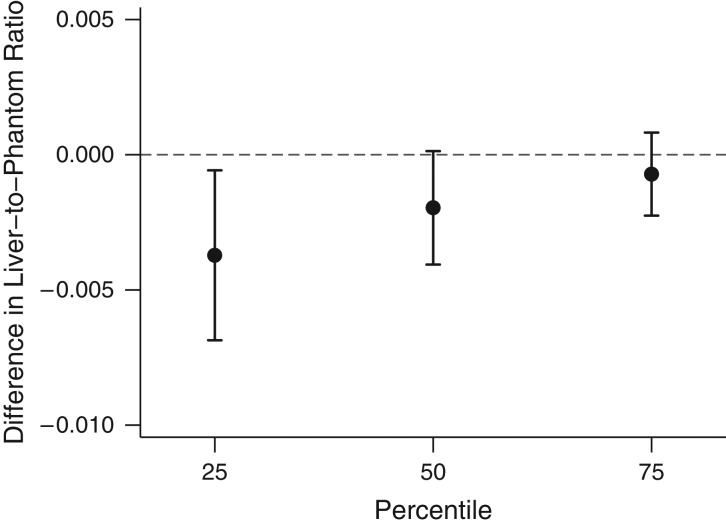
We separately examined the associations between PM2.5 concentration and distance to major roadways with the 25th, 50th, and 75th percentile of the LPR distribution using quantile regression models. The results showed that the 25th percentile of the LPR distribution shifts more than the 75th percentile for the same increase in distance to major roadways, which suggested stronger associations between proximity and liver fat among participants who had more liver fat than among those who had less liver fat (Figure 2).
Figure 2.
Associations between distance to the nearest major roadway and the 25th, 50th, and 75th percentiles of the distribution of liver-to-phantom ratio in participants from the Multi-Detector Computed Tomography Study, Boston, Massachusetts, 2002–2005. Models adjusted for age at MDCT scan and (age at MDCT scan)2, sex, cigarette-smoking status (current, former, or never), pack-years of smoking, alcohol intake, educational level, usual occupation, physical activity, antihypertensive medication use, statin use, quartile of median household income in the participant's census tract in 2000, median value of owner-occupied housing units in the census tract, population density (population/km2) in the census tract, cardiovascular disease, diabetes, and an exam identifier. Results were scaled to approximate comparing participants who lived 58 m from the nearest major roadway with those who lived 416 m from the nearest major roadway. Bars: 95% confidence intervals.
The age- and sex-adjusted analyses showed similar association pattern to that of the full-adjustment model (Tables 2 and 3). The observed associations did not differ by median age, sex, or educational attainment (Web Tables 1 and 2, available at https://academic.oup.com/aje). Results from sensitivity analyses are summarized in Web Table 3. Adding body mass index to the models, excluding men who reported >14 drinks/week and women who reported >7 drinks/week, or excluding participants with cardiovascular disease or diabetes did not change our results substantially. The associations were not altered when using the 2003–2005 average PM2.5 concentrations, adjusting for time covariates, or restricting analyses to those with complete measurements of both PM2.5 concentration and distance. Excluding participants who moved between Offspring Study examinations 6 and 7 did not materially alter our results. The associations were slightly attenuated after including participants who lived ≥1,000 m from major roadways in the analyses (Web Table 4).
DISCUSSION
In the present study, we observed more liver fat and a higher prevalence of hepatic steatosis (LPR ≤ 0.33) among participants who lived closer to major roadways than among those who lived further away. We found no association between residential-based estimates of annual average PM2.5 and liver fat. Participants with more liver fat appeared to have stronger associations between residential proximity to a major roadway and liver fat than did those with less liver fat. The associations observed in our study were comparable in magnitude to a recent report of the association between liver fat and consuming sugar-sweetened beverages (median = 1 drink/week) compared with nonconsumers (5).
Our present study extended findings in animal studies to a large cohort of adults. Reports from controlled animal studies suggested positive associations between PM2.5 concentration and overall and abdominal obesity (6, 7) as well as liver-fat accumulation (8–10). For example, after exposing mice fed high-fat chow to PM2.5 for 6 weeks (85 μg/m3, 6 hours/day), Tan et al. (9) found a higher degree of hepatic inflammation and fibrosis than that found in mice that were exposed to filtered air. In another study, mice fed with regular chow that were exposed to PM2.5 for 10 weeks (74.6 μg/m3, 6 hours/day) showed a nonalcoholic steatohepatitis–like phenotype, with disrupted hepatic glycogen storage, glucose tolerance, and insulin resistance (10). However, the PM2.5 concentrations in the controlled animal studies were higher than the ambient PM2.5 levels in our study, and the relevance of animal studies to assessing the associations of ambient air pollution with hepatic steatosis in humans is unclear.
Individuals with diabetes may be more susceptible to associations between air pollution and liver fat. In an animal study, Tomaru et al. (8) found fatty changes in livers of diabetic obese mice, but not among nondiabetic mice, after intratracheally administering 100 μg of diesel exhaust particles every 2 weeks for 12–18 weeks. We did not have sufficient statistical power to examine whether the observed associations differed by diabetes status. However, excluding participants with cardiovascular disease or diabetes did not materially change our results.
In the present study, we found positive associations between living closer to a major roadway and more liver fat, but the positive associations were not observed for the annual average PM2.5 concentrations. This discrepancy might be explained by differences in these 2 exposure metrics. The distance to a major roadway relates to near-road exposures more closely than the satellite model–based PM2.5 predictions; it represents multiple traffic-related factors such as vehicle emissions (both particulate and gaseous pollutants), road dust, traffic noise, traffic light, and possible psychological stress induced by these near-road exposures (43, 44). In the present study, distance to major roadways was weakly correlated with 2003 annual average PM2.5 concentrations (r = −0.2; P < 0.0001). On the other hand, the model-based PM2.5 concentrations include sources beyond local traffic (27). Both local and regional emission sources contribute to the air pollution levels. For example, the primary air pollutant black carbon was most likely from local traffic, residential heating, and cooking, while the sulfate particles were likely transported from other regions. As in any observational study, our findings might be influenced by residual confounding that was highly correlated with living closer to a roadway and more liver fat but not with PM2.5 predictions. However, we adjusted for a large set of potential confounders in our models, including area-level socioeconomic position. Additionally, our study region was in the northeastern United States, where the air pollution levels were relatively low; as shown in Figure 1, the 2003 annual average PM2.5 concentration was 10.6 μg/m3, with a standard deviation of 1.4 μg/m3 and an interquartile range of 1.4 μg/m3. The relatively low levels of PM2.5 and small amount of variation in exposure may contribute to the null associations in our study.
There are several limitations to our study that should be considered. Participants enrolled in the MDCT study were generally healthy, predominantly white individuals of European ancestry and were middle-aged. As a result, our observations may not be generalizable to populations of different age groups, ethnicities, socioeconomic positions, or lifestyles. As in any cross-sectional observational study, we cannot exclude the possibility of residual confounding, unmeasured confounding, or uncertainty of temporality. Results from our study should not be used to infer causality. However, we have adjusted for potential confounders, including demographic characteristics, lifestyle, and individual and area-level socioeconomic position in our models. We did not adjust for diet in our analyses. Diet could be a confounder of the association between air pollution and liver-fat accumulation; however, to the extent that diet is related to other social factors, we partially accounted for it by including several individual and area-level measures of socioeconomic position in the models. Information on lifestyle factors was collected from self-reported questionnaires, and there may be some misclassification or measurement errors, which are likely nondifferential.
Our study also had several strengths. The study population was composed of participants enrolled in relatively large and well-characterized cohorts. Data were collected using standardized protocols for physical examinations and MDCT scan. We constructed models that adjusted for a robust set of potential confounders, including demographic characteristics, lifestyle, and individual and area-level measures of socioeconomic position. We also adjusted for individual physical activity in the analyses. We employed a novel spatiotemporal model to estimate annual average PM2.5 concentrations at participants’ home addresses. We used MDCT, a technique with high interreader and intrareader reliability, to quantify liver-fat attenuation. Finally, the assessment of air pollution and liver fat were performed independently of each other.
In summary, we found that living closer to a major roadway was associated with more liver fat (lower LPR) and higher prevalence of hepatic steatosis (LPR ≤ 0.33). However, we observed no association of residential-based annual average PM2.5 concentrations with liver fat. Future longitudinal studies with repeated liver-fat measurements are warranted to confirm or refute our findings, and to extend these results by examining progression of liver-fat accumulation in relation to ambient air pollution.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Wenyuan Li, Kirsten S. Dorans, Elissa H. Wilker, Joel Schwartz, Murray A. Mittleman); Cardiovascular Epidemiology Research Unit, Division of Cardiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts (Wenyuan Li, Kirsten S. Dorans, Elissa H. Wilker, Mary B. Rice, Murray A. Mittleman); Division of Pulmonary, Critical Care and Sleep Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts (Mary B. Rice); Division of Gastroenterology, Boston Medical Center, Boston University School of Medicine, Boston, Massachusetts (Michelle T. Long); Framingham Heart Study (National Heart, Lung, and Blood Institute), Framingham, Massachusetts (Michelle T. Long, Caroline S. Fox); Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Joel Schwartz, Petros Koutrakis, Diane R. Gold); Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Brent A. Coull); Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Diane R. Gold); and Division of Intramural Research, National Heart, Lung, and Blood Institute, Bethesda, Maryland (Caroline S. Fox).
This work was supported by the National Heart, Lung and Blood Institute (grants HHSN268201500001I, N01-HC 25195 and T32HL007575); the US Environmental Protection Agency (grants RD-834798 and RD-835872); and the National Institutes of Environmental Health Sciences (grants P01 ES09825, K23ES026204, R00 ES022243, and P30ES000002).
The contents are solely the responsibility of the grantee and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the US Department of Health and Human Services; or the US Environmental Protection Agency. Further, the US Environmental Protection Agency does not endorse the purchase of any commercial products or services mentioned in the publication.
Conflicts of interest: E.H.W. received nonfinancial support from Servier Laboratories, Neuilly-sur-Seine, France. C.S.F. owns stock in and receives salary from Merck. The other authors report no conflicts.
REFERENCES
- 1. Martin-Dominguez V, Gonzalez-Casas R, Mendoza-Jimenez-Ridruejo J, et al. . Pathogenesis, diagnosis and treatment of non-alcoholic fatty liver disease. Rev Esp Enferm Dig. 2013;105(7):409–420. [DOI] [PubMed] [Google Scholar]
- 2. Than NN, Newsome PN. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239(1):192–202. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Koenig AB, Abdelatif D, et al. . Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Blissett D, Blissett R, et al. . The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. [DOI] [PubMed] [Google Scholar]
- 5. Ma J, Fox CS, Jacques PF, et al. . Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol. 2015;63(2):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun Q, Yue P, Deiuliis JA, et al. . Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119(4):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu X, Yavar Z, Verdin M, et al. . Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30(12):2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomaru M, Takano H, Inoue K, et al. . Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int J Mol Med. 2007;19(1):17–22. [PubMed] [Google Scholar]
- 9. Tan HH, Fiel MI, Sun Q, et al. . Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J Immunotoxicol. 2009;6(4):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng Z, Xu X, Zhang X, et al. . Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol. 2013;58(1):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Z, Xu X, Zhong M, et al. . Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol. 2011;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu X, Liu C, Xu Z, et al. . Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124(1):88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mendez R, Zheng Z, Fan Z, et al. . Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Transl Res. 2013;5(2):224–234. [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JW, Park S, Lim CW, et al. . The role of air pollutants in initiating liver disease. Toxicol Res. 2014;30(2):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tarantino G, Capone D, Finelli C. Exposure to ambient air particulate matter and non-alcoholic fatty liver disease. World J Gastroenterol. 2013;19(25):3951–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomao E, Baccolo TP, Sacchi L, et al. . Harm to the liver among employees of the Municipal Police Force. Int J Environ Health Res. 2002;12(2):145–151. [DOI] [PubMed] [Google Scholar]
- 17. Markevych I, Wolf K, Hampel R, et al. . Air pollution and liver enzymes. Epidemiology. 2013;24(6):934–935. [DOI] [PubMed] [Google Scholar]
- 18. Pan WC, Wu CD, Chen MJ, et al. . Fine particle pollution, alanine transaminase, and liver cancer: a Taiwanese Prospective Cohort Study (REVEAL-HBV). J Natl Cancer Inst. 2016;108(3):djv341. [DOI] [PubMed] [Google Scholar]
- 19. Kim KN, Lee H, Kim JH, et al. . Physical activity– and alcohol-dependent association between air pollution exposure and elevated liver enzyme levels: an Elderly Panel Study. J Prev Med Public Health. 2015;48(3):151–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Browning JD, Szczepaniak LS, Dobbins R, et al. . Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. [DOI] [PubMed] [Google Scholar]
- 21. Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Speliotes EK, Massaro JM, Hoffmann U, et al. . Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23(6):894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kannel WB, Feinleib M, McNamara PM, et al. . An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110(3):281–290. [DOI] [PubMed] [Google Scholar]
- 24. Splansky GL, Corey D, Yang Q, et al. . The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165(11):1328–1335. [DOI] [PubMed] [Google Scholar]
- 25. Fox CS, Massaro JM, Hoffmann U, et al. . Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 26. Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. [DOI] [PubMed] [Google Scholar]
- 27. Kloog I, Chudnovsky AA, Just AC, et al. . A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ. 2014;95:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rice MB, Ljungman PL, Wilker EH, et al. . Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham Heart Study. Am J Respir Crit Care Med. 2015;191(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilker EH, Preis SR, Beiser AS, et al. . Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015;46(5):1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou Y, Levy JI. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. 2007;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwasaki M, Takada Y, Hayashi M, et al. . Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78(10):1501–1505. [DOI] [PubMed] [Google Scholar]
- 32. Long MT, Wang N, Larson MG, et al. . Nonalcoholic fatty liver disease and vascular function: cross-sectional analysis in the Framingham heart study. Arterioscler Thromb Vasc Biol. 2015;35(5):1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 34. Elias PK, Elias MF, D'Agostino RB, et al. . Alcohol consumption and cognitive performance in the Framingham Heart Study. Am J Epidemiol. 1999;150(6):580–589. [DOI] [PubMed] [Google Scholar]
- 35. Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139(8):857–861. [PubMed] [Google Scholar]
- 36. Loucks EB, Lynch JW, Pilote L, et al. . Life-course socioeconomic position and incidence of coronary heart disease: the Framingham Offspring Study. Am J Epidemiol. 2009;169(7):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenbloom JI, Wilker EH, Mukamal KJ, et al. . Residential proximity to major roadway and 10-year all-cause mortality after myocardial infarction. Circulation. 2012;125(18):2197–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilker EH, Mostofsky E, Lue SH, et al. . Residential proximity to high-traffic roadways and poststroke mortality. J Stroke Cerebrovasc Dis. 2013;22(8):e366–e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dubowsky SD, Suh H, Schwartz J, et al. . Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114(7):992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li W, Wilker EH, Dorans KS, et al. . Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham Heart Study. J Am Heart Assoc. 2016;5(5):e002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bind MA, Peters A, Koutrakis P, et al. . Quantile regression analysis of the distributional effects of air pollution on blood pressure, heart rate variability, blood lipids, and biomarkers of inflammation in elderly American Men: the Normative Aging Study. Environ Health Perspect. 2016;124(8):1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koenker RW, Bassett G. Regression quantiles. Econometrica. 1978;46(1):33–50. [Google Scholar]
- 43. Jerrett M, McConnell R, Wolch J, et al. . Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health. 2014;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McConnell R, Shen E, Gilliland FD, et al. . A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children's Health Study. Environ Health Perspect. 2015;123(4):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.