Abstract
The common and severe psychiatric disorders, including major depressive disorder (MDD) and bipolar disorder (BD), are associated with inflammation, oxidative stress and changes in lipid metabolism. Those pathways are implicated in the premature development of vascular and metabolic comorbidities, which account for considerable morbidity and mortality, including increased dementia risk. During endoplasmic reticulum stress, the soluble epoxide hydrolase (sEH) enzyme converts anti-inflammatory fatty acid epoxides generated by cytochrome p450 enzymes into their corresponding and generally less anti-inflammatory, or even pro-inflammatory, diols, slowing the resolution of inflammation. The sEH enzyme and its oxylipin products are elevated post-mortem in MDD, BD and schizophrenia. Preliminary clinical data suggest that oxylipins increase with symptoms in seasonal MDD and anorexia nervosa, requiring confirmation in larger studies and other cohorts. In rats, a soluble sEH inhibitor mitigated the development of depressive-like behaviors. We discuss sEH inhibitors under development for cardiovascular diseases, post-ischemic brain injury, neuropathic pain and diabetes, suggesting new possibilities to address the mood and cognitive symptoms of psychiatric disorders, and their most common comorbidities.
Keywords: omega-3 fatty acids, soluble epoxide hydrolase, lipidomics, depression, drug development, ER stress, NLRP3, inflammasome, metabolism, mitochondrial dysfunction, dementia, neuroprogression, neurodegeneration, obesity, diabetes, heart disease, stroke
1. Inflammation and anti-inflammatory agents in depression
The phenomenon of increased inflammation in MDD has been well-established, with most evidence stemming from the measurement of cytokine messengers in peripheral blood, notably interleukin-6 (IL-6), tumor necrosis factor (TNF) and others (Dowlati et al., 2009; Kohler et al., 2017). In MDD, treatment with antidepressants, specifically the selective serotonin reuptake inhibitors, may reduce TNF and IL-6 (Hannestad et al., 2011) suggesting that some cytokines may be transient state markers (Goldsmith et al., 2016). These inflammatory signals appear to be relevant to mood states across the spectra of common and severe psychiatric disorders, including MDD, BD and schizophrenia (Goldsmith et al., 2016), due in part to production of psychoactive tryptophan metabolites that result from cytokine signaling (Schwarcz et al., 2012).
Despite this evidence, support for anti-inflammatory agents as antidepressants has been mixed, showing generally minimal clinical benefit. The strongest evidence seems to support the use of celecoxib, a selective cyclooxygenase 2 inhibitor, when used as an adjunct to monoaminergic antidepressants (Kohler et al., 2014). Specifically, in those with inflammatory comorbidity, treatment with anti-cytokine therapies has been shown to benefit mood symptoms (Kappelmann et al., 2016), and a study in treatment resistant depression suggested that the anti-TNF antibody infliximab may have mood benefits only in patients who have evidence of elevated peripheral inflammatory activity (i.e. C-reactive protein [CRP]) (Raison et al., 2013).
Although inflammation may be involved in psychiatric disorders, the evidence overwhelmingly indicates high heterogeneity in this relationship (Kohler et al., 2007), likely indicating that particular subsets of patients may benefit from anti-inflammatory treatments. Such treatments will require more accurate and specific quantitative biomarkers that can be reproduced reliably between laboratories to inform their use. The extensive variability in the measurement of cytokine levels within and between laboratories (Noble et al., 2008), and substantial heterogeneity between studies comparing cytokines between patients to controls (Kohler et al., 2007), or comparing patients to themselves pre- vs. post-treatment (Hannestad et al., 2011), suggests suboptimal utility as diagnostic or prognostic biomarkers. Considerably less work has examined the possibility of lipid metabolic biomarkers, although prostaglandins measured quantitatively have shown promise (Calabrese et al., 1986; Nishino et al,1989.; Ohishi et al., 1989). There exists a clear need to identify specific markers of inflammation in depressive disorders, the sources of heterogeneity in related inflammatory pathways, and the underlying causes. There is always the worry that blood or urinary biomarkers whether cytokine or lipid may not reflect levels in the central nervous system.
2. Inflammatory, vascular and metabolic comorbidities of depression
There has been much speculation about the causes of increased inflammation in depression, including effects of stress, poor nutrition, physical inactivity, obesity, smoking, gut permeability, disturbances in commensal gut microbiota, mitochondrial dysfunction, autoimmity, and sleep disturbances (Anderson 2017; Berk et al.,2013; Robertson et al., 2017). In a meta-analysis, Howren et al. showed that CRP elevations in MDD were mediated by obesity (Howren et al., 2017), which is highly prevalent in MDD and a risk factor for the development of diabetes and cardiovascular diseases that contribute prominently to the excess morbidity and mortality associated with psychiatric disorders (de Melo et al., 2017).
The increased risk for the development of cardiovascular disease associated with MDD and BD has been recognized recently in a consensus statement from the American Heart Association (Goldstein et al., 2015). Emerging evidence suggests that this risk begins in early life presentations, manifest as elevated cardiovascular and metabolic risk factors such as higher BMI, cardiac pulse pressure, and IL-6 (Hatch et al., 2017), and increased cognitive vulnerability to those risk factors. In one study, elevated triglycerides and diastolic blood pressure were associated with poorer executive function in adolescents with BD, a diathesis which was not identified in unaffected adolescents (Naiberg et al., 2016). In young male adults with depression, subclinical atherosclerosis, as indicated by intima-media thickening of the carotid arteries, was found (Elovainio et al., 2005), and in mid- and later-life, depressive disorders carry an increased risk of heart disease and stroke (Pan et al., 2011; Van der Kooy et al., 2007).
While causality is difficult to discern, elevations in inflammatory markers may increase over time with exposure to a mood disorder, mediated largely by changes in lifestyle factors including lack of physical activity (Figure 1). In a study of healthy elderly, depressive symptoms contributed to increased IL-6 and CRP over time, but the reverse effect was not observed (Stewart et al., 2009). Similarly, in a study of outpatients with coronary artery disease, depressive symptoms predicted increased IL-6 and CRP over 5 years, but baseline IL-6 and CRP did not predict subsequent depressive symptoms. Moreover, the prospective association could be explained by physical inactivity, BMI and smoking (Duivis et al., 2011). Therefore, the epidemiology does not unilaterally support a causal relationship for inflammation leading to depression; however, inflammation is now well-known to be involved in the development of obesity, atherosclerosis and type 2 diabetes (Libby et al., 2009; Winer et al., 2014), which are commonly comorbid with depression. In BD, adipose accumulation is accelerated during episodes (Fiedorowicz et al., 2015) and increased adiposity is seen in MDD across the lifespan, from adolescence (Coryell et al., 2016) to older adults with heart disease (Swardfager et al., 2010). Therefore, these comorbidities are likely contributors to findings of increased inflammation in MDD and BD. Logically, anti-inflammatory agents might then be targeted to mitigate the effects of depressive disorders on cardiac and metabolic health. The question remains as to which specific inflammatory pathways might be useful both for diagnosis and therapy.
Figure 1. Hypothetical relationships between depressive disorders, vascular risk factors and cognitive vulnerability mediated by inflammatory activity and oxidative stress.
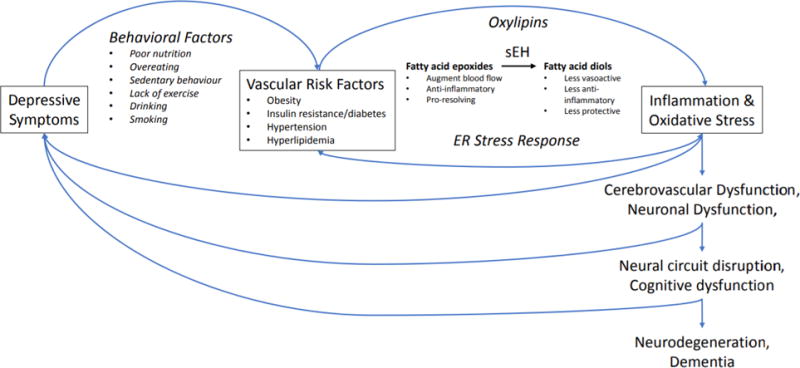
Anti-inflammatory lipid mediators (e.g. fatty acid epoxides) are metabolized by soluble epoxide hydrolase (sEH) into less beneficial (less bioactive) and sometimes even pro-inflammatory or cytotoxic oxylipin diol species. Increased sEH activity in the brain and vasculature in depressive disorders may confer vulnerability to vascular risk factors that affect neurovascular coupling/cerebral blood flow/brain metabolism and contribute to cognitive and mood symptoms, and predispose cerebrovascular disease that contribute to neuroprogression and cognitive decline.
Identifying particular inflammatory pathways elevated in psychiatric disorders that support cardiovascular disease progression has been challenging. Inflammation promotes atherosclerosis via a number of mechanisms, some involving TNF or P-selectin activation of vascular endothelial cells, white blood cell infiltration, and promotion of vascular smooth muscle cell proliferation by inflammatory signaling or platelet derived factors (Libby, 2002). Widely used cardiovascular preventative antiplatelet therapy with low-dose aspirin may prevent the onset of depression, particularly in older men with elevated homocysteine (Almeida et al., 2012), and some evidence suggests that adjunctive aspirin may speed the onset of antidepressant action (Mendlewicz et al., 2006). Accordingly, certain platelet derived lipid species, have been associated with depressive symptoms in coronary artery disease patients (Mazereeuw et al., 2015), and with poorer cognitive performance in depressed CAD patients (Mazereeuw et al., 2014). These preliminary results suggest that some platelet-mediated inflammatory lipid pathways may be related to depressive symptoms (Mazereeuw et al., 2013), but further lipidomic studies will be needed to confirm which species may be most relevant.
Considerable attention has been paid to the omega-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are often found to be lower in MDD (summarized in (Otoki et al., 2017)), and which can have anti-inflammatory, antidepressant and cardioprotective properties (Kromhout et al., 2012; Mocking et al., 2016). However, the clinical benefits of omega-3 PUFA treatment have been modest and highly variable (Mocking et al., 2016), which might necessitate precision medicine approaches, such as biomarkers to support their use, such as measures of oxidative stress (Mazereeuw et al., 2017) or structural lipids impacted by changes in omega-3 fatty acid metabolism (Otoki et al., 2017). An alternative approach would be to consider novel treatments that specifically target dysregulation within the pathways that contribute to reductions in omega-3 PUFAs, and perhaps more importantly, to changes in their bioactive metabolites.
3. Inflammatory, anti-inflammatory and pro-resolving lipid species
As shown in Figure 2, PUFAs such as arachidonic acid (AA), EPA and DHA are metabolized by cyclooxygenases (COX) (Smith et al., 2000; Wada et al., 2007), lipoxygenases (LOX) (Funk, 2001; Yamamoto et al., 1988) or cytochrome P450 enzymes (Fer et al., 2008; Morisseau et al., 2010; Spector and Kim, 2015) to produce a plethora of ‘oxylipin’ lipid mediators that regulate inflammation and resolution pathways in tissues. Fatty acid epoxides, derived from AA, EPA and DHA through the cytochrome p450 pathway are potent anti–inflammatory mediators (Capozzi et al., 2016; Morin et al., 2008; Morin et al., 2010; Morisseau et al., 2010; Node et al., 2001), likely acting via G protein-coupled receptors (GPCRs) present in the target tissues (Ding et al., 2014; Liu et al., 2016). These GPCRs and the enzymes within the biosynthetic cascades of the PUFAs that activate them, offer a new and vast arena for the development of novel pharmacotherapies.
Figure 2. Enzymatic synthesis of oxylipins from arachidonic acid (AA; 20:4 n-6), eicosapentaenoic acid (EPA; 20:5 n-3) and docosahexaenoic acid (DHA; 22:6 n-3).
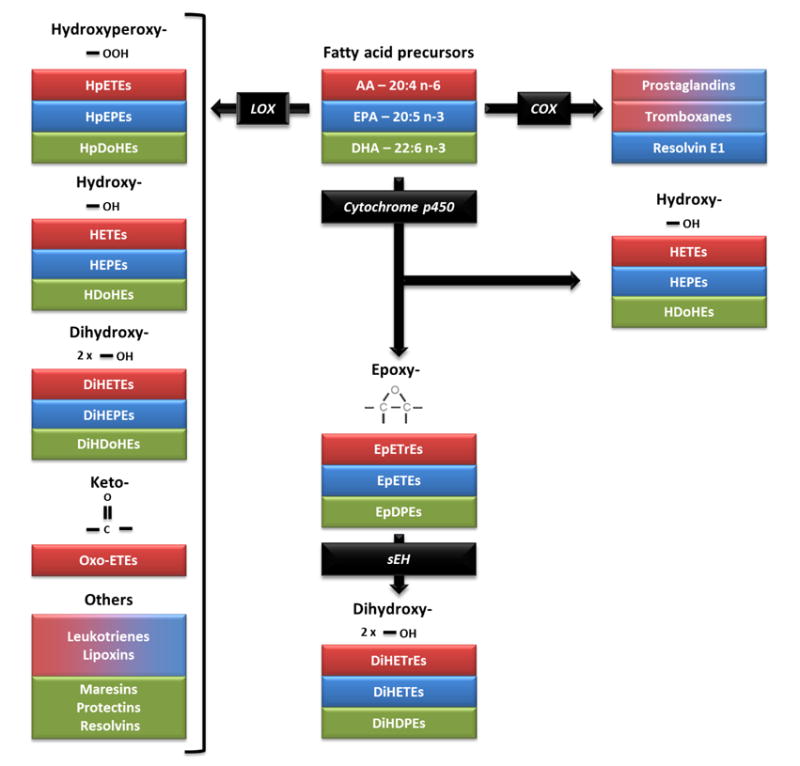
Polyunsaturated fatty acids, including AA, EPA and DHA, are precursors to oxidized fatty acid metabolites, commonly known as oxylipins. In this figure, fatty acid precursors and their respective metabolites share the same color code. Prostaglandins, thromboxanes and resolvins are produced from AA and EPA though the cyclooxygenase pathway (COX) pathway. The lipoxygenase (LOX) pathway produces hydroperoxy-metabolites, such as hydroperoxyeicosatetraenoic acids (HpETEs), hydroperoxyeicosapentaenoic acids (HpEPEs) and hydroperoxydocosahexaenoic acids (HpDoHEs), that are rapidly converted into hydroxy-metabolites, including hydroxyeicosatetraenoic acids (HETEs), hydroxyeicosapentaenoic acids (HEPEs) or hydroxydocosahexaenoic acids (HDoHEs). Hydroxy-metabolites from the LOX pathway can then be converted into dihydroxy metabolites, such as dihydroxyeicosatetraenoic acids (DiHETEs), dihydroxyeicosapentaenoic acids (DiHEPEs) or dihydroxydocosahexaenoic acids (DiHDoHEs), and into keto-metabolites, such as oxo-eicosatetraenoic acids (oxo-ETEs). Leukotrienes and lipoxins from AA and EPA, as well as maresins, protectins and resolvins from DHA are also synthesized through the LOX pathway. Cytochrome p450 enzymes are oxidative enzymes that generate hydroxy-metabolites on activated or terminal carbons or epoxides at olefinic bonds. Epoxides generated by Cytochromie p450s include epoxyeicosatrienoic acids (EpETrEs), epoxyeicosatetraenoic acids (EpETEs) and epoxydocosapentaenoic acids (EpDPEs). These largely anti-inflammatory epoxy-metabolites can be converted largely by soluble epoxide hydrolase (sEH) into their respective diols, dihydroxyeicosatrienoic acids (DiHETrEs), dihydroxyeicostetraenoic acids (DiHETEs) and dihydroxydocosapentaenoic acids (DiHDPES).
Fatty acid epoxides are hydrolyzed by soluble epoxide hydrolase (sEH) and converted to their respective diols (Morisseau et al, 2010.; Zeldin et al., 1993; Zeldin et al., 1995). These diols have been reported as being mostly inactive or less active compared to the epoxide metabolites in regulating cardiovascular function (Lee et al, 1999.; Node et al., 1999) and inflammation (Capozzi et al., 2016; Morin et al., 2008; Node et al., 1999). For this reason, interest has grown in sEH inhibitors as therapeutic agents for several inflammation-related pathologies (Shen and Hammock, 2012), such as myocardial infarction (Kompa et al., 2013; Xu et al., 2013), atherosclerosis (Li et al., 2016; Shen et al., 2015; Ulu et al., 2008; Zhang et al., 2009), inflammatory pain (Inceoglu et al., 2015; Rose et al., 2010; Wagner et al., 2011; Wagner et al., 2013), neuropathic pain (Wagner et al., 2014; Wagner et al., 2017) and metabolic syndrome (Iyer et al., 2012; Luria et al., 2011).
Inhibitors of sEH have yet to pass regulatory approval and attach themselves to a clinical indication, but in vitro and in vivo studies have generated promising results. Some of the leading candidates have already entered/passed early-phase clinical trials (see Table 1). These leading molecular entities include a urea (Table 1: Chemical AR928; also UC1153) or an amide group (Table 1: GSK2256294), which bind directly to the active sites of sEH as transition state mimics. sEH inhibitors of other structures have been identified and described previously, including those having a carbamate structure (Morisseau et al., 1999; Lee et al., 2014).
Table 1.
Structures of sEH inhibitors commonly in research, and three compoundsα used in clinical trials.
| Compound* | Structure | IC50 for human sEH# | Comments |
|---|---|---|---|
| AUDA, UC700 |
|
3nM <11nM> |
Early sEH inhibitor with high lipophilicity and high melting point designed to mimic the 14, 15-EET structure. It is also a PPAR alpha agonist and it would be expected, from its structure, to be biologically active as an EET mimic. All lipophilic high melting sEH inhibitors are only available biologically in true solution in an organic co-solvent. |
| AEPU, UC950 |
|
1nM <5nM> |
A water miscible sEH inhibitor with low melting point, and activity on the sEH from many species. Rapidly penetrates cell membranes, protein binding is low, and it was designed for rapid metabolism |
| t-TUCB, UC1728 |
|
900pM <1nM> |
A lipophilic sEH inhibitor with broad potency across many species. Improved pharmacokinetics compared with AUDA due to removal of the metabolically labile adamantane group. Commonly used in equine and companion animal studies. Lipophilic and high melting point. |
| TPPU, UC1770 |
|
600pM <10nM> |
Widely used and commercially available sEH inhibitor of moderate water solubility. A lipophilic and high melting point compound, which must be administered in an organic co-solvent. High target occupancy. Currently the most commonly used model sEH inhibitor. Most piperidines have low potency outside of primate and rodent species. |
| AR9281α, APAU, UC1154 |
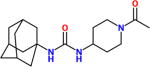
|
15nM <6nM> |
No off-target effects seen in Phase I and II clinical trials in hypertensive – pre-diabetic patients run by Arête Therapeutics. More water soluble than most piperidine sEH inhibitors. Very short half-life in most species. It shows poor target occupancy. Like UC950, it is an excellent tool if a short half-life water soluble compound is needed. |
| TCCα |
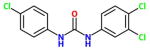
|
13 nM <21nM> {353nM} |
Triclocarban is an old topical antimicrobial commonly used in cosmetics and hard soaps. It is lipophilic and has a short half life due to N-glycosylation. It was successful in a double blind 30 person clinical trial as a topical with diclofenac with diabetic neuropathy. Note the very poor activity on murine enzyme. The study was run by a collaboration between Sphaera and Synthia Dermatec. |
| GSK2256294α |
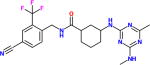
|
400pM [27pM] |
A lipophilic high melting compound run in three Phase I trials, and with some efficacy data on cigarette induced pulmonary disease. Good pharmacokinetics and no adverse effects noted. It is available for experimental use. The pM potency was reported by GSK using a different substrate. |
Several synonyms are used in the literature for the compounds.
IC50 can be highly reproducible but the value depends upon the substrate structure and its concentration as well as other incubation conditions. Unless otherwise noted by <>, {} or [] these values were determined with the spectral substrate CMNPC and the pure human recombinant sEH. Many sEH inhibitors are so powerful that they violate Michaelis-Menton assumptions. Most are transition state mimics showing slow – tight binding kinetics so that the target occupancy driven by kinetic koff appears the best predictor of in vivo efficacy. IC50 on the rat is noted by <>, and IC50 on the mouse is noted by {}.
Due to the anti-inflammatory effects of CYP450 derived lipid epoxides and the less beneficial effects of the sEH derived diols, activation of sEH in depressive disorders suggests a plausible new link. Since cytokines tend to vary between patients (Hannestad et al., 2011), the question before the field is whether oxylipins, in particular fatty acid epoxides and their respective diols, might inform a more specific and targetable inflammatory status in depressive states, and therefore may be more useful in predicting response to current therapies, or in identifying potential new treatments targetting oxylipin diols derived from sEH.
A recent meta-analysis of omega-3 PUFA data demonstrated that EPA-rich omega-3 PUFA may be recommended for the adjunctive treatment of depression (Sarris et al., 2016). In a variety of bioassays, and particularly in inflammation driven models, epoxides of EPA and DHA have proven to have anti-inflammatory activities (Kodani et al., 2015). Given the crucial role of sEH, and to a lesser extent the microsomal epoxide hydorolase (Marowsky et al., 2017), in the metabolism of the anti-inflammatory omega-3 epoxides, it is likely that omega-3 PUFA (particularly EPA), in combination with a sEH inhibitor, would be a novel therapeutic approach for depression (Hashimoto, 2016).
4. Soluble epoxide hydrolase in psychiatric disorders
As discussed, the search for the source of chronic, low-grade inflammation in psychiatric disorders, and for inflammatory mediators of the relationships between psychiatric disorders and adverse cardiovascular and metabolic outcomes, has been met with mixed results. Emerging evidence suggests that sEH activity is elevated in several psychiatric disorders. The ratios of sEH-derived products to their respective substrates (i.e. diol:epoxide ratio) were significantly elevated in anorexia nervosa, an eating disorder characterized by an aversion to fat consumption (Shih et al., 2016). In one small preliminary study of participants with MDD with seasonal pattern (also known as seasonal affective disorder), increased plasma concentrations of fatty acid diols produced by the sEH pathway were reported within-subjects during depressive episodes (Hennebelle et al., 2017). These two studies provide the first evidence in vivo of increased sEH activity in psychiatric disorders, encouraging replication in additional cohorts.
Studies on post-mortem brain also reported increased sEH protein expression in parietal cortex of patients suffering from major depressive disorder, bipolar disorder and schizophrenia (Ren et al., 2016). Consistent with that evidence, pharmacological targeting of sEH was found to reduce inflammation and resolve behavioral impairments in animal models of depression, pain, and schizophrenia (Ma et al., 2013; Ren et al., 2016). Inflammation-induced and social defeat stress models of depression are both characterized by an increase expression of sEH in mouse prefrontal cortex and hippocampus. In these models, a single administration or a chronic intake of a potent sEH inhibitor prevents depression-like behavior such as increased immobility in the tail suspension and forced swim tests, or reduced sucrose preference (Ren et al., 2016). The sEH knock-out mice confer resilience to social defeat stress, identifying a role of sEH in stress resilience (Ren et al., 2016). A single administration of sEH inhibitor has also been reported to reduce hyperlocomotion and restore prepulse inhibition in a phencyclidine (PCP)-induced model of schizophrenia (Ma et al., 2013). Those findings suggest that sEH inhibitors offer potential as therapeutic drugs, which may alleviate stress-responsive symptoms across psychiatric disorders.
Inhibiting sEH activity has also shown promise for other neurological disorders such as epilepsy and seizure. Mouse seizure models are characterized by an increased sEH expression in hippocampus accompanied by an increased in inflammatory markers such as IL-1β and IL-6 (Hung et al., 2015). Administration of sEH inhibitors prevented neuroinflammation and reduced the number and duration of seizures in mouse models (Hung et al., 2015; Inceoglu et al., 2013; Vito et al., 2014). Decreasing neuronal excitability may reduce excitotoxicity that results from excessive calcium influx due to overstimulation of NMDA receptors, which has been proposed as a mediator of inflammation-induced depressive symptoms and neurodegeneration in mood disorders (O’Connor et al., 2009; Schwarcz et al., 2012; Swardfager et al., 2009).
The potential benefits of sEH inhibition in psychiatry might also be mediated partially by direct effects on neurotransmission. Inhibition of sEH has been reported to enhance prefrontal cortex neuronal synaptic neurotransmission, increase field excitatory postsynaptic potentials, the input-output curve, and long-term potentiation in extracellular recordings, by increasing expression of glutamate receptors, including NMDA, AMPA and mGluRs (Wu et al., 2015). sEH inhibition also promotes axonal growth in cultured sensory and cortical neurons (Abdu et al., 2011). The sEH inhibitors therefore offer promise to probe neuronal, vascular and inflammatory processes relevant to the symptoms, progression and complications of psychiatric disorders.
5. Potential benefits of sEH inhibition in protection against depression comorbidities
5.1 Cardiovascular & Cerebrovascular
The peripheral and cerebral vasculature may be vulnerable in psychiatric disorders, including both the large and small blood vessels (Naiberg et al., 2017). The sEH enzyme was identified as a susceptibility factor for heart failure because it increased hydrolysis of cardioprotective epoxyeicosanoid levels (Monti et al., 2008). By genetic knock-out or chemical inhibition of sEH, protection can be afforded in various models of brain and vascular injury, pain, lung and kidney disease, and the development of hypertension and diabetes (Imig and Hammock, 2009; Ingraham et al., 2011). Other inflammatory comorbidities such as asthma are also common in depression (Boudreau et al., 2014; Lavoie et al., 2006), and lipoxin activity in asthma has been related to sEH (Ono et al., 2014). The many potential protective mechanisms related to sEH inhibition remain under investigation, but they have been related to vascular endothelial function, neuroprotection, angiogenesis, anti-platelet actions, reduced oxidative stress, attenuated inflammatory responses to ischemia, and resolution of inflammation (Iliff and Alkayed, 2009). Protective mechanisms of sEH inhibitors may also involve attenuating hyperlipidemia, macrophage and monocyte infiltration into atherosclerotic lesions, and monocyte adhesion to endothelial cells activated by inflammation (notably TNF) and platelets (Li et al., 2016).
Potentially relevant to observations of accelerated thickening of the large vessels in depressive disorders, polymorphisms in sEH genes have been associated with the risk of ischemic stroke in humans (Fava et al., 2010; Gschwendtner et al., 2008). sEH is overexpressed in human atherosclerotic plaque, and it promoted carotid-artery injury induced neointima formation in a rat model by enhancing vascular smooth muscle cell proliferation and migration in response to platelet derived growth factor signaling (Wang et al., 2015). In addition to risk of stroke, sEH may be involved in protection from the resulting ischemic damage. Pharmacological inhibition or genetic knock-out of sEH affords robust protection against ischemic brain injury and increase neuronal survival (Koerner et al., 2007; Yuan et al., 2016; Zhang et al., 2007; Zhang et al., 2008). Pharmacological inhibition of sEH can attenuate astrocyte infiltration, glial scar formation, microglial activation, neuronal apoptosis, infarct volume, blood flow deficiency and behavioral deficits following experimental cerebral ischemia (Dorrance et al., 2005; Qu et al., 2015; Simpkins et al., 2009; Stulc, 1989; Taguchi et al., 2016).
Histopathological evidence from the Oregon Brain Aging Study suggests that elevated sEH may also be involved in vulnerability to disease of the cerebral small vessels, since sEH products were elevated in brain tissue from people with vascular cognitive impairment and vascular endothelial sEH protein levels were elevated in the small vessels of affected tissues (Nelson et al., 2014). It is possible that sEH expression could exacerbate dysfunction of the small vessels, leading to psychiatric symptoms and cognitive deficits due to cardiovascular risk factor-induced perturbations in vascular homeostasis even prior to observable structural brain abnormalities (Vanella et al., 2015).
There is considerable evidence to support the notion that disruption of the neural circuitry of reward is involved in the pathophysiology of mood disorders (Russo and Nestler, 2013). Changes in communication between the nodal structures within reward systems and multiple other networks have been documented in functional MRI studies. The grey matter in the nodal structures depends on its ability to couple increased demand for oxygen and glucose on activation with vasodilatory signals that augment local blood volume. The potential for sEH to attenuate vasodilation when overexpressed (Chadderdon et al., 2016), and hence impair neurovascular coupling, is notable, particularly since sEH is present in the brain and cerebral vasculature affecting the concentrations of vasoactive arachidonic acid metabolites (Nelson et al., 2014). Emerging evidence suggests that microstructural changes in the white matter connecting nodal grey matter structures may also underlie disruption of neural networks (Versace et al., 2013), which would also be consistent with changes in arterioles and the small vessels that penetrate and supply blood to the white matter. The biological bases for neural circuit disruption in mood disorders are likely to be multifactorial. The sEH hypothesis would be consistent with observations of the impact of inflammation on reward systems (Swardfager et al., 2016).
5.2 Neurocognition and Neuroprogression in Depressive Disorders
Deficits in neurocognition detected commonly in MDD and BD are underappreciated but common and important contributors to the burden of mood disorders (Millan et al., 2012). The severity of deficits can increase with the number and severity of successive mood episodes, suggesting a neuroprogressive phenomenon (Berk et al., 2011). The most common cognitive deficits in MDD and BD, including a propensity for poorer executive function, attention, and psychomotor processing speed, resemble those seen in older people due to cerebrovascular disease, which often manifests as hyperintensities in the white matter visible on structural MRI (Hachinski et al., 2006).
Throughout the lifespan, there appears to be a trifecta of cardiovascular dysregulation, depression and cognitive vulnerability. Substantiating the vulnerability of the cerebral vasculature in mood disorders, white matter hyperintensities, particularly in the frontal regions, are more common in BD than unaffected individuals (Aylward et al., 1994; Beyer et al., 2009). Some studies have reported associations between white matter hyperintensities and psychiatric disorders, including BD, MDD and conduct disorder/attention deficit disorder in early life, including in children 12 years of age (Lyoo et al., 2002). Moreover, recent evidence suggests that the small vessels may be particularly vulnerable to vascular risk factors and that these changes may be related to executive function in adolescents with bipolar disorder, as indicated by photography of the retinal blood vessels (Naiberg et al., 2016). Together the cognitive and imaging findings might suggest that the common and severe mood disorders are associated with increased vascular risk factors and a vulnerability of the cerebral vasculature thereto.
There appears to be an interaction between depression and metabolic dysfunction in predicting brain vulnerability, as indicated by increased risks of dementia (Katon et al., 2015), and vascular cognitive impairment (particularly impairments in executive function) (Swardfager and MacIntosh, 2017), in those with both depression and diabetes later in life. In adult BD studies, elevated BMI or insulin resistance appears to moderate the course of the disease and response to treatment (Calkin et al., 2009; Calkin et al., 2015). Diabetes is known to increase the risk of depression (Rotella and Mannucci, 2013a), and vice versa (Rotella and Mannucci, 2013b), although the consequences of metabolic dysfunction on the course and severity of MDD are not as well documented as those in BD. Conversely, the impact of depression on diabetes outcomes appears to be indirect, mediated by health behaviors and specific symptom domains (Carter and Swardfager, 2016). For instance, anhedonia can be particularly difficult to treat pharmacologically, and it appears to have a detrimental impact on glycemic control and mortality in type 2 diabetes that is mediated by decreased physical activity (Nefs et al., 2016). Anhedonia has been related to inflammation (Swardfager et al. 2016) and to small vessel disease (Lavretsky et al., 2008). Neuroprotective benefits of sEH inhibition, particularly given the vasoactive properties of sEH substrates, their protective effects against vascular risk factors, and their metabolic benefits, suggest that they may impact the course of neurocognitive decline.
5.3 Obesity & insulin resistance
Since inflammation in psychiatric disorders may arise in part due to increased rates of obesity (Howren et al., 2009), it may be relevant that sEH inhibitors have been shown to modulate inflammation in adipose tissue in obese mice (Lopez-Vicario et al., 2015); specifically, inhibition of sEH favoured the polarization of macrophages towards an anti-inflammatory M2 phenotype, which is known to protect against the development of insulin resistance and type 2 diabetes (Wang et al., 2014). As reviewed previously (Luther and Brown, 2016), sEH increases in animal models of obesity and diabetes, whereas the epoxylipid sEH substrates promote islet cell function and peripheral tissue insulin sensitivity. Clinically, genetic polymorphisms the she gene EPHX2, and circulating epoxy lipid concentrations, correlate with insulin sensitivity (Luther and Brown, 2016). Moreover, obesity and diabetes are risk factors for cardiovascular disease, and sEH inhibition can improve coronary endothelial function, prevent cardiac remodeling and reduce diastolic dysfunction in obese insulin-resistant mice (Roche et al., 2015). Thus, sEH may have beneficial roles both in maintaining glucose homeostasis, and in protecting the vasculature from the consequences of obesity and diabetes (Kodani and Hammock, 2015; Zuloaga et al., 2014).
Since physical inactivity is a prominent risk factor in depression, it is especially notable that evidence from non-human primates suggests that sEH may be involved in the mechanism by which physical inactivity contributes to deficits in insulin resistance and glucose-stimulated small vessel vasodilation (Chadderdon et al., 2015). Specifically, the ratio of sEH-derived diols to fatty acid epoxide substrates was increased after forced physical inactivity, and this correlated with microvascular insulin resistance and the inability to dilate and augment blood volume. Exercise can have anti-depressant effects, but those effects have been small and inconsistent (Rethorst et al., 2009), suggesting the need to identify molecules that could augment its benefits. Strategies that might be of benefit include augmenting cytochrome p450-derived sEH substrates, or sEH inhibitors, which may be able to augment neurovascular responses in those with sEH activation or increasing the omega-3 to omega-6 ratio in the diet.
6. Perspectives and Synthesis
Adopting a Mediterranean diet has been shown to be beneficial in the prevention of cardiovascular disease and dementia. Recently, a small randomized controlled trial of a modified Mediterranean diet demonstrated antidepressant effects (Jacka et al., 2017). This finding is of interest because the diet has been shown to encourage the formation of endogenous lipid electrophiles (e.g. 10-nitrooctadec-9-enoic acid) that inhibit sEH (Charles et al., 2014). In mice, inhibition of sEH by endogenous nitrolipids seems to be responsible for some of the beneficial effects of the Mediterranean diet (e.g. antihypertensive effects) (Charles et al., 2011). Notably, clinical evidence also suggests that the Mediterranean diet can modify markers of inflammation and endoplasmic reticulum (ER) stress (Yubero-Serrano et al., 2012).
Increasingly, it appears that sEH inhibitors, and mimics of epoxylipids, are effective in situations where the ER stress response is activated. For example, ER stress increases in neuropathic pain and the epoxyeicosatrienoic acids that reduce ER stress markers tend to be effective against neuropathic pain. Moreover, agents apart from epoxylipids that reduce neuropathic pain also tend to reduce ER stress (Inceoglu et al., 2015). As the intracellular hub for protein synthesis, the ER is responsible for supporting cellular homeostasis with protein and lipid synthesis. The ER stress response, a cascade of kinase and phosphatase activation, can signal apoptosis when the ER becomes overwhelmed with excessive demand for protein folding or elimination of misfolded proteins.
Evidence in support of heightened ER stress in MDD is now accumulating. For instance, peripheral expression of BiP, EDEM1, CHOP, and XBP1, the major indicators of the ER stress response, were elevated 1.34-fold to 1.68-fold in community dwelling individuals with MDD (Nevell et al., 2014). Increased ER stress proteins GRP78, GRP94, and calreticulin, have been found in the temporal cortex in MDD post-mortem, and GRP78 appears to be affected by antidepressants (Bown et al., 2000). In animal studies, rats that developed learned helplessness in the inescapable shock paradigm had upregulated GRP78, GRP94, ATF6, and XBP-1 compared to rats who were not subjected to shock, or those that did not develop learned helplessness (Timberlake and Dwivedi, 2015). Collectively, these and other studies have suggested a role for ER stress in the development of depressive disorders due to deficiencies in resilience to chronic stress.
ER stress is activated by reactive oxygen species, which can arise from mitochondrial dysfunction or hyperglycemia, leading some authors to propose that central insulin and peroxisome proliferator-activated receptor-γ (PPAR-γ) systems may be targeted in depression (Gold et al., 2013). It was also noted that a state of innate immune activity, termed “parainflammation”, tends to accompany the ER stress response, which might account for some of the chronic low-grade inflammatory activity seen in MDD and BD (Gold et al., 2013). The inflammatory findings would be consistent with ER stress stimulating inflammasome activity via NLRP3 (Bronner et al., 2015), an important sensor of metabolic dysfunction increasingly thought to link oxidative stress and inflammation in depressive and neurodegenerative disorders, and in type 2 diabetes, obesity and cardiovascular diseases that are commonly comorbid with depressive disorders (Singhal et al., 2014). Since reactive oxygen species are a major factor in activating the ER stress response, one could consider an axis of effects as mitochondrial dysfunction increase ROS, which in turn feed back to cause more mitochondrial dysfunction and also drive the ER stress response from a homeostatic mechanism towards autophagy, apoptosis and inflammation. The demonstrated impact of the epoxylipids and of the sEH inhibitors on processes underlying each of these conditions, suggests a plausible new mechanism by which these harmful cascades may be interrupted. Autophagy, apoptosis and inflammation are implicated in not only psychiatric disorders, but also in neurodegeneration, for which psychiatric disorders are a risk factor.
7. Conclusions
Collectively, the data suggest a need for further work to validate preliminary findings of increased epoxide hydrolase activity in psychiatric disorders. Oxylipins are detectable in peripheral blood and LC-MS/MS oxylipin assays are now available, offering quantitative measures of cytochrome p450 and sEH products that can be standardized between laboratories for clinical studies. Due to the emerging picture of concomitant neural, vascular and metabolic insult in depressive disorders, and their cumulative impact on cognitive and functional decline, protection of the brain and vasculature afforded by dietary and pharmacological sEH inhibition in animal studies to date suggests a unique opportunity to explore new anti-inflammatory therapeutics in psychiatry that address both the symptoms of the disorders and their long-term sequelae.
Figure 3.
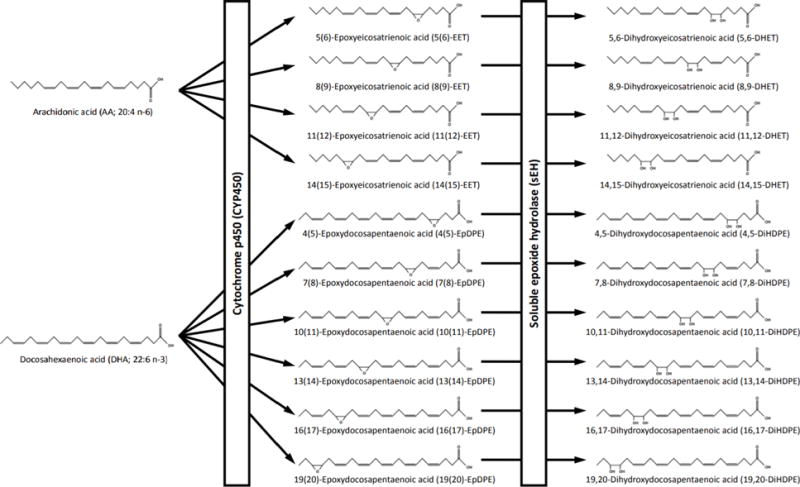
Biotransformation of arachidonic acid and docosahexaenoic acid into epoxides by cytochrome p450s, and subsequent biotransformation to corresponding diols by sEH.
Highlights.
Soluble epoxide hydrolase (sEH) metabolizes anti-inflammatory lipids
sEH activation may be a link between mood disorders and inflammation
sEH inhibitors are under development for vascular, nerve and metabolic disorders
sEH-dervied oxylipins are elevated in the brain and blood in mood disorders
inhibiting sEH mitigated the development of depressive-like behaviors in rats
inhibiting sEH may protect against inflammatory comorbidities in psychiatry
Acknowledgments
Sources of support: Diabetic Complications Consortium 16GRU3711, Alzheimer’s Association & Brain Canada AARG501466, NSERC RGPIN-2017-0692 and Canadian Partnership for Stroke Recovery, and Centre for Collaborative Drug Discovery (WS); AMED (KH); NIEHS/R01 ES002710 (BDH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdu E, Bruun DA, Yang D, Yang J, Inceoglu B, Hammock BD, Alkayed NJ, Lein PJ. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2011;117:632–642. doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Flicker L, Yeap BB, Alfonso H, McCaul K, Hankey GJ. Aspirin decreases the risk of depression in older men with high plasma homocysteine. Transl Psychiatry. 2012;2:e151. doi: 10.1038/tp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G. Linking the biological underpinnings of depression: Role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2017 doi: 10.1016/j.pnpbp.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, Peyser CE, Pearlson GD. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151:687–693. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yucel M, Gama CS, Dodd S, Dean B, Magalhaes PV, Amminger P, McGorry P, Malhi GS. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience and biobehavioral reviews. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer JL, Young R, Kuchibhatla M, Krishnan KR. Hyperintense MRI lesions in bipolar disorder: A meta-analysis and review. Int Rev Psychiatry. 2009;21:394–409. doi: 10.1080/09540260902962198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau M, Bacon SL, Ouellet K, Jacob A, Lavoie KL. Mediator effect of depressive symptoms on the association between BMI and asthma control in adults. Chest. 2014;146:348–354. doi: 10.1378/chest.13-1796. [DOI] [PubMed] [Google Scholar]
- Bown C, Wang JF, MacQueen G, Young LT. Increased temporal cortex ER stress proteins in depressed subjects who died by suicide. Neuropsychopharmacology. 2000;22:327–332. doi: 10.1016/S0893-133X(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nunez G, He Y, Yin XM, O’Riordan MX. Endoplasmic Reticulum Stress Activates the Inflammasome via NLRP3- and Caspase-2-Driven Mitochondrial Damage. Immunity. 2015;43:451–462. doi: 10.1016/j.immuni.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese JR, Skwerer RG, Barna B, Gulledge AD, Valenzuela R, Butkus A, Subichin S, Krupp NE. Depression, immunocompetence, and prostaglandins of the E series. Psychiatry research. 1986;17:41–47. doi: 10.1016/0165-1781(86)90040-5. [DOI] [PubMed] [Google Scholar]
- Calkin C, van de Velde C, Ruzickova M, Slaney C, Garnham J, Hajek T, O’Donovan C, Alda M. Can body mass index help predict outcome in patients with bipolar disorder? Bipolar disorders. 2009;11:650–656. doi: 10.1111/j.1399-5618.2009.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin CV, Ruzickova M, Uher R, Hajek T, Slaney CM, Garnham JS, O’Donovan MC, Alda M. Insulin resistance and outcome in bipolar disorder. Br J Psychiatry. 2015;206:52–57. doi: 10.1192/bjp.bp.114.152850. [DOI] [PubMed] [Google Scholar]
- Capozzi ME, Hammer SS, McCollum GW, Penn JS. Epoxygenated Fatty Acids Inhibit Retinal Vascular Inflammation. Sci Rep. 2016;6:39211. doi: 10.1038/srep39211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J, Swardfager W. Mood and metabolism: Anhedonia as a clinical target in Type 2 diabetes. Psychoneuroendocrinology. 2016;69:123–132. doi: 10.1016/j.psyneuen.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Chadderdon SM, Belcik JT, Bader L, Kievit P, Grove KL, Lindner JR. Vasoconstrictor eicosanoids and impaired microvascular function in inactive and insulin-resistant primates. International journal of obesity. 2016;40:1600–1603. doi: 10.1038/ijo.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles RL, Burgoyne JR, Mayr M, Weldon SM, Hubner N, Dong H, Morisseau C, Hammock BD, Landar A, Eaton P. Redox regulation of soluble epoxide hydrolase by 15-deoxy-delta-prostaglandin J2 controls coronary hypoxic vasodilation. Circ Res. 2011;108:324–334. doi: 10.1161/CIRCRESAHA.110.235879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, Morisseau C, Hammock BD, Freeman BA, Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2014;111:8167–8172. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell WH, Butcher BD, Burns TL, Dindo LN, Schlechte JA, Calarge CA. Fat distribution and major depressive disorder in late adolescence. J Clin Psychiatry. 2016;77:84–89. doi: 10.4088/JCP.14m09169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo LGP, Nunes SOV, Anderson G, Vargas HO, Barbosa DS, Galecki P, Carvalho AF, Maes M. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2017 doi: 10.1016/j.pnpbp.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Ding Y, Fromel T, Popp R, Falck JR, Schunck WH, Fleming I. The biological actions of 11,12-epoxyeicosatrienoic acid in endothelial cells are specific to the R/S-enantiomer and require the G(s) protein. The Journal of pharmacology and experimental therapeutics. 2014;350:14–21. doi: 10.1124/jpet.114.214254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46:842–848. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A Meta-Analysis of Cytokines in Major Depression. Biological psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Duivis HE, de Jonge P, Penninx BW, Na BY, Cohen BE, Whooley MA. Depressive Symptoms, Health Behaviors, and Subsequent Inflammation in Patients With Coronary Heart Disease: Prospective Findings From the Heart and Soul Study. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.10081163. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Keltikangas-Jarvinen L, Kivimaki M, Pulkki L, Puttonen S, Heponiemi T, Juonala M, Viikari JS, Raitakari OT. Depressive symptoms and carotid artery intima-media thickness in young adults: the Cardiovascular Risk in Young Finns Study. Psychosom Med. 2005;67:561–567. doi: 10.1097/01.psy.0000170340.74035.23. [DOI] [PubMed] [Google Scholar]
- Fava C, Montagnana M, Danese E, Almgren P, Hedblad B, Engstrom G, Berglund G, Minuz P, Melander O. Homozygosity for the EPHX2 K55R polymorphism increases the long-term risk of ischemic stroke in men: a study in Swedes. Pharmacogenetics and genomics. 2010;20:94–103. doi: 10.1097/FPC.0b013e3283349ec9. [DOI] [PubMed] [Google Scholar]
- Fer M, Dreano Y, Lucas D, Corcos L, Salaun JP, Berthou F, Amet Y. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471:116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG, Andersen LE, Persons JE, Calarge C. Rapid adipose deposition with mood disorders. Ann Clin Psychiatry. 2015;27:283–288. [PMC free article] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gold PW, Licinio J, Pavlatou MG. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-gamma systems. Mol Psychiatry. 2013;18:154–165. doi: 10.1038/mp.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, Stoney CM, Wasiak H, McCrindle BW, American Heart Association, A., Hypertension, Obesity in Youth Committee of the Council on Cardiovascular Disease in the, Y. Major Depressive Disorder and Bipolar Disorder Predispose Youth to Accelerated Atherosclerosis and Early Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132:965–986. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- Gschwendtner A, Ripke S, Freilinger T, Lichtner P, Muller-Myhsok B, Wichmann HE, Meitinger T, Dichgans M. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with an increased risk of ischemic stroke in white Europeans. Stroke. 2008;39:1593–1596. doi: 10.1161/STROKEAHA.107.502179. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Soluble epoxide hydrolase: a new therapeutic target for depression. Expert opinion on therapeutic targets. 2016;20:1149–1151. doi: 10.1080/14728222.2016.1226284. [DOI] [PubMed] [Google Scholar]
- Hatch JK, Scola G, Olowoyeye O, Collins JE, Andreazza AC, Moody A, Levitt AJ, Strauss BH, Lanctot KL, Goldstein BI. Inflammatory Markers and Brain-Derived Neurotrophic Factor as Potential Bridges Linking Bipolar Disorder and Cardiovascular Risk Among Adolescents. J Clin Psychiatry. 2017;78:e286–e293. doi: 10.4088/JCP.16m10762. [DOI] [PubMed] [Google Scholar]
- Hennebelle M, Otoki Y, Yang J, Hammock BD, Levitt AJ, Taha AY, Swardfager W. Altered soluble epoxide hydrolase-derived oxylipins in patients with seasonal major depression: An exploratory study. Psychiatry research. 2017;252:94–101. doi: 10.1016/j.psychres.2017.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hung YW, Hung SW, Wu YC, Wong LK, Lai MT, Shih YH, Lee TS, Lin YY. Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy. Brain Behav Immun. 2015;43:118–129. doi: 10.1016/j.bbi.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Alkayed NJ. Soluble Epoxide Hydrolase Inhibition: Targeting Multiple Mechanisms of Ischemic Brain Injury with a Single Agent. Future Neurol. 2009;4:179–199. doi: 10.2217/14796708.4.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, Hammock BD. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci U S A. 2015;112:9082–9087. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life sciences. 2006;79:2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, Hackett E, Hwang SH, Lee KS, Rogawski MA, Morisseau C, Hammock BD. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS One. 2013;8:e80922. doi: 10.1371/journal.pone.0080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham RH, Gless RD, Lo HY. Soluble epoxide hydrolase inhibitors and their potential for treatment of multiple pathologic conditions. Curr Med Chem. 2011;18:587–603. doi: 10.2174/092986711794480212. [DOI] [PubMed] [Google Scholar]
- Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, Brown L. Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp Diabetes Res. 2012;2012:758614. doi: 10.1155/2012/758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, Castle D, Dash S, Mihalopoulos C, Chatterton ML, Brazionis L, Dean OM, Hodge AM, Berk M. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial) BMC Med. 2017;15:23. doi: 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W, Pedersen HS, Ribe AR, Fenger-Gron M, Davydow D, Waldorff FB, Vestergaard M. Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA psychiatry. 2015;72:612–619. doi: 10.1001/jamapsychiatry.2015.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodani SD, Hammock BD. The 2014 Bernard B. Brodie award lecture-epoxide hydrolases: drug metabolism to therapeutics for chronic pain. Drug Metab Dispos. 2015;43:788–802. doi: 10.1124/dmd.115.063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci. 2007;27:4642–4649. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta psychiatrica Scandinavica. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Kompa AR, Wang BH, Xu G, Zhang Y, Ho PY, Eisennagel S, Thalji RK, Marino JP, Jr, Kelly DJ, Behm DJ, Krum H. Soluble epoxide hydrolase inhibition exerts beneficial anti-remodeling actions post-myocardial infarction. Int J Cardiol. 2013;167:210–219. doi: 10.1016/j.ijcard.2011.12.062. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H. Fish oil and omega-3 fatty acids in cardiovascular disease: do they really work? Eur Heart J. 2012;33:436–443. doi: 10.1093/eurheartj/ehr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie KL, Bacon SL, Barone S, Cartier A, Ditto B, Labrecque M. What is worse for asthma control and quality of life: depressive disorders, anxiety disorders, or both? Chest. 2006;130:1039–1047. doi: 10.1378/chest.130.4.1039. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Zheng L, Weiner MW, Mungas D, Reed B, Kramer JH, Jagust W, Chui H, Mack WJ. The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry. 2008;23:1040–1050. doi: 10.1002/gps.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Lu T, Weintraub NL, VanRollins M, Spector AA, Shibata EF. Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes. J Physiol. 1999;519(Pt 1):153–168. doi: 10.1111/j.1469-7793.1999.0153o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu Y, Zhang X, Lv H, Pang W, Sun X, Gan LM, Hammock BD, Ai D, Zhu Y. Optimized inhibitors of soluble epoxide hydrolase improve in vitro target residence time and in vivo efficacy. J Med Chem. 2014;57:7016–7030. doi: 10.1021/jm500694p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu Y, Zhang X, Lv H, Pang W, Sun X, Gan LM, Hammock BD, Ai D, Zhu Y. Inhibition of soluble epoxide hydrolase alleviated atherosclerosis by reducing monocyte infiltration in Ldlr(−/−) mice. J Mol Cell Cardiol. 2016;98:128–137. doi: 10.1016/j.yjmcc.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Qian ZY, Xie F, Fan W, Nelson JW, Xiao X, Kaul S, Barnes AP, Alkayed NJ. Functional screening for G protein-coupled receptor targets of 14,15-epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat. 2016 doi: 10.1016/j.prostaglandins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, Claria J. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci U S A. 2015;112:536–541. doi: 10.1073/pnas.1422590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci U S A. 2011;108:9038–9043. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther JM, Brown NJ. Epoxyeicosatrienoic acids and glucose homeostasis in mice and men. Prostaglandins Other Lipid Mediat. 2016;125:2–7. doi: 10.1016/j.prostaglandins.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Lee HK, Jung JH, Noam GG, Renshaw PF. White matter hyperintensities on magnetic resonance imaging of the brain in children with psychiatric disorders. Comprehensive psychiatry. 2002;43:361–368. doi: 10.1053/comp.2002.34636. [DOI] [PubMed] [Google Scholar]
- Ma M, Ren Q, Fujita Y, Ishima T, Zhang JC, Hashimoto K. Effects of AS2586114, a soluble epoxide hydrolase inhibitor, on hyperlocomotion and prepulse inhibition deficits in mice after administration of phencyclidine. Pharmacol Biochem Behav. 2013;110:98–103. doi: 10.1016/j.pbb.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Meyer I, Erismann-Ebner K, Pellegrini G, Mule N, Arand M. Beyond detoxification: a role for mouse mEH in the hepatic metabolism of endogenous lipids. Archives of toxicology. 2017;91:3571–3585. doi: 10.1007/s00204-017-2060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereeuw G, Herrmann N, Andreazza AC, Scola G, Ma DW, Oh PI, Lanctot KL. Oxidative stress predicts depressive symptom changes with omega-3 fatty acid treatment in coronary artery disease patients. Brain Behav Immun. 2017;60:136–141. doi: 10.1016/j.bbi.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Mazereeuw G, Herrmann N, Bennett SA, Swardfager W, Xu H, Valenzuela N, Fai S, Lanctot KL. Platelet activating factors in depression and coronary artery disease: a potential biomarker related to inflammatory mechanisms and neurodegeneration. Neuroscience and biobehavioral reviews. 2013;37:1611–1621. doi: 10.1016/j.neubiorev.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Mazereeuw G, Herrmann N, Xu H, Blanchard AP, Figeys D, Oh PI, Bennett SA, Lanctot KL. Platelet activating factors are associated with depressive symptoms in coronary artery disease patients: a hypothesis-generating study. Neuropsychiatr Dis Treat. 2015;11:2309–2314. doi: 10.2147/NDT.S87111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereeuw G, Herrmann N, Xu H, Figeys D, Oh PI, Bennett SA, Lanctot KL. Platelet-activating factors are associated with cognitive deficits in depressed coronary artery disease patients: a hypothesis-generating study. Journal of neuroinflammation. 2014;11:119. doi: 10.1186/1742-2094-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. International Clinical Psychopharmacology. 2006;21:227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, Dubois B, Geyer MA, Goodwin GM, Gorwood P, Jay TM, Joels M, Mansuy IM, Meyer-Lindenberg A, Murphy D, Rolls E, Saletu B, Spedding M, Sweeney J, Whittington M, Young LJ. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhe HG, Schene AH. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry. 2016;6:e756. doi: 10.1038/tp.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gosele C, Heuser A, Fischer R, Schmidt C, Schirdewan A, Gross V, Hummel O, Maatz H, Patone G, Saar K, Vingron M, Weldon SM, Lindpaintner K, Hammock BD, Rohde K, Dietz R, Cook SA, Schunck WH, Luft FC, Hubner N. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet. 2008;40:529–537. doi: 10.1038/ng.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C, Sirois M, Echave V, Albadine R, Rousseau E. 17,18-epoxyeicosatetraenoic acid targets PPARgamma and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. American journal of respiratory cell and molecular biology. 2010;43:564–575. doi: 10.1165/rcmb.2009-0155OC. [DOI] [PubMed] [Google Scholar]
- Morin C, Sirois M, Echave V, Gomes MM, Rousseau E. EET displays anti-inflammatory effects in TNF-alpha stimulated human bronchi: putative role of CPI-17. American journal of respiratory cell and molecular biology. 2008;38:192–201. doi: 10.1165/rcmb.2007-0232OC. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. Journal of lipid research. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA. 1999;96:8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiberg MR, Hatch JK, Selkirk B, Fiksenbaum L, Yang V, Black S, Kertes PJ, Goldstein BI. Retinal photography: A window into the cardiovascular-brain link in adolescent bipolar disorder. J Affect Disord. 2017;218:227–237. doi: 10.1016/j.jad.2017.04.066. [DOI] [PubMed] [Google Scholar]
- Naiberg MR, Newton DF, Collins JE, Dickstein DP, Bowie CR, Goldstein BI. Elevated triglycerides are associated with decreased executive function among adolescents with bipolar disorder. Acta psychiatrica Scandinavica. 2016;134:241–248. doi: 10.1111/acps.12603. [DOI] [PubMed] [Google Scholar]
- Nefs G, Pop VJ, Denollet J, Pouwer F. Depressive symptoms and all-cause mortality in people with type 2 diabetes: a focus on potential mechanisms. Br J Psychiatry. 2016 doi: 10.1192/bjp.bp.114.154781. [DOI] [PubMed] [Google Scholar]
- Nelson JW, Young JM, Borkar RN, Woltjer RL, Quinn JF, Silbert LC, Grafe MR, Alkayed NJ. Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins Other Lipid Mediat. 2014;113–115:30–37. doi: 10.1016/j.prostaglandins.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevell L, Zhang K, Aiello AE, Koenen K, Galea S, Soliven R, Zhang C, Wildman DE, Uddin M. Elevated systemic expression of ER stress related genes is associated with stress-related mental disorders in the Detroit Neighborhood Health Study. Psychoneuroendocrinology. 2014;43:62–70. doi: 10.1016/j.psyneuen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ueno R, Ohishi K, Sakai T, Hayaishi O. Salivary prostaglandin concentrations: possible state indicators for major depression. Am J Psychiatry. 1989;146:365–368. doi: 10.1176/ajp.146.3.365. [DOI] [PubMed] [Google Scholar]
- Noble JE, Wang L, Cerasoli E, Knight AE, Porter RA, Gray E, Howe C, Hannes E, Corbisier P, Wang J, Wu L, Altieri I, Patriarca M, Hoffman A, Resch-Genger U, Ebert B, Voigt J, Shigeri Y, Vonsky MS, Konopelko LA, Gaigalas AK, Bailey MJ. An international comparability study to determine the sources of uncertainty associated with a non-competitive sandwich fluorescent ELISA. Clin Chem Lab Med. 2008;46:1033–1045. doi: 10.1515/CCLM.2008.182. [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi K, Ueno R, Nishino S, Sakai T, Hayaishi O. Increased level of salivary prostaglandins in patients with major depression. Biological psychiatry. 1988;23:326–334. doi: 10.1016/0006-3223(88)90283-1. [DOI] [PubMed] [Google Scholar]
- Ono E, Dutile S, Kazani S, Wechsler ME, Yang J, Hammock BD, Douda DN, Tabet Y, Khaddaj-Mallat R, Sirois M, Sirois C, Rizcallah E, Rousseau E, Martin R, Sutherland ER, Castro M, Jarjour NN, Israel E, Levy BD, National Heart, L., Blood Institute’s Asthma Clinical Research, N. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med. 2014;190:886–897. doi: 10.1164/rccm.201403-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otoki Y, Hennebelle M, Levitt AJ, Nakagawa K, Swardfager W, Taha AY. Plasma Phosphatidylethanolamine and Triacylglycerol Fatty Acid Concentrations are Altered in Major Depressive Disorder Patients with Seasonal Pattern. Lipids. 2017 doi: 10.1007/s11745-017-4254-1. [DOI] [PubMed] [Google Scholar]
- Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu YY, Yuan MY, Liu Y, Xiao XJ, Zhu YL. The protective effect of epoxyeicosatrienoic acids on cerebral ischemia/reperfusion injury is associated with PI3K/Akt pathway and ATP-sensitive potassium channels. Neurochemical research. 2015;40:1–14. doi: 10.1007/s11064-014-1456-2. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Ma M, Ishima T, Morisseau C, Yang J, Wagner KM, Zhang JC, Yang C, Yao W, Dong C, Han M, Hammock BD, Hashimoto K. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci U S A. 2016;113:E1944–1952. doi: 10.1073/pnas.1601532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39:491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- Robertson RC, Seira Oriach C, Murphy K, Moloney GM, Cryan JF, Dinan TG, Paul Ross R, Stanton C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145. [DOI] [PubMed] [Google Scholar]
- Roche C, Besnier M, Cassel R, Harouki N, Coquerel D, Guerrot D, Nicol L, Loizon E, Remy-Jouet I, Morisseau C, Mulder P, Ouvrard-Pascaud A, Madec AM, Richard V, Bellien J. Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice. Am J Physiol Heart Circ Physiol. 2015;308:H1020–1029. doi: 10.1152/ajpheart.00465.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, Hammock BD. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013a;74:31–37. doi: 10.4088/JCP.12r07922. [DOI] [PubMed] [Google Scholar]
- Rotella F, Mannucci E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res Clin Pract. 2013b;99:98–104. doi: 10.1016/j.diabres.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am J Psychiatry. 2016;173:575–587. doi: 10.1176/appi.ajp.2016.15091228. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Hammock BD. Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem. 2012;55:1789–1808. doi: 10.1021/jm201468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Peng H, Peng R, Fan Q, Zhao S, Xu D, Morisseau C, Chiamvimonvat N, Hammock BD. Inhibition of soluble epoxide hydrolase in mice promotes reverse cholesterol transport and regression of atherosclerosis. Atherosclerosis. 2015;239:557–565. doi: 10.1016/j.atherosclerosis.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih PB, Yang J, Morisseau C, German JB, Zeeland AA, Armando AM, Quehenberger O, Bergen AW, Magistretti P, Berrettini W, Halmi KA, Schork N, Hammock BD, Kaye W. Dysregulation of soluble epoxide hydrolase and lipidomic profiles in anorexia nervosa. Mol Psychiatry. 2016;21:537–546. doi: 10.1038/mp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174:2086–2095. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT. Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci. 2014;8:315. doi: 10.3389/fnins.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Spector AA, Kim HY. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta. 2015;1851:356–365. doi: 10.1016/j.bbalip.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulc J. Extracellular transport pathways in the haemochorial placenta. Placenta. 1989;10:113–119. doi: 10.1016/0143-4004(89)90012-x. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Herrmann N, Dowlati Y, Oh PI, Kiss A, Walker SE, Lanctot KL. Indoleamine 2,3-dioxygenase activation and depressive symptoms in patients with coronary artery disease. Psychoneuroendocrinology. 2009;34:1560–1566. doi: 10.1016/j.psyneuen.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Herrmann N, Marzolini S, Saleem M, Farber SB, Kiss A, Oh PI, Lanctot KL. Major depressive disorder predicts completion, adherence, and outcomes in cardiac rehabilitation: a prospective cohort study of 195 patients with coronary artery disease. J Clin Psychiatry. 2010 doi: 10.4088/JCP.09m05810blu. [DOI] [PubMed] [Google Scholar]
- Swardfager W, MacIntosh BJ. Depression, Type 2 Diabetes, and Poststroke Cognitive Impairment. Neurorehabilitation and neural repair. 2017;31:48–55. doi: 10.1177/1545968316656054. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS. Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neuroscience and biobehavioral reviews. 2016;64:148–166. doi: 10.1016/j.neubiorev.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Taguchi N, Nakayama S, Tanaka M. Single administration of soluble epoxide hydrolase inhibitor suppresses neuroinflammation and improves neuronal damage after cardiac arrest in mice. Neurosci Res. 2016;111:56–63. doi: 10.1016/j.neures.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Timberlake MA, 2nd, Dwivedi Y. Altered Expression of Endoplasmic Reticulum Stress Associated Genes in Hippocampus of Learned Helpless Rats: Relevance to Depression Pathophysiology. Frontiers in pharmacology. 2015;6:319. doi: 10.3389/fphar.2015.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, Weiss RH. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–323. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- Vanella L, Canestraro M, Lee CR, Cao J, Zeldin DC, Schwartzman ML, Abraham NG. Soluble epoxide hydrolase null mice exhibit female and male differences in regulation of vascular homeostasis. Prostaglandins Other Lipid Mediat. 2015;120:139–147. doi: 10.1016/j.prostaglandins.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Andreazza AC, Young LT, Fournier JC, Almeida JR, Stiffler RS, Lockovich JC, Aslam HA, Pollock MH, Park H, Nimgaonkar VL, Kupfer DJ, Phillips ML. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry. 2013 Jan;29 doi: 10.1038/mp.2012.188. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vito ST, Austin AT, Banks CN, Inceoglu B, Bruun DA, Zolkowska D, Tancredi DJ, Rogawski MA, Hammock BD, Lein PJ. Post-exposure administration of diazepam combined with soluble epoxide hydrolase inhibition stops seizures and modulates neuroinflammation in a murine model of acute TETS intoxication. Toxicol Appl Pharmacol. 2014;281:185–194. doi: 10.1016/j.taap.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, Smyth EM, Wilson SJ, FitzGerald GA, Garavito RM, Sui de X, Regan JW, Smith WL. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- Wagner K, Gilda J, Yang J, Wan D, Morisseau C, Gomes AV, Hammock BD. Soluble epoxide hydrolase inhibition alleviates neuropathy in Akita (Ins2 Akita) mice. Behav Brain Res. 2017;326:69–76. doi: 10.1016/j.bbr.2017.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Dong H, Yang J, Hwang SH, Jones P, Morisseau C, Hammock BD. Comparative efficacy of 3 soluble epoxide hydrolase inhibitors in rat neuropathic and inflammatory pain models. Eur J Pharmacol. 2013;700:93–101. doi: 10.1016/j.ejphar.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Gill SS, Hammock BD. Epoxygenated fatty acids and soluble epoxide hydrolase inhibition: novel mediators of pain reduction. J Agric Food Chem. 2011;59:2816–2824. doi: 10.1021/jf102559q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Yang J, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. J Pain. 2014;15:907–914. doi: 10.1016/j.jpain.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Opland D, Tsai S, Luk CT, Schroer SA, Allison MB, Elia AJ, Furlonger C, Suzuki A, Paige CJ, Mak TW, Winer DA, Myers MG, Jr, Woo M. Pten deletion in RIP-Cre neurons protects against type 2 diabetes by activating the anti-inflammatory reflex. Nat Med. 2014;20:484–492. doi: 10.1038/nm.3527. [DOI] [PubMed] [Google Scholar]
- Wang Q, Huo L, He J, Ding W, Su H, Tian D, Welch C, Hammock BD, Ai D, Zhu Y. Soluble epoxide hydrolase is involved in the development of atherosclerosis and arterial neointima formation by regulating smooth muscle cell migration. Am J Physiol Heart Circ Physiol. 2015;309:H1894–1903. doi: 10.1152/ajpheart.00289.2015. [DOI] [PubMed] [Google Scholar]
- Winer DA, Winer S, Chng MH, Shen L, Engleman EG. B Lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell Mol Life Sci. 2014;71:1033–1043. doi: 10.1007/s00018-013-1486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HF, Yen HJ, Huang CC, Lee YC, Wu SZ, Lee TS, Lin HC. Soluble epoxide hydrolase inhibitor enhances synaptic neurotransmission and plasticity in mouse prefrontal cortex. Journal of biomedical science. 2015;22:94. doi: 10.1186/s12929-015-0202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DY, Davis BB, Wang ZH, Zhao SP, Wasti B, Liu ZL, Li N, Morisseau C, Chiamvimonvat N, Hammock BD. A potent soluble epoxide hydrolase inhibitor, t-AUCB, acts through PPARgamma to modulate the function of endothelial progenitor cells from patients with acute myocardial infarction. Int J Cardiol. 2013;167:1298–1304. doi: 10.1016/j.ijcard.2012.03.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ueda N, Ehara H, Maruyama T, Yokoyama C, Kaneko S, Yoshimoto T, Komatsu N, Watanabe K, Hattori A, et al. Biochemical studies on mammalian lipoxygenases. Ann N Y Acad Sci. 1988;524:12–26. doi: 10.1111/j.1749-6632.1988.tb38527.x. [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu J, Dong R, Zhu J, Tao C, Zheng R, Zhu S. 14,15-epoxyeicosatrienoic acid promotes production of brain derived neurotrophic factor from astrocytes and exerts neuroprotective effects during ischaemic injury. Neuropathol Appl Neurobiol. 2016;42:607–620. doi: 10.1111/nan.12291. [DOI] [PubMed] [Google Scholar]
- Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuniga O, Delgado-Lista J, Gutierrez-Mariscal FM, Perez-Martinez P, Delgado-Casado N, Cruz-Teno C, Tinahones FJ, Villalba JM, Perez-Jimenez F, Lopez-Miranda J. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67:3–10. doi: 10.1093/gerona/glr167. [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem. 1993;268:6402–6407. [PubMed] [Google Scholar]
- Zeldin DC, Wei S, Falck JR, Hammock BD, Snapper JR, Capdevila JH. Metabolism of epoxyeicosatrienoic acids by cytosolic epoxide hydrolase: substrate structural determinants of asymmetric catalysis. Arch Biochem Biophys. 1995;316:443–451. doi: 10.1006/abbi.1995.1059. [DOI] [PubMed] [Google Scholar]
- Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan SK, Gless R, Webb HK, Wang YX. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol. 2009;29:1265–1270. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga KL, Krasnow SM, Zhu X, Zhang W, Jouihan SA, Shangraw RE, Alkayed NJ, Marks DL. Mechanism of protection by soluble epoxide hydrolase inhibition in type 2 diabetic stroke. PLoS One. 2014;9:e97529. doi: 10.1371/journal.pone.0097529. [DOI] [PMC free article] [PubMed] [Google Scholar]


